Abstract
Chitosan is a bio-functional polysaccharide that has a great potential for applications in various fields owing to its chemical functional groups which can be easily modified to achieve specific goals. Chitosan-based nanomaterials are gaining immense interest from researchers due to their versatile physicochemical and biological properties. In the present chapter, we give a complete overview of the preparation strategies of chitosan nanoparticles, including both novel and green methods. Moreover, we have systematically summarized the modification strategies of chitosan for improving their water solubility, biocompatibility, mechanical properties, and antimicrobial activity, which will help the researchers pick the most appropriate strategy for its particular application.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
1 Introduction
The research interest in natural polymers has been rising for the last three decades. The worldwide market for chitosan derivatives has grown at a rate of approximately 6.5% approximately over a period of 5 years, and in 2024, it is anticipated to reach 53 million USD, owing to the increasing investments in newer drug developments, applications in the biomedical field, as well as applications like detoxification of water and wastewater. There is also a growing interest in the use of bio-degradable chitosan products such as fertilizers. Key areas of interest include physical moderation of polysaccharides to increase their applications in the mechanisms of biological activity of the enzymatic methods, polymers, chemical, and products of their physical, enzymatic, chemical, or degradation; and the molecular and biochemical characterization of chitosanolytic and chitinolytic enzymes synthesized by numerous organisms. Chitin deacetylases can be used to bio-convert chitin to chitosan. The aforementioned enzymatic reaction has many advantages over the traditional process used in the conversion, namely, the production of chitosan with the desired degree of deacetylation and higher molecular weight. Chitosanases and chitinases are two other enzymes involved in chitosan and chitin conversion. The various functional groups of chitosan can be moderated using a large number of ligands. The amino group functional group within ligands can be used in a variety of chemical reactions, including metal chelation, sulfonation, alkylation, carboxymethylation, grafting acetylation, quaternization, and so on. Hydroxyethylation, carboxymethylation, sulfonation, and phosphorylation can also be used to modify hydroxyl groups. A plethora of effective strategies for chitosan and its derivatives have been created in order to broaden their applications by increasing their antimicrobial activity and water solubility. Betaine, nitric oxide (NO) releasing donors, a quaternary phosphonium salt, and metal ions, for example, have been added to chitosan derivatives to improve their antimicrobial activity. Various solid materials with good adsorptive properties have been used to improve the heat resistance, strength, and adsorptive activity of chitosan. Chitosan in combination with activated carbon performs well in the removal of some inorganic and metallic pollutants from an aqueous medium. This chapter aims to present recent research on a wide range of chitosan modification strategies [30].
2 Structures and Properties of Chitosan
2.1 Structure
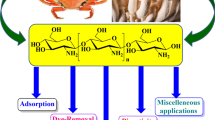
Chitosan’s structure (Fig. 1) is very similar to that of cellulose, which is made up of hundreds to thousands of –(1-4) linked D-glucose units [57]. The hydroxyl group at the C-2 position of cellulose has been replaced by an acetamide group in the chitosan structure. Chitosan, also known as –(1-4) linked 2-amino-2-deoxy—D-glucopyranose, is an N-deacetylated chitin derivative obtained by converting the acetamide groups into primary amino groups [2].
However, chitin deacetylation is never complete, so some chitosan or deacetylated chitin still contains acetamide groups. Chitosan and chitin both contain 5–8% nitrogen, in the form of acetylated amine groups in chitin and primary aliphatic amine groups in chitosan [11] On each repeat unit of chitosan, there are primary and secondary hydroxyl groups, as well as an amine group on each deacetylated unit. These reactive groups can easily change the physical and mechanical properties of chitosan [26].
The presence of amine groups in chitosan is advantageous because it allows for various biological functions as well as the use of modification reactions [3]. Chitosan’s excellent properties, such as biocompatibility, biodegradability, bioactivity, non-toxicity, and good absorption, make it an excellent alternative to synthetic polymers [12, 67].
2.2 Physiochemical Properties
Chitosan is well-known for its chelation, solubility, viscosity, mucoadhesive nature, film formation, polyoxysalt formation, and polyelectrolyte behavior. The unbranched and linear forms of chitosan have high viscosity and can be customized by adjusting the deacetylation conditions. Chitosan is distinguished by its low crystalline region content and a high degree of deacetylation. Several factors influence the physicochemical properties of chitosan, including the degree of deacetylation (DD), crystallinity, MW, and degradation methods [19]. Commercial chitosan is classified into two types based on its MW: high MW chitosan and low MW chitosan. High MW chitosan has a molecular weight (MW) of 190–375 kDa and a DD of >75%, whereas low MW chitosan has a MW of 20–190 kDa and a DD of 75%. It was discovered that the rate of chitosan degradation is inversely proportional to DD and is affected by the order and distribution of acetyl groups. Every glycosidic residue contains three reactive positions, one amino group, and two hydroxyl groups. The amino group is very important because it is responsible for the cationic nature of chitosan as well as the regulation of its various physiochemical properties. It is also pH sensitive. Chitosan dissolves and forms soluble cationic polysaccharides due to the pH-responsive amino groups protonated at lower pH. At a pH greater than 6, the amino group is deprotonated, rendering chitosan insoluble. Its solubility is also affected by acetyl group positioning along the chain, deacetylation methods, and ionic strength. Chitosan exhibits chelating properties for several metal ions at an acidic pH, which occurs at a pH = 7 or by electrostatic attraction on protonated ionic groups.
2.3 Biological Properties
According to studies, chitosan is a non-toxic, biocompatible, and biodegradable polymer [4, 36, 62]. Chitosan and its derivatives showcase exceptional biological properties like anti-inflammatory [10, 37], anti-tumor, anti-bacterial [23, 72], anti-fungal [72], hemostatic [76], and analgesic [5, 76]. Chitosan has a powerful anti-bacterial effect on a wide variety of pathogenic bacteria due to its cationic nature, which allows negatively charged proteins and lipids in the bacterial cell wall to interact. Chitosan diffusion into the cell membrane causes membrane permeability to expand and disrupt, resulting in cytoplasmic portion leakage and bacterial cell death. Various in-vitro experiments revealed that chitosan's antibacterial function may be due to its DNA binding capacity. When chitosan comes into contact with bacteria nuclei, it binds to DNA and prevents mRNA synthesis [17, 38].
Chitosan’s antifungal properties are due to an electrostatic reaction with the negatively charged phospholipids in the cell membrane. Chitosan can penetrate the cell and prevent DNA/RNA synthesis, resulting in cell death when the cell membrane is disrupted. The DD and MW play an important role in regulating chitosan’s antifungal properties. Chitosan’s antifungal effect is generally enhanced when the DD is higher and the MW is lower [71]. Chitosan has anti-inflammatory properties because it inhibits the release of interleukin-8 (IL-8) and tumor necrosis factor (TNF) from mast cells [53]. Chitosan is superior to NSAIDs (the conventional medications for various inflammatory conditions). It has no gastric side effects because the free amino groups form a defensive shield over the stomach. Chitosan can also be used to heal connective tissues. When chitosan is acid hydrolyzed, glucosamine monosaccharides are formed, which form the proteoglycan structural units of connective tissues and cartilage, assisting in tissue repair. Chitosan derivatives with anti-tumor activity are known as chit oligosaccharides. According to some researchers, the anti-tumor effects were caused by the increased activity of natural killer lymphocytes. Chit oligosaccharides are thought to cause T-cell differentiation and proliferation by stimulating lymphocyte-activating factors. Some research suggests that chit oligosaccharides effectively boost immune responses and modulate the functions of immunocompetent cells [47]. Chitosan (poly-N-acetyl glucosamine) exhibits a hemostatic effect by accelerating erythrocyte accumulation [63]. Chitosan’s positive charge promotes erythrocyte adhesion, fibrinogen absorption, and platelet adhesion and activation. Chitosan’s hemostatic property is due to its polycationic content and non-specific plasma membrane binding [73]. Several in-vitro studies have revealed that the main analgesic effect of chitosan is achieved by lowering the concentration of inflammatory mediators (bradykinin) at the site of the injury. It also absorbs protons released at the site of inflammation to control pain [50].
3 Some Common Chitosan Derivatives
-
A.
Mono-Carboxymethylated Chitosan (MCC)
MCC is a polyampholyte capable of forming viscous-elastic gels with anionic macromolecules at neutral pH or in aqueous environments. MCC appears to be less potent than the quaternized derivative of chitosan. It improves the permeation and absorption of low molecular-weight heparin (LMWH; an anionic polysaccharide) across intestinal epithelial cells. Because they cause cell membrane damage, chitosan derivatives have no effect on the viability of intestinal epithelial cells. Permeation increases due to an increase in the number of pores formed in the cell membrane, and there is no need to alter epithelial cell viability [29, 69].
-
B.
N-Succinyl Chitosan
It is made by inserting succinyl groups into the chitosan terminals of glucosamine units. In a succinyl chitosan molecule, –NH3+ and –COO− form polyionic complexes. At various pH levels, this derivative is water-soluble [60]. N-succinyl-chitosan exhibits distinct properties in vivo and in vitro, including low toxicity, biocompatibility, and long-term retention in the body. N-succinyl-chitosan is useful as a drug carrier because it can be conjugated with various drugs to avoid complications during chemotherapy [29, 32, 33, 51, 65].
-
C.
N-Acetylated Chitosan
N-acetylated chitosan is used as a gene delivery carrier to overcome the gastrointestinal tract’s morphological and physiological barriers to target gene expression. Gene delivery to the intestine via N-acetylated chitosan is more efficient than chitosan alone. This finding is especially significant in the duodenum, where the LacZ gene is most effectively expressed using N-acetylated chitosan. The IL-10 gene is also successfully transferred to the intestines when mixed with N-acetyl chitosan. As a result, plasmid DNA can be delivered to the intestines orally using N-acetylated chitosan as a carrier. As a result, we can create a dietary dose system to deliver a DNA vaccine for the treatment of gastrointestinal diseases [28, 29].
-
D.
N, N-Di carboxymethyl Chitosan
This derivative is produced by the alkylation of chitosan with monochloroacetic acid at 90 °C and pH 8–8.5. This water-soluble derivative is a chelating agent that can be used to treat osteogenesis and other chelating applications [25, 29].
-
E.
Thiolated Chitosan Conjugate
This chitosan derivative was created by covalently attaching isopropyl-S-acetylthioacetimidate to chitosan. This derivative has in situ gelling properties, making it a promising novel tool for a variety of drug delivery systems [15, 29].
-
F.
Polyethylene Glycol-Crosslinked N-Methylene Phosphonic Chitosan (NMPC)
By reductive animation, NMPC is modified with polyethylene glycol-aldehyde (PEG-CHO) of varying molecular weight. The cross-linking of NMPC with PEG-COH chains increases hygroscopicity and water swelling. This cross-linked NMPC derivative is suitable as a medical material item due to its film-forming capacity and swelling properties [15, 29].
-
G.
Graft-Copolymerization of Chitosan
Radical polymerization is used to create this derivative with 4-(6-methacryloxyhexyloxy)-4′-nitrobiphenyl. Graft-copolymerization is carried out in a homogeneous environment using Azobisisobutyronitrile (AIBN) as an initiator and 2% acetic acid as a solvent [13, 13].
-
H.
N-lauryl-Carboxymethyl-Chitosan
The hydrophobic moieties are provided by chitosan with lauryl groups attached to amino groups, and the hydrophilic moieties are provided by carboxymethyl groups attached to hydroxy groups (N lauryl-carboxymethyl-chitosan (LCC)). Taxol is found to solubilize in LCC by forming micelles with particle sizes less than 100 nm. In vitro hemolysis testing revealed that LCC is less likely to cause membrane damage than polysorbate 80 as an intravenous surfactant [29, 42].
-
I.
Galactosylated, Chitosan-Graft-Poly (Vinyl Pyrrolidone)
GCPVP (galactosylated chitosan-graft-poly (vinyl pyrrolidone)) has been identified as a potential hepatocyte-targeting gene carrier. GCPVP alone and GCPVP/DNA complex have negligible cytotoxicity regardless of GCPVP concentration or charge ratio, but GCPVP/DNA complex has a slightly cytotoxic effect on HepG2 cells when a higher charge ratio and Ca2+ are used. It is established using confocal laser scanning microscopy that the ASGPR of hepatocytes and endocytosis by an interaction between galactose ligands of GCPVP is the major route of transfection of GCPVP/F plasmid complexes [29, 48].
-
J.
Chitosan–Glutathione (GSH) Conjugate
This derivative improves the mucoadhesive and permeation properties of chitosan. The novel thiolated chitosan appears to be a promising multifunctional excipient for various drug delivery systems due to the strong permeation enhancing effect of the chitosan–GSH conjugate/GSH system and the improved cohesive and mucoadhesive properties [29, 44].
4 Methods of Synthesis of Chitosan Nanoparticles
In 1994, Ohya et al., for the first time, described the classification of chitosan NPs for the systemic administration of a chemotherapeutic drug [49]. Following that, the chitosan NPs have been extensively explored by the researchers leading to the development of different methods for their preparation. Various important preparation strategies are mentioned in Fig. 2, and their advantages and drawbacks are depicted in Fig. 3. A brief description of these methods is given below.
4.1 Emulsification and Cross-linking
It is the first method documented in the literature for the preparation of chitosan NPs in which the amino group of chitosan and the aldehyde group of the crosslinking agent has been employed. In this method, an emulsion and an oil phase are prepared. The emulsion consists of an aqueous solution of chitosan while the oil phase comprises span 80 as a stabilizer, toluene, and glutaraldehyde as a crosslinker [27, 49]. On intense mixing of these two phases, the droplets are formed after crosslinking which forms the basis of NPs. The NPs can be separated from the emulsion by centrifugation, multiple washings, and vacuum drying. This approach is, however, no longer employed because of the evident toxicity of glutaraldehyde [75].
4.2 Reversed Micelles Method
It is another method based on covalent crosslinking in which the production of chitosan NPs is assisted by water-in-oil reverse micelle structures (Fig. 4). This method resolved the inconveniences associated with the conventional methods based on glutaraldehyde as a non-harmful solvent and a crosslinker are employed in this strategy [20, 31]. The aqueous phase consisting of chitosan and glutaraldehyde is mixed with the organic phase which comprises an organic solvent and a lipophilic surfactant. The chitosan NPs are formed via crosslinking within the chitosan-containing core of the micelle.
4.3 Precipitation-Based Methods
NPs formed by precipitation methods are generally larger than 600–800 nm. Two precipitation-based methods which have been described in the literature are:
-
The phase inversion precipitation method: This method is based on the combination of emulsification and precipitation. In the presence of a stabilizer, an oil-in-water emulsion is prepared using an organic phase and an aqueous solution of chitosan. After the application of high-pressure homogenization, the emulsion is separated from the methylene chloride by evaporation, causing acetone to diffuse out of the droplets and NPs to precipitate simultaneously [75].
-
The emulsion-droplet coalescence method: In this process, the precipitation of NPs is induced by the coalescence of two water-in-oil emulsions. The continuous phase for two emulsions, one with chitosan and another with NaOH, is prepared by mixing liquid paraffin and sorbitan sesquiloleate. The chitosan-containing emulsion is prepared by applying high-speed homogenization. Following the mixing of two emulsions, NaOH diffuses into the ultrafine droplets which lower the solubility of chitosan, inducing NP formation and precipitation [24].
4.4 Ionic Gelation
It is one of the most preferred strategies for the preparation of chitosan NPs primarily because it does not involve the use of toxic crosslinkers or solvents. This method was first described by Calvo et al. in 1997 [8]. This technique follows the principle of ionic crosslinking, which occurs when the oppositely charged groups are present. Firstly, chitosan is added to the aqueous solution of an acid (generally acetic acid) followed by the addition of an aqueous solution of sodium tripolyphosphate (TPP) under intense stirring [75]. The diffusion of anionic molecules and the cationic chitosan molecules takes place leading to crosslinking and ultimately the formation of NPs (Fig. 5). Although various anionic crosslinkers like glutaraldehyde can be used to synthesize chitosan NPs, TPP is preferred because of its biocompatibility and biodegradability [59].
4.5 Self-assembly
This method is extensively employed for the formation of NPs. It is based on numerous interactions which take place at the same time. The nature of interactions can be any of the following [56, 78]:
-
Electrostatic
-
Hydrophobic
-
Hydrogen bonding
-
Van der Waals forces
The self-assembly approach may either involve complex formation between chitosan and the natural anionic molecules or the modification of hydrophobicity of chitosan via grafting. NPs produced by this approach is highly suitable for the encapsulation of lipophilic and hydrophilic drugs [56].
4.6 Top-Down Approach
In this approach, two steps are involved in the preparation of nanoparticles:
-
(i)
Acid hydrolysis: Acid hydrolysis of chitin is carried out in the presence of a strong acid like HCl to form chitin nanocrystals.
-
(ii)
Deacetylation: This step involves treatment with alkali (NaOH) to form chitosan NPs, which are basically the chitin NPs with a deacetylation level of more than 60% [74].
The formation of chitin NPs via the top-down method is illustrated in Fig. 6.
5 Green Synthesis of Chitosan Nanoparticles
The biological/green methods for the extraction of chitosan are becoming highly prevalent due to the various drawbacks of chemical extraction techniques such as [64]:
-
Alteration of the physico-chemical properties of chitin
-
High cost of purification processes
-
Presence of some chemicals in the wastewater effluents
In biological synthesis, enzymes and micro-organisms are employed for the extraction of chitin and the recovery of chitosan. The various advantages of the biological extraction of chitin are highlighted in Fig. 7. Khanafari and co-workers compared the chemical and biological methods of extraction of chitin from shrimp shells and showed the superiority of the biological method over the chemical one because the structural integrity of chitin was preserved when it was extracted through the biological method [35]. In a study, the extraction of chitin from shrimp shells and fungi was investigated by Teng et al. in which they used a one-pot fermentation process where the proteins were hydrolyzed into amino acids by fungal proteases [68]. Younes et al. extensively reviewed the methods of preparation of chitosan from marine sources and their future outlooks [79].
Spray drying is one of the green preparation routes for the preparation of chitosan NPs. In this method, chitosan is dissolved in the aqueous acetic acid and the resulting solution is passed through a nozzle, keeping the temperature range between 120 and 150 °C, leading to the formation of chitosan NPs. Spray drying can also be used to obtain magnetic chitosan NPs [22].
Supercritical-CO2-assisted solubilization and atomization (SCASA) is a green method in which only water and CO2 are used during the preparation process. This technique is devoid of any acid or harmful solvents. After about 48 h of the dissolution of CO2 in water, the chitosan solution is fed to a fluidized bed, and NPs are formed which are collected by a filter placed on the fluidized bed [75]. The publications associated with the “green synthesis” of chitin/chitosan-based nanomaterials in the literature are very few. As discussed earlier, chitosan nanoparticles are frequently prepared by the ionic gelation method in which the ionic crosslinking takes place between chitosan and TPP. Recently, Gadkari et al. reported the green synthesis of antimicrobial chitosan NPs, which were prepared by chemical crosslinking between chitosan and a cinnamaldehyde, an eco-friendly bactericidal agent [18]. Purified chitin films and nano fibres with high molecular weight were successfully obtained by Qin et al. by employing eco-friendly ionic liquids [55]. Green synthesis of Ag-Ch nanocomposites was reported in which chitosan served as a reducing agent as well as a stabilizing agent, using NaOH as an accelerator. The as-prepared nanocomposite was shown to have antimicrobial activity against E. coli and S. aureus bacteria [66].
6 Modification Strategies of Chitosan
There are different scopes of modifications of chitosan that are depicted in Fig. 8.
6.1 Chitosan Modification to Produce Functional Derivatives
There are various types of chitosan derivatives that are produced by modification and are presented in Fig. 9.
6.1.1 Quaternized Chitosan Derivatives
According to numerous publications, it is possible to modify the positive (NH3+) charge of chitosan in order to make it soluble over a wide pH range and in a neutral or a little alkaline medium. This is one method for increasing the solubility of chitosan in water. At pH 6.5, chitosan is positively charged, whereas quaternized chitosan remains permanently positively charged at pH > 6.5. Under basic conditions, a quaternization reaction occurs between chitosan and alkyl iodide. The most well-known quaternized chitosan is N,N,N-trimethyl chitosan chloride (TMC), which has been described for a variety of applications. TMC is produced through two consecutive reactions. The first involves N-methyl-2-pyrrolidinone (NMP), which is used as a solvent in alkaline conditions (NaOH) in the presence of methyl iodide and chitosan, and the second involves the replacement of iodide ions with chloride ions using anionic exchange resin. By varying the carbon length of alkyl halides, different types of quaternized chitosan can be easily obtained [7].
6.1.2 N-Acyl Chitosan Derivatives
Chitosan becomes aquaphobic through the N-Acylation process, which involves implanting various fatty acids. The reaction is an addition of amide to fatty acid –COOH groups and chitosan –NH2 groups. Acyl halide or acid anhydride is the chemical reagents used in N-Acylation. This acylation is typically carried out in pyridine/chloroform, pyridine, and methanol/acetic acid/water. Nonetheless, because the chitosan repeating unit contains two reactive –hydroxyl groups, this reaction can produce O-alkyl chitosan. Many researchers recommend replacement with trityl groups, in place of chitosan’s primary –OH groups to avoid O-acylation. Because of the formation of chitosan chloroacyl, this process improves the N-Acylation step. N-Acyl chitosan has been produced using a variety of acid anhydrides [7].
6.1.3 Oxy-Chitosan Derivatives
Many researchers have looked into the production of chitouronic acid sodium (carboxylated chitin or chitosan) which is soluble in water using TEMPO-an organic catalyst used in the oxidation of –OH functions into –CHO in NaOCl and NaBr conditions. TEMPO is well-known for its use in regioselectively oxidizing the primary hydroxyl groups of various polysaccharides [7]. TEMPO is used to make oxy-chitosan derivatives, specifically 6-oxy-chitosan. Chitouronic sodium salts are typically made from shrimp or fungal cell chitin that has been pre-treated (chemically or enzymatically). A new class of carboxylated chitosan with high biocompatibility on human keratocytes was produced from Trichoderma and Aspergillus fungal biomass. C6-oxy-chitosan, a novel bioactive derivative, has shown to be efficient against activity Leishmania. Recently, an environmentally friendly process has been developed for the C6 oxidation of chitosan using a TEMPO system to produce chitosan soluble in water has been developed [7].
6.1.4 Chitosan’s Cross-linked Derivatives
The cross-linking of chitosan requires the use of very particular chemical agents to get the chains linked together, which ultimately results in a 3-D macromolecular network. Chitosan is cross-linked in the presence of –CHO derivates, such as glyoxal, glutaraldehyde (GTA), or formalin with covalent bonds, to produce chitosan-based hydrogels. The cross-linking reaction with chitosan involves the formation of a Schiff base (imine). The most researched cross-linking agent is GTA [7]. It is cheap, readily available, and synthetic. In the presence of labile hydrogen, the hired response is a condensation response among the aldehyde group and a primary amine group from the chitosan chain. However, GTA is toxic, and natural GTA substitutes are being investigated for use in the production of chitosan hydrogel. Figure 3 depicts the chitosan cross-linking reactions. Citric acid was used as a cross-linking agent in the preparation of chitosan/polyvinyl alcohol (PVA) membranes. This cross-linking strategy was investigated in order to create biomaterials for hemodialysis membranes [7]. Cross-linking citric acid and chitosan was expected to incorporate carboxylate groups (COO) into biomaterials, increasing the bioactive sites on the chitosan membrane for biomolecule transport (urea, creatinine, etc.). PVA was used to improve the crosslinked chitosan membrane’s mechanical efficiency and hydrophobicity. Figure 10 depicts the main cross-linking chitosan strategies.
6.1.5 Chitosan with Low Molecular Weight (LMW) and Chito Oligosaccharide (COS)
Because of its excessive viscosity, HMW chitosan is difficult to be applied commercially. Because of the production of COS and the low molecular weight of chitosan, a reduction in its molecular weight is an effective idea for addressing viscosity issues and improving its biological properties [7]. Oligosaccharides are defined as oligomers with a degree of polymerization ranging from 2 to 10, but some higher degrees of polymerization are classified as LMW. COS and LMW chitosan are primarily produced using enzymatic, chemical, and physical methods. The reduction of MW via enzymatic, chemical, or physical processes is linked to improved chitosan water or acetic acid solubility. Depolymerization of chitosan is primarily accomplished through chitosanolysis of acid, which is the most used method for producing COS and LMW chitosan. Using HCl, HNO2, H2O2, and potassium persulfate in chitosanolysis are examples of chemical processes. Sonication, microwave irradiation, gamma irradiation, or a thermal procedure are examples of physical processes. Enzymatic processes employ both specific enzymes and nonspecific enzymes like chitinase, chitosanase and pepsin, cellulase, pronase, lysozyme, papain, hemicellulase, or pectinase, respectively. However, the main issues with enzymatic depolymerization are the costs [7]. High-pressure homogenization (HPH), plasma, and the use of zeolites as adsorbents to purify acid hydrolysis COS and LMW chitosan have all been described as new methods for reducing the molecular mass of chitosan. Furthermore, electrochemical processes for depolymerizing chitosan have been developed.
6.2 Enhanced Anti-microbial Performance
6.2.1 Introducing Cationic Compounds
Although the degree of de-acetylation, molecular weight, and content of the NH2 residual group can influence the anti-microbial activity of chitosan derivatives, the same of chitosan derivatives is primarily dependent on the positively charged NH2 group, which can interfere with and destroy the negative bacterial membrane, bind bacterial DNA, and block protein synthesis [14]. The higher the positive charge, the greater the microbial activity. Increasing positive electricity can improve the antimicrobial activity of chitosan derivatives:
-
A.
NH2 Direct Quaternization
Quaternary ammonium salt is the most commonly used anti-microbial group in chitosan derivatives [1]. NH2’s direct quaternization group was carried out primarily to improve the anti-microbial activity of the chitosan derivates, which can be tuned by varying the ratio of the cationic groups present. Following research, it was discovered that the N-trimethyl group was the most important factor in improving the anti-microbial activity of chitosan derivatives [14]. By varying the trimethylation, acetylation, and acylation, the chitosan derivative with the best anti-microbial activity, better than S. aureus and E. coli, could be obtained.
-
B.
Grafting onto Chitosan
By grafting QAS, and betaine, chitosan derivatives’ antimicrobial activity can be improved. Combining acetate chitosan and C12–C18 alkyl aminopropyl betaine demonstrated a broader anti-microbial spectrum and superior anti-microbial activity than either compound alone. Despite the fact that glycine betaine contains QAS, its anti-bacterial mechanism differs from traditional QAS due to chelation between the NH2 group and the trace amount of metal ions (Ca2+, Na+) that are required for bacteria to maintain their stability and metabolism of the cell, which can destroy the stability and inhibit the growth of cell membrane [16]. As a result, retaining the necessary amounts of NH2 and OH groups during the chemical modification of chitosan is required [14].
-
C.
Grafting Polymerization from Chitosan
Through grafting copolymerization of chitosan acrylamide and methacrylamidopropyl trimethyl ammonium chloride, PQAS can be incorporated into the chitosan skeleton. The produced chitosan derivatives can destroy the bacteria membrane at just a concentration of 14 mg/L. Similarly, anti-microbial chitosan-based polymerized nanoparticles with positive surface charges were synthesized by grafting N-trimethyl aminoethyl methacrylate chloride or methyl methacrylate and N-dimethyl aminoethyl methacrylate hydrochloride from chitosan [14].
6.2.2 Neutral Anti-microbial Compound Conjugation
-
A.
Polypeptide
Another way of producing chitosan peptide conjugate (CPC) with improved antimicrobial activity is to integrate it with an antimicrobial peptide (CGGG(KLAKLAK)2) and an enzyme-cleavable peptide (GPLGVRGC). The morphology of CPC can be enhanced due to the peeling off of the protective PEG layer induced by the presence of gelatinase from bacteria, resulting in excellent anti-microbial activity with a minimum inhibitory concentration value of about 7×10−6 M based on the disruption of hydrophobic/hydrophilic balance.
-
B.
NO Donor
Chitosan can be used as a loading matrix for NO, an anti-biofilm agent [58] to create a derivative that can slowly release NO by sparging chitosan derivatives with NO gas Planktonic cells treated with NO-loaded chitosan show a decrease in colony-forming units, which could be useful in wound dressing. NO can be loaded on chitosan-poly(amidoamine) via reaction with its secondary NH2 group, yielding chitosan-poly(amidoamine)/NONOate with a loading amount of 1.7 mol/mg and excellent antimicrobial and wound healing activity.
6.2.3 Metal Coordination
By coordinating chitosan with Ag+ ions, a construction based on the synergistic impact of chitosan and Ag+ ions was recently done [9], Moldable hydrogel with an antibacterial action against S. aureus and E. coli. The Ag+ ions can be complexed with chitosan and then photo reduced or electro reduced in situ [77]. The anti-microbial effect was greatly enhanced by the synergistic effect of chitosan and metal ions, which stabilized the metal through coordination and by increasing its positive charge, which further promoted its interaction with negatively charged bacteria. Cu(II) complexes formed by chelating O-carboxymethyl chitosan (CMCS) Schiff bases with Cu2+ ions have significantly higher antifungal activity against Phytophthora capsici than the original chitosan [54]. The easy release of copper cations from the complex, caused by the benzene ring’s space steric hindrance, results in an effective anti-fungal performance.
6.2.4 Physical Integration
The physical blending of chitosan derivatives with antibiotics is another common method to increase the anti-microbial activity of chitosan derivatives. Through the solgel transition process, a polyelectrolyte composite hydrogel based on chitosan/CMchitosan/AgNPs demonstrates long-term antibacterial efficacy against S. aureus and P. aeruginosa [77]. Even while direct combination with antibiotics can enhance chitosan’s anti-microbial performance, slow-release antibiotics may increase the probability of microorganism resistance, which requires careful consideration in future research. Based on the synergistic impact of CNC rod and polycationic CS, chitosan can be mixed with cellulose nanocrystals (CNCs) to generate a hydrophobic spray-coating composite with increased antimicrobial action. CNC can cause bacterial membrane rupture, allowing chitosan derivatives with protonated NH2 groups to enter the cell, resulting in bacterial death [70].
6.3 Improved Water Solubility
For the improvement of solubility in the water of chitosan under a basic and neutral environment, hydrophilic compounds with reactive groups can be conjugated with chitosan through amide and hydroxyl groups [14].
6.3.1 Modification of the Amine Group
Hydrophilic compounds with –COOH, –CHO, –OCN, and epoxy easily bind to the backbone of the chitosan with the aid of coupling reactions with its very reactive amide group. By grafting Brij-S20-succinic anhydride to chitosan, the solubility in the water of chitosan can be enhanced. Brij-S20-succinic anhydride disrupts the inter/intra molecular bonds, lowering crystallinity and increasing solubility. Chitosan complex 1-(4-(2-aminoethyl) phenoxy) zinc(II) phthalocyanine (ZnPcN) [46] on conjugation with acid groups of CMCS, and amide groups of phthalocyanine, improves solubility in the water because the conjugate’s absorption intensity ratio is double than that of ZnPcN used alone, and agglomeration in water is reduced. When the amide group of chitosan reacts with the acid group of gluconic acid, the generated sugar-carrying chitosan is soluble under various pH settings due to the hydrophilicity of the gluconyl group and the N-acetyl group [14].
6.3.2 Hydroxyl Group Modification
The OH group of chitosan reacts with halo hydrocarbon or sulfonyl chloride to produce CMCS, which has better solubility in the water [40]. The solubility in the water of chitosan can also be improved by the reaction of its hydroxyl group with N-acetyl ethylenediamine. It is evident that solubility in water of chitosan can be enhanced by the replacement of the hydrophilic group chemically [14].
6.4 Better Biocompatibility
Biocompatibility is very important, especially in cases where biomaterials have a direct contact with human organs or tissues [14]. It relates to how specific tissues react to different elements.
6.4.1 Organic Modification
-
A.
Introduction of Carboxyl Group
By inserting a carboxyl group into the molecular structure of chitosan derivates, the toxicity of protonated cationic amide or groups in chitosan derivates can be minimized, resulting in CMCS, which is non-toxic, biocompatible, and biodegradable [14].
-
B.
Introduction of Betaine
Zwitterionic betaine can combine huge amounts of water to form a hydration layer through hydrogen bonding and electrostatic contact [21, 34, 80]. The water of hydration between proteins and zwitterionic molecule imposes substantial repulsions against nonspecific protein adsorption because it restricts the movement of water adsorbed at the surface of glycine betaine. With the addition of betaine, the derivative exhibits effective antimicrobial characteristics, anti-nonspecific protein adsorption, and anti-platelet adhesiveness [14].
-
C.
PEGylation
Because of its high hydrophilicity and chain flexibility, PEG has been used to alter chitosan derivatives to minimize toxicity and nonspecific protein adsorption, resulting in a volume exclusion effect [14]. Crosslinking chitosan with aldehyde terminated PEG or grafting activated monomethoxy poly(ethylene glycol) to chlorinated N-phthaloyl chitosan can be used to make the PEG-modified chitosan derivative [43].
-
D.
Introduction of Polycaprolactone
Biocompatible polycaprolactone (PCL) can promote cell proliferation and adhesion [41], as a result, it can be utilized to improve the rate of healing and the quality of neonatal tissue. In comparison to PHBHHx or chitosan-g-PCL/PHBHHx, the chitosan-g-PCL and poly(3-hydroxybutyrate-co-3-hydroxyhexanoate) (PHBHHx) hybrid have a rougher surface and smaller grain sizes, which can increase cell adhesion and proliferation, resulting in higher initial cell survival.
-
E.
Introduction of Gelatin
Mixed –OH groups modified biocompatible boron nitride nanotubes (BNNTs) with chitosan to form a slow degradative chitosan/BNNT-OH scaffold that showed improved cellular proliferation and adhesion compared to chitosan, based on the increased mechanical performance and pore size of biocompatible boron nitride nanotubes (BNNTs) [6]. The discontinuousness and hydrophilicity of water-swollen gelatin nanospheres are responsible for the deposited derivatives with controlled wettability and surface roughness. The ratio of chitosan and harmless gelatin nanospheres in the derivative can be changed to alter protein adsorption and cell adhesion [14].
6.4.2 Inorganic Modification
-
A.
Carbon/Boron Materials
Some inorganic compounds, such as graphene oxide, can improve chitosan biocompatibility by enhancing its surface shape, roughness, and pole size, which encourages cell attachment and proliferation at the surface of chitosan derivatives [14].
Mixed OH groups modified biocompatible boron nitride nanotubes (BNNTs) with chitosan to form a slow degradative chitosan/BNNT-OH scaffold that showed improved cellular proliferation and adhesion compared to chitosan, based on the increased mechanical performance and pore size of biocompatible boron nitride nanotubes (BNNTs) [14].
-
B.
Metal Ions
By combining biocompatible Zn2+ ions with chitosan, the resulting porous CS2ZnAlg microspheres can further increase [Zn2+] [52]. When compared with the control group, the hemostasis time of the chitosan/Zn2+ derivative was drastically reduced (134 5 s) (over 600 s) [14]. Following the termination of bleeding, the chitosan/zinc derivative shapes dark red aggregates of blood cells, platelets, fibrins, and microspheres near the wound site. Chitosan’s positive charge can reendow its mucoadhesive activity because it merges with negatively charged red blood cells.
Enhanced Mechanical Property
Chitosan’s mechanical performance can be bettered if it forms a great number of networks either by physical or by chemical crosslinking [61] or in combination with metal nanoparticles [39]. The physically crosslinked hydrogel double-network (PCDHN) of chitosan/Na alginate (SA)/calcium ions (Ca2+) is much better than a physical single network of hydrogel cross-linked via electrostatic interactions, and 10 times better than chitosan/SA hydrogel. The creation of a double network through the use of hydrogen bonding can also strengthen chitosan derivatives. Poly(vinyl alcohol) CS [45] (CPH) DN hydrogel has exceptional tensile strength, elongation at break, and compressive strength thanks to physical crosslinking. The adsorption of fracture energy by chitosan’s first physical network results in CPH’s outstanding excellent mechanical quality, while the second poly vinyl alcohol network maintains the hydrogel’s shape. Furthermore, the biocompatible chitosan-based hydrogel with surface mineralization can enhance the differentiation of bone marrow stem cells [14].
7 Conclusions
Chitosan, one of the most available biopolymers, has been extensively studied and used in diverse fields due to its biodegradability, biocompatibility, non-toxicity, antimicrobial, and antifungal properties. However, its broad applications are limited by its poor solubility and insufficient mechanical strength. To this, chemical modification has been demonstrated to be an effective approach. After their first description over two decades ago, Chitosan NPs have acquired a significant position in various industries like pharma and agriculture. Various methods for the modification and preparation of chitosan NPs have been developed. Sometimes, the NP synthesis requires the use of toxic substances which may adversely affect the environment. To overcome this issue, green preparation techniques stand out as an alternative. These methods are eco-friendly as well as cost-effective. Also, these are devoid of toxic chemicals and high energy, pressure, etc., are not required. Moreover, the synthesis of chitosan NPs using biological methods (using microorganisms like bacteria, fungi, and algae) is even more appealing since it is rapid, non-toxic, and environment-friendly. However, there are scarce reports available on the green synthesis of NPs. The development of novel and innovative green methods for the synthesis of chitosan NPs is a domain where more research and developments are expected in the future.
Abbreviations
- BNNT:
-
Boron nitride nanotube
- Ch:
-
Chitosan
- COS:
-
Chito oligosaccharide
- GTA:
-
Glutaraldehyde
- HMW:
-
High molecular weight
- LCC:
-
N lauryl-carboxymethyl-chitosan
- LMW:
-
Low molecular weight
- NPs:
-
Nanoparticles
- PCDHN:
-
Physically crosslinked hydrogel double-network
- PCL:
-
Polycaprolactone
- PEG:
-
Polyethylene glycol
- QAS:
-
Quaternized ammonium salt
- TEMPO:
-
(2,2,6,6-tetramethylpiperidin-1-yl)oxyl
- TPP:
-
Sodium tripolyphosphate
References
Abu Elella M (2021) Synthesis and potential applications of modified Xanthan gum. J Chem Eng Res Updates 8:73–97
Aranaz I et al (2012) Functional characterization of chitin and chitosan. Curr Chem Biol 3(2):203–230
Azmana M et al (2021) A review on chitosan and chitosan-based bionanocomposites: promising material for combatting global issues and its applications. Int J Biol Macromol 185(June):832–848
Bagheri-Khoulenjani S, Taghizadeh SM, Mirzadeh H (2009) An investigation on the short-term biodegradability of chitosan with various molecular weights and degrees of deacetylation. Carbohyd Polym 78(4):773–778
Bano I et al (2017) Chitosan: a potential biopolymer for wound management. Int J Biol Macromol 102:380–383
Boccaccini AR et al (2010) Electrophoretic deposition of biomaterials. J Royal Soc Interface 7(Suppl 5):S581–S613
Brasselet C et al (2019) Modification of chitosan for the generation of functional derivatives. Appl Sci (Switzerland) 9(7)
Calvo P, Remuñán-López C, Vila-Jato JL, Alonso MJ (1997) Novel hydrophilic chitosan-polyethylene oxide nanoparticles as protein carriers. J Appl Polym Sci 63(1):125–132
Cao J et al (2018) Dual physical crosslinking strategy to construct moldable hydrogels with ultrahigh strength and toughness. Adv Func Mater 28:1800739
Chang S-H et al (2019) Effect of chitosan molecular weight on anti-inflammatory activity in the RAW 264.7 macrophage model. Int J Biol Macromol 131:167–175
Chebotok EN, Yu V, Novikov, Konovalova IN (2006)Depolymerization of chitin and chitosan in the course of base deacetylation.Russ J Appl Chem 79(7):1162–1166
Croisier F, Jérôme C (2013) Chitosan-based biomaterials for tissue engineering. Eur Polymer J 49(4):780–792
Dal Pozzo A et al (2000)Preparation and characterization of poly(ethyleneglycol)-crosslinked reacetylated chitosans.Carbohyd Polym 42:201–206
Ding S, Wang Y, Li J, Chen S (2021) Progress and prospects in chitosan derivatives: modification strategies and medical applications. J Mater Sci Technol 89:209–224
Ding W, Lian Q, Samuels RJ, Polk MB (2003)Synthesis and characterization of a novel derivative of chitosan.Polymer 44:547–556
Dutta PK, Tripathi S, Mehrotra GK, Dutta J (2009)Perspectives for chitosan based antimicrobial films in food applications.Food Chem 114(4):1173–1182
Elsabee MZ, Abdou ES (2013) Chitosan based edible films and coatings: a review. Mater Sci Eng C Mater Biol Appl 33(4):1819–41
Gadkari RR et al (2019) Green synthesis of chitosan-cinnamaldehyde cross-linked nanoparticles: characterization and antibacterial activity. Carbohyd Polym 226:115298. https://doi.org/10.1016/j.carbpol.2019.115298
Ghosh A, Ali M (2012) Studies on physicochemical characteristics of chitosan derivatives with dicarboxylic acids. J Mater Sci 47:1196–1204
Grenha A (2012) Chitosan nanoparticles: a survey of preparation methods. J Drug Target 20(4):291–300
Hu Z et al (2018) Investigation of the effects of molecular parameters on the hemostatic properties of chitosan. Molecules (Basel, Switzerland) 23(12)
Huang HY, Shieh YT, Shih CM, Twu YK (2010) Magnetic chitosan/iron (II, III) oxide nanoparticles prepared by spray-drying. Carbohyd Polym 81(4):906–910. https://doi.org/10.1016/j.carbpol.2010.04.003
Ibañez-Peinado D, Ubeda-Manzanaro M, Martínez A, Rodrigo D (2020) Antimicrobial effect of insect chitosan on Salmonella typhimurium, Escherichia coli O157:H7 and Listeria monocytogenes survival. PLoS ONE 15:1–14, 12 Dec 2020
Ichikawa H, Tokumitsu H, Miyamoto M, Fukumori Y (2007) Nanoparticles for neutron capture therapy of cancer. In: 6 Nanotechnologies for the life sciences
Inamdar N, Mourya VK, Tiwari A (2010) Carboxymethyl chitosan and its applications. Adv Mater Lett 1:11–33
Islam S, Rahman Bhuiyan MA, Islam MN (2017) Chitin and chitosan: structure, properties and applications in biomedical engineering. J Polym Environ 25(3):854–866
Jameela SR, Kumary TV, Lal AV, Jayakrishnan A (1998) Progesterone-loaded chitosan microspheres: a long acting biodegradable controlled delivery system. J Control Release 52:17–24. Jameela1998.Pdf
Jintapattanakit A et al (2007) Peroral delivery of insulin using chitosan derivatives: a comparative study of polyelectrolyte nanocomplexes and nanoparticles. Int J Pharm 342(1–2):240–249
Hemant Yadav KS, Joshi GB, Singh MN, HG Shivakumar (2010) Naturally occurring chitosan and chitosan derivatives: a review.Current Drug Therapy 6(1):2–11
Kaczmarek MB et al (2019) Enzymatic modifications of chitin, chitosan, and chitooligosaccharides. Front Bioeng Biotechnol 7(SEP)
Kafshgari MH et al (2012) Preparation of alginate and chitosan nanoparticles using a new reverse micellar system. Iran Polym J (English Edition) 21(2):99–107
Kato Y, Onishi H, Machida Y (2000) Evaluation of N-succinyl-chitosan as a systemic long-circulating polymer. Biomaterials 21(15):1579–1585
Kato Y, Onishi H, Machida Y (2005) Contribution of chitosan and its derivatives to cancer chemotherapy. In Vivo (Athens, Greece) 19(1):301–310
Keefe AJ, Jiang S (2011) Poly(zwitterionic)protein conjugates offer increased stability without sacrificing binding affinity or bioactivity. Nat Chem 4(1):59–63
Khanafari A, Marandi R, Sanatei S (2008) Recovery of chitin and chitosan from shrimp waste by chemical and microbial methods. Iran J Environ Health Sci Eng 5(1):19–24
Kim I-Y et al (2008) Chitosan and its derivatives for tissue engineering applications. Biotechnol Adv 26(1):1–21
Kim S (2018) Competitive biological activities of chitosan and its derivatives: antimicrobial, antioxidant, anticancer, and anti-inflammatory activities. Int J Polym Sci 2018:1708172 [Tao Y (ed)]
Kong M, Chen XG, Xing K, Park HJ (2010) Antimicrobial properties of chitosan and mode of action: a state of the art review. Int J Food Microbiol 144(1):51–63
Krawczak P (2019) Editorial corner—a personal view: polymer composites: evolve towards multifunctionality or perish. Express Polym Lett 13(9):771
Kritchenkov AS et al (2020) Efficient reinforcement of chitosan-based coatings for ricotta cheese with non-toxic, active, and smart nanoparticles. Prog Org Coat 145(April):105707
Lee K-W et al (2007) Poly(propylene fumarate) bone tissue engineering scaffold fabrication using stereolithography: effects of resin formulations and laser parameters. Biomacromolecules 8(4):1077–1084
Leitner VM, Walker GF, Bernkop-Schnürch A (2003) Thiolated polymers: evidence for the formation of disulphide bonds with mucus glycoproteins. Eur J Pharm Biopharm Official Journal of Arbeitsgemeinschaft fur Pharmazeutische Verfahrenstechnik e.V 56(2):207–214
Li Y, Wang X, Wei Y, Tao L (2017) Chitosan-based self-healing hydrogel for bioapplications. Chin Chem Lett 28(11):2053–2057
Lin Y, Chen Q, Luo H (2007) Preparation and characterization of N-(2-carboxybenzyl)chitosan as a potential PH-sensitive hydrogel for drug delivery. Carbohyd Res 342(1):87–95
Lin Z et al (2021) Preparation of chitosan/calcium alginate/bentonite composite hydrogel and its heavy metal ions adsorption properties. Polymers 13(11)
Liu S et al (2022) Biocompatible gradient chitosan fibers with controllable swelling and antibacterial properties. Fibers Polym 23(1):1–9
Lodhi G et al (2014) Chitooligosaccharide and its derivatives: preparation and biological applications. Biomed Res Int 2014:654913
Miwa A et al (1998) Development of novel chitosan derivatives as micellar carriers of taxol. Pharm Res 15(12):1844–1850
Ohya Y, Shiratani M, Kobayashi H, Ouchi T (1994) Release behavior of 5-fluorouracil from chitosan-gel nanospheres immobilizing 5-fluorouracil coated with polysaccharides and their cell specific cytotoxicity. J Macromol Sci Part A 31(5):629–642
Okamoto Y et al (2002) Analgesic effects of chitin and chitosan. Carbohyd Polym 49(3):249–252
Onishi H, Takahashi H, Yoshiyasu M, Machida Y (2001) Preparation and in vitro properties of N-succinylchitosan- or carboxymethylchitin-mitomycin C conjugate microparticles with specified size. Drug Dev Ind Pharm 27(7):659–667
Pan M et al (2018) Porous chitosan microspheres containing zinc ion for enhanced thrombosis and hemostasis. Mater Sci Eng C Mater Biol Appl 85:27–36
Park BK, Kim M-M (2010) Applications of chitin and its derivatives in biological medicine. Int J Mol Sci 11(12):5152–5164
Potara M et al (2011) Synergistic antibacterial activity of chitosan-silver nanocomposites on Staphylococcus aureus. Nanotechnology 22(13):135101
Qin Y, Xingmei L, Sun N, Rogers RD (2010)Dissolution or extraction of Crustacean shells using ionic liquids to obtain high molecular weight purified chitin and direct production of chitin films and fibers.Green Chem 12(6):968–997
Quiñones JP, Peniche H, Peniche C (2018) Chitosan based self-assembled nanoparticles in drug delivery. Polymers 10(3):1–32
Rahman Bhuiyan MA et al (2017) Chitosan coated cotton fiber: physical and antimicrobial properties for apparel use. J Polym Environ 25(2):334–342
Reighard KP et al (2017) Role of nitric oxide-releasing chitosan oligosaccharides on mucus viscoelasticity. ACS Biomater Sci Eng 3(6):1017–1026
Riegger BR et al (2018) Chitosan nanoparticles via high-pressure homogenization-assisted miniemulsion crosslinking for mixed-matrix membrane adsorbers. Carbohyd Polym 201:172–181. https://doi.org/10.1016/j.carbpol.2018.07.059
Sannan T, Kurita K, Iwakura Y (2003) Studies on chitin, 2. Effect of deacetylation on solubility. Die Makromolekulare Chemie 177:3589–3600
Serhan M et al (2019) Total iron measurement in human serum with a smartphone. In: AIChE annual meeting, conference proceedings, Nov 2019
Shariatinia Z (2019) Pharmaceutical applications of chitosan. Adv Coll Interface Sci 263:131–194
Singh R, Shitiz K, Singh A (2017) Chitin and chitosan: biopolymers for wound management. Int Wound J 14(6):1276–1289
Sivanesan I et al (2021) Nanoforms/nanocomposites for drug delivery applications, 1–21
Song Y, Onishi H, Nagai T (1993) Pharmacokinetic characteristics and antitumor activity of the N-succinyl-chitosan-mitomycin C conjugate and the carboxymethyl-chitin-mitomycin C conjugate. Biol Pharm Bull 16(1):48–54
Susilowati E, Maryani, Ashadi (2019) Green synthesis of silver-chitosan nanocomposite and their application as antibacterial material. J Phys Conf Ser 1153(1)
Tan H, Chu C, Payne K, Marra K (2009) Injectable in situ forming biodegradable chitosan-hyaluronic acid based hydrogels for cartilage tissue engineering. Biomaterials 30:2499–2506
Teng WL et al (2001) Concurrent production of chitin from shrimp shells and fungi. Carbohyd Res 332(3):305–316
Thanou M, Verhoef JC, Junginger HE (2001) Oral drug absorption enhancement by chitosan and its derivatives. Adv Drug Deliv Rev 52(2):117–126
Tyagi P et al (2019) High-strength antibacterial chitosan-cellulose nanocrystal composite tissue paper. Langmuir ACS J Surf Colloids 35(1):104–112
Verlee A, Mincke S, Stevens CV (2017) Recent developments in antibacterial and antifungal chitosan and its derivatives. Carbohyd Polym 164:268–283
Wang N et al (2020) Antibacterial effect of chitosan and its derivative on Enterococcus faecalis associated with endodontic infection. Exp Ther Med 19(6):3805–3813
Wang YW et al (2019) Biological effects of chitosan-based dressing on hemostasis mechanism. Polymers 11(11)
Wijesena RN et al (2015) A method for top down preparation of chitosan nanoparticles and nanofibers. Carbohyd Polym 117:731–738. https://doi.org/10.1016/j.carbpol.2014.10.055
Yanat M, Schroën K (2021) Preparation methods and applications of chitosan nanoparticles; with an outlook toward reinforcement of biodegradable packaging. React Funct Polym 161(Feb)
Yang J et al (2008) Effect of chitosan molecular weight and deacetylation degree on hemostasis. J Biomed Mater Res Part B Appl Biomater 84(1):131–137
Yang J et al (2020) Preparation of a chitosan/carboxymethyl chitosan/AgNPs polyelectrolyte composite physical hydrogel with self-healing ability, antibacterial properties, and good biosafety simultaneously, and its application as a wound dressing. Compos B Eng 197:108139
Yang Y et al (2014) Advances in self-assembled chitosan nanomaterials for drug delivery. Biotechnol Adv 32(7):1301–1316.https://doi.org/10.1016/j.biotechadv.2014.07.007
Younes I et al (2012) Chitin and chitosan preparation from shrimp shells using optimized enzymatic deproteinization. Process Biochem 47(12):2032–2039. https://doi.org/10.1016/j.procbio.2012.07.017
Zhang L et al (2013) Zwitterionic hydrogels implanted in mice resist the foreign-body reaction. Nat Biotechnol 31(6):553–556
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2022 The Author(s), under exclusive license to Springer Nature Singapore Pte Ltd.
About this chapter
Cite this chapter
Mansi, Gulati, S., Amar, A. (2022). Strategies for Synthesis and Chemical Modifications of Chitosan-Based Nanocomposites: A Versatile Material with Extraordinary Potential for Diverse Applications. In: Gulati, S. (eds) Chitosan-Based Nanocomposite Materials. Springer, Singapore. https://doi.org/10.1007/978-981-19-5338-5_2
Download citation
DOI: https://doi.org/10.1007/978-981-19-5338-5_2
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-19-5337-8
Online ISBN: 978-981-19-5338-5
eBook Packages: Physics and AstronomyPhysics and Astronomy (R0)