Abstract
This chapter provides an overview of the prospects of using electromagnetic fields (EMFs), with a specific focus on static magnetic fields, for treatment of human disease. The information provided covers the underlying basis for widespread skepticism surrounding “magnetotherapy”—which in part is deserved based on overinflated claims by its practitioners over the past two centuries (or even longer). On the other hand, a compelling scientific foundation is in place to propel nascent efforts to use magnetotherapy from a questionable niche medical practice into the mainstream; a goal of this chapter is to provide a summary of this information using specific (but non-comprehensive) examples of human ailments that are expected (based on current information) to benefit from magnetic field treatment.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
15.1 Introduction
Therapies that involve exposure to electromagnetic fields (EMFs) date back to the inception of practical methods to harness and exploit magnetism and electricity. Anecdotal folklore suggests that the subset of these therapies using time-invariant (i.e., static) magnetic fields (SMFs) extend back two or even 3000 years [perhaps even to 1000 BC (Mourino 1991)], when “lodestones” were thought to have the ability to draw disease out of a person’s body (Zyss 2008; Palermo 2015). Jumping forward, by the early sixteenth century (AD) the Swiss physician Paracelsus was using magnets to treat epilepsy, diarrhea, and hemorrhage, and in the mid eighteenth century, the Austrian doctor Franz Mesmer had opened a healing salon in Paris to treat the untoward effects of the body’s innate “animal magnetism” (Mourino 1991). With the advent of electricity as a power source, EMFs were added to the healing repertoire and were being used to assist bone healing as early as the mid-nineteenth century, with definitive literature reports verifying efficacy appearing in the 1970s (Bassett et al. 1974a, b).
Since World War II, magnetic field therapy (usually referred to as “magnetotherapy” in this chapter) has flourished across the globe—albeit unevenly with various levels of acceptance in different countries—with an estimated two million recipients each year (Markov 2009). Magnetotherapy has many attractive features, including its relatively low cost compared to many current treatment modalities, its typically noninvasive nature, and its established safety record (with obvious exceptions, such as individuals with medical device implants such as pacemakers or insulin pumps). On the other hand, magnetotherapy has a long-standing reputation for quackery. To give one example of the origins of this reputation, by the late nineteenth century, Thatcher’s Chicago Magnetic Company (a mail order outfit) claimed that “magnetism properly applied will cure every curable disease no matter what the cause” (Macklis 1993).
Today, similar overblown rhetoric from some quarters continues to obscure the valid scientific underpinnings of magnetotherapy. In part, magnetotherapy remains controversial because its opponents persist in making polarized blanket statements that categorically reject the possibility of beneficial health effects, while many proponents of magnetotherapy promise miracle cures for long lists of disparate ailments. The reality almost certainly lies between these extremes, and the purpose of this chapter is to provide an overview of what is currently known about human magnetic field therapy, what is not known, and what needs to be known (and done) to move this field forward.
15.2 Overview of Electromagnetic Field (EMF) Treatment Modalities
Although somewhat arbitrary, EMF therapeutic modalities are generally categorized in five categories as outlined by Markov (though some classification schemes give six categories) in an excellent synopsis of the influence of magnetic fields on human health (Markov 2014). These categories are briefly discussed below.
15.2.1 Low-Frequency Sine Waves
Low-frequency sine wave (LFS) electromagnetic fields are based on predominant commercially supplied electricity sources, which are 60 Hz in North American and generally 50 Hz in Europe and Asia (Markov 2014). One use of LFS is as an alternative to high-frequency fields in deep brain stimulation for the treatment of epilepsy (Goodman 2005; Goodman et al. 2005). Another potential application is for the treatment of cancer (Blackman 2012); more broadly, efforts are underway to use diverse frequencies of EMFs including SMFs to treat cancer (Zimmerman et al. 2012).
15.2.2 Pulsed Electromagnetic Fields (PEMFs)
Pulsed electromagnetic fields (PEMFs) are low-frequency fields with specific wave shapes and amplitudes (Markov 2014). PEMF treatment was introduced clinically in the 1970s by Bassett and colleagues, who used a specific biphasic low-frequency signal for bone healing, particularly for the treatment of delayed fractures (Bassett et al. 1974a, b). Although reports continue to appear questioning the efficacy of PEMF therapy (Rose and Bryan-Frankson 2008), transcranial magnetic stimulation devices have been approved by the US Food and Drug Administration (FDA) for patients not responsive to chemical antidepressants (Martiny et al. 2010; Anonymous 2011). In addition, there are a profusion of PEMF devices that are sold and marketed as FDA-registered “wellness devices”; these products, however, are not permitted to claim efficacy for treating disease (Anonymous 2015).
There are, nevertheless, several studies that do indicate various forms of therapeutic effectiveness for PEMF beyond solely bone healing and the treatment of depression. A study in 2019 by Elshiwi and coworkers indicated that PEMF can improve the clinical outcomes of physical therapy when used alongside it as a treatment for lower back pain (Elshiwi et al. 2019). PEMF also has shown therapeutic potential for the treatment of rheumatoid arthritis and other diseases characterized by chronic inflammation and immune dysfunction (Ross et al. 2019). Finally, recent studies concluded that PEMF has a pro-osteogenic and pro-chondrogenic effect on mesenchymal stem cells, and thus could be used in the field of regenerative medicine to improve grafting and tissue repair (Varani et al. 2021).
15.2.3 Pulsed Radiofrequency Fields (PRFs)
Pulsed radiofrequency field (PRF) therapy refers to a technique where radio frequency oscillations are generated at a defined rate of pulses per second with frequencies ranging from 1.0 × 104 to 3.0 × 1011 Hz. Therapeutically, PRFs offer an alternative to continuous radiofrequency (CRF) therapy, which has been used since the 1970s and offers the advantage of pain control without tissue destruction (Byrd and Mackey 2008). These therapies typically utilize frequencies between 300 and 750 kHz, are now delivered to precise locations in the body by catheter, and as mentioned, are used in two primary modalities: in continuous mode, these devices are designed to produce deep heat, while in pulsed (non-thermal) mode, which uses short (e.g., 20 ms) high-voltage bursts followed by a longer (e.g., 480 ms) silent phase to allow for heat dissipation, they are used for soft tissue stimulation (Markov 2014). Thermal PRF (i.e., CFR) therapy delivers high current focally to ablate the tissue of interest (e.g., a tumor or cardiac tissues that trigger arrhythmias) by heating to temperatures of 60–80 °C, resulting in focal tissue destruction (Byrd and Mackey 2008).
It remains controversial whether nonthermal PRF truly avoids biological effects due to heating; for example, although temperatures stay at or below 42 °C minimizing cell death or tissue destruction, heat shock response nonetheless could be triggered. Resolving this ambiguity will ultimately be necessary to fully define the biochemical mechanism of therapeutic responses associated with PRF therapy. Despite uncertainty over mechanism (and even efficacy), PRF is being used to treat a growing list of indications which are typically oriented toward amelioration of pain, including axial pain, radicular pain, facial pain, inguinal pain and orchialgia, and miscellaneous pain syndromes (Byrd and Mackey 2008).
15.2.4 Transcranial Magnetic/Electric Stimulation (TMS)
Transcranial magnetic stimulation (TMS) involves applying very short magnetic pulses of up to 8 Tesla to selected portions of the brain (Markov 2014). During TMS, a magnetic field generator is placed in proximity to the head of the person receiving the treatment (Groppa et al. 2012). The coil produces electric currents in the region of the brain just under the coil through electromagnetic induction. TMS can be used to diagnose connections between the brain and a muscle to evaluate damage from several indications, including stroke, multiple sclerosis, amyotrophic lateral sclerosis, movement disorders, motor neuron disease, and injuries (Groppa et al. 2012).
Therapeutically, TMS has been evaluated for conditions such as movement disorders, stroke, amyotrophic lateral sclerosis, multiple sclerosis, epilepsy, consciousness disorders, tinnitus, depression, anxiety disorders, obsessive-compulsive disorder, schizophrenia, craving/addiction, and motor conversion (Lefaucheur et al. 2014). In particular, more recent findings continue to support the therapeutic benefit of TMS as an option for otherwise treatment-resistant depression (Garnaat et al. 2018) and depression in adolescents (Croarkin and MacMaster 2018). Additionally, a 2019 study by Philip and coworkers indicated that one form of TMS, intermittent theta-burst stimulation (iTBS), is likely to be effective for the treatment of post-traumatic stress disorder (Philip et al. 2019). As of 2020, TMS has been approved as a therapeutic method for the treatment of two psychiatric disorders: major depressive disorder and obsessive-compulsive disorder, with approval by the FDA in 2008 and 2018, respectively (Iglesias 2020).
In a 2020 review, Lefaucheur and coauthors concluded that there is sufficient evidence to accept “definite efficacy” for the analgesic effect of high-frequency (HF) TMS of the primary motor cortex (M1) contralateral to the pain, the antidepressant effect of HF-TMS of the left dorsolateral prefrontal cortex (DLPFC), and LF-TMS of contralesional M1 in post-acute stroke. “Probable efficacy” is proposed for several indications, including but not limited to the antidepressant effect of low-frequency (LF) TMS of the right DLPFC (as well as the left for Parkinson’s disease patients specifically), HF-TMS of the left DLPFC for the treatment of fibromyalgia pain, HF-TMS of bilateral M1 regions for motor impairment, and iTBS to treat spasticity in multiple sclerosis patients. Finally, TMS achieves “possible efficacy” in a number of indications, including LF-TMS of the left temporoparietal cortex for auditory-verbal hallucinations and of the auditory cortex for chronic tinnitus (Lefaucheur et al. 2014).
15.2.5 Static/Permanent Magnetic Fields (SMF)
Static magnetic fields—that is, time-invariant magnetic fields—are a feature of various permanent magnets; alternatively, they can be generated by passing direct current (DC) through a coil (Markov 2014). These fields are the primary focus of this book, with detailed description of the underlying physics provided elsewhere; in this chapter, SMFs will be discussed based on their field strengths with Sect. 15.3.1 covering weak fields in the range of the Earth’s magnetic field (<0.65 gauss or ~65 μT). Section 15.3.2 will discuss the absence of these fields, which by default make a convincing case that humans can detect and (subconsciously) respond to weak magnetic fields. Finally, Sect. 15.3.3 will provide an overview of the therapeutic use of more powerful moderate strength fields that range up to ~1 T (one Tesla or 10,000 gauss). Strong fields above one Tesla are rarely used in magnetotherapy per se, but people are exposed to these field strengths during magnetic resonance imaging (MRI), generally without any discernible impact on health.
15.2.6 “Non-therapeutic” Electromagnetic Field (EMF) Exposure Allays Safety Concerns
Over the past century or so, humans have been increasingly subject to inadvertent exposure from man-made EMFs. For example, the rise of metal industries, welding processes, and certain electrified train systems in the late nineteenth centuries resulted in significant exposure for workers and even bystanders to SMFs; in 1921, Drinker and Thomson asked the question “Does the magnetic field constitute an industrial hazard?” and concluded that it didn’t (Hartwig et al. 2009). Over the years as new “EMF”-based threats have emerged (Tucker and Schmitt 1978), such as living under high-voltage power lines or the ubiquitous adoption of cell phones, which have raised fears of childhood and brain cancers, have been met with detailed scrutiny that have ruled out clear-cut evidence of harm. Ultimately meta-analysis of many such studies has cast doubt on the idea that EMF exposure causes any measurable detriment to human health, acting as a helpful baseline for establishing the safety of magnetotherapy. On the other hand, the (general) lack of deleterious effect of EMFs has also been used to cast doubt on whether beneficial effects are possible as well, based on the assumption that these fields likely have no meaningful impact on human health; a substantial portion of this chapter either directly or indirectly addresses this fallacy.
15.3 Biomedical Effects of Static Magnetic Field Therapies Categorized by Field Strength
15.3.1 “DIY” Treatments with Low to Moderate Strength Static Magnetic Fields Are Widespread But Unproven
The largest segment of extant “magnetic therapies” falls into the do-it-yourself (DIY) category, where individuals use various types of permanent magnets that provide continuous SMF exposure. This modality of magnetotherapy is used to treat a wide range of ailments, with a quick internet search (conducted in January, 2017, but similar results have been obtained for at least 20 years) including magnetic bedding pads, magnets embedded in pillows, magnetic shoe insoles, magnetic back belts, magnetic leg and arm supports, magnetic bracelets, magnetic finger and toe rings, and multipurpose magnetic pads that can be customized to wear on virtually any part of the body. Note that no specific weblinks are provided here for several reasons. First, any particular commercial link is apt to be quickly out of date; second, this publication wishes to avoid the appearance of endorsing any particular product; and finally, to spur any interested reader to perform their own search for “magnetic therapy products” (or similar terms). Such a search will almost certainly provide—above and beyond many sites selling these products—numerous links running the gamut from “debunking” the entire idea of magnetotherapy and mocking consumers for falling for a billion-dollar “scam”—reportedly a conservative value for annual sales of these products, which was reported almost 20 years ago (Weintraub 1999)—to enthusiastic endorsements for efficacy against a broad gamut of human diseases; increasingly, products are coming available to treat one’s pets as well.
Intuition alone makes a powerful case that many DIY magnetotherapy efforts are likely misguided and minimally effective. Even if the magnets used are “high-quality” (e.g., constructed from latest neodymium-based alloys) as advertised, with field strengths reported in the range of tens to hundreds gauss (i.e., up two to three orders of magnitude stronger than the Earth’s magnetic field), one key issue is that magnets themselves are NOT therapeutic. This point is discussed by Markov (2009) who describes how the term “magnetic therapy” is a misnomer. Instead, he emphasizes that the therapeutic effects of magnets emanate from the fields they generate and the subsequent interaction of these fields with the target tissue or organ in a person (note that the use of the “magnetotherapy” in this chapter implicitly denotes magnetic field therapy). In this regard, it is critical to note that field strength decreases exponentially with distance from the surface of a permanent magnet (for example, by ~2 orders of magnitude in only a few millimeters for magnets in the range of hundreds of gauss), and therefore, field strength is negligible in deep tissue that would need to be penetrated to have an effect on many of the conditions purportedly treated with magnetotherapy.
One example of this pitfall is provided by a report where commercial magnetic wraps had no effect on blood circulation in horses (Steyn et al. 2000) or pain perception in people (Kuipers et al. 2007), which—because the field strengths used did not penetrate effectively into tissue to the depth where the target vessels or nerves were located—were not surprising results. More trivially, but still important, magnets placed in clothing or otherwise attached via wrappings that surround the body provide inconsistent magnetic field exposure to the intended target tissue if the clothing or wrapping is loose or not applied and worn consistently from day to day. An illustration of this point is that the field strength of a 500 gauss magnet can be as little as 1 gauss only 1 or 2 cm away from the magnet’s surface. As a result, determination of dose—a key parameter in determining medical efficacy—is typically impossible to determine with any degree of accuracy in DIY magnetotherapy (Markov 2009).
15.3.2 Hypomagnetic Fields (HMF)—Evidence for Magnetotherapy by Default?
Interestingly, the impact of weak to moderately strong SMF on human health perhaps has been demonstrated most convincingly by default; that is, by observing the effects of the absence of geomagnetic-strength magnetic fields. These studies have exploited a century of efforts to develop materials designed to shield sensitive equipment from magnetic fields, such as submarine telegraph cables, electric power transformers, cathode ray tubes, and magnetic phonograph cartridges. To achieve the required shielding, “mu-metals” have been developed that have a representative composition of ~77% nickel, 16% iron, 5% copper, and 2% chromium or molybdenum (Jiles 1998). In essence, a mu-metal is a high-permeability alloy that does not block magnetic fields per se, but instead provides a path for the magnetic field lines to go around the area intended to be shielded. Details on magnetic field shielding are largely beyond the scope of this discussion, but more information can be found online (e.g., in technical documents provided by vendors of magnetic shield products such as http://www.magnetic-shield.com/pdf/how_do_magnetic_shields_work.pdf). For this discussion, the key point is that products exist that can effectively shield objects from ambient magnetic fields that, for practical purposes, can isolate a research subject from a background (generally the Earth’s) magnetic field. Geomagnetic field shielding produces what has come to be known as “hypomagnetic fields” (HMFs).
In the past few years, a provocative set of experimental results have emerged, indicating that HMF has numerous biological and biomedical effects across species—including humans. For example, long-term HMF exposure is associated with embryonic malformation in insects (Wan et al. 2014), amphibians [e.g., newts (Asashima et al. 1991) and frogs (Mo et al. 2012)], and rodents [e.g., mice (Fesenko et al. 2010)], as well as abnormal DNA methylation in murine embryonic stem cells (Baek et al. 2019). Additional effects of HMF have been described in rodents, including inhibition of stress-induced analgesia (Prato et al. 2005), decreased noradrenaline release (Choleris et al. 2002; Zhang et al. 2007), and impairment of learning and neurogenesis in the hippocampus (Zhang et al. 2021). Learning defects have been described in birds (Xu et al. 2003) and Drosophila (Zhang et al. 2004). Finally, the negative impact of HMF has been reported to extend to humans; these effects have often and most convincingly been deduced from space flight, where the geomagnetic field is negligible in strength because it is generally not practical to confine a person to an artificially shielded HMF area. These studies have shown HMF effects in humans that include perturbed circadian rhythms (Wever 1970; Bliss and Heppner 1976) and weakened cognitive function (Binhi and Sarimov 2009).
The generally deleterious effects of HMF across several biological processes in many species, including the still-speculative but nevertheless plausible observations in people, have strengthened the case that weak magnetic fields do have legitimate biomedical relevance. For example, it appears that GMFs keep us healthy and contribute to normal physiology. Extrapolating from these observations, it has been hypothesized that because a lack of magnetic fields is harmful, field strengths stronger than the Earth’s magnetic field might exacerbate and extend the beneficial impact of GMF exposure. A parallel drawn from pharmacology is that many natural drugs, such as aspirin or the antioxidant resveratrol, must be consumed at much higher levels to have a medical effect than a person can reasonably obtain from natural consumption (Scott et al. 2012). Similarly, arguments have been made—abutted with claims that humans evolved when the Earth’s magnetic field was as much as an order of magnitude stronger than it is today—the earth’s magnetic field is constantly waxing and waning, and even reverses polarity on a millions-of-years time scale (Mori et al. 2013), an event associated with mass extinctions (Lipowski and Lipowska 2006)—that to achieve maximum benefits from magnetotherapy, stronger magnetic fields should be used.
15.3.3 Stronger Magnetic Fields—Impacts on Human Health
15.3.3.1 Moderate Strength Static Magnetic Field Therapy
The benefits (or necessity) of using stronger than GMF-strength fields for human therapy have spurred efforts to use static magnetic fields much stronger than afforded naturally by today’s geomagnetic fields. In some cases, these strategies involve “DIY” efforts with magnets in the tens to hundreds of milli-Tesla range, but, as discussed above, these efforts are likely ineffective for treatments that require deep penetration of tissue. As an alternative, medical devices, often from Europe, that create stronger electromagnetic fields have been marketed. The US FDA generally permits these for “general wellness” (Anonymous 2015) while prohibiting claims for efficacy for treatment of any specific medical indication.
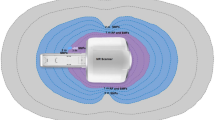
In some cases, proponents of magnetic therapy are pursuing more rigorous evidence of efficacy. One example is provided by continuing efforts of Joe Kirschvink and colleagues to demonstrate that humans are affected by externally applied magnetic field in ways that are medically relevant (Hand 2016). Another example of moving forward with therapeutic intervention is provided by the Advanced Magnetic Research Institute (AMRi) that has developed a “Magnetic Molecular Energizer™” (MME) device (Bonlie 2001) capable of producing SMFs of 0.3–0.5 T that completely penetrate the human body in an ~20 cm radius (Fig. 15.1). Based on the assumption that the “biosensor” for magnetic reception is located directly in the diseased or damaged tissue, a patient is positioned with the magnetic field centered on the affected area. Double-blind clinical trials seemingly showed efficacy against lower back pain (ClinicalTrials.gov Identifier: NCT00325377) and possibly against symptoms of diabetic neuropathy (ClinicalTrials.gov Identifier: NCT00134524). The results of these studies, however, were difficult to interpret because positive outcomes were not statistically different from placebo-treated patients, who also experienced marked improvement (Dean Bonlie, personal communication). These clinical studies illustrate two recurring themes in efforts to establish clinical efficacy for magnetotherapy; first, therapeutic effectiveness is most well established for pain perception (the subject of these tests) and second, the placebo effect is often overwhelming in magnetotherapy; both of these points are further elaborated in Sect. 15.5.3, below.
The Molecular Magnetic Energizer™ (MME) device and illustration of a patient during treatment. (a) The MME [as illustrated in U.S. Patent documents (Bonlie 2001)] consists of two major elements: a magnetic field generator (32) for producing a treating magnetic field and a patient support (34) for positioning a patient within the magnetic field. The magnetic field generator consists of a magnetic circuit (35) having an upper electromagnet (36) and a lower electromagnet (38) separated by a gap (40) on their adjacent pole faces (42) and connected by a C-shaped core (44) (or “C-core”) on their opposing poles (46). In the embodiment shown, C-core has a circular cross-section with an 8-inch (20.3 cm) diameter. The electromagnets are wired in parallel with a power supply to create magnetic fields of the same sense. For example, the positive pole of the upper electromagnet 36 would face the negative pole of the lower electromagnet 38 (or vice versa). (b) A patient is shown positioned in the MME device in a supine position; it should be noted that the magnetic field generator apparatus can be rotated and otherwise adjusted via parts 48, 50, and 52 to accommodate patients who prefer to be treated in other positions, for example, lying on their side. (Image from public website: http://www.amri-intl.com/)
15.3.3.2 Higher Strength Static Magnetic Field Exposure
Strong fields above 1 Tesla are rarely used in magnetotherapy per se, but people are routinely exposed to field strengths of 1.3 (and now up to 3) T during magnetic resonance imaging (MRI). As of 2016, over 150 million people have undergone MRI procedures, with ~ten million undergoing examination each year (Anonymous 2016). Overall, it is accepted that MRI has little, if any, discernible impact on health, either beneficial or deleterious (Schenck 2000). Based on this apparent lack of response, SMFs are generally regarded to be safe by regulatory agencies such as the US Food and Drug Administration (FDA) (Anonymous 2015). Upon comprehensive review of the literature available described the in vivo and ex vivo effects of SMFs, Hartwig and coauthors confirmed that >1 T SMFs that accompany MRI are rarely harmful (Hartwig et al. 2009), with the possible exception of inconclusive reports where exposure led to acute neurobehavioral effects, such as eye-hand coordination speed and visual and auditory working memory problems (De Vocht et al. 2006) and a non-statistically significant increase in spontaneous abortions in MRI workers (Evans et al. 1993). It should be noted that these reports dealt with MRI workers and, no doubt based on warnings raised by these speculative studies, safety standards have been tightened and follow-up and continuing problems have not been reported.
15.4 Prospects for Therapeutic Areas
Magnetotherapy has been applied to almost any imaginable human ailment. For example, MedicineNet (http://www.medicinenet.com/script/main/art.asp?articlekey=22961) summarizes conditions claimed to be diagnosed or treated using magnetic field therapy (largely through the “DIY” methods mentioned above) to include arthritis, cancer, circulatory disorders, diabetic neuropathy, fibromyalgia, HIV/AIDS, immune dysfunction, infection, inflammation, insomnia, multiple sclerosis, muscle pain, neuropathy, pain, rheumatoid arthritis, sciatica, and stress, as well as to increase energy and prolong life. The above-mentioned AMRi Corporation, which utilizes stronger strength SMF therapy, is investigating the treatment of ailments that range from spinal cord injury, brain injury, stroke impairment, multiple sclerosis, muscular dystrophy, cerebral palsy, Parkinson’s disease, Alzheimer’s disease, congestive heart failure, to orthopedic conditions involving bone and joint repair. As described in Sect. 15.5 below, many find it implausible that a “one size fits all” treatment could be effective against so many indications, and this doubt in part contributes to disbelief in therapeutic efficacy for magnetic field exposure. However, as discussed next, pain perception, blood flow, and effects on the cardiovascular system, as well as the impact on stem cells and cells found in the neurological system, provide a compelling scientific basis for beneficial effects of SMFs that, if carefully and rigorously translated to the clinic, hold legitimate promise for human therapy.
15.4.1 Pain Perception
A substantial body of evidence has accumulated showing that exposure to EMFs affects pain sensitivity (nociception) and pain inhibition (analgesia); in particular, acute exposure to various EMFs has been shown to inhibit analgesia in many studies (Del Seppia et al. 2007). In some studies, however, depending on the duration, intensity, frequency, and repeated nature of EMF exposure, increased analgesia has actually been observed (Del Seppia et al. 2007). While many of these studies—conducted in diverse organisms ranging from snails to mice to people—have involved time-varying fields, there is also substantial evidence that SMFs can affect pain perception. These findings have most convincingly come from HMF studies where mice apparently detect and respond to the absence of the ambient geomagnetic field.
In a pioneering study, mice experienced a maximum analgesic response after 4–6 days of exposure (Prato et al. 2005). Follow-up studies showed a more complex biphasic response, where geomagnetic shielding for 1 h per day for 10 consecutive days initially decreased the pain threshold over the first 2 days, followed by a sharp increase peaking by the fifth day, with a return to pre-exposure values within 8 days (Del Seppia et al. 2007). Interestingly, the kinetics of this response roughly mirror an in vitro cell-based assay response to moderate strength SMF (Wang et al. 2009) described in more detail below in Sect. 15.4.3. It was found more recently that the application of transcranial static magnetic field stimulation (tSMS) to the primary motor cortex (M1) and primary somatosensory cortex (S1) of humans may affect cortical processing of pain, making this a possible noninvasive method for the treatment of chronic pain alongside other standard-of-care treatments (Kirimoto et al. 2018).
15.4.2 Blood Flow/Vascularization
As discussed in more detail in Chap. 4, beneficial effects of magnetotherapy in humans often have been attributed to improved blood flow. Although many of the Internet claims in this regard are nonsensical—for example, the idea that a magnetic field attracts the iron in the blood is based on the misconception that hemoglobin is ferromagnetic. Instead, iron in oxygenated blood is diamagnetic, which means there is a real but almost negligible force repelling the blood; on the other hand, deoxygenated blood is paramagnetic, which means there will be a similarly almost negligible force attracting the blood (Zborowski et al. 2003). Either way, these effects are dwarfed by thermal motion and the ambient flow of the blood (as discussed in more detail in Chap. 4).
Nevertheless, there is evidence—although inconclusive because of many conflicting or inconclusive studies—that magnetic fields can legitimately modulate blood flow in humans (or other mammals). As an aside, some negative results can be accounted for by the trivial explanation that the magnetic fields used were not strong enough to penetrate deeply into the tissue where the target blood vessels were used. One example with horses was mentioned above (Steyn et al. 2000). Similarly, a study using 500 gauss (0.05 T) fields to measure blood flow in the forearms of healthy young men was equally ineffective (Martel et al. 2002); this is not surprising because field strength would be two to three orders of magnitude lower at the location of the targeted blood vessels embedded in tissue. A ~tenfold larger field (4042 gauss, or ~0.4 T), by contrast, did statistically affect blood flow in treated fingers (Mayrovitz and Groseclose 2005); interestingly, this effect was actually a reduction in blood flow, converse to what is generally thought to be therapeutically beneficial.
A set of studies in rabbits using similar strength fields (i.e., ~0.18–0.25 T) also showed legitimate effects of SMFs on blood flow (Xu et al. 1998; Okano and Ohkubo 2001; Gmitrov et al. 2002). These three studies demonstrated a biphasic response of blood flow where exposure enhanced vasodilation when the vessels were vasoconstricted and enhanced vasoconstriction in vessels that were vasodilated; in other words, the SMFs appeared to work to maintain circulatory homeostasis and “normalize” vascular function. A conceptually similar normalization effect was observed in mice where the impact of surgical intervention that would otherwise cause luminal diameter expansion in vascular networks was abrogated by continual exposure to SMFs over 4–7 days (Morris and Skalak 2007). Together, these studies suggest that while SMF exposure does have an interesting effect on blood flow, it likely is not mediated through magnetic or inductive effects on iron containing molecules (hemoglobin) or cells (RBCs) per se.
Instead, therapeutic effects on blood flow are likely mediated by “non-canonical” mechanisms (i.e., not magnetite, chemomagnetic sensing, or inductive mechanisms, which are the three molecular mechanisms found throughout nature in many diverse organisms, as discussed in detail in Chap. 4). Another interesting feature of these studies is that field strengths of greater than ~0.1 T (1000 gauss) were needed for efficacy; as mentioned, the simple explanation is that weaker field strengths could not penetrate deeply enough into tissue to reach the intended site of action (i.e., the blood vessels themselves). Another explanation (again as discussed in more detail in Chap. 4) is that field strengths of ~0.2 T or higher can alter the biophysical properties of lipid assemblies (Braganza et al. 1984). As a result, the properties of lipid biolayers (i.e., biological membranes) are affected in ways that putatively explain many phenomena observed in magnetotherapy. For example, changes in ion flux could reasonably be explained by allosteric changes to ion channels brought about changes to the biophysical properties of membranes, rather than the less plausible explanation that SMF directly affects the movement of ions (i.e., through an inductive or “Hall effect,” which has sometimes been postulated to explain the mechanism of magnetotherapy). Similarly, changes to signal pathway activity can be explained by the effects of magnetic field exposure on the biophysical properties of membranes, as discussed below for neural cells. Both of these topics are discussed in the next section in the context of studies performed in the author’s laboratory.
In addition to affecting blood flow, some studies have shown that SMF can play a role in angiogenesis and improvement of vascularization, generally in combination with a nanocomposite scaffold. For example, a 2016 study indicated that murine osteoblasts stimulated by a combination of SMF and magnetic nanoparticle scaffolding promoted angiogenesis in endothelial cells, predominantly indicated by the expression of angiogenesis-related genes and the formation of capillary tubes (Yun et al. 2016). Another, later, study showed that tissues were vascularized more quickly in cell-containing nanocomposite hydrogels when those hydrogels were exposed to SMF, suggesting that SMF has a vasculogenic effect on engineered bone grafts (Filippi et al. 2019). Even more recently, it was found that exosomes derived from bone mesenchymal stem cells stimulated with magnetic nanoparticles and SMF can promote angiogenesis and improve wound healing (Wu et al. 2020, 2021).
15.4.3 Evidence for Treatment of Neurological Disease and Neural Regeneration
In a study that was inspired by the need to find a scientific basis for coalescing evidence that magnetic field therapy may be a viable treatment option for neurological ailments via moderate strength fields (i.e., 0.1–1 Tesla), we treated the PC12 rat adrenal pheochromocytoma cell line with ~0.25 T SMFs. PC12 cells display metabolic features of Parkinson’s disease (PD) (Blum et al. 2000; Meng et al. 2007), such as possessing intracellular substrates for dopamine (DA) synthesis, metabolism, and transport, and they also abundantly express adenosine A2A receptors (e.g., A2AR) implicated in PD (Kobayashi et al. 1998). In these studies, we showed that SMF treatment reproduced several responses elicited by ZM241385, a selective A2AR antagonist; SMF exposure also counteracted several PD-relevant endpoints exacerbated by A2AR agonist CGS21680 in a manner similar to ZM241385 (Wang et al. 2010). These results raise the intriguing hypothesis that SMF can reproduce the effects of a promising class of non-dopaminergic PD drugs (i.e., ZM241385 and analogues) in a noninvasive manner, and more broadly, holds potential for ameliorating additional neurological disorders such as Alzheimer’s and Huntington’s diseases through modulation of A2AR (Takahashi et al. 2008).
In a second study from the author’s laboratory, SMF-mediated responses associated with transient interleukin-6 (IL-6) signaling in human embryonic cells [the hEBD LVEC line (Shamblott et al. 2001)] translated into changes observable at the whole cell level (Wang et al. 2009). The responses observed in these cells began very rapidly after SMF exposure began, first observed within 15–30 min in increased transcription of mRNA for IL-6, with actual secretion of this pro-inflammatory cytokine increasing for the next 2–4 days.
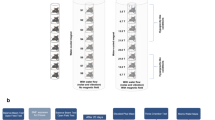
Because IL-6 guides differentiation of neural stem cells primarily to astrocytes (Taga and Fukuda 2006)—which is generally a medically unwanted outcome because hyperproliferation of this cell type leads to scar formation rather than regeneration—we investigated whether evidence of astrocytogenesis was seen in SMF-treated cells. Interestingly, responses consistent with astrocyte differentiation (i.e., slowed proliferation and morphological changes) expected from IL-6 exposure were not seen; neither were biochemical markers of astrocyte differentiation (Fig. 15.2a). Instead, markers found in neurons (Fig. 15.2b) and oligodendrocytes were manifest (Fig. 15.2c, d), indicating that the other pathways modulated by SMFs [nine other signaling pathways besides IL-6 were affected by SMF exposure in this study (Wang et al. 2009)] tuned—and in fact reversed—the usual, and most often unwanted, pro-inflammatory activity of IL-6. Ultimately, if oligodendrocyte formation can be promoted in vivo by SMF treatment without concomitant scar-forming astrocyte enhancement, this capability could lead to noninvasive therapies for conditions such as multiple sclerosis (MS) that are linked to oligodendrocyte pathologies.
SMF treatment reverses astrocyte differentiation in hEBD LVEC (human embryonic) cells. In these experiments, cells were treated with 4.0 ng/mL IL-6 and exposed to SMFs (control cells received neither stimuli) and the monolayers were co-stained with Oregon Green 488 phalloidin to visualize actin, the nuclear dye DAPI (blue), and one of the following markers (red). In Panel (a), the GFAP astrocyte marker was absent from both the control and treated cells (IL-6 treatment alone causes up-regulation, not shown). Panel (b) shows the neuron marker NEF. Panels (c) and (d) show expression of the pre-oligodendrocyte markers (c) Vim and (d) Gal-C, respectively, upon combined IL-6 and SMF treatment. Images were obtained by confocal microscopy, using identical exposure settings for each set of photographs. [Reprinted from (Wang et al. 2009), open access]
Other studies outside the author’s laboratory have also investigated the role of SMF in the treatment of neurological disease, as well as in neural growth and regeneration. For example, it has been found that moderate-intensity SMF promotes the differentiation of oligodendrocyte precursor cells into oligodendrocytes and the secretion of neurotrophic factors (Prasad et al. 2017), as well as increasing neurosphere formation and proliferation of neural progenitor cells (Ho et al. 2019). However, SMF in this same intensity range can also have a negative impact on astrocytes, adversely affecting their viability and decreasing their mitochondrial function (da Costa et al. 2021). Examples of research into SMF specifically for the treatment of neurological disorders include a 2018 study by Rivadulla and coauthors that demonstrated a reduction in epileptiform cortical activity in both a rat and monkey (Rivadulla et al. 2018), and a slightly earlier study by Dileone et al. that indicates that SMF modulates cortical activity in a dopamine-dependent manner in Parkinson’s disease patients (Dileone et al. 2017).
15.4.4 Stem Cells
One of the most notable recent developments in the study of biological SMF application is the usage of SMF for stem cell engineering, in which SMF is used as a method of regulating stem cell fate. This emerging application for SMF has particularly been used for chondrogenic, osteogenic, and adipogenic stem cells, and it has been speculated that SMF can be used to induce synthesis and secretion of extracellular microvesicles from stem cells for regenerative medicine as well (Marycz et al. 2018). For example, it has been shown that bone mesenchymal stem cells stimulated with magnetic nanoparticles and SMF can produce exosomes that promote both osteogenesis and angiogenesis (Wu et al. 2021). Human mesenchymal stem cell proliferation, alignment, and expression of stemness marker genes can also be affected by SMFs of up to 24 mT, indicating that SMF can be used as a tool to induce differentiation (Sadri et al. 2017). Adipose-derived stem cells, meanwhile, have been studied for the purpose of improving cardiac regeneration. Stem cells have been preloaded with superparamagnetic iron oxide particles and exposed to SMF, resulting in increased retention of cardiac cells and improved recovery of heart function (Wang et al. 2016).
Some other forms of stem cell research involving SMFs have been conducted that do not involve the utilization of chondrogenic, osteogenic, or adipogenic stem cells. For example, moderate-intensity SMF has been found to improve proliferative activity in neural progenitor cells in mice, as well as increasing neurosphere formation, as mentioned above in Sect. 15.3.3 (Prasad et al. 2017). Stem cell-related applications of SMF are just beginning to emerge, but they are already showing promise in the field of regenerative medicine for multiple different cell types.
15.4.5 Other Therapeutic Areas for Static Magnetic Field
There are a variety of other, less prominent therapeutic applications that have been researched for SMF as well. SMF has been studied for use in the treatment of Type II diabetes and other redox-related metabolic diseases (Carter et al. 2020), the investigation of effects on radiotherapeutic endpoints when combined with ionizing radiation (Mohajer 2019), and understanding the impact of magnetic fields on orthodontic movement of teeth and regeneration of oral tissue (Lew et al. 2021), among others. It is possible that, in the future, other applications of SMF therapy will become prominent research topics, such as SMF application to stem cell research (Sect. 15.4.4).
15.5 Pitfalls with SMF Clinical Studies and Acceptance of Magnetotherapy
15.5.1 Hyperbolic and Ambiguous Claims vs. Outright Rejection of Magnetotherapy
It can be a daunting task to precisely match treatment parameters to various pathological indications even for long-standing medicines. For example, it has taken a century to understand how to fully exploit aspirin as a medicine; indeed, some aspects of this drug remain poorly understood. For example, at a pharmacological level, the need for esterase processing of aspirin is not fully elucidated (Lavis 2008). However, much is known about some aspects of aspirin, including the impact of administration duration on dosage effectiveness, as well as how it appears to reduce the risk of cardiovascular disease. On the other hand, no evidence exists that aspirin is effective against many other conditions, for example, pancreatic cancer or neurological disorders such as Alzheimer’s disease. Aspirin is again used here to illustrate pitfalls—and lessons to be learned—for magnetic field therapy. Aspirin, if tested against a wrong medical indication—or at the wrong dose or duration—could easily be shown to have no effect, but this does not mean that it has no benefit for other ailments. Similarly, magnetic field therapy should not be considered to be debunked if a certain treatment modality shows no effect against a certain ailment; indeed, to the contrary, careful compiling of conditions that do not work could be extremely helpful in guiding treatments toward diseases and other ailments where the magnetotherapy does work.
Unfortunately, the efficacy of magnetic therapy has been clouded by ambiguity that results in large part from study design, as illustrated by a review of over 50 studies approximately a decade ago (Colbert et al. 2007, 2009). In these studies, only two provided sufficiently detailed experimental protocols to actually reproduce the work; although a more recent systematically analyzed compilation of studies does not appear to be available, anecdotal perusal of the literature over the past decade suggests that the problem of incomplete reporting of experimental conditions persists up to today. As Markov forcefully editorializes, until parameters used in magnetic field therapy—starting at a very basic terminology level to overcome confusion over semantic differences between “magnetic therapy” and “magnetic field therapy” (i.e., magnets themselves have no therapeutic effect but the fields they produce do)—magnetotherapy is apt to remain marginalized and not fully accepted by the mainstream scientific and medical communities (Markov 2009). Indeed, Markov (and his colleagues) have been trying to educate about these issues for at least two decades, and in that vein, has proposed a set of parameters that must be considered and clearly defined; these endpoints are discussed next in Sect. 15.5.2.
15.5.2 Parameters Necessary to be Controlled in Magnetotherapy
The variety of commercially available EMF devices—often with poorly characterized and sometimes misrepresented field strength specifications—makes it difficult to compare the physical and engineering characteristics of any particular device used in any reported study, thus providing significant obstacles for analysis of clinical efficacy. Markov outlines a set of parameters that must be controlled, defined, and reported to be able to evaluate magnetotherapy outcomes (Markov 2009); these are:
-
Type of field.
-
Intensity or induction.
-
Spatial gradient (dB/dx).
-
Localization.
-
Time of exposure.
-
Depth of penetration.
-
Temporary change (dB/dt).
-
Frequency.
-
Pulse shape.
-
Component (electric or magnetic).
An attraction of SMF therapy is that the latter four parameters (indicated in italics) are not in play, thereby simplifying evaluation of this therapeutic modality, and in theory, increasing the reproducibility of the studies.
15.5.3 The Placebo Effect
As already alluded to above, pain response was the only medical outcome where magnetic fields unambiguously had a beneficial therapeutic effect, based on the bulk of the literature reviewed by Del Seppia and coauthors a decade ago (Del Seppia et al. 2007). Many of the relevant studies were performed in animals, often rodents, where there presumably is no placebo effect, but in humans, the placebo response cannot be discounted so easily. Indeed, difficulties in establishing benefits of magnetic therapy result in part from designing experiments that account for the placebo effect. For example, a study from 1978 describes “the extreme cleverness with which perceptive individuals unintentionally used subtle auxiliary clues to develop impressive records of apparent magnetic field detection” (Tucker and Schmitt 1978). Of course, in many cases, not even “extreme cleverness” is necessary for a test subject to figure out whether they are part of the placebo control arm of a study, because real magnets have a propensity to attract loose magnetically susceptible objects such as paper clips.
As discussed earlier, evidence suggests that deep-penetrating SMFs of at least 0.2 T are required to affect the biophysical properties of membranes (Braganza et al. 1984) implicated in therapeutic responses in humans at the cell level (Wang et al. 2009, 2010). The only plausible way to deliver these fields in a deeply penetrating manner is to use electrical coils to generate the required moderate strength (e.g., 0.3–0.5 T) magnetic fields. One example of such an instrument is the MME device (Fig. 15.1) developed by AMRi (Bonlie 2001), which requires seven miles of copper coils situated above and below a patient (the entire apparatus is close two stories in height). In theory, pitfalls that befall efforts to conduct controlled clinical trials using DIY-type wearable magnets (such as attracting or not attracting) loose paperclips during everyday activities can be avoided by strictly monitoring the treatment environment. In reality, however, when in operation, electricity running through the device needed to generate the SMFs creates a perceptible humming noise, making it obvious whether or whether or not actual treatment is underway. As a result, control subjects in double-blind clinical studies (ClinicalTrials.gov Identifier: NCT00325377 and NCT00134524) were subject to record MME device noise. Interestingly—and perhaps unsurprisingly—a large placebo effect was observed in these studies that plausibly can be explained by the belief of control subjects that they were undergoing legitimate SMF exposure.
The placebo effect—evidenced by sham-treated test subjects experiencing improvement to long-standing conditions (lower back pain and diabetic neuropathy) that were not responsive to conventional medical treatment at rates comparable to SMF-treated individuals—illustrates the growing realization that placebo treatment is not equivalent to “no treatment.” Briefly, placebo effect depends on belief in the effectiveness of the treatment; in fact, the opposite “nocebo” effect has been proposed where a patient who disbelieves in a treatment may experience a worsening of symptoms (Kennedy 1961). Of note, “belief” is a rather ambiguous concept, but can theoretically be converted into physiological modulation through opioid neurotransmitters whose endogenous production is controlled by the brain.
The placebo effect can be powerful, with attempts to objectively measure its contribution to medical intervention overall ranging from 30 to 40% of overall observed effects of a medicine. The impact of the placebo effect varies among treatment modalities and disease conditions, with one of the stronger responses reported for the effects of antitussive medicines in patients with acute upper respiratory tract infections. In these patients, 85% of the reduction in coughing was linked to the placebo effect, whereas only 15% was linked to the actual physiological effects of the pharmacological agents (Eccles 2002). It appears that the placebo effect might be equally pervasive and influential in response to SMF treatments and, in a lesson being learned from psychiatry (Horgan 2013), the field should consider embracing—rather than being embarrassed by—this aspect of magnetotherapy.
15.6 Concluding Comments
This chapter describes various modes of EMF therapy, with the main focus on SMFs. Up to now, this therapeutic modality has both shown promise and been downplayed, in part due to over-enthusiastic claims by its practitioners. Accordingly, strict guidelines have been proposed to maintain “quality control” when patients are being treated with magnetotherapy in efforts to rigorously establish efficacy against specific medical indications, several of which are mentioned and described in some detail (e.g., pain perception and management, blood flow and vascularization, neurological regeneration, and stem cell differentiation in Sect. 15.4).
References
Anonymous (2011) Guidance for industry and FDA staff—class II special controls guidance document: repetitive transcranial magnetic stimulation (rTMS) systems. US Food and Drug Administration Document number 1728. http://www.fda.gov/MedicalDevices/DeviceRegulationandGuidance/GuidanceDocuments/ucm265269.htm
Anonymous (2015) General wellness: policy for low risk devices—guidance for industry and Food and Drug Administration staff. U.S. Food and Drug Administration Document number 1300013: http://www.fda.gov/downloads/medicaldevices/deviceregulationandguidance/guidancedocuments/ucm429674.pdf
Anonymous (2016) Information for patients. International Society for Magnetic Resonance in Medicine. http://www.ismrm.org/resources/information-for-patients/
Asashima M, Shimada K, Pfeiffer CJ (1991) Magnetic shielding induces early developmental abnormalities in the newt, Cynops pyrrhogaster. Bioelectromagnetics 12:215–224
Baek S, Choi H, Park H, Cho B, Kim S, Kim J (2019) Effects of a hypomagnetic field on DNA methylation during the differentiation of embryonic stem cells. Sci Rep 9(1):1333. https://doi.org/10.1038/s41598-018-37372-2
Bassett CA, Pawluk RJ, Pilla AA (1974a) Acceleration of fracture repair by electromagnetic fields. A surgically noninvasive method. Ann N Y Acad Sci 238:242–262
Bassett CA, Pawluk RJ, Pilla AA (1974b) Augmentation of bone repair by inductively coupled electromagnetic fields. Science 184(4136):575–577
Binhi VN, Sarimov RM (2009) Zero magnetic field effect observed in human cognitive processes. Electromagn Biol Med 28:310–315
Blackman CF (2012) Treating cancer with amplitude-modulated electromagnetic fields: a potential paradigm shift, again? Br J Cancer 106(2):241–242
Bliss VL, Heppner FH (1976) Circadian activity rhythm influenced by near zero magnetic field. Nature 261(5559):411–412
Blum D, Torch S, Nissou MF, Benabid AL, Verna JM (2000) Extracellular toxicity of 6-hydroxydopamine on PC12 cells. Neurosci Lett 283:193–196
Bonlie DR (2001) Treatment using oriented unidirectional DC magnetic field. U.S.P. Office United States 6210317
Braganza LF, Blott BH, Coe TJ, Melville D (1984) The superdiamagnetic effect of magnetic fields on one and two component multilamellar liposomes. Biochim Biophys Acta 801(1):66–75
Byrd D, Mackey S (2008) Pulsed radiofrequency for chronic pain. Curr Pain Headache Rep 12(1):37–41
Carter CS, Huang SC, Searby CC, Cassaidy B, Miller MJ, Grzesik WJ, Piorczynski TB, Pak TK, Walsh SA, Acevedo M, Zhang Q, Mapuskar KA, Milne GL, Hinton AO, Guo D-F, Weiss R, Bradberry K, Taylor EB, Rauckhorst AJ, Dick DW, Akurathi V, Falls-Hubert KC, Wagner BA, Carter WA, Wang K, Norris AW, Rahmouni K, Buettner GR, Hansen JM, Spitz DR, Abel ED, Sheffield VC (2020) Exposure to static magnetic and electric fields treats type 2 diabetes. Cell Metab 32(4):561–574. https://doi.org/10.1016/j.cmet.2020.09.012
Choleris E, Del Seppia C, Thomas AW, Luschi P, Ghione G, Moran GR, Prato FS (2002) Shielding, but not zeroing of the ambient magnetic field reduces stress-induced analgesia in mice. Proc Biol Sci 269(1487):193–201
Colbert AP, Markov MS, Souder JJ (2007) Static magnetic field therapy: dosimetry considerations. J Altern Complement Med 14(5):577–582
Colbert A, Markov M, Sauder J (2009) Static magnetic field therapy: methodological challenges to conducting clinical trials. Environmentalist 29(2):177–185
Croarkin PE, MacMaster FP (2019) Transcranial magnetic stimulation for adolescent depression. Child Adolesc Psychiatr Clin N Am 28(1):33–43. https://doi.org/10.1016/j.chc.2018.07.003
da Costa CC, Martins LA, Koth AP, Ramos JM, Guma FT, de Oliveira CM, Pedra NS, Fischer G, Helena ES, Gioda CR, Sanches PR, Junior AS, Soares MS, Spanevello RM, Gamaro GD, de Souza IC (2021) Static magnetic stimulation induces changes in the oxidative status and cell viability parameters in a primary culture model of astrocytes. Cell Biochem Biophys 79(4):873–885. https://doi.org/10.1007/s12013-021-01015-7
De Vocht F, Stevens T, van Wendel-de-Joode B, Engels H, Kromhout H (2006) Acute neurobehavioral effects of exposure to static magnetic fields: analyses of exposure–response relations. J Magn Reson Imaging 23:291–297
Del Seppia C, Ghione S, Luschi P, Ossenkopp KP, Choleris E, Kavaliers M (2007) Pain perception and electromagnetic fields. Neurosci Biobehav Rev 31(4):619–642
Dileone M, Carrasco-López MC, Segundo-Rodriguez JC, Mordillo-Mateos L, López-Ariztegui N, Alonso-Frech F, Catalan-Alonso MJ, Obeso JA, Oliviero A, Foffani G (2017) Dopamine-dependent changes of cortical excitability induced by transcranial static magnetic field stimulation in parkinson’s disease. Sci Rep 7(1):1–7. https://doi.org/10.1038/s41598-017-04254-y
Eccles R (2002) The powerful placebo in cough studies? Pulmon Pharmacol Ther 15(3):303–308
Elshiwi AM, Hamada HA, Mosaad D, Ragab IMA, Koura GM, Alrawaili SM (2019) Effect of pulsed electromagnetic field on nonspecific low back pain patients: a randomized controlled trial. Braz J Phys Ther 23(3):244–249. https://doi.org/10.1016/j.bjpt.2018.08.004
Evans JA, Savitz DA, Kanal E, Gillen J (1993) Infertility and pregnancy outcome among magnetic resonance imaging workers. J Occup Med 35:1191–1195
Fesenko EE, Mezhevikina LM, Osipenko MA, Gordon RY, Khutzian SS (2010) Effect of the “zero” magnetic field on early embryogenesis in mice. Electromagn Biol Med 29:1–8
Filippi M, Dasen B, Guerrero J, Garello F, Isu G, Born G, Ehrbar M, Martin I, Scherberich A (2019) Magnetic nanocomposite hydrogels and static magnetic field stimulate the osteoblastic and vasculogenic profile of adiposederived cells. Biomaterials 223:119468. https://doi.org/10.1016/j.biomaterials.2019.119468
Garnaat SL, Yuan S, Wang H, Philip NS, Carpenter LL (2018) Updates on transcranial magnetic stimulation therapy for major depressive disorder. Psychiatr Clin North Am 41(3):419–431. https://doi.org/10.1016/j.psc.2018.04.006
Gmitrov J, Ohkubo C, Okano H (2002) Effect of 0.25 T static magnetic field on microcirculation in rabbits. Bioelectromagnetics 3(3):224–229
Goodman JH (2005) Low frequency sine wave stimulation decreases the incidence of kindled seizures. In: Corcoran ME, Moshé SL (eds) Advances in behavioral biology. Springer, Boston, p 55
Goodman JH, Berger RE, Tcheng TK (2005) Preemptive low-frequency stimulation decreases the incidence of amygdala-kindled seizures. Epilepsia 46(1):1–17
Groppa S, Oliviero A, Eisen A, Quartarone A, Cohen LG, Mall V, Kaelin-Lang A, Mima T, Rossi S, Thickbroom GW, Rossini PM, Ziemann U, Valls-Solé J, Siebner HR (2012) A practical guide to diagnostic transcranial magnetic stimulation: report of an IFCN committee. Clin Neurophysiol 123(5):858–882
Hand E (2016) What and where are the body’s magnetometers? Science 352(6293):1510–1511
Hartwig V, Giovannetti G, Vanello N, Lombardi M, Landini L, Simi S (2009) Biological effects and safety in magnetic resonance imaging: a review. Int J Environ Res Public Health 6(6):1778–1798
Ho SY, Chen IC, Chen YJ, Lee CH, Fu CM, Liu FC, Liou HH (2019) Static magnetic field induced neural stem/progenitor cell early differentiation and promotes maturation. Stem Cells Int 2019:8790176. https://doi.org/10.1155/2019/8790176
Horgan J (2013) Psychiatrists, instead of being embarrassed by placebo effect, should embrace it, author says. Scientific American Cross-Check. https://blogs.scientificamerican.com/cross-check/psychiatrists-instead-of-being-embarrassed-by-placebo-effect-should-embrace-it-author-says/
Iglesias AH (2020) Transcranial magnetic stimulation as treatment in multiple neurologic conditions. Curr Neurol Neurosci Rep 20(1):1. https://doi.org/10.1007/s11910-020-1021-0
Jiles DC (1998) Introduction to magnetism and magnetic materials, 2nd edn. CRC Press, Boca Raton
Kennedy WP (1961) The nocebo reaction. Med World 95:203–205
Kirimoto H, Tamaki H, Otsuru N, Yamashiro K, Onishi H, Nojima I, Oliviero A (2018) Transcranial static magnetic field stimulation over the primary motor cortex induces plastic changes in cortical nociceptive processing. Front Hum Neurosci 12:63. https://doi.org/10.3389/fnhum.2018.00063
Kobayashi S, Conforti L, Pun RY, Millhorn DE (1998) Adenosine modulates hypoxia-induced responses in rat PC12 cells via the A2A receptor. J Physiol 508:95–107
Kuipers NT, Sauder CL, Ray CA (2007) Influence of static magnetic fields on pain perception and sympathetic nerve activity in humans. J Appl Physiol 102(4):1410–1415
Lavis LD (2008) Ester bonds in prodrugs. ACS Chem Biol 3(4):203–206
Lefaucheur JP, André-Obadia N, Antal A, Ayache S, Baeken C, Benninger DH, Cantello RM, Cincotta M, de Carvalho M, De Ridder D, Devanne H, Di Lazzaro V, Filipović SR, Hummel FC, Jääskeläinen SK, Kimiskidis VK, Koch G, Langguth B, Nyffeler T, Oliviero A, Padberg F, Poulet E, Rossi S, Rossini PM, Rothwell JC, Schönfeldt-Lecuona C, Siebner HR, Slotema CW, Stagg CJ, Valls-Sole J, Ziemann U, Paulus W, Garcia-Larrea L (2014) Evidence-based guidelines on the therapeutic use of repetitive transcranial magnetic stimulation (rTMS). Clin Neurophysiol 125(11):2150–2206
Lew W-Z, Feng S-W, Lee S-Y, Huang H-M (2021) The review of bioeffects of static magnetic fields on the oral tissue-derived cells and its application in regenerative medicine. Cell 10(10):2662. https://doi.org/10.3390/cells10102662
Lipowski A, Lipowska D (2006) Long-term evolution of an ecosystem with spontaneous periodicity of mass extinctions. Theory Biosci 125(1):67–77
Macklis R (1993) Magnetic healing, quackery and the debate about the health effects of electromagnetic fields. Ann Med 118(5):376–383
Markov MS (2009) What need to be known about the therapy with static magnetic fields. Environmentalist 29:169–176
Markov MS (2014) Electromagnetic fields and life. J Electr Electron Syst 3:119
Martel GF, Andrews SC, Roseboom CG (2002) Comparison of static and placebo magnets on resting forearm blood flow in young, healthy men. J Orthop Sports Phys Ther 32(10):518–524
Martiny K, Lunde M, Bech P (2010) Transcranial low voltage pulsed electromagnetic fields in patients with treatment-resistant depression. Biol Psychiatry 68(2):163–169
Marycz K, Kornicka K, Röcken M (2018) Static Magnetic Field (SMF) as a regulator of stem cell fate – new perspectives in regenerative medicine arising from an underestimated tool. Stem Cell Rev Rep 14(6):785–792. https://doi.org/10.1007/s12015-018-9847-4
Mayrovitz HN, Groseclose EE (2005) Effects of a static magnetic field of either polarity on skin microcirculation. Microvasc Res 69(1–2):24–27
Meng H, Li C, Feng L, Cheng B, Wu F, Wang X, Li Z, Liu S (2007) Effects of Ginkgolide B on 6-OHDA-induced apoptosis and calcium over load in cultured PC12. Int J Dev Neurosci 25:509–514
Mo WC, Liu Y, Cooper HM, He RQ (2012) Altered development of Xenopus embryos in a hypogeomagnetic field. Bioelectromagnetics 33:238–246
Mohajer JK, Nisbet A, Velliou E, Ajaz M, Schettino G (2019) Biological effects of static magnetic field exposure in the context of MR-guided radiotherapy. Br J Radiol 92(1094):20180484. https://doi.org/10.1259/bjr.20180484
Mori N, Schmitt D, Wicht J, Ferriz-Mas A, Mouri H, Nakamichi A, Morikawa M (2013) Domino model for geomagnetic field reversals. Phys Rev E 87(1):012108
Morris CE, Skalak TC (2007) Chronic static magnetic field exposure alters microvessel enlargement resulting from surgical intervention. J Appl Phys 103(2):629–636
Mourino MR (1991) From Thales to Lauterbur, or from the lodestone to MR imaging: magnetism and medicine. Radiology 180(3):593–612
Okano H, Ohkubo C (2001) Modulatory effects of static magnetic fields on blood pressure in rabbits. Bioelectromagnetics 22:408–418
Palermo E (2015) Does magnetic therapy work? Live Science. http://www.livescience.com/40174-magnetic-therapy.html
Philip NS, Barredo J, Aiken E, Larson V, Jones RN, Shea MT, Greenberg BD, van 't Wout-Frank, M. (2019) Theta-burst transcranial magnetic stimulation for posttraumatic stress disorder. Am J Psychiatry 176(11):939–948. https://doi.org/10.1176/appi.ajp.2019.18101160
Prasad A, Teh DBL, Blasiak A, Chai C, Wu Y, Gharibani PM, Yang IH, Phan TT, Lim KL, Yang H, Liu X, All AH (2017) Static magnetic field stimulation enhances oligodendrocyte differentiation and secretion of neurotrophic factors. Sci Rep 7(1):6743. https://doi.org/10.1038/s41598-017-06331-8
Prato FS, Robertson JA, Desjardins D, Hensel J, Thomas AW (2005) Daily repeated magnetic field shielding induces analgesia in CD-1 mice. Bioelectromagnetics 26(2):109–117
Rivadulla C, Aguilar J, Coletti M, Aguila J, Prieto S, Cudeiro J (2018) Static magnetic fields reduce epileptiform activity in anesthetized rat and monkey. Sci Rep 8(1):1–10. https://doi.org/10.1038/s41598-018-33808-x
Rose REC, Bryan-Frankson BA (2008) Is there still a role for pulsed electromagnetic field in the treatment of delayed unions and nonunions. Internet J Orthop Surg 10(1):e5574. https://doi.org/10.5580/e5574
Ross CL, Ang DC, Almeida-Porada G (2019) Targeting mesenchymal stromal cells/pericytes (mscs) with pulsed electromagnetic field (PEMF) has the potential to treat rheumatoid arthritis. Front Immunol 10:266. https://doi.org/10.3389/fimmu.2019.00266
Sadri M, Abdolmaleki P, Abrun S, Beiki B, Samani FS (2017) Static magnetic field effect on cell alignment, growth, and differentiation in human cord-derived mesenchymal stem cells. Cell Mol Bioeng 10(3):249–262. https://doi.org/10.1007/s12195-017-0482-y
Schenck JF (2000) Safety of strong, static magnetic fields. J Magn Reson Imaging 12:2–19
Scott E, Steward WP, Gescher AJ, Brown K (2012) Resveratrol in human cancer chemoprevention—choosing the ‘right’ dose. Mol Nutr Food Res 56(1):7–13
Shamblott MJ, Axelman J, Littlefield JW, Blumenthal PD, Huggins GR, Cui Y, Cheng L, Gearhart JD (2001) Human embryonic germ cell derivatives express a broad range of developmentally distinct markers and proliferate extensively in vitro. Proc Natl Acad Sci U S A 98:113–118
Steyn PF, Ramey DW, Kirschvink J, Uhrig J (2000) Effect of a static magnetic field on blood flow to the metacarpus in horses. J Am Vet Med Assoc 2000(217):6
Taga T, Fukuda S (2006) Role of IL-6 in the neural stem cell differentiation. Clin Rev Allergy Immunol 28(3):249–256
Takahashi RN, Pamplona FA, Prediger RDS (2008) Adenosine receptor antagonists for cognitive dysfunction: a review of animal studies. Front Biosci 13:2614–2632
Tucker RD, Schmitt OH (1978) Tests for human perception of 60 Hz moderate strength magnetic fields. IEEE Trans Biomed Eng 25(6):509–518
Varani K, Vincenzi F, Pasquini S, Blo I, Salati S, Cadossi M, De Mattei M (2021) Pulsed electromagnetic field stimulation in osteogenesis and chondrogenesis: signaling pathways and therapeutic implications. Int J Mol Sci 22(2):809. https://doi.org/10.3390/ijms22020809
Wan GJ, Jiang SL, Zhao ZC, Xu JJ, Tao XR, Sword GA, Gao YB, Pan WD, Chen FJ (2014) Bio-effects of near-zero magnetic fields on the growth, development and reproduction of small brown planthopper, Laodelphax striatellus and brown planthopper, Nilaparvata lugens. J Insect Physiol 68:7–15
Wang Z, Sarje A, Che P-L, Yarema KJ (2009) Moderate strength (0.23–0.28 T) static magnetic fields (SMF) modulate signaling and differentiation in human embryonic cells. BMC Genomics 4(10):356
Wang Z, Che P-L, Du J, Ha B, Yarema KJ (2010) Static magnetic field exposure reproduces cellular effects of the Parkinson’s disease drug candidate ZM241385. PLoS One 5(11):e13883
Wang J, Xiang B, Deng J, Lin H-Y, Zheng D, Freed DH, Arora RC, Tian G (2016) Externally applied static magnetic field enhances cardiac retention and functional benefit of magnetically iron-labeled adipose-derived stem cells in infarcted hearts. Stem Cells Transl Med 5(10):1380–1393. https://doi.org/10.5966/sctm.2015-0220
Weintraub M (1999) Magnetic biostimulation in painful peripheral neuropathy: a novel intervention—a randomized, double-placebo crossover study. Am J Pain Manag 9:8–17
Wever R (1970) The effects of electric fields on circadian rhythmicity in men. Life Sci Space Res 8:177–187
Wu D, Kang L, Tian J, Wu Y, Liu J, Li Z, Wu X, Huang Y, Gao B, Wang H, Wu Z, Qiu G (2020) Exosomes derived from bone mesenchymal stem cells with the stimulation of Fe3O4 nanoparticles and static magnetic field enhance wound healing through upregulated miR-21-5p. Int J Nanomedicine 15:7979–7993. https://doi.org/10.2147/IJN.S275650
Wu D, Chang X, Tian J, Kang L, Wu Y, Liu J, Wu X, Huang Y, Gao B, Wang H, Qiu G, Wu Z (2021) Bone mesenchymal stem cells stimulation by magnetic nanoparticles and a static magnetic field: release of exosomal mir-1260a improves osteogenesis and angiogenesis. J Nanobiotechnol 19(1):1–19. https://doi.org/10.1186/s12951-021-00958-6
Xu S, Okano H, Ohkubo C (1998) Subchronic effects of static magnetic fields on cutaneous microcirculation in rabbits. In Vivo 12(4):383–389
Xu ML, Wang XB, Li B, Li DF, Jiang JC (2003) Long-term memory was impaired in one-trial passive avoidance task of day-old chicks hatching from hypomagnetic field space. Chin Sci Bull 48:2454–2457
Yun H-M, Ahn S-J, Park K-R, Kim M-J, Kim J-J, Jin G-Z, Kim H-W, Kim E-C (2016) Magnetic nanocomposite scaffolds combined with static magnetic field in the stimulation of osteoblastic differentiation and bone formation. Biomaterials 85:88–98. https://doi.org/10.1016/j.biomaterials.2016.01.035
Zborowski M, Ostera GR, Moore LR, Milliron S, Chalmers JJ, Schechter AN (2003) Red blood cell magnetophoresis. Biophys J 84(4):2638–2645
Zhang B, Lu H, Xi W, Zhou X, Xu S, Zhang K, Jiang J, Li Y, Guo A (2004) Exposure to hypomagnetic field space for multiple generations causes amnesia in Drosophila melanogaster. Neurosci Lett 371(2–3):190–195
Zhang X, Li JF, Wu QJ, Li B, Jiang JC (2007) Effects of hypomagnetic field on noradrenergic activities in the brainstem of golden hamster. Bioelectromagnetics 28:155–158
Zhang Y, Cao L, Varga V, Jing M, Karadas M, Li Y, Buzsáki G (2021) Cholinergic suppression of hippocampal sharp-wave ripples impairs working memory. Proc Natl Acad Sci U S A 118(15):e2016432118. https://doi.org/10.1073/pnas.2016432118
Zimmerman JW, Pennison MJ, Brezovich I, Yi N, Yang C, Ramaker R, Absher D, Myers RM, Kuster N, Costa FP, Barbault A, Pasche B (2012) Cancer cell proliferation is inhibited by specific modulation frequencies. Br J Cancer 2012(106):307–313
Zyss T (2008) Magnetotherapy. Neuro Endocrinol Lett 29(Suppl 1):161–201
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2023 The Author(s), under exclusive license to Springer Nature Singapore Pte Ltd.
About this chapter
Cite this chapter
Epler, P., Yarema, K.J. (2023). Prospects, Pitfalls, and Opportunities for Human Static Magnetic Field Therapy. In: Zhang, X. (eds) Biological Effects of Static Magnetic Fields. Springer, Singapore. https://doi.org/10.1007/978-981-19-8869-1_15
Download citation
DOI: https://doi.org/10.1007/978-981-19-8869-1_15
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-19-8868-4
Online ISBN: 978-981-19-8869-1
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)