Abstract
In the current situation, almost every industrial and transportation activities are dependent on fossil fuels for their energy requirements. Fossil fuels are an overpriced and non-renewable source of energy. Besides these fossil fuels, combustion emits greenhouse gases that cause air pollution and global warming. Nowadays, microalgae-originated biofuels gained huge attention from researchers as they are expected to be a potential alternative to conventional fuels. Microalgal biofuel sector considers various strategies to enhance lipid content and biomass productivity in different habitats. To attain high lipid content from microalgae, lipid triggering circumstances are required to be optimized. This chapter summarizes various cultivation conditions, including pH, light intensity, and temperature for raised lipid accumulation inside microalgal cells. Different levels of physical factors affecting microalgal growth and lipid yield have been discussed in this chapter. The influence of the cultivation conditions such as CO2 concentration, temperature, light colour, and light intensity on lipid accumulation is evaluated comprehensively. Also, very recent progress and research studies on microalgal biomass and biodiesel production are discussed and summarized.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
6.1 Introduction
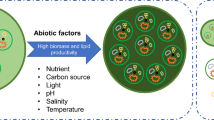
In the present, continuous depletion in fossil fuel reservoirs causes scarcity of energy resources. Conventional fuels will be going to become exhausted and unable to satisfy the growing demand for energy. Continuously inflated fuel prices, global warming, and greenhouse gas emission bring the requirement to upgrade sustainable energy resources. Algae-derived biofuels are alternative to replace conventional fuels. Third-generation biofuels include microalga biomass for the production of ethanol, butanol, and biodiesel. Algae have huge potential to replace first- and second-generation agricultural feedstocks as microalgae are rich in carbohydrate-lipid content and have a rapid growth rate and high biomass yield (Verma and Mishra 2020). In fourth-generation biofuels, upcoming advances bring the metabolic and gene engineering approaches to design custom microalgae to increase biofuel production (Abdullah et al. 2019; Moravvej et al. 2019). Brazil, Germany, France, Sweden, and the United States are leading countries in the consumption and development of biofuels (Adenle et al. 2013). It is observed that genetically modified algae shown significant enhancement in biomass, lipid accumulation, and carbon capturing capacity (Beacham et al. 2017; Shuba and Kifle 2018; Dutta et al. 2014; Levitan et al. 2014). Algal species are found in many shapes and sizes, extending from single-cell microalgae to multicellular filaments macroalgae in different aquatic habitats (Shafik et al. 2015). Most of the algal species are found in extreme environmental conditions in various aquatic habitats (Adeniyi et al. 2018). Based on their cellular organization and development, algae are classified into cyanobacteria and eukaryotes. Cyanobacteria are differentiated by the lack of chloroplasts and an adequate nucleus. Based on cell wall composition, storage products, and pigments, eukaryotic algae can be classified into Chrysophyceae, Chlorophyceae, Euglenophyceae, Phaeophyceae, Pyrrophyceae, Rhodophyceae, and Xanthophyceae. Algae achieve its economic significance in biofuel generation, bioremediation, wastewater treatment, nutraceuticals, animal feed, and biofertilizer (Sirakov et al. 2015; Suparmaniam et al. 2019; Pulz and Gross 2004). Additionally, algae are rich in several metabolites, including fatty acids, nutraceuticals, proteins, pigments, and vitamins (Sirakov et al. 2015; Pulz and Gross 2004). Under optimized conditions, algae can grow rapidly and produce two to ten-fold higher lipid content as compared to soybean, jatropha, and rapeseed (Rawat et al. 2013; Chisti 2007; Lam and Lee 2011; Tsukahara and Sawayama 2005). This chapter summarizes various cultivation conditions, including pH, light intensity, and temperature for raised lipid accumulation inside microalgal cells. Different levels of physical factors affecting microalgal growth and lipid yield have been discussed in this chapter; the impact of the culture conditions like CO2 concentration, temperature, light colour, and light intensity on lipid accumulation is evaluated comprehensively. Also, very recent progress and research studies on microalgal biomass and biodiesel production are discussed and summarized. Figure 6.1 represents crucial factors affecting microalgae culture and ultimately affect biofuel production.
6.2 Microalgal Cultivation
Algae have higher growth rates, photosynthetic levels, and CO2 sequestering efficiency. Microalgae are significantly considered organisms for reducing the nutrient load (nitrogen and phosphorous) from agricultural, industrial, municipal, and domestic wastewater. They consist of considerable quantities of fatty acids that yield sustainable biodiesel after transesterification. Microalgal cultivation for lipid recovery and biofuel generation is a moderate process. Meanwhile, they need quite simple light and aeration arrangements to grow. However, some factors alter the optimum growth and total lipid yield in microalgae. These factors include macro- and micronutrients (concentration and availability), CO2, pH, light (photoperiod/intensity), and temperature. Algae have higher growth rates, photosynthetic levels, and CO2 sequestering efficiency. Microalgae are significantly considered organisms for reducing the nutrient load (N and P) from industrial, agricultural, municipal, and domestic wastewater. They consist of considerable quantities of fatty acids that yield sustainable biodiesel after transesterification. Microalgal biomass cultivation for biodiesel production is a moderate process; meanwhile, they need quite simple light and aeration arrangements to grow. Some factors like nutrients availability, pH, temperature, CO2, photoperiod, and light intensity might affect biomass yield and lipid accumulation inside microalgal cells. Among all these conditions, light and temperature are primarily significant for algal growth. A temperature that ranges from 20 to 30 °C is preferable for most microalgal species. Highly raised temperature results in physiological adaptations in microalgal cells that cause lesser unsaturated fatty acid production (Van Wagenen et al. 2012). Biodiesel properties like cetane number, heating point, oxidative stability, lubricity, and melting point are primarily based on the type of precursor fatty acid. Palmitoleic acid, stearic acid, linolenic acid, oleic acid, linoleic acid, and palmitic acid are the most suitable fatty acids for biodiesel generation (Zheng et al. 2013). The lipid synthesis pathway in microalgae might be altered in several limiting conditions and synthesize a higher amount of triacylglycerol (TAG) in lipid bodies (Hu et al. 2008). It is necessary to observe loads of microalgal species for their high lipid productivity; then selected algal species are optimized for different factors to attain maximum biodiesel production. The cultivation system is an essential factor as it affects the biofuel yield and photosynthesis efficiency of microalgae. Generally, algal cultivation is preferred in two ways: (1) open cultivation system and (2) closed cultivation system. Photobioreactors (PBR) are a closed system for microalgal cultivation. Inside PBR, microalgal cultural conditions as light intensity, aeration, and stirring are customized according to the microalgal species (Liao et al. 2018; Lee and Lee 2016). Glass or plastic material is used to construct PBR with different designs such as flat plate, airlift, and tubular bioreactor and bubble column (Brennan and Owende 2010). Airlift bioreactor is widely appreciated to eliminate the chance of contamination and to obtain high algal biomass (Huang et al. 2010). High cost, oxygen build-up, biofouling, overheating, cell damage, and difficulties in scale-up are major limitations of PBR (Adeniyi et al. 2018; Brennan and Owende 2010). In comparison, open cultivation systems like the shallow pond, unstirred pond, raceway pond, closed pond, and circular pond are less optimized but provide affordable, lower maintenance cost, less operational cost, and easy scale-up. Open systems are highly affected by water evaporation, temperature, and light variability. PBR exhibited highly customized growth conditions and ensured the availability of adequate light intensity; hence, PBR provides a higher biomass productivity rate than the open system (Leite et al. 2013). CO2 consumption as a carbon source is the major pros of utilizing photoautotrophic systems. Furthermore, chances of contamination are significantly less while using photoautotrophs (Mata et al. 2010). A combined design of an open pond and closed PBR system is applied to achieve elevated biomass productivity and excellent nutrient removal. A combined setup of closed and open systems is termed as hybrid algal cultivation method, which is adequate for massive microalga cultivation (Razzak et al. 2017; Rawat et al. 2013). Hybrid systems are customized to eliminate drawbacks of open systems and to minimize the operational cost of photobioreactors. Such a hybrid cultivation system preferred the initial stage of microalgal culture inside photobioreactors, and then it will be transferred to ponds (Schenk et al. 2008) (Figs. 6.2 and 6.3).
Concerning metabolic activity, algal cells can be classified into main categories that are heterotrophic, photoautotrophic, autotrophic, and mixotrophic (Daliry et al. 2017). The heterotrophic mode of nutrition needs the presence of organic carbon as a substrate, whereas photoheterotrophs need both light and organic carbon to survive (Chen and Durbin 1994). Autotrophic microalgae convert inorganic source of carbon into chemical energy in the availability of sunlight, while mixotrophic algae can survive in both heterotrophic and autotrophic modes dependent on the presence/absence of carbon source and light (Burkholder et al. 2008). Scarsella et al. (2010) found that mixotrophy nutrition mode in microalgae is suitable for yielding maximum lipid and biomass. Heterotrophic metabolism has a better growth rate than autotrophic metabolism (Martinez et al. 1991).
6.3 Impact of Cultural Conditions on the Algal Lipid Content
Photoautotrophs like green algae can synthesize proteins, lipids, carbohydrates, and proteins. Lipid composition in algae is highly affected by its life cycle, whereas variation in lipid content depends on the cultivation medium and some other physical factors (Scarsella et al. 2010). Temperature, pH level, photoperiod, light intensity, and CO2 highly affect photosynthesis in green algae (Deng et al. 2014). Figure 6.4 depicts impact of stress condition on algal cells.
6.3.1 Effect of Temperature
It is evident to evaluate the influence of temperature to achieve high microalgal growth rate at broad-scale outdoor cultivation systems. Microalgal culture in outdoor open ponds is expected to undergo extreme surrounding temperatures and produce high algal biomass for economic lipid production. Surrounding temperature is a crucial parameter that affects lipid composition and content in algal cells (Ras et al. 2013). Temperature modifies metabolic pathways and causes acceleration or retardation of biochemical reaction rate in algal cells (Afify et al. 2010). The dependency of biochemical reactions rate on temperature can be described by Arrhenius function and Q10 (temperature coefficient). Q10 is a factor by which any biological processes or biochemical reaction rate increases if the temperature is raised by 10 units (Teoh et al. 2010). The relationship among temperature and algal growth rate follows the Arrhenius relationship. Q10 and Arrhenius function are equally expected to achieve accelerated growth rates with rising temperatures. However, microalgal growth rate accelerates up to an optimum temperature, but crossing that optimum value can cause retardate growth (Suzuki and Takahashi 1995; Montagnes and Franklin 2001). Table 6.1 present different studies observing the effect of temperature on different algal species. It has been seen that raised temperatures in Nannochloropsis salina and Ochromonas Danica cultivation cause correspondence increase in their lipid content, whereas Chlorella sorokiniana behave contradictory and show no variation in lipid content corresponding to raised temperature (Fakhry and El Maghraby 2015). Kalacheva et al. (2002) use Botryococcus braunii cultivation to observe the effect of different temperatures (18 °C, 25 °C, and 32 °C) on cellular lipid content. At 25 °C (optimum temperature), lipid content was estimated up to 22%, whereas crossing 32 °C cause 5% less cellular lipid content than optimum temperature (Kalacheva et al. 2002). Similarly, 25 °C is the optimum growth temperature for Chlorella vulgaris and Nannochloropsis oculata. A study found that increasing temperature from 20 to 25 °C Nannochloropsis oculata cultivation results in the twofold increment of lipid accumulation (7.90–14.92%) (Converti et al. 2009). However, in the case of C. vulgaris, raising temperature more than optimum (from 25 to 30 °C) causes approximately 7% reduction (14.71–5.90%) in lipid content (Converti et al. 2009). Researchers found that temperatures ranging from 15 to 35 °C might be effective in raising lipid accumulation inside microalgal cells (Converti et al. 2009; Kalacheva et al. 2002; Fakhry and El Maghraby 2015). Some studies were performed to observe the additive impact of nutrient availability and temperature on the cellular lipid contents of algae. To examine the synergistic effect of temperature and nutrient availability, microalgal species such as Chromulina ochromonoides, Dunaliella tertiolecta, Isochrysis galbana, Nannochloropsis oculata, Odontella aurita, and Thalassiosira pseudonana were cultivated at 10 and 20 °C with two different conditions (nutrient starved and sufficient) (Roleda et al. 2013; Shuping et al. 2010). Results revealed that adding multiple stress conditions would not be able to give additional impact to enhance lipid content, and only the nutrient-deficient condition is favourable to improve lipid accumulation (Shuping et al. 2010; Roleda et al. 2013). A study on microalgae was performed to analyse the impact of temperature on fatty acid composition. Nannochloropsis oculata and Tetraselmis subcordiformis were cultivated under several temperatures ranging from 15 to 35 °C (Wei et al. 2015). Results showed that high temperatures cause more MUFA accumulation than PUFA and neutral lipids (Wei et al. 2015). Dunaliella salina, a marine microalga, was studied under a temperature range shifting from 30 to 12 °C to analyse temperature shifting effect on lipid composition. Under lower temperatures, unsaturated fatty acid accumulation is raised by 20% (Sharma et al. 2012). Generally, low temperatures lead to high membrane fluidity, more unsaturated fatty acids, and less saturated fatty acids associated with algal cell adaptation in stress conditions (Mathimani and Nair 2016). Regardless of cellular organization, eukaryotic green microalgae B. braunii, C. vulgaris, and prokaryotic algae S. platensis showed variation in lipid yield over raised temperatures. B. braunii, C. vulgaris, and S. platensis accumulated a lower amount of unsaturated fatty acids as compared to saturated fatty acids at high temperatures (Sushchik et al. 2003). Researchers are not able to conclude a similar trend of temperature variation for its impact on lipid accumulation in algal cells. High temperature enhances lipid accumulation as a storage product of algal cells due to its maximum use (Sayegh and Montagnes 2011). Stress conditions are applied to the activity of photosystem II when microalgal cultivation is done at more than optimum temperature and results in a lower yield of biomass and lipid (Sheng et al. 2011), whereas at extreme temperatures, microalgal growth is obstructed due to enzyme denaturation and termination of metabolic reactions (Renaud et al. 2002).
6.3.2 Effect of Light Intensity
Alga is a widespread photosynthetic living being. Light is a vital aspect of the autotrophic activity, photosynthesis, and growth of microalgal species. Algal cells consist of numerous photon harvesting pigments, where chlorophyll ‘a’ and chlorophyll ‘b’ are most abundant pigments that have sensitivity towards red and blue wavelengths. Light is a prominent aspect for algal growth. Algae can grow at different photoperiods, wavelengths, and light intensities; however, different light conditions may cause the difference in their photosynthetic activity and lipid accumulation. In addition, light intensity might influence the lipid yield and alteration in the fatty acid composition (Liu et al. 2008). In a study, a Cladophora species (filamentous algae) was cultivated using extreme light intensities. Results revealed that the composition of polar phospholipids was significantly reduced, whereas triacylglyceride accumulation was enhanced (Napolitano 1994). Table 6.2 present different studies observing the influence of light intensity on several algal species. Similarly, Desmarestia viridis was cultivated under varying light intensities. When light intensity shift from 700 to 1500 μmol m−2 s−1, 63% biomass reduction was observed (Gordillo et al. 1998a, b). Total lipid content is amplified when Desmarestia viridis was cultivated under dark conditions; however, triglyceride contents are reduced simultaneously (Smith et al. 1993). An increment in extracellular polysaccharide content was observed while raising proton flux density (Iqbal and Zafar 1993). Algae that are grown under enhanced intensity result in more lipid accumulation. Scenedesmus sp. was cultivated in 250–400 μmol m−2 s−1 range of light intensity and showed enhanced lipid accumulation (Liu et al. 2012). Despite that, two marine algal species Nannochloropsis and Chlorella sp. were grown under an extreme photon density of 10,000 lx, demonstrating lower lipid production (Cheirsilp and Torpee 2012). de Mooij et al. (2016) cultivate C. reinhardtii to study its productivity in different light colour. C. reinhardtii cultivation done inside a photobioreactor has light intensity of 1500 μmol m−2 s−1, where yellow light results in highest productivity (54 g m−2 day−1) as compared to red and blue light (de Mooij et al. 2016). Krzemińska et al. (2014) use five different algae (B. braunii, N. conjuncta, N. texensis, N. terrestris, and S. obliquus) to observe consequences of two different photoperiods (12:12 light/dark hour cycle and 24-h uninterrupted illumination) on biomass productivity and growth rate. 12:12 light/dark hour cycle was favourable to stimulate growth rate in Neochloris sp., while continuous stimulation is found effective for higher growth rates in case of S. obliquus and B. braunii (Krzemińska et al. 2014). Also, a study observes the effect of continuous illumination on the fatty acid composition of a green microalga Chlorella minutissima; however, there is no significant difference occurred in fatty acid composition at a light intensity of 200–400 μE m−2 s−1 (Tang et al. 2011). Although several findings relate the fatty acid composition to the light intensity, it was observed that PUFA content was dropped down with raised light intensity (Juneja et al. 2013). Continuous illumination, longer photoperiods, and high photon intensity reduce MUFA and PUFA content and elevate saturated fatty acid levels (Al-Qasmi et al. 2012). Thalassiosira pseudonana cultivated in 12:12 h light/dark photoperiod revealed lower PUFA and elevated SFA and MUFA levels (Brown et al. 1996). Inconsistently, intensified photon flux of 50–150 μmol m−2 s−1 light intensity increases fatty acid accumulation in algal cells (Schnurr et al. 2016). No considerable alteration occurs in the fatty acid profile when raising the photon flux beyond 300–600 μmol m−2 s−1 (Schnurr et al. 2016). Cuellar-Bermudez et al. (2015) studied the combined effect of CO2 and light intensity on the fatty acid composition of Synechocystis sp. PCC6803. Total lipid content enhanced when Synechocystis sp. cultivated under 1920 μmol m−2 s−1 light intensity with 3% CO2 concentration; however increasing light illumination does not affect fatty acid composition (Cuellar-Bermudez et al. 2015). It is considered that growth and lipid yield is varying with different algal strains. Beyond the threshold limit of light, intensity triggers lamellae disruption within the chloroplast and destined photoinhibition or inactivation of CO2 fixing enzymes (Juneja et al. 2013). Extreme illumination intensities (beyond threshold level) disrupt the photosynthetic receptor system and trigger photoinhibition (Wahidin et al. 2013). Availability of essential fatty acids, different pigments, and dark respiration rate are the severe factors behind the physiological photo-acclimatization, whereas changes in volume, number, and density of photosynthetic apparatus can cause morphological photo-acclimation (Fábregas et al. 2004). It is found that desaturated chloroplast membranes of algae can exceed the limitation of low light intensity (Mock and Kroon 2002). Prevention of photoinhibition and dissipation of excess photon energy could assist by NADH and ATP generated during photosynthesis. Also, triacylglyceride synthesis needs high NADH and ATP levels; hence the carbon flux is generated during photosynthesis when microalgal cells are cultivated under extreme light conditions (Solovchenko et al. 2008; Liu et al. 2012). Despite this, when algal cells absorb the surplus level of excitation energy, it results in photodamage (destruction of photoreceptors of PS II); however, polypeptides of the reaction centre are more stable during extreme light exposure (He et al. 2001). When Synechocystis sp. PCC6883 was acclimatized under extreme light intensities, plastoquinone oxidation takes place, and the PS I/PS II ratio reaches up to 5, which was advantageous to minimize photodamage (Cuellar-Bermudez et al. 2015). The light could provoke algal culture growth, chloroplast membrane development, and lipid synthesis; hence light is a crucial factor for lipid accumulation in microalgal cells, but light intensity/photoperiod requirements are dependent and varies with algal strains.
6.3.3 Effect of Salinity
Salinity (NaCl concentration) is the vital factor that modifies the composition of biochemical compounds inside microalgal cells. Too low or high salinity exposure of algal cells cause alteration in their natural growth rate and biochemical composition. Studies revealed that high salinity can cause increased lipid content in algae (Zhila et al. 2011; Renaud and Parry 1994). In a study, marine algae Dunaliella was cultivated under different saline concentrations ranging from 0.4 to 4 M; results reported an increase in SFA and MUFA with increased salinity up to 4 M (Xu and Beardall 1997). Similarly, an increase in triglyceride (40–56%) and lipid content (60–67%) was reported when Dunaliella tertiolecta was cultivated under saline concentration raised from 0.5 to 1.0 M NaCl (Takagi and Yoshida 2006). Botryococcus braunii cultivation under high salinity level results in higher growth rate, with increase in lipid and carbohydrate content (Rao et al. 2007). Correspondingly, Botryococcus braunii was cultivated in two salinity conditions (without NaCl and with 0.50 NaCl). This study reported higher lipid content in a media with 0.50 NaCl; however, carbohydrates, proteins, and pigments were less (Ben-Amotz et al. 1985). A study reported reduced protein content whereas no variation in lipid and carbohydrate content in Botryococcus braunii under high saline levels. However, growth rate was reduced as algal cells was unable of adapt in high saline concentration (Vazquez-Duhalt and Arredondo-Vega 1991). Accordingly, another study supported reduced protein content per algal cells in Tetraselmis suecica with increased NaCl concentration (Fabregas et al. 1984).
6.3.4 Effect of Nutrients
Varying nutrient composition using different nutrient limitations could be helpful to identify which nutrient or substrate affect algal growth up to what extent. In general, Michaelis-Menten equation described nutrient uptake rate. At optimal culture conditions, algal growth is proportional to the utilization rate of highly limiting substrate. N and P are crucial macronutrients for metabolism and growth of microalgal cells. Nitrogen is basic element to form nucleic acid and proteins. Similarly, phosphate plays a major role as energy currency in the form of ATP in all living beings. Despite that, phosphorous is key element found in RNA, DNA, and phospholipids. Limitation of nitrogen and phosphorus will shift metabolic system of microalgal cells. Starvation of N and P shifts the lipid metabolism and tends towards lipid storage instead of membrane lipid synthesis. Hence the total lipid content increases in microalgae under N- and P-starved conditions (Hirano et al. 1997). Specific discussion on all limiting substrate is reviewed below.
6.3.4.1 Carbon
C, H, and O are crucial non-mineral nutrients. Carbon is most essential nutrient for algal growth, photosynthesis, and reproduction. Carbon uptake is generally fixed by three ways: (1) as energy resource; (2) as respiration; and (3) as a raw material for cell formation (Berman-Frank and Dubinsky 1999). Lower carbon fixing rate results in lesser growth rate in microalgal cells. Algal cells need inorganic carbon substrate for photosynthesis which is available in the form of carbon dioxide (CO2) or carbonate in autotrophic mode of nutrition. CO2 dissolved in water are convertible into carbonate or bicarbonate depending on nutrient composition, pH, and temperature. With rise in pH level, carbonate content rises as compared to carbon dioxide and bicarbonate (Chen and Durbin 1994). Riebesell et al. (2000) observe Emiliania huxleyi to analyse the influence of CO2 concentration on lipid composition. CO2 concentration significantly influences the composition of PUFA and alkenones. Study revealed low concentration of CO2 tends to increase PUFA 22:6 (n-3), while high CO2 concentration raises 14:0 fatty acids composition (Riebesell et al. 2000). A study reported that increased CO2 concentration results in enhanced unsaturation and fatty acids content (Tsuzuki et al. 1990). Similarly, in Dunaliella salina, increased CO2 levels leads to elevated level of fatty acid accumulation (Muradyan et al. 2004).
6.3.4.2 Nitrogen
Nitrogen is readily consumed by algal cell; algae consume the inorganic source of nitrogen existing in media and turn it into biochemical compounds to carry out their physiological processes appropriately (Ross et al. 2018; Yang et al. 2017). However, it was studied that nitrogen-starved condition leads to high lipid biosynthesis level and enhanced triglyceride accumulation with lesser protein component (Wei et al. 2020; Heraud et al. 2005; Wang et al. 2009). So, the higher lipid and lower protien content could be obtained at the disbursement of algal growth rate (Converti et al. 2009; Li et al. 2008), under nitrogen-limited conditions to redirect their photosynthetic carbon into carbohydrate synthesis (Hu 2004). Nitrogen-depleted media can also cause lower oxygen generation, lesser CO2 fixation, and decreased tissue and chlorophyll content (Kolber et al. 1988). In a study, sugar phosphates and ammonium were additionally supplied into the growth media of Chlorella pyrenoidosa where an increase in amino acid was observed (Holm-Hansen et al. 1959). In algal photosystem II, phycobilisomes act as antennae for light harvesting. In red algae and cyanobacteria, nitrogen-limited culture media cause degradation of phycobilisomes (Collier and Grossman 1992). Photosynthesis remains persistent (with reduced rate) until nitrogen concentration depleted below a threshold level. Spirulina platensis cells were grown under high CO2 availability with nitrogen-depleted medium and depict very less carbon fixation efficiency (Gordillo et al. 1998a, b). Nitrogen depletion may cause variation in enzyme levels inside algal cells and cause lipid synthesis however simultaneously reducing chlorophyll synthesis that generates higher carotenoid accumulation (Zhang et al. 2017).
6.3.4.3 Phosphorus
Phosphorus is a crucial element for adequate growth and metabolism in microalgal cells. Besides nitrogen, phosphorus is primary limiting macronutrient for microalgal cells (Ren et al. 2017). Phosphorus limitation generally cause lesser than needful rate of light assimilation for carbon fixation; also it will result in reduced level of substrate synthesis during Calvin-Benson cycle (Barsanti and Gualtieri 2005). Phosphorus limitation will be an advantageous condition to gain high lipid accumulation in algal cells. In a study, Scenedesmus sp. is grown at 2.0 mg L−1 of initial phosphorus concentration and then reduced it to 0.1 mg L−1 early phosphorus concentration. It was observed that reduction of phosphorus concentration attains increment in total lipid accumulation to 53% (at 0.1 mg L−1) from 23% (at 2.0 mg L−1) (Xin et al. 2010). Phosphatidylglycerol is a glycerolipid present in chloroplast membrane. It also has significant roles in cell growth, keeping satisfactory levels of chlorophyll protein complex and PS II function. In a study, Chlamydomonas reinhardtii was grown in a phosphorus-limited medium; results depict decreased phosphatidylglycerol content (Sato et al. 2000). In phosphorus-deficient medium, protein and chlorophyll a content tends to reduce and thus enhanced relative carbohydrates amount in algal cells (Healey and Hendzel 1979). Like nitrogen deficiency, phosphorus limitation tends to reduced phycobilisome content (Collier and Grossman 1992). In Selenastrum minutum, phosphorus-deprived condition causes reduction in respiration rate of algal cells (Theodorou et al. 1991).
6.3.4.4 Micronutrients
Trace metals are micronutrients required by living cells in very less quantity (less than 4 ppm), and these trace metals are very crucial for physiology of algal cells. Trace metals commonly include cobalt, copper, manganese, iron, zinc, and nickel (Facey et al. 2019). For algal cells, trace metal accessibility is dependent on free ion concentration, i.e. it is independent of dissolving trace metal concentration in the culture media (Parent et al. 1996). High level of trace metal in culture media can cause reduction in antioxidant levels, cell membrane damage, and photosynthesis impairment; similarly deficiency of these can also inhibit cell growth. Iron plays significant role as catalyst redox reactions of electron transport system, nitrogen assimilation, and photosynthesis in algal cells (Rueler and Ades 1987). Iron deficiency causes reduction in photosynthetic electron transport, which further reduces NADPH availability. Iron deficiency causes reduction in ferredoxin availability and substitutes ferredoxin with flavodoxin (McKay et al. 1999). Subsequently, ferredoxin substitution could be unfavourable because the catalytic activity of flavodoxin is very slow and not comparable and efficient as ferredoxin (McKay et al. 1999). Iron-limited media can bring chlorophyll-deficit condition inside algal cells (Greene et al. 1992). In C. vulgaris, effect of elevated iron concentration has been observed in a study and remarks enhanced lipid content (Liu et al. 2008), whereas iron-deficient condition can cause depletion in carotenoid composition (van Leeuwe and Stefels 1998). Algal cell membrane consists of many functional groups like phosphate, carboxylic, and sulfhydryl groups. Such functional groups act as binding site for metal ions. Most of the metabolic reactions will be inhibited by the presence of some non-essential elements like chromium, lead, and cadmium. A very small concentration of these metals can cause severe toxicity within algal cells. Similarly, presence of excess concentration of essential metals like zinc, nickel, and copper can also cause cellular toxicity (Campanella et al. 2001; Kennish 1992; Rai and Mallick 1993).
6.3.5 Effect of CO2
Carbon dioxide (CO2) is accessible in the atmosphere and emitted continuously from different industrial sources, vehicles, and power plants. Increasing levels of CO2 in atmosphere will be harmful for living beings. Algal cells are naturally contributing to mitigate CO2 by carbon capturing and carbon sequestration. At high CO2 concentration, Chlorella sp. shows higher rate of photosynthesis (Singh and Singh 2014). Mainly CO2 concentration affects lipid content and microalgal growth rate. Some researchers inspected the impact of CO2 concentration on fatty acid, lipid composition, and biomass growth in different algae. In a study, C. vulgaris, Scenedesmus sp., and B. braunii are chosen to observe the effect of different CO2 concentrations (Yoo et al. 2010). At 10% CO2 level, after 2 weeks, B. braunii gain 26.55 mg L−1 biomass productivity and 5.51 mg L−1 day−1 of total lipid productivity, whereas Chlorella vulgaris obtain biomass productivity of 104.76 mg L−1 day−1 and lipid productivity of 6.91 mg L−1 day−1 (Yoo et al. 2010). On similar culture conditions and 10% CO2 levels, Scenedesmus sp. obtains biomass productivity of 217.50 mg L−1 day−1 and lipid productivity of 20.65 mg L−1 day−1 (Yoo et al. 2010). In the same analysis, Yoo et al. (2010) investigate effect of flue gases comprising 5.5% CO2 levels on C. vulgaris, Scenedesmus sp., and B. braunii. Results show enhanced biomass productivity in all three species, whereas Scenedesmus sp. shows 3.7-fold increment in the total lipid productivity (Yoo et al. 2010). In a study, B. braunii 765 was cultured with 20% CO2 availability; findings reported increasing CO2 levels from 2% to 20% cause enhanced biomass productivity and total lipid productivity varies from 10.41% to 12.71% by weight (Ge et al. 2011). With an environmental perspective, Phaeodactylum tricornutum and Nannochloropsis salina were cultured using desulphated and untreated flue gas with inorganic source of CO2. Result observed no significant changes in biomass obtained from P. tricornutum and N. salina, although the presence of some toxic non-essential metals like mercury, vanadium, and nickel in flue gas can affect the biomass productivity (Moheimani 2013). A study was performed on D. viridis which was cultured in nitrogen deficit and high CO2 availability simultaneously. Result has shown that lipid composition in D. viridis is very sensitive towards nitrogen-deficient condition (in presence of high CO2 concentration) (Gordillo et al. 1998a, b).
6.3.6 Effect of pH
pH of any culture media is the essential factor in any metabolic process, so in the microalgal growth, pH plays major role in metal speciation, nutrient availability, and enzymatic activities within the microalgal cells. Any changes in pH levels may directly influence enzymatic actions and further affected different metabolic pathways like photosynthesis and lipid synthesis (Jin et al. 2016). However, response of algal cells and its metabolic process towards optimum pH or at other pH range is different and strain specific (Moheimani 2013). Varying pH affects lipid and biomass yield in microalgal cells. In a study, during inoculation initial pH of algal growth media was kept neutral because availability of carbon species is depending on the pH. After some time, algal cells consume inorganic carbon present in media, and hence pH of the growth media gradually increases (Rai et al. 2015). Effect of numerous inorganic carbon sources and pH on Chlorella sp. and T. suecica CS-187 was observed for lipid and biomass productivity. At pH 7.5, T. suecica gained a biomass productivity of 320 mg L−1 day−1 and lipid productivity of 92 mg L−1 day−1, whereas at pH 7.0, Chlorella sp. obtained biomass productivity of 407 mg L−1 day−1 and lipid productivity of 99 mg L−1 day−1 (Moheimani 2013). In a study, growth media was set with different pH ranging from 6.0 to 10.0 to observe effect of pH on the lipid accumulation and biomass productivity in N. salina (Bartley et al. 2014). Outcomes revealed that pH 8 to pH 9 depicts maximum growth rates in N. salina (Bartley et al. 2014). In a study, C. vulgaris cultivated in heterotrophic mode at optimum pH (7.5) in sulphur-deficient condition obtain lipid content of 53.43% and maximum specific growth rate of 0.541 days−1. Cell death occurs in C. vulgaris at pH 3, 4, and 11, whereas at pH 9.5 algal cells start to aggregate (Sakarika and Kornaros 2016). In a different study Chlorella sp. was grown at varying pH levels ranging from 5 to 11 to observe impact of pH on biomass growth and lipid content (Zhang et al. 2014). After a month, highest lipid content was 32.8% (lipid yield of 168 mg L−1) and was noticed at pH 7.0, whereas at pH 5.0, maximum triacylglyceride content was 63% (Zhang et al. 2014). For Dunaliella salina pH 11.5 and Dunaliella acidophila pH 3 is found as optimum pH (Varshney et al. 2015).
6.4 Future Aspects
In this chapter, we discussed influence of different culture conditions like nutrients, and environmental factors like light, pH, CO2 availability, temperature, etc., on algal biomass and lipid content. Tremendous efforts have been done by the researchers to understand behaviour of algal biomass growth for lipid production and nutrient recovery from wastewater. These efforts include enhanced optimization of the environmental factors and its effects on algal cell growth and composition. With the progress made by biotechnology research and bioinformatics software, it is feasible to acquire an enormous information from genetic data. It is possible now to trace molecular interaction and its effect on metabolism. Genome sequences of Chlorella vulgaris, Chlamydomonas reinhardtii, and Dunaliella salina are known (Smith et al. 2010; Merchant et al. 2007; Blanc et al. 2010). Predictive models and automated control systems for optimum culture condition would be effective solution for huge scale algal biomass and fatty acid production (Coşgun et al. 2021). Understanding of critical factors affecting algal production will be beneficial for successful and sustainable biomass production for renewable fuel generation.
6.5 Conclusions
In present scenario, increasing demands of conventional fuels bring necessity of alternative sustainable energy resources. Biodiesel, biobutanol, biohydrogen, and bioethanol production from microalgae is promising alternative for fossil fuels. However, lipid yield is still not sufficient to meet biodiesel production as per demand. Hence, most research work is inclined towards enhanced lipid production by altering different culture conditions such different light colours, light intensities, photoperiods, temperature, pH, and inorganic carbon sources. So, this chapter give a comprehensive glance by discussing influence of critical factors for increasing lipid content, fatty acid composition, and biomass growth of microalgae.
Abbreviations
- ATP:
-
Adenosine triphosphate
- CO2:
-
Carbon dioxide
- MUFA:
-
Monounsaturated fatty acid
- NADH :
-
Nicotinamide adenine dinucleotide
- PBR:
-
Photobioreactor
- PS I:
-
Photosystem I
- PS II:
-
Photosystem II
- PUFA:
-
Polyunsaturated fatty acid
- Q10 :
-
Temperature coefficient
- SFA:
-
Saturated fatty acid
- TAG:
-
Triacylglycerol
References
Abdullah B, Muhammad SAFAS, Shokravi Z, Ismail S, Kassim KA, Mahmood AN, Aziz MMA (2019) Fourth generation biofuel: a review on risks and mitigation strategies. Renew Sust Energ Rev 107:37–50
Adeniyi OM, Azimov U, Burluka A (2018) Algae biofuel: current status and future applications. Renew Sust Energ Rev 90:316–335
Adenle AA, Haslam GE, Lee L (2013) Global assessment of research and development for algae biofuel production and its potential role for sustainable development in developing countries. Energy Policy 61:182–195
Afify AEMM, Shalaby EA, Shanab SM (2010) Enhancement of biodiesel production from different species of algae. Grasas Aceites 61(4):416–422
Al-Qasmi M, Raut N, Talebi S, Al-Rajhi S, Al-Barwani T (2012) A review of effect of light on microalgae growth. In: Proceedings of the world congress on engineering, vol 1(4)
Barsanti L, Gualtieri P (2005) Algae: anatomy, biochemistry, and biotechnology. CRC Press
Bartley ML, Boeing WJ, Dungan BN, Holguin FO, Schaub T (2014) pH effects on growth and lipid accumulation of the biofuel microalgae Nannochloropsis salina and invading organisms. J Appl Phycol 26(3):1431–1437
Beacham TA, Sweet JB, Allen MJ (2017) Large scale cultivation of genetically modified microalgae: a new era for environmental risk assessment. Algal Res 25:90–100
Ben-Amotz A, Tornabene TG, Thomas WH (1985) Chemical profile of selected species of microalgae with emphasis on lipids 1. J Phycol 21(1):72–81
Berman-Frank I, Dubinsky Z (1999) Balanced growth in aquatic plants: Myth or reality? Phytoplankton use the imbalance between carbon assimilation and biomass production to their strategic advantage. Bioscience 49(1):29–37
Blanc G, Duncan G, Agarkova I, Borodovsky M, Gurnon J, Kuo A et al (2010) The Chlorella variabilis NC64A genome reveals adaptation to photosymbiosis, coevolution with viruses, and cryptic sex. Plant Cell 22(9):2943–2955
Brennan L, Owende P (2010) Biofuels from microalgae—a review of technologies for production, processing, and extractions of biofuels and co-products. Renew Sust Energ Rev 14(2):557–577
Brown MR, Dunstan GA, Norwood SJ, Miller KA (1996) Effects of harvest stage and light on the biochemical composition of the diatom Thalassiosira pseudonana 1. J Phycol 32(1):64–73
Burkholder JM, Glibert PM, Skelton HM (2008) Mixotrophy, a major mode of nutrition for harmful algal species in eutrophic waters. Harmful Algae 8(1):77–93
Campanella L, Cubadda F, Sammartino MP, Saoncella AJWR (2001) An algal biosensor for the monitoring of water toxicity in estuarine environments. Water Res 35(1):69–76
Cheirsilp B, Torpee S (2012) Enhanced growth and lipid production of microalgae under mixotrophic culture condition: effect of light intensity, glucose concentration and fed-batch cultivation. Bioresour Technol 110:510–516
Chen CY, Durbin EG (1994) Effects of pH on the growth and carbon uptake of marine phytoplankton. Mar Ecol Prog Ser 109:83–83
Chisti Y (2007) Biodiesel from microalgae. Biotechnol Adv 25(3):294–306
Collier JL, Grossman AR (1992) Chlorosis induced by nutrient deprivation in Synechococcus sp. strain PCC 7942: not all bleaching is the same. J Bacteriol 174(14):4718–4726
Converti A, Casazza AA, Ortiz EY, Perego P, Del Borghi M (2009) Effect of temperature and nitrogen concentration on the growth and lipid content of Nannochloropsis oculata and Chlorella vulgaris for biodiesel production. Chem Eng Process Process Intensif 48(6):1146–1151
Coşgun A, Günay ME, Yıldırım R (2021) Exploring the critical factors of algal biomass and lipid production for renewable fuel production by machine learning. Renew Energy 163:1299–1317
Cuellar-Bermudez SP, Romero-Ogawa MA, Vannela R, Lai YS, Rittmann BE, Parra-Saldivar R (2015) Effects of light intensity and carbon dioxide on lipids and fatty acids produced by Synechocystis sp. PCC6803 during continuous flow. Algal Res 12:10–16
Daliry S, Hallajisani A, Mohammadi RJ, Nouri H, Golzary A (2017) Investigation of optimal condition for Chlorella vulgaris microalgae growth. Global J Environ Sci Manage 3(2):217–223
de Mooij T, de Vries G, Latsos C, Wijffels RH, Janssen M (2016) Impact of light color on photobioreactor productivity. Algal Res 15:32–42
Deng YL, Kuo MY, Juang YJ (2014) Development of flow through dielectrophoresis microfluidic chips for biofuel production: Sorting and detection of microalgae with different lipid contents. Biomicrofluidics 8(6):064120
Dutta K, Daverey A, Lin JG (2014) Evolution retrospective for alternative fuels: First to fourth generation. Renew Energy 69:114–122
Fabregas J, Abalde J, Herrero C, Cabezas B, Veiga M (1984) Growth of the marine microalga Tetraselmis suecica in batch cultures with different salinities and nutrient concentrations. Aquaculture 42(3-4):207–215
Fábregas J, Maseda A, Domínguez A, Otero A (2004) The cell composition of Nannochloropsis sp. changes under different irradiances in semicontinuous culture. World J Microbiol Biotechnol 20(1):31–35
Facey JA, Apte SC, Mitrovic SM (2019) A review of the effect of trace metals on freshwater cyanobacterial growth and toxin production. Toxins 11(11):643
Fakhry EM, El Maghraby DM (2015) Lipid accumulation in response to nitrogen limitation and variation of temperature in Nannochloropsis salina. Bot Stud 56(1):1–8
Ge Y, Liu J, Tian G (2011) Growth characteristics of Botryococcus braunii 765 under high CO2 concentration in photobioreactor. Bioresour Technol 102(1):130–134
Gim GH, Ryu J, Kim MJ, Kim PI, Kim SW (2016) Effects of carbon source and light intensity on the growth and total lipid production of three microalgae under different culture conditions. J Ind Microbiol Biotechnol 43(5):605–616
Gordillo FJ, Goutx M, Figueroa FL, Niell FX (1998a) Effects of light intensity, CO2 and nitrogen supply on lipid class composition of Dunaliella viridis. J Appl Phycol 10(2):135–144
Gordillo FJ, Jiménez C, Figueroa FL, Niell FX (1998b) Effects of increased atmospheric CO2 and N supply on photosynthesis, growth and cell composition of the cyanobacterium Spirulina platensis (Arthrospira). J Appl Phycol 10(5):461–469
Greene RM, Geider RJ, Kolber Z, Falkowski PG (1992) Iron-induced changes in light harvesting and photochemical energy conversion processes in eukaryotic marine algae. Plant Physiol 100(2):565–575
He Q, Dolganov N, Björkman O, Grossman AR (2001) The high light-inducible polypeptides in Synechocystis PCC6803: expression and function in high light. J Biol Chem 276(1):306–314
Healey FP, Hendzel LL (1979) Indicators of phosphorus and nitrogen deficiency in five algae in culture. J Fish Board Canada 36(11):1364–1369
Heraud P, Wood BR, Tobin MJ, Beardall J, McNaughton D (2005) Mapping of nutrient-induced biochemical changes in living algal cells using synchrotron infrared microspectroscopy. FEMS Microbiol Lett 249(2):219–225
Hirano A, Ueda R, Hirayama S, Ogushi Y (1997) CO2 fixation and ethanol production with microalgal photosynthesis and intracellular anaerobic fermentation. Energy 22(2-3):137–142
Holm-Hansen O, Nishida K, Moses V, Calvin M (1959) Effects of mineral salts on short-term incorporation of carbon dioxide in Chlorella. J Exp Bot 10(1):109–124
Hu Q (2004) Environmental effects on cell composition, vol 1. Blackwell Science Ltd., Oxford, pp 83–93
Hu Q, Sommerfeld M, Jarvis E, Ghirardi M, Posewitz M, Seibert M, Darzins A (2008) Microalgal triacylglycerols as feedstocks for biofuel production: perspectives and advances. Plant J 54(4):621–639
Huang G, Chen F, Wei D, Zhang X, Chen G (2010) Biodiesel production by microalgal biotechnology. Appl Energy 87(1):38–46
Iqbal M, Zafar SI (1993) Effects of photon flux density, CO2, aeration rate, and inoculum density on growth and extracellular polysaccharide production by Porphyridium cruentum. Folia Microbiol 38(6):509–514
Jin J, Dupré C, Legrand J, Grizeau D (2016) Extracellular hydrocarbon and intracellular lipid accumulation are related to nutrient-sufficient conditions in pH-controlled chemostat cultures of the microalga Botryococcus braunii SAG 30.81. Algal Res 17:244–252
Juneja A, Ceballos RM, Murthy GS (2013) Effects of environmental factors and nutrient availability on the biochemical composition of algae for biofuels production: a review. Energies 6:4607–4638
Kalacheva GS, Zhila NO, Volova TG, Gladyshev MI (2002) The effect of temperature on the lipid composition of the green alga Botryococcus. Microbiology 71(3):286–293
Kennish MJ (1992) Ecology of Estuaries. Anthropogenic effects. CRC Press Inc., Boca Raton, FL
Kolber Z, Zehr J, Falkowski P (1988) Effects of growth irradiance and nitrogen limitation on photosynthetic energy conversion in photosystem II. Plant Physiol 88(3):923–929
Krzemińska I, Pawlik-Skowrońska B, Trzcińska M, Tys J (2014) Influence of photoperiods on the growth rate and biomass productivity of green microalgae. Bioprocess Biosyst Eng 37(4):735–741
Lam MK, Lee KT (2011) Renewable and sustainable bioenergies production from palm oil mill effluent (POME): win–win strategies toward better environmental protection. Biotechnol Adv 29(1):124–141
Lee OK, Lee EY (2016) Sustainable production of bioethanol from renewable brown algae biomass. Biomass Bioenergy 92:70–75
Leite GB, Abdelaziz AE, Hallenbeck PC (2013) Algal biofuels: challenges and opportunities. Bioresour Technol 145:134–141
Levitan O, Dinamarca J, Hochman G, Falkowski PG (2014) Diatoms: a fossil fuel of the future. Trends Biotechnol 32(3):117–124
Li Y, Horsman M, Wang B, Wu N, Lan CQ (2008) Effects of nitrogen sources on cell growth and lipid accumulation of green alga Neochloris oleoabundans. Appl Microbiol Biotechnol 81(4):629–636
Liao Q, Chang JS, Herrmann C, Xia A (eds) (2018) Bioreactors for microbial biomass and energy conversion. Springer
Liu ZY, Wang GC, Zhou BC (2008) Effect of iron on growth and lipid accumulation in Chlorella vulgaris. Bioresour Technol 99(11):4717–4722
Liu J, Yuan C, Hu G, Li F (2012) Effects of light intensity on the growth and lipid accumulation of microalga Scenedesmus sp. 11-1 under nitrogen limitation. Appl Biochem Biotechnol 166(8):2127–2137
Martinez F, Ascaso C, Orus MI (1991) Morphometric and stereologic analysis of Chlorella vulgaris under heterotrophic growth conditions. Ann Bot 67(3):239–245
Mata TM, Martins AA, Caetano NS (2010) Microalgae for biodiesel production and other applications: a review. Renew Sust Energ Rev 14(1):217–232
Mathimani T, Nair BB (2016) Evaluation of microalga for biodiesel using lipid and fatty acid as a marker—a central composite design approach. J Energy Inst 89(3):436–446
McKay RML, La Roche J, Yakunin AF, Durnford DG, Geider RJ (1999) Accumulation of ferredoxin and flavodoxin in a marine diatom in response to Fe. J Phycol 35(3):510–519
Merchant SS, Prochnik SE, Vallon O, Harris EH, Karpowicz SJ, Witman GB, Grossman AR (2007) The Chlamydomonas genome reveals the evolution of key animal and plant functions. Science 318(5848):245–250
Mock T, Kroon BM (2002) Photosynthetic energy conversion under extreme conditions—II: the significance of lipids under light limited growth in Antarctic Sea ice diatoms. Phytochemistry 61(1):53–60
Moheimani NR (2013) Inorganic carbon and pH effect on growth and lipid productivity of Tetraselmis suecica and Chlorella sp (Chlorophyta) grown outdoors in bag photobioreactors. J Appl Phycol 25:387–398
Montagnes DJS, Franklin DJ (2001) Effect of temperature on diatom volume, growth rate, and carbon and nitrogen content: reconsidering some paradigms. Limnol Oceanogr 46:2008–2018
Moravvej Z, Makarem MA, Rahimpour MR (2019) The fourth generation of biofuel. In: Second and third generation of feedstocks. Elsevier, pp 557–597
Muradyan EA, Klyachko-Gurvich GL, Tsoglin LN, Sergeyenko TV, Pronina NA (2004) Changes in lipid metabolism during adaptation of the Dunaliella salina photosynthetic apparatus to high CO2 concentration. Russ J Plant Physiol 51(1):53–62
Napolitano GE (1994) The relationship of lipids with light and chlorophyll measurements in freshwater algae and periphyton 1. J Phycol 30(6):943–950
Nzayisenga JC, Farge X, Groll SL, Sellstedt A (2020) Effects of light intensity on growth and lipid production in microalgae grown in wastewater. Biotechnol Biofuels 13(1):1–8
Parent L, Twiss MR, Campbell PG (1996) Influences of natural dissolved organic matter on the interaction of aluminum with the microalga Chlorella: a test of the free-ion model of trace metal toxicity. Environ Sci Technol 30(5):1713–1720
Pulz O, Gross W (2004) Valuable products from biotechnology of microalgae. Appl Microbiol Biotechnol 65(6):635–648
Rai LC, Mallick N (1993) Heavy metal toxicity to algae under synthetic microcosm. Ecotoxicology 2(4):231–242
Rai MP, Gautom T, Sharma N (2015) Effect of salinity, pH, light intensity on growth and lipid production of microalgae for bioenergy application. OnLine J Biol Sci 15(4):260
Rao AR, Dayananda C, Sarada R, Shamala TR, Ravishankar GA (2007) Effect of salinity on growth of green alga Botryococcus braunii and its constituents. Bioresour Technol 98(3):560–564
Ras M, Steyer JP, Bernard O (2013) Temperature effect on microalgae: a crucial factor for outdoor production. Rev Environ Sci Biotechnol 12(2):153–164
Rawat I, Kumar RR, Mutanda T, Bux F (2013) Biodiesel from microalgae: a critical evaluation from laboratory to large scale production. Appl Energy 103:444–467
Razzak SA, Ali SAM, Hossain MM, deLasa H (2017) Biological CO2 fixation with production of microalgae in wastewater—a review. Renew Sust Energ Rev 76:379–390
Ren L, Wang P, Wang C, Chen J, Hou J, Qian J (2017) Algal growth and utilization of phosphorus studied by combined mono-culture and co-culture experiments. Environ Pollut 220:274–285
Renaud SM, Parry DL (1994) Microalgae for use in tropical aquaculture II: effect of salinity on growth, gross chemical composition and fatty acid composition of three species of marine microalgae. J Appl Phycol 6(3):347–356
Renaud SM, Thinh LV, Lambrinidis G, Parry DL (2002) Effect of temperature on growth, chemical composition and fatty acid composition of tropical Australian microalgae grown in batch cultures. Aquaculture 211(1–4):195–214
Riebesell U, Revill AT, Holdsworth DG, Volkman JK (2000) The effects of varying CO2 concentration on lipid composition and carbon isotope fractionation in Emiliania huxleyi. Geochim Cosmochim Acta 64(24):4179–4192
Roleda MY, Slocombe SP, Leakey RJ, Day JG, Bell EM, Stanley MS (2013) Effects of temperature and nutrient regimes on biomass and lipid production by six oleaginous microalgae in batch culture employing a two-phase cultivation strategy. Bioresour Technol 129:439–449
Ross ME, Davis K, McColl R, Stanley MS, Day JG, Semião AJ (2018) Nitrogen uptake by the macro-algae Cladophora coelothrix and Cladophora parriaudii: Influence on growth, nitrogen preference and biochemical composition. Algal Res 30:1–10
Rueler JG, Ades DR (1987) The role of iron nutrition in photosynthesis and nitrogen assimilation in SCENEDESMUS QUADRICAUDA (Chlorophyceae) 1. J Phycol 23(3):452–457
Sakarika M, Kornaros M (2016) Effect of pH on growth and lipid accumulation kinetics of the microalga Chlorella vulgaris grown heterotrophically under sulfur limitation. Bioresour Technol 219:694–701
Sato N, Hagio M, Wada H, Tsuzuki M (2000) Environmental effects on acidic lipids of thylakoid membranes. Biochem Soc Trans 28(6):912–914
Sayegh FA, Montagnes DJ (2011) Temperature shifts induce intraspecific variation in microalgal production and biochemical composition. Bioresour Technol 102(3):3007–3013
Scarsella M, Belotti G, De Filippis P, Bravi M (2010) Study on the optimal growing conditions of Chlorella vulgaris in bubble column photobioreactors. Chem Eng 20:85–90
Schenk PM, Thomas-Hall SR, Stephens E, Marx UC, Mussgnug JH, Posten C, Hankamer B (2008) Second generation biofuels: high-efficiency microalgae for biodiesel production. Bioenergy Res 1(1):20–43
Schnurr PJ, Espie GS, Allen GD (2016) The effect of photon flux density on algal biofilm growth and internal fatty acid concentrations. Algal Res 16:349–356
Shafik HM, Saad MG, El-Serehy HA (2015) Impact of nitrogen regime on fatty acid profiles of Desmodesmus quadricaudatus and Chlorella sp. and ability to produce biofuel. Acta Bot Hungar 57(1-2):205–218
Sharma KK, Schuhmann H, Schenk PM (2012) High lipid induction in microalgae for biodiesel production. Energies 5(5):1532–1553
Sheng J, Kim HW, Badalamenti JP, Zhou C, Sridharakrishnan S, Krajmalnik-Brown R, Vannela R (2011) Effects of temperature shifts on growth rate and lipid characteristics of Synechocystis sp. PCC6803 in a bench-top photobioreactor. Bioresour Technol 102(24):11218–11225
Shuba ES, Kifle D (2018) Microalgae to biofuels: ‘promising’ alternative and renewable energy, review. Renew Sust Energ Rev 81:743–755
Shuping Z, Yulong W, Mingde Y, Kaleem I, Chun L, Tong J (2010) Production and characterization of bio-oil from hydrothermal liquefaction of microalgae Dunaliella tertiolecta cake. Energy 35(12):5406–5411
Singh SP, Singh P (2014) Effect of CO2 concentration on algal growth: a review. Renew Sust Energ Rev 38:172–179
Sirakov I, Velichkova K, Stoyanova S, Staykov Y (2015) The importance of microalgae for aquaculture industry. Review. Int J Fish Aquat Stud 2(4):81–84
Smith RE, Cavaletto JF, Eadie BJ, Gardner WS (1993) Growth and lipid composition of high Arctic ice algae during the spring bloom at Resolute, Northwest Territories, Canada. Mar Ecol Prog Ser:19–29
Smith DR, Lee RW, Cushman JC, Magnuson JK, Tran D, Polle JE (2010) The Dunaliella salina organelle genomes: large sequences, inflated with intronic and intergenic DNA. BMC Plant Biol 10(1):1–14
Solovchenko AE, Khozin-Goldberg I, Didi-Cohen S, Cohen Z, Merzlyak MN (2008) Effects of light intensity and nitrogen starvation on growth, total fatty acids and arachidonic acid in the green microalga Parietochloris incisa. J Appl Phycol 20(3):245–251
Suparmaniam U, Lam MK, Uemura Y, Lim JW, Lee KT, Shuit SH (2019) Insights into the microalgae cultivation technology and harvesting process for biofuel production: a review. Renew Sust Energ Rev 115:109361
Sushchik NN, Kalacheva GS, Zhila NO, Gladyshev MI, Volova TG (2003) A temperature dependence of the intra-and extracellular fatty-acid composition of green algae and cyanobacterium. Russ J Plant Physiol 50(3):374–380
Suzuki Y, Takahashi M (1995) Growth response of several diatom species isolated from various environments to temperature. J Phycol 31:880–888
Takagi M, Yoshida T (2006) Effect of salt concentration on intracellular accumulation of lipids and triacylglyceride in marine microalgae Dunaliella cells. J Biosci Bioeng 101(3):223–226
Tang H, Chen M, Garcia MED, Abunasser N, Ng KS, Salley SO (2011) Culture of microalgae Chlorella minutissima for biodiesel feedstock production. Biotechnol Bioeng 108(10):2280–2287
Teoh ML, Chu WL, Phang SM (2010) Effect of temperature change on physiology and biochemistry of algae: a review. MJS 29(2):82–97
Theodorou ME, Elrifi IR, Turpin DH, Plaxton WC (1991) Effects of phosphorus limitation on respiratory metabolism in the green alga Selenastrum minutum. Plant Physiol 95(4):1089–1095
Tsukahara K, Sawayama S (2005) Liquid fuel production using microalgae. J Jpn Pet Inst 48(5):251–259
Tsuzuki M, Ohnuma E, Sato N, Takaku T, Kawaguchi A (1990) Effects of CO2 concentration during growth on fatty acid composition in microalgae. Plant Physiol 93(3):851–856
van Leeuwe MA, Stefels J (1998) Effects of iron and light stress on the biochemical composition of Antarctic Phaeocystis sp. (Prymnesiophyceae). II. Pigment composition. J Phycol 34(3):496–503
Van Wagenen J, Miller TW, Hobbs S, Hook P, Crowe B, Huesemann M (2012) Effects of light and temperature on fatty acid production in Nannochloropsis salina. Energies 5(3):731–740
Varshney P, Mikulic P, Vonshak A, Beardall J, Wangikar PP (2015) Extremophilic micro-algae and their potential contribution in biotechnology. Bioresour Technol 184:363–372
Vazquez-Duhalt R, Arredondo-Vega BO (1991) Haloadaptation of the green alga Botryococcus braunii (race A). Phytochemistry 30(9):2919–2925
Verma M, Mishra V (2020) An introduction to algal biofuels. In: Microbial strategies for techno-economic biofuel production. Springer, Singapore, pp 1–34
Wahidin S, Idris A, Shaleh SRM (2013) The influence of light intensity and photoperiod on the growth and lipid content of microalgae Nannochloropsis sp. Bioresour Technol 129:7–11
Wang ZT, Ullrich N, Joo S, Waffenschmidt S, Goodenough U (2009) Algal lipid bodies: stress induction, purification, and biochemical characterization in wild-type and starchless Chlamydomonas reinhardtii. Eukaryot Cell 8(12):1856–1868
Wei L, Huang X, Huang Z (2015) Temperature effects on lipid properties of microalgae Tetraselmis subcordiformis and Nannochloropsis oculata as biofuel resources. Chin J Oceanol Limnol 33(1):99–106
Wei Z, Wang H, Li X, Zhao Q, Yin Y, Xi L et al (2020) Enhanced biomass and lipid production by co-cultivation of Chlorella vulgaris with Mesorhizobium sangaii under nitrogen limitation. J Appl Phycol 32(1):233–242
Xin L, Hong-Ying H, Ke G, Ying-Xue S (2010) Effects of different nitrogen and phosphorus concentrations on the growth, nutrient uptake, and lipid accumulation of a freshwater microalga Scenedesmus sp. Bioresour Technol 101(14):5494–5500
Xu XQ, Beardall J (1997) Effect of salinity on fatty acid composition of a green microalga from an Antarctic hypersaline lake. Phytochemistry 45(4):655–658
Yang J, Gao H, Glibert PM, Wang Y, Tong M (2017) Rates of nitrogen uptake by cyanobacterially-dominated assemblages in Lake Taihu, China, during late summer. Harmful Algae 65:71–84
Yoo C, Jun SY, Lee JY, Ahn CY, Oh HM (2010) Selection of microalgae for lipid production under high levels carbon dioxide. Bioresour Technol 101(1):S71–S74
Zhang Q, Wang T, Hong Y (2014) Investigation of initial pH effects on growth of an oleaginous microalgae Chlorella sp. HQ for lipid production and nutrient uptake. Water Sci Technol 70(4):712–719
Zhang P, Li Z, Lu L, Xiao Y, Liu J, Guo J, Fang F (2017) Effects of stepwise nitrogen depletion on carotenoid content, fluorescence parameters and the cellular stoichiometry of Chlorella vulgaris. Spectrochim Acta A Mol Biomol Spectrosc 181:30–38
Zheng Y, Li T, Yu X, Bates PD, Dong T, Chen S (2013) High-density fed-batch culture of a thermotolerant microalga Chlorella sorokiniana for biofuel production. Appl Energy 108:281–287
Zhila NO, Kalacheva GS, Volova TG (2011) Effect of salinity on the biochemical composition of the alga Botryococcus braunii Kütz IPPAS H-252. J Appl Phycol 23(1):47–52
Acknowledgement
The authors of the manuscript are thankful to the Indian Institute of Technology (BHU) Varanasi, Varanasi, for extending their technical and financial support.
Conflict of Interest
The authors have declared no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2023 The Author(s), under exclusive license to Springer Nature Singapore Pte Ltd.
About this chapter
Cite this chapter
Verma, M., Mishra, V. (2023). Influence of Culture Conditions on the Microalgal Biomass and Lipid Accumulation. In: Srivastava, N., Mishra, P.K. (eds) Technological Advancement in Algal Biofuels Production. Clean Energy Production Technologies. Springer, Singapore. https://doi.org/10.1007/978-981-19-6806-8_6
Download citation
DOI: https://doi.org/10.1007/978-981-19-6806-8_6
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-19-6805-1
Online ISBN: 978-981-19-6806-8
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)