Abstract
Innovation is important for the healthcare system advancement, in order to continue delivering the high-quality care at an affordable cost to the society. It can be achieved by nonconventional thinking, tapping into creative minds, and extensive collaborative work to make better use of existing facilities and designing new technologies. Nanorobotics is such an innovation that can revolutionize the current face of medicine and biomedical sciences with their state-of-the-art technology. Improved outcomes of nanorobots-based treatments for diabetes, drug delivery for pancreatic and ovarian cancer, and laparoscopic treatment of skin cancer have already been reported. This book chapter will cover the recent advancements of this emerging field with their biomedical applications.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
12.1 Introduction of Nanorobotics: Magic Bullets of Future
Innovation is important for the advancement of medicine and healthcare segment in order to continue delivering the high-quality care at an affordable cost to the society. It can be achieved by unconventional thinking, tapping into creative minds, and extensive collaborative work to make better use of existing facilities and designing new technologies. Nanorobotics is such an innovation that can revolutionize the current face of drug delivery systems and biomedical sciences with its state-of-the-art technology. Nanorobots are futuristic concept, and by definition, nanorobots are electromechanical systems in size range of 10−9 nanometers and are functionally capable of carrying out humanly impossible tasks with precision at nanoscale dimensions. Typically miniaturized computers, onboard sensing devices, motors, power supplies, and manipulators are basic component of a nanorobot. The construction of nanorobots is a complex engineering process and still in its infancy, but with the emerging new technologies and major research going on in this area, soon nanorobots will be a significant reality with their real-world applications, and they will be introduced in every field of industry, especially in the medical industry. Improved outcomes of nanorobots based drug delivery for ovarian and pancreatic cancer have already been reported. This book chapter will cover the recent advancements in this emerging field with emphasis on their applications in cancer therapy. Although this concept is in its infancy but the technology of nanorobotics is on the right direction and healthcare and medical sectors will be the immediate beneficiary. The promising future applications of robotic science are attracting government interest in many countries (Reppesgaard 2002; Wieland III 2004; Cavalcanti et al. 2004). Recently the US National Science Foundation has launched a program on “Scientific Visualization” on how nanorobotics can be portrayed by supercomputers. In 2015 itself, nanosystems and embedded nanodevices had a market projection of 1 trillion US$, while global market of nanobots has CAGR of 21% and is expected to reach US$ 100 billion in 2023 from US$ 74 billion in 2016. IBM, PARC, Hewlett Packard, Bell Laboratories, and Intel Corp. are few companies involved in nanoelectronics fabrication. With technological advancement in molecular computing, processing of logic task is possible by nano-bio-computers (Adleman 1995; Hagiya 2000), which is a promising first step to enable future nanoprocessors with increased complexity. Nano-kinetic devices and miniaturized sensing devices are the stepping stone for nanorobotics operation and locomotion (Sun et al. 2001; Stracke et al. 2000).
Apparently, nanorobotics sounds a new word, but in the early 1900s, German Nobel laureate Paul Ehrlich laid the foundation of this field by virtually describing an in vivo missile kind of therapy module that would deliver the drug at specific site to cure the diseases. Ehrlich named this concept as “Magische Kugel,” meaning “Magic Bullet,” which would have numerous applications in treatment of diseases, including cancer. In those times, it was just hypothetical, but with the onset of nanotechnology advancements, we now have commercial magic bullets or nano-based multifunctional targeted drug delivery systems to perform the task described by Dr. Ehrlich.
Nanotechnology advancements are the major driving force behind the fast pace of nanorobotics. Although it’s a highly interdisciplinary field, but better understanding of nanofabrication and emergence of nano electronics and NEMS have enabled scientists to develop different nanodevices. Currently this field is in its infancy, but several substantial steps have been taken by great researchers all over the world who are contributing to this ever challenging and exciting field. The ability to manipulate matter at the nanoscale is one core application for which nanorobots could be the technological solution (Ummat et al. 2005a, b).
Although in many references, different nanoparticles were also included while describing nanorobotics task in different fields involving defense, medical, and environmental applications, in this chapter we only focused on basics related with emergence of nanorobots and their possible medical applications as nanorobots are better substitutes of nanoparticles in performing cell repair, programmed drug release, and fighting infections in vivo. Apparently these nanodevices will build a better future with their enormous applications in medicine and biomedical engineering.
12.1.1 Origin of Nanorobots: From Fiction to Reality
The concept of nanorobots is a technological breakthrough that would evolve new therapy modules and many in vivo applications that are beyond imagination in current medical scenario. Science fiction movies and novels have already talked about such devices long back in 1966, where in a movie “The Fantastic Voyage,” a submarine was shrunk up to the limit to be injected into bloodstream for repair tasks. Although it was pure imagination, with the immense technological advancement in material science, MEMS, microfabrication, and biomolecular science, researchers are working to materialize this important aspect of device fabrication from micro to nano level that will be a boon for medical sciences to send minuscule nanorobots inside the human body for repair tasks. The concept of bio-nanorobotics is now evolved as micro devices in surgery and medical treatments are frequently used and improving clinical procedures in recent years (Murphy et al. 2007; Cavalcanti et al. 2008a, b, c). Catheterization is one such successful biomedical device application for heart and intracranial surgery (Roue 2002; Ikeda et al. 2005; Fann et al. 2004).
Although technical advancements in this field have shown a promising picture of the future, the real-time applications are still far from reality. In recent years, many new ideas have come into existence. Nanorobotics is a multidisciplinary field and needs combined efforts of specialists from many fields of science and technology to work achieving the common target of nurturing this field to maturity. If we look a little back in time, Sir Richard Feynman in his famous lecture “There is plenty of room at the bottom” discussed about the possibility of creating tiny objects mimicking tasks of biological cells in vivo (Feynman 1996). This thought provoking speech of Dr. Feynman turned into reality two decades later, when Eric Drexler suggested that construction of nanodevices is possible from biological parts to inspect and repair the cells of a living human being. Today’s molecular machine systems and molecular manufacturing foundation were laid by Drexler’s book on Nanosystems: Molecular Machinery, Manufacturing, and Computation (Drexler 1992; Freitas Jr 2005a, b; Saxena et al. 2015).
Owing to its high precision, efficacy robustness, affordability, and rapid mode of action, molecular nanotechnology (MNT) or nanorobotics would enhance the limits of existing branches of science. With the advent of MNT, in future, direct in vivo surgery on individual human cells by deploying large numbers of microscopic medical nanorobots would be possible (Freitas Jr 2005a, b).
12.1.2 Nature: The Inspiration Behind Nanorobotics
Nanorobotics is an emerging interdisciplinary field based on nanotechnology and nanofabrication and governs the applications and control of nanodevices. This field is still in its infancy despite all hypes as any robotic device with all artificial components hasn’t been generated yet. “Nature” is the prime inspiration of designing nanorobots because natural processes are immensely optimized in terms of energy and material usage that inspires human quest of designing artificial in vitro and in vivo nanodevices to perform tasks equivalent to natural biological processes.
Protein and DNAs are the natural molecular machines of nature to accomplish various synthetic mechanisms and are deployed to execute several cellular tasks, from moving payload to catalyzing reactions and as cellular information carrier. With the scientific advancement, today we have a far better understanding of working principles and mechanisms that is the prime governing factor to use the natural machines (proteins and DNA), or creating artificial ones, using nature’s modules. An optimum assembly of bio or biosimilar component would form nanodevices having multiple degrees of freedom and ability to apply force and to interact with objects in the nanoscale world. By definition, “Nanorobots are ‘smart’ structures with the ability to perform actuation, sensing, signaling, information processing, intelligence, manipulation, and swarm behavior at nanoscale (10−9 m)” (Khulbe 2014). These devices would be an amalgamation of nature as well as man-made micromachines to generate motion, force, or signal and biological functions, i.e., payload delivery, cellular repair tasks, etc., in response to the specific physiochemical stimuli inside human or animal bodies (Ummat et al. 2005a, b).
Based on the specific need and requirement, nanodevices with individual or combinatorial properties (swarm intelligence and cooperative behavior) can be fabricated for actuation to perform desired tasks at the nanoscale. Basic characteristics for a nanorobot to function may include:
-
(i)
Swarm intelligence: decentralization and distributive intelligence
-
(ii)
Self-assembly and replication
-
(iii)
Information processing and programmability at nano level
-
(iv)
External interface to control and monitor nanorobotic tasks (Ummat et al. 2005b)
High efficiency and consistency is the main advantage of using nature’s machine components as proposed by Kinosita et al. (Kinosita et al. 2000). While as in conventional macromachines, forces and motions are generated to accomplish specific tasks, in similar fashion, bio-nanomachines can also be explored to deploy nano-objects to fabricate other nanomachines to perform tasks at cellular levels. Figure 12.1 represents the nature machines and their artificial counterpart and their functions.
12.1.3 Advantage of Nanorobots over Conventional Medical Techniques
Human civilization has done a remarkable progress in all walks of life, and technological advancements are leading us toward a whole new era of mesmerizing discoveries. We have done a significant progress in medical sciences, and new methods have been developed for providing a better understanding of the vitals as well as to aid diagnosis like CT scan, MRI, endoscopy, etc. But as old order changes yielding place of new, all technology inevitably has to be phased out sometime, and as our current medical advancements overcome the drawbacks of their predecessors, nanorobotics would also surpass many drawbacks of today’s medical technology including incisions that relate to painful surgical procedures, with better success rate of sophisticated surgeries with no harm to patients. With the advent of new technological era with robotics, IOT, AI, and machine learning impacting healthcare, conventional techniques of investigation and diagnosis would adapt with the new changes, and we have already seen robotic surgical procedures taking place globally. Basically a surgical robot is a self-powered, computer-controlled device that can be programmed to aid in the positioning and manipulation of surgical instruments, enabling the surgeon to carry out more complex tasks (Gomez 2004). These systems function as master-slave manipulators that work as remote extensions completely governed by the surgeon. Till date two master-slave systems have received approval by the US Food and Drug Administration (FDA): (i) da Vinci Surgical System (Intuitive Surgical, Mountain View, California) (http://www.intuitivesurgical.com/products/da_vinci.htm.; Ballantyne and Moll 2003) and (ii) the ZEUS system (Computer Motion, Goleta, California) (Marescaux and Rubino 2003). Apart from these macro robotic applications, miniaturized nanodevices would defend the body from inside owing to their biocompatibility and nature-inspired architecture. DNA origami-based applications are just an example of how nanorobots would change the healthcare arena. Owing to their tiny architecture and biocompatibility, in vivo applications of nanorobots would provide an upper edge in many aspects of their conventional healthcare practices, i.e., less recovery time, minimal pain, possibility to monitor the treatment regimen, and possibility of rapid self-action in case of emergency. Targeted payload delivery to the specific site for precise therapy and self-excretion and disintegration after completion of task, if required (Bhat 2014).
12.2 Nanorobots: Components and Functions
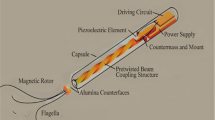
Nanorobotics is an innovative approach in the development of nature-inspired devices at nanometer scale to perform desired task inside the body and hence known as robots. In its present stage of infancy, nanorobotics is still a theoretical concept. Nanorobots architecture follows the techniques of molecular modeling based on “Energy Minimization” on the hyper surfaces of the bio-modules; “Hybrid Quantum-Mechanical” and “Molecular Mechanical method”; “Empirical Force field” methods; and “Maximum Entropy Production” in least time. Figure 12.2 is showing the circuit diagram of nanorobots. The basic components and design attributes of nanorobots to perform specific task are:
12.2.1 Size and Shape
The basic shape and size that would be less than the size of the blood vessel to traverse into it without damage or blocking the blood flow. A spherical ball like nanorobots can be the simplest structure without self-propulsion mechanisms that could be injected into the bloodstream to navigate their paths through the reservoir. Nano-bearings and nano-gears were the most convenient parts to be fabricated as reported by Drexler et al. He proposed an overlap-repulsion bearing design using both ball-and-stick and space-filling representations. His proposed bearing had 206 atoms of carbon, silicon, oxygen, and hydrogen, with a small shaft that rotates within a ring sleeve measuring 2.2 nm in diameter. Following Fig. 12.3 a, b shows design of both ball-and-stick and space-filling representations with end views and exploded views. In this model, atoms of the shaft were arranged in a 6-fold symmetry and the ring had a 14-fold symmetry to provide low energy barriers during shaft rotation (Drexler 1992; Freitas Jr 2005a, b).
12.2.2 Sensors and Actuators
Nanorobots should have the ability to sense temperature, pressure, fluid type and analysis, path, and position while performing the assigned task. In the past decade, scientists have successfully fabricated miniaturized silicon complementary metal oxide semiconductor (CMOS)-based motion sensor, but now to further downscale the process, nanowire-based CMOS devices have been fabricated, and this circuit assembly can achieve maximal efficiency for applications regarding chemical changes (Curtis et al. 2006; Balasubramanian et al. 2005; Zhang et al. 2004), lesser self-heating, and thermal coupling for CMOS functionality (Fung and Li 2004). Risveden et al. developed a region ion-selective bioelectronic nanosensor and observed that low energy consumption and high sensitivity are prime benefits of nano-sensors (Risveden et al. 2007). With the advancement of nanotechnology and nanofabrication, different versions of nano-enabled CMOS sensors are proposed based on carbon nanotubes and other nanomaterials, while researchers have developed new advanced manufacturing techniques, i.e., silicon-on-insulator (SOI) technology to assemble high-performance logic sub-90 nm circuits as described and patented by Park et al. (Park et al. 2005), while Bernstain et al. provided solution to deal with bipolar effect and hysteretic variations based on SOI structures (Bernstein et al. 2003). Although the groundbreaking 10 nm circuits are still far from reality, 45 nm of these nano CMOS ICs represent breakthrough technology devices that are currently being utilized in products (Cavalcanti et al. 2008a, b, Cavalcanti et al. 2007).
12.2.3 Power Generation
Power generation ability is another pillar for a nanorobot device to perform its assigned operations and tasks. At the nanoscale, low power in few pico-watts or micro watts would be needed. Potential means of generating power for the nanobots are:
-
(a)
Power from fluid flow or counter-current motion
-
(b)
Power derived from the reservoir temperature
-
(c)
Power from friction with rock fabrics
-
(d)
Downhole fuel cell generation from in-situation hydrocarbon
-
(e)
Use of downhole recharges station (Freitas 1999; Roundy et al. 2003; Liu et al. 2004)
Few previous reports provide possible applicable solutions to power generation based on CMOS as combination of CMOS with active telemetry and power supply is the most effective and secure way to ensure energy as long as necessary to keep the nanorobot in operation (Cavalcanti et al. 2003). Firstly, Mohseni et al. (2005) described the use of CMOS for active telemetry by developing implantable microelectrodes arrays for wireless in vivo brain signal recording of spontaneous neural activity at 96.2 MHz from the auditory cortex of a live marmoset monkey at numerous transmission distances ranging from 10 to 50 cm with signal-to-noise ratios in the range of 8.4–9.5 dB. Similarly, Sauer et al. developed a generic chip that can be used to power and interface with an implanted sensor. The chip had dual voltage regulators, used to supply both digital and analog sources. Both voltages were designed to be 3.3 V and nano-circuits with resonant electric properties can operate as a chip providing an electromagnetic energy supplying 1.7 mA, sufficient to operate many tasks with minimal losses during transmission (Sauer et al. 2005). Another possible way for power generation and controlling is radio frequency (RF)-based telemetry procedures as RF-based telemetry procedures have demonstrated good results in patient monitoring and power transmission through inductive coupling reported in many previous reports (Ghovanloo and Najafi 2004; Eggers et al. 2000a, b; Kermani et al. 2006).
RFID-based biomedical applications are already there, i.e., pacemaker, wearable devices and smart fabrics, etc. RF-based power generation would be an extension of this technology for miniaturized nanorobotics devices. In an estimate, ~1 μW of energy can be saved in resting state of nanorobots. Heating is the major drawback of RFID technique, and in vivo environment, only real applications would tell the efficacy of this widely explored technique, although uploading control software in mobile phones for power and data transmission could be an alternative as proposed by Ahuja and Myers (2006).
12.2.4 Propulsion, Control, and Navigation
Nanorobots are designed to perform in vivo operations, and depending on their nanometer dimensions, in the bloodstream their navigation is an important aspect to be considered. As nanorobots are the nature-inspired structures, their control mechanisms are also inspired by nature, and specifically to understand nanorobot motion in vivo, bacterial motions are well-studied for the design and construction of nanorobots. Nature has developed its own innovative mechanisms to overcome the motion-related hurdles in nano-sized environment, i.e., low inertia, high viscous forces, low efficiency, and low convective motion. It’s a fact that motion by beating of cilia and flagella at 30 μm/sec with 1% efficiency costs 2x10−8 erg/s in bacterial movement that shows the energy efficacy of the nature machines (Sharma and Mittal 2008; Purcell 1977). For bio organisms, swimming or flying is preferred over walking or crawling to overcome viscosity in nano-domains, while many cells are able to crawl on a solid substrate to which they stick using adhesive forces. Generally, three prime processes are responsible for microbial motion: (i) formation and protrusion of a thin lamellipod in front of the cell, (ii) adhesion of the lamellipod to the substrate, and (iii) its retraction at the rear, pulling the cell forward (Joanny et al. 2003). As described by Squires and Brady (2005), bacterial motion efficacy depends on the “Peclet number (Pe): ratio of time taken to cover a distance (l) at velocity (v) with a diffusion constant (D) and flagella,” and cilia could work only if Pe is greater than unity. While for nanorobots owing to their tiny sizes, it’s a difficult task to obtain same efficiency with same parameters and nanorobots are supposed to work in different environments, other propulsion mechanisms would also come into picture and “diffusion” may be a more effective choice in comparison to convective motion. Moreover in nano-domains, the nonrigid nature of nanorobots is also to be taken into account. The nonrigidity of nanomechanisms can be described by “bead-spring models” (Higdon 1979), “slenderness theory,” “Kirchhoff’s rod theory,” and a combination of “resistive force theory” (Kim and Powers 2004; Powers 2002). For nanobots of size less than 600 nm, diffusion (random walk) due to thermal agitation (Brownian motion) is prime mode of propulsion to move small objects in fluid at room temperature as reported first by Purcell in 1977. Later Feringa et al. provided a detailed account of generating controlled movement of objects. They developed a model based on two enantiomers of a bistable molecule that would function as the two distinct states in a molecular information storage system and designed a chiroptical molecular switches and light-driven unidirectional rotary motors. In another report, his group constructed molecular machinery or the incorporation of the light-driven motor into multifunctional systems (Feringa 2001; Feringa et al. 2002). Regarding control of propulsion, two possible mechanisms have been proposed.
12.2.4.1 External Control Mechanisms
As clear from title itself, application of external potential fields control the dynamics of the nanorobot in its work environment. MRI is the preferred choice of researchers as an external control mechanism for guiding the nano particles. Martel et al. have developed a robotic platform that uses magnetic resonance imaging (MRI) for sending information to a controller responsible for the real-time control and navigation of untethered magnetic carriers, nanorobots, and/or magnetotactic bacteria (MTB) having a wireless robotic arm, sensors, and therapeutic agents, toward preset paths in the blood vessels to perform specific remote tasks (Martel et al. 2009).
In another approach, Farahani et al. designed an “adaptive controller” to optimize the motion of nanorobots within the blood vessel. The simulation showed that the proposed control method, by identifying the functional characterization of nanorobots such as transport capability, biocompatibility, planning, receiving, and generating signals, had good performance and high efficiency in controlling the nanorobot motion toward the predetermined reference trajectory path by the signal received from the damaged area, with an error of about 0.01 μm (Farahani and Farahani 2016; Rao et al. 2014).
12.2.4.2 Internal Control Mechanisms
The internal control mechanism can be both passive and active. In passive control, nanorobots could be associated with biomolecules for biochemical sensing and selective binding of various biomolecules. These mechanisms are well explored in designing nano delivery systems. However, a static behavior without any change during the assigned task is the prime limitation of passive control. In this regard, active control mechanisms are better alternatives to control the nanorobots effectively in dynamic environment. Internal control modules can be the best choice in this regard, and fabrication of inbuilt molecular computers can solve the problem to program the nanorobots to act smartly based on the situations in vivo by varying their conduct. Molecular computers could be utilized to achieve this target. Leonard Adleman (from the University of Southern California) has introduced DNA computers a decade ago to solve a mathematical problem by utilizing DNA molecules. These DNA structures acted as switches, by changing their position and can be used to perform the logical operations that make computer calculations possible. Researchers at Harvard and Bar-Ilan University in Israel have built different nanoscale robots that can interact with each other, using their DNA switches to react and produce different signals. A bimolecular computer for cancer detection and treatment has recently been developed by Benenson et al. (2001). This device consisted of an input and output module to act together and can be used to disease diagnosis and drug release in response to cure that disease. They explored novel concept of software (made up of DNAs) and hardware (made up of enzymes) molecular elements. It’s a kind of generalized device with vast applicability of identifying any disease having a particular pattern of gene expression associated with it (Rao et al. 2014).
12.2.5 Data Storage and Transmission
Data transmission from implantable devices can be very useful for the monitoring of patients. RF is a very promising solution for implanted devices via inductive coupling. In addition to gathering power, RF can also be used to create a two-way link owing to its ability to send data back again to base station (Irazoqui-Pastor et al. 2003; Akin et al. 1998). Sauer et al. have reported a power harvesting chip to provide power, control signals, and a data link for an accompanied sensor to function and can be coupled with other devices. It is a well-known fact that RF energy between 1 and 10 MHz penetrates the body with minimum energy loss (Finkenzeller 2003). So they fabricate their inductive link to operate at a frequency of 4 MHz, where the chip supplies up to 2 mA at 3.3 volts. The chip was designed to let data transmission back to the module that broadcasts the power to eliminate need for a physical connection.
“Acoustic communication” is another alternative to communicate between longer distances. It can detect low energy consumption as compared to light communication approaches. Freitas et al. evaluated the feasibility of in vivo ultrasonic communication for micron-size robots broadcasting into various types of tissues. Frequencies between 10 MHz and 300 MHz gave the best transaction between efficient acoustic generation and attenuation for communication over 100 microns distance. They observed that power available from ambient oxygen and glucose in the bloodstream supported communication rates of about 10,000 bits/second between micron-sized robots. The acoustic pressure fields needed for this communication would not damage nearby tissue, and short bursts at significantly higher power could be explored for therapeutic usage (Hogg and Freitas 2012).
Although optical communication has also been explored with faster rates of data transmission, high-energy demands of this mode make it not the preferred choice for medical nanorobotics (Vasilescu et al. 2005). Chemical signaling is another approach of transmission, reported by Cavalcanti et al. (2005) in nanorobots for some teamwork coordination. To overcome the limitations, CMOS with submicron system on chip (SoC) design uses extremely low-power consumption for nanorobots communicating collectively at longer distances through acoustic sensors. Integrated sensors for data transfer can also be a good alternate to communicate with implanted devices, where a bunch of nanorobots may be equipped with single-chip RFID CMOS-based sensors as reported by Panis et al. (2004).
12.3 Types of Nanorobots
Nanorobots would be the future of medicine and all fields including surgery would be benefitted. As a broad classification, two types of nanorobots are organic and inorganic. The organic nanorobots are bio-nanorobots fabricated using biological entities like viruses and bacterial DNA cells and are more biocompatible in general, while inorganic nanobots are of synthesized proteins and others types of artificial material. These are more toxic and not suitable for direct use without encapsulation. In another way, nanorobots can be classified based on their assembly: “positional assembly” and “self-assembly.” In self-assembly, the arm of a miniature robot or a microscopic set is used to pick the molecules and assemble manually. In positional assembly, the billions of molecules are put together and automatically get assembled based on their natural affinities into the desired configuration (Kharwade et al. 2013; Venkatesan and Jolad 2010; Merina 2010).
Apart from these categorizations, there are four types of nanorobots that were conceptualized by Robert A. Freitas Jr as artificial blood:
-
(i)
Respirocytes
-
(ii)
Microbivores
-
(iii)
Clottocytes
-
(iv)
Chromallocyte
12.3.1 Respirocyte
Numerous conceptual designs of medical nanorobots have been reported, but in 1998, first theoretical design, describing a hypothetical artificial mechanical red blood cell or “respirocyte” made of 18 billion precisely arranged structural atoms, was given by Freitas (1999). These were proposed replica of red blood cells or erythrocytes which is a blood-borne spherical 1 μm diamondoid 1000-atmosphere pressure vessel with reversible molecule-selective pumps. Respirocyte would mimic the oxygen and carbon dioxide transport functions of erythrocytes and gets their power to function by endogenous serum glucose (Manjunath and Kishore 2014; Freitas 2009).
Each respirocyte had three types of rotors with different functions: one rotor to release the stored oxygen while traveling through the body, second rotor seizes all the carbon dioxide in the bloodstream which are released at the lungs, while the third one utilizes the glucose from the bloodstream as fuel source (Freitas 2009).
This artificial cell was more efficient than the RBCs (red blood cells) in its ability to supply 236 times more oxygen to the tissues per unit volume than RBCs (red blood cells) and would be useful for patients suffering from anemia, in rapid treatment for asphyxia (e.g., monoxide poisoning), as a backup for tissue oxygenation for heart and surgical patients, for site-specific deoxygenation of tumors, and as support for other nanorobots by releasing oxygen as and when needed during in vivo operations (Freitas 2009).
12.3.2 Microbivore Nanorobots
Another conceptual design for the nanorobotic artificial phagocytes described by Freitas et al. was “microbivores” as a surveillance nanorobot to identify and eliminate unwanted pathogens including bacteria, viruses, or fungi by digesting them using a combination of onboard mechanical and artificial enzymatic systems. Microbivore could be considered as an artificial phagocyte having primary function to obliterate pathogens found in human blood and considered as “guardian of bloodstream.” Nanorobots recognize a target microbes by contacting its surface antigen markers same as nano delivery systems and uses “digest and discharge” mechanism for removal of pathogens and could be a preferred treatment for sepsis. Basic architecture of microbivore consists of:
-
(i)
An array of reversible binding sites
-
(ii)
An array of telescoping grapples
-
(iii)
A morcellation chamber
-
(iv)
Digestion chamber (Eshaghian-Wilner 2009)
It is an oblate spheroidal device for nanomedical applications with 3.4 μm diameter along its major axis and 2.0 μm diameter along its minor axis, precisely organized by 610 billion atoms in a 12.1 μm3 geometric volume. These nanobots consume up to 200 pW power to digest trapped microbes. A detailed description of these nanorobots is given elsewhere (Manjunath and Kishore 2014; Freitas Jr 2005a, b, 2009).
12.3.3 Clottocyte Nanorobots
Clottocytes were designed with a unique biological capability of “instant hemostasis” in approximately 1 second (Freitas Jr 2005a, b; Manjunath and Kishore 2014). Clottocyte nanorobots mimic platelet in our blood. When there is a wound, platelet forms a clot to stop the blood flow; in similar way, clottocyte nanorobots form a fiber-like structure around the wound to stop the blood flow. These are spherical nanorobots powered by serum-oxyglucose, approximately 2 μm in diameter containing a fiber mesh specific to the blood group to release it at the site of action to create a clot. The response time of clottocyte is 100–1000 times faster than the natural hemostatic system (Eshaghian-Wilner 2009).
The basic requirement for optimal performance of these nanorobots is consistent communication protocols to control the coordinated mesh release from neighboring clottocytes and to regulate multidevice-activation radius within the local clottocyte population.
12.3.4 Chromallocyte (Mobile Cell Repair Nanorobots)
Chromallocyte nanorobots are hypothetical mobile cell repair nanorobots. These nanorobots were designed to replace entire chromosome. They were the most advanced nanorobots, proposed by Freitas et al. (2005) to perform chromosome replacement therapy (CRT). In CRT, the entire chromatin content of the nucleus in a living cell is extracted and promptly replaced with a new set of prefabricated chromosomes which have been artificially manufactured as defect-free copies of the originals.
A single lozenge-shaped 69 micron3 chromallocyte has dimensions of 4.18 μm and 3.28 μm along cross-sectional diameters and 5.05 μm length and consumes 50–200 pW in normal operation, and during outmessaging, a maximum of 1000 pW would be needed (Manjunath and Kishore 2014; Freitas Jr 2005a, b).
12.4 Medical Application of Nanorobots
Although nanorobotics is still in its infancy, the advantages associated with proposed designs and ability to perform complex tasks with high precision make them potential candidates and better alternatives for conventional therapies. Some advantages over conventional methods are listed below:
-
1.
Targeted therapy would be possible with no damage to adjacent tissues.
-
2.
Less posttreatment care required.
-
3.
Considerably less recovery time.
-
4.
Continuous monitoring and diagnosis from the inside would be possible.
-
5.
Rapid response to a sudden change.
It is obvious that nanorobots have more applicability in healthcare sector based on their design and size attributes. The development of nanorobots provides remarkable advances in diagnosis and treatment of various complex diseases. Nano-based drug delivery applications have already been reported by many researchers worldwide. These robots deliver specific drug to a target site inside the human body. Although fully mechanized self-sufficient nanorobots are distant dream at present, with the rapid advancements in related fields of electronics, biotechnology, and microfabrication, we would witness their application in treatment of complex medical problems soon.
In this segment, we would focus on in vivo usage of nanorobots in treatment and diagnosis and their potentially entailing benefits in the form of new therapy modules that were otherwise impossible to attain. In few reviews, detailed accounts of nanorobotics in vivo applications are mentioned (Soto and Chrostowski 2018).
12.4.1 Nanorobots in Cancer Treatment
Cancer is defined as a diseased condition where there is an uncontrolled growth of abnormal cells taking place and which may spread to the whole body within short time span. Cancer can be cured with current medical technologies, but many times late diagnosis and adverse side effects of chemotherapy are the limiting factor for an efficient treatment regimen, targeted drug delivery only to cancer cells is still not fully adopted by clinicians, and with conventional therapy, healthy tissue damage can’t be prevented.
Nanorobots designed for targeted therapy can work more precisely in releasing the payload at tumor site only after identifying the cancer tissue with no peripheral impact on healthy ones. First described by Freitas Jr., “pharmacytes” are a class of nanorobot enabled to deliver cytocidal agents to tumor site on a cell-by-cell basis. Moreover, unmetabolized cytocidal molecules would be engulfed by pharmacytes after initiating treated cells to be transported out of the patient’s body that would mitigate posttreatment collateral damage. There are two mechanisms to deliver the payload at target site; direct or by progressive cytopenetration based on the specific molecules to attach with cancer cell surface receptors to promote cell death by activating death receptors, i.e., CD95L ligand that binds to the extracellular domains of three CD95 death receptors), TNF or lymphotoxin alpha (binds to CD120a), Apo3L ligand aka TWEAK (binds to DR3), or Apo2L ligand aka TRAIL (binds to DR4 and DR5) (Freitas Jr 2005a, b).
In another approach, Douglas et al. devised an autonomous DNA nanorobot to transport molecular payloads to targeted cells after picking signals from cell surface for activation and structure reconfiguring for payload delivery. These devices have been tested for lymphoma and leukemia, consisted of series of DNA strands linked in 2D chains and folded into 3D structures, that could selectively open and close. Their nanobots carried two molecular cargos: a gold nanoshell and an antibody fragment with a DNA hinge at one end and a DNA latch on the other (Douglas et al. 2012). DNA origami-based nanorobots for drug delivery systems were put forth by Harvard University researchers, and till now many applications have been reported based on their biocompatibility and drug delivery efficacy. DNA origami is a technique “to create complex three-dimensional shapes from a single-stranded DNA molecule via self-assembly through Watson–Crick base pairing.” With the advancement of computational design tools, we can easily predict the durability of conformational changes in long DNA scaffolds in the presence of short DNA sequences that act as “staples” and “fasteners” to stabilize the final conformation. Till date many applications have been reported, and a detailed account can be obtained from many exciting reviews (Udomprasert and Kangsamaksin 2017; Linko et al. 2015; Baig et al. 2018; Zhang et al. 2014)
Few researchers have investigated the performance of DNA origami nanostructures inside the living insects, i.e., Blaberus discoidalis, by hemocoel injection and concluded that these DNA nanorobots can properly function inside living systems (Amir et al. 2014; Arnon et al. 2016). As reported by Perrault and Shih that after i.v. injection into mice, a lipid bilayer-encapsulated nanostructure remained in blood circulation significantly longer than its free form (Perrault and Shih 2014). Li et al. (2018) also reported a DNA “nanorobot” functionalized with target molecules for precise delivery of an active drug only at tumor site. In another approach, Rudchenko et al. (2013) described a molecular automata based on strand displacement cascades directed by antibodies to analyze and sorting of cells based on their surface receptors as inputs while a unique T molecular tag on the cell surface of a specific subpopulation of lymphocytes within human blood cells prepare final output of a molecular automation. This technique was advantageous in identifying the cells with no unique distinguishing feature.
Park et al. came up with a breakthrough idea of “bacteriobot,” where nontoxic bacteria, i.e., Salmonella typhimurium, after genetic modification were explored to attract chemicals released by cancer cells and delivery of anticancer drugs at target site. The bacteria were engineered to have receptors for higher migration velocity toward tumor cell lysates than normal cells using flagellar motion as shown in Fig. 12.4 a, b.
Schematic representation of bacteriobots (a, b) structure of S. typhimurium-attached PS microbeads under confocal laser scanning microscope, (c) in vivo application of bacteriobots in mice (Park et al. 2013)
In this series, few other reports are also there where Akin et al. described bio-hybrid nanobots for payload delivery based on ‘Listeria monocytogenes’ to deliver nanoparticles containing genes and proteins, within a mouse, and gene expression was monitored through differences in the luminescence produced within the different mouse organs based on the payload delivery (Akin et al. 2007), where as a step further, Taherkhani et al. (2014) in vitro coupled “magnetotactic” bacteria which produce magnetic iron oxide nanoparticles naturally with liposomes loaded with therapeutic agents. Later these bacteria were guided with an external magnetic field to deliver the drug-loaded liposomes in vivo to a mouse tumor site (Felfoul et al. 2016).
Recently, Hoop et al. explored magnetically guided nanorobots for the delivery of fluorouracil to check its efficacy in tumor reduction in a mice model. This guided method of drug delivery allowed the nanorobotic platform to dispense more amount of the therapeutic agent in a localized area of the tumor as compare to conventional medicine (Hoop et al. 2018). These are few recent but promising applications of in vivo cancer therapy by nanorobots with a bright picture of more efficient future cancer therapies. In very few cases, real-time bioimaging was also achieved via nanorobots. A dual imaging approach was explored by Yan et al. to detect biodegradable magnetic microhelix nanorobots in mice. Fluorescence imaging provided the whereabouts of nanorobots inside the subcutaneous tissue and intraperitoneal cavity of a mouse, while magnetic resonance-based imaging was used to detect the nanorobot’s position inside the mouse’s stomach, as shown in Fig. 12.5a–d. These approaches can be useful for obtaining good quality images for cancer sites as well as monitoring treatment regimen.
In vivo imaging of magnetically propelled microrobot. (a) Scanning electron microscopy (SEM) and fluorescence images of the helical, (b) diagrammatic representation of microbots in mouse subcutaneous tissues and (c) inside the mouse stomach. (Yan et al. 2017) (e) schematic of microrobots for enhanced payloads retention in the gastrointestinal tract. (f) Micrograph illustrating the bubble generation at the end of the microrobot responsible for locomotion. (g) Ex vivo fluorescent images of the gastrointestinal tract retention of the dye rhodamine (i, control; ii: after 6 h, and iii; after 12 h of administration) (Li et al. 2016)
12.4.2 Nanorobots in Diagnosis and Treatment of Diabetes
Diabetes is basically body’s inability to process blood glucose, and thus glucose levels in the bloodstream are the prime factors for the diagnosis and treatment of diabetes. Cavalcanti et al. (2008a, b, c) designed novel nanobot for efficient diabetes control based on an alarm system. These nanorobots flew with the RBCs through the bloodstream detecting the glucose levels and whenever glucose attains critical levels it triggers an alarm in nanorobot through the cell phone to alert the patient. At a typical glucose concentration, the nanorobots keep the glucose levels ranging around 130 mg/dl as a target for the blood glucose levels (BGLs). Data storage to cell phone was also possible with these tiny entities by RF signals to keep the records of glucose levels for further clinical investigations of patients.
Recently a nanotech-based approach is described by MIT researchers where nanoparticles not only sensed glucose levels in the body but also secreted the appropriate amount of insulin to get it under control. Gu et al. prepared a nanonetwork loaded with insulin and glucose-metabolizing enzyme. This 3D scaffold dissociated when needed to release insulin in hyperglycemic condition, and later polymeric matrix was degraded. A single nanoparticle injection could maintain the glucose in the blood (200 mg/dL) up to 10 days (Gu et al. 2013). For glucose monitoring the nanorobots use an embedded chemosensor that involves the modulation of human SGLT3 protein glucosensor activity (Wright et al. 2005).
12.4.3 Nanorobots in Retention of Payloads and Wound Healing
Apart from delivery of drug molecules, retention of the drug or cells is also a promising feature of nanobots for sustained drug release in special cases of pain management, bacterial infection treatment, etc. Wang’s group is pioneer in proposing biodegradable zinc and magnesium powered microrobots that utilized gastric and intestinal fluids as fuels to withhold payload in the stomach and intestinal tissues (Gao et al. 2015; Esteban-Fernández de Ávila et al. 2017a, Soto and Chrostowski 2018) and explored these nanorobots for pH neutralization of the gastric fluid (Li et al. 2017) and treatment of bacterial infection (Helicobacter pylori) in the stomach (Esteban-Fernández de Ávila et al. 2017b).
To achieve retention of the microrobot, different mechanisms were applied like direct piercing of the surrounding tissue, or by an improvement in mass transport and nucleation by generation of gas bubbles. Li et al. designed magnesium-based microrobots with built-in “delay activation.” They used polymeric coatings to activate the microrobot motion based on their thickness or environmental pH conditions and got dissolved at neutral pH of the intestinal fluids to activate the microrobot (Li et al. 2016) as shown in Fig. 12.5e–g. In a recent approach described by Karshalev et al. nanorobots were incorporated in a pill matrix to streamline their administration with existing pharmaceutical protocols (Karshalev et al. 2018; Soto and Chrostowski 2018).
In wound healing also nanorobots have applications as reported by Baylis et al. where thrombin was delivered at target site to pause the bleeding of wounds in the vasculature of mouse and pig models by chemically propelled calcium carbonate-based microrobots. The distribution of nanorobots at wound region can be explained by “lateral propulsion,” “buoyant rise,” and “convection” (Baylis et al. 2015). Another laser-based wound sealing approach with locomotive microrobot was explored by He et al. Localized collagen denaturation and melting were initiated by laser generated high temperature followed by temperature decrease that allowed condensation and wound closure (He et al. 2016; Soto and Chrostowski 2018).
12.4.4 Surgery, Biopsy, and Thrombolysis by Nanorobots
Other potential implementation of nanorobots would be in biopsy/surgery. Although few in vitro platforms have been proposed for nano/micro high precision surgery, none have not been converted to in vivo models yet (Kwan et al. 2015; Soto et al. 2015). Gultepe et al. have used their microrobots having star-shaped grippers for easy access to narrow channels in the body and to expunge tissue samples from a pig bile duct (Gultepe et al. 2013). For in vivo surgical application, researchers have developed a module based on magnetic microrobots for controlled navigation inside the eye of a living rabbit (Ullrich et al. 2013; Pokki et al. 2017).
Apart from clot formation, nanorobots have the applicability to remove the clots in narrow arteries reported by Cheng et al. Magnetically actuated nanorobots loaded with tissue plasminogen activator were intravenously injected and sent to the blood clot location. With externally controlled rotations of nanorobots, an interaction of the tissue plasminogen activator molecule with the blood clot interface resulted in thrombolysis (Cheng et al. 2014). More recently, Hu et al. developed a new strategy by incorporating plasminogen activator into porous magnetic iron oxide (Fe3O4)-microrods for targeted thrombolytic therapy in ischemic stroke for mechanical destruction of clot induced by distal middle cerebral artery occlusion (Hu et al. 2018). This approach was a major breakthrough not only for the treatment of ischemic stroke but also a vast implication on other fatal thrombotic diseases such as myocardial infarction and pulmonary embolism. Soto and Chrostowski (2018) have covered most of the recent nanorobotics applications in detail.
12.5 Future and Commercialization Aspects of Nanorobots
The field of nanorobotics is gradually achieving maturity with the advancement of supporting fields of microfabrication, electronics, nanotechnology, and medicine. Despite promising medical applications and new therapy modules with many exciting new findings based on nanorobots, few key issues have to be addressed before their full-fledged real-world applications. In current scenario, major challenges with nanorobots in vivo applications can be summarized as:
-
(a)
Lack of sensing and actuation
-
(b)
Integration and control mechanism inefficiency
-
(c)
Lack of understanding of chemistry and biological principles at nanoscale
-
(d)
Clinical risk associated with in vivo application of nanorobots
-
(e)
Toxicity on long-term retention and proper clearance mechanisms
-
(f)
Hurdles in commercialization
Nanorobotics is a highly interdisciplinary platform and its inputs and collaborations from many fields are desired to take this from infancy to maturity. For toxicity and retention issues can be handled by manufacturing of the micro/nanostructure with biocompatible materials but yet a long way to get the necessary approvals for their medicinal usage and applications in vivo. Apart from these pros and cons of nanorobot, this field has a bright future with great impact on lives of millions of people that makes it mandatory to thoroughly consider the commercialization prospects of nanorobotics (Soto and Chrostowski 2018).
Lab to market strategy for this new stream needs enormous efforts to prove its clinical efficacy, validations, and regulatory approvals and to design a strong commercialization plan. Pharmaceutical companies and insurance providers are the target customers for nanorobots with matching areas of interests of reduced costs with more productivity and considering the immense potential of nanorobots this is very much possible. Till date, very few active companies are working toward the commercialization of nanorobots for use in medical applications. Major market player in nanorobotics are Ginkgo Bioworks (USA), EV Group (Austria), Bruker (USA), Imina Technologies (Switzerland), Oxford Instruments (UK), JEOL (Japan), Xidex (USA), Klocke Nanotechnik (Germany), Toronto Nano Instrumentation (Canada), Park Systems (South Korea), and few more.
There are few technical hurdles to be overcome before commercial production and large-scale application of nanorobots. First is the mass fabrication of nonentities with desired efficacy. To overcome this researchers have suggested special nano modules for inspecting, testing, and transporting of nanorobotic designs (Wang and Zhang 2017; Lu et al. 2017; Zhang et al. 2018). Production costs and market penetration can be achieved once sorting out technical difficulties. To keep intellectual property rights of the new techniques developed by key players in the market is one way to keep the initial hefty investment cost under control that would in turn open a gateway to launch a strong business model with recognizable revenue generation streams. In recent years, many patents have been filed ranging from fabrication methods commonly used for parallel mass fabrication, power generation, and tracking of nanorobots to their in vivo intercellular applications (Natan and Mallouk 2007; Odell et al. 2017; Martel and Felfoul 2018).
There may be many possible approaches to explore this technology for wide clinical applications by integrating with conventional medical procedures. Despite everything, there is no doubt about the benefits of nanorobots in medicine, and once established in near future, this technology would be a boon for human civilization.
References
Adleman LM (1995) On constructing a molecular computer. In: Lipton RJ, Baum E (eds) DNA based computers, DIMACS 27. American Mathematical Society, Providence, RI, pp 1–21
Ahuja SP, Myers JR (2006) A survey on wireless grid computing. J Supercomput 37:3–21
Akin T, Najafi K, Bradley RM (1998) A wireless implantable multichannel digital neural recording system for a micromachined sieve electrode. IEEE J Solid State Circuits 33:109–118
Akin D, Sturgis D, Ragheb K, Sherman D, Burkholder K, Robinson JP et al (2007) Bacteria-mediated delivery of nanoparticles and cargo into cells. Nat Nanotechnol 2:441–449
Amir Y, Ben-Ishay E, Levner D, Ittah S, Abu-Horowitz A, BacheleT I (2014) Universal computing by DNA origami robots in a living animal. Nat Nanotechnol 9:353–357
Arnon S, Dahan N, Koren A, Radiano O, Ronen M, Yannay T et al (2016) Thought-controlled nanoscale robots in a living host. PLoS One 11:e0161227
Baig MS, Babar U, Arshad U, Ullah MA (2018) DNA origami and bionanotechnology: an efficacious tool for modern therapeutics and drug delivery. Int J Dev Res 8:824660–824669
Balasubramanian A, Bhuva B, Mernaugh R, Haselton FR (2005) Si-based sensor for virus detection. IEEE Sensors J 5:340–344
Ballantyne GH, Moll F (2003) The da Vinci telerobotic surgical system: the virtual operative field and telepresence surgery. Surg Clin 83:1293–1304
Baylis JR, Yeon JH, Thomson MH, Kazerooni A, Wang X, John AES, Piret JM (2015) Self-propelled particles that transport cargo through flowing blood and halt hemorrhage. Sci Adv 1:e1500379
Benenson Y, Paz-Elizur T, Adar R, Keinan E, Livneh Z, Shapiro E (2001) Programmable and autonomous computing machine made of biomolecules. Nature 414:430–434.
Bernstein K, Chuang CT, Joshi R, Puri R (2003) Design and CAD challenges in sub-90nm CMOS technologies. International conference on computer aided design, 129–136
Bhat AS (2014) Nanobots: the future of medicine. Int J Manag Eng Sci 5:44–49
Cavalcanti A (2003) Assembly automation with evolutionary nanorobots and sensor-based control applied to nanomedicine. IEEE Trans Nanotechnol 2:82–87
Cavalcanti A, Rosen L, Kretly LC, Rosenfeld M, Einav S (2004) Nanorobotic challenges in biomedical applications, design and control. International conference on electronics, circuits and systems, 447–450. IEEE
Cavalcanti A, Hogg T, Kretly LC (2005) Transducers development for nanorobotic applications in biomedical engineering. IEEE NDSI Conference on nanoscale devices and system integration, Houston TX, USA
Cavalcanti A, Shirinzadeh B, Freitas RA Jr, Hogg T (2007) Nanorobot architecture for medical target identification. Nanotechnology 19:015103. (15pp)
Cavalcanti A, Shirinzadeh B, Kretly LC (2008a) Medical nanorobotics for diabetes control. Nanomedicine 4:127–138
Cavalcanti A, Shirinzadeh B, Zhang M, Kretly L (2008b) Nanorobot hardware architecture for medical defense. Sensors 8:2932–2958
Cheng R, Huang W, Huang L, Yang B, Mao L, Jin K et al (2014) Acceleration of tissue plasminogen activator-mediated thrombolysis by magnetically powered nanomotors. ACS Nano 8:7746–7754
Curtis AS, Dalby M, Gadegaard N (2006) Cell signaling arising from nanotopography: implications for nanomedical devices. Nanomedicine (Lond) 1:67–72
Drexler KE (1992) Nanosystems: molecular machinery, manufacturing, and computation. Wiley, New York, pp 21–26
Douglas SM, Bachelet I, Church GM (2012) A logic-gated nanorobot for targeted transport of molecular payloads. Science 335:831–834
Eggers T, Marscher C, Marschner U, Clasbrummel B, Laur R, Binder J (2000a) Advanced hybrid integrated low-power telemetric pressure monitoring system for biomedical application. In International conference on micro electro mechanical systems, 23–37
Eggers T, Marschner C, Marschner U, Clasbrummel B, Laur R, Binder J (2000b) Advanced hybrid integrated low-power telemetric pressure monitoring system for biomedical applications. In Proceedings IEEE thirteenth annual international conference on micro electro mechanical system, 329–334. IEEE
Eshaghian-Wilner MM (ed) (2009) Bio-inspired and nanoscale integrated computing, vol 1. Wiley, New York
Esteban-Fernández de Ávila B, Angsantikul P, Li J, Lopez-Ramirez MA, Ramírez-Herrera DE, Thamphiwatana S et al (2017a) Micromotor-enabled active drug delivery for in vivo treatment of stomach infection. Nat Commun 8:272
Esteban-Fernández de Ávila B, Angsantikul P, Li J, Gao W, Zhang L, Wang J (2017b) Micromotors go in vivo: from test tubes to live animals. Adv Funct Mater 28:1705640
Fann JI, St. Goar FG, Komtebedde J, Oz MC, Block PC, Foster E et al (2004) Beating heart catheter-based edge-to-edge mitral valve procedure in a porcine model: efficacy and healing response. Circulation 110:988–993
Farahani A, Farahani A (2016) An adaptive controller for motion control of nanorobots inside human blood vessels. Biosci Biotechnol Res Commun 9:546–552
Felfoul O, Mohammadi M, Taherkhani S, De Lanauze D, Xu YZ, Loghin D et al (2016) Magneto-aerotactic bacteria deliver drug-containing nanoliposomes to tumour hypoxic regions. Nat Nanotechnol 11:941–947
Feringa BL (2001) In control of motion: from molecular switches to molecular motors. Acc Chem Res 34:504–513
Feringa BL, Koumura N, Van Delden RA, MKJ TW (2002) Light-driven molecular switches and motors. Appl Phys A 75:301–308
Feynman RP (1996) There is plenty of room at the bottom. Eng Sci 23:22–26
Finkenzeller K, RFID handbook, Wiley, Oxford, 2003
Freitas RA Jr (1999) Nanomedicine vol I Basic Capabilities Landes Bioscience. https://www.nanomedicine.com
Freitas RA Jr (2005a) Current status of nanomedicine and medical nanorobotics. J Comput Theor Nanosci 2:1–25
Freitas RA Jr (2005b) Microbivores: artificial mechanical phagocytes using digest and discharge protocol. J Evol Technol 14:1–52
Freitas RA Jr (2009) Medical nanorobotics: the long term goal for nanomedicine. In: Schulz MJ, Vesselin N, Shanov (eds) Nanomedicine design of particles, sensors, motors, implants, robots and devices. Artech House, Norwood Ma, pp 367–392
Fung CK, Li WJ (2004) Ultra-low-power polymer thin film encapsulated carbon nanotube thermal sensors. 4th IEEE conference on nanotechnology, 158–160.IEEE
Gao W, Dong R, Thamphiwatana S, Li J, Gao W, Zhang L, Wang J (2015) Artificial micromotors in the mouse’s stomach: a step toward in vivo use of synthetic motors. ACS Nano 9:117–123
Ghovanloo M, Najafi K (2004) A wideband frequency-shift keying wireless link for inductively powered biomedical implants. IEEE Trans Circuit Syst I Reg Pap 51:2374–2383
Gomez G (2004) Sabiston textbook of surgery, 17th edn. Elsevier Saunders, Philadelphia, PA. Emerging technology in surgery: informatics, Electronics, Robotics
Gu Z, Aimetti AA, Wang Q, Dang TT, Zhang Y, Veiseh O et al (2013) Injectable nano-network for glucose-mediated insulin delivery. ACS Nano 7:4194–4201
Gultepe E, Randhawa JS, Kadam S, Yamanaka S, Selaru FM, Shin EJ et al (2013) Biopsy with thermally-responsive untethered microtools. Adv Mater 25:514–519
Hagiya M (2000) From molecular computing to molecular programming. In International workshop on DNA-based computers, 89–102. Springer
He W, Frueh J, Hu N, Liu L Gai M, He Q (2016) Guidable thermophoretic Janus micromotors containing gold nanocolorifiers for infrared laser assisted tissue welding. Advanced Science 3(12):1600206
Higdon JJL (1979) A hydrodynamic analysis of flagellar propulsion. J Fluid Mech 90:685–711
Hogg T, Freitas RA Jr (2012) Acoustic communication for medical nanorobots. Nano Communication Networks 3:83–102
Hoop M, Ribeiro AS, Rösch D, Weinand P, Mendes N, Mushtaq F et al (2018) Mobile magnetic nanocatalysts for bioorthogonal targeted cancer therapy. Adv Funct Mater 28:1705920
Hu J, Huang S, Zhu L, Huang W, Zhao Y, Jin K, ZhuGe Q (2018) Tissue plasminogen activator-porous magnetic microrods for targeted thrombolytic therapy after ischemic stroke. ACS Appl Mater Inter 10:32988–32997
Ikeda S, Arai F, Fukuda T, Kim EH, Negoro M, Irie K, Takahashi I (2005) In vitro patient-tailored anatomical model of cerebral artery for evaluating medical robots and systems for intravascular neurosurgery. In International conference on intelligent robots and systems, 1558–1563
Irazoqui-Pastor P, Mody I, Judy JW (2003) In-vivo EEG recording using a wireless implantable neural transceiver. In First international IEEE EMBS conference on neural engineering, 2003.Conference proceedings, 622–625. IEEE
Joanny JF, Jülicher F, Prost J (2003) Motion of an adhesive gel in a swelling gradient: a mechanism for cell locomotion. Phys Rev Lett 90:168102
Karshalev E, Esteban-Fernández de Ávila B, Beltrán-Gastélum M, Angsantikul P, Tang S, Mundaca-Uribe R, Zhang F, Zhao J, Zhang L, Wang J (2018) Micromotor pills as a dynamic Oral delivery platform. ACS Nano 12(8):8397–8405
Kermani BG, Mueller J, Hall LC, Nagle HT, Scarantino CW (2006) Methods, systems, and associated implantable devices for dynamic monitoring of physiological and biological properties of tumors US Patent Specification 7010340
Kharwade M, Nijhawan M, Modani S (2013) Nano robots: a future medical device in diagnosis and treatment. Res J Pharm, Biol Chem Sci 4:1299–1307
Khulbe P (2014) Nanorobots: a review. IJPSR 5(6):2164–2173
Kim M, Powers TR (2004) Hydrodynamic interactions between rotating helices. Phys Rev E 69:061910
Kinosita K, Yasuda R, Noji H, Adachi K (2000) A rotary molecular motor that can work at near 100% efficiency. Philos Trans R Soc Lond B Biol Sci 355:473–489
Kwan JJ, Myers R, Coviello CM, Graham SM, Shah AR, Stride E et al (2015) Ultrasound-propelled nanocups for drug delivery. Small 11:5305–5314
Li J, Thamphiwatana S, Liu W, Esteban-Fernández de Ávila B, Angsantikul P, Sandraz E et al (2016) Enteric micromotor can selectively position and spontaneously propel in the gastrointestinal tract. ACS Nano 10:9536–9542
Li J, Angsantikul P, Liu W, Esteban-Fernández de Ávila B, Thamphiwatana S, Xu M et al (2017) Micromotors spontaneously neutralize gastric acid for pH-responsive payload release. Angew Chem Int Ed 56:2156–2161
Li S, Jiang Q, Liu S, Zhang Y, Tian Y, Song C et al (2018) A DNA nanorobot functions as a cancer therapeutic in response to a molecular trigger in vivo. Nat Biotechnol 36:258
Linko V, Ora A, Kostiainen MA (2015) DNA nanostructures as smart drug-delivery vehicles and molecular devices. Trends Biotechnol 33:586–594
Liu W, Van Wyk JD, Odendaal WG (2004) Design and evaluation of integrated electromagnetic power passives with vertical surface interconnections. In Nineteenth annual IEEE applied power electronics conference and exposition, 2:958–963. IEEE
Lu X, Soto F, Li J, Li T, Liang Y, Wang J (2017) Topographical manipulation of microparticles and cells with acoustic microstreaming. ACS Appl Mater Interfaces 9(44):38870–38876
Manjunath A, Kishore V (2014) The promising future in medicine: nanorobots. Biomed Sci Eng 2:42–47
Marescaux J, Rubino F (2003) The ZEUS robotic system: experimental and clinical applications. Surg Clin 83:1305–1315
Martel S, Felfoul O (2018) U.S. Patent No. 9,905,347. U.S. Patent and Trademark Office, Washington, DC
Martel S, Felfoul O, Mathieu JB, Chanu A, Tamaz S, Mohammadi M et al (2009) MRI-based medical nanorobotic platform for the control of magnetic nanoparticles and flagellated bacteria for target interventions in human capillaries. Int J Robot Res 28:1169–1182
Merina RM (2010) Use of nanorobots in heart transplantation. INTERACT, 265–268. IEEE
Mohseni P, Najafi K, Eliades SJ, Wang X (2005) Wireless multichannel biopotential recording using an integrated FM telemetry circuit. IEEE Trans Neural Syst Rehabil Eng 13:263–271
Murphy D, Challacombe B, Nedas T, Elhage O, Althoefer K, Seneviratne L, Dasgupta P (2007) Equipment and technology in robotics. Arch Esp Urol 60:349–354
Natan MJ, Mallouk TE (2007) U.S. Patent No. 7,225,082. U.S. Patent and Trademark Office, Washington, DC
Odell L, Nacev AN, Weinberg IN (2017) U.S. Patent No. 9,833,170. U.S. Patent and Trademark Office, Washington, DC
Panis C, Hirnschrott, U, Farfeleder S, Krall A, Laure G, Lazian W, Nurmi J (2004) A scalable embedded DSP core for SoC applications. International symposium on system-on-chip proceedings, 85–88. IEEE
Park JG, Lee GS, Lee SH (2005) U.S. Patent No. 6,884,694. U.S. Patent and Trademark Office, Washington, DC
Park SJ, Park SH, Cho S, Kim DM, Lee Y, Ko SY, Park S (2013) New paradigm for tumor theranostic methodology using bacteria-based microrobot. Sci Rep 3:3394
Perrault SD, Shih WM (2014) Virus-inspired membrane encapsulation of DNA nanostructures to achieve in vivo stability. ACS Nano 8:5132–5140
Pokki J, Ergeneman O, Chatzipirpiridis G, Lühmann T, Sort J, Pellicer E et al (2017) Protective coatings for intraocular wirelessly controlled microrobots for implantation: Corrosion, cell culture, and in vivo animal tests. J Biomed Mater Res B Appl Biomater 105:836–845
Powers TR (2002) Role of body rotation in bacterial flagellar bundling. Phys Rev E 65:040903
Purcell EM (1977) Life at low Reynolds number. Am J Phys 45:3–11
Rao TVN, Saini HS, Prasad PB (2014) Nanorobots in Medicine-A New Dimension in Bio Nanotechnology. Int J Sci Eng Comp Technol 4:74–79
Reppesgaard L (2002) Nanobiotechnologie: Die Feinmechaniker der Zukunftnutzen Biomaterial alsWerkstoff. Computer Zeitung 36:22
Risveden K, Pontén JF, Calande N, Willander M, Danielsson B (2007) The region ion sensitive field effect transistor, a novel bioelectronics nanosensor. Biosens Bioelectron 22:3105–3112
Roue CC (2002) Aneurysm liner. 6350270US, Feb.
Roundy S, Wright PK, Rabaey JM (2003) Energy scavenging for wireless sensor networks. Norwel:45–47
Rudchenko M, Taylor S, Pallavi P, Dechkovskaia A, Khan S, Butler VP Jr et al (2013) Autonomous molecular cascades for evaluation of cell surfaces. Nat Nanotechnol 8:580–586
Sauer C, Stanacevic M, Cauwenberghs G, Thakor N (2005) Power harvesting and telemetry in CMOS for implanted devices. IEEE Trans Circuit Syst I Reg Pap 52:2605–2613
Saxena S, Pramod BJ, Dayananda BC, Nagaraju K (2015) Design, architecture and application of nanorobotics in oncology. Indian J Cancer 52:236
Sharma NN, Mittal RK (2008) Nanorobot movement: Challenges and biologically inspired solutions. Int J Smart Sens Intell Syst 1:87–109
Soto F, Martin A, Ibsen S, Vaidyanathan M, Garcia-Gradilla V, Levin Y, Wang J (2015) Acoustic microcannons: toward advanced microballistics. ACS Nano 10(1):1522–1528
Soto F, Chrostowski R (2018) Frontiers of medical micro/nanorobotics: in vivo applications and commercialization perspectives towards clinical uses. Front Bioeng Biotechnol 6:1–12
Stracke R, Böhm KJ, Burgold J, Schacht HJ, Unger E (2000) Physical and technical parameters determining the functioning of a kinesin-based cell-free motor system. Nanotechnology 11(2):52–56
Sun J, Gao M, Feldmann J (2001) Electric field directed layer-by-layer assembly of highly fluorescent CdTe nanoparticles. J Nanosci Nanotechnol 1:133–136
Squires TM, Brady JF (2005) A simple paradigm for active and nonlinear microrheology. Phys Fluids 17:073101
Taherkhani S, Mohammadi M, Daoud J, Martel S, Tabrizian M (2014) Covalent binding of nanoliposomes to the surface of magnetotactic bacteria for the synthesis of self-propelled therapeutic agents. ACS Nano 8:5049–5060
Udomprasert A, Kangsamaksin T (2017) DNA origami applications in cancer therapy. Cancer Sci 108:1535–1543
Ullrich F, Bergeles C, Pokki J, Ergeneman O, Erni S, Chatzipirpiridis G et al (2013) Mobility experiments with microrobots for minimally invasive intraocular surgery. Invest Ophthalmol Vis Sci 54:2853–2863
Ummat A, Dubey A, Sharma G, Mavroidis C (2005a) Nanorobotics – fractal navigator
Ummat A, Dubey A, Mavroidis C (2005b) Bio-nanorobotics: a field inspired by nature. Biomimetics:219–246
Vasilescu I, Kotay K, Rus D, Dunbabin M, Corke P (2005) Data collection, storage, and retrieval with an underwater sensor network. In Proceedings of the 3rd international conference on Embedded networked sensor systems 154–165. ACM
Venkatesan M, Jolad B (2010) Nanorobots in cancer treatment. INTERACT, 258–264. IEEE
Wang J, Zhang L (2017) U.S. Patent Application No. 15/356, 977
Wieland III CF (2004) Is the US nanotechnology investment paying off? Small Times Magazine, 4(1)
Wright EM, Sampedro AD, Hirayama BA, Koepsell H, Gorboulev V, Osswald C (2005) Novel glucose sensor. United States patent US 0267154
Yan X, Zhou Q, Vincent M, Deng Y, Yu J, Xu J, Xu T, Tang T, Bian L, Wang Y-XJ, Kostarelos K, Zhang L (2017) Multifunctional biohybrid magnetite microrobots for imaging-guided therapy. Science Robotics 2(12):eaaq1155
Zhang M, Sabharwal CL, Tao W, Tarn TJ, Xi N, Li G (2004) Interactive DNA sequence and structure design for DNA nanoapplications. IEEE Trans Nanobioscience 3:286–292
Zhang Q, Jiang Q, Li N, Dai L, Liu Q, Song L et al (2014) DNA origami as an in vivo drug delivery vehicle for cancer therapy. ACS Nano 8:6633–6643
Zhang Z, Dai C, Huang JY, Wang X, Liu J, Ru C et al (2018) Robotic immobilization of motile sperm for clinical intracytoplasmic sperm injection. IEEE Trans Biomed Eng 62:2620–2628
Acknowledgment
Authors are thankful to DBT-BIRAC for the grant and NCL Innovation Park, Pune, for the support to carry out the research work.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2020 Springer Nature Singapore Pte Ltd.
About this chapter
Cite this chapter
Gandhi, M., Joshi, P.N. (2020). Nanorobots for In Vivo Monitoring: The Future of Nano-Implantable Devices. In: Chandra, P., Prakash, R. (eds) Nanobiomaterial Engineering. Springer, Singapore. https://doi.org/10.1007/978-981-32-9840-8_12
Download citation
DOI: https://doi.org/10.1007/978-981-32-9840-8_12
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-32-9839-2
Online ISBN: 978-981-32-9840-8
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)