Abstract
Coronavirus disease 2019 (COVID-19), caused by the continuously evolving novel Severe Acute Respiratory Syndrome Coronavirus-2 (SARS-CoV-2), has been a global health concern since the start of pandemic. Apart from other COVID-19 associated complications, emerging studies have suggested autoimmune manifestations following SARS-CoV-2. Additionally, autoantibodies have been detected in COVID-19 patients. COVID-19 can lead to autoimmune manifestations, through various mechanisms such as molecular mimicry, bystander activation, cytokine storm, generation of autoantibodies, and genetic susceptibility. Several autoimmune diseases like Guillain-Barre syndrome, immune thrombocytopenic purpura, autoimmune hemolytic anemia, autoimmune thyroid diseases, Kawasaki disease, and Alopecia areata have been linked with COVID-19. Additionally, autoimmune diseases have been linked with the increased risk of severe illness and mortality after SARS-CoV-2 infection. Therefore, understanding the pathophysiology of COVID-19 associated autoimmunity can aid in management and treatment of COVID-19.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
1 Introduction
Coronavirus disease 2019 (COVID-19) caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) is still prevalent even after 2 years of pandemic. It has affected almost all countries with around 450 million confirmed cases and over 6 million deaths as of March 14, 2022 (Singhal 2020; Shoenfeld 2020; Liu et al. 2021; Yazdanpanah and Rezaei 2022). Despite swift advancements in COVID-19 vaccine development, the SARS-CoV-2 virus is still a major health concern. Additionally, delayed or inadequate treatment causes major proportion of COVID-19 associated mortality (Yazdanpanah and Rezaei 2022). Clinical features, host genetic factors, co-morbidities, and autoimmune complications have been suggested to increase the risk of severe illness and mortality post-SARS-CoV-2 infection (Haberman et al. 2020; Freites Nuñez et al. 2020; Tan et al. 2021). Therefore, better risk stratification, clinical management, and better understanding of autoimmune complications after COVID-19 infection are required for COVID-19 management.
Autoimmune diseases are characterized by loss of immune tolerance leading to aberrant immune response against the hosts own tissues (Wang et al. 2015). The prevalence of autoimmune diseases is about 3–5% worldwide (Leslie and Hawa 1994; Wang et al. 2015). As viruses can induce type II and IV hypersensitivity, there is a possibility for COVID-19-mediated autoimmunity (Lin and Askonas 1981). Moreover, since the beginning of COVID-19 pandemic, various reports have been suggested for the appearance of COVID-19 manifestations after SARS-CoV-2 infection (Yazdanpanah and Rezaei 2022). Moreover, COVID-19 causes an hyperinflammatory state which results in autoimmune complications (Yazdanpanah and Rezaei 2022). Additionally, studies suggest that SARS-CoV-2 infection can lead to various autoimmune disease like Miller Fisher syndrome, Antiphospholipid antibodies (APS), thrombosis, Guillain-Barre syndrome (GBS), systemic lupus erythematosus (SLE), Kawasaki disease, autoimmune hemolytic anemia, and idiopathic thrombocytopenic purpura (ITP) (Galeotti and Bayry 2020; Ehrenfeld et al. 2020; Cavalli et al. 2020; McMillan et al. 2021; Liu et al. 2021). In addition, the autoimmune complications arising from SARS-CoV-2 infection suggest COVID-19 as a classical example for autoimmune/inflammatory syndrome induced by adjuvants (ASIA syndrome) also known as “Shoenfeld’s syndrome” (Halpert and Shoenfeld 2020).
The SARS-CoV-2 infection can lead to autoimmunity through various mechanisms such molecular mimicry, bystander activation, cytokine storm, production of autoantibodies, and genetic susceptibility (Moran and Prendergast 2001; Ercolini and Miller 2009; Smatti et al. 2019; Ragab et al. 2020; Icenogle 2020; Liu et al. 2021; Bergamaschi et al. 2021a, b). Moreover, autoimmune complications after SARS-CoV-2 infection can lead to increased disease severity (Haberman et al. 2020; Freites Nuñez et al. 2020; Tan et al. 2021). However, in the current scenario a far better understanding regarding the autoimmune complications after SARS-CoV-2 infection is needed; therefore this chapter discusses the different autoimmune conditions that develop after SARS-CoV-2 infection and the possible mechanisms involved to establish a possible association of COVID-19 and autoimmunity.
2 Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2)
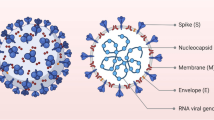
Coronaviruses are diverse group of viruses, belonging to the Coronaviridae family (González et al. 2003). It infects different animals and causes mild to severe respiratory illness in humans (Hu et al. 2020). The name coronavirus was derived from the Greek word crown refereeing to crown or corona like appearance (House et al. 2021). They are enveloped RNA viruses with club like spikes on their surfaces (Fig. 3.1). Its laboratory diagnosis is generally done by detecting viral RNA by real time PCR. The viruses mutate rapidly, and account for almost 15% of common cold (House et al. 2021). In 2002 and 2012, two highly pathogenic coronaviruses strains: severe acute respiratory syndrome coronavirus (SARS-CoV) and Middle East respiratory syndrome coronavirus (MERS-CoV), emerged causing deadly respiratory illnesses (Hu et al. 2020). At the end of 2019, SARS-CoV-2 pandemic emerged from Wuhan city of China. The virus spreads rapidly and surpassed SARS and MERS infections quickly. The outbreak become a global threat to public health (House et al. 2021). The SARS-CoV-2 virus was successfully isolated from Wuhan sea food market in the first week of January (Kumar and Malviya 2020). The SARS-CoV-2 viral structure consists of helical nucleocapsid with 30 kb plus stranded RNA genome. The viral RNA interacts with nucleocapsid (N) protein, and the virus structure consists of structural spike protein (S), membrane protein (M) and envelope protein (E) (Fig. 3.1) (Wang et al. 2020).
The general mechanism by which the virus enters host cells is similar to other coronaviruses. The virus enters the host cells by attachment of S-protein to the angiotensin-converting enzyme 2 receptor (ACE2), thus the SARS-CoV-2 virus has a tropism to pulmonary, hepatic, gastrointestinal, and renal human cells (Santos et al. 2020). The interaction of viral S protein to ACE2 receptors triggers viral endocytosis and endosome formation. The S protein consists of S1 and S2 subunits, and the proteolytic cleavage of S1 protein by cellular proteases exposes S2 fusion peptide allowing the fusion of viral envelope to endosome membrane and release of capsid to the cell cytoplasm (Santos et al. 2020; Walls et al. 2020). The positively stranded viral RNA consisting of ORF1a and ORF1b are translated to produce non-structural proteins (NSPs) (Santos et al. 2020). The NSPs then form replication complex responsible for replication, RNA synthesis and sub-genomic RNA formation (Santos et al. 2020; Chen et al. 2020). The sub-genomic RNA is translated to produce S, E, and M structural proteins (Li et al. 2020). The replicated viral genome is encapsulated by the N protein and assembled with the structural proteins to form the complete virion. The complete virion is transported through cellular vesicles to the cell surface and released out by exocytosis (Santos et al. 2020; Li et al. 2020).
3 Virus Infection and Autoimmunity
Autoimmune diseases are characterized as an aberrant immune responses, resulting due to recognition of self-antigens as non-self-antigens (Giri et al. 2022). There are more than 80 autoimmune diseases, and their prevalence ranges from 3% to 5% worldwide (Ercolini and Miller 2009; Wang et al. 2015). Autoimmune diseases such as rheumatoid arthritis (RA), systemic lupus erythematosus (SLE), multiple sclerosis (MS), type 1 diabetes mellitus (T1DM), etc. have been a global health concern and contribute significantly to the mortality and morbidity associated with autoimmune diseases (Wang et al. 2015). There is no cure for most autoimmune diseases, and the current treatment options generally only provide symptomatic reliefs (Chandrashekara 2012). Moreover, despite recent advancements in the field, the exact etiology for autoimmune diseases is unclear. Several factors like genetics, epigenetics, stress, environmental factors have been suggested to trigger the autoimmune response (Costenbader et al. 2012). Apart from these, studies also suggest that multiple factors including infections, nutrition, stress, microbiota, tobacco, smoke, pharmaceutical agents, hormones, ultraviolet light, heavy metals, vaccines can trigger the development of autoimmune diseases (Shoenfeld et al. 2000; Costenbader et al. 2012).
Viruses are considered to be one of the major environmental trigger responsible for the development of autoimmunity. Multiple mechanisms such as molecular mimicry, bystander activation, cross-reaction, epitope spreading, cytokine storm have been suggested to play a crucial role in viral infection induced autoimmunity (Ercolini and Miller 2009; Smatti et al. 2019; Liu et al. 2021). Moreover, viruses can induce Type II and IV hypersensitivity (Lin and Askonas 1981) and can cause ASIA syndrome (Halpert and Shoenfeld 2020). Additionally, the viral infections induced autoimmunity has been observed in experimental autoimmune encephalomyelitis (EAE) model of multiple sclerosis (MS), West Nile virus (WNV)-mediated myasthenia gravis (MG), Theiler’s murine encephalomyelitis virus-CNS autoimmunity, Epstein–Barr virus (EBV), and measles virus induced MS and SARS-CoV-2 virus induced autoimmunity (Miller et al. 1997; Tucker and Andrew Paskauskas 2008; Constantinescu et al. 2011; Lünemann 2012; Getts et al. 2013; Leis et al. 2014; Smatti et al. 2019; Galeotti and Bayry 2020; Ehrenfeld et al. 2020; Cavalli et al. 2020; McMillan et al. 2021; Liu et al. 2021).
3.1 SARS-CoV-2 Induced Autoimmunity
Emerging studies have suggested COVID-19 patients develop various autoimmune manifestations like GBS, ITP, RA, SLE, T1DM, Miller Fisher syndrome, Kawasaki disease, Autoimmune thyroid diseases, Antiphospholipid antibodies, Vitiligo, Alopecia areata, Cold agglutinin syndrome thrombosis, APS and autoimmune hemolytic anemia (Galeotti and Bayry 2020; Ehrenfeld et al. 2020; Cavalli et al. 2020; Tung et al. 2021; McMillan et al. 2021; Ruggeri et al. 2021; Liu et al. 2021; Post et al. 2021; Edwards et al. 2021; Bhagat et al. 2021; Dewanjee et al. 2021; Tammaro et al. 2022). Moreover, studies have also found that patients develop a range of autoantibodies and cytokine storm post-COVID-19 (McMillan et al. 2021; Ryabkova et al. 2021; Chang et al. 2021), suggesting that SARS-CoV-2 infection can trigger autoimmunity (Halpert et al. 2021; Dotan and Shoenfeld 2021; Dotan et al. 2021a). Additionally, the autoimmune complications arising from SARS-CoV-2 infection suggest COVID-19 as a classical example for ASIA syndrome (Halpert and Shoenfeld 2020). Multiple mechanisms like molecular mimicry, cytokine storm, production of autoantibodies, and genetic susceptibility can result in SARS-CoV-2 induced autoimmunity (Moran and Prendergast 2001; Ercolini and Miller 2009; Smatti et al. 2019; Ragab et al. 2020; Icenogle 2020; Liu et al. 2021; Bergamaschi et al. 2021a, b). These mechanisms are discussed in the following sections.
3.1.1 Molecular Mimicry
Molecular mimicry occurs when infectious agents such as viruses present antigens similar to host self-antigens (Arango et al. 2013). Thus, the cross-reaction between SARS-CoV-2 viral antigens and self-antigens can activate self-reactive T and B mediated autoimmune response (Arango et al. 2013). Experimental evidence suggests that viruses such as Herpes simplex virus (HSV), Epstein–Barr virus (EBV), and cytomegalovirus (CMV) can trigger various autoimmune diseases through molecular mimicry (Moran and Prendergast 2001; Smatti et al. 2019). Additionally, recent studies have also suggested the role of EBV and CMV in the development of vitiligo—skin autoimmune disease (Doğan et al. 2014; Dwivedi et al. 2018).
Previously, human coronaviruses CoV-229E and HCoV-OC43 have been linked with MS (Salmi et al. 1982; Stewart et al. 1992; Talbot et al. 1996; Arbour et al. 2000; Moody et al. 2021). Furthermore, cross reactive T cells immune response has been detected between myelin and HCoV-OC43 antigens (Arbour et al. 2000; Moody et al. 2021). Similarly, SARS-CoV-1 has been found to be associated with autoimmune diseases by cross reactivity (Wang et al. 2004; Moody et al. 2021). Patients with autoimmune diseases such as RA, SLE, Sjogren’s syndrome were found positive for SARS-CoV-1 antibodies, despite lacking any previous SARS-CoV-1 infections (Wang et al. 2004; Moody et al. 2021). Therefore, these studies indicate that like other coronaviruses, SARS-CoV-2 can also lead to autoimmune diseases.
In addition, the spike glycoprotein of SARS-CoV-2 virus has structural resemblances with mammalian proteomes (Kanduc and Shoenfeld 2020). The study suggested SARS-CoV-2 proteome and the human proteins PARP14, PARP9, and MACROD1, may potentially behave as molecular mimics, which may result in activation of autoreactive CD8+ and CD4+ T cells mediated autoimmune response (Kanduc and Shoenfeld 2020). Moreover, the study found that NSP3 protein consist of an LKH tripeptide which is homologous with human proteome and these homologous regions may prompt autoreactive B cells to produce antibodies for these epitopes (Kanduc and Shoenfeld 2020). Furthermore, molecular mimicry between SARS-CoV-2 and the female reproductive system has been reported (Dotan et al. 2021b). It has also been reported that molecular mimicry between SARS-CoV-2 antigens and neural antigens can lead to GBS (Shoraka et al. 2021) (Table 3.1). Similarly, molecular mimicry of SARS-CoV-2 antigens with Ankyrin-1 (Ank-1) protein has been suggested to cause Autoimmune hemolytic anemia (Angileri et al. 2020). Apart from these, molecular mimicry may be involved in autoimmune manifestations like ITP, SLE, and APS, post-COVID-19 (Table 3.1) (Bhattacharjee and Banerjee 2020; Tung et al. 2021; Gracia-Ramos et al. 2021). It has been suggested that such molecular mimics through resident antigen presenting cells can promote autoreactive T and B cells response (Fig. 3.2) (Kanduc and Shoenfeld 2020). Thus, molecular mimicry could be a potential mechanism contributing to SARS-CoV-2 associated autoimmune complications (Kanduc and Shoenfeld 2020).
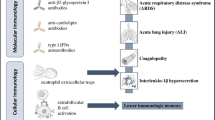
Role of SARS-CoV-2 infection in development of autoimmunity. SARV-CoV-2 through molecular mimicry and bystander activation can promote autoreactive T and B cells response. Additionally, cytokine storm can lead to increased pro-inflammatory cytokines which further contribute to activation of Th1 response. Moreover, TLR sensing of viral RNA through resident antigen presenting cells can activate self-reactive adaptive response. Furthermore, the increased levels of autoantibodies detected in COVID-19 can result in tissue damage. Therefore, COVID-19 patients may develop various autoimmune diseases through various mechanisms such as molecular mimicry, bystander activation, cytokine storm, and production of autoantibodies
3.1.2 Bystander Activation
Bystander activation is an another crucial mechanism by which SARS-CoV-2 infection can cause autoimmunity (Arango et al. 2013). Here the inflammatory response against SARS-CoV-2 can contribute to activation of self-reactive T and B cells (Fig. 3.1) (Arango et al. 2013); thus these non-antigenic activation can lead to an autoimmune response (Pacheco et al. 2019). The autoimmune response mediated by SARS-CoV-2 can be triggered by pathogen-associated molecular patterns (PAMPs), cytokines and chemokines. Viral infections such as Influenza virus, EBV, and CMV trigger bystander activation leading to autoimmune response mediated by self-reactive T cells, B cells, NK cells, and DCs (Fujinami et al. 2006; Arango et al. 2013; Jung et al. 2017; Dwivedi et al. 2018; Pacheco et al. 2019).
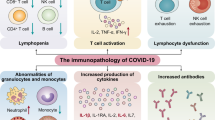
The evidence for SARS-CoV-2 infection mediated autoimmunity through bystander activation is suggested in patients with persistent COVID-19. The patients with persistent COVID-19 have highly activated innate immune cells (Phetsouphanh et al. 2022). Additionally, the patients had elevated expression of pro-inflammatory cytokines like type I IFN. Moreover, this elevated immune response was persistent for 8 months post-COVID-19 (Phetsouphanh et al. 2022). Additionally, patients with severe COVID-19 had delayed bystander CD8+ T cell immune response (Table 3.1) (Bergamaschi et al. 2021a, b). Furthermore, this immune and inflammatory abnormalities have been found to be persistent in severe disease for more than 60 days post-COVID-19 (Table 3.1) (Bergamaschi et al. 2021a, b). In addition, evidence of heterologous T cell immunity between bacterial pathogens and SARS-CoV-2 further suggests that bystander activation is mediated by SARS-CoV-2 infection (Eggenhuizen et al. 2022). Overall, the above mentioned studies suggest that SARS-CoV-2 infection can trigger autoimmune response by bystander activation.
3.1.3 Cytokine Storm
Another mechanism by which SARS-CoV-2 infection can cause autoimmunity is cytokine storm (Ragab et al. 2020; Icenogle 2020). It is also called cytokine release syndrome, which is a life-threatening inflammatory syndrome involving elevated levels of cytokines and hyper immune cells activation (Ragab et al. 2020; Icenogle 2020). The severely ill COVID-19 patients have been characterized by elevated cytokines, which is suggestive of immune dysregulation (Huang et al. 2020; Tay et al. 2020; Qin et al. 2020). The clinical features of severe COVID-19 suggested an increased levels of IL-2, IL-17, IP10, MCP1, TNF-α, IL-1, and IFN-γ cytokines (Table 3.1) (Huang et al. 2020). Furthermore, these increased pro-inflammatory cytokines led to activation of Th1 response and resulted in cytokine storm (Ragab et al. 2020; Icenogle 2020). The increase in cytokines also led to infiltration of macrophages, neutrophils, and T cells from the circulation into the site of infection (Ragab et al. 2020; Icenogle 2020), indicating that the elevated immune response can lead to tissue and organ damage. In addition, severe COVID-19 patients have been characterized by persistent inflammatory and persistent CD8+ T cells response (Bergamaschi et al. 2021a, b). One recent study has suggested for higher levels of IL-6 in mortality cases (Ruan et al. 2020). Overall, the above mentioned studies suggest that increased cytokine levels post COVID-19 can lead to increased Th1 response and increased infiltration of immune cells to the site of infection, which might lead to COVID-19 mediated autoimmunity (Fig. 3.1) (Ragab et al. 2020; Icenogle 2020).
3.1.4 Autoantibodies
Increased levels of autoantibodies have been detected in COVID-19 patients with severe disease (Bastard et al. 2020; Zhou et al. 2020; Zuo et al. 2020; Vlachoyiannopoulos et al. 2020). Previous study suggested the presence of antinuclear antigen (ANA) antibodies, anti-SSA/Ro antibodies, anti-SSA/Ro antibodies, anti-scl-70 antibodies, and anti-U1-RNP antibodies in severely ill COVID-19 patients (Table 3.1) (Vlachoyiannopoulos et al. 2020). The study reported that 70% of COVID-19 patients had at least one of the systemic autoimmune rheumatic disease (Vlachoyiannopoulos et al. 2020); among which the most common antibodies are antiphospholipid antibodies (APLs) (Zuo et al. 2020; Xiao et al. 2020; Franke et al. 2021). These APLs are generally associated with APS (Xiao et al. 2020). Moreover, severe COVID-19 patients had the presence of antibodies against phospholipids (Zuo et al. 2020; Xiao et al. 2020; Franke et al. 2021). Severe COVID-19 patients with neurological symptoms also reported to exhibit autoantibodies against neuronal targets (Franke et al. 2021). Moreover, antibodies have been found against cytokines such as IFN-γ, GM-CSF, IL-6, and IL-10 (Table 3.1) (Bastard et al. 2020). One study has suggested that the LKH tripeptide of NSP3 protein homologous with human proteome may prompt autoreactive B cells to produce antibodies for these epitopes (Kanduc and Shoenfeld 2020). Thus, the presence of autoantibodies suggests that severe SARS-CoV-2 infection can result in autoimmune reaction (Fig. 3.1).
3.1.5 Innate Immune Mechanisms
The SARS-CoV-2 RNA can be recognized by DCs which can further activate innate immune response (Fig. 3.1) (Velikova and Georgiev 2021). Additionally, the single stranded mRNA recognized by PAMPs like Toll-like receptors (TLRs) such as TLR3, TLR7, and TLR8 can prime the innate immune response to produce pro-inflammatory cytokines like IFN-γ (Wang et al. 2021; Talotta 2021). Furthermore, the DCs can then induce T and B cell response which can contribute to the autoimmune response (Table 3.1) (Wang et al. 2021; Talotta 2021; Velikova and Georgiev 2021).
3.1.6 Genetic Susceptibility
HLA-B*15:27 and HLA-DRB1*04:06 have been found to be risk alleles for COVID-19 susceptibility (Yu et al. 2021). Additionally, HLA-A*11 and HLA-B*40 have also been linked with COVID-19 infection (Table 3.1) (Warren and Birol 2020; Littera et al. 2020; Lorente et al. 2021; Yu et al. 2021). Interestingly, HLA-DRB1*04:06 has been found to be risk factor for autoimmune diseases (Table 3.1) (Sun et al. 2019; Zhao et al. 2019). These findings indicate a possible correlation between autoimmune diseases and COVID-19 severity and mortality (Yu et al. 2021). In addition, DRB1 and DQA1 have been associated with circulating IL-6 levels, which is a key inflammatory marker for COVID-19 severity (Ahluwalia et al. 2021). Overall, these findings indicate that individuals vulnerable to SARS-CoV-2 infection may also be susceptible to autoimmune diseases (Yu et al. 2021). However, studies analyzing such associations are scarce and future genetic association studies are warranted to establish the correlation between SARS-CoV-2 infection and autoimmune diseases.
4 Similarities Between COVID-19 Manifestations and Autoimmunity
Various clinical presentations extending from asymptomatic infection to lethal respiratory failure are observed in patients with COVID-19 disease caused by SARS-CoV-2. Moreover, it is also observed that many patients experience other long-term symptoms after the initial onset often extending beyond the original organ involved and this phenomena is known as post-acute sequelae of COVID-19 (PASC) (Knight et al. 2021). Apart from various post-COVID manifestations such as respiratory, cardiac, musculoskeletal, endocrine, etc., one of the key manifestations observed is the development of a detrimental immune reaction against self-tissue antigens (Mehandru and Merad 2022). Here, we discuss some of the major autoimmune diseases and syndromes reported to be associated with COVID-19, so far.
5 Autoimmune Complications of COVID-19
5.1 Guillain-Barre Syndrome
Guillain-Barré syndrome (GBS) is a severe immune mediated neuropathy with a global incidence of around 1–2 cases per million in a year. It is caused due to autoimmune damage to the peripheral nervous system characterized by rapidly developing motor weakness. It is believed to be triggered by a prior respiratory or gastrointestinal infection in most of the cases (Bragazzi et al. 2021). Hence, there could be fair chances of GBS onset post-COVID-19 infection. The first case of GBS associated with SARS-CoV-2 infection was reported in China in a 61-years-old woman. Neurological examination revealed symmetric weakness and areflexia in both legs; however, in this case a pattern of para-infectious profile was observed instead of post-infection (Zhao et al. 2020). Various researchers around the world have reported the incidence of GBS in patients with COVID-19 (Yazdanpanah and Rezaei 2022). Till date, around 90% of GBS cases were reported in individuals above 50 years of age and almost two third of them were diagnosed after 2 weeks of SARS-CoV-2 infection. It is noteworthy that the clinical manifestations and severity in these GBS patients were like non-COVID GBS patients (Ramos-Casals et al. 2021).
5.2 Immune Thrombocytopenic Purpura
Immune thrombocytopenic purpura (ITP) is a hematological autoimmune disorder characterized by autoantibody mediated destruction of platelets resulting in increased bleeding risk (Cooper et al. 2021). The clinical course is often acute and severe in pediatric patients whereas around 64% of chronic cases are observed in adults. ITP has been reported to be associated with several viral infections such as EBV, CMV, HIV, and HCV (Liebman 2008; Elalfy and Nugent 2016). Reports have suggested the incidence of ITP in patients with SARS-CoV-2 infection, predominantly affecting patients of more than 50 years age displaying ITP manifestations such as purpura and mucosal bleeding (Ramos-Casals et al. 2021). In around 20% of cases, ITP occurred 3 weeks after COVID-19 onset; however, about 7% of ITP cases were also observed in asymptomatic COVID-19 patients. COVID-19 associated ITP could be due to the underlying immune dysregulation, genetic predisposition, and other mechanisms such as molecular mimicry, cryptic antigen expression, epitope spreading, etc. (Bhattacharjee and Banerjee 2020) (Table 3.2).
5.3 Kawasaki Disease
Kawasaki disease (KD), first reported by Japanese physician Tomisaku Kawasaki, is an acute febrile systemic vasculitides predominantly occurring in childhood (Beom Kim 2019). KD is manifested by inflammatory changes to the endothelial walls of arteries including coronary arteries, and coronary artery lesions may lead to serious complications as coronary artery ectasia/dilatation, coronary artery aneurysm, and acute myocardial infarction (Newburger et al. 2004). The onset of KD is generally between 6 months and 5 years of age with common symptoms such as fever, skin rash, diffuse mucosal inflammation, non-exudative conjunctivitis, cervical lymphadenopathy, etc. (Minich et al. 2007; Gatterre et al. 2012). Many SARS-CoV-2 infected children in UK were reported with systemic inflammatory syndrome features, similar to KD (Martinez et al. 2020). A few other cases of critically ill SARS-CoV-2 infected children presenting characteristics of systemic inflammation and some features of KD were also reported. Verdoni et al., have reported that among ten, almost half of the COVID-19 infected children presented classical KD like manifestations and remaining were identified with incomplete KD (Verdoni et al. 2020).
5.4 Autoimmune Thyroid Diseases
Autoimmune thyroid diseases (AITDs), affecting about 2–5% of the population, are among the most prevalent autoimmune disorders, mainly comprising of Graves’ Disease (GD) and Hashimoto Thyroiditis (HT) (Dayan and Daniels 1996; Simmonds and Gough 2004). They are characterized by the loss of immune tolerance in addition to humoral and cell-mediated autoimmune response against thyroid gland (Dayan and Daniels 1996; Simmonds and Gough 2004). Interestingly, Vojdani et al., have demonstrated that SARS-CoV-2 antibodies cross reacted with different human tissues including thyroid (Vojdani et al. 2021). It has been observed that even patients with mild COVID-19 symptoms have shown complications of AITDs. In a cohort study, Lui et al., have reported thyroid dysfunctions in COVID-19 patients. They noticed that during the convalescence period, the incidence of thyroiditis was rare; however, they observed an imbalance in thyroid function test and detected anti-thyroid antibodies in these patients (Lui et al. 2021). In another report, Feghali et al., presented three cases of thyroid dysfunction which developed few weeks after the convalescence period of SARS-CoV-2 infection in patients without any previous history of thyroid disease. They observed a 38 years old woman developed hypothyroidism after 6 weeks of SARS-CoV-2 infection, a 33 years old female developed Grave’s disease after 8 weeks of SARS-CoV-2 infection and another case of 41 years old female developed thyroiditis 6 weeks post SARS-CoV-2 infection (Feghali et al. 2021). It has been speculated that the thyroid related anomalies may occur due to a direct or indirect effects of SARS-CoV-2 infection on the gland. The plausible development of chronic thyroid autoimmunity and hypothyroidism has been also anticipated because of either a prior subacute thyroiditis or a viral trigger of autoimmunity in susceptible people (Ruggeri et al. 2021) (Table 3.2).
5.5 Rheumatoid Arthritis
Rheumatoid arthritis (RA) is an autoimmune disorder characterized by inflammatory changes of the synovial tissue of joints, cartilage and bone. RA is prevalent in around 0.06–1.27% population worldwide. Aberrations in the cellular and humoral immune response result in generation of autoantibodies in addition to lymphocyte infiltration into the synovium (Thanapati et al. 2017; Giri et al. 2021a; Almutairi et al. 2021). RA is a chronic multisystem autoinflammatory disorder manifested by joint pain, synovitis, stiffness, and muscle wasting around the involved joints (Mohammed 2020). Around 15% of the patients infected with SARS-Cov-2 were observed to have arthralgia at some point. Mukarram and colleagues have reported a case series of five patients who developed bilaterally symmetrical polyarthritis, without any previous history of any rheumatic disease. Moreover, the musculoskeletal manifestations were phenotypically like RA. Interestingly, these patients responded well to low-dose glucocorticoids and disease-modifying anti-rheumatic drugs (DMARDs) (Mukarram et al. 2021). A few other studies have also reported presence of anti-citrullinated protein antibodies (ACPA) and flaring of RA in patients infected with SARS-CoV-2 (Vlachoyiannopoulos et al. 2020; Perrot et al. 2021). However, there is still a debate on the association of COVID-19 and RA that whether they are actually associated or mere a coincidence. Derksen et al., carried out a detailed investigation on 61 patients and observed that the seroprevalence of ACPA is not significantly higher post SARS-CoV-2 infection and the patients demonstrating polyarthritis were resembling regular patients with RA and hence, they speculated that RA after COVID-19 may be coincidence rather than connected (Derksen et al. 2021).
5.6 Systemic Lupus Erythematosus
Systemic lupus erythematosus (SLE) is a chronic autoimmune disorder with inconsistent manifestations predominantly affecting women (Barber et al. 2021). It is characterized by an overall loss of self-tolerance along with autoreactive T and B cell activation resulting in generation of pathogenic autoantibodies leading to tissue injury (Choi et al. 2012). Patients affected with SLE had posed a serious concern during the COVID-19 pandemic as they are already susceptible to infections because of their immune system and the related organ damage in addition to ongoing immunosuppressive treatments (Ehrenfeld et al. 2020). However paradoxically, immunosuppressants were identified as a means of reducing inflammation and likelihood of acute respiratory distress syndrome (ARDS) in COVID-19 patients (Horisberger et al. 2020). While the anti-viral immunity is essential for protection against COVID-19, a hysterical pro-inflammatory cytokine storm may lead to damaging the lungs and other organs resulting in substantial increase in morbidity and mortality (Spihlman et al. 2020). Several cases of SLE patients have been reported of getting COVID-19 (Gartshteyn et al. 2020). However, reports of SLE manifestations after COVID-19 infections are limited. Zamani et al., have reported a case of 39 years old Persian male displaying SLE manifestations following SARS-CoV-2 infection, without any prior history of SLE (Zamani et al. 2021).
5.7 Type 1 Diabetes
Type 1 diabetes (T1D) is one of the most prevalent autoimmune disorders caused by combination of genetic and environmental triggers leading to immune mediated destruction of β-cells causing in lifelong dependency on exogenous insulin (Gan et al. 2012). Several viral infections such as coxsackievirus, cytomegalovirus and enterovirus, have been reported to be associated with T1D (Pak et al. 1988; Stene and Rewers 2012; Eizirik and Op de Beeck 2018). However, there is no clear spectrum of SARS-CoV-2 and T1D association and only a few studies have been reported which are needed to be interpreted carefully. A few studies have reported ketosis and induced diabetic ketoacidosis (DKA) in diabetic patients with SARS-CoV-2 infection; however, overall diabetic manifestations were not clear (Firouzabadi et al. 2020; Dehghani Firouzabadi et al. 2020). Some other studies have stated the development of diabetes and serious metabolic complications in COVID-19 patients (Chee et al. 2020; Heaney et al. 2020). Nevertheless, there are very scarce reports substantiating T1D and COVID-19 association and the possible reasons could be the younger age of T1D patients, lower prevalence of T1D, and high numbers of CD8+ T cells in T1D which may play a defensive role against SARS-COV2 infection (Chowdhury and Goswami 2020).
5.8 Vitiligo
Vitiligo is one of the most common pigmentary skin disorders characterized by circumscribed white patches in the skin resulting due to the autoimmune destruction of melanocytes from the epidermis (Dwivedi et al. 2013a; Bergqvist and Ezzedine 2020). The exact pathomechanism is not clear; however, it is proposed that oxidative stress might be an initial trigger generating endoplasmic reticulum (ER) stress which subsequently might instigate and exacerbate of anti-melanocyte immune response (Laddha et al. 2013; Jadeja et al. 2021). Both humoral and cell-mediated autoimmune responses have been found to be involved in melanocyte destruction (El-Gayyar et al. 2020; Giri et al. 2020a, b). Moreover, studies have suggested the role of IFN-γ and TNF-α cytokines in melanocytes destruction (Laddha et al. 2012; Dwivedi et al. 2013b; Harris 2015; Giri et al. 2021b). Unlike the other autoimmune disorders discussed in this chapter, there are not much evidence of association between vitiligo and COVID-19 to the best of our knowledge except one recent report by Herzum et al. (2022). They report a case of 45-year-old woman presented with well demarcated milky-white patches developed after 2 weeks of SARS-CoV-2 infection. On the follow up studies it was observed that the lesions were stabilized after 1 month of initial progression (Herzum et al. 2022). It is noteworthy that the protection against the viral infection and disease onset significantly rely on functional innate and adaptive immunity in addition to the interferon signaling pathways. Based on the fact that in generalized vitiligo (GV) there is a shift of the immune response towards adaptive type 1 (IFN-γ and CD8+ T cells) and innate immune responses, it was speculated that patients with GV may clear SARS-CoV-2 infection more effectively and reduce the risk of COVID-19 development. However, this hypothesis needs to be validated by further studies in this direction (Post et al. 2021) (Table 3.2). On the other hand, other studies propose that in case of COVID-19, vitiligo autoimmunity may affect the cytokine storm-related disease burden. Moreover, study by Adlen and Henzy reported a significant difference in COVID-19 manifestations in patients with other autoimmune co-morbidity such as vitiligo (Aidlen and Henzy 2021).
5.9 Alopecia areata
Alopecia areata (AA) is an autoimmune dermatological condition wherein immune system attacks hair follicles, resulting in a non-scarring form of hair loss (Fukuyama et al. 2022). The predominance of AA accounted higher in patients aged 10–25 years (Juárez-Rendón et al. 2017). Pathogenesis of AA includes autoimmune response against hair follicles along with factors affecting our daily lifestyle (Minokawa et al. 2022). In AA, lymphocytic cells infiltrate around the peribulbar region of hair follicles which results in patchy loss of hair follicles (Guo et al. 2015). COVID-19 emergence is reported to be associated with alopecia in infected patients, including some cases of AA. Different studies performed worldwide have reported both manifestation of new-onset AA and progression of pre-existing AA condition after SARS-CoV-2 infection. Patients between age of 13 and 56 years and without previous medical history of AA have shown new-onset AA with significant patchy hair loss on scalp. An observational study carried out using questionnaire included survey of 389 patients, which reported recurrence of AA in 44% patients following SARS-CoV-2 infection (Christensen and Jafferany 2022). However, a retrospective study including 32 patients with mild-to-moderate COVID-19 has given controversial data; which shows no acceleration of AA symptoms after 6 months of SARS-CoV-2 infection (Rudnicka et al. 2021). These studies suggest a complex relationship between AA and COVID-19 and requires further investigations to understand the association between COVID-19 and AA (Christensen and Jafferany 2022).
5.10 Cold Agglutinin Syndrome (CAS)
Cold agglutinin syndrome is another rare autoimmune hematological disorder, which accounts for 25% of all autoimmune hemolytic anemia (AIHA) cases. The autoantibodies, also known as cold agglutinins, agglutinate red blood cells (RBCs) at 4 °C. It is observed to be predominant in patients aged 51–96 years (Berentsen and Tjønnfjord 2012). Cold agglutination is described to be associated with viral infections including rubella virus, HIV, influenza viruses, varicella-zoster virus (VZV), and Epstein–Barr virus (EBV) (Kaur et al. 2021). After COVID-19 pandemic, cold agglutinin syndrome is also reported to be clinically important in SARS-CoV-2 infection as one of the hematological manifestations. In vivo hemolysis due to presence of cold agglutinins was diagnosed in two SARS-CoV-2 infected men. The antibodies reacted at cold body temperatures with RBCs of patient and donor. In these cases, refractory septic shock, hypoxic respiratory failure and progressive thrombocytopenia were developed during COVID-19 disease (Jensen et al. 2020). Other case-studies have also reported that following the SARS-CoV-2 infection, patients have developed CAS with low levels of hemoglobin, elevated levels of bilirubin and lactate dehydrogenase as well as abnormality in other blood parameters. These reports indicate that detection of the hemolytic patterns in SARS-CoV-2 infected patients and management of the severe disease complications are necessary (Patil et al. 2020; Maslov et al. 2020; Huscenot et al. 2020).
5.11 Antiphospholipid Syndrome
Antiphospholipid syndrome (APS), also called “Hughes syndrome,” is a thrombo-inflammatory disorder, characterized by formation of blood clots in veins and arteries. Antiphospholipid autoantibodies (aPL), specifically anticardiolipin antibodies (aCL), anti-β2 glycoprotein-I (β2GPI) antibodies, and lupus anticoagulant (LA) are considered as markers of APS (Cervera 2017). Antiphospholipid syndrome is found to be entangled with 33% cases of SLE (Knight and Kanthi 2022). In addition, APS is associated infections, drug and immune system related disease, and metastatic tumors. The frequency of antiphospholipid autoantibodies falls between 1% and 5% in population. Moreover, aPL crosslink with surface protein of platelets and endothelial cells, propelled by procoagulant and anticoagulant reactions, which further evolves to thromboembolic lesions (Cervera 2017). Evidences indicate that COVID-19 affects immune system and cardiovascular system, in additive manner to other multiorgan systemic disease. In a clinical investigation, serum samples of 29 patients with severe COVID-19 were tested. The study reported that 20 patients out of 29 exhibited presence of several systemic autoantibodies including antibodies against aCL (IgG/IgM), a-β2GPI (IgG/IgM), p-ANCA, and c-ANCA (Vlachoyiannopoulos et al. 2020). In another study of 56 COVID-19 cases, increased serum concentration of aCL was observed in severe COVID-19 patients compared to moderate infection, suggesting for risk of thromboembolic events (Bertin et al. 2020). Additionally, in study of 66 COVID-19 patients 47% exhibited positive result for IgA aCL (25.8%) and IgG aβ2GP1 (18.2%) (Xiao et al. 2020). These data revealed that circulating levels of aPLs are clinically important to understand development and severity of COVID-19 associated immunothrombosis.
6 Autoimmune Disease: A Risk Factor for Severe COVID-19?
Previous studies have suggested for development of autoimmune diseases after SARS-CoV-2 infection (Galeotti and Bayry 2020; Ehrenfeld et al. 2020; Cavalli et al. 2020; McMillan et al. 2021; Liu et al. 2021). However, the role of autoimmune disease in severe COVID-19 is controversial. Since the COVID-19 pandemic has begun, many autoimmune disease patients have suspended their therapies due to the fear of immune suppression. However, it might lead to worsening of autoimmune conditions (Liu et al. 2021). Therefore, a better understanding of the role of autoimmune diseases on COVID-19 development is required to improve COVID-19 and autoimmune disease management.
Initial studies had found that patients with autoimmune disease were not a risk factor for COVID-19 (Zen et al. 2020). However, multicentric study from China found that autoimmune disease patients may be at an increased risk of COVID-19 development (Zhong et al. 2020). Moreover, a Spanish study found that autoimmune disease patients might be at a greater risk for severe COVID-19 (Pablos et al. 2020). Additionally, patients with rheumatic inflammatory disease have been found to be at a greater risk towards development of severe pneumonia (Bachiller-Corral et al. 2021). Similarly, patients with autoantibodies against ACE2 and angiotensin type-1 receptors have been found to be at an increased risk for COVID-19 severity (Rodriguez-Perez et al. 2021). Recently, a meta-analysis study suggested that patients with autoimmune diseases had an increased risk of COVID-19 (Akiyama et al. 2021). Studies have also suggested for the presence of autoantibodies in severe COVID-19 patients (Bastard et al. 2020; Zhou et al. 2020; Zuo et al. 2020; Vlachoyiannopoulos et al. 2020). Although the studies correlating COVID-19 in autoimmune patients are scarce, the findings suggest that autoimmunity might play a critical role in increasing COVID-19 severity.
7 Conclusions
COVID-19 pandemic has had significant global health impact. Similar to autoimmune diseases, COVID-19 manifestations causes immune system mediated tissue and organ damage. SARVS-CoV-2 infection leads to various autoimmune conditions like Guillain-Barre syndrome, Immune thrombocytopenic purpura, Kawasaki disease, Autoimmune thyroid diseases, Rheumatoid Arthritis, Systemic Lupus Erythematosus, Type 1 Diabetes, Vitiligo, Alopecia areata, Cold agglutinin syndrome, Antiphospholipid syndrome through various mechanisms such as molecular mimicry, bystander activation, cytokine storm, production of autoantibodies, etc. Furthermore, studies have also suggested a correlation between autoimmune diseases and risk of developing severe COVID-19. However, future studies must investigate the relationship between severe COVID-19 and autoimmune diseases. Moreover, future studies must also investigate the genetic association between COVID-19 and autoimmune diseases. Additionally, the studies must characterize the risk for COVID-19 development in patients having pre-existing autoimmune diseases, which might be helpful in treatment and management of both COVID-19 and autoimmune disease patients.
References
Ahluwalia TS, Prins BP, Abdollahi M et al (2021) Genome-wide association study of circulating interleukin 6 levels identifies novel loci. Hum Mol Genet 30:393–409. https://doi.org/10.1093/HMG/DDAB023
Aidlen D, Henzy J (2021) Assessment of correlations between risk factors and symptom presentation among defined at-risk groups following a confirmed COVID-19 diagnosis. medRxiv:2021.11.30.21267029. https://doi.org/10.1101/2021.11.30.21267029
Akiyama S, Hamdeh S, Micic D, Sakuraba A (2021) Prevalence and clinical outcomes of COVID-19 in patients with autoimmune diseases: a systematic review and meta-analysis. Ann Rheum Dis 80:384–391. https://doi.org/10.1136/annrheumdis-2020-218946
Almutairi K, Nossent J, Preen D, Keen H, Inderjeeth C (2021) The global prevalence of rheumatoid arthritis: a meta-analysis based on a systematic review. Rheumatol Int 41:863–877. https://doi.org/10.1007/S00296-020-04731-0
Amirfakhryan H (2020) Kawasaki-like disease in children with COVID-19: a hypothesis. Med Hypotheses 143:110117. https://doi.org/10.1016/J.MEHY.2020.110117
Angileri F, Légaré S, Marino Gammazza A, Conway de Macario E, Macario AJL, Cappello F (2020) Is molecular mimicry the culprit in the autoimmune haemolytic anaemia affecting patients with COVID-19? Br J Haematol 190:e92–e93. https://doi.org/10.1111/BJH.16883
Arango M-T, Shoenfeld Y, Cervera R, Anaya J-M (2013) Infection and autoimmune diseases. El Rosario University Press, Bogota
Arbour N, Day R, Newcombe J, Talbot PJ (2000) Neuroinvasion by human respiratory coronaviruses. J Virol 74:8913–8921. https://doi.org/10.1128/JVI.74.19.8913-8921.2000
Bachiller-Corral J, Boteanu A, Garcia-Villanueva MJ et al (2021) Risk of severe COVID-19 infection in patients with inflammatory rheumatic diseases. J Rheumatol 48:1098–1102. https://doi.org/10.3899/jrheum.200755
Barber MRW, Drenkard C, Falasinnu T et al (2021) Global epidemiology of systemic lupus erythematosus. Nat Rev Rheumatol 17:515–532. https://doi.org/10.1038/S41584-021-00668-1
Bastard P, Rosen LB, Zhang Q et al (2020) Autoantibodies against type I IFNs in patients with life-threatening COVID-19. Science 370:eabd4585. https://doi.org/10.1126/SCIENCE.ABD4585
Beom Kim G (2019) Reality of Kawasaki disease epidemiology. Kor J Pediatr 62:292–296. https://doi.org/10.3345/KJP.2019.00157
Berentsen S, Tjønnfjord GE (2012) Diagnosis and treatment of cold agglutinin mediated autoimmune hemolytic anemia. Blood Rev 26:107–115. https://doi.org/10.1016/J.BLRE.2012.01.002
Bergamaschi L, Mescia F, Turner L et al (2021a) Delayed bystander CD8 T cell activation, early immune pathology and persistent dysregulation characterise severe COVID-19. medRxiv:2021.01.11.20248765. https://doi.org/10.1101/2021.01.11.20248765
Bergamaschi L, Mescia F, Turner L et al (2021b) Longitudinal analysis reveals that delayed bystander CD8+ T cell activation and early immune pathology distinguish severe COVID-19 from mild disease. Immunity 54:1257–1275.e8. https://doi.org/10.1016/J.IMMUNI.2021.05.010
Bergqvist C, Ezzedine K (2020) Vitiligo: a review. Dermatology 236:1–22. https://doi.org/10.1159/000506103
Bertin D, Brodovitch A, Beziane A et al (2020) Anticardiolipin IgG autoantibody level is an independent risk factor for COVID-19 severity. Arthritis Rheumatol 72:1953–1955. https://doi.org/10.1002/ART.41409
Bhagat YV, Hussien S, Queenan H, Michael MB (2021) Exacerbation of secondary cold agglutinin syndrome in the setting of SARS-CoV-2. Cureus 13:e19387. https://doi.org/10.7759/CUREUS.19387
Bhattacharjee S, Banerjee M (2020) Immune thrombocytopenia secondary to COVID-19: a systematic review. SN Compr Clin Med 2:2048–2058. https://doi.org/10.1007/S42399-020-00521-8
Biswas S, Ghosh R, Mandal A et al (2022) COVID-19 induced miller fisher syndrome presenting with autonomic dysfunction: a unique case report and review of literature. Neurohospitalist 12:111–116. https://doi.org/10.1177/19418744211016709
Bragazzi NL, Kolahi AA, Nejadghaderi SA et al (2021) Global, regional, and national burden of Guillain–Barré syndrome and its underlying causes from 1990 to 2019. J Neuroinflammation 18:1–11. https://doi.org/10.1186/S12974-021-02319-4/FIGURES/5
Cavalli E, Bramanti A, Ciurleo R et al (2020) Entangling COVID-19 associated thrombosis into a secondary antiphospholipid antibody syndrome: diagnostic and therapeutic perspectives (Review). Int J Mol Med 46:903–912. https://doi.org/10.3892/ijmm.2020.4659
Cervera R (2017) Antiphospholipid syndrome. Thromb Res 151(Suppl 1):S43–S47. https://doi.org/10.1016/S0049-3848(17)30066-X
Chandrashekara S (2012) The treatment strategies of autoimmune disease may need a different approach from conventional protocol: a review. Indian J Pharm 44:665–671
Chang SE, Feng A, Meng W et al (2021) New-onset IgG autoantibodies in hospitalized patients with COVID-19. Nat Commun 12:5417. https://doi.org/10.1038/s41467-021-25509-3
Chee YJ, Ng SJH, Yeoh E (2020) Diabetic ketoacidosis precipitated by Covid-19 in a patient with newly diagnosed diabetes mellitus. Diabetes Res Clin Pract 164:108166. https://doi.org/10.1016/J.DIABRES.2020.108166
Chen WH, Strych U, Hotez PJ, Bottazzi ME (2020) The SARS-CoV-2 vaccine pipeline: an overview. Curr Trop Med Rep 7:61–64. https://doi.org/10.1007/S40475-020-00201-6
Choi J, Kim ST, Craft J (2012) The pathogenesis of systemic lupus erythematosus-an update. Curr Opin Immunol 24:651–657. https://doi.org/10.1016/J.COI.2012.10.004
Chowdhury S, Goswami S (2020) COVID-19 and type 1 diabetes: dealing with the difficult duo. Int J Diab Dev Ctries 40:315–320. https://doi.org/10.1007/S13410-020-00846-Z
Christensen RE, Jafferany M (2022) Association between alopecia areata and COVID-19: a systematic review. JAAD Int 7:57–61. https://doi.org/10.1016/J.JDIN.2022.02.002
Constantinescu CS, Farooqi N, O’Brien K, Gran B (2011) Experimental autoimmune encephalomyelitis (EAE) as a model for multiple sclerosis (MS). Br J Pharmacol 164:1079–1106. https://doi.org/10.1111/J.1476-5381.2011.01302.X
Cooper N, Kruse A, Kruse C et al (2021) Immune thrombocytopenia (ITP) World Impact Survey (I-WISh): impact of ITP on health-related quality of life. Am J Hematol 96:199. https://doi.org/10.1002/AJH.26036
Costenbader KH, Gay S, Alarcón-Riquelme ME, Iaccarino L, Doria A (2012) Genes, epigenetic regulation and environmental factors: which is the most relevant in developing autoimmune diseases? Autoimmun Rev 11:604–609. https://doi.org/10.1016/j.autrev.2011.10.022
Dayan CM, Daniels GH (1996) Chronic autoimmune thyroiditis. N Engl J Med 335:99–107. https://doi.org/10.1056/NEJM199607113350206
Dehghani Firouzabadi M, Dehghani Firouzabadi F, Goudarzi S, Jahandideh H, Roomiani M (2020) Has the chief complaint of patients with COVID-19 disease changed over time? Med Hypotheses 144:109974. https://doi.org/10.1016/J.MEHY.2020.109974
Derksen VFAM, Kissel T, Lamers-Karnebeek FBG et al (2021) Onset of rheumatoid arthritis after COVID-19: coincidence or connected? Ann Rheum Dis 80:1096–1098. https://doi.org/10.1136/ANNRHEUMDIS-2021-219859
Dewanjee S, Kandimalla R, Kalra RS et al (2021) COVID-19 and rheumatoid arthritis crosstalk: emerging association, therapeutic options and challenges. Cells 10:3291. https://doi.org/10.3390/CELLS10123291
Doğan Z, Özdemir P, Ekşioğlu M, Filik L (2014) Relationship between Helicobacter pylori infection and vitiligo: a prospective study. Am J Clin Dermatol 15:457–462. https://doi.org/10.1007/s40257-014-0087-3
Dotan A, Shoenfeld Y (2021) [COVID-19 and autoimmune diseases]. Harefuah. 160:62–67
Dotan A, Muller S, Kanduc D, David P, Halpert G, Shoenfeld Y (2021a) The SARS-CoV-2 as an instrumental trigger of autoimmunity. Autoimmun Rev 20:102792. https://doi.org/10.1016/j.autrev.2021.102792
Dotan A, Kanduc D, Muller S, Makatsariya A, Shoenfeld Y (2021b) Molecular mimicry between SARS-CoV-2 and the female reproductive system. Am J Reprod Immunol 86:e13494. https://doi.org/10.1111/aji.13494
Dwivedi M, Laddha NC, Arora P, Marfatia YS, Begum R (2013a) Decreased regulatory T-cells and CD4(+) /CD8(+) ratio correlate with disease onset and progression in patients with generalized vitiligo. Pigment Cell Melanoma Res 26:586–591. https://doi.org/10.1111/pcmr.12105
Dwivedi M, Laddha NC, Shah K, Shah BJ, Begum R (2013b) Involvement of interferon-gamma genetic variants and intercellular adhesion molecule-1 in onset and progression of generalized Vitiligo. J Interf Cytokine Res 33:646–659. https://doi.org/10.1089/jir.2012.0171
Dwivedi M, Laddha N, Begum R (2018) Viral causes of vitiligo: a new perspective for vitiligo pathogenesis. Viral Immunol 2:1–4
Edwards AE, Vathenen R, Henson SM, Finer S, Gunganah K (2021) Acute hyperglycaemic crisis after vaccination against COVID-19: a case series. Diabet Med 38:e14631. https://doi.org/10.1111/DME.14631
Eggenhuizen PJ, Ng BH, Chang J et al (2022) Heterologous immunity between SARS-CoV-2 and pathogenic bacteria. Front Immunol 13:155. https://doi.org/10.3389/FIMMU.2022.821595/BIBTEX
Ehrenfeld M, Tincani A, Andreoli L et al (2020) Covid-19 and autoimmunity. Autoimmun Rev 19:102597. https://doi.org/10.1016/j.autrev.2020.102597
Eizirik DL, Op de Beeck A (2018) Coxsackievirus and type 1 diabetes mellitus: the Wolf’s footprints. Trends Endocrinol Metab 29:137–139. https://doi.org/10.1016/J.TEM.2017.12.002
Elalfy MS, Nugent D (2016) Viruses, anti-viral therapy, and viral vaccines in children with immune thrombocytopenia. Semin Hematol 53(Suppl 1):S70–S72. https://doi.org/10.1053/j.seminhematol.2016.04.021
El-Gayyar M, Helmy M, Amer E, Elsaied M, Gaballah M (2020) Antimelanocyte antibodies: a possible role in patients with vitiligo. Indian J Dermatol 65:33. https://doi.org/10.4103/IJD.IJD_344_18
Ercolini AM, Miller SD (2009) The role of infections in autoimmune disease. Clin Exp Immunol 155:1–15. https://doi.org/10.1111/J.1365-2249.2008.03834.X
Feghali K, Atallah J, Norman C (2021) Manifestations of thyroid disease post COVID-19 illness: report of Hashimoto thyroiditis, Graves’ disease, and subacute thyroiditis. J Clin Transl Endocrinol Case Rep 22:100094. https://doi.org/10.1016/J.JECR.2021.100094
Firouzabadi FD, Firouzabadi MD, Ghalehbaghi B, Jahandideh H, Roomiani M, Goudarzi S (2020) Have the symptoms of patients with COVID-19 changed over time during hospitalization? Med Hypotheses 143:110067. https://doi.org/10.1016/J.MEHY.2020.110067
Franke C, Ferse C, Kreye J et al (2021) High frequency of cerebrospinal fluid autoantibodies in COVID-19 patients with neurological symptoms. Brain Behav Immun 93:415. https://doi.org/10.1016/J.BBI.2020.12.022
Freites Nuñez DD, Leon L, Mucientes A et al (2020) Risk factors for hospital admissions related to COVID-19 in patients with autoimmune inflammatory rheumatic diseases. Ann Rheum Dis 79:1393–1399. https://doi.org/10.1136/ANNRHEUMDIS-2020-217984
Fujinami RS, von Herrath MG, Christen U, Whitton JL (2006) Molecular mimicry, bystander activation, or viral persistence: infections and autoimmune disease. Clin Microbiol Rev 19:80–94. https://doi.org/10.1128/CMR.19.1.80-94.2006
Fukuyama M, Ito T, Ohyama M (2022) Alopecia areata: current understanding of the pathophysiology and update on therapeutic approaches, featuring the Japanese Dermatological Association guidelines. J Dermatol 49:19–36. https://doi.org/10.1111/1346-8138.16207
Galeotti C, Bayry J (2020) Autoimmune and inflammatory diseases following COVID-19. Nat Rev Rheumatol 16:413–414. https://doi.org/10.1038/s41584-020-0448-7
Gan MJ, Albanese-O’Neill A, Haller MJ (2012) Type 1 diabetes: current concepts in epidemiology, pathophysiology, clinical care, and research. Curr Probl Pediatr Adolesc Health Care 42:269–291. https://doi.org/10.1016/J.CPPEDS.2012.07.002
Gartshteyn Y, Askanase AD, Schmidt NM et al (2020) COVID-19 and systemic lupus erythematosus: a case series. Lancet Rheumatol 2:e452. https://doi.org/10.1016/S2665-9913(20)30161-2
Gatterre P, Oualha M, Dupic L et al (2012) Kawasaki disease: an unexpected etiology of shock and multiple organ dysfunction syndrome. Intensive Care Med 38:872–878. https://doi.org/10.1007/S00134-012-2473-8
Getts DR, Chastain EML, Terry RL, Miller SD (2013) Virus infection, antiviral immunity, and autoimmunity. Immunol Rev 255:197–209. https://doi.org/10.1111/IMR.12091
Giri PS, Dwivedi M, Laddha NC, Begum R, Bharti AH (2020a) Altered expression of nuclear factor of activated T cells, forkhead box P3, and immune-suppressive genes in regulatory T cells of generalized vitiligo patients. Pigment Cell Melanoma Res 33:566–578. https://doi.org/10.1111/pcmr.12862
Giri PS, Dwivedi M, Begum R (2020b) Decreased suppression of CD8+ and CD4+ T cells by peripheral regulatory T cells in generalized vitiligo due to reduced NFATC1 and FOXP3 proteins. Exp Dermatol 29:759–775. https://doi.org/10.1111/EXD.14157
Giri P, Shah F, Gupta B et al (2021a) Genetic association of interleukin-4 VNTR polymorphism with susceptibility to rheumatoid arthritis in south gujarat population. Gene Rep 25:101322
Giri PS, Begum R, Dwivedi M (2021b) Meta-analysis for association of TNFA-308(G > A) SNP with vitiligo susceptibility. Gene 809:146027. https://doi.org/10.1016/j.gene.2021.146027
Giri PS, Shah F, Dwivedi MK (2022) Probiotics and prebiotics in the suppression of autoimmune diseases. In: Probiotics in the prevention and management of human diseases. Academic Press, London, pp 161–186. https://doi.org/10.1016/B978-0-12-823733-5.00019-2
González JM, Gomez-Puertas P, Cavanagh D, Gorbalenya AE, Enjuanes L (2003) A comparative sequence analysis to revise the current taxonomy of the family Coronaviridae. Arch Virol 148:2207–2235. https://doi.org/10.1007/s00705-003-0162-1
Gracia-Ramos AE, Martin-Nares E, Hernández-Molina G (2021) New onset of autoimmune diseases following COVID-19 diagnosis. Cells 10:3592. https://doi.org/10.3390/CELLS10123592
Guo H, Cheng Y, Shapiro J, McElwee K (2015) The role of lymphocytes in the development and treatment of alopecia areata. Expert Rev Clin Immunol 11:1335–1351. https://doi.org/10.1586/1744666X.2015.1085306
Haberman R, Axelrad J, Chen A et al (2020) Covid-19 in immune-mediated inflammatory diseases — case series from New York. N Engl J Med 383:85–88. https://doi.org/10.1056/NEJMC2009567/SUPPL_FILE/NEJMC2009567_DISCLOSURES.PDF
Halpert G, Shoenfeld Y (2020) SARS-CoV-2, the autoimmune virus. Autoimmun Rev 19:102695. https://doi.org/10.1016/j.autrev.2020.102695
Halpert G, Watad A, Tsur AM et al (2021) Autoimmune dysautonomia in women with silicone breast implants. J Autoimmun 120:102631. https://doi.org/10.1016/j.jaut.2021.102631
Harris JE (2015) IFN-γ in vitiligo, is it the fuel or the fire? Acta Derm Venereol 95:643–644. https://doi.org/10.2340/00015555-2137
Heaney AI, Griffin GD, Simon EL (2020) Newly diagnosed diabetes and diabetic ketoacidosis precipitated by COVID-19 infection. Am J Emerg Med 38:2491.e3. https://doi.org/10.1016/J.AJEM.2020.05.114
Herzum A, Micalizzi C, Molle MF, Parodi A (2022) New-onset vitiligo following COVID-19 disease. Ski Heal Dis 2:e86. https://doi.org/10.1002/SKI2.86
Horisberger A, Moi L, Ribi C, Comte D (2020) Impact of COVID-19 pandemic on SLE: beyond the risk of infection. Lupus Sci Med 7:408. https://doi.org/10.1136/lupus-2020-000408
House NNC, Palissery S, Sebastian H (2021) Corona viruses: a review on SARS, MERS and COVID-19. Microbiol Insights 14:11786361211002481. https://doi.org/10.1177/11786361211002481
Hu B, Guo H, Zhou P, Shi Z-L (2020) Characteristics of SARS-CoV-2 and COVID-19. Nat Rev Microbiol 19(3):141–154. https://doi.org/10.1038/s41579-020-00459-7
Huang C, Wang Y, Li X et al (2020) Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 395:497–506. https://doi.org/10.1016/S0140-6736(20)30183-5
Huscenot T, Galland J, Ouvrat M, Rossignol M, Mouly S, Sène D (2020) SARS-CoV-2-associated cold agglutinin disease: a report of two cases. Ann Hematol 99:1943. https://doi.org/10.1007/S00277-020-04129-9
Icenogle T (2020) COVID-19: infection or autoimmunity. Front Immunol 11:2055. https://doi.org/10.3389/FIMMU.2020.02055/BIBTEX
Jadeja SD, Mayatra JM, Vaishnav J, Shukla N, Begum R (2021) A concise review on the role of endoplasmic reticulum stress in the development of autoimmunity in vitiligo pathogenesis. Front Immunol 11:3817
Jensen CE, Wilson S, Thombare A, Weiss S, Ma A (2020) Cold agglutinin syndrome as a complication of Covid-19 in two cases. Clin Infect Pract 7:100041. https://doi.org/10.1016/J.CLINPR.2020.100041
Juárez-Rendón KJ, Sánchez GR, Reyes-López M et al (2017) Alopecia Areata. Current situation and perspectives. Arch Argent Pediatr 115:E404–E411. https://doi.org/10.5546/AAP.2017.ENG.E404
Jung AL, Herkt CE, Schulz C et al (2017) Legionella pneumophila infection activates bystander cells differentially by bacterial and host cell vesicles. Sci Rep 7:6301. https://doi.org/10.1038/s41598-017-06443-1
Kanduc D, Shoenfeld Y (2020) Molecular mimicry between SARS-CoV-2 spike glycoprotein and mammalian proteomes: implications for the vaccine. Immunol Res 68:310–313. https://doi.org/10.1007/s12026-020-09152-6
Kaur J, Mogulla S, Khan R, Krishnamoorthy G, Garg S (2021) Transient cold agglutinins in a patient with COVID-19. Cureus 13:e12751. https://doi.org/10.7759/CUREUS.12751
Knight JS, Kanthi Y (2022) Mechanisms of immunothrombosis and vasculopathy in antiphospholipid syndrome. Semin Immunopathol 44:347. https://doi.org/10.1007/S00281-022-00916-W
Knight JS, Caricchio R, Casanova J-L et al (2021) The intersection of COVID-19 and autoimmunity. J Clin Invest 131:e154886. https://doi.org/10.1172/JCI154886
Kumar D, Malviya R (2020) Corona virus: a review of COVID-19. EJMO 4:8. https://doi.org/10.14744/ejmo.2020.51418
Laddha NC, Dwivedi M, Begum R et al (2012) Tumor necrosis factor a promotor polymorphism and nonsegmental vitiligo: a molecular susceptibility marker in Egyptian women. PLoS One 3:1–17. https://doi.org/10.1111/ced.12446
Laddha NC, Dwivedi M, Mansuri MS et al (2013) Vitiligo: interplay between oxidative stress and immune system. Exp Dermatol 22:245–250. https://doi.org/10.1111/exd.12103
Leis AA, Szatmary G, Ross MA, Stokic DS (2014) West nile virus infection and myasthenia gravis. Muscle Nerve 49:26–29. https://doi.org/10.1002/MUS.23869
Leslie RD, Hawa M (1994) Twin studies in auto-immune disease. Acta Genet Med Gemellol 43:71–81. https://doi.org/10.1017/s000156600000297x
Li X, Geng M, Peng Y, Meng L, Lu S (2020) Molecular immune pathogenesis and diagnosis of COVID-19. J Pharm Anal 10:102–108. https://doi.org/10.1016/J.JPHA.2020.03.001
Liebman HA (2008) Viral-associated immune thrombocytopenic purpura. Hematology 2008:212–218. https://doi.org/10.1182/ASHEDUCATION-2008.1.212
Lin YL, Askonas BA (1981) Biological properties of an influenza A virus-specific killer T cell clone. Inhibition of virus replication in vivo and induction of delayed-type hypersensitivity reactions. J Exp Med 154:225–234. https://doi.org/10.1084/JEM.154.2.225
Littera R, Campagna M, Deidda S et al (2020) Human leukocyte antigen complex and other immunogenetic and clinical factors influence susceptibility or protection to SARS-CoV-2 infection and severity of the disease course. The Sardinian experience. Front Immunol 11:605688. https://doi.org/10.3389/FIMMU.2020.605688
Liu Y, Sawalha AH, Lu Q (2021) COVID-19 and autoimmune diseases. Curr Opin Rheumatol 33:155–162. https://doi.org/10.1097/BOR.0000000000000776
Lorente L, Martín MM, Franco A et al (2021) HLA genetic polymorphisms and prognosis of patients with COVID-19. Med Intensiva 45:96–103. https://doi.org/10.1016/J.MEDIN.2020.08.004
Lui DTW, Lee CH, Chow WS et al (2021) Insights from a prospective follow-up of thyroid function and autoimmunity among COVID-19 survivors. Endocrinol Metab 36:582–589. https://doi.org/10.3803/ENM.2021.983
Lünemann JD (2012) Epstein-Barr virus in multiple sclerosis: a continuing conundrum. Neurology 78:11–12. https://doi.org/10.1212/WNL.0B013E318241F2B3
Martinez OM, Bridges ND, Goldmuntz E, Pascual V (2020) The immune roadmap for understanding multi-system inflammatory syndrome in children: opportunities and challenges. Nat Med 26(12):1819–1824. https://doi.org/10.1038/s41591-020-1140-9
Maslov DV, Simenson V, Jain S, Badari A (2020) COVID-19 and cold agglutinin hemolytic anemia. TH Open 4:e175–e177. https://doi.org/10.1055/S-0040-1715791
McMillan P, Dexhiemer T, Neubig RR, Uhal BD (2021) COVID-19—a theory of autoimmunity against ACE-2 explained. Front Immunol 12:499
Mehandru S, Merad M (2022) Pathological sequelae of long-haul COVID. Nat Immunol 23:194–202. https://doi.org/10.1038/s41590-021-01104-y
Miller SD, Vanderlugt CL, Begolka WS et al (1997) Persistent infection with Theiler’s virus leads to CNS autoimmunity via epitope spreading. Nat Med 3:1133–1136. https://doi.org/10.1038/NM1097-1133
Minich LLA, Sleeper LA, Atz AM et al (2007) Delayed diagnosis of Kawasaki disease: what are the risk factors? Pediatrics 120:e1434. https://doi.org/10.1542/PEDS.2007-0815
Minokawa Y, Sawada Y, Nakamura M (2022) Lifestyle factors involved in the pathogenesis of alopecia areata. Int J Mol Sci 23:1038. https://doi.org/10.3390/IJMS23031038
Mohammed RHA (2020) Introductory Chapter: Rheumatoid arthritis - overview of current facts and strategies. In: Rheumatoid arthritis - other perspectives towards a better practice. IntechOpen, London. https://doi.org/10.5772/INTECHOPEN.92771
Moody R, Wilson K, Flanagan KL, Jaworowski A, Plebanski M (2021) Adaptive immunity and the risk of autoreactivity in COVID-19. Int J Mol Sci 22:8965. https://doi.org/10.3390/IJMS22168965
Moran AP, Prendergast MM (2001) Molecular mimicry in Campylobacter jejuni and Helicobacter pylori lipopolysaccharides: contribution of gastrointestinal infections to autoimmunity. J Autoimmun 16:241–256. https://doi.org/10.1006/jaut.2000.0490
Mukarram MS, Ishaq Ghauri M, Sethar S, Afsar N, Riaz A, Ishaq K (2021) COVID-19: an emerging culprit of inflammatory arthritis. Case Rep Rheumatol 2021:1–8. https://doi.org/10.1155/2021/6610340
Newburger JW, Takahashi M, Gerber MA et al (2004) Diagnosis, treatment, and long-term management of Kawasaki disease: a statement for health professionals from the Committee on Rheumatic Fever, Endocarditis and Kawasaki Disease, Council on Cardiovascular Disease in the Young, American Heart Association. Circulation 110:2747–2771. https://doi.org/10.1161/01.CIR.0000145143.19711.78
Pablos JL, Galindo M, Carmona L et al (2020) Clinical outcomes of hospitalised patients with COVID-19 and chronic inflammatory and autoimmune rheumatic diseases: a multicentric matched cohort study. Ann Rheum Dis 79:1544–1549. https://doi.org/10.1136/annrheumdis-2020-218296
Pacheco Y, Acosta-Ampudia Y, Monsalve DM, Chang C, Gershwin ME, Anaya J-M (2019) Bystander activation and autoimmunity. J Autoimmun 103:102301. https://doi.org/10.1016/j.jaut.2019.06.012
Pak CY, Mcarthur RG, Eun HM, Yoon JW (1988) Association of cytomegalovirus infection with autoimmune type 1 diabetes. Lancet 2:1–4. https://doi.org/10.1016/S0140-6736(88)92941-8
Patil NR, Herc ES, Girgis M (2020) Cold agglutinin disease and autoimmune hemolytic anemia with pulmonary embolism as a presentation of COVID-19 infection. Hematol Oncol Stem Cell Ther. https://doi.org/10.1016/J.HEMONC.2020.06.005
Perrot L, Hemon M, Busnel JM et al (2021) First flare of ACPA-positive rheumatoid arthritis after SARS-CoV-2 infection. Lancet Rheumatol 3:e6. https://doi.org/10.1016/S2665-9913(20)30396-9
Phetsouphanh C, Darley DR, Wilson DB et al (2022) Immunological dysfunction persists for 8 months following initial mild-to-moderate SARS-CoV-2 infection. Nat Immunol 23:210–216. https://doi.org/10.1038/s41590-021-01113-x
Post NF, Luiten RM, Wolkerstorfer A, Bekkenk MW, Böhm M (2021) Does autoimmune vitiligo protect against COVID-19 disease? Exp Dermatol 30:1254–1257
Qin C, Zhou L, Hu Z et al (2020) Dysregulation of immune response in patients with coronavirus 2019 (COVID-19) in Wuhan, China. Clin Infect Dis 71:762–768. https://doi.org/10.1093/CID/CIAA248
Ragab D, Salah Eldin H, Taeimah M, Khattab R, Salem R (2020) The COVID-19 cytokine storm; what we know so far. Front Immunol 11:1446. https://doi.org/10.3389/FIMMU.2020.01446/BIBTEX
Ramos-Casals M, Brito-Zerón P, Mariette X (2021) Systemic and organ-specific immune-related manifestations of COVID-19. Nat Rev Rheumatol 17(6):315–332. https://doi.org/10.1038/s41584-021-00608-z
Rodriguez-Perez AI, Labandeira CM, Pedrosa MA et al (2021) Autoantibodies against ACE2 and angiotensin type-1 receptors increase severity of COVID-19. J Autoimmun 122:102683. https://doi.org/10.1016/j.jaut.2021.102683
Ruan Q, Yang K, Wang W, Jiang L, Song J (2020) Correction to: clinical predictors of mortality due to COVID-19 based on an analysis of data of 150 patients from Wuhan, China (Intensive Care Medicine, (2020), 46, 5, (846-848), 10.1007/s00134-020-05991-x). Intensive Care Med 46:1294–1297. https://doi.org/10.1007/S00134-020-06028-Z/FIGURES/1
Rudnicka L, Rakowska A, Waskiel-Burnat A, Kurzeja M, Olszewska M (2021) Mild-to-moderate COVID-19 is not associated with worsening of alopecia areata: a retrospective analysis of 32 patients. J Am Acad Dermatol 85:723–725. https://doi.org/10.1016/J.JAAD.2021.05.020
Ruggeri RM, Campennì A, Deandreis D et al (2021) SARS-COV-2-related immune-inflammatory thyroid disorders: facts and perspectives. Expert Rev Clin Immunol 17:737–759. https://doi.org/10.1080/1744666X.2021.1932467
Ryabkova VA, Churilov LP, Shoenfeld Y (2021) Influenza infection, SARS, MERS and COVID-19: cytokine storm - the common denominator and the lessons to be learned. Clin Immunol 223:108652. https://doi.org/10.1016/j.clim.2020.108652
Salmi A, Ziola B, Hovi T, Reunanen M (1982) Antibodies to coronaviruses OC43 and 229E in multiple sclerosis patients. Neurology 32:292–295. https://doi.org/10.1212/WNL.32.3.292
Santos IA, Grosche VR, Bergamini FRG, Sabino-Silva R, Jardim ACG (2020) Antivirals against coronaviruses: candidate drugs for SARS-CoV-2 treatment? Front Microbiol 11:1818. https://doi.org/10.3389/FMICB.2020.01818
Shoenfeld Y (2020) Corona (COVID-19) time musings: our involvement in COVID-19 pathogenesis, diagnosis, treatment and vaccine planning. Autoimmun Rev 19:102538
Shoenfeld Y, Aharon-Maor A, Sherer Y (2000) Vaccination as an additional player in the mosaic of autoimmunity. Clin Exp Rheumatol 18:181–184
Shoraka S, Ferreira MLB, Mohebbi SR, Ghaemi A (2021) SARS-CoV-2 infection and Guillain-Barré syndrome: a review on potential pathogenic mechanisms. Front Immunol 12:674922. https://doi.org/10.3389/FIMMU.2021.674922
Simmonds MJ, Gough SCL (2004) Unravelling the genetic complexity of autoimmune thyroid disease: HLA, CTLA-4 and beyond. Clin Exp Immunol 136:1. https://doi.org/10.1111/J.1365-2249.2004.02424.X
Singhal T (2020) A review of coronavirus disease-2019 (COVID-19). Indian J Pediatr 87:281–286. https://doi.org/10.1007/s12098-020-03263-6
Smatti MK, Cyprian FS, Nasrallah GK, Al Thani AA, Almishal RO, Yassine HM (2019) Viruses and autoimmunity: a review on the potential interaction and molecular mechanisms. Viruses 11:762. https://doi.org/10.3390/v11080762
Spihlman AP, Gadi N, Wu SC, Moulton VR (2020) COVID-19 and systemic lupus erythematosus: focus on immune response and therapeutics. Front Immunol 11:2861. https://doi.org/10.3389/FIMMU.2020.589474/BIBTEX
Stene LC, Rewers M (2012) Immunology in the clinic review series; focus on type 1 diabetes and viruses: the enterovirus link to type 1 diabetes: critical review of human studies. Clin Exp Immunol 168:12. https://doi.org/10.1111/J.1365-2249.2011.04555.X
Stewart JN, Mounir S, Talbot PJ (1992) Human coronavirus gene expression in the brains of multiple sclerosis patients. Virology 191:502–505. https://doi.org/10.1016/0042-6822(92)90220-J
Sun Y, Liu H, Yang B et al (2019) Investigation of the predisposing factor of pemphigus and its clinical subtype through a genome-wide association and next generation sequence analysis. J Eur Acad Dermatol Venereol 33:410–415. https://doi.org/10.1111/JDV.15227
Talbot PJ, Paquette JS, Ciurli C, Antel JP, Ouellet F (1996) Myelin basic protein and human coronavirus 229E cross-reactive T cells in multiple sclerosis. Ann Neurol 39:233–240. https://doi.org/10.1002/ANA.410390213
Talotta R (2021) Do COVID-19 RNA-based vaccines put at risk of immune-mediated diseases? In reply to “potential antigenic cross-reactivity between SARS-CoV-2 and human tissue with a possible link to an increase in autoimmune diseases”. Clin Immunol 224:108665. https://doi.org/10.1016/j.clim.2021.108665
Tammaro A, Adebanjo GAR, Parisella FR, Luzi F, Scarabello A (2022) Hair and nail manifestations of COVID-19. J Cosmet Dermatol 21:1339. https://doi.org/10.1111/JOCD.14774
Tan EH, Sena AG, Prats-Uribe A et al (2021) COVID-19 in patients with autoimmune diseases: characteristics and outcomes in a multinational network of cohorts across three countries. Rheumatology 60:SI37–SI50. https://doi.org/10.1093/RHEUMATOLOGY/KEAB250
Tay MZ, Poh CM, Rénia L, MacAry PA, Ng LFP (2020) The trinity of COVID-19: immunity, inflammation and intervention. Nat Rev Immunol 20:363–374. https://doi.org/10.1038/S41577-020-0311-8
Thanapati S, Ganu M, Giri P et al (2017) Impaired NK cell functionality and increased TNF-α production as biomarkers of chronic chikungunya arthritis and rheumatoid arthritis. Hum Immunol 78:370–374. https://doi.org/10.1016/j.humimm.2017.02.006
Tucker WG, Andrew Paskauskas R (2008) The MSMV hypothesis: measles virus and multiple sclerosis, etiology and treatment. Med Hypotheses 71:682–689. https://doi.org/10.1016/J.MEHY.2008.06.029
Tung ML, Tan B, Cherian R, Chandra B (2021) Anti-phospholipid syndrome and COVID-19 thrombosis: connecting the dots. Rheumatol Adv Pract 5:rkaa081. https://doi.org/10.1093/RAP/RKAA081
Velikova T, Georgiev T (2021) SARS-CoV-2 vaccines and autoimmune diseases amidst the COVID-19 crisis. Rheumatol Int 41:509–518. https://doi.org/10.1007/S00296-021-04792-9/FIGURES/1
Verdoni L, Mazza A, Gervasoni A et al (2020) An outbreak of severe Kawasaki-like disease at the Italian epicentre of the SARS-CoV-2 epidemic: an observational cohort study. Lancet 395:1771–1778. https://doi.org/10.1016/S0140-6736(20)31103-X
Vlachoyiannopoulos PG, Magira E, Alexopoulos H et al (2020) Autoantibodies related to systemic autoimmune rheumatic diseases in severely ill patients with COVID-19. Ann Rheum Dis 79:1661–1663. https://doi.org/10.1136/ANNRHEUMDIS-2020-218009
Vojdani A, Vojdani E, Kharrazian D (2021) Reaction of human monoclonal antibodies to SARS-CoV-2 proteins with tissue antigens: implications for autoimmune diseases. Front Immunol 11:3679. https://doi.org/10.3389/FIMMU.2020.617089/BIBTEX
Walls AC, Park YJ, Tortorici MA, Wall A, McGuire AT, Veesler D (2020) Structure, function, and antigenicity of the SARS-CoV-2 spike glycoprotein. Cell 181:281–292.e6. https://doi.org/10.1016/J.CELL.2020.02.058
Wang Y, Sun S, Shen H et al (2004) Cross-reaction of SARS-CoV antigen with autoantibodies in autoimmune diseases. Cell Mol Immunol 1:304–307
Wang L, Wang FS, Gershwin ME (2015) Human autoimmune diseases: a comprehensive update. J Intern Med 278:369–395
Wang M-Y, Zhao R, Gao L-J, Gao X-F, Wang D-P, Cao J-M (2020) SARS-CoV-2: structure, biology, and structure-based therapeutics development. Front Cell Infect Microbiol 10:587269. https://doi.org/10.3389/FCIMB.2020.587269
Wang Z, Schmidt F, Weisblum Y et al (2021) mRNA vaccine-elicited antibodies to SARS-CoV-2 and circulating variants. Nature 592:616–622. https://doi.org/10.1038/s41586-021-03324-6
Warren RL, Birol I (2020) Retrospective in silico HLA predictions from COVID-19 patients reveal alleles associated with disease prognosis. medRxiv:2020.10.27.20220863. https://doi.org/10.1101/2020.10.27.20220863
Xiao M, Zhang Y, Zhang S et al (2020) Antiphospholipid antibodies in critically ill patients with COVID-19. Arthritis Rheumatol 72:1998–2004. https://doi.org/10.1002/ART.41425
Yazdanpanah N, Rezaei N (2022) Autoimmune complications of COVID-19. J Med Virol 94:54–62. https://doi.org/10.1002/JMV.27292
Yu X, Ho K, Shen Z et al (2021) The association of human leukocyte antigen and COVID-19 in Southern China. Open Forum Infect Dis 8:ofab410. https://doi.org/10.1093/OFID/OFAB410
Zamani B, Moeini Taba SM, Shayestehpour M (2021) Systemic lupus erythematosus manifestation following COVID-19: a case report. J Med Case Rep 15:1–4. https://doi.org/10.1186/S13256-020-02582-8/FIGURES/2
Zen M, Fuzzi E, Astorri D et al (2020) SARS-CoV-2 infection in patients with autoimmune rheumatic diseases in northeast Italy: a cross-sectional study on 916 patients. J Autoimmun 112:102502. https://doi.org/10.1016/j.jaut.2020.102502
Zhao Y, Zhao Y, Zhang Y, Zhang L (2019) HLA-II genes are associated with outcomes of specific immunotherapy for allergic rhinitis. Int Forum Allergy Rhinol 9:1311–1317. https://doi.org/10.1002/ALR.22384
Zhao H, Shen D, Zhou H, Liu J, Chen S (2020) Guillain-Barré syndrome associated with SARS-CoV-2 infection: causality or coincidence? Lancet Neurol 19:383–384. https://doi.org/10.1016/S1474-4422(20)30109-5
Zhong J, Shen G, Yang H et al (2020) COVID-19 in patients with rheumatic disease in Hubei province, China: a multicentre retrospective observational study. Lancet Rheumatol 2:e557–e564. https://doi.org/10.1016/S2665-9913(20)30227-7
Zhou Y, Han T, Chen J et al (2020) Clinical and autoimmune characteristics of severe and critical cases of COVID-19. Clin Transl Sci 13:1077–1086. https://doi.org/10.1111/CTS.12805
Zuo Y, Estes SK, Ali RA et al (2020) Prothrombotic autoantibodies in serum from patients hospitalized with COVID-19. Sci Transl Med 12:eabd3876. https://doi.org/10.1126/SCITRANSLMED.ABD3876
Acknowledgments
This work was supported by grants to Dr. Mitesh Dwivedi {Grant no. ECR/2017/000858} and {CRG/2021/002419} Science & Engineering Research Board, Department of Science & Technology (SERB-DST), New Delhi, India. We are grateful to Uka Tarsadia University, Maliba Campus, Tarsadi, Gujarat, India for providing the facilities needed for the preparation of this chapter.
Author information
Authors and Affiliations
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2022 The Author(s), under exclusive license to Springer Nature Singapore Pte Ltd.
About this chapter
Cite this chapter
Giri, P.S., Jadeja, S.D., Shoenfeld, Y., Dwivedi, M.K. (2022). COVID-19 and Autoimmunity. In: Dwivedi, M.K., Sankaranarayanan, A., Kemp, E.H., Shoenfeld, Y. (eds) Role of Microorganisms in Pathogenesis and Management of Autoimmune Diseases. Springer, Singapore. https://doi.org/10.1007/978-981-19-4800-8_3
Download citation
DOI: https://doi.org/10.1007/978-981-19-4800-8_3
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-19-4799-5
Online ISBN: 978-981-19-4800-8
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)