Abstract
In all organisms, the excretory system maintains the internal aqueous and ionic environment for homeostasis. The organs of the renal system, constituted by paired kidneys, ureters, urinary bladder and urethra, help in the excretion of waste products and hydrogen ions, harmful drugs and toxins. Nephrons, the kidney’s functional units, are concerned with the glomerular filtration, tubular reabsorption and tubular secretion to form urine. Glomerular filtration is a passive, non-selective process where fluids and electrolytes are filtered through the three layers of the glomerular membrane into Bowman’s space under the influence of physical forces. Kidneys maintain relatively constant renal blood flow and glomerular filtration rate (GFR) within a wide range of mean systemic arterial pressure by autoregulatory mechanisms. Tubular reabsorption is a highly selective process by which water and solutes are reabsorbed to the peritubular capillaries. The concentration of urine occurs by the countercurrent multiplier and countercurrent exchange systems existing in the hypertonic renal medullary interstitium. Urine formed in the kidneys is conveyed to the urinary bladder through ureters for temporary storage and removed from it periodically through the urethra by a process called micturition. The avian excretory system is structurally and functionally modified to avoid excess water loss and eliminate the major nitrogenous waste product, the uric acid.
Graphical Abstract

Description of the graphic: The renal system is structurally organised (1) to eliminate end products of metabolism and other foreign waste materials. The process of urine formation (2) involves glomerular filtration, tubular reabsorption and tubular secretion. Glomerular filtration (3) is controlled by physical forces acting across the glomerular capillary wall and autoregulated by the kidney. Tubular reabsorption (4) is a highly selective process that occurs passively and actively. The tubular filtrate is concentrated by countercurrent exchange and countercurrent multiplier mechanisms (5). The urine formed is voided out by a process known as micturition (6). The avian renal system (7) is also structurally and functionally modified to eliminate the major nitrogenous end product, uric acid
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
FormalPara Learning Objectives-
Importance of excretory system and physiological functions of the kidney
-
General and functional ultrastructural morphology of the renal system
-
The process of urine formation includes glomerular filtration, tubular reabsorption and secretion
-
Urine concentration mechanisms following countercurrent multiplier and countercurrent exchange mechanisms
-
Renal function tests
-
Urinary bladder and its functions
-
Peculiarities of avian excretion
1 Introduction
Maintaining an internal aqueous environment with consistent water and solute composition is vital for homeostasis. Loss of water and electrolytes during digestion, metabolism, thermoregulation and elimination of waste products always creates imbalances in fluid and electrolyte composition in the internal environment. In animals, the urinary or renal excretory system counteracts such imbalances by effectively eliminating metabolic wastes and selective retention of water and solutes. Thus, the renal system is considered a major excretory system in the body. The other systems involved in excretion are the respiratory system, digestive system and integumentary system and have a minor role in animals.
Protein and nucleic acid metabolism always produces nitrogenous waste products in the body. Ammonia (NH3), a potentially toxic base that can bind with protons to become an ammonium ion (NH4+), constitutes the major end product of protein metabolism. If present in excess, these ions can also interfere with Na+/K+ ATPase substituting for K+, causing morphological changes in neurons, interrupted ion conduction and disrupted neurotransmitter metabolism. Thus, NH3 and NH4+ must either be highly diluted and rapidly excreted or be converted into a less toxic form. The most common of these forms are urea and uric acid.
Most aquatic animals are ammonotelic, and they rely on ammonia excretion through gills in the most diluted form as water is freely available for them. Most terrestrial mammals used metabolic energy to convert ammonia to urea using ATP in the liver and excreted through kidneys, known as ureotelic. Urea is 10–100 times less toxic than ammonia. Thus, it can be accumulated in too much higher concentrations and has the benefit of removing two nitrogens per molecule. It takes about ten times less water to excrete a given amount of nitrogen as urea than ammonia. In reptiles, birds and insects, ammonia is converted to uric acid (uricotelic) before excretion. It requires more ATP for the production of uric acid but is less toxic as it is highly insoluble and has the added benefit of removing four nitrogens per molecule. It is converted as urates into the hindgut and excreted in a semisolid form. It takes about 50–100 times less water to excrete a given amount of nitrogen as uric acid than ammonia. Compared to urea, which is essentially infinitely soluble in solution, uric acid is highly insoluble (precipitates at concentrations greater than about 0.4 mM). Urea and uric acid are excreted through renal system, which is constituted by kidneys and other organs with functional and structural modifications in mammals and birds.
2 Physiological Functions of the Kidney
-
Excretion of waste products of metabolism and unwanted foreign materials: The major metabolic waste products include urea, creatinine, bilirubin, uric acid and metabolites of protein, nucleic acid, haemoglobin and various hormones. Kidneys also aid in eliminating pesticides, drugs and food additives that enter by different means to the body.
-
Maintenance of water and electrolyte balances: The kidneys can alter either the rates of absorption or excretion or the rates of both water and ions like sodium, potassium, chloride, calcium, hydrogen, magnesium and phosphate to regulate the fluid and electrolyte balance in the body.
-
Regulation of arterial pressure: Arterial pressure is maintained on a long-term basis by adjusting the kidneys’ excretion of sodium and water. Angiotensin II, a vasoactive peptide, has a vital role in the short-term maintenance of blood pressure produced in the body with the help of renal proteolytic enzyme renin.
-
Acid-base regulation: Kidneys can regulate excess hydrogen ions and acids, like sulphuric and phosphoric acids, to maintain the pH of body fluids.
-
Erythropoietin production: In hypoxic conditions, kidneys can produce hormone-like erythropoietin (EPO), which stimulates erythrocyte production.
-
Calcium homeostasis by calcitriol formation: The kidneys can produce the metabolically active derivative of vitamin D-1,25, dihydroxy cholecalciferol (calcitriol or vitamin D3). It is essential in maintaining the normal calcium deposition in bones and reabsorption of calcium from the gastrointestinal tract.
-
Glucose synthesis: When the blood glucose level lowers, kidneys can synthesise glucose by gluconeogenesis.
3 Functional Morphology of Kidney
The mammalian urinary system has a pair of bean-shaped kidneys located on the dorsal side of the lower abdominal cavity on either side of the vertebral column (Fig. 9.1). Both kidneys together constitute 0.4% of the body weight. Each kidney is externally covered by a thick connective tissue renal capsule and internally divided into two parts, the outer cortex and an inner medulla. The hilum is an indentation seen on the medial aspect of the kidney through which pass renal blood vessels (renal artery and vein), lymphatics and nerves. The medulla has triangular structures called pyramids or papillae extending into calyces (singular calyx) channels. The minor calyces drain urine from the tubules of each papilla and discharge it to the major calyces. The calyces open into a wide conical central cavity called the pelvis. Some mammalian species like humans, dogs, small ruminants and rabbits have uni-pyramidal kidneys, whereas the kidneys of large ruminants and pigs are multi-pyramidal in nature.
There are two tubular structures called ureters carrying urine from the pelvis to the urinary bladder for temporary storage. Urine is removed from the urinary bladder periodically through the urethra.
The nephrons are the basic functional units of the kidney. Several nephrons of a kidney bind together by connective tissue. The number of nephrons in each kidney varies in different species of mammals, for example, about 4 million in cattle, 1.25 million in pigs, 1 million in humans, 0.5 million in dogs and 0.25 million in cats. Each nephron has a renal corpuscle and a renal tubule. The renal corpuscle comprises a tuft of capillaries called glomerulus surrounded by a cup-shaped Bowman’s capsule (Fig. 9.2). The Bowman’s capsule encloses a space between the two walls (visceral and parietal) of it called Bowman’s space, which continues as the renal tubule.
Schematic diagram showing the major features of the renal corpuscle. (a) Renal corpuscle, (b) structure of glomerular capillary, (c) enlarged structure of glomerular capillary wall showing the intercellular pores (fenestrations) between the endothelial cells lining the glomerular capillary, acellular basement membrane and filtration slits situated between the foot processes of the podocytes constituting the visceral layer of Bowman’s capsule
The renal tubule has three structurally and functionally different divisions; the proximal convoluted tubule (PCT) located in the cortex, the loop of Henle (with its thin descending limb, thin ascending limb and thick ascending limb) located in the medulla and the distal convoluted tubule (DCT) present in the cortex. In mammals, up to 8 DCTs coalesce to form a collecting duct (CT), which runs back to the medulla (Fig. 9.3). The portion of the CT that lies in the cortex is the cortical collecting duct, which dips into the medulla called the medullary collecting duct. The collecting ducts plunge deep into the medulla to evacuate their contents to the renal pelvis.
In mammals, there are two types of nephrons: cortical nephrons and juxtamedullary nephrons (Fig. 9.4). The glomerulus of cortical nephrons lies in the peripheral cortex, and that of the juxtamedullary nephrons lies in the cortex’s inner layer, adjacent to the medulla. The arrangement of glomeruli in the cortex gives the cortex a granular appearance.
The position of the loops of Henle varies in two types of nephrons; the terminal hairpin loop of Henle dips slightly into the medulla, whereas the longer loops of juxtamedullary nephrons pass deep into the medulla.
3.1 Renal Blood Supply
In a human being with a 70 kg body weight, 1100 mL of blood flows through both kidneys per minute, constituting 22% of cardiac output. The arterial blood reaches the kidney through the renal artery through the hilum. It then branches progressively to form the interlobar arteries, arcuate arteries, interlobular arteries (radial arteries) and short afferent arterioles, one of which supplies blood to the capillary tuft of each nephron. The capillaries of the glomerulus reunite at the exit point as efferent arteriole. The efferent arteriole then subdivides into the second capillaries called peritubular capillaries surrounding the proximal and distal convoluted tubules in the renal cortex. These capillaries ultimately rejoin to form the vessels of the venous system, which run parallel to the arteriolar vessels and progressively form the interlobular vein, arcuate vein, interlobar vein and renal vein, which leaves the kidney at the hilus. The peritubular capillaries of juxtamedullary nephrons form hairpin loops in close association with the loops of Henle. These vascular hairpins are known as vasa recta. When passing through the medulla, the collecting ducts of both cortical and juxtamedullary nephrons always run parallel in proximity to the ascending and descending loops of Henle of juxtamedullary nephrons and vasa recta. The parallel arrangement gives the striated appearance to the medulla.
3.2 Juxtaglomerular Apparatus
The thick ascending limb of the loop of Henle enters the cortex from the medulla, passes between the afferent and efferent arterioles and continues as a distal tubule (Fig. 9.5). The epithelial cells of the tubules that come in contact with the afferent and efferent arterioles facing the angle between the blood vessels are collectively known as macula densa. The presence of epithelial cells in the densa marks the beginning of the distal tubule. The specialised granular smooth muscle cells of afferent arterioles that contact macula densa are called juxtaglomerular (JG) cells. From these cells, proteolytic enzyme renin is produced. Mesangial cells and the matrix secreted by the mesangial cells occupy the space between the afferent arteriole, efferent arteriole, macula densa and glomerular capillaries. Those cells located between the arterioles and macula densa are lacis cells or extraglomerular mesangial cells. Mesangial cells exhibit contractile property to aid blood flow through glomerular capillaries. Besides, they also secrete prostaglandins, maintain basement membrane and possess phagocytic properties. The macula densa, juxtaglomerular cells and lacis cells are together known as the juxtaglomerular (JG) apparatus. This apparatus has functional importance in renal haemodynamics and glomerular filtration rate (GFR).
3.3 Nerve Supply
Sympathetic fibres of the autonomous nervous system enter the kidneys through the hilus along with the renal artery and vein and innervate blood vessels of the kidney, nephron segments and JG cells. Motor activity of these nerves produces alterations in renal haemodynamics and composition of the tubular fluid. The kidney contains afferent sensory nerve fibres sensing stretch, located mainly in the renal pelvic wall. The renal pelvic pressure increases due to the accumulation of urine, and it causes stretching of the pelvis wall that stimulates the mechanoreceptors present in between the smooth muscles. This sensation results in the activation of sensory afferent nerves of the ipsilateral kidney, which in turn causes decreased contralateral motor renal nerve activity. The diminished efferent renal sympathetic nerve activity in the contralateral kidney causes excess sodium ions and water excretion as a compensatory mechanism. The renorenal reflex coordinates the functions of the two kidneys and thus facilitates the physiological regulation of sodium and water balance to maintain homeostasis.
3.4 Ultrastructure of Glomerulus
The glomerular capillary membrane has three layers, the innermost highly fenestrated endothelial layer, next to that a basement membrane and an outermost epithelial layer made of podocytes (Fig. 9.6). Podocytes are flattened epithelial cells with foot processes, which interdigitate with adjacent foot processes, leaving filtration slits in between and encircling the complete glomerular tuft. The basement membrane is composed of collagen and glycoprotein, and the collagen provides structural strength to the membrane. The glycoproteins repel the plasma proteins, if present, with their negative charge. Less than 1% of albumin is completely excluded from the filtrate usually.
4 Urine Formation
The physiological events involved in urine formation can be categorised into three stages: glomerular filtration, tubular reabsorption and tubular secretion. The urine is formed and excreted due to the combining effect of the three stages.
4.1 Glomerular Filtration
Glomerular filtration is a passive, non-selective process where fluids and electrolytes are filtered through the three layers of the glomerular membrane into Bowman’s space under the influence of specific physical forces. It is called non-selective and passive because filtration is indiscriminate without expending energy. The fluid collected in the Bowman’s space is called glomerular filtrate. The amount of glomerular filtrate formed per minute is known as the glomerular filtration rate (GFR). The composition of this filtrate is similar to blood except for the presence of blood cells and plasma proteins. The plasma flow rate through both the kidneys per minute is called renal plasma flow (RPF). Usually, about 20% of plasma that enters the glomeruli is filtered, producing 170 L of glomerular filtrate with an average GFR of 125 mL/min or 180 L/24 h by both kidneys in humans. The fraction of renal plasma flow that is filtered is called the filtration fraction:
The amount of any substance present in plasma reaching the kidneys per minute is the plasma load for that substance. The plasma load that filters into the capsular space is known as the tubular load of the substance.
The physical forces involved in glomerular filtration are (1) glomerular capillary hydrostatic pressure that favours filtration, (2) colloid osmotic pressure (COP) of plasma proteins that oppose filtration, (3) hydrostatic pressure of fluid in the Bowman’s capsule which opposes the filtration and (4) Bowman’s capsule osmotic pressure that favours the filtration. The glomerulus’ hydrostatic capillary pressure is higher than the pressure in other capillaries because the amount of blood that enters through the wider afferent arteriole is subjected to the increased resistance offered by the comparatively narrow efferent arteriole. The difference in diameter of the arterioles makes more or less the same capillary blood pressure all along the capillary tuft of the glomerulus. The hydrostatic pressure of the glomerular capillary was estimated to be about 60 mmHg, which was higher than the capillary pressure elsewhere. It forms the major driving force of fluid from the glomerulus to the Bowman’s space. The COP of plasma proteins (32 mm of Hg) of glomerular capillary blood and hydrostatic pressure of capsular fluid (18 mm of Hg) together exert an opposing force of filtration of magnitude 50 mm of Hg. The COP of capsular fluid is negligible because very little amount of protein is present in the capsular space and the fluid remains in the capsular space for a very short duration due to the forward propulsion by the hydrostatic pressure. So, the net pressure of filtration becomes 10 mm of Hg (60 − 50 mm of Hg).
Besides the net filtration pressure, GFR also depends on the glomerular capillary surface area and the permeability or the hydraulic conductivity of the capillary membrane. The product of these two factors is known as the filtration coefficient (Kf). The value of Kf cannot be estimated directly but can be calculated as
So, from the values mentioned above, Kf can be calculated as 125/10 = 12.5 mL/min/mm of Hg of filtration pressure.
Chronic uncontrolled hypertension and diabetes mellitus affect the glomerulus’s permeability characteristics, reducing the Kf value and thereby reducing GFR.
4.2 Physiologic Control of GFR and Renal Blood Flow
Among the physical forces controlling filtration, plasma colloid osmotic pressure and hydrostatic pressure of capsular fluid are usually not regulated for controlling glomerular filtration. Instead, by extrinsic sympathetic nerves, humoral factors and autoregulatory mechanisms, the hydrostatic pressure of glomerular capillaries can be controlled to optimise GFR.
In acute conditions, like severe haemorrhage and ischaemia, sympathetic stimulation causes vasoconstriction of renal arterioles resulting in reduced GFR. Apart from that, it can also contract the mesangial cells, reducing the surface area of the glomerulus participating in filtration resulting in the reduction of Kf value and GFR. Under normal resting conditions, the role played by these nerves in the regulation of GFR is meagre.
Humoral factors, like epinephrine, norepinephrine and endothelin, cause vasoconstriction and reduction in GFR, whereas prostaglandins (PGE2 and PGI2) and bradykinin cause vasodilatation, an increased renal blood flow and increase in GFR. Angiotensin II is a peptide showing preferential vasoconstrictor property with the efferent arteriole of the kidney. This peptide is produced from angiotensinogen, a plasma protein. Angiotensinogen is converted to angiotensin I on proteolytic cleavage with renin of the JG apparatus. Angiotensin I in the lungs is converted to angiotensin II by the angiotensin-converting enzyme.
-
Autoregulation: Autoregulation is a process involving various intrinsic mechanisms by which the kidneys maintain relatively constant renal blood flow and GFR within a wide range of mean systemic arterial pressure. Two mechanisms are mainly involved, myogenic response and tubuloglomerular feedback.
-
Myogenic response: When the blood enters the afferent arteriole, the stretch receptors of smooth muscles on the walls of the blood vessels experience increased or decreased tension depending on the hydrostatic pressure exerted by blood on the walls. The increased pressure within the vessel causes the vessel wall to stretch which inherently contracts the vessel wall to restrict the blood flow. At the same time, the inherent relaxation of an unstretched afferent arteriole increases blood flow when the pressure decreases.
-
Tubuloglomerular feedback: Tubuloglomerular feedback is another mechanism of autoregulation to maintain optimum filtration pressure when the kidney experiences altered perfusion pressure. When GFR increases due to elevated glomerular hydrostatic pressure, macula densa cells sense an increase in the concentration of Na+ and Cl− in the tubular fluid. This sensitisation of macula densa cells, by some unknown means, constricts the afferent arteriole lowering hydrostatic pressure and also contracts the mesangium lowering the filtration surface area. Both these effects reduce GFR. Macula densa cells can sense the reduced concentration of Na+ and Cl− in the tubular fluid when GFR decreases due to lower hydrostatic pressure. Then renin is released from the JG cells, ultimately causing the formation of angiotensin II. Angiotensin II selectively constricts the efferent tubule offering more resistance to blood flow, and thus GFR is increased.
Know More……
Transmembrane Proteins in Excretion
Polycystin 1 and polycystin 2 are the two transmembrane proteins coded by PKD1 and PKD2 genes expressed in different locations of renal epithelial cells, including the primary cilia present on the apical membrane of these cells. These cilia protrude from the cells to the lumen and act as tubular fluid flow sensors. They transduce the alterations in the tubular flow into a cellular response regulating fluid and electrolyte transport. Whenever increased tubular fluid flow occurs, there will be bending of cilia, which activates PKD1/PKD2-dependent calcium ion influx into the cell and results in potassium ion secretion. It is also reported that functional loss of polycystins results in renal cyst formation.
4.3 Tubular Reabsorption
The water and solutes of the tubular fluids when transported to the peritubular capillaries throughout the nephron, including the collecting duct, are called tubular reabsorption. The substances reabsorbed across the tubular epithelial layer reach the renal interstitial, are then absorbed into the peritubular capillaries and finally get into the systemic circulation. Unlike glomerular filtration, tubular reabsorption is a highly selective process. The transportation occurs by two routes, the transcellular route across the epithelial cells and the paracellular route across the junctional spaces. Tubular reabsorption can either be active or passive. Water is normally reabsorbed by a passive diffusion process in a concentration gradient through both transcellular and paracellular routes.
Along with water, soluble solutes like potassium, magnesium and chloride ions and organic solvents are also taken to the interstitium through a process known as solvent drag. About 65% of filtered sodium, chloride, bicarbonate, magnesium and potassium and almost all filtered amino acids and glucose are absorbed through PCT. The epithelial cells of PCT are provided with numerous mitochondria for meeting the increased metabolic demand associated with transport mechanisms. The microvilli’s extensive surface area provided on the luminal surface also favours bulk reabsorption.
Capillary dynamics in the peritubular capillaries favours reabsorption by bulk flow. In the peritubular capillaries, hydrostatic pressure and COP are 17 and 30 mm of Hg, respectively, whereas in the interstitial fluid, the values are 6 and 10 mm of Hg, respectively. Hence, the reabsorption pressure (30 + 6 = 36 mmHg) exceeds filtration pressure (17 + 10 = 27 mmHg) by 9 mmHg (36 − 27 = 9 mmHg), favouring reabsorption to peritubular capillaries. The tubular fluid remains isosmotic with plasma as both solutes and water are reabsorbed.
The amount of a substance filtered through the glomerular filtrate and presented to the tubule per minute is known as the tubular load of that particular substance. The maximum rate at which the substance is reabsorbed from the tubular lumen to the peritubular fluid is the tubular transport maximum (Tm). The renal threshold is the plasma concentration of a substance at which it first appears in the urine. The property of tubules to increase reabsorption by the increased tubular load due to increased GFR is known as glomerulotubular balance.
Na+-K+ ATPase present on the basolateral side of the tubular epithelial cells hydrolyses ATP. The released energy is used to transport sodium ions from the tubular cells to the interstitium. Potassium ions are taken in return into the interior of the cells from the interstitial space. This is known as primary active transport. The increased transport of sodium ions out of the cell creates an intracellular potential of −70 mV. This negative potential and decreased intracellular sodium concentration favour sodium diffusion into the cell from the tubular fluid in a concentration gradient. The energy released during primary active transport is used to transport another substance known as secondary active transport. For example, glucose or amino acid is transported along with sodium. Hence, secondary active transport is known as co-transport. If a secondary secretion occurs along with primary active sodium transport, that is known as counter-transport. The inward influx of sodium ions accompanied by the outflow of hydrogen ions is an example of counter-transport.
Sodium reabsorption mainly (65%) occurs at the proximal PCT as co-transport and counter-transport. But chloride-driven Na+ transport takes place from the distal portions of the PCT. In the first two modes of transportation, sodium-coupled carrier molecules are involved, whereas in the chloride-driven Na+ transport, both Cl− and Na+ ions are transported through the leaky tight junctions. About 25% of Na+ present in the tubular fluid is absorbed in the thick ascending limb of both cortical and medullary segments of the loop of Henle. Sodium is transported by co-transport using Na+-K+-2Cl− carriers present on the luminal surface of the loop of Henle. Nearly 5% tubular Na+ is absorbed from the proximal segment of the distal tubule along with Cl− co-transport. The second half of the distal tubule has principal cells and intercalated cells.
The principal cells reabsorb sodium and water from the lumen and secrete potassium ions into the lumen. The intercalated cells absorb potassium ions and secrete hydrogen ions. The principal cells are the site of action of the adrenal cortical hormone, aldosterone. The proteolytic enzyme renin is released from JG cells, and it affects the production of angiotensin II, which stimulates the adrenal cortex to release aldosterone. Aldosterone favours sodium reabsorption by increasing the number of Na+-K+ ATPase on the basolateral membrane of tubular epithelial cells. Aldosterone also increases potassium secretion by principal cells. The remaining 5% of sodium ions in the tubular fluid are absorbed under the control of aldosterone, depending on the body’s requirement. Atrial natriuretic peptide (ANP) inhibits the renal absorption of sodium by exerting its influence on the principal cells.
The excess positive charge generated due to absorption of sodium ions is neutralised up to 75% by chloride ion transport. Chloride transport occurs through tight junctions, and chloride can also be passively absorbed in a concentration gradient at the time of solvent drag. From the thick limb of the loop of Henle and the proximal segment of the distal tubule, chloride ions are absorbed by way of secondary active transport or co-transport with sodium.
Glucose and amino acids are reabsorbed by co-transport with sodium ions. They are released from the carrier molecules and transported to the peritubular space by facilitated diffusion inside the cells. In human beings, if GFR is 125 mL and plasma concentration of glucose is 1 mg/mL (100 mg/dL), tubular load of glucose will be 125 × 1 = 125 mg/min. The transport maximum or tubular maximum (Tm) value for glucose is estimated to be an average of 375 mg/min for an adult human being. It is the maximum milligrams per minute at which a substance is transported from the tubular lumen to the interstitial fluid. Beyond the Tm value, the same increment in the serum level of a substance will be excreted through urine. When the blood glucose level increases to 2 mg/mL from 1 mg/mL, the tubular load becomes 250 mg/min. In this stage, a trace amount of glucose may appear in urine because some individual nephrons may have lower Tm values, and some other nephrons may not absorb to their maximum capacity. At a tubular load of 375 mg, both kidneys’ nephrons absorb glucose at their maximum capacity. The increased urinary concentration of any substance will reflect the increased plasma level of that substance. The Tm value for amino acids is 1.5 mM/min (Table 9.1).
Lower thresholds may occur in diabetes in cats, but stress causes hyperglycaemia and glycosuria.
Proteins of molecular weight less than 69,000 will be entirely absorbed from the PCT by active pinocytosis. Inside the cells, they will be degraded by cellular lysozyme to amino acids and these amino acids are transported through the basolateral membrane to the peritubular space. The peptides are hydrolysed at the luminal brush border, and the amino acids formed are transported by co-transport.
When water is reabsorbed osmotically, that will facilitate urea transport to the peritubular space in a concentration gradient. In the inner medullary collecting ducts, facilitated diffusion occurs through urea transporters. Half of the amount filtered will be reabsorbed, and the remaining half is excreted through urine. Since the tubular membrane is impermeable to creatinine, the amount filtered will be excreted as a whole through urine.
Water is reabsorbed extensively from the PCT. Although the descending thin segment of the loop of Henle is permeable to water, it is highly impermeable to solutes. The ascending segments (including both thin and thick) are highly impermeable to water. In the presence of vasopressin or antidiuretic hormone (ADH), the late distal tubule and the collecting ducts are made permeable to water (Fig. 9.7). Whenever the extracellular fluid volume decreases or osmolarity increases, ADH is released from the posterior pituitary. The water reabsorption by ADH is mediated through an intracellular protein called aquaporin-2 (AQP-2). When ADH binds to the plasma membrane receptors of late distal tubules, collecting tubules and collecting ducts, there will be increased formation of cAMP, activation of protein kinases and translocation of intracellular AQP-2 proteins from the interior to the plasma membrane. Fusion of AQP-2 to the luminal plasma membrane results in the opening of water channels in these regions, resulting in water entry to the interior of the cell. Water exits the cell through a different water channel (either AQP-3 or AQP-4) permanently positioned at the basolateral border and then enters the blood, in this way being reabsorbed.
ADH-mediated water reabsorption from renal tubules. Dehydration causes the release of vasopressin (ADH) from the posterior pituitary, which attaches to the basolateral plasma membrane of epithelial cells of the late distal tubule, collecting tubule and collecting duct. This attachment causes the translocation of intracellular water channels AQP-2 to the luminal membrane increasing water permeability
About 50% of the plasma calcium is ionised, and the remainder binds to the plasma proteins or exists in combination with anions such as phosphate. So, the glomerulus can filter only about 50% of the plasma calcium. Usually, about 99% of the filtered calcium is reabsorbed by the tubules, and only about 1% of the filtered calcium is excreted. About 65% of the filtered calcium is reabsorbed in the proximal tubule, about 25–30% is reabsorbed in the loop of Henle and 4–9% is reabsorbed in the distal and collecting tubules. Parathyroid hormone increases calcium reabsorption, especially from the distal tubules, and magnesium reabsorption from the loop of Henle and decreases phosphate reabsorption by PCT.
Know More……
Aquaporins
Aquaporins (AQPs) constitute a family of proteins located in the plasma membrane and mediate water transport. A total of 13 proteins (AQP1–12Α, Β) are included in the AQP family in humans. Though the majority of AQPs facilitate water reabsorption, AQPs (like AQP3, AQP7 and AQP9) also play a significant role in glycerol transportation, thus being referred to as aquaglyceroporins. Because of AQPs’ fundamental roles in essential water homeostasis, the distribution of AQPs was initially regarded as ubiquitous from prokaryotes to eukaryotes.
Renal excretion of magnesium is increased markedly during increased magnesium ion levels but decreases to almost nil during times of its depletion in the blood. Regulation of magnesium excretion is achieved significantly by changing tubular reabsorption. The proximal tubule usually reabsorbs only about 25% of the filtered magnesium. The loop of Henle is the primary site of reabsorption, where about 65% of the filtered load of magnesium is reabsorbed. Only a minimal amount (less than 5%) of the filtered magnesium is reabsorbed in the distal and collecting tubules. Increased extracellular fluid magnesium concentration, extracellular volume expansion and increased extracellular fluid calcium concentration may increase magnesium excretion.
4.4 Tubular Secretion
Hydrogen ions are secreted to the PCT in return to sodium ions, produced by the dissociation of H2CO3 formed by the hydration of CO2 inside the cell. The intercalated cells of the late distal tubule and cortical and medullary collecting ducts secrete hydrogen ions by an active hydrogen-ATPase mechanism. The principal cells of distal and cortical collecting tubule segments of nephrons secrete potassium under the influence of aldosterone whenever dietary potassium intake is high. Organic acids and bases, like bile salts, urates and catecholamines, are secreted from the proximal tubule. In addition, some waste products of metabolism, harmful drugs and toxins are also secreted to the tubular fluid of PCT.
5 Urine Concentration
The kidneys can dilute or concentrate urine without altering the amounts of solutes reabsorbed or excreted. Kidneys can eliminate excess water in the body even by diluting urine to the lowest limit of 50 mOsm/L. At the same time, if the body faces acute dehydration, kidneys can conserve water by increasing the osmolarity of urine excreted to about 1200–1400 mOsm/L in humans. In these two conditions, extracellular fluid osmolarity is maintained at the normal level of 300 mOsm/L. A human being of 70 kg body weight must excrete a minimum 600 mOsm of solutes every day. At the maximum osmotic concentration of 1200 mOsm/L, the obligatory minimum quantity of urine that must be produced is 600 mOsm/1200 mOsm/L = 0.5 L. A desert animal, an Australian hopping mouse, can concentrate urine up to 10,000 mOsm/L, whereas an aquatic animal beaver can concentrate only up to 500 mOsm/L and has the minimum urine-concentrating capacity.
The capacity of the kidney to concentrate urine is attributed to the special arrangement of juxtamedullary nephrons with their loops of Henle dipping deep into the hyperosmotic medullary interstitium. Two mechanisms are involved in the process of urine concentration. One creates hypertonicity of the medulla by the countercurrent multiplier system, and the other maintains it by the countercurrent exchange system.
5.1 Countercurrent Multiplier System
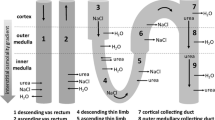
The descending loop of Henle carries the tubular fluid downward from the cortex to the medulla. The tubule’s U-shaped arrangement enables the fluid to flow in opposite directions (countercurrent) in the two tubules (Fig. 9.8). In the thick ascending limb of the loop of Henle, sodium is actively transported from the tubular fluid to the peritubular space. Although chloride, potassium and other ions are co-transported with sodium, the thick ascending limb is impermeable to water. Passive reabsorption of sodium and chloride ions also takes place from the thin ascending limb of the loop of Henle. The descending limb is permeable to the water simultaneously and impermeable to solutes. Since the medullary interstitium gets more and more concentrated with the sodium chloride diffused from the ascending limb, there is increased osmotic outflow of water from the descending limb as it dips deep into the medulla. More sodium chloride will be added to the descending limb from the proximal tubule as a continuous process. The newly arrived sodium chloride gets added to the already existing sodium chloride in the interstitium, thus multiplying the osmotic concentration of the medullary interstitium. Hence, the physiological processes involved in the multiplication of medullary interstitial hypertonicity with specialised transport mechanisms of solutes and water when the countercurrent flow of tubular fluid occurs through the loop of Henle are known as countercurrent multiplier system.
Countercurrent multiplier system. The tubular fluid, when it enters the descending limb, becomes progressively more concentrated due to loss of water. But the ascending limb pumps out Na+, K+ and Cl− ions, and the filtrate becomes hypo-osmotic. The water removed from the tubule enters the vasa recta
The fluid leaving the loop of Henle has an osmolarity of 100 mOsm/L, which is about one-third of plasma osmolarity. Suppose further reabsorption of water is not taking place under the influence of ADH. In that case, the osmolarity can even become 50 mOsm/L since additional reabsorption of solutes occurs from the distal tubule and collecting ducts. The plasma concentration of ADH influences water reabsorption from the late distal tubules, collecting tubules and ducts. ADH-dependent water reabsorption is more pronounced in the cortical collecting tubules than in the collecting ducts. The limited absorption of water in the medullary collecting duct also helps to maintain hypertonic medullary interstitium.
5.2 Countercurrent Exchange System
Countercurrent exchange system is a U-shaped blood vessel (vasa recta) that parallels Henle’s loop. Blood flow through the vasa recta constitutes 5% of total renal blood flow, meeting the metabolic demands of the interstitium, at the same time preserving the hypertonicity of the renal medulla. The colloid osmotic pressure of plasma proteins and hydrostatic pressure of blood flowing through these capillaries are favoured by reabsorption. As the descending limb dips deep into the medulla, it loses water and solutes, increasing its tonicity. When the blood in the ascending limb flows in the opposite direction, it gains water and loses solutes, gradually decreasing tonicity.
6 Obligatory Urine Volume
The maximal concentrating ability of the kidney decides to a minimum how much urine volume must be excreted each day to eliminate the body of waste products of metabolism and ions that are ingested. A normal 70 kg human being excretes about 600 mOsm solute in 1 day. Maximal urine concentration is 1200 mOsm/L, characterised by the minimal volume of urine called the obligatory urine volume. It can be calculated as 600 mOsm/day/1200 mOsm/L = 0.5 L/day. This minimal loss of volume in the urine contributes to dehydration and water loss from the skin, respiratory tract and gastrointestinal tract, especially when the animal is deprived of water.
7 Urea Recycling
The thick ascending limb of the loop of Henle, distal tubule and cortical collecting ducts (top and middle portions of collecting ducts) are impermeable to urea. When ADH concentration of plasma is very high, water reabsorption from the distal parts of the nephron takes place at a faster rate, resulting in urine concentration. The innermost portion of the collecting duct contains many membrane transporters for urea called UT-As, upregulated by vasopressin. The concentration difference favours urea diffusion out of the duct into the interstitial fluid, creating a high urea concentration in the inner medullary interstitial fluid. When the kidney is forming maximum concentrated urine, the urea contributes to about 50% of the osmolarity of the medullary interstitium (Fig. 9.9). A portion of the urea reabsorbed from the inner medullary ducts diffuses into the thin loop of Henle and is re-circulated. This re-circulation helps the concentration of urea to be excreted so that excretion is possible with minimum water loss. A high-protein diet always increases the capacity to concentrate urine because of the comparatively more urea formation as a nitrogenous waste. Malnutrition always reduces the ability to concentrate urine.
Know More……
Shipwreck Victims
The maximum osmotic concentration of the renal medulla is 1200 mOsm/L. The seawater has an osmolarity between 1000 and 1200 mOsm/L with a sodium chloride concentration of about 3.0–3.5%. If an individual drinks 1 L of seawater with an osmotic concentration of 1200 mOsmol/L, that would provide a total sodium intake of 1200 mOsm/L. So, for every litre of seawater drunk, a volume of 2 L of urine would be voided out to get rid of 1200 mOsm of solutes from the body in addition to other obligatory solutes such as urea. The shipwreck victims would have a net fluid loss of 1 L for every litre of seawater drunk, explaining the rapid dehydration in such individuals.
8 Renal Clearance
Renal clearance of a substance is plasma volume that completely clears a particular substance per minute.
If a substance is freely filtered but not reabsorbed or secreted, the plasma clearance rate will equal the glomerular filtration rate (GFR). But no endogenous substance is completely cleared from plasma through the kidneys. For example, the glucose is freely filtered, complete reabsorption takes place usually and glucose clearance will be zero. Since 50% of filtered urea is reabsorbed, the plasma clearance of urea is 50% of GFR. The H+ is freely filtered as well as secreted but not reabsorbed. So, the plasma clearance rate of H+ is always greater than GFR.
An exogenous biologically inert substance, inulin obtained from tubers of a plant, is neither reabsorbed nor secreted but freely filtered. So, inulin clearance rate will be a measure of GFR. Determination of plasma inulin clearance requires the continuous intravenous infusion of inulin to maintain a constant plasma concentration. Because of this reason, renal clearance of an endogenous substance, creatinine (end product of muscle metabolism), is usually used clinically to give a rough estimate of GFR. Creatinine is freely filtered, not reabsorbed but slightly secreted. So, creatinine clearance is not an absolute reflection of GFR but gives a close approximation.
A substance must be freely filtered, and the remaining should be secreted for it to be completely cleared from total plasma reaching kidneys because GFR constitutes only 20% of renal plasma flow. Such a substance can only be used for the measurement of renal plasma flow. But no known substance is completely cleared from plasma. However, para-aminohippuric acid (PAH) is the only substance that is cleared up to 90% from plasma. So, renal PAH clearance can be used to approximate renal plasma flow measurement.
8.1 Assessment of Renal Function
The renal function tests may be classified into (1) tests which measure glomerular filtration rate and (2) tests which study the tubular function. In clinical biochemistry, certain physical and chemical constituents are checked when reporting on a urine sample (Table 9.2). The physical constituents have volume, appearance, odour, colour and specific gravity. The chemical characteristics usually checked are pH, proteins, blood, reducing sugars (glycosuria), ketone bodies, bile pigments and nonprotein nitrogen compounds like urea, creatinine and uric acid. Clearance tests are usually performed to assess the GFR. The measurement of specific gravity or osmolality indicates tubular function. The tubular efficiency for urine concentration can be evaluated using water deprivation and ADH response tests.
8.2 Diuresis
It is the increased urine formation by the kidneys resulting in excessive urination. The reasons for diuresis are increased water intake, action of diuretic drugs and certain diseases such as diabetes mellitus and diabetes insipidus. In diabetes mellitus, the extra glucose present in the tubular fluid build-up increases osmotic pressure and causes osmotic diuresis. In diabetes insipidus, impaired water reabsorption occurs due to lack of sufficient ADH and excessive water loss through urine. The increased water loss may lead to dehydration and polydipsia.
9 Micturition
The process of bladder emptying is known as micturition or urination. The wall of the mammalian urinary bladder is made up of smooth muscle layers of the detrusor muscle. The smooth muscle fibres extend in all directions and fuse so that low-resistant electrical pathways exist from one muscle to another. The epithelial lining of the bladder is an impermeable transitional epithelium that can accommodate the changes in the bladder size. The highly folded bladder wall can flatten out to increase bladder storage capacity. In animals, the small triangular area on the dorsal aspect of the urinary bladder (posterior aspect in human beings) lying immediately above the bladder neck is trigone. The ureters enter the bladder at an oblique angle in the trigone to form a functional valve to prevent the backflow of urine at the time of bladder filling (Fig. 9.10).
The urethra is a tubular structure conveying urine from the neck of the bladder to the exterior. It has two sphincters, an internal urethral sphincter and an external urethral sphincter. Since the internal sphincter is a continuation of the smooth muscle fibres of the detrusor muscle, it is involuntary in action. Its peculiar anatomical arrangement keeps it closed until the pressure inside the bladder exceeds a critical threshold. The external sphincter is lying beyond the bladder and is composed of skeletal muscle, which is under cortical control by a voluntary motor neurone.
Micturition reflex is an autonomic spinal cord reflex (involving the spinal cord’s sacral segment) initiated by the stretch of the receptors in the bladder wall during filling. Afferent impulses are sent through the sensory fibres of pelvic nerves and efferent impulses through the parasympathetic fibres of the same nerve. Thus, contraction of the detrusor muscle begins, and no special mechanism is needed to open the internal sphincter; changes in the shape of the bladder during contraction mechanically pull the internal sphincter open. When urine reaches the neck of the bladder and the external sphincter, afferent impulses are sent to another reflex centre located in the pons. Efferent impulses from this centre (through the pudendal nerve) prevent contraction of the bladder and relaxation of the external sphincter allowing the urine to flow through the urethra. Once the micturition reflex begins, it is self-regenerative, and as the bladder continues to fill, the generation of reflex becomes more frequent and powerful. The external sphincter relaxes when the pressure exceeds the critical threshold, and urine gets voided out. Voluntary inhibition of micturition is possible by the tonic contraction of the external sphincter until a convenient time comes with the involvement of cortical centres of the brain.
10 Descriptive Terms
The normal condition of storing urine in the bladder during filling is known as urinary continence. Urinary incontinence is the frequent dribbling of urine due to improper functioning of the external sphincter. Polyuria is increased urine output, and oliguria is decreased urine output. The condition of no urine output is known as anuria. Dysuria is difficult or painful urination, and stranguria is painful, drop-by-drop and slow discharge of urine.
Uraemia is a clinical state in which the blood urea nitrogen level, an indicator of nitrogen waste products, is elevated. It results due to certain factors, like (1) renal failure; (2) increased production of urea in the liver due to a high-protein diet, drugs and increased breakdown of protein; (3) decreased elimination of urea due to reduced blood flow to the kidney, and obstruction of urinary tract; (4) dehydration; and (5) chronic infection of the kidney.
Uraemia is a severe condition that can even become fatal because very high nitrogen in the blood is toxic to the body. Symptoms of uraemia include mental confusion, loss of consciousness, decreased urine production, dry mouth, debility, paleness of skin or pallor, bleeding problems, increased heart rate (tachycardia), oedema and increased thirst. Treatment includes dialysis or a kidney transplant.
11 Avian Renal Physiology
The avian urinary system consists of a pair of kidneys and ureters that transport urine to the urodeum of the cloaca. The urinary bladder is absent in birds. The kidney lies in a cavity formed by the ventral surface of the synsacrum. The mass of the two kidneys is proportional to (body mass)0.9 or 0.8% or 1.8% of the body weight. The external appearance of the kidney is elongated and tri-lobed with anterior, middle and posterior divisions. Within each division, the kidney is divided into numerous subunits.
In avian species, a dual afferent blood supply is present in the kidney via a ‘high-pressure’ (160/120 mm of Hg) renal artery and a ‘low-pressure’ (25 mm of Hg) supply via a renal portal system (RPS). It is estimated that 1/2 to 2/3rd of blood supplied to avian kidneys is through the renal portal system. The renal artery supplies glomerular areas of the kidney; peritubular areas are partly by efferent glomerular arterioles and also by the venous return from the legs communicating with the RPS. The magnitude of the renal portal supply reaching the peritubular regions appears to be controlled by a smooth muscle valve called the renal portal valve. When the valves close by contraction, the peritubular areas of kidney are perfused with blood. When the valve is opened, the blood is directly shunted to posterior vena cava.
The functional unit of the kidney is the nephron. Avian kidney has two kinds of nephrons. One kind is reptilian type with no loops of Henle, located in the cortex, and another is mammalian type with long- or short-length loops located in the medulla (Fig. 9.11). Only a small percentage of nephrons (15–25%) are mammalian-type nephrons in birds.
Avian kidneys usually alternate between the uses of mammalian- and reptilian-type nephrons. When birds concentrate urine, they opt for mammalian-type nephrons and completely shut down about 80% of the reptilian-type nephrons. Thus, the GFR is reduced to 40% of the normal value. During diuresis, 75% of the filtrate comes from the reptilian-type nephrons.
The role of the kidney in the bird is filtration, absorption and secretion, as in the case of other vertebrates. They filter water and water-soluble substances from the blood, including waste products of metabolism and ions, and are voided out through urine. Kidneys also have an important role in conserving body water and reabsorption of needed substances, viz. glucose.
Blood enters nephrons via afferent arterioles, as in the case of mammals. In the glomerulus, the blood under high pressure gets filtered through the walls of the capillaries and the capsule walls. The filtrate entering the proximal tubules is plasma without protein since protein molecules are generally not filtered due to their large size. In the proximal convoluted tubules, the vital substances in the filtrate, such as vitamins and glucose, are reabsorbed into the blood. The kidney tubules can reabsorb almost 98% of the glucose that filters into the tubule even in a carbohydrate-rich dietary state.
Birds can conserve body water by producing urine of more osmotic concentration than plasma, as in the case of mammals. But the urine concentration capacity is limited in birds compared to mammals. On water deprivation, mammals can concentrate urine 5–10 times more than plasma; some mammals can do it about 20–25 times. But birds in water deprivation can only produce 1.4–2.8 times more concentrated urine than plasma. This ‘concentrating capacity’ is a feature of the medullary cones.
Solutes, like sodium chloride, are actively transported out of the ascending limb of the loop of Henle, and they become concentrated in the medulla (medullary cones). Unlike in mammals, only sodium chloride is responsible for maintaining medullary hypertonicity in birds. When the filtrate passes through the osmotic gradient in the medulla, water gradually leaves the tubules by osmosis, and the filtrate becomes concentrated. Because only the looped mammalian nephrons contribute to the intramedullary osmotic gradient, the presence of reptilian (loopless) nephrons limits the ability of the kidneys to produce hyperosmotic urine. Thus, the birds have limited urine concentration ability than mammals.
Usually, more water accompanies the solutes that travel from the kidneys through the ureters to the cloaca due to the reduced capacity of avian kidneys to concentrate urine (compared to mammals). A mechanism exists in water-deprived birds for reducing the amount of water leaving the kidneys. When dehydration occurs, the pituitary gland releases a hormone called arginine vasotocin (AVT) into the blood. AVT causes a decrease in the glomerular filtration rate in the avian kidneys, reducing the amount of water moving from the blood into the kidney tubules. Besides, AVT aids in the opening of protein water channels called aquaporins and thus increases the permeability of the walls of collecting ducts to water. Due to the increased permeability of the collecting ducts, more water leaves by osmosis out of the collecting ducts to the hypertonic medullary cones. From there, water is reabsorbed by kidney capillaries. Studies suggest that the effectiveness of AVT in reducing urine production or water reabsorption varies among species, but, in general, AVT is considered to be less effective in conserving water than the mammalian equivalent antidiuretic hormone (ADH). Therefore, water-deprived birds tend to lose more water from the kidneys than similar sized water-deprived mammals.
Uric acid is the end product of nitrogen metabolism in terrestrial reptiles and birds. In these species, the embryo’s development takes place in eggs that have shells impermeable to water. So, the embryo is provided only with a limited supply of water. Hence, these organisms deposit excretory products as insoluble substances that do not require water to minimise water usage. Although uric acid is freely filtered at the glomerulus, secretion in the tubules accounts for 90% of the uric acid excreted in the urine of birds. Precipitation of uric acid occurs when the quantity of uric acid present in the tubules exceeds its solubility. Uric acid sediment moves through the tubules and appears as a white coagulum in the urine. Since uric acid is not present in the solution, it does not contribute to the osmotic pressure of the urine and thus avoids the obligatory loss of water.
Salt glands of birds are supposed to be evolved from the nasal glands of reptiles. They lie immediately under the skin in supraorbital depressions of the frontal bone in the skull of Charadriiform birds. Still, in other birds, they may be located above the palate or within the orbit of the eye (Fig. 9.12). The marine birds (and some desert and Falconiform birds) secrete excess sodium chloride via the salt glands using less water than is consumed, thus saving water. So, birds are not physiologically affected by the high salt load.
Salt glands have a countercurrent blood flow system to remove and concentrate salt ions from the blood. It is paired and crescent-shaped glands. Each gland contains several longitudinal lobes approximately 1 mm in diameter, and each lobe contains a central duct from which radiated thousands of tubules are enmeshed in blood capillaries. These tiny capillaries carry blood along the tubules of the gland, which have walls just one cell thick and form a simple barrier between the salty fluid within the tubules and the bloodstream. The salt excretion occurs in this gland.
Learning Outcomes
-
Metabolic waste products are eliminated, and water and electrolytes are retained in the body at optimum concentrations by effective glomerular filtration, tubular reabsorption and tubular secretion occurring inside the kidney’s nephrons.
-
The kidney can auto-regulate its functions by inherent mechanisms, like myogenic response and tubulo glomerular fluid. The countercurrent multiplier and countercurrent exchange systems are responsible for creating and maintaining graded hypertonicity in the medullary interstitium, which is essential for concentrating urine. Urea recycling also helps to maintain medullary hypertonicity.
-
The urine formed is conveyed to the urinary bladder through ureters for temporary storage until it is voided out by the process of micturition.
-
Structural and functional modifications are present in the avian renal system to eliminate the major metabolic end product of birds, the uric acid. Salt glands provide a means of eliminating excess salt from the body, especially in marine species of birds.
Exercise
Objective Questions
-
Q1.
Inulin clearance study is used to measure _______.
-
Q2.
The plasma concentration of a substance at which it first appears in urine is known as ______.
-
Q3.
Which type of collecting duct cells is involved in acid secretion?
-
Q4.
Which condition stimulates the kidney to release erythropoietin?
-
Q5.
Which is the major force favouring filtration across the glomerular capillary wall?
-
Q6.
Which water channel is responsible for ADH-induced water reabsorption from the collecting duct?
-
Q7.
Which tubular part of the nephron is completely impermeable to water?
-
Q8.
Which hormone increases renal reabsorption of calcium?
-
Q9.
What is the major end product of nitrogen metabolism in birds?
-
Q10.
The property of tubules to increase reabsorption following the increased tubular load due to increased GFR is known as ______.
-
Q11.
Which mechanism helps glucose reabsorption from PCT?
-
Q12.
What are the target cells of aldosterone in renal tubules?
-
Q13.
Which organic compounds are responsible for 50% renal medullary interstitial hypertonicity?
-
Q14.
Where are the urea transporters located?
-
Q15.
The bulk of glomerular filtrate is reabsorbed in which part of the nephron?
-
Q16.
PAH clearance is used to study in ______.
-
Q17.
What amount of mammalian-type nephrons are present in the birds?
-
Q18.
The mode of Na+ reabsorption from Henle’s loop is ______.
-
Q19.
Which proteolytic enzyme affects the release of aldosterone after secreting from JG cells?
-
Q20.
Which specialised tubular epithelial cells are involved in monitoring sodium ion concentration in the tubular fluid?
Subjective Questions
-
Q1.
What is tubuloglomerular feedback?
-
Q2.
Describe the dynamics of glomerular filtration.
-
Q3.
Explain micturition.
-
Q4.
How does ADH-mediated water reabsorption occur in the kidney?
-
Q5.
How does sodium reabsorption occur from the renal tubules?
-
Q6.
Describe the urine concentration mechanisms.
-
Q7.
What is urea recycling?
-
Q8.
Describe renal clearance.
-
Q9.
Write the speciality of avian excretion.
-
Q10.
Write the dynamics of tubular reabsorption.
Answers to Objective Questions
-
A1.
GFR
-
A2.
Renal threshold
-
A3.
Intercalated cells
-
A4.
Hypoxia
-
A5.
Hydrostatic pressure of capillary blood
-
A6.
Aquaporin-2
-
A7.
Thin portion of the loop of Henle
-
A8.
PTH
-
A9.
Uric acid
-
A10.
Glomerulotubular balance
-
A11.
Co-transport/symport/secondary active transport
-
A12.
Principal cells
-
A13.
Urea
-
A14.
Collecting duct
-
A15.
Proximal tubule
-
A16.
Renal plasma flow
-
A17.
15–25%
-
A18.
Co-transport with K+ and Cl−
-
A19.
Renin
-
A20.
Macula densa
Keywords for Answer to Subjective Questions
-
A1.
Filtration pressure, macula densa, afferent arteriole, mesangium
-
A2.
Hydrostatic pressure, colloid osmotic pressure, net filtration pressure
-
A3.
Micturition reflex, reflex centres of pons, relaxation of external sphincter
-
A4.
Dehydration, ADH, aquaporin-2
-
A5.
Co-transport, counter-transport, chloride-driven sodium transport, transport from DCT
-
A6.
Countercurrent multiplier, countercurrent exchange
-
A7.
Inner medullary collecting duct, urea transporters, interstitial hypertonicity
-
A8.
GFR, inulin clearance, creatinine clearance, renal plasma flow, PAH clearance
-
A9.
Uric acid, reptilian and mammalian nephrons, AVT, renal portal system
-
A10.
Hydrostatic pressure, colloid osmotic pressure, capillary blood, interstitial fluid
Further Reading
Hall JE (2011) Urine concentration and dilution: regulation of extracellular fluid osmolarity and sodium concentration. In: Hall JE (ed) Guyton and Hall textbook of medical physiology, 12th edn. Elsevier Saunders, Philadelphia, pp 345–360
Magouliotis DE, Tasiopoulou VS, Svokos AA, Svokos KA (2020) Aquaporins in health and disease. Adv Clin Chem 98:149–171
Parrah JD, Moulvi BA, Gazi MA, Makhdoomi DM, Athar H, Din MU, Dar S, Mir AQ (2013) Importance of urinalysis in veterinary practice – a review. Vet World 6(9):640–646. https://doi.org/10.14202/vetworld.2013.640-646
Reece WO (2009) The urinary system. In: Reece WO (ed) Functional anatomy and physiology of domestic animals, 4th edn. Wiley-Blackwell, pp 344–347
Reece WO (ed) (2015) Duke’s physiology of domestic animals. Wiley
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2023 The Author(s), under exclusive license to Springer Nature Singapore Pte Ltd.
About this chapter
Cite this chapter
Beena, V. (2023). Excretory Physiology. In: Das, P.K., Sejian, V., Mukherjee, J., Banerjee, D. (eds) Textbook of Veterinary Physiology. Springer, Singapore. https://doi.org/10.1007/978-981-19-9410-4_9
Download citation
DOI: https://doi.org/10.1007/978-981-19-9410-4_9
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-19-9409-8
Online ISBN: 978-981-19-9410-4
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)