Abstract
Synthetic scaffolds made from electrospun fibres have showed to be a novel method to solve issues related to shortage of donors and other complications. Pure gelatin methacrylate (GelMA) has showed to have excellent compatibility in tissue engineering applications as it closely resembles properties of native extracellular matrix. However, studies of electrospinning of GelMA largely use GelMA blends. In this study, pure GelMA fibres were successfully produced using electrospinning method. To facilitate development of pure GelMA fibers using electrospinning method as well as optimize the process, alterations to significant parameters of electrospinning, including concentration of solution, voltage of power supply, flow rate and temperature was done. It was observed that temperature is a significant parameter in the electrospinning of pure GelMA. A correlation between concentration and fibre diameter was observed that further emphasises the wide usage of electrospun GelMA fibers in tissue engineering application as fibers of differing diameters and mechanical strengths can be produced. This study sets the foundation for the usage of electrospun pure GelMA scaffolds in tissue engineering applications.
Access provided by Autonomous University of Puebla. Download conference paper PDF
Similar content being viewed by others
Keywords
1 Introduction
As Singapore and the world faces societal problems like ageing population leading to increasing healthcare demands, there is a need to find effective and low-cost solutions to such problems. With problems associated with the shortage of donor organs, donor site morbidity and complications, risk of disease transmission and immuno-rejection problems, tissue engineered scaffolds have been developed as a novel perspective for organ repair.
Electrospinning is a fiber production method which uses electric force to draw charged threads of polymer solutions. It has emerged as a new scaffold fabrication method. The underlying rationale of using microfibres for scaffolding is based on the principle that electrospun fibers can mimic the physical structure of the native extracellular matrix (ECM). From the biological viewpoint, almost all of the tissues and organs, such as bone, nerve, blood vessel, ligament, tendon, and cartilage, are synthesized and hierarchically organized into fibrous structure [1,2,3,4,5]. The scaffolds made by electrospinning exhibit better cellular attachment, growth and differentiation compared with those made by other techniques. The large specific surface area provided by a low-dimension fibrous structure, which facilitates cell adhesivity to the electrospun fibers.
Gelatin methacrylate (GelMA) hydrogel, chemically modified gelatin, has been widely used for various biomedical applications due to its suitable biological properties and tunable physical characteristics. GelMA hydrogels closely resemble the aforementioned properties of native extracellular matrix (ECM) because of the presence of cell-attaching and matrix metalloproteinase responsive peptide motifs, which allow cells to proliferate and spread in GelMA-based scaffolds. GelMA is also versatile from a processing perspective. It crosslinks when exposed to light irradiation to form hydrogels with tunable mechanical properties [6].
Hydrogel electrospinning is recent invention. Hydrogels are difficult to spin as they have limited solubility in organic solvents, thus aqueous solvents are used. However, the combination of water’s low volatility and hydrogel material’s affinity for water slows the drying process for the polymer jet, resulting in deposition of wet material rather than dry polymer fibers. GelMA based electrospinning has largely been using GelMA blends with other polymers such as polyvinyl alcohol (PVA) or nanoparticles, and not using pure GelMA. This is due to pure GelMA’s high affinity to water, making electrospinning challenging. However, there is a need to produce pure GelMA electrospun fibers due the excellent compatibility of pure GelMA in tissue engineering applications coupled with the effectiveness of electrospinning in producing synthetic scaffolds.
2 Aims and Objectives
We aim to create a pure GelMA fibrous structure that can be cell laden as electrospun fibers have shown to have excellent cellular attachment, growth and differentiation. Due to the lack of studies on the electrospinning of pure GelMA despite its excellent compatibility in producing tissue engineered scaffolds, this study aims to optimize parameters of electrospinning in order to produce pure GelMA fibers.
3 Methods and Materials
-
A.
Characterisation of GELATIN METHACRYLATE (GelMA)
In order to make the GelMA solution for electrospinning, solutions were prepared by weighing GelMA and adding deionized water. The concentration of the solution was determined by the mass of GelMA in 0.5 ml of deionized water. The solutions was stirred at 50 °C using a heated magnetic stirrer, in order to ensure dissolution of GelMA and a homogenous mixture.
-
B.
Electrospinning Process
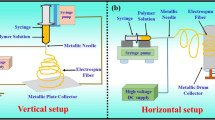
The electrospinning method is a process that creates nanofibres through an electrically charged jet of polymer solution. A basic electrospinning setup (Fig. 1 [7]) consists of a syringe to hold polymer solution (often connected to a syringe pump), two electrodes and a DC voltage supply in the kV range.
Before electrospinning the needle was kept in a hot water bath of 50 °C, to ensure that the GelMA solution does not dry up when flowing through the needle.
Parameters of electrospinning are of paramount importance as they affect production of fibres and fibre morphology. Significant parameters include, viscosity and surface tension, voltage of power supply, distance between syringe and grounded surface, temperature, and flow rate of solution from syringe.
The following parameters are of significance when electrospinning polymers.
-
(1)
Suitable Solvent to dissolve polymer
-
(2)
Vapour Pressure of the solvent so that the solvent
-
(i)
Evaporates quickly enough for fibre to maintain its integrity
-
(ii)
Evaporates not too quickly to allow the fibre to harden before it reaches nanometre range
-
(i)
-
(3)
Viscosity and Surface Tension of the solvent
-
(i)
Not too large to prevent the jet from forming
-
(ii)
Not be too small to allow the polymer solution to flow freely from the syringe
-
(i)
-
(4)
Power supply should be adequate to overcome the viscosity and surface tension of polymer solution to form and sustain the jet of polymer from syringe
-
(5)
Distance between syringe and grounded surface should not be too small to create sparks between the electrodes but should be large enough for the solvent to evaporate in time for fibres to form
-
(6)
Ambient Parameters Temperature fluctuations can cause fluctuations in viscosity and surface tension of solution. Low humidity may dry the solvent and increase the rate of solvent evaporation. On the contrary, high humidity will lead to the thick fiber diameter.
4 Results and Discussion
-
A.
Optimising Parameters for Electrospinning of GelMA
-
(1)
Temperature of GelMA Solution
-
(1)
Since hydrogels like GelMA have a high affinity for water, the solution exist as gels at room temperature. This high viscosity solution does not produce fibres when electrospun as it results in the hard ejection of jets from solution. Generally, there is a linear relationship between concentration of solution and viscosity. However, in the case of GelMA solutions, even low concentration such as 10% solutions exist as a gel at room temperature. In order for electrospinning to occur, the mixture must exist as a solution to allow the evaporation of water and also the ejection of jets from the solution.
We found that heating the solution to an optimal temperature led to a significant increase in the success rate of fibre production.
As seen by Figs. 2, 3 and 4, the fibre morphology has drastically changed as temperature was increased. At room temperature wet gel was deposited, while at the optimal temperature (56 °C) smooth fibre were produced. At a high temperature (70 °C), beaded fibre were produced which is not desirable. At a very high temperature, viscosity was very low and surface temperature of solvent was very high which leads to low extensional viscosity, thus leading to bead formation. Thus, temperature is a hugely important controllable variable to note in the electrospinning of GelMA.
-
(2)
Concentration and Flow Rate of GelMA Solution
Concentration is an important parameter to consider in the electrospinning of all polymers. When the concentration is very low, polymeric micro (nano)-particles will be obtained and electrospray occurs instead of electrospinning because of low viscosity and high surface tensions of the solution [8]. As the concentration is little higher, a mixture of beads and fibers will be obtained.
Generally, lower flow rate is recommended as the polymer solution will get enough time for evaporation. If the flow rate is very high, bead fibres with thick diameter will form rather than the smooth fibres with thin diameter owing to the short drying time prior to reaching the collector and low stretching forces. Flow rate is very important in the electrospinning of GelMA due to the low volatility of water, thus a lower flow rate ensures enough time for the evaporation of water and formation of polymer fibres. Generally, fibre production occurs when flowrate is between 0.5 and 1.0 ml/hr. In this study, flow rates of 0.6 and 0.8 ml/hr were tested.
For a certain concentration of GelMA solution and a certain flow rate, the temperature was increased until the mixture existed as a liquid solution rather than a gel. Moreover, in the electrospinning setup, the voltage was increased until formation of fibre was observed.
For all the experiments, the diameter of the needle was constant and the distance between the needle and grounded surface was maintained at 7 cm (Fig. 5).
In order to verify reproducibility, it was ensured that fiber collection with the parameters was repeatable at least twice.
Table 1 in Appendix shows the optimal conditions for electrospinning GelMA solutions of various concentrations as well as the respective fibre morphologies. As seen by the results in Table 1, fibre morphology significantly changed with change in concentration and change in feed rate. Generally, a lower feed rate produced more desirable fibres which had less beads and were smoother. Generally, an increase in concentration led to larger and less smooth fibres however, fibres produced were long and continuous as compared to the short and detached fibres produced using 10% and 12% GelMA solutions.
The temperature and voltage was optimized to the respective concentrations. Increase in concentration led to a more viscous solution thus a higher temperature was needed to ensure that the GelMA remained in solution form rather than in gel form. However, in the case of 20% GelMA solution, a high temperature of 70 °C was needed to maintain the solution in liquid form, causing the anomalous beading and discontinuous wet fibres. A very high concentration of 20% failed to produce dry fibres.
-
B.
Fiber Morphology
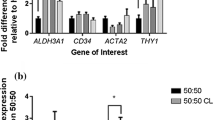
The diameters significantly vary with concentration of solution, however, there was no significant change in diameter due to change in flow rate. Figures 6, 7, 8 and 9 show the distribution of fibre diameters for 10, 12, 15 and 18% solution respectively. Generally, an increase in concentration led to a increase in average diameter.
This is of utmost importance for the application of GelMA electrospun structures in tissue engineering as engineering of different types of tissues require fibres different morphologies, for example, skeletal muscles require scaffolds to have high mechanical strength and larger fibre diameter.
5 Conclusion and Recommendations for Future Work
Electrospinning of pure GelMA under optimized conditions proved to be a novel method for tissue engineering applications due to the cost effectiveness of electrospinning, tunable fiber morphologies as well as the biocompatibility and mechanical properties of GelMA. Compared to other biocompatible polymers such as Polyvinyl alcohol (PVA), GelMA has a promising remarkable compatibility for a wide spectrum of applications. This study of optimization of noteworthy parameters specifically for the electrospinning of pure GelMA builds the foundation for the development of pure GelMA structures for various tissue engineering applications, tissue engineered scaffolds in particular.
In the future, studies should test the cell attachment, growth and differentiation in the pure GelMA fibrous structures formed using the different concentrations and flow rates, to further determine the optimal concentration for various tissue engineering applications.
References
Nishida, T., Yasumoto, K., Otori, T., & Desaki, J. (1988). The network structure of corneal fibroblasts in the rat as revealed by scanning electron microscopy. Investigative Ophthalmology & Visual Science, 29(12), 1887–1890.
Li, X., Gao, H., Uo, M., et al. (2009). Effect of carbon nanotubes on cellular functions in vitro. Journal of Biomedical Materials Research A, 91(1), 132–139.
Li, X., Liu, H., Niu, X., et al. (2012). The use of carbon nanotubes to induce osteogenic differentiation of human adipose-derived MSCs in vitro and ectopic bone formation in vivo. Biomaterials, 33(19), 4818–4827.
Li, X. M., Wang, L., Fan, Y. B., Feng, Q. L., Cui, F. Z., & Watari, F. (2013). Nanostructured scaffolds for bone tissue engineering. Journal of Biomedical Materials Research A, 101(8), 2424–2435.
Kadler, K. E., Holmes, D. F., Trotter, J. A., & Chapman, J. A. (1996). Collagen fibril formation. Biochemical Journal, 316(1), 1–11.
Kan, Y., Trujillo-de Santiago, G., Alvarez, M. M., Tamayol, A., Annabi, N., & Khademhosseini, A. (2015). Synthesis, properties, and biomedical applications of gelatin methacryloyl (GelMA) hydrogels. Retrieved from https://www.sciencedirect.com/science/article/pii/S014296121500719X.
Salles, V., Seveyrat, L., Fiorido, T., Hu, L., Galineau, J., Eid, C. … Guyomar, D. Synthesis and characterization of advanced carbon-based nanowires—Study of composites actuation capabilities containing these nanowires as fillers. Retrieved from https://www.intechopen.com/books/nanowires-recent-advances/synthesis-and-characterization-of-advanced-carbon-based-nanowires-study-of-composites-actuation-capa.
Deitzel, J. M., Kleinmeyer, J., Harris, D., & Beck Tan, N. C. (2011). The effect of processing variables on the Morphology of electrospun snanofibers and textiles. Polymer, 42(1), 261–272.
Acknowledgements
Srushti Sakhardande would like to express our sincere her sincere thanks to Assistant Professor Zhang Yilei for introducing and supervising her on this project as well as allow her to conduct my experiments in the lab at the School of Mechanical and Aerospace Engineering at NTU. She would also like to thank Vivek Damodar for his guidance and support in supervising this research and helping her with his expertise in electrospinning.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Appendix
Appendix
See Table 1.
Rights and permissions
Copyright information
© 2019 Springer Nature Singapore Pte Ltd.
About this paper
Cite this paper
Sakhardande, S., Yilei, Z. (2019). Optimization of the Electrospinning Process to Create Pure Gelatin Methacrylate Microstructures for Tissue Engineering Applications. In: Guo, H., Ren, H., Bandla, A. (eds) IRC-SET 2018. Springer, Singapore. https://doi.org/10.1007/978-981-32-9828-6_3
Download citation
DOI: https://doi.org/10.1007/978-981-32-9828-6_3
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-32-9827-9
Online ISBN: 978-981-32-9828-6
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)