Abstract
In 1959, physicist Richard Feynman gave a speech entitled “The Problem of Manipulating and Controlling Things at a Tiny Level,” creatively proposing the concept that “the whole encyclopedia is written on a needle,” which became the earliest scientific prediction of nanotechnology. Nanotechnology provides an innovative impetus for seven basic disciplines, including physics, materials, chemistry, energy science, life science, pharmacology and toxicology, and engineering, which has become a prominent source of manufacturing technology for transformative industries. Nanorobots, with virtue of advanced chips and nanotechnology, new intelligent nanomaterials that carry out rapid movement, perform multiple complex tasks at the nanoscale, and accurately build and manipulate objects at the atomic level, are crucial to various functions like the therapeutic development of precision medicine, signaling, sensor detection, and other fields. Nanorobots in medicine advance the progress of medical treatment and improve the quality of life of patients on account of its promising approaches in effectiveness. In this chapter, the current trends and promising opportunities for nanorobots in drug delivery, surgery, and biosensing will be summarized.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
2.1 Introduction
“Nanorobots” is an emerging technology in engineering. The development of nanorobots belongs to the category of “molecular nanotechnology (referred to as MNT),” which is based on the biological principles of the molecular level as a design prototype and design and manufacture of “functional molecular devices” that can operate on nanospace (Wan et al. 2020). A nanorobot is a multifunctional, controllable machine made from inorganic or polymeric nanomaterials and then modified with biomimetic materials (Zhou et al. 2021). At present, there is no strict definition of the size of nanorobots in the world; size between 100 nanometer and 1000 nanometer is used to be called as nanorobots (Patel et al. 2006). The structures of nanorobots have aroused enormous interest among researchers and engineers in nanobiology (Garcia-Gradilla et al. 2013; Li et al. 2014). The first generation of nanorobots is an organic combination of biological systems and mechanical systems, which can be injected into blood vessels for health examination and disease treatment, while it can also be used to repair human organs, perform cosmetic surgery, and install normal DNA from genes instead of harmful DNA in genes in order to make the body function properly. The second generation of nanorobots is a nanoscale molecular device assembled directly from atoms or molecules with specific functions, while the third generation will contain nanocomputers, which are devices that can perform human-machine dialogue (Sobczak et al. 2012; van den Heuvel and Dekker 2007; Seeman 2003). Recently, the Indian Institute of Technology in Guwahati has creatively developed a third-generation (3G) self-propelled robot consisting of chemically modified multi-walled carbon nanotubes (CNTs) that can achieve multi-mode self-driving in response to acidic, alkaline, magnetic, and light stimulation. Meanwhile, dye degradation can also be effectively realized by inducing dye degradation through advanced oxidation (Mitra et al. 2020) (Fig. 2.1). Compared to conventional robots, nanorobots provide advantages over their suitability and great biocompatibility for applications in drug delivery, surgery, and biosensing, which improve the safety and efficacy of drugs for targeted hard-to-reach cells and tissues (Orive et al. 2003; Hu et al. 2020; Mertz 2018; Chen et al. 2019a). Owing to small size, nanorobots are able to move and perform operations in microscopic environments that are not accessible to traditional devices. For example, nanorobots can enter microfluidic chips to micro-manipulate and assemble microstructures, enter the natural passages or blood vessels of organisms for detection and drug delivery, and even enter the interior of a single cell to measure the nucleus (Zhou et al. 2021; Solovev et al. 2012; Sitti et al. 2015). Nanorobots allow scientists to control their populations to change configurations through narrow pipes and reach targeted locations to release drugs (Mitragotri et al. 2014). Nanorobots hold considerable application in various fields, especially for advancing diagnosis and treatment of disease, which are able to precisely kill cancer cells, unclog blood clots, remove fat deposits within the arteries, and clean general surgical procedures (Bucolo et al. 2022; Lee et al. 2022; Lueg and Jungo 2021). The concept of treatment with the tool of nanorobots has been proposed for decades (Jager et al. 2000; Morgado et al. 2019). Richard Feynman (Olsman and Goentoro 2018) pioneered the concept of using nanorobots to treat diseases in the field of medicine; with the continuous advancement of industrial manufacturing technology, nanotechnology provides a novel approach, as it can be applied in targeted drug delivery and disease treatment, making significant progress in the treatment of some complex diseases (Mak et al. 2014; Ewart et al. 2018). Meanwhile, 3D printing provides the benefit in the field of nanorobots, which performs a series of operations on atomic and cellular structures at the molecular level that humans are unable to manipulate (Wei et al. 2020; Kim et al. 2020; Li et al. 2020; Khan et al. 2018). Nanorobots are used to pass through the vitreous body, a colorless, transparent colloidal substance that fills the eyeball between the retina and the crystal, which delivery drugs into the retina to treat aging-related diseases, such as macular degeneration (Kim et al. 2013). A golden nanorobot covered by a mixed coating constructed of platelets and blood cell membranes was developed creatively by scientists at the University of California, which was 25 times thinner than a human hair and travelled at a speed of 35 microns per second in ultrasonically powered blood. When they crossed the bloodstream by ultrasound, the platelet membranes trap bacteria, and the blood cell membranes trap toxins. Platelet and blood cell membrane coatings, on the other hand, can also prevent proteins from attaching to nanorobots, preventing them from being attacked by other cells (Wu et al. 2019). From a medical point of view, nanorobots are a potential therapeutic approach to enter specifically target in the human body. Once reaching the target, they can carry out executive function, such as delivering drugs or getting real-time insights into how the drugs are working. After completing these goals, the nanorobot can be degraded without leaving a trace rapidly (Mostaghaci et al. 2017; Hosseinidoust et al. 2016; Li et al. 2018). Overall, nanorobots provide unlimited possibilities for intelligent sensing detection, controllable drug delivery, precision collaborative medicine, and other large health fields. However, their practical applications face a variety of problems. For example, considering that only a few tenths of a human hair and locating in human body is difficult them the controlled autonomous movement of nanorobots in the human body has always been a problem; if they entered the blood vessels, they are wrapped and impacted by blood, etc. and faced with the challenge in uncontrolled movement (Schuerle et al. 2019).
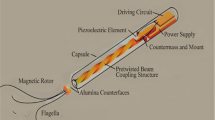
Schematic of a CNT-bot composed of a –COOH-functionalized MWCNT cluster doped with a ferrous (Fe2+) salt and magnetite nanoparticles (FeONPs) before being coated with a Mg film. (Figure was reproduced with permission from Mitra et al. 2020)
2.2 Design of Nanorobots
For nearly two decades, researchers have been trying to perform biomedical tasks such as drug-targeted delivery, cancer treatment, and microsurgery by designing nanorobots that can convert energy such as chemical energy, magnetic energy, electrical energy, light energy, and ultrasound energy in the environment into mechanical motion. However, how to improve the biocompatibility, anti-blood fouling ability, and drug-carrying capacity of nanorobots and the therapeutic effect of cancer cells are the challenges faced by nanorobots for future biomedical applications. In recent years, the research of self-driven synthetic nanomachines has aroused widespread interest from scientists around the world, because they are expected to enter the human body to travel freely in the blood and perform micro-scale tasks, such as active drug delivery and precision treatment of tumors. However, how to achieve self-driven nanomotors to quickly open cell membranes or carry drugs into the inside of cells still faces huge challenges. Considering the following four aspects is required special considerations in order to obtain a micro-scale robot: (Wan et al. 2020) the composition and structure of nanorobots, biocompatibility and biodegradability; (Zhou et al. 2021) the function, such as we can load the robot with certain drugs, proteins, fluorescent dyes to achieve certain applications or positioning functions; (Patel et al. 2006) driving control, such as magnetism, light, ultrasound and other different energy forms; (Garcia-Gradilla et al. 2013) in vivo tracking methods: such as in shallow tissues, fluorescence imaging tracking, and in deep tissues or organs, ultrasound imaging or magnetic resonance imaging and other techniques can reach. After fully considering the above factors, we can use micro−/nanorobotics to handle with many complex problems in the field of biomedicine. Compared with passively diffused colloidal particles or nanocarriers, nanorobots induce distinctive initiative, which is conducive to optimizing and enhancing the directional enrichment of carriers at the culprit lesion site (Fournier-Bidoz et al. 2005; Mei et al. 2011; Karshalev et al. 2018; Teo et al. 2016).
Challenges in the development process mainly of nanorobots are energy, drive, and control. Traditional chemotherapy was more limited in clinical implementation, and chemotherapy drugs spread widely in the patient’s body, making it difficult for the targeted tissue to reach the optimal therapeutic concentration of the drug, and the causal distribution of the drug may cause greater toxic side effects (Kumar 2011; Deshpande et al. 2015). Therefore, nanorobots with drive-response systems have great application prospects in the process of drug delivery. Drive-response system is the “engine” in the nanoscale, which can convert other forms of energy into the energy that drives nanorobots to work (Mathesh et al. 2022; Ortiz-Rivera et al. 2018). Unlike macroscopic robots, nanorobots cannot connect external wires or carry batteries to power, nor can they load motors to generate motion. In addition, in the microscopic environment, how to observe and wirelessly control nanorobots according to instructions to move and operate is also a difficult problem to overcome. In response to these problems, recent progress on nanorobots that scientists have carried out mainly (Li et al. 2016; Chatzipirpiridis et al. 2015; Yang et al. 2020; Fan et al. 2021) includes light-driven (Nature 2022; Kirui et al. 2014; Peng et al. 2022), thermal-driven (Pinan Basualdo et al. 2021; Barbot et al. 2019; Ongaro et al. 2017), chemically driven (Mitra et al. 2020; Xuan et al. 2014; Sanchez et al. 2015), and microbial robots’ in vivo tracking and localization. In 2013, a research team at the University of Georgia in the United States developed a magnetically driven nanorobot that accelerates thrombolysis by facilitating the delivery of tissue plasminogen activators; in the same year, Pumera’s team first studied the toxic effects of a self-propelled nanorobot on human cells, making the nanorobot a solid step toward clinical application (Cheng et al. 2014; Chng et al. 2014). Cai Lintao’s team selected ocean-derived magnetotropic bacteria (AMB-1) as a template, used Michael’s addition reaction to load nano-photosensitizers onto the surface of bacteria, and built an intelligent micro−/nanorobot (AI microrobot), which realized magnetron navigation, tumor penetration, and photothermal ablation in mice through magnetic/light sequence manipulation (Deng et al. 2020; Chen et al. 2019b). An AI micro−/nanorobot driven by sequential magnetism and light triggered, and used to achieve active targeting of cancer treatment, was designed by Cai Lintao’s team at the Shenzhen Institute of Advanced Technology of the Chinese Academy of Sciences for the treatment of tumors. By implanting an AI micro−/nano-biological robot in a tumor patient, it can automatically swim to the tumor foci site and eliminate the tumor, and then the robot itself can be absorbed by the human body without causing any damage to health. Using the magnetic and hypoxia integrated targeting of micro−/nano-biological robots, after breaking through the complex physiological barrier with photosensitizer into the tumor interior, the remote near-infrared laser trigger is used to generate local high temperature, and the visualized and precise treatment of tumors is realized in the experiment (Xing et al. 2021).
Recently, using liquid metal gallium as the material, the research team realized the mass fabrication of the white blood cell membrane surface camouflage liquid metal gallium needle-like swimming nanorobot by combining nanoporous template plastic forming and cell membrane coating technology. This liquid metal swimming nanorobot that combines driving motion, deformation-fusion, drug loading, photothermal absorption, and anti-blood fouling capabilities is expected to provide new research ideas for the design of a new generation of swimming nanorobots (Wang et al. 2020). A new type of thrombolytic drug delivery strategy has been designed, which developed an open space C-shaped magnetic drive system with functions such as laser positioning and ultrasound imaging navigation to achieve in vivo applications (Wang et al. 2021a). Nanorobots with chemical response as driving forces have received great attention and are currently a common driving method (Lyu et al. 2021). Hydrogen peroxide was used as a fuel, which decomposed to release oxygen bubbles, creating a thrust that drove nanorobots to swim in the liquid. However, current designs are primarily based on complex three-dimensional (3D) structures with limited accessible surface area at catalytic reaction sites, requiring higher fuel concentrations to drive. The team at Deakin University in Australia recently designed an enzyme-based nanorobot that can be actively driven at ultra-low fuel concentration (0.003% H2O2) by studying the weak interaction of the two-dimensional nanostructure-enzyme interface and designing, constructing, and regulating the catalytic reaction dynamics of biological enzymes at the single-molecule level (Mathesh et al. 2022). The controlled surface chemistry of the two-dimensional enzyme nanostructure, the high specific surface area, and the scalable synthesis method make it ideal for the development of efficient nanorobots. Ultrasonic response serves an important role during driving nanorobots work in vivo. The development of a needle-type acoustic-electrical composite electrode realized the generalized cluster control of tubular and spherical nanorobots and the controllable switching of motion modes. Through the electrolyzed water bubble generation technology, a micron-sized bubble vibration core was formed at the electrode tip, which resonated with ultrasonic field excitation, and then formed a locally enhanced sound flow field, driving the neighboring nanorobot to quickly disperse to other areas. However, after removing the bubbles, the acoustic-electrical composite electrode could be used as an acoustic isolator, providing a fixed boundary to block the propagation of acoustic energy in the fluid and then forming an energy potential trap around the electrode, driving a wide range of dispersed nanorobots to enrich below the electrode. Surprisingly, these two functional modes of the acoustic-electrical composite electrode did not have coupling interference under different ultrasonic frequency excitation. Although the current limitation of this study was that it cannot effectively drive large nanorobots with a diameter of more than 30 μm to carry out cluster movements, the clustered control platform achieved a high degree of integration with existing electrochemical detection platforms, providing a possibility for the realization of active ultrasensitivity detection technology based on nanorobots (Lu et al. 2021). In the intricate body, only the nanorobots that drive the system cannot discern the direction and complete the task of targeting tumors (Yuan et al. 2017). Therefore, it is necessary to carry a navigation system at the same time. At present, scientists mainly use magnetic fields, infrared rays, ultrasonic, and other signals with strong penetration and directionality to guide the direction of movement of nanorobots. Combined with the emerging in vivo imaging technology, it is expected to realize the remote and precise control of nanorobots (Soto et al. 2022). Meanwhile, a “tracker” for tumor cells for the nanorobot has been installed, tumor cells often have specific protein receptors that are overexpressed on the surface, and ligands that specifically recognize these receptors can be accurately identified and bound to tumor cells by connecting them on the surface of the nanorobot (Zhang et al. 2021a). The team of Fudan University in China cleverly used the simultaneous (alkenyl) radical polymerization reaction and (trimethoxysilane) hydrolyzed polymerization reaction in the precursor to achieve selective island growth of silicon on the surface of polystyrene nanoparticles and then obtained a nanorobot with a selective hollow structure after wrapping polydopamine and calcination at high temperature. In addition, parameters such as the size and quantity of island silicones can be precisely adjusted, and then multifunctional nanorobots with adjustable topologies can be synthesized according to different needs, and the super-assembly strategy can achieve rapid movement, intelligent drug delivery, and collaborative precision treatment of cancer (Xie et al. 2022).
The response system of nanorobots is also an urgent consideration for their application. For example, nanorobots containing various response elements gushed out in tumor-specific microenvironments. Responding element remains stable in blood circulation and normal tissues, while a selective response occurs in the tumor microenvironment and releases the drug. It not only ensures the fixed-point delivery of drugs but also realizes the specific treatment of tumors. A piece of DNA sequence capable of binding highly selectively to the target tumor is used as a “drug release switch” for the nanorobot, enabling it to release the drug at the tumor site at a fixed point (Li et al. 2018). The Shanghai Institute of Materia Medica of the Chinese Academy of Sciences has designed and prepared a drug-carrying nanorobot based on Boolean operation, which will dissociate different drugs for acidic, reducing, and special enzymes to achieve drug release response (Hou et al. 2020).
It is worth mentioning that nano-manipulation and intelligence need to be considered in the application of nanorobots. Nano-manipulation technology is the precise manipulation of a tiny object at the micro- or nanoscale (Drinkwater 2016; Nelson et al. 2010). Intelligence is an important step in the practical application of nanorobots, which aims to use simple machines to explore and make decisions in complex environments (Ceylan et al. 2017). Tang et al. prepared a nanorobot of TiO2 nanotree, which will react with photocells under light, generate anions and cations at the opposite ends of the nanotree, form an electric field, and then promote the forward movement of the nanotree under the action of the electric field. By adjusting the electric potential and the direction of light, an asymmetrical concentration field/photoelectric field can be generated, and the nanotree is deflected, thus achieving different dimensions of freedom (Dai et al. 2016).
The unique advantages of nanorobots in self-driving, in vivo navigation, and intelligent response give it a promising future in drug delivery, surgery, and sensing.
2.3 Application
2.3.1 Drug Delivery
The traditional treatment of diseases is mainly based on drugs, but due to the lack of precious targeting, the concentration of lesions in the body is insufficient, causing poor treatment effect, and even the drug is diffuse, resulting in damage to healthy tissues and causing side effects in patients (Orive et al. 2003; Zelikin et al. 2016). Some scholars have made statistics on drug delivery in the past 30 years and found that after about 12 hours of traditional delivery, less than 1% of the drugs reached the target location. This means that the vast majority of medications have been lost on the road (Yao et al. 2012). Therefore, the construction of a new type of drug active transportation channel has become a research hotspot in the industry (Suri et al. 2016; Wang et al. 2016). As mentioned, nanorobots are subject to controlled driving forces and navigation and response systems, which attract the attention of the research field (Nelson et al. 2010; Torne et al. 2013). A variety of micro- and nanodrug carrier robots rely on self-propulsion movements to cross the barrier of multiple biological barriers to send drugs to the bottom of the eyeball or deep in the brain tissue, so that the thorny medical problems such as glaucoma, epilepsy, glioblastoma, stroke hemiplegia, and so on can be solved (Lee et al. 2012; Fu et al. 2021). Since 2004, a variety of chemical and external physics (such as photoelectromagnetic heat)-driven swimming micro−/nanorobots have emerged in the industry, which can swim efficiently in water. These micro- and nanorobots can not only swim efficiently in water or other biological fluids but also control their movement behavior with the help of chemistry, light, magnetism, etc., so that they can reach the specified location according to people’s wishes (Douglas et al. 2012). However, the human internal environment is very complex, especially in the body; there are also a variety of biological barriers such as blood-brain barriers and blood-eye barriers, which protect the human body from the invasion of foreign bacteria and viruses at the same time, but also prevent these robots from accurately delivering drugs to the patient area (Gallego and Cena 2020; Pourgholi et al. 2016).
The early swimming micro−/nanorobots were basically composed of components such as microelectromechanical systems, and their own materials were mainly metals, metal oxides, and artificial polymers. After micro−/nanorobots enter the body, they cannot be degraded at first, so they have great danger; secondly, these metals and metal polymers are human exogenous substances and have poor biocompatibility; once they enter the body, they will trigger the “alarm” of the immune system and then be surrounded by immune cells; and before they reach the lesion, they may have been strangled by the human immune system (Zhang et al. 2004). As early as 2010, the first swimming nanorobot research and development team in China, applying chemical methods, for the first time to assemble atoms into micro−/nanostructures, successfully performed controlled swimming under the chemical field or external light and magnetic field and even directly guided to the target cell (Bayrac et al. 2017). Scientists innovatively proposed that the magnetic drug carrier gel is covered with a “camouflage” coat using the E. coli membrane, thereby increasing the amount of phagocytosis of the magnetic drug-carrying particles by neutrophils, manipulating the neutrophil microrobot to enrich the brain, and the neutrophil robot that reaches the brain relies on its tendency function, crosses the blood-brain barrier along the inflammatory factors released by the glioma, and finally reaches the glioma patient area and releases the drug, realizing the drive precision target under the exogenous magnetic field. The strategy also improves the biocompatibility of the swimming micro−/nanorobot and also prevents the leakage of drugs contained in the magnetic nanogel (Zhang et al. 2021a) (Fig. 2.2). By binding DNA aptamers outside the nanorobot, nucleolin (a protein specifically expressed on tumor-associated endothelial cells) and thrombin in its lumen can be bound. In this design, the nucleoside targeting aptamer is both a targeting domain and a molecular trigger mechanically opened by nanorobots. Thus, thrombin in the body is exposed and activates coagulation at the site of the tumor, thereby inhibiting the growth and exacerbation of the tumor (Li et al. 2018). The white blood cell membrane modified on the surface of the nanorobot can accelerate the entry into cancer cells through specific recognition with cancer cells, while the liquid metal swimming nanorobot loaded with the anti-cancer drug doxorubicin shows good cancer cell drug and photothermal combination therapy ability (Wang et al. 2020).
Schematic of active therapeutics of dual-responsive neutrobots in vivo. (Figure was reproduced with permission from Zhang et al. 2021a)
In nanorobots carrying drug targets into the body, drugs are trapped on the surface of micro−/nanorobots by electrostatic action (Soto et al. 2020). Electrostatics has been reported to act as a positively charged antimicrobial drug by ultrasound into negatively charged polypyrrole-polystyrene sulfonate fragments on nanorobots. Moreover, the electrostatic interaction is stable at pH 7. Under different acid-base conditions, drug release can be carried out. This method proposes a unique trigger release mechanism based on electrochemical stimulation (Manesh et al. 2010; Li et al. 2017). In addition, the use of electrochemical stimulation as a release mechanism is further expanded by loading the therapeutic payload with bismuth coating. The surface of the ultrasonically propulsion porous nanowire is functionally performed with an anionic coating that allows doxorubicin electrostatic loading into a micro−/nanorobot structure. The porous part is responsible for increasing the drug load and promoting the release through the photothermal effect of near-infrared light radiation (Gao et al. 2021).
2.3.2 Surgery
At present, the miniaturized surgical tools or medical devices used in large-scale surgeries in clinical practice, although relatively small in size, still do not have the ability to enter places that catheters and blades cannot reach, hindering the progress of surgery. Surgery using micro−/nanorobots may reach areas of the body that cannot be reached by catheters or invasive surgeries, allowing tissues to be sampled or treatment payloads to be drilled deep into diseased tissues (Xu and Liu 2021; Saravana and Vijayalakshmi 2006). In addition, the potential to reduce the risk of infection and recovery time is a distinct advantage of nanorobots (Agrahari et al. 2020).
Nanorobots can target any tissue part of the body and perform corresponding operations at the cellular level, thus assisting doctors in achieving more accurate, flexible, and controllable minimally invasive surgery (Zhang et al. 2022). The micro−/nanotechnology tools currently used in minimally invasive surgery include nano-drills, micro-clamps, and micro-bullets. Among them, the micro-clamp can pass through the body’s narrowest capillaries to capture and move out of the cells in the tissue (Agrahari et al. 2020).
Studies have shown that nanorobots can penetrate thick biological tissues with the help of magnetic fields and ultrasonic waves, and on this basis, nanorobots carrying magnetic fields and ultrasonic control systems have been developed. Magnetically driven microrobot implants have been successful in eye surgery in animals (Tabatabaei et al. 2016), while ultrasound-driven nanomachines can perform precision surgeries at the single-cell level or even at the subcellular level (Chechetka et al. 2016). Both studies suggest that effective control systems combined with nanorobots can accurately position themselves in surgery and efficiently advance the application of nanorobots in surgery (Nguyen et al. 2017).
Neurosurgery has always had pain points such as small surgical space and difficulty in positioning, and because surgery generally requires operation of specific parts of neural tissue, the operation needs to be very precise. Surgeons generally struggle to achieve the required precision (Bland 2005). In the mid-1980s, PUMA robots were first used in neurosurgery (Zollinger 1991; Barnes 2016). According to the preoperative image of the intracranial lesion, the surgeon enters the coordinates of the lesion into the robot and applies the robot-guided needle for biopsy and other operations. In 1985, Kwoh et al. used the position information obtained from images by the PUMA206 robot for the first time to stereotaxicize patients and perform biopsy surgery (Kwoh et al. 1988). According to the preoperative image of the intracranial lesion, the surgeon enters the coordinates of the lesion into the robot and applies the robot-guided needle for biopsy and other operations. This is the first clinical application of neurosurgical robots.
Therefore, the use of robots to do precise movements on the basis of medical image guidance has become the preferred surgical method of most doctors. Spiral nanorobots made of iron-coated silica capable of being controlled by low-intensity magnetic fields and able to track its movements reach depths during root canal treatment to a depth that is not possible during current clinical treatment, thus helping to completely kill bacteria deep inside the dentin tubules to increase the success rate of root canal treatment (Dasgupta et al. 2022) (Fig. 2.3). A good association of the internal structures of the brain with the external surgical framework can be achieved through medical images. In related clinical applications, such robots are mainly used as an aid to stereoscopic frame positioning, using 3D image guidance and positioning surgical tools to achieve intracranial target targets. At present, neurosurgical robots are mainly used for brain surgery, biopsy, Parkinson’s disease targeted stimulation, epilepsy electrode measurement, removal of cysts or hematoma emptying, and other operations (Benabid et al. 1987).
Schematic diagram of the distribution of dental spiral magnetic nanorobots under oscillating field. (Figure was reproduced with permission from Dasgupta et al. 2022)
At present, neurosurgery robotic systems have evolved from stereotactic surgery to microsurgery and even remote surgery. With the rapid development of multimedia and information network technology, high-speed networks and virtual reality systems based on effective computer graphics provide technical support for remote human-machine communication, making long-distance surgery a reality, and surgery can be completed by surgeons controlling the robot at the surgical site through remote control operating systems in different places.
2.3.3 Biosensing
Recently, nanoporous sensors have become an important tool for solution-based analysis of key components of single-molecule life, including nucleic acids, proteins, polysaccharides, and a large number of biomolecules that play an important role in life and healthcare (Rahman et al. 2021). Nanosensors are nanoscale in shape or sensitivity, or the interaction distance between the sensor and the substance or object to be detected is in the nanometer range (Chinnadayyala et al. 2019; Fu and Ma 2020; Ding et al. 2002). Nanosensors have sub-micron sizes, transducers, probes, or nano−/microsystems, their physicochemical properties and their detection sensitivity to biomolecules or cells are greatly improved, the detection reaction time is also greatly shortened, and high-throughput real-time detection and analysis can be realized (Polidori et al. 2013; Miyashita et al. 2009). Nanosensors mainly use electricity. When materials are physically manipulated, nanosensors change their conductivity, triggering a detectable signal response that ultimately analyzes and measures the response (Liu et al. 2021). Nanotechnology sensors are mainly divided into nanochemical and biological sensors, nano-gas sensors, and other types of nanosensors, such as pressure, temperature, and flow (Lee and Wong 2009; Mishra et al. 2019). At present, nanochemical and biological sensors are clinically diagnosing cancer, cardiovascular disease, etc. in an early stage (Benjaminsen et al. 2011). Due to the attached surface of the gas sensor, a nano-coating is used as a sensitive material to improve the sensitivity and performance of the sensor, usually metal oxide semiconductor nanoparticles, carbon nanotubes, and two-dimensional nanofilms (Forier et al. 2014). The adsorbed gas molecules interact with carbon nanotubes, change their Fermi energy level to cause a significant change in their macroscopic resistance, and detect the gas composition by detecting changes in their resistance, which can be used as a gas sensor (Sondergaard et al. 2014).
Compared with traditional sensors, nanosensors have many significant characteristics, such as high sensitivity, low power consumption, low cost, and multifunctional integration, due to their ability to operate at the atomic and molecular scales, making full use of the unique properties of nanomaterials, Raman spectral effects, catalytic efficiency, conductivity, strength, hardness, toughness, superplasticity, and superparamagnetism (Fulaz et al. 2019; Desai et al. 2013). The sensor made of nanotechnology, the size is reduced, the accuracy is improved, the performance is greatly improved, the nanosensor is standing on the atomic scale, which greatly enriches the theory of the sensor, promotes the production level of the sensor, and broadens the application field of the sensor. Nanosensors have now been widely developed in the fields of biology, chemistry, mechanics, aviation, and military.
Sensors made using nanotechnology can be used for the early diagnosis, monitoring, and treatment of diseases, making early diagnosis of various cancers a display (Aylott 2003). For example, when performing blood tests, when the antibodies to cancer cells present in the sensor encounter the corresponding antigen, the current in the sensor changes, and the type and concentration of cancer cells in the blood can be judged through this current change (Fu and Ma 2020). In the monitoring of ovarian cancer, aromatic ligands capable of reacting with volatile organic compounds associated with ovarian cancer are first selected, forming an integrated cross-reaction induction array with gold nanoparticles and flexible striped polyimide films (Salvati et al. 2015). When a patient with ovarian cancer exhales gas through gold nanoparticles, the specific volatile organic compounds in them react with ligands on the array, causing measurable changes in resistance. This bendable sensor has a high 80% accuracy rate in ovarian cancer diagnosis (Williams et al. 2018). A special odor is produced by human respiration, which consists of specific volatile organic compounds that characterize hypoglycemia. An array of nanosensors was developed to detect this odor and could transmit hypoglycemic signals to relatives for patient safety (Lemmerman et al. 2020). In addition to medical diagnosis, nanosensing is also used in microelectronics information technology and national defense security (Zhang et al. 2021b). Nano-gold materials are the core materials of nanosensors, which are widely used in test strips, and their size, shape, and self-assembly behavior directly affect the performance of visualization (Wang et al. 2021b). Through the development of bioinformatic chips, nanorobots, and brain-computer interface devices embedded in nanosensors, foreign militaries can effectively improve human memory, reaction ability, and visual and auditory sensitivity and improve the battlefield perception and rapid disposal ability of combatants (Wang 2018).
Nanosensors have revolutionized the need for portable analytical tools with selectivity, stability, and other characteristics in every industry. Nanosensors also have high sensitivity for greater accuracy. They can easily interact at the nanoscale. Small, durable, lightweight, and portable, nanosensors enable better sensitivity, power, speed, and detection range due to the ratio of surface area to volume (Du et al. 2012). Compared to other types of sensors, nanosensors have shorter response times, are faster, and can be analyzed in real time (Li et al. 2018). In addition, nanosensors require a smaller sample volume for analysis and cause minimal interference with the observed material or process. They can also detect multiple things at the same time, making them devices with multiple functions (Fig. 2.4).
2.4 Conclusion
Nanotechnology has provided new ways and means for drug delivery and disease treatment, making significant progress in the treatment of some of the most difficult diseases to overcome. With the help of nanocarriers, drugs can overcome the biological barriers of the human body, directly reach the lesion area through artificial manipulation, and reduce the damage to other tissues, while increasing the local drug concentration to enhance the therapeutic effect, nanorobots are undoubtedly the most representative technology for drug delivery with the help of nanocarriers. Nano-scale adjustment kills mutated cancerous cells, through the guidance of external lasers, accurate calculations to find cancerous cells with radiation exceeding standards, and the use of advanced biological cell lysis technology to dissolve possible diseased cells into chemical molecular elements, and through the accurate verification of specific sensor systems, the cell components are successfully entered into healthy cells, completing the conversion of necrotic cells and successful healthy cells. In short, the unique advantages of nanobots with self-driving, in vivo navigation, and intelligent response make it have broad prospects in tumor treatment. In addition, in the future, nanorobots are also expected to shine in medical fields such as in vivo imaging, immunotherapy, and minimally invasive surgery. On the other hand, as a new drug carrier system, nanorobots also face challenges such as low biocompatibility, insufficient in vivo experimental data, and high thresholds for industrial production. At present, most nanorobots are still in the verification stage of in vitro experiments or animal models, and there is still a certain distance from real clinical applications. We expect that with the painstaking research of scientists, nanorobots will be able to overcome these problems and bring revolutionary progress to medicine.
References
Agrahari V, Agrahari V, Chou ML, Chew CH, Noll J, Burnouf T. Intelligent micro−/nanorobots as drug and cell carrier devices for biomedical therapeutic advancement: promising development opportunities and translational challenges. Biomaterials. 2020;260:120163. https://doi.org/10.1016/j.biomaterials.2020.120163.
Aylott JW. Optical nanosensors--an enabling technology for intracellular measurements. Analyst. 2003;128(4):309–12. https://doi.org/10.1039/b302174m.
Barbot A, Tan H, Power M, Seichepine F, Yang GZ. Floating magnetic microrobots for fiber functionalization. Sci Robot. 2019;4(34):eaax8336. https://doi.org/10.1126/scirobotics.aax8336.
Barnes SA. Reply to “Women in academic surgery: why is the playing field still not level?” Appearing in the American Journal of Surgery, Feb. 2016, volume 211, number 2. Am J Surg. 2016;212(2):366. https://doi.org/10.1016/j.amjsurg.2016.03.002.
Bayrac C, Eyidogan F, Avni OH. DNA aptamer-based colorimetric detection platform for salmonella Enteritidis. Biosens Bioelectron. 2017;98:22–8. https://doi.org/10.1016/j.bios.2017.06.029.
Benabid AL, Cinquin P, Lavalle S, Le Bas JF, Demongeot J, de Rougemont J. Computer-driven robot for stereotactic surgery connected to CT scan and magnetic resonance imaging. Technological design and preliminary results. Appl Neurophysiol. 1987;50(1–6):153–4. https://doi.org/10.1159/000100701.
Benjaminsen RV, Sun H, Henriksen JR, Christensen NM, Almdal K, Andresen TL. Evaluating nanoparticle sensor design for intracellular pH measurements. ACS Nano. 2011;5(7):5864–73. https://doi.org/10.1021/nn201643f.
Bland KI. Hiram Polk and the American journal of surgery. Am J Surg. 2005;190(2):338–43. https://doi.org/10.1016/j.amjsurg.2005.05.037.
Bucolo M, Bucolo G, Buscarino A, Fiumara A, Fortuna L, Gagliano S. Remote ultrasound scan procedures with medical robots: towards new perspectives between medicine and engineering. Appl Bionics Biomech. 2022;2022:1072642. https://doi.org/10.1155/2022/1072642.
Ceylan H, Giltinan J, Kozielski K, Sitti M. Mobile microrobots for bioengineering applications. Lab Chip. 2017;17(10):1705–24. https://doi.org/10.1039/c7lc00064b.
Chatzipirpiridis G, Ergeneman O, Pokki J, Ullrich F, Fusco S, Ortega JA, et al. Electroforming of implantable tubular magnetic microrobots for wireless ophthalmologic applications. Adv Healthc Mater. 2015;4(2):209–14. https://doi.org/10.1002/adhm.201400256.
Chechetka SA, Yuba E, Kono K, Yudasaka M, Bianco A, Miyako E. Magnetically and near-infrared light-powered supramolecular nanotransporters for the remote control of enzymatic reactions. Angew Chem Int Ed Engl. 2016;55(22):6476–81. https://doi.org/10.1002/anie.201602453.
Chen Z, Wang Z, Gu Z. Bioinspired and biomimetic nanomedicines. Acc Chem Res. 2019a;52(5):1255–64. https://doi.org/10.1021/acs.accounts.9b00079.
Chen F, Zang Z, Chen Z, Cui L, Chang Z, Ma A, et al. Nanophotosensitizer-engineered salmonella bacteria with hypoxia targeting and photothermal-assisted mutual bioaccumulation for solid tumor therapy. Biomaterials. 2019b;214:119226. https://doi.org/10.1016/j.biomaterials.2019.119226.
Cheng R, Huang W, Huang L, Yang B, Mao L, Jin K, et al. Acceleration of tissue plasminogen activator-mediated thrombolysis by magnetically powered nanomotors. ACS Nano. 2014;8(8):7746–54. https://doi.org/10.1021/nn5029955.
Chinnadayyala SR, Park J, Le HTN, Santhosh M, Kadam AN, Cho S. Recent advances in microfluidic paper-based electrochemiluminescence analytical devices for point-of-care testing applications. Biosens Bioelectron. 2019;126:68–81. https://doi.org/10.1016/j.bios.2018.10.038.
Chng EL, Zhao G, Pumera M. Towards biocompatible nano/microscale machines: self-propelled catalytic nanomotors not exhibiting acute toxicity. Nanoscale. 2014;6(4):2119–24. https://doi.org/10.1039/c3nr04997c.
Dai B, Wang J, Xiong Z, Zhan X, Dai W, Li CC, et al. Programmable artificial phototactic microswimmer. Nat Nanotechnol. 2016;11(12):1087–92. https://doi.org/10.1038/nnano.2016.187.
Dasgupta D, Peddi S, Saini DK, Ghosh A. Mobile nanobots for prevention of root canal treatment failure. Adv Healthc Mater. 2022;11:e2200232. https://doi.org/10.1002/adhm.202200232.
Deng G, Peng X, Sun Z, Zheng W, Yu J, Du L, et al. Natural-killer-cell-inspired Nanorobots with aggregation-induced emission characteristics for near-infrared-II fluorescence-guided glioma theranostics. ACS Nano. 2020;14(9):11452–62. https://doi.org/10.1021/acsnano.0c03824.
Desai AS, Chauhan VM, Johnston AP, Esler T, Aylott JW. Fluorescent nanosensors for intracellular measurements: synthesis, characterization, calibration, and measurement. Front Physiol. 2013;4:401. https://doi.org/10.3389/fphys.2013.00401.
Deshpande A, Mailis-Gagnon A, Zoheiry N, Lakha SF. Efficacy and adverse effects of medical marijuana for chronic noncancer pain: systematic review of randomized controlled trials. Can Fam Physician. 2015;61(8):e372–81.
Ding Z, Quinn BM, Haram SK, Pell LE, Korgel BA, Bard AJ. Electrochemistry and electrogenerated chemiluminescence from silicon nanocrystal quantum dots. Science. 2002;296(5571):1293–7. https://doi.org/10.1126/science.1069336.
Douglas SM, Bachelet I, Church GM. A logic-gated nanorobot for targeted transport of molecular payloads. Science. 2012;335(6070):831–4. https://doi.org/10.1126/science.1214081.
Drinkwater BW. Dynamic-field devices for the ultrasonic manipulation of microparticles. Lab Chip. 2016;16(13):2360–75. https://doi.org/10.1039/c6lc00502k.
Du G, Moulin E, Jouault N, Buhler E, Giuseppone N. Muscle-like supramolecular polymers: integrated motion from thousands of molecular machines. Angew Chem Int Ed Engl. 2012;51(50):12504–8. https://doi.org/10.1002/anie.201206571.
Ewart L, Dehne EM, Fabre K, Gibbs S, Hickman J, Hornberg E, et al. Application of microphysiological systems to enhance safety assessment in drug discovery. Annu Rev Pharmacol Toxicol. 2018;58:65–82. https://doi.org/10.1146/annurev-pharmtox-010617-052722.
Fan F, Jin L, Yang L. pH-sensitive nanoparticles composed solely of membrane-disruptive macromolecules for treating pancreatic cancer. ACS Appl Mater Interfaces. 2021;13(11):12824–35. https://doi.org/10.1021/acsami.0c16576.
Forier K, Raemdonck K, De Smedt SC, Demeester J, Coenye T, Braeckmans K. Lipid and polymer nanoparticles for drug delivery to bacterial biofilms. J Control Release. 2014;190:607–23. https://doi.org/10.1016/j.jconrel.2014.03.055.
Fournier-Bidoz S, Arsenault AC, Manners I, Ozin GA. Synthetic self-propelled nanorotors. Chem Commun (Camb). 2005;41(4):441–3. https://doi.org/10.1039/b414896g.
Fu Y, Ma Q. Recent developments in electrochemiluminescence nanosensors for cancer diagnosis applications. Nanoscale. 2020;12(26):13879–98. https://doi.org/10.1039/d0nr02844d.
Fu X, Chen T, Song Y, Feng C, Chen H, Zhang Q, et al. mRNA delivery by a pH-responsive DNA Nano-hydrogel. Small. 2021;17(29):e2101224. https://doi.org/10.1002/smll.202101224.
Fulaz S, Hiebner D, Barros CHN, Devlin H, Vitale S, Quinn L, et al. Ratiometric imaging of the in situ pH distribution of biofilms by use of fluorescent mesoporous silica nanosensors. ACS Appl Mater Interfaces. 2019;11(36):32679–88. https://doi.org/10.1021/acsami.9b09978.
Gallego L, Cena V. Nanoparticle-mediated therapeutic compounds delivery to glioblastoma. Expert Opin Drug Deliv. 2020;17(11):1541–54. https://doi.org/10.1080/17425247.2020.1810015.
Gao Y, Wei F, Chao Y, Yao L. Bioinspired soft microrobots actuated by magnetic field. Biomed Microdevices. 2021;23(4):52. https://doi.org/10.1007/s10544-021-00590-z.
Garcia-Gradilla V, Orozco J, Sattayasamitsathit S, Soto F, Kuralay F, Pourazary A, et al. Functionalized ultrasound-propelled magnetically guided nanomotors: toward practical biomedical applications. ACS Nano. 2013;7(10):9232–40. https://doi.org/10.1021/nn403851v.
Guided by the light, nimble nanovehicles make special deliveries. Nature. 2022;601(7894):487. https://doi.org/10.1038/d41586-022-00115-5.
Hosseinidoust Z, Mostaghaci B, Yasa O, Park BW, Singh AV, Sitti M. Bioengineered and biohybrid bacteria-based systems for drug delivery. Adv Drug Deliv Rev. 2016;106(Pt A):27–44. https://doi.org/10.1016/j.addr.2016.09.007.
Hou B, Zhou L, Wang H, Saeed M, Wang D, Xu Z, et al. Engineering stimuli-activatable boolean logic prodrug nanoparticles for combination cancer immunotherapy. Adv Mater. 2020;32(12):e1907210. https://doi.org/10.1002/adma.201907210.
Hu M, Ge X, Chen X, Mao W, Qian X, Yuan WE. Micro/nanorobot: a promising targeted drug delivery system. Pharmaceutics. 2020;12(7):665. https://doi.org/10.3390/pharmaceutics12070665.
Jager EW, Inganas O, Lundstrom I. Microrobots for micrometer-size objects in aqueous media: potential tools for single-cell manipulation. Science. 2000;288(5475):2335–8. https://doi.org/10.1126/science.288.5475.2335.
Karshalev E, Esteban-Fernandez de Avila B, Wang J. Micromotors for “chemistry-on-the-Fly”. J Am Chem Soc. 2018;140(11):3810–20. https://doi.org/10.1021/jacs.8b00088.
Khan FA, Narasimhan K, Swathi CSV, Mustak S, Mustafa G, Ahmad MZ, et al. 3D printing technology in customized drug delivery system: current state of the art, prospective and the challenges. Curr Pharm Des. 2018;24(42):5049–61. https://doi.org/10.2174/1381612825666190110153742.
Kim S, Qiu F, Kim S, Ghanbari A, Moon C, Zhang L, et al. Fabrication and characterization of magnetic microrobots for three-dimensional cell culture and targeted transportation. Adv Mater. 2013;25(41):5863–8. https://doi.org/10.1002/adma.201301484.
Kim E, Jeon S, An HK, Kianpour M, Yu SW, Kim JY, et al. A magnetically actuated microrobot for targeted neural cell delivery and selective connection of neural networks. Sci Adv. 2020;6(39):eabb5696. https://doi.org/10.1126/sciadv.abb5696.
Kirui DK, Koay EJ, Guo X, Cristini V, Shen H, Ferrari M. Tumor vascular permeabilization using localized mild hyperthermia to improve macromolecule transport. Nanomedicine. 2014;10(7):1487–96. https://doi.org/10.1016/j.nano.2013.11.001.
Kumar SP. Cancer pain: a critical review of mechanism-based classification and physical therapy management in palliative care. Indian J Palliat Care. 2011;17(2):116–26. https://doi.org/10.4103/0973-1075.84532.
Kwoh YS, Hou J, Jonckheere EA, Hayati S. A robot with improved absolute positioning accuracy for CT guided stereotactic brain surgery. IEEE Trans Biomed Eng. 1988;35(2):153–60. https://doi.org/10.1109/10.1354.
Lee YH, Wong DT. Saliva: an emerging biofluid for early detection of diseases. Am J Dent. 2009;22(4):241–8.
Lee H, Lytton-Jean AK, Chen Y, Love KT, Park AI, Karagiannis ED, et al. Molecularly self-assembled nucleic acid nanoparticles for targeted in vivo siRNA delivery. Nat Nanotechnol. 2012;7(6):389–93. https://doi.org/10.1038/nnano.2012.73.
Lee JH, Lee JM, Hwang J, Park JY, Kim M, Kim DH, et al. User perception of medical service robots in hospital wards: a cross-sectional study. J Yeungnam Med Sci. 2022;39(2):116–23. https://doi.org/10.12701/yujm.2021.01319.
Lemmerman LR, Das D, Higuita-Castro N, Mirmira RG, Gallego-Perez D. Nanomedicine-based strategies for diabetes: diagnostics, monitoring, and treatment. Trends Endocrinol Metab. 2020;31(6):448–58. https://doi.org/10.1016/j.tem.2020.02.001.
Li J, Gao W, Dong R, Pei A, Sattayasamitsathit S, Wang J. Nanomotor lithography. Nat Commun. 2014;5:5026. https://doi.org/10.1038/ncomms6026.
Li H, Zhang J, Zhang N, Kershaw J, Wang L. Fabrication and wireless micromanipulation of magnetic-biocompatible microrobots using microencapsulation for microrobotics and microfluidics applications. J Microencapsul. 2016;33(8):712–7. https://doi.org/10.1080/02652048.2016.1234514.
Li J, Esteban-Fernandez de Avila B, Gao W, Zhang L, Wang J. Micro/nanorobots for biomedicine: delivery, surgery, sensing, and detoxification. Sci Robot. 2017;2(4):eaam6431. https://doi.org/10.1126/scirobotics.aam6431.
Li S, Jiang Q, Liu S, Zhang Y, Tian Y, Song C, et al. A DNA nanorobot functions as a cancer therapeutic in response to a molecular trigger in vivo. Nat Biotechnol. 2018;36(3):258–64. https://doi.org/10.1038/nbt.4071.
Li D, Liu C, Yang Y, Wang L, Shen Y. Micro-rocket robot with all-optic actuating and tracking in blood. Light Sci Appl. 2020;9:84. https://doi.org/10.1038/s41377-020-0323-y.
Liu M, Qiu JG, Ma F, Zhang CY. Advances in single-molecule fluorescent nanosensors. Wiley Interdiscip Rev Nanomed Nanobiotechnol. 2021;13(5):e1716. https://doi.org/10.1002/wnan.1716.
Lu X, Wei Y, Ou H, Zhao C, Shi L, Liu W. Universal control for micromotor swarms with a hybrid Sonoelectrode. Small. 2021;17(44):e2104516. https://doi.org/10.1002/smll.202104516.
Lueg C, Jungo V. Mobile remote presence robots for medical consultation and social connectedness. Stud Health Technol Inform. 2021;281:999–1003. https://doi.org/10.3233/SHTI210328.
Lyu X, Liu X, Zhou C, Duan S, Xu P, Dai J, et al. Active, yet little mobility: asymmetric decomposition of H2O2 is not sufficient in propelling catalytic micromotors. J Am Chem Soc. 2021;143(31):12154–64. https://doi.org/10.1021/jacs.1c04501.
Mak IW, Evaniew N, Ghert M. Lost in translation: animal models and clinical trials in cancer treatment. Am J Transl Res. 2014;6(2):114–8.
Manesh KM, Cardona M, Yuan R, Clark M, Kagan D, Balasubramanian S, et al. Template-assisted fabrication of salt-independent catalytic tubular microengines. ACS Nano. 2010;4(4):1799–804. https://doi.org/10.1021/nn1000468.
Mathesh M, Bhattarai E, Yang W. 2D active nanobots based on soft nanoarchitectonics powered by an ultralow fuel concentration. Angew Chem Int Ed Engl. 2022;61(7):e202113801. https://doi.org/10.1002/anie.202113801.
Mei Y, Solovev AA, Sanchez S, Schmidt OG. Rolled-up nanotech on polymers: from basic perception to self-propelled catalytic microengines. Chem Soc Rev. 2011;40(5):2109–19. https://doi.org/10.1039/c0cs00078g.
Mertz L. Tiny conveyance: micro- and Nanorobots prepare to advance medicine. IEEE Pulse. 2018;9(1):19–23. https://doi.org/10.1109/MPUL.2017.2772118.
Mishra N, Trivedi A, Gajdhar SK, Bhagwat H, Khutwad GK, Mall PE, et al. Correlation of blood glucose levels, salivary glucose levels and oral colony forming units of Candida albicans in type 2 diabetes mellitus patients. J Contemp Dent Pract. 2019;20(4):494–8.
Mitra S, Roy N, Maity S, Bandyopadhyay D. Multimodal chemo−/magneto−/phototaxis of 3G CNT-bots to power fuel cells. Microsyst Nanoeng. 2020;6:19. https://doi.org/10.1038/s41378-019-0122-x.
Mitragotri S, Burke PA, Langer R. Overcoming the challenges in administering biopharmaceuticals: formulation and delivery strategies. Nat Rev Drug Discov. 2014;13(9):655–72. https://doi.org/10.1038/nrd4363.
Miyashita M, Ito N, Ikeda S, Murayama T, Oguma K, Kimura J. Development of urine glucose meter based on micro-planer amperometric biosensor and its clinical application for self-monitoring of urine glucose. Biosens Bioelectron. 2009;24(5):1336–40. https://doi.org/10.1016/j.bios.2008.07.072.
Morgado PI, Palacios M, Larrain J. In situ injectable hydrogels for spinal cord regeneration: advances from the last 10 years. Biomed Phys Eng Express. 2019;6(1):012002. https://doi.org/10.1088/2057-1976/ab52e8.
Mostaghaci B, Yasa O, Zhuang J, Sitti M. Bioadhesive bacterial microswimmers for targeted drug delivery in the urinary and gastrointestinal tracts. Adv Sci (Weinh). 2017;4(6):1700058. https://doi.org/10.1002/advs.201700058.
Nelson BJ, Kaliakatsos IK, Abbott JJ. Microrobots for minimally invasive medicine. Annu Rev Biomed Eng. 2010;12:55–85. https://doi.org/10.1146/annurev-bioeng-010510-103409.
Nguyen VD, Zheng S, Han J, Le VH, Park JO, Park S. Nanohybrid magnetic liposome functionalized with hyaluronic acid for enhanced cellular uptake and near-infrared-triggered drug release. Colloids Surf B Biointerfaces. 2017;154:104–14. https://doi.org/10.1016/j.colsurfb.2017.03.008.
Olsman N, Goentoro L. There‘s (still) plenty of room at the bottom. Curr Opin Biotechnol. 2018;54:72–9. https://doi.org/10.1016/j.copbio.2018.01.029.
Ongaro F, Scheggi S, Ghosh A, Denasi A, Gracias DH, Misra S. Design, characterization and control of thermally-responsive and magnetically-actuated micro-grippers at the air-water interface. PLoS One. 2017;12(12):e0187441. https://doi.org/10.1371/journal.pone.0187441.
Orive G, Hernandez RM, Rodriguez Gascon A, Dominguez-Gil A, Pedraz JL. Drug delivery in biotechnology: present and future. Curr Opin Biotechnol. 2003;14(6):659–64. https://doi.org/10.1016/j.copbio.2003.10.007.
Ortiz-Rivera I, Mathesh M, Wilson DA. A supramolecular approach to nanoscale motion: polymersome-based self-propelled nanomotors. Acc Chem Res. 2018;51(9):1891–900. https://doi.org/10.1021/acs.accounts.8b00199.
Patel GM, Patel GC, Patel RB, Patel JK, Patel M. Nanorobot: a versatile tool in nanomedicine. J Drug Target. 2006;14(2):63–7. https://doi.org/10.1080/10611860600612862.
Peng J, Chen F, Liu Y, Zhang F, Cao L, You Q, et al. A light-driven dual-nanotransformer with deep tumor penetration for efficient chemo-immunotherapy. Theranostics. 2022;12(4):1756–68. https://doi.org/10.7150/thno.68756.
Pinan Basualdo FN, Bolopion A, Gauthier M, Lambert P. A microrobotic platform actuated by thermocapillary flows for manipulation at the air-water interface. Sci Robot. 2021;6(52):eabf1571. https://doi.org/10.1126/scirobotics.abd3557.
Polidori D, Sha S, Ghosh A, Plum-Morschel L, Heise T, Rothenberg P. Validation of a novel method for determining the renal threshold for glucose excretion in untreated and canagliflozin-treated subjects with type 2 diabetes mellitus. J Clin Endocrinol Metab. 2013;98(5):E867–71. https://doi.org/10.1210/jc.2012-4205.
Pourgholi F, Hajivalili M, Farhad JN, Kafil HS, Yousefi M. Nanoparticles: novel vehicles in treatment of glioblastoma. Biomed Pharmacother. 2016;77:98–107. https://doi.org/10.1016/j.biopha.2015.12.014.
Rahman M, Sampad MJN, Hawkins A, Schmidt H. Recent advances in integrated solid-state nanopore sensors. Lab Chip. 2021;21(16):3030–52. https://doi.org/10.1039/d1lc00294e.
Salvati E, Stellacci F, Krol S. Nanosensors for early cancer detection and for therapeutic drug monitoring. Nanomedicine (Lond). 2015;10(23):3495–512. https://doi.org/10.2217/nnm.15.180.
Sanchez S, Soler L, Katuri J. Chemically powered micro- and nanomotors. Angew Chem Int Ed Engl. 2015;54(5):1414–44. https://doi.org/10.1002/anie.201406096.
Saravana KR, Vijayalakshmi R. Nanotechnology in dentistry. Indian J Dent Res. 2006;17(2):62–5. https://doi.org/10.4103/0970-9290.29890.
Schuerle S, Soleimany AP, Yeh T, Anand GM, Haberli M, Fleming HE, et al. Synthetic and living micropropellers for convection-enhanced nanoparticle transport. Sci Adv. 2019;5(4):eaav4803. https://doi.org/10.1126/sciadv.aav4803.
Seeman NC. DNA in a material world. Nature. 2003;421(6921):427–31. https://doi.org/10.1038/nature01406.
Sitti M, Ceylan H, Hu W, Giltinan J, Turan M, Yim S, et al. Biomedical applications of untethered mobile milli/microrobots. Proc IEEE Inst Electr Electron Eng. 2015;103(2):205–24. https://doi.org/10.1109/JPROC.2014.2385105.
Sobczak JP, Martin TG, Gerling T, Dietz H. Rapid folding of DNA into nanoscale shapes at constant temperature. Science. 2012;338(6113):1458–61. https://doi.org/10.1126/science.1229919.
Solovev AA, Xi W, Gracias DH, Harazim SM, Deneke C, Sanchez S, et al. Self-propelled nanotools. ACS Nano. 2012;6(2):1751–6. https://doi.org/10.1021/nn204762w.
Sondergaard RV, Henriksen JR, Andresen TL. Design, calibration and application of broad-range optical nanosensors for determining intracellular pH. Nat Protoc. 2014;9(12):2841–58. https://doi.org/10.1038/nprot.2014.196.
Soto F, Wang J, Ahmed R, Demirci U. Medical micro/Nanorobots in precision medicine. Adv Sci (Weinh). 2020;7(21):2002203. https://doi.org/10.1002/advs.202002203.
Soto F, Karshalev E, Zhang F, Esteban Fernandez de Avila B, Nourhani A, Wang J. Smart materials for microrobots. Chem Rev. 2022;122(5):5365–403. https://doi.org/10.1021/acs.chemrev.0c00999.
Suri GS, Kaur A, Sen T. A recent trend of drug-nanoparticles in suspension for the application in drug delivery. Nanomedicine (Lond). 2016;11(21):2861–76. https://doi.org/10.2217/nnm-2016-0238.
Tabatabaei SN, Tabatabaei MS, Girouard H, Martel S. Hyperthermia of magnetic nanoparticles allows passage of sodium fluorescein and Evans blue dye across the blood-retinal barrier. Int J Hyperth. 2016;32(6):657–65. https://doi.org/10.1080/02656736.2016.1193903.
Teo WZ, Zboril R, Medrik I, Pumera M. Fe(0) Nanomotors in ton quantities (10(20) units) for environmental remediation. Chemistry. 2016;22(14):4789–93. https://doi.org/10.1002/chem.201504912.
Torne S, Darandale S, Vavia P, Trotta F, Cavalli R. Cyclodextrin-based nanosponges: effective nanocarrier for tamoxifen delivery. Pharm Dev Technol. 2013;18(3):619–25. https://doi.org/10.3109/10837450.2011.649855.
van den Heuvel MG, Dekker C. Motor proteins at work for nanotechnology. Science. 2007;317(5836):333–6. https://doi.org/10.1126/science.1139570.
Wan M, Wang Q, Wang R, Wu R, Li T, Fang D, et al. Platelet-derived porous nanomotor for thrombus therapy. Sci Adv. 2020;6(22):eaaz9014. https://doi.org/10.1126/sciadv.aaz9014.
Wang H. Plasmonic refractive index sensing using strongly coupled metal nanoantennas: nonlocal limitations. Sci Rep. 2018;8(1):9589. https://doi.org/10.1038/s41598-018-28011-x.
Wang F, Yang S, Yuan J, Gao Q, Huang C. Effective method of chitosan-coated alginate nanoparticles for target drug delivery applications. J Biomater Appl. 2016;31(1):3–12. https://doi.org/10.1177/0885328216648478.
Wang D, Gao C, Zhou C, Lin Z, He Q. Leukocyte membrane-coated liquid metal nanoswimmers for actively targeted delivery and synergistic chemophotothermal therapy. Research (Wash D C). 2020;2020:3676954. https://doi.org/10.34133/2020/3676954.
Wang L, Wang J, Hao J, Dong Z, Wu J, Shen G, et al. Guiding drug through interrupted bloodstream for potentiated thrombolysis by C-shaped magnetic actuation system in vivo. Adv Mater. 2021a;33(51):e2105351. https://doi.org/10.1002/adma.202105351.
Wang Z, Murphy A, O'Riordan A, O’Connell I. Equivalent impedance models for electrochemical nanosensor-based integrated system design. Sensors (Basel). 2021b;21(9):3259. https://doi.org/10.3390/s21093259.
Wei T, Liu J, Li D, Chen S, Zhang Y, Li J, et al. Development of magnet-driven and image-guided degradable microrobots for the precise delivery of engineered stem cells for cancer therapy. Small. 2020;16(41):e1906908. https://doi.org/10.1002/smll.201906908.
Williams RM, Lee C, Galassi TV, Harvey JD, Leicher R, Sirenko M, et al. Noninvasive ovarian cancer biomarker detection via an optical nanosensor implant. Sci Adv. 2018;4(4):eaaq1090. https://doi.org/10.1126/sciadv.aaq1090.
Wu Z, Li L, Yang Y, Hu P, Li Y, Yang SY, et al. A microrobotic system guided by photoacoustic computed tomography for targeted navigation in intestines in vivo. Sci Robot. 2019;4(32):eaax0613. https://doi.org/10.1126/scirobotics.aax0613.
Xie L, Liu T, He Y, Zeng J, Zhang W, Liang Q, et al. Kinetics-regulated interfacial selective superassembly of asymmetric smart nanovehicles with tailored topological hollow architectures. Angew Chem Int Ed Engl. 2022;61(12):e202200240. https://doi.org/10.1002/anie.202200240.
Xing JH, Yin T, Li SM, Xu TT, Ma AQ, Chen Z, et al. Sequential magneto-actuated and optics-triggered biomicrorobots for targeted cancer therapy. Adv Funct Mater. 2021;31(11):ARTN 2008262. https://doi.org/10.1002/adfm.202008262.
Xu K, Liu B. Recent progress in actuation technologies of micro/nanorobots. Beilstein J Nanotechnol. 2021;12:756–65. https://doi.org/10.3762/bjnano.12.59.
Xuan M, Shao J, Lin X, Dai L, He Q. Self-propelled Janus mesoporous silica nanomotors with sub-100 nm diameters for drug encapsulation and delivery. ChemPhysChem. 2014;15(11):2255–60. https://doi.org/10.1002/cphc.201402111.
Yang Y, Arque X, Patino T, Guillerm V, Blersch PR, Perez-Carvajal J, et al. Enzyme-powered porous micromotors built from a hierarchical micro- and mesoporous UiO-type metal-organic framework. J Am Chem Soc. 2020;142(50):20962–7. https://doi.org/10.1021/jacs.0c11061.
Yao L, Zhao X, Li Q, Zu Y, Fu Y, Zu B, et al. In vitro and in vivo evaluation of camptothecin nanosuspension: a novel formulation with high antitumor efficacy and low toxicity. Int J Pharm. 2012;423(2):586–8. https://doi.org/10.1016/j.ijpharm.2011.11.031.
Yuan S, Holmqvist F, Kongstad O, Jensen SM, Wang L, Ljungstrom E, et al. Long-term outcomes of the current remote magnetic catheter navigation technique for ablation of atrial fibrillation. Scand Cardiovasc J. 2017;51(6):308–15. https://doi.org/10.1080/14017431.2017.1384566.
Zelikin AN, Ehrhardt C, Healy AM. Materials and methods for delivery of biological drugs. Nat Chem. 2016;8(11):997–1007. https://doi.org/10.1038/nchem.2629.
Zhang Y, Sun C, Kohler N, Zhang M. Self-assembled coatings on individual monodisperse magnetite nanoparticles for efficient intracellular uptake. Biomed Microdevices. 2004;6(1):33–40. https://doi.org/10.1023/b:bmmd.0000013363.77466.63.
Zhang H, Li Z, Gao C, Fan X, Pang Y, Li T, et al. Dual-responsive biohybrid neutrobots for active target delivery. Sci Robot. 2021a;6(52) https://doi.org/10.1126/scirobotics.aaz9519.
Zhang L, Guo Y, Hao R, Shi Y, You H, Nan H, et al. Ultra-rapid and highly efficient enrichment of organic pollutants via magnetic mesoporous nanosponge for ultrasensitive nanosensors. Nat Commun. 2021b;12(1):6849. https://doi.org/10.1038/s41467-021-27100-2.
Zhang Z, Wang L, Chan TKF, Chen Z, Ip M, Chan PKS, et al. Micro-/nanorobots in antimicrobial applications: recent Progress, challenges, and opportunities. Adv Healthc Mater. 2022;11(6):e2101991. https://doi.org/10.1002/adhm.202101991.
Zhou H, Mayorga-Martinez CC, Pane S, Zhang L, Pumera M. Magnetically driven micro and nanorobots. Chem Rev. 2021;121(8):4999–5041. https://doi.org/10.1021/acs.chemrev.0c01234.
Zollinger RM. A salute to the American journal of surgery on its 100th birthday. Am J Surg. 1991;161(2):191–3. https://doi.org/10.1016/0002-9610(91)91128-6.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2023 The Author(s), under exclusive license to Springer Nature Switzerland AG
About this chapter
Cite this chapter
Ye, Q., Sun, J. (2023). Nanorobots for Drug Delivery, Surgery, and Biosensing. In: Lim, KT., Abd-Elsalam, K.A. (eds) Nanorobotics and Nanodiagnostics in Integrative Biology and Biomedicine. Springer, Cham. https://doi.org/10.1007/978-3-031-16084-4_2
Download citation
DOI: https://doi.org/10.1007/978-3-031-16084-4_2
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-031-16083-7
Online ISBN: 978-3-031-16084-4
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)