Abstract
Phenolic compounds are the largest group of plant secondary metabolites. Plants synthesize phenolic compounds utilizing precursor via shikimic pathway through phenylpropanoid pathway. Among phenolic compounds, flavonoids are significant having significant function in various plant physiological processes. Formation of reactive oxygen species (ROS) is one of the strategies used by plants under stress. However, excess production of ROS has negative impact on cellular processes. In this scenario, phenolic compounds assist plants by scavenging ROS as they are excellent antioxidants. Besides their antioxidant ability, they also act as metal chelators and active screeners increasing plant tolerance against extremes of environments. Phenolic compounds act as direct shield and absorb excess light, thus providing protection from UV and damage to biological entities (mainly DNA) from photodamage. Anthocyanins are important group of phenolic compounds having important role in protecting plants from photoinhibition and protecting photosynthetic complex. Moreover, phenolic compounds like most of flavonoids and salicylic acid act as signaling molecules. Flavonoids are important rhizospheric signaling molecules targeting plant beneficial microbes to flourish and benefiting plants, and in legumes, some flavonoids initiate nod factor for nodulation, an important process for plant–rhizobial interaction.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
3.1 Introduction
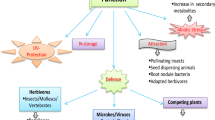
During stress, plants experience reduction in the optimum physiological parameters leading to limited plant growth and development. Plants, in their ecological niches, are exposed to continuous variations in their environment, including excessive salt or water, heavy metals, drought, and high-intensity radiations (Pedrol et al. 2006). Major abiotic stresses involve temperature variations (low or high temperature), water stress (drought or flood), variation in radiations, mineral deficiency, or excess, etc. This induces the production of various reactive oxygen species (ROS) such as hydroxyl radical (OH•) superoxide (O2•–), singlet oxygen (1O2), and hydrogen peroxide (H2O2) that causes oxidative damage to plants and adversely reduces crop quality and productivity (Sachdev et al. 2021). Being sessile, plants maintain certain metabolic and structural adjustments to cope up with stress conditions by changing expression of stress-related genes. This developmental plasticity involves production of different metabolites that help plants to improve their growth under unfavorable environment. Metabolic signaling is the major key to regulate multiple physiological adjustments as a defense response against external stimuli. In this regard, endogenous hormonal signaling triggers the secretion of various primary and secondary metabolites to re-program the physiological activities of cell (Ahmed et al. 2020). Plastic nature of plants in terms of their competitiveness and endurance under various environmental conditions indicates the presence of complex regulatory mechanism. This regulatory system coordinate and control the perception of external stimuli, their transduction, and synthesis of various protective metabolites such as terpenes, alkaloids, and phenolics. Accumulation of these metabolites ensures plant survival and protection against oxidative damage under unfavorable conditions (Kumar et al. 2020a; Tuladhar et al. 2021). Phenols are the key metabolites actively participating in plant defense mechanism. Antioxidant ability of phenols makes them competitive ROS scavenging agent. In addition to this, phenolics also regulate the development of plant-rhizobacterial associations that passively enhance plant immunity and competence under stress conditions. Biology of phenolics in plant system and their interactions with other molecules to regulate plant defense is the key for understanding plant developmental plasticity. Therefore, translation of these molecular signals and thoughtful knowledge of their interactions is necessary. This chapter focuses on the key aspects of abiotic stresses effecting plant biology, their response, and stress-induced activeness of phenolic compounds in plant defense.
3.2 Phenolic Biofactories
3.2.1 Phenolic Compounds
Phenolic compounds (or polyphenols) are the plant special metabolites that play significant part in plant survival and defense processes during environmental variations. They play critical role in plant development by contributing to lignin and pigment biosynthesis, hence providing structural integrity to them. Phenols are characterized as aromatic metabolites with the presence of single or multiple hydroxyl groups, elaborated with amino, methyl, glycosyl, or methoxy groups (Tyagi et al. 2020). Regarding their structure, they range from simple (salicylic acid) to complex polymers (suberin, lignin). The major phenolic groups are anthocyanins, tannins, flavonoids, and hydroxycinnamic acids (HCAs). Flavonoids are extensively distributed and most common among all phenolic compounds with four thousand known molecules having subgroups, isoflavones, flavones, and its derivatives 2,3-dihydroxyflavone (Kumar et al. 2020a; Šamec et al. 2021). Some phenolic compounds are abundantly found among kingdom Plantae such as chlorogenic acid, while others are taxonomically limited to particular genera making them biomarkers for taxonomic studies such as isoflavones which are found exclusively in legumes, and some even are restricted to specific plant organs such as anthocyanins which give bright color to fruits and flowers and protect young leaves from photodamage (Lattanzio 2013). Coumarins, styrylpyrones, stilbenes, and furanocoumarins are the phenolic compounds with restricted taxonomic distribution (Grace 2005). In addition, some polyphenols such as proanthocyanidins and hydrolyzable tannins are restricted only to early developmental stages of plants. They abundantly found only in woody plants (Lattanzio 2013). Hence, presence of phenolic compounds varies according to environmental conditions, species type, organ, and even the developmental stage of plant. This wide distribution of phenolic compounds defines their structural and functional diversity.
3.2.2 Types of Polyphenols
The heterogenous nature and structures of phenols provide various ways of classification. Several classes of phenols based on their fundamental structure and carbon chains are phenols and benzoquinones (C6), catechol melanins (C6)n, phenolic acids (C6–C1), acetophenones and phenylacetic acid (C6–C2), isocoumarins/coumarins, phenylpropenes, chromones and hydroxycinnamic acids (C6–C3), lignans and neolignans (C6–C3)2, lignin (C6–C3)n, naphthoquinones (C6–C4), xanthones (C6–C1–C6), stilbenes and anthraquinones (C6–C2–C6), flavonoids, neoflavanoids and isoflavonoids (C6–C3–C6), bi-/tri-flavonoids (C6–C3–C6)2/3, and condensed tannins (C6–C3–C6)n (Ahmed et al. 2020). Phenols may also be categorized as “performed phenolics” which are usually produced during plant developmental and growth processes and “induced phenolics” which are produced because of external biotic or abiotic stress or during any mechanical or physical injury (Tak and Kumar 2020). As flavonoids are abundant in nature and frequently present in leaves and fruit peel, therefore, phenolics are also categorized as flavonoids and non-flavonoids. Flavonoids are further divided into six subgroups, i.e., flavonones, flavonols (kaempferol, isorhamnetin, myricetin, and quercetin), flavones, anthocyanidins, isoflavones, and flavan-3-ols. Non-flavonoids are divided into tannins, stilbenes, phenolic acids (gallic acid), and lignans. Among them gallic acid is important as it is the precursor for other phenolic compounds like stilbenes and hydrolyzable tannins (Aviles-Gaxiola et al. 2020; de la Rosa et al. 2019). On the other hand, phenols can also be categorized based on their occurrence in nature, i.e., shortly distributed (simple phenols, hydroquinones, and derivatives of benzoic acid), widely distributed (coumarins, flavonoids and its derivatives, and phenolic acids), and polymers (lignin and tannins). Lastly, based on their existence in plants, phenolic substances are classified as “soluble” (mostly low molecular weight tannins, flavonoids, and phenols), and “insoluble” forms (condensed tannins and phenolic acids) (Ahmed et al. 2020).
3.2.3 Biosynthetic Pathways
Various interconnected signals participate in regulating the synthesis of phenolic compounds. These include both plant developmental and environmental signals. MYBs (V-myb myeloblastosis viral oncogene homolog) are the transcription factors (TFs) involve in multiple gene expression including biosynthesis of phenolic compounds (Tyagi et al. 2020). Various MYB TFs are involved in biosynthesis of polyphenols, as activators or repressors. One gene can regulate multiple MYB proteins. Important transcription factors involved in phenylpropanoid pathways are WRKY, MYB, bHLH, WD40, and MADS box proteins (Kołton et al. 2022). Sometimes phenolic biosynthesis is also regulated with plant growth regulators, such as ethylene and abscisic acid (ABA) trigger anthocyanin production (Koyama et al. 2018). Phenolic biosynthesis has long been associated with stress stimuli. Elevated ROS levels, under stress, trigger gene expression of various antioxidants including polyphenols. Under certain environmental conditions or during sensitive developmental stages, plant experience oxidative stress due to reduced activity of ROS scavengers. The increased ROS is due to electrons leakage to O2 from electron transport chain (ETC) in mitochondria and chloroplast or during photorespiration in peroxisomes (Grace 2005). Polyphenols are biogenetically synthesized through shikimate/phenylpropanoid pathway, malonate/polyketide pathway, or mevalonate pathway (Fig. 3.1) (Bhattacharya et al. 2010). Majority of polyphenols (hydroxycinnamic acids HCAs) are synthesized through phenylpropanoid pathways. It involves the synthesis of cinnamic acid from phenylalanine precursor using key enzyme “phenylalanine ammonia lyase” (PAL). Synthesis of cinnamic acid is critical point during secondary metabolic process (Saltveit 2017; Ledesma-Escobar et al. 2019). HCAs also act as precursors for the biosynthesis of other phenolics including flavonoids. Flavonoid biosynthesis uses the intermediate products of both phenylpropanoid and polyketide pathways. Chalcone synthase (CHS) catalyzes the major step that connects phenylpropanoid and flavonoid pathways. Flavonoid pathway is initiated with the condensation of p-coumaroyl-CoA with acetate via malonyl-CoA. This involves the synthesis of various flavonoids such as flavonols, chalcones, anthocyanins, flavones, catechins, and proanthocyanidins (Kołton et al. 2022). Phenolic compounds accumulate and incorporate within cell wall to fight against stress.
Overview of various pathways involve in the biosynthesis of phenolic compounds showing their biosynthetic relations. PAL phenyl ammonia lyase, TAL tyrosin ammonia lyase, ICS isochorismate synthase, 4CL 4-coumarate CoA ligase, C4H cinnamate-4-hydroxylase, CHS chalcone synthase, CH1 chalcone isomerase, 1FS isoflavone synthase, FS flavonol synthase, DFR flavan-3,4-diol, ANS anthocyanidin synthase
3.2.4 Phenolics in Plant Biology: Physiological Impact
Diversity of structural and distributional pattern of polyphenols defines their multifunctional properties. Phenolic compounds play principle role in various physiological processes to improve plant tolerance under sub-optimal conditions. They act as key participant in protecting plants against environmental stresses especially against ultraviolet (UV) radiations. They have antioxidant properties that improve plant adaptability. In addition, polyphenols have versatile functions such as antibiotic, antimicrobial, anti-nutritional activity, metal chelators, and signaling agents, hence provide competitive advantage to plants (Sharma et al. 2019). They greatly influence numerous physiological processes involve in developmental processes for instance synthesis of photosynthetic pigment, seed germination, flowering, senescence, and cell division (Tanase et al. 2019). Phenolic substances also possess significant taxonomic and evolutionary importance besides their key role in various metabolic and physiological processes. Metabolomic studies of various phenolic pathways display evolutionary diversification of plants (Delgoda and Murray 2017). Furthermore, some phenolics have ecological importance as they maintain and modulate fauna diversity. Polyphenols also regulate soil nutrient mineralization by effecting the activity of decomposers. Moreover, phenolic compounds present in root exudates alter soil physiochemical properties and increase nutrient uptake by plants. They increase soil porosity and absorption site which enhance nutrient mobilization. Various phenolic compounds (flavonoids, gallic acid, or ferulic acid) control spore germination, fungal growth, and plant microbial associations (both bacterial and mycorrhizal) (Lattanzio 2013). In addition, polyphenols are also involved in signal transduction and act as signaling molecules (e.g., salicylic acid (SA) and flavonoids) during several plant physiological processes. Flavonoids act as chemoattractant as well as chemorepellent for a variety of rhizopheric microbes and initiate nodulation process. In the same way, SA stimulates root development, induces flowering and stomatal closure, reduces transpiration, regulates geotropism, and inhibits fruit ripening (Mohamed et al. 2020). SA triggers signaling pathways that regulate gene expression of phenylpropanoid biosynthesis-related genes (Fig. 3.1). SA triggers CHS activity, hence, regulating phenolic compounds biosynthesis (Mohamed et al. 2020). Furthermore, SA also interacts with signaling pathways of phytohormones such as ethylene, auxins, and jasmonic acid (Vlot et al. 2009).
3.3 Plant Metabolic Adjustments Under Abiotic Stress
Plants have highly efficient mechanism to sense, response, and adapt according to varying environmental conditions. Regulation of various mechanisms at cellular and molecular level enables plants to tolerate and survive through uncomfortable conditions. Initiation of various stress-induced transcriptional factors regulates complex network of stress-related genes to synthesize proteins involved in various plant defensive mechanisms (Fraire-Velázquez and Balderas-Hernández 2013). The stimulus of abiotic stress received by cell receptors initiates series of various interconnected responses at multiple levels of cellular organization. These responses include signal sensation, transduction, RNA processing, biosynthesis of respective proteins, and its post-translational modifications (PTMs). PTMs play central role in stress signaling as they regulate protein activity at various cellular levels. Stress stimulus is sensed in various cellular compartments including cell membrane, cell wall, cytoplasm, mitochondria, and nucleus. Stress signal is sent to regulatory proteins (Ca2+, protein kinases) that trigger alternative gene transcription and protein translation resulting in the production of modified proteins having key role in stress response (Zhang et al. 2022). Stress encourages ROS production which transduce signal to nucleus via mitogen-activated protein kinases (MAPKs) to initiate stress-related pathways and gene expression (activates TFs including WRKY, MYB, bHLH) leading to expression of stress-related genes including formation of polyphenols (Naing and Kim 2021). However, under certain conditions, overproduction of ROS becomes difficult to control by scavengers leading to oxidative damage. Peroxisomes, mitochondria, and chloroplasts are the primary ROS-producing bodies. ROS causes irreversible oxidation of amino acids, reduces CO2 fixation, and interacts with photosynthetic apparatus. ROS react with chlorophyll and form chlorophyll triple state that rapidly generates singlet oxygen and damage photosynthetic system especially PSII that ultimately reduces photosynthetic efficiency and in longer term, cause cell death (Sharma et al. 2019). Plants have established natural ROS scavenging systems (enzymatic and non-enzymatic) to maintain cellular redox homeostasis and reduce cytotoxic effect of ROS. Polyphenols especially phenolic acid, anthocyanins, and flavonoids play an important role in balancing cellular redox levels (Martinez et al. 2016).
Plant stress response involves re-allocation of resources between growth and defense systems. Metabolic adjustments and phenolic pattern of plants have been defined by resource allocation via dialogue between plant and its environment. Accumulation of secondary metabolites by activation of secondary metabolism during stress conditions is due to shifting of energy from primary to secondary metabolic pathways. Suppression of primary metabolism leads to suppressed primary growth but enable plant to acclimatize to the environment and make them more competent during unfavorable conditions. Carbon flux from primary to secondary metabolic pathways increases secondary metabolite production, and as a result, cells accumulate various metabolites and growth regulators such as jasmonic acid, abscisic acid (ABA), and ethylene (Lattanzio 2013). ABA synthesis is the first defensive strategy used by plants which play central role in stress signal management in plants. It converts adverse environmental stress signals into gene expression and transmits stress stimuli from source to the whole plant for its respective response (Pedrol et al. 2006). ABA also interacts with other stress-related transcriptional factors including those involved in phenolic biosynthesis especially anthocyanin (González-Villagra et al. 2019). Anthocyanins have antioxidant activity and are the key photoprotective agents that protect photosynthetic apparatus (PS II) of plants during radiation stress (Liu et al. 2018). Moreover, stress-induced metabolic switching also leads to the accumulation of low molecular weight osmolytes especially proline. Accumulation of compatible solutes during osmotic stress helps plants to maintain cell turgidity, restore redox metabolism, and protect and stabilize cellular proteins. Proline accumulation transfers energy toward biosynthetic pathways of phenolic compounds through OPP pathway (Lattanzio 2013). Oxidation of NADPH during proline synthesis boosts oxidative pentose phosphate (OPP) pathway activity by providing precursors for the biosynthesis phenolic compounds. Hence, oxidation of NADPH and production of NADP+ during proline synthesis by pentose phosphate pathway cause interaction and facilitation of simultaneous polyphenol biosynthesis (Lattanzio 2013).
3.4 Phenolics Are Strong Antioxidants
Presence of OH group attached to six carbon aromatic ring makes phenols a weak acid. Electron donating ability of phenols makes them strong antioxidants, hence excellent ROS scavengers. Lower electron reduction ability of phenolic radicals and their lesser reactivity than oxygen radicals make them strong scavengers. Most of the plant phenolics have radical scavenging properties; however, flavonoids, HCAs, and anthocyanins have efficient radical scavenging ability. Among flavonoids, the strongest scavenging ability is present in anthocyanidins cyanidin, flavonol quercetin, epigallocatechin gallate, delphinidin, and flavan-3-ols epicatechin gallate. Moreover, phenolics with ortho-dihydroxy structures readily donate electron because of additional OH group at ortho position (catechol structure) as compared to monohydroxy structures, thereby, are least efficient antioxidants (Grace 2005). This additional OH group lowers electron reduction potential by 300–400 mV and increases stability of respective phenoxyl radical. Phenolics with catechol structures are readily oxidized and hence have better scavenging ability. Furthermore, in flavonoids 2,3 double bond and OH group at position 3 in C ring and presence of OH at 3 and 5 positions in A ring enhances antioxidant property. In the same way, chlorogenic acid, gallic acid, and its methyl esters also possess antioxidant property, but salicylic acid do not have significant antioxidant property. Higher molecular phenolics like tannins also possess strong antioxidant ability. Beside strong antioxidants, phenolic compounds directly protect DNA and lipids from ROS, hence acting as shield against oxidative damage. Phenolic compounds mostly flavonoids and HCAs are highly efficient against lipid peroxidation and low-density lipoprotein oxidation as they are active chain breaking antioxidants and metal chelators (Grace 2005). Moreover, flavonoids also oxidize alkyl peroxyl radicals. Hence, flavonoids present near surfaces protect lipids from oxidation. Anthocyanins are the most potent antioxidants with higher abilities to quench free radical species. Anthocyanins are water soluble phenolic compounds that give characteristic colors (blue, purple, or red) to flowers and fruits. Anthocyanins act as antioxidants as well as screening agents which protect plants from visible light and shield photosynthetic apparatus from extreme lights (Liu et al. 2018). The antioxidant ability of anthocyanins mainly based on the extent of hydroxylation at B ring. This hydroxylation increases the antioxidant capacity of anthocyanins. Moreover, glycosylated forms of anthocyanins have reduced metal chelating, electron donating, and delocalizing ability due to which they have reduced radical scavenging ability as compared to aglycone form. Sugar units at 3 and 5 positions lower antioxidant ability (Zhao et al. 2014).
3.5 Plant Phenolics Under Variable Environmental Conditions
3.5.1 Osmotic Stress
Osmotic stress is the most significant among all abiotic stresses affecting plant physiological activities. Water deficiency (drought) and excess (flood) both cause the generation of ROS in plant cells. As a result, synthesis of polyphenols is enhanced due to de novo synthesis of the enzymes involved in phenylpropanoid pathway. The concentration of phenolic compounds may increase up to 10 mM in some cells. Increased activity of PAL, CHS, CH1, and flavanone 3-hydroxylase (F3H) enzymes due to overexpression of TFs is the key reason for aggregation of various phenolic substances, e.g., anthocyanin, flavonol, caffeic acid, ferulic acid, cinnamic acid, quercetin, kaempferol, and asp-coumaric acid in plant cells (Wang et al. 2018). Anthocyanins and other flavonoids accumulate in vacuole, close to ROS production sites and inhibit the chain reaction by deactivating radicals with their OH groups to avoid ROS accumulation. Thereby, reducing stress and maintaining osmotic balance within cell (Zhang et al. 2019; Kumar et al. 2020b), overexpression of key genes involved biosynthesis of anthocyanin such as dihydroflavonol 4-reductase and and MYB TFs such as Myeloblastosis 5A and Myeloblastosis A1 (MybA1) boost anthocyanin UDP-glucose: flavonoid 3-O-glucosyl transferase (UFGT) production (González-Villagra et al. 2019). Anthocyanins act as antioxidants in maintaining cellular oxidative homeostasis to ensure osmotic balance. Thus, there exists a positive association between anthocyanin aggregation and osmotic homeostasis (Waseem et al. 2019; Naing and Kim 2021). Similarly, upregulation of 5-enolpyruvylshikimate-3-phosphate (EPSP) synthase (key enzyme in shikimate pathway) leads to accumulation of catechin, vanillic acid, ferulic acid, chlorogenic acid, gallic acid, rutin, and benzoic acid in some plants under drought (Ghimire et al. 2017). Drought stress induces alteration in various gene expressions involved in biosynthesis of phenolic compounds and their overexpression causing phenolic accumulation under stress that acts as antioxidants and prevents redox damage (Fig. 3.2).
3.5.2 Temperature Stress
Deviation from optimum temperatures (chilling/freezing or high temperature) results in alteration of physiological activities in plants affecting various developmental processes and causes stress. Temperature below freezing point causes freezing injuries and formation of intracellular ice crystals that disrupt cellular activities and create osmotic potential gradient. Similarly, heat stress affects photosynthetic activities and causes oxidative stress (Kumar et al. 2020a; Jan et al. 2021). Also, high temperature causes reduction in enzymatic activities. Thermal interruptions liberate cytosolic ATP into extracellular matrix which is then received by receptors present on plasma membrane of other cells, as a result, increase in cellular Ca2+ concentrations occurs that trigger NADPH oxidase, thus activating ROS production (Jacobo-Velázquez et al. 2011). Hence, temperature stress affects plant photosynthetic activities and causes generation of ROS. Thermal alterations cause increased activity of PAL that accumulate phenolics which get incorporated in plant cell wall (lignin and suberin) and protect plants by inducing tolerance against cold stress (Chalker-Scott and Fuchigami 2018). Cell wall-associated phenolics have great influence in cold stress resistance in plants. Suberin and lignin act as barrier and maintain osmotic potential gradient. Similarly, accumulation of farinose in aerial parts of leaves minimizes formation of ice crystals in plant cell. Hence, change in composition of phenolic content would increase plasma membrane adhesion to cell wall, thus reducing membrane disruption during stress. Coumaric acid and caffeic acid protect microfilaments of cytoskeleton, thus protecting cell from disruption during heat stress (Commisso et al. 2016). Increased accumulation of various other phenolic substances, e.g., chlorogenic acid, anthocyanins, gallic acid, and ferulic acid significantly acts as stress protectants and improves plant resistance against temperature stress (Kumar et al. 2020c). Low temperature causes photoinhibition of photosynthesis by inhibiting assimilatory reactions and affecting electron transport resulting in overexcitation of photosynthetic apparatus, thus affecting plant photosystems especially PSII and causing photoinhibitory damage. Overexpression of PAL, CHS, and upregulation of TFs regulates anthocyanin accumulation in plant epidermis that acts as photobarrier and protects photosynthetic apparatus. Both HCAs and anthocyanins protect plant cell under low temperature by protecting chlorophyll and maintain plant photosynthetic capacity by regulating photosynthetic genes (Grace 2005). Hence, low temperature induces upregulation of anthocyanin-producing TFs. Moreover, anthocyanins also scavenge stress induce ROS to maintain normal cellular oxidation levels. Association between low temperature stress tolerance, ROS scavenging, anthocyanin accumulation, and upregulation of TFs is also found existing (Jiang et al. 2019). In addition, C-repeat binding factor 1 (CBF1) is also involved in upregulation of anthocyanin biosynthetic genes by regulating glycosyltransferases (genes involved in anthocyanin pathways) (Naing and Kim 2021). Similarly, during high temperatures, plants accumulate caffeoylquinic acid (Alegria et al. 2012).
3.5.3 Radiation Stress
Overexposure to radiation (visible light) also leads to photoinhibition. Plants have specialized proteins and photoreceptors to sense the intensity, quality, and duration of light. These photoreceptors perceive light of specific wavelength over a continuous spectral range with the help of chromophore (Aguirre-Becerra et al. 2021). In the same way, exposure to UV has led to photoinduction of DNA and degradation of chlorophyll. Radiation stress influences the light harvesting complex, photosystem II, electron transport chain, and acceptor sites of PSII. Moreover, long-term exposure to high light intensities leads to overproduction of intercellular ROS (Kumar et al. 2020c). Plants have various mechanisms to prevent overexcitation of photosynthetic apparatus. A strong correlation between high light intensity and phenylpropanoid biosynthesis exists. Plants synthesize phenolic compounds in response to oxidative pressure caused by overproduction of ROS. Biosynthesis of phenolic substances and expression of various genes involved in phenylpropanoid pathway have been strongly influenced by intensity and quality of light. Blue light influences the accumulation of malonyl-CoA and coumaroyl CoA that ultimately enhances phenolic production to scavenge ROS. Similarly, red light is involved in the stimulation of anthocyanin production (Liu et al. 2016; Qian et al. 2016). Light stress causes the accumulation of various phenolic compounds, for instance anthocyanins, catechins, HCAs, flavonols, chlorogenic acid, rutin, caffeic acid (Grace 2005). Light-dependent regulation of PAL, CHS, and F3H causes accumulation of anthocyanins and flavonoids. UV-B exposure has led to the upregulation of almost 121 genes involved in phenylpropanoid pathway including PAL and CHS (Rodríguez-Calzada et al. 2019). Flavonoids, anthocyanins, and HCAs have high UV absorbing capacity, hence act as screening agents. Anthocyanins, flavonoids (kaempferol derivatives), phenolic acid esters accumulate in epidermal cells and protect mesophyll from extreme light (Naikoo et al. 2019). Besides this, flavonoids also protect DNA from photodamage. Plant primary and secondary metabolic pathways are coordinated in response to UV stress. UV-B exposure also enhances the expression of primary metabolic genes that are directly related to phenylpropanoid pathways such as that involved in pentose phosphate pathway and shikimate pathway (Aguirre-Becerra et al. 2021).
3.5.4 Salinity Stress
In the presence of salinity, plant cells start accumulating Na+ and Cl− that disturb cellular homeostasis and cause nutritional imbalance due to inhibition of water and nutrient uptake. This imbalance initiates the production of ROS which can act as signaling molecule in maintaining cellular redox mechanism and triggering MYB TFs that regulates anthocyanin synthesis but under severe stress, overproduction of ROS can damage chlorophyll and negatively affect photosynthetic rate. Anthocyanin acts as antioxidant agent as it can absorb and neutralize free radicals and decompose peroxides to limit the damage by ROS. Moreover, anthocyanins have ability to chelate cellular ions, hence protecting the cell from cytotoxicity caused by ionic imbalance (Naing and Kim 2021). Upregulation of key genes (PAL, CHS) associated with anthocyanin biosynthesis is the key factor in salinity-induced anthocyanin accumulation. Beside anthocyanins, the predominant phenolics that accumulate under salinity stress are p-coumaric acid, isoorientin, vitexin, vanillin, rutin, orientin, oleuropein, ferulic acid, and protocatechuic acid (Jamalian et al. 2013; Grzeszczuk et al. 2018). Stress hormones also trigger phenolic compound accumulation under salinity stress. For example, jasmonic acid and its derivatives also indirectly take part in phenolic accumulation in plants by enhancing activity of PAL and other enzymes participating in phenylpropanoid pathway (Lim et al. 2012). In the same way, abscisic acid also influences phenolic accumulation in plants. Ferulic acid provides support and strength to plant cell wall and causes cell elongation (Minh et al. 2016). Furthermore, SA also participates in mitigating salinity stress in plants. SA improves photosynthetic activity and ascorbates and guaiacol peroxidase activities and accumulation of various osmolytes including proline, etc. (Mohamed et al. 2020). Under salinity stress, salicylic acid is also involved in regulation of GST gene expression and enhances antioxidant transcription and activity of enzymes involved in ascorbate-GSH pathway (Li et al. 2013). Moreover, SA also restores membrane potential and prevents salinity-induced ionic flux through GORK channel, thus improving salinity resistance in plants (Jayakannan et al. 2013).
3.5.5 Mineral Stress
Optimum concentrations of various metals are important for normal plant physiological activities; however, at higher concentrations, they negatively affect cellular activities and retard plant growth. This can also limit CO2 fixation, thereby disrupting photosynthetic activities. Metals can also interact with mitochondrial ETC and cause the formation of ROS (Ai et al. 2018). Major metal toxicity includes production of ROS, inactivation of metabolic enzymes, and ionic substitutions (Kumar et al. 2020c). Metal toxicity in plants triggers secondary metabolite production as they have strong antioxidant and metal chelating ability, thus protecting plants from metal stress. Heavy metal stress causes regulation of various anthocyanin biosynthetic genes including F3H, CH1, DFR, CHS; these genes regulate anthocyanin production (Handa et al. 2019). Anthocyanins act as metal chelators, hence protecting plants from metal toxicity. Activation of PAL and CHS is dominant in most cases; however, some other anthocyanin-related genes such as F3H, MYBL2, and TT8 are also the reason behind stress-induced phenolic accumulation (Imtiaz et al. 2018). The predominant phenolic acids accumulating in presence of high concentrations of heavy metal are chlorogenic acid, rutin, cinnamic acid, and epigallocatechin. Moreover, excess Cu also increases various enzymatic activities such as shikimate dehydrogenase, ascorbate peroxidase, cinnamyl alcohol dehydrogenase, and polyphenyl oxidase as a result accumulation of ferulic acid, protocatechuic acid, vanillic acid, chlorogenic acid, p-OH benzoic, p-coumaric acid, and syringic acid is prominent (Aviles-Gaxiola et al. 2020). Similarly, mineral deficiency also causes cellular stress that regulates transcriptional regulatory genes involved in phenylpropanoid pathway. For example, vanillic acid, trans-cinnamic acid, and p-coumaric acid are prominent under K-deficiency; similarly, chlorogenic acid and rutin are dominant phenolics during nitrogen stress. The responsible genes involved in their accumulation are PAL, CHS, and F3H (Aviles-Gaxiola et al. 2020).
3.6 Phenolic Signaling
Phenolic compounds present in root exudates act as chemotactic signal for rhizospheric microbial community. Rhizobacteria can oxidize aromatic compounds released by root exudates. Phenolic acids are alternate carbon source for diazotrophs and serve as precursor for the biosynthesis of phenolic lipids. Phenolic acids are released from emerging roots in leguminous plants during seed germination and favor the soil Rhizobium community to accumulate near roots, thus providing competitive advantage for them (Mandal et al. 2010). Polyphenols, mainly flavonoids, can serve as chemoattractant and promote growth of selected rhizobacteria. Leguminous plants release various phenolic compounds such as flavones, flavonols, isoflavonoids, vanillin that serve as chemoattractant for rhizobia. These signals regulate nod genes and develop legume rhizobial symbiosis. Moreover, phenolic acids also protect growing cells of nodules from oxidative damage during developmental phase. However, some phenolic compounds act as repressor for nod regulation (Mierziak et al. 2014). Phenolic compounds with free OH group interact with legume rhizobia signaling and regulate nodulation factor (nod) to initiate nodule formation. For instance, genistein and daidzein induce nod in Bradyrhizobium japonicum but inhibit in Sinorhizobium meliloti. Similarly, in Rhizobium leguminosarum, naringenin stimulates this factor whereas quercetin represses nod factor (Mandal et al. 2010). Moreover, endogenous phenolics such as p-coumaric acid, 4-hydroxybenzaldehyde, and protocatechuic acid stimulate bacterial IAA (auxin) production efficiency, hence making plant more competent. In addition, caffeic acid, p-coumaric acid, and ferulic acid interact with root formation in concentration-dependent manner and inhibit growth of roots (Chamkhi et al. 2021).
3.7 Conclusion
Phenolic compounds are important for healthy plant activities under variable environmental conditions. Knowledge about various polypropanoid pathways and factors interacting with them is important to develop stress-tolerant plant species. There is a need to study the factors involved in the regulation of gene expression regarding biosynthesis of phenolic compounds. Changing environmental conditions severely affects our agricultural products. Thus, in this case, extensive study of phenolic compounds and their practical application would assist in the development of strong agricultural and horticultural systems by producing stress-tolerant plant species.
References
Aguirre-Becerra H, Vazquez-Hernandez MC, Saenz de la OD et al (2021) Role of stress and defense in plant secondary metabolites production. In: Bioactive natural products for pharmaceutical applications. Springer, Cham, pp 151–195
Ahmed A, Tariq A, Habib S (2020) Interactive biology of auxins and phenolics in plant environment. In: Plant phenolics in sustainable agriculture. Springer, Singapore, pp 117–133
Ai TN, Naing AH, Yun BW et al (2018) Overexpression of RsMYB1 enhances anthocyanin accumulation and heavy metal stress tolerance in transgenic petunia. Front Plant Sci 9:1388
Alegria C, Pinheiro J, Duthoit M et al (2012) Fresh-cut carrot (cv. Nantes) quality as affected by abiotic stress (heat shock and UV-C irradiation) pre-treatments. LWT Food Sci Technol 48(2):197–203
Aviles-Gaxiola S, Olivo-Vázquez G, Cabanillas-Bojórquez LA et al (2020) Plants as biofactories for phenolic compounds. In: Plant phenolics in sustainable agriculture. Springer, Singapore, pp 467–500
Bhattacharya A, Sood P, Citovsky V (2010) The roles of plant phenolics in defence and communication during Agrobacterium and Rhizobium infection. Mol Plant Pathol 11(5):705–719
Chalker-Scott L, Fuchigami LH (2018) The role of phenolic compounds in plant stress responses. In: Low temperature stress physiology in crops. CRC Press, Boca Raton, pp 67–80
Chamkhi I, Benali T, Aanniz T et al (2021) Plant-microbial interaction: the mechanism and the application of microbial elicitor induced secondary metabolites biosynthesis in medicinal plants. Plant Physiol Biochem 167:269–295
Commisso M, Toffali K, Strazzer P et al (2016) Impact of phenylpropanoid compounds on heat stress tolerance in carrot cell cultures. Front Plant Sci 7:1439
de la Rosa LA, Moreno-Escamilla JO, Rodrigo-García J et al (2019) Phenolic compounds. In: Yahia EM, Carrillo-Lopez A (eds) Postharvest physiology and biochemistry of fruits and vegetables. Woodhead Publishing, Duxford, pp 253–271
Delgoda R, Murray J (2017) Evolutionary perspectives on the role of plant secondary metabolites. In: Pharmacognosy. Elsevier, New York, pp 93–100
Fraire-Velázquez S, Balderas-Hernández VE (2013) Abiotic stress in plants and metabolic responses. Abiotic stress—plant responses and applications in agriculture. InTech, Rijeka, pp 25–48
Ghimire BK, Seong ES, Yu CY et al (2017) Evaluation of phenolic compounds and antimicrobial activities in transgenic Codonopsis lanceolata plants via overexpression of the γ-tocopherol methyltransferase (γ-tmt) gene. S Afr J Bot 109:25–33
González-Villagra J, Cohen JD, Reyes-Díaz MM (2019) Abscisic acid is involved in phenolic compounds biosynthesis, mainly anthocyanins, in leaves of Aristotelia chilensis plants (Mol.) subjected to drought stress. Physiol Plant 165(4):855–866
Grace SC (2005) Phenolics as antioxidants. In: Antioxidants and reactive oxygen species in plants. Blackwell, Oxford, pp 141–168
Grzeszczuk M, Salachna P, Meller E (2018) Changes in photosynthetic pigments, total phenolic content, and antioxidant activity of Salvia coccinea Buc’hoz ex Etl. induced by exogenous salicylic acid and soil salinity. Molecules 23(6):1296–1307
Handa N, Kohli SK, Sharma A et al (2019) Selenium modulates dynamics of antioxidative defence expression, photosynthetic attributes and secondary metabolites to mitigate chromium toxicity in Brassica juncea L. plants. Environ Exp Bot 161:180–192
Imtiaz M, Mushtaq MA, Nawaz MA et al (2018) Physiological and anthocyanin biosynthesis genes response induced by vanadium stress in mustard genotypes with distinct photosynthetic activity. Environ Toxicol Pharmacol 62:20–29
Jacobo-Velázquez DA, Martínez-Hernández GB, Rodríguez SDC et al (2011) Plants as biofactories: physiological role of reactive oxygen species on the accumulation of phenolic antioxidants in carrot tissue under wounding and hyperoxia stress. J Agric Food Chem 59(12):6583–6593
Jamalian S, Gholami M, Esna-Ashari M (2013) Abscisic acid-mediated leaf phenolic compounds, plant growth and yield is strawberry under different salt stress regimes. Theor Exp Plant Phys 25(4):291–299
Jan R, Asaf S, Numan M et al (2021) Plant secondary metabolite biosynthesis and transcriptional regulation in response to biotic and abiotic stress conditions. Agronomy 11(5):968
Jayakannan M, Bose J, Babourina O et al (2013) Salicylic acid improves salinity tolerance in Arabidopsis by restoring membrane potential and preventing salt-induced K+ loss via a GORK channel. J Exp Bot 64:2255–2268
Jiang L, Tian X, Li S et al (2019) The AabHLH35 transcription factor identified from Anthurium andraeanum is involved in cold and drought tolerance. Plants 8:216
Kołton A, Długosz-Grochowska O, Wojciechowska R et al (2022) Biosynthesis regulation of folates and phenols in plants. Sci Hortic 291:110561
Koyama R, Roberto SR, De Souza RT et al (2018) Exogenous abscisic acid promotes anthocyanin biosynthesis and increased expression of flavonoid synthesis genes in Vitis vinifera × Vitis labrusca table grapes in a subtropical region. Front Plant Sci 9:323
Kumar S, Abedin MM, Singh AK et al (2020a) Role of phenolic compounds in plant-defensive mechanisms. In: Plant phenolics in sustainable agriculture. Springer, Singapore, pp 517–532
Kumar S, Bhushan B, Wakchaure GC et al (2020b) Plant phenolics under water-deficit conditions: Biosynthesis, accumulation, and physiological roles in water stress alleviation. In: Plant phenolics in sustainable agriculture. Springer, Singapore, pp 451–465
Kumar M, Tak Y, Potkule J, Choyal P et al (2020c) Phenolics as plant protective companion against abiotic stress. In: Plant phenolics in sustainable agriculture. Springer, Singapore, pp 277–308
Lattanzio V (2013) Phenolic compounds: introduction. Nat Prod 50:1543–1580
Ledesma-Escobar CA, Priego-Capote F, Luque de Castro MD (2019) Relevance and analysis of Citrus flavonoids. In: Polyphenols in plants. Elsevier, London, pp 133–150
Li G, Peng X, Wei L et al (2013) Salicylic acid increases the contents of glutathione and ascorbate and temporally regulates the related gene expression in salt-stressed wheat seedlings. Gene 529:321–325
Lim JH, Park KJ, Kim BK et al (2012) Effect of salinity stress on phenolic compounds and carotenoids in buckwheat (Fagopyrum esculentum M.) sprout. Food Chem 135(3):1065–1070
Liu H, Chen Y, Hu T et al (2016) The influence of light-emitting diodes on the phenolic compounds and antioxidant activities in pea sprouts. J Funct Foods 25:459–465
Liu Y, Tikunov Y, Schouten RE et al (2018) Anthocyanin biosynthesis and degradation mechanisms in Solanaceous vegetables: a review. Front Chem 6:52
Mandal SM, Chakraborty D, Dey S et al (2010) Phenolic acids act as signaling molecules in plant-microbe symbioses. Plant Signal Behav 5(4):359–368
Martinez V, Mestre TC, Rubio F et al (2016) Accumulation of flavanols’ over hydroxycinnamic acids favors oxidative damage protection under abiotic stress. Front Plant Sci 7:838
Mierziak J, Kostyn K, Kulma A et al (2014) Flavonoids as important molecules of plant interactions with the environment. Molecules 19(10):16240–16265
Minh L, Khang D, Ha PTT et al (2016) Effects of salinity stress on growth and phenolics of rice (Oryza sativa L.). Int Lett Nat Sci 57:1–10
Mohamed HI, El-Shazly HH, Badr A et al (2020) Role of salicylic acid in biotic and abiotic stress tolerance in plants. In: Plant phenolics in sustainable agriculture. Springer, Singapore, pp 533–554
Naikoo MI, Dar MI, Raghib F et al (2019) Role and regulation of plants phenolics in abiotic stress tolerance: an overview. In: Plant signaling molecules. Woodhead Publishing, Duxford, pp 157–168
Naing AH, Kim CK (2021) Abiotic stress-induced anthocyanins in plants: their role in tolerance to abiotic stresses. Physiol Plant 172(3):1711–1723
Pedrol N, González L, Reigosa MJ (2006) Allelopathy and abiotic stress. In: Allelopathy. Springer, Dordrecht, pp 171–209
Qian H, Liu T, Deng M et al (2016) Effects of light quality on main health-promoting compounds and antioxidant capacity of Chinese kale sprouts. Food Chem 196:1232–1238
Rodríguez-Calzada T, Qian M, Strid Å et al (2019) Effect of UV-B radiation on morphology, phenolic compound production, gene expression, and subsequent drought stress responses in chili pepper (Capsicum annuum L.). Plant Physiol Biochem 134:94–102
Sachdev S, Ansari SA, Ansari MI et al (2021) Abiotic stress and reactive oxygen species: generation, signaling, and defense mechanisms. Antioxidants 10(2):277
Saltveit ME (2017) Synthesis and metabolism of phenolic compounds. In: Fruit and vegetable phytochemicals. Wiley-Blackwell, Ames, pp 89–100
Šamec D, Karalija E, Šola I et al (2021) The role of polyphenols in abiotic stress response: the influence of molecular structure. Plants 10(1):118
Sharma A, Shahzad B, Rehman A et al (2019) Response of phenylpropanoid pathway and the role of polyphenols in plants under abiotic stress. Molecules 24(13):2452
Tak Y, Kumar M (2020) Phenolics: a key defence secondary metabolite to counter biotic stress. In: Plant phenolics in sustainable agriculture. Springer, Singapore, pp 309–329
Tanase C, Bujor OC, Popa VI et al (2019) Phenolic natural compounds and their influence on physiological processes in plants. In: Watson RR (ed) Polyphenols in plants, 2nd edn. Academic Press, Cambridge, MA, pp 45–58
Tuladhar P, Sasidharan S, Saudagar P (2021) Role of phenols and polyphenols in plant defense response to biotic and abiotic stresses. In: Biocontrol agents and secondary metabolites. Woodhead Publishing, Duxford, pp 419–441
Tyagi K, Shukla P, Rohela GK (2020) Plant phenolics: their biosynthesis, regulation, evolutionary significance, and role in senescence. In: Plant phenolics in sustainable agriculture. Springer, Singapore, pp 431–449
Vlot AC, Dempsey MA, Klessig DF (2009) Salicylic acid, a multifaceted hormone to combat disease. Annu Rev Phytopathol 47:177–206
Wang F, Ren G, Li F et al (2018) A chalcone synthase gene AeCHS from Abelmoschus esculentus regulates flavonoid accumulation and abiotic stress tolerance in transgenic Arabidopsis. Acta Physiol Plant 40:97
Waseem M, Rong X, Li Z et al (2019) Dissecting the role of a basic helix-loop-helix transcription factor, SlbHLH22, under salt and drought stresses in transgenic Solanum lycopersicum L. Front Plant Sci 10:734
Zhang Q, Zhai J, Shao L et al (2019) Accumulation of anthocyanins: an adaptation strategy of Mikania micrantha to low temperature in winter. Front Plant Sci 10:104
Zhang H, Zhu J, Gong Z et al (2022) Abiotic stress responses in plants. Nat Rev Genet 23:104–119
Zhao CL, Chen ZJ, Bai XS et al (2014) Structure-activity relationships of anthocyanidin glycosylation. Mol Divers 18:687–700
Author information
Authors and Affiliations
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2023 The Author(s), under exclusive license to Springer Nature Singapore Pte Ltd.
About this chapter
Cite this chapter
Tariq, A., Ahmed, A. (2023). Genetic Basis of Phenolics in Abiotic Stress Management. In: Lone, R., Khan, S., Mohammed Al-Sadi, A. (eds) Plant Phenolics in Abiotic Stress Management. Springer, Singapore. https://doi.org/10.1007/978-981-19-6426-8_3
Download citation
DOI: https://doi.org/10.1007/978-981-19-6426-8_3
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-19-6425-1
Online ISBN: 978-981-19-6426-8
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)