Abstract for Section 10.1
Kidneys and their evolutionary precursor organs are indisputable for the survival of mammalians to invertebrates like Drosophila. These organs are responsible for clearing of water-soluble exogenous and also endogenous metabolites with a potential for toxicity in settings of overload. Such substances are for instance ammonium ions (from protein metabolism), ureic acid (from nucleic acid metabolism), and creatinine (end product of muscle proteins). These substances are called urophenic. In situations of kidney failure these substances are retained and cause severe toxicity in the patients (uremia). Further, water and ions are secreted which have important functions in cells: Na+ (sodium), K+ (potassium), HCO3 − (bicarbonate), and phosphate and calcium. Especially Na+ ions are recognized by specialized kidney tubular cells and indicate the volume state of the organism.
At the same time, the body must retain its homeostasis and retain its crucial fluids and molecules. The kidney is thus a major checkpoint for blood clearance, blood homeostasis, blood volume, blood pressure, and its pH.
Abstract for Section 10.2
Main functions of the urinary system are elimination of metabolic wastes that otherwise accumulate and harm the integrity of the body, maintenance of homeostasis and osmoregulation, acid-base regulation and hormone production. Different species have developed different urinary sytems to adapt to the environment they are living in.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
10.1 Basic Physiology of the Human Kidney
10.1.1 Abstract
Kidneys and their evolutionary precursor organs are indisputable for the survival of mammalians to invertebrates like Drosophila. These organs are responsible for clearing of water-soluble exogenous and also endogenous metabolites with a potential for toxicity in settings of overload. Such substances are for instance ammonium ions (from protein metabolism), ureic acid (from nucleic acid metabolism), and creatinine (end product of muscle proteins). These substances are called urophenic. In situations of kidney failure these substances are retained and cause severe toxicity in the patients (uremia). Further, water and ions are secreted which have important functions in cells: Na+ (sodium), K+ (potassium), HCO3 − (bicarbonate), and phosphate and calcium. Especially Na+ ions are recognized by specialized kidney tubular cells and indicate the volume state of the organism.
At the same time, the body must retain its homeostasis and retain its crucial fluids and molecules. The kidney is thus a major checkpoint for blood clearance, blood homeostasis, blood volume, blood pressure, and its pH.
10.1.2 Introduction
Whereas in mammalians the intestine preferentially secretes lipophilic substances, hydrophilic substances are preferentially secreted by the renal system, i.e., the kidneys. In the first part of this chapter predominantly the highly developed kidneys in humans will be discussed which are analogous to kidneys of other mammalian species. Basic principle No. 1 is filtration through semipermeable membranes. This means that water and small ions should be able to trespass, whereas molecules of a molecular mass above 68 kDa should be retained. This means that the major plasma protein albumin or other proteins only appears in the urine in situations where the permeability of the filter increases, like in inflammation. This is called proteinuria and is associated with loss of the oncotic pressure in the blood vessels. In consequence, fluid is lost to the interstitial space of the body and causes edema. The urine may be milky and/or cloudy. Further, immunoglobulins (160 kDa and more) and all cellular compounds (red and white blood cells) should be retained in physiological condition. In case the permeability increases further, red blood cells may be found in urine (hematuria).
However, also the increased amount of a substance in the blood may lead to urinary filtration. The renal threshold of glucose (RTG) is 160–180 mg/dL. Above that glucose is found in urine. Also the volume of the urine will be much higher due to the osmotic capacity of glucose. As a consequence, diabetic patients with insufficiently controlled blood glucose levels run the risk of exsiccosis.
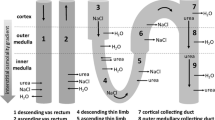
The volume passing a filter is also dependent on the pressure on the membrane. The kidneys are located in short distance to the abdominal aorta from where they get oxygen-rich blood (Fig. 10.1a). In fact 20 % of the cardiac output, i.e., 1,500 L blood per day run into the kidneys in an adult person. From this volume, 10 % are actually filtered to form the primary urine, i.e., 150 L/day. As the output urine, however, is only 1.2–1.8 or 2 L per day, significant concentration must take place in between. The ion concentration in the beginning is 300 mosm/L and is enhanced to 1,200 mosm/L in the end. This can be pursued by the so-called countercurrent principle in a specialized tubulus part—the loop of Henle (Fig. 10.1b, c). This is a special feature, which especially mammalian kidneys have developed in order to retain and control volume. The countercurrent principle relies on the active, energy-dependent transport of Na+ ions from primary urine into the renal interstitium. When water passes by in other segments of the tubes, it follows the osmotic gradient and thus can be reabsorbed. Concentration has taken place. In fact, 99 % of the primary urine is reabsorbed in healthy condition.
Kidney anatomy and function. (a) The kidneys are tightly associated to the aorta where they may sense volume disturbances and immediately counteract them. Directly on top of the kidneys the adrenal glands are located which help in volume regulation by providing aldosterone. Further, regular blood pressure along the aorta is needed to produce primary urine, which is concentrated in the renal medulla by the countercurrent principle. The ureters transport the concentrated urine into the bladder as a transient reservoir (© [Martha Kosthorst]—Fotolia.com); (b) Scheme of the microanatomy of a nephron (© [krishnacreations]—Fotolia.com); (c) physiological function of a nephron: The afferent arteries transport blood into the glomerulum. The Bowman’s capsule is used as a collector of primary urine which then enters the proximal tubes and Henle’s loop, being surrounded by a tight capillary net. During its transport the urine is concentrated in the medulla and reaches via distal tubes the collecting tubes being connected to the renal pelvis and ureters (© [Alila Medical Images]—Fotolia.com)
There may, however, be pathologic conditions where reabsorption is disturbed. This would result in polyuria (too much volume lost by urine—exsiccosis), or oliguria (too little urine is produced) or anuria (no urine). The latter will result in an intoxication of the organism with uriphenic substances.
10.1.3 Microanatomy of the Kidneys: The Nephron
So far, we have summarized the basic principles of kidney function, which are filtration and concentration. These are guaranteed by a highly specialized microanatomy. The smallest functional unit in the kidney is the nephron, of which 300,000 to 1 million exist in a human kidney; in mouse for instance 11,000 only (Fig. 10.1b). A nephron is the results of a collaborative action between one arteria and one urine-collecting tube, and their synapse is called a glomerulum. This is a small tulip-shaped bowel hosting the arteria, receiving the filtrate and forwarding it to a single tube. The incoming vessel (vas afferens) multiplies in several branches to increase the effective filter surface, before they assemble again to the vas efferens (outgoing vessel). An inner endothelial cell layer, a basal membrane, and so-called podocytes around compose each vessel. One can imagine that these multilayer ultrafilters can be easily hit by aggregates exceeding 70 kD, which stick to the filter and cause inflammation often affecting the whole organ—glomerulonephritis. Needless to say that secondarily the filter capacity may thereby be severely affected. In physiologic conditions, however, primary urine is filtered and then collected by a long single tube, finally assembling with others in the so-called collecting tubes. During its tube journey, urine is further concentrated. Whereas within the glomerulus the pressure is high (15 mmHg), the pressure in the tubal system decreases significantly allowing a number of selective or passive reabsorption and excretion processes.
10.1.4 The Embryonic Kidney Development
From the early embryonic development on, soon after yolk sac and amnionic cavity have been established and cells are differentiated to ectoderm, endoderm, and mesoderm, the kidneys start to develop from the so-called Wolffian duct from mesoderm layer. The first developmental forms of it, termed pronephros and mesonephros, are transient in mammalians, birds, and reptiles. Only the third form, metanephros, persists and starts building up the excretory system from gestation week 5 on. The Wolffian duct on the caudal side fuses with the hindgut to form a primitive cloaca from where urine and products of the intestine are excreted. Urine production starts from week 10 on by the embryo.
The location of the metanephros is dorsal, along the neural tube and the aorta, but outside the perinatal cavity. The metanephros forms separated functional units, called renculi of which 10–20 exist in humans; in, e.g., guinea pig only one. This causes a lobulated surface typical for human embryonal kidneys, and existing also in other mammalian species (see below). From weeks 9–12 on the lobulated surface changes to a smooth one, and the developing kidneys ascent from the caudal to the lumbar region. During this journey which starts at week 7–9, they form loose multiple supply connections with the aorta which move cranially finally forming the renal arteria. The ureters are urine transporter tubes. They collect urine from the renal pelvis, where urine from the kidney nephrons is pooled. On the other end ureters make contact to the cloaca and later bladder. The ureters follow the kidneys during their ascent. As the urine system develops in parallel to the genital system they are together called urogenital system. During the ascent of the metanephros for instance, the male testes follow and later have to descend again along the ureters (see Sect. 10.2) in order to find their final position outside the body. The scrotum temperature of only 32 °C is optimal for the maturation of, at least human, semen. Some problems during the kidney and testes up- and down movements occur quite often causing malpositions and consecutive organ failure. Finally, after additional rotation the kidneys find their final position at term. Further, during the development a septum is formed to separate the hindgut/rectum from the urogenital sinus where primordial bladder with associated rudimental Wolffian duct and ureters are formed. In male mammalians the last distance of the urogenital system, where semen and urine are released, still indicates the common developmental history.
10.1.5 Some Nephrons Participate in Volume Regulation by Autoregulation
As outlined above, a nephron is the minimal functional unit of the kidney. Glomerular capillaries and collecting tube system constitute it. There are two different types of nephrons with different functions. Type I is residing in the renal cortex where 99 % of the incoming plasma volume arrives. Therefore, this type of nephrons is an effective sensor of blood pressure and volume arriving. These cortical nephrons are also used for regulating the blood flow and thus the filtration rate in the kidneys. This is called myogenic autoregulation: Active change in arterial blood pressure may result in vasoconstriction (fall in renal plasma flow (RPF) ⟹ less filtration ⟹ retaining of fluid ⟹ elevation of blood pressure) or vasodilatation (higher filtration rate, lowering of volume and blood pressure). Vasoconstriction may occur due to nerval irritation, irritation of vasomotoric centers in the brain stem, hypoxia, stress (catecholamines such as adrenalin), or during work. This is reasonable as during body activity higher blood pressure and circulation rate are needed for full oxygen supply of organs. However, often after stress situations retained urine is released with satisfaction. Vasodilating conditions causing enhanced diuresis on the other hand are bacterial endotoxins causing fever (pyrogens) or protein-rich food.
The net release of urine is thus dependent on the capillary pressure, +50 mmHg, which is countered by the oncotic pressure of plasma proteins in the vessels (−25 mmHg), and the pressure of the Bowman’s capsule (−10 mmHg) (Fig. 10.1c). This causes a net filtration pressure of +15 mmHg in healthy condition. Factors that influence that are (1) changes in permeability, for instance by precipitated immune complexes which block the filter, or (2) reduced filter area, for instance due to loss of parenchyma. In diagnostics it is important to have measures for the filtration capacity of the kidney. This can be achieved by measuring substances, which trespass the filter. Experimentally, para-amino hippuric acid (PAH) can be used to determine the arterio-venous difference, also called RPF, as it is excreted almost completely (90 %) and only to 10 % reabsorbed. Inulin is a small molecule which is freely filtrated through the glomeruli. It can thus be used to determine the glomerular filtration rate which should be 125 mL/min, resulting in 150–180 L primary urine per day. The formula would be
However, for diagnosis it is more practicable to use body metabolites for determination which also are secreted easily, such as creatinine. For this, urine has to be collected from the patient during 24 h. The normal range of creatinine excretion is 500–2,000 mg/day for a healthy adult person, but depends also on sex and age.
10.1.6 Some Nephrons Participate in Volume Regulation by the Endocrine Network
The second type of nephrons are expanding from the cortex deep into the medulla. They are building up the osmotic gradient using Na+ ions. These nephrons are therefore important sensors of the Na+ contents in the urine. Excess Na+ ion levels at the level of distal tubes indicate that too little fluid is trespassing as a possible result of volume reduction by low fluid intake, volume loss by bleeding or other types of shock, or volume loss by excessive diarrhea or vomiting. This organ lying there is called the juxtaglomerular apparatus (juxta = close to). It consists of Na+-sensing cells of the distal tube forming the Macula densa, which are surrounded by vascular mesangium cells and in direct connection with juxtaglomerular endocrine cells. This is a major checkpoint for volume control: As a response to lowered GFR and hence lowered Na+ absorption, but also to sympathic stimuli (stress), these endocrine cells produce renin. It is released directly to the glomerular capillaries to direct their contraction state. Renin is further distributed to the whole organism to trigger a cascade of consecutive hormonal regulatory circles. Angiotensinogen from the liver is converted to angiotensin I by renin. The lung provides then angiotensin converting enzyme which produces Angiotensin II, which strongly enhances blood pressure, by multiple mechanisms: It augments sympathic activity, tubular Na+-reabsorption, and the release of aldosterone, a further regulatory hormone, from the adrenal gland, and enhances the secretion of ADH, the antidiuretic hormone from the pituitary gland. Together, this system is called the renin–angiotensin–aldosterone system (RAAS). It is an important target of modern anti-hypertonic medications, such as the angiotensinogen-converting enzyme (ACE)-inhibitors. The highest level in this hierarchy is taken in by aldosterone.
Aldosterone is a product of the suprarenal glands’ cortex and thus is ready to instruct the kidneys from short distance with immediate effect. It induces reabsorption of Na+-ions (and hence water) from the tubes, especially proximal Henle’s tubes. In exchange, K+-ions are pumped out. In diseases with hyperaldosteronism, such as benign (Conns’ disease) or malignant tumors of the adrenal glands, this leads to hypertonia with concomitant hypokalemia associated with muscular weakness and a risk for bradycardia and atrioventricular block in the heart. In addition, alkalosis occurs, as with the K+ (potassium) ions also H+ (protons) are lost (see below).
The antidiuretic hormone (ADH) induces water reabsorption by inducing H2O channels in the collecting tubes which leads to a concentration of urine (antidiuresis). ADH is derived from the posterior pituitary gland (hypophysis) under the control of the corticotropin releasing factor (or hormone; CRH) from the hypothalamus (see Chap. 8). It is predominantly released upon signals from baroreceptors for low blood pressure, and signals from the hypothalamus for hyperosmolality (concentration, high ion strength) of the blood plasma, such as may occur in settings of extensive evaporation of body water, in heat or reduced drinking. Tumors in the hypophysis typically may lead to hyposecretion of ADH which leads to excessive diuresis of up to 20 L per day. This is a life threatening condition due to exsiccosis. From an evolutionary point of view, CRF is well conserved even in insects and may function itself as an ADH (De Loof et al. 2012).
10.1.7 Other Endocrine Functions of the Kidney
From the above it is clear that the kidney interacts with several other organs in regulating one of the most important body functions: volume regulation.
In addition to the secretion of the hormone renin, renal tubes are also a source of prostaglandins. They are formed from membrane phospholipids by the phospholipase A enzyme which first produces arachidonic acid which then can be transformed to either leukotrienes by the enzyme lipoxygenase or prostaglandins by the enzymes cyclooxygenase-1 or -2 (COX-1, COX-2). In the kidney, like in other epithelial cells (e.g., in the stomach, see Chap. 9), especially prostaglandin E has a regulatory function (Breyer et al. 1998). It inhibits the reabsorption of Na+ ions (and osmotically that of H2O) in the renal tube and, thereby, enhances urine volume. This is one mechanism for enhanced diuresis in bacterial infections. Therefore, it counterregulates the RAAS and decreases blood volume and pressure. In therapy of pain and inflammation COX-2 inhibitors are used which counteract this and may lead to hypertension.
Erythropoietin (EPO) is a hormonal product directly of the kidneys. Its secretion by interstitial fibroblasts nearby peritubular capillaries is stimulated by hypoxia and consecutive release of hypoxia-induced factor (HIF) by the liver and the gastrointestinal tract. Therefore, several organ systems cooperate to protect the organism from hypoxic damage. When EPO reaches the bone marrow, site of blood cell production and maturation, it binds to EPO receptors of pro-erythrocytes (precursors of red blood cells). These cells still have nuclei and DNA and can thus enhance the production of hemoglobin. In consequence, hemoglobin-rich erythrocytes leave the bone marrow and are ready to transport more oxygen. This is a physiological mechanism to adapt to hypoxic conditions, such as stays in higher altitude. This phenomenon is used in sports to enhance the capacity of oxygen transport in athletes. This mechanism can also be misused by EPO doping.
The kidney takes also a critical role in vitamin D3 metabolism by a hydroxylation step. Vitamin D3 (Calcitriol, 1α,25(OH)2-Cholecalciferol) is today mostly regarded as a steroidal hormone (D-hormone) because it is synthesized in the skin from 7-dehydro cholesterol upon UV irradiation (Präcalciol); besides it can be taken up by nutrition. Next, in the liver hydroxylation at position 25 takes place, followed by a second hydroxylation at position 1α in the kidney. The active 1α,25-Dihydroxyvitamin D2 is from there distributed into the body where it specifically binds to vitamin D-receptor (VDR). It induces Ca2+ uptake from the intestine, supports the mineralization of bones and immune defense, and it suppresses malignant cellular growth. Reduced D-hormone levels are associated with a higher rate of colon carcinoma (Deeb et al. 2007). In case of chronic kidney failure such as during glomerulonephritis, the final activation of D-hormone is missing and results in low calcium levels. As a counterregulatory mechanism, the parathyroid gland secretes parathormone (PTH). This results in hyperplasia of the parathyroid organs, the so-called secondary hyperparathyroidism. Importantly, PTH activates osteoclasts to resolve calcium from bones. Chronic kidney failure thus typically results in renal osteodystrophic syndrome with massive decalcification of bones associated with pain and an enhanced risk for untypical fractures. The specific function of the osteoclasts in this context is discussed in Chap. 3.
10.1.8 The Kidney Controls the Acid–Base Balance
The urine is not only concentrated by the passage through tubes but also a pH shift takes place. Depending on the metabolism, pH values between 4.5 and 8.0 may be physiologic for human urine. This indicates that active processes may regulate the H+ concentration. A key enzyme for this is carboanhydrase. This enzyme transforms H2O and CO2, which are present in all cells of the body, to H+ ions and HCO3− (bicarbonate) ions. Thereby, two aims can be fulfilled: (1) excretion of protons to the luminal (urine) side (in exchange with Na+ of the proximal and distal tubular cells); (2) secretion of HCO3 − ions back to the blood. Bicarbonate ion secretion is dependent on the function of the Na+–K+ ATPase (an energy-dependent ion pump). When one HCO3 – leaves the cell to the blood, one K+ potassium ion is taken up to the inside of the cell. Thereby, the kidneys contribute to the so-called buffer capacity of the blood: HCO3− ions released to the blood may collect free protons and be transformed to H2CO3. Carbonic acid is very instable and immediately dissociates again to H2O and CO2 again, two harmless substances. In case of renal insufficiency, when both the proximal and distal tubular functions are disturbed this leads to metabolic acidosis and hyperkalemia. Hyperkalemia leads to depolarization of the membrane potential of cells, most significant on neuromuscular cells. Hyperkalemia thus leads to atrioventricular conduction problems, resulting in arrhythmia up to asystole by AV-block.
10.2 Comparative Aspects of the Urinary System
10.2.1 Abstract
Main functions of the urinary system are elimination of metabolic wastes that otherwise accumulate and harm the integrity of the body, maintenance of homeostasis and osmoregulation, acid-base regulation and hormone production. Different species have developed different urinary sytems to adapt to the environment they are living in.
10.2.2 Basic Principles of the Elimination of Metabolic Wastes in Different Species
End products of metabolism that need to be eliminated are carbon dioxide, water, and species-specific residuals of the protein metabolism. After degradation of proteins to amino acids several potential destinies are feasible.
For production of energy amino acids can be metabolized in the citric acid cycle, with CO2 (carbon dioxide) and H2O (metabolic water) as end products. Carbon dioxide is exhaled via the lungs while water is either reused or eliminated as described in section homeostasis and osmoregulation.
Amino acids can also be used in the muscle metabolism with creatinine as final waste. Creatinine is apparent in all vertebrates and entirely filtered and eliminated by the urinary system.
Furthermore, amino acids can be incorporated as nuclear bases (pyrimidine and purine bases) in DNA and RNA which form species-specific end products during degradation. While pyrimidine bases are degraded to ammonia that is water-soluble and easily excreted in urine, purine bases are degraded in species-specific ways. While invertebrates, larval stages of amphibians, and most fish may also reduce purines to ammonia, adult amphibians and some other fish are only able to reduce purines to allantoic acids. Most mammals degrade purines to allantoin, while primates, guinea pigs, and humans together with reptiles and birds reduce purines only up to the stage of uric acid. Therefore, some residual energy is lost with these excretes.
The normal pathways are jeopardized in case of Dalmatians: dogs normally degrade their purine to the allantoin stage—however, this breed is affected by a genetic defect that inhibits complete degradation and purine metabolism ends with uric acid. This leads to pathologically high contents of uric acid, posing a risk to developing uroliths.
All other nitrogenous residuals are eliminated in a species-specific way. From all amino acids the nitrogen part has to be removed from the carbon skeleton before the latter can be used for energy production. The removed nitrogen is forming ammonia (NH3) which is toxic and therefore has to be eliminated as fast as possible from the body. In water living species the excretion is not a problem as ammonia is highly soluble in water and easily diffuses as ammonia or ammonium (NH4) via the gills or other surfaces (ammonotelic species). In land living species excretion of ammonia in water stands against the attempt to save and reabsorb water and therefore only low amounts leave the body as ammonium ions with the urine, while for the majority detoxification is necessary. Detoxification is done by conversion to relatively safe substances like urea or uric acid. Amphibians and mammals that generally live in moderate habitats produce urea (ureotelic species). Birds, reptiles, and many insects that are also able to live in arid areas only excrete uric acid (uricotelic species). The advantage of producing uric acid is the possibility to excrete it in more or less solid form so only limited water is necessary. For fetal development, elimination of the nontoxic uric acid is advantageous too, as it can be stored in a space saving manner within the egg shell. However, the disadvantage is the great energy consumption for conversion and loss of nitrogenous compounds.
10.2.3 Possible Ways of Dealing with Unwanted Substances
There are different ways of removing unwanted substances from the metabolism: storage, immediate excretion, and temporal storage with subsequent excretion.
The simplest form of dealing with metabolic wastes is storing detoxified end products within the body. An example is the nucleobase guanine, which is stored as photonic crystal in the skin of fish and is responsible for the metallic look (Levy-Lioret et al. 2008). Sharks even store urea in the muscles and therewith become isotone with the surrounding sea water. In case the shark is fished, the urea transforms to ammonia and develops a bad smell that only slowly disappears by evaporation.
Immediate excretion is seen in aquatic species where substances can diffuse to the water surrounding the animal. Examples are gills of fish that are able to expel ammonia from the body or the highly permeable skin of amphibians like frogs that has an active transport system for sodium.
The simplest example of temporal storage is the contractile vacuole of unicellular organisms. Mainly water and soluble substances are stored in vacuoles that fuse with the cell membrane after a while and eliminate the content to the outer medium.
The most widely used way of excretion is via specialized organs for excretion. The general blueprint consists of convoluted canals with great surface area. In many cases temporal storage is performed in addition.
10.2.3.1 Excretion in Invertebrates
In invertebrates like annelids and molluscs there are dead end- (Protonephridium) and open-systems, which are in contact with the circulatory system (Metanephridium). Ciliated or flame cells produce a fluid flow in a canal system that opens with nephridopores to the outside from each segment of the body. In insects and spiders the Malphigian tubules are the main site of excretion. They are located in close relationship to the alimentary canal and are surrounded by hemolymph. Functionally, an active transport of hydrogenic and potassium ions is followed by a passive influx of water and nitrogenous wastes. Uric acid is precipitated in the intestine and water reabsorbed before excretion with the feces.
10.2.3.2 Excretion in Vertebrates
Vertebrates developed a highly specialized excretory organ: the kidney—with the nephron as functional unit for urine production. A nephron is composed of a renal corpuscle (glomerulus and capsule) and the adjacent tubular system. While the first is for filtration of blood to primary urine, the latter has the function of secretion, reabsorption, and excretion.
From comparative studies of development it is believed that the kidney of the earliest vertebrates extended the length of the coelomic cavity and showed tubules arranged in segments resembling the invertebrate nephridium. This ancestral kidney is called archinephros and is not apparent as a functional form in adult vertebrates. Nevertheless, archinephros can be seen up until now during development of embryonic hagfish. Each tubule opens to the coelomic cavity with a nephrostome and collects the filtrate in the archinephric duct. The adult hagfish already shows a pronephros that derives only from the cranial parts of the archinephros and shows a reduced number of tubules. The mesonephros is a functional kidney of embryos of reptiles, birds, and mammals, only seen during development and not occurring in adult animals. It further develops to an opisthonephros in fish and amphibians or a metanephros in amniotes.
The opisthonephros is based on the caudal aspects of the mesonephros and is built of glomeruli and many coiled opisthonephric tubules. Fresh water fish have larger glomeruli than fish from salt water. The opisthonephric duct conducts both urinary excretes and sperm derived from colocalized testis.
In contrast, the metanephros of reptiles, birds, and mammals that also derives from caudal parts of the mesonephros develops an additional and separated duct for transportation of urinary excretes only: the ureter. The ureter ends either in the cloaca or in the urinary bladder (mammals)—an organ for temporal storage.
The metanephric kidney of reptiles shows multiple lobulations and a multitude of branches of the ureter. As nephrons are only in the cortical region concentration of urine is only possible in isosmotic situation. Final water reabsorption is performed by the cloaca epithelium. For lizards number of nephrons is speculated to range between 3,000 and 30,000.
Kidneys of birds are normally lobulated in three parts and tightly secured underneath the synsacrum and ilium. Probably due to the high metabolic rate of birds and the resulting metabolic wastes, a high number of nephrons are prevalent: in chicken about 200,000. Avian nephrons can be classified into two types: reptilian-type nephrons located in the cortex and mammalian-type nephrons in the medullary regions. The latter are with about 10–40 % the minority and show larger, more complex corpuscle and a loop of Henle. The produced uric acid is passed into the cloaca and transported back up to the caeca. In the intestine more water can be reabsorbed before final elimination together with the feces.
The mammalian kidney is located retroperitoneal in the lumbar region, with the right kidney in a slightly more cranial position in many species (except humans). The kidney consists of a fibrous tissue capsule and a lobulated parenchyma, which can be divided into a cortical and a medullary region arranged in pyramids. The tip of each pyramid is called papilla renalis and collects the urine into the renal pelvis. The lobes of the kidney fuse to a varying level, which leads to a different kidney morphology in mammals (Fig. 10.2). When papillae are not fused the renal pelvis is partitioned in a multitude of calices (Fig. 10.2a). Nephrons are the functional units of the kidney and their basic structure is similar in all mammals. While corpuscles with glomeruli (network of capillaries) and Bowman’s capsule as well as the contorted tubular parts are located in the cortex (Fig. 10.3c), the medulla mainly contains the loop of Henle for concentration purposes. The number of nephrons differs between species. Pig kidneys consist of 1,000,000 nephrons (comparable to humans) while cattle have up to 4,000,000 nephrons and mice only possess about 10,000–15,000. Not only number but also concentration capacities of nephrons differ. The psammomys, a gerbil, has only about 100 nephrons however, with very long tubules, which can most efficiently concentrate the urine as an adaptation to its arid habitat. Concentration capacity is highest in the loop of Henle within the medulla due to the counterflow principle disposed here. In animals with especially long loops of Henle also the papilla is prolonged—reaching into the upper part of the ureter in some gerbils and white-tailed antelope squirrels. In all mammals the renal pelvis or calices join into the ureter and finally flow into the bladder.
Top view on cast specimen of large uriniferous structures and on plastinated kidneys. Color changes are due to original blood content of the specimen and fixation procedure. (a) Cast specimen of renal calices (cow) and renal pelvis (dog); (b) degree of fusion differs between species, left: lobulated (sulcated) bovine kidney, right: smooth canine kidney; (c) form also differs between species, left: oval-shaped porcine kidney, right: heart-shaped equine kidney. Preparations: Institute of Anatomy, Histology and Embryology, Vetmeduni Vienna
Macro- and microscopic cross sections of the kidney. (a) Detail of midsagittal plane of a bovine kidney showing a lobe with the pyramid consisting of cortex (co) and medulla (me). Both portions are partially fused to the neighboring pyramid. Renal papil (p) ends in the renal calix (ca). (b) Histological section of the cortex of a rat kidney showing a glomerulum (g), Bowman’s capsule (bc), the afferent arteriole (a), and proximal (pt) and distal (dt) tubules, H&E stain, 40× magnification. Preparations: Institute of Anatomy, Histology and Embryology, Vetmeduni Vienna
The bladder is a hollow organ used for temporal storage of the constantly flowing urine until micturition. It can be divided into a corpus and a neck region. The composition of the bladder wall secures the great capacity of the bladder to shrink and expand, depending on the contending volume, as well as the deliberate micturition. The urethra connects the bladder with the outside and develops in a sex-specific fashion. Female animals have a very short urethra. In males a long urethra is present located within the penis. In male pigs and ruminants there is a sigmoid flexure of the urethra (see Chap. 12). The outer exit of the urethra is a lot smaller in males than in females, which increases the risk of urinary stones to be stuck in male individuals. Especially in small ruminants the ostium urether externum is situated on an elongation of the penis tip. This portion is called processus urethralis and increases the risk of problematic situations from urinary stones in male goats/sheep even more.
10.2.4 Homeostasis and Osmoregulation
Homeostasis is defined as the ability to maintain the inner system stable, independent of the environmental situation. Therefore, a regulation system and feedback mechanisms are needed.
Concerning homeostasis of water, the osmoregulation system controls uptake and loss of water and solved substances. The need of the animal to maintain this system to a constant level becomes clear by recapitulating the principle of osmosis: In a semipermeable membrane the water is diffusing to the compartment with higher concentration of solutes. Especially animals living in the water have to deal with this phenomenon because of diffusion of water and salt. There are two types of adaption: animals that conform to the surrounding situation are called osmoconformers, and those who regulate their inner osmolarity: osmoregulators. Osmoconformers are mainly invertebrates and some fish living in the deep sea, which try to adjust their inner osmolarity to the outside situation. To maintain isotonicity with the sea water sharks, for example, accumulate urea in their body fluids and muscles and only exchange certain ions with the surrounding water. As a constant internal situation is necessary for optimal functioning and their body is not able to react to changes very well, those animals are restricted to small habitats with relatively stable conditions. As the majority of habitats are inconstant in their conditions, also most vertebrates are osmoregulators that try to maintain a constant inner milieu independent of the environmental situation. Therefore, mechanisms have evolved to regulate inner water and solute concentrations. In aquatic species one has to distinguish between fresh and sea water animals: Fresh water fish are hypertonic in comparison to the water around and therefore water is constantly absorbed via the skin, gills, and together with food. To maintain high levels of salt concentrations fresh water fish have to take up ions by active transport systems and at the same time try to lose much water by highly diluted urine. Salt water fish are living in an outer milieu showing a high osmotic pressure. The internal fluids are lower in concentration than the outer sea water and additional salts are taken up with the food. Water is lost via diffusion through skin and gills. So there is great need to take up enough water and excrete ions in highly concentrated urine.
All land living animals are osmoregulators. They have limited access to water and need to reduce water loss. Mechanisms therefore are reduction of evaporation by body surface, reabsorption within the body, increase of water intake, or production of metabolic water. For the excretion of crystalline uric acid less water is needed compared to urea—therefore, reptiles and birds are more prone to live in arid regions. In some migrating bird species the metabolic water alone is sufficient for the function of kidneys. Another important aspect is to protect the fetus from evaporation of water—which is believed to be a very important prerequisite for the evolution of land living species. Eggs either are laid in moist surroundings, like in mosquitoes, or have an eggshell. In mammals amnions contain embryonic fluids and membranes to protect the embryo from drying out.
10.2.5 Acid–Base Regulation
Another function of the urinary tract is the homeostasis of the pH value. Two organ systems are responsible to maintain the acid–base homeostasis: the kidneys and the lungs. The lungs contribute by regulating the carbon dioxide through breathing. The kidneys have two very important roles. They pump the hydrogen in the urine and reabsorb the bicarbonate.
10.2.6 Hormone Production
A hormone of special interest in comparative aspects is erythropoietin (EPO) as it is present in all mammals and was recently demonstrated to be also involved in red blood cell development in fish and amphibians (Nogawa-Kosaka et al. 2011; Paffett-Lugassy et al. 2007). In mammals, three body sites produce EPO: In the central nervous system EPO is produced by the astrocytes and has also paracrine effects. The liver also contributes to the EPO production, but the majority of the plasma EPO is produced by the kidney. In case of hypoxemia, the kidneys produce EPO, which leads to increased erythrocyte production in the bone marrow and an increased erythrocyte content in the blood. In cats, there are many chronic diseases where the kidneys tempt to fail. As a consequence affected cats develop anemia. The cats show pale mucous membranes and have a decreased number of erythrocytes in the blood. A current treatment option for this condition is to transfer cat patients feline EPO genes (Beall et al. 2000).
Another important hormone affecting kidney function is aldosterone. It is derived from the adrenal glands (see Chap. 8), acts on the distal tubules and collecting ducts, and stimulates sodium reabsorption, water retention, and potassium secretion. In mice there is strain-specific, sex-specific, and generation-dependent aldosterone secretion (Spyroglou et al. 2012).
Parathyroid hormone from the parathyroid glands is upregulated if calcium levels in the blood are decreased. As already outlined above in Sect. 10.1, this hormone promotes the kidney to produce vitamin D to reabsorb calcium from the intestine. This is especially important for dairy cows and other lactating animals, because the daily body turnover of calcium increased threefold during lactation.
References
Beall CJ, Phipps AJ, Mathes LE, Stromberg P, Johnson PR (2000) Transfer of the feline erythropoietin gene to cats using a recombinant adeno-associated virus vector. Gene Ther 7:534–539
Breyer MD, Zhang Y, Guan YF, Hao CM, Hebert RL, Breyer RM (1998) Regulation of renal function by prostaglandin E receptors. Kidney Int Suppl 67:S88–S94
De Loof A, Lindemans M, Liu F, De Groef B, Schoofs L (2012) Endocrine archeology: do insects retain ancestrally inherited counterparts of the vertebrate releasing hormones GnRH, GHRH, TRH, and CRF? Gen Comp Endocrinol 177(1):18–27
Deeb KK, Trump DL, Johnson CS (2007) Vitamin D signalling pathways in cancer: potential for anticancer therapeutics. Nat Rev Cancer 7:684–700
Levy-Lioret A, Pokroy B, Levavi-Sivan B, Leiserowitz L, Weiner S, Addadi L (2008) Biogenic guanine crystals from the skin of fish may be designed to enhance light reflectance. Cryst Growth Des 8:507–511
Nogawa-Kosaka N, Sugai T, Nagasawa K, Tanizaki Y, Meguro M, Aizawa AY, Maekawa S, Adachi M, Kuroki R, Kato T (2011) Identification of erythroid progenitors induced by erythropoietic activity in Xenopus laevis. J Exp Biol 214:921–927
Paffett-Lugassy N, Hsia N, Fraenkel PG, Paw B, Leshinsky I, Barut B, Bahary N, Caro J, Handin R, Zon LI (2007) Functional conservation of erythropoietin signaling in zebrafish. Blood 110:2718–2726
Spyroglou A, Sabrautzki S, Rathkolb B, Bozoglu T, Hrabé de Angelis M, Reincke M, Bidlingmaier M, Beuschlein F (2012) Gender-, strain-, and inheritance-dependent variation in aldosterone secretion in mice. J Endocrinol 215:375–381
Further Reading
Von Engelhardt W, Breves G (2010) Physiologie der Haustiere, 3rd edn. Enke, Stuttgart
Foelsch UR, Kochsiek K, Schmidt RF. Pathophysiologie. Springer
Hickman CP Jr, Roberts LS, Keen SL, Eisenhour DJ, Larson A, L’Anson H (2011) Integrated principles of zoology, 15th edn. McGraw-Hill, New York
Hildebrand M (1974) Analysis of vertebrate structure, 1st edn. Wiley, New York
Krück F (2000) Pathophysiologie/Pathobiologie, 2nd edn. Urban und Schwarzenberg, München
Nickel R, Schummer A, Seiferle E (2004) Lehrbuch der Anatomie der Haustiere: Anatomie der Vögel, Band V, 3rd edn. Paul Parey, Stuttgart
Reece WO (2009) Functional anatomy and physiology of domestic animals, 4th edn. Wiley-Blackwell, Aimes
Reece JB, Taylor MR, Simon EJ, Dickey JL (2012) Campbell biology—concepts & connections, 7th edn. Pearson, San Francisco
Stark D (1982) Vergleichende Anatomie der Wirbeltiere auf evolutionsbiologischer Grundlage, 1st edn. Springer, Berlin
Paulev P-E, Zubieta-Calleja G, eds. Textbook in medical physiology and pathophysiology. Essentials and clinical problems, 2nd edn. Chapter 30
Treuting PM, Dintzis SM (2012) Comparative anatomy and histology—a mouse and human atlas, 1st edn. Elsevier, Waltham
Author information
Authors and Affiliations
Corresponding authors
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2014 Springer-Verlag Wien
About this chapter
Cite this chapter
Jensen-Jarolim, E., Schöpper, H., Gabner, S. (2014). Volume and Clearance: Kidneys and Excretory Systems. In: Jensen-Jarolim, E. (eds) Comparative Medicine. Springer, Vienna. https://doi.org/10.1007/978-3-7091-1559-6_10
Download citation
DOI: https://doi.org/10.1007/978-3-7091-1559-6_10
Published:
Publisher Name: Springer, Vienna
Print ISBN: 978-3-7091-1558-9
Online ISBN: 978-3-7091-1559-6
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)