Abstract
Recent cancer treatments have entered a new era with novel types of immunotherapies. In particular, immune checkpoint signals mediated by the immunosuppressive cofactors programmed cell death 1 (PD-1) and PD-1 ligand 1 (PD-L1), are the most promising targets for new cancer treatments.
Several clinical trials of various types of gynecologic cancers have been completed and revealed a modest antitumor effect with monotherapy with immune checkpoint inhibitors (ICIs; anti-PD-1 antibody and/or anti-PD-L1 antibody). However, genetic and/or molecular biomarker-selected endometrial cancer and cervical cancers are more promising for treatment with ICIs. Some ICIs have been approved by the FDA and the combination of ICIs with other agents has yielded good results in trials for these cancers. Therefore, the selection of patients who would benefit from ICI immunotherapy is quite important.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
6.1 Introduction
In the last decade, cancer treatment has been revolutionized by new types of immunotherapies, mainly immune checkpoint inhibitors (ICIs) such as anti-programmed cell death 1 (PD-1) antibodies and/or anti-PD ligand 1 (PD-L1) antibodies (Table 6.1), which have become standard treatments for several advanced solid tumors [1, 2] (Table 6.2). Based on the mechanism of action of these agents, several biomarkers to measure the response to treatment have been investigated in clinical trials and have led to the approval of ICI-based treatments [3, 4]. More recently, some clinical trials using ICI monotherapy have demonstrated promising antitumor effects for gynecologic cancers such as mismatch repair deficient (MMRd) or microsatellite instability (MSI)-high cases of endometrial cancers and PD-L1-expressing cervical cancer [5]. However, gynecologic cancers, particularly ovarian cancer, represent a heterogenous subgroup of histologies, and thus their responses to ICIs cannot be fully predicted using known biomarkers. Therefore, the optimal biomarkers for specific subtypes of patients with cancer are urgently required [4].
Conversely, combination immunotherapies with other antitumor therapies such as chemotherapy, targeted therapy, radiotherapy, or other immunotherapies have been expected to enhance the antitumor effect of ICIs in gynecologic cancers, and some of these have demonstrated promising synergistic effects.
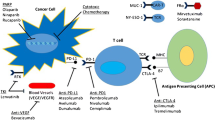
This chapter highlights the mechanism of action of ICIs and recent clinical trials of ICIs used to treat gynecologic cancers, including specific molecular- or genetic-based personalized immunotherapies (Fig. 6.1).
6.2 PD-1 Signal
PD-1 (CD279) is an immunosuppressive co-inhibitory molecule that belongs to the CD28 family of receptors on T cells. PD-1 was discovered in 1992 and is known to be an induced molecule on T cells undergoing apoptosis [6]. Additional studies demonstrated PD-1 expression on mature hematopoietic cells such as T and B cells, as well as monocytes, following activation [7]. The cognate ligands for PD-1 are the B7-family molecules, PD-L1 (CD274, B7-H1), and PD-L2 (CD273, B7-H2). PD-L1 is expressed in human tonsils, placenta, monocytes, and lungs, where it plays a role in immune tolerance. PD-L2 is mainly expressed in dendritic cells (DCs) under normal physiological conditions [8]. The PD-1/PD-L1/L2 signaling pathway has been shown to control excessive autoimmune and inflammatory responses. This pathway plays a key role in immune homeostasis, together with the B7–1/2/CTLA-4 signaling pathway described above [9]. The CTLA-4 signaling pathway is primarily involved in the process of antigen presentation in lymph nodes, whereas the key role of the PD-1 signaling pathway is to suppress the immune response to target cancer cells in peripheral tissue.
6.3 PD-1 Signal Inhibitors
Several clinical trials have utilized humanized anti-PD-1 antibodies (pembrolizumab, nivolumab, dostarlimab-gxly, cemiplimab-rwlc, and balstilimab) and anti-PD-L1 antibodies (atezolizumab, durvalumab, avelumab) for solid tumors as PD-1 signal inhibitors (Table 6.1). Subsequently, some antibodies have been approved by the FDA for gynecologic cancers (Table 6.2). Pembrolizumab, an anti-PD-1 antibody, was first approved for melanoma in 2014 and dostarlimab-gxly, another anti-PD-1 antibody, has been approved for mismatch repair deficient (MMRd) recurrent or advanced solid tumors. To date, at least 200 clinical studies have been carried out using some type of PD-1 signal inhibitors in gynecologic cancers (ClinicalTrials.gov) [10].
6.4 Personalized PD-1 Signal Inhibitors
Based on the mechanism of action of PD-1 signal inhibitors, several biomarkers for the response to treatment have been investigated in various clinical trials, including gynecologic cancers, and this has led to the approval of PD-1 signal inhibitors. Recent clinical trials of PD-1 signal inhibitors for gynecological cancer have demonstrated promising results in endometrial cancers and cervical cancers. Because gynecological cancers represent a heterogeneous group of tumors, the optimal biomarkers for a specific type of cancer have not yet been fully determined; some biological biomarkers known to be useful for the treatment of gynecologic cancers are shown in Fig. 6.1.
6.5 PD-L1 Expression
PD-L1 protein expression is a predictive biomarker of the efficacy of PD-1 inhibitors in several types of cancer including cervical cancer, but not in endometrial cancer and ovarian cancer, as demonstrated in previous immunotherapeutic clinical trials [11, 12]. PD-L1 gene amplification has been reported in 0.7% of 100,000 cases of more than 100 types of solid tumors, and specifically in 2.7% (10/374) cases of cervical cancer. Furthermore, although the number of cases is small, the response rate (RR) to PD-1 pathway inhibitors was 66.7% (6 responses) in 9 solid tumors with PD-L1 gene amplification [10], and the same has been reported in multiple cancer types including lung cancer and breast cancer. Therefore, PD-1 pathway inhibitors are often recommended for cancers with high PD-L1 gene expression including cervical cancers, and not for endometrial and ovarian cancers.
In the KEYNOTE-158 trial of pembrolizumab in previously treated recurrent cervical cancer, the RR was 14.6% in patients with high PD-L1 expression and 0% in patients with low expression. The FDA approved pembrolizumab for PD-L1–positive recurrent cervical cancer in 2018 [13]. The KEYNOTE-826 trial studied the use of pembrolizumab in untreated metastatic/advanced cervical cancer and combined standard chemotherapy with bevacizumab with or without pembrolizumab. This study found that both progression-free survival and overall survival improved in the pembrolizumab group, and following this, the FDA approved pembrolizumab as a first-line treatment for PD-L1 positive cervical cancer in 2022 [14]. Furthermore, in the randomized phase III EMPOWER-Cervical 1/GOG-3016/ENGOT-cx9 study with the novel anti-PD-1 antibody cemiplimab, cemiplimab demonstrated significantly longer overall survival compared to chemotherapy [11]. In the RaPiDS trial, another anti-PD-1 antibody, balstilimab, also demonstrated that the objective response rate (ORR) in PD-L1-positive patients was 20% and 8% in PD-L1 negative patients. The combination immunotherapy of balstilimab and the CTLA-4 inhibitor zalifrelimab resulted in an ORR of 27% and 11% in the PD-L1-positive and PD-L1-negative cohorts, respectively [15].
On the other hand, previous clinical trials of PD-1 signal inhibitors have shown no significant effect on PD-L1 expression in other gynecologic cancers [16].
6.6 Microsatellite Instability
Recent reports identified the frequency of genetic mutations derived from high microsatellite instability (MSI-High) with DNA mismatch repair deficiency (MMRd) in cancer cells as a candidate biomarker [17]. Many mutated antigens (called neoantigens) produced by MSI expressed on the surface of cancer cells are recognized by T cells and B cells as foreign antigens, either directly or through the APC system. Cancer cells exposed to IFN-γ released from activated T cells express PD-L1, thereby establishing an acquired immune resistance [18]. In this case, PD-1 signal inhibitors are more likely to be effective.
The frequency of MMRd /MSI-High in cancer varies by cancer type. In an analysis of 12,019 patients with MSI with 32 different types of cancer, MSI-High was identified in patients with 24 different carcinomas (2.2%). Endometrial cancer was the most common (17%) cancer with MSI-High, while ovarian cancer (3%) and cervical cancer were rare [5]. Therefore, MMRd/MSI-High endometrial cancer has become the focus of research as a good target for PD-1 signal inhibitors.
In the KEYNOTE-058 study of pembrolizumab in MMRd/MSI-High solid tumors, a high response rate was reported for MSI-H endometrial cancer in 28 of 49 patients (RR: 57%) and also for MMRd/MSI-High ovarian cancer in 5 of 15 patients (RR: 33%). The FDA has approved pembrolizumab for MMRd/MSI-High solid tumors across cancer types [19]. In addition to pembrolizumab, several other PD-1 pathway inhibitors such as dostarlimab-gxly (PD-1) and the antibodies avelumab (PD-L1) and durvalumab (PD-L1) have also been shown to be significantly more effective in patients with MMRd or MSI-High [20,21,22].
On the other hand, the KEYNOTE146 trial (Phase I/II), which investigated the efficacy of combination therapy of pembrolizumab with the multi-kinase inhibitor lenvatinib, showed a high RR of 57%, and was approved by the FDA for microsatellite stable (MSS)/mismatch repair proficient (MMRp) in 2019. The KEYNOTE-775/309 trial (phase III) demonstrated pembrolizumab in combination with lenvatinib prolonged overall survival, regardless of MMR abnormality [23].
6.7 Tumor Mutational Burden (TMB)
Recent comprehensive genetic mutation analysis of cancer tissues using next generation sequencing has revealed that, among the same cancer types, patients with high somatic gene mutations (tumor mutational burden-high: TMB-High) have high immunogenicity (immunoreactivity) due to the release of neoantigens, and the PD-1 pathway is induced by immune homeostatic reactions. In this situation, PD-1 pathway inhibitors reactivate the antitumor effect with increased immune cell infiltration into the tumor [24].
The KEYNOTE-158 trial of pembrolizumab in multiple solid tumors showed that the RR of the TMB-High group (TMB ≥10 mut/Mb, n = 102) was 29%, while that of the Non-TMB-High group (n = 688) was 6% [25]. In 2020, the FDA approved pembrolizumab for TMB-High solid tumors (≥10 mut/Mb), including gynecologic cancers.
The threshold of 10 mut/Mb of TMB-High is open to some debate. Recent research has demonstrated that not all types of TMB-High tumors such as brain tumors, unselected colon cancer, and esophageal cancer have demonstrated a favorable response to PD-1 signal inhibitors [26]. As for gynecological cancers, TMB-H predicts good responses in endometrial cancer but not in cervical cancer and ovarian cancer [27, 28].
6.8 Genomic Instability Score
Genomic Instability Score (GIS) is an algorithmic measurement of loss of heterozygosity, telomeric allelic imbalance, and large-scale state transitions by a next generation sequencing-based in vitro diagnostic test of tumor tissue specimens [29]. The results of the test are used to aid the identification of patients with ovarian cancer with positive homologous recombination deficiency status, who are eligible for treatment with poly (ADP-ribose) polymerase (PARP) inhibitors. It is thought that GIS-high will become a good biomarker of combination immunotherapy for ovarian cancer [30]. In the phase II study of the PARP inhibitor olaparib with the anti-PD-L1 antibody durvalumab (MEDIOLA doublet cohort) for germline BRCA-mutated platinum-sensitive relapsed (PSR) ovarian cancer, high ORR (72%) and disease control rates (81%) were observed with 19% of complete response (CR) cases [31]. Additionally, in the MEDIOLA triplet cohort, the triple combination immunotherapy of olaparib, durvalumab, and the anti-VEGF antibody bevacizumab for non-gBRCAm PSR ovarian cancer demonstrated an incredible antitumor effect in biomarker selected patients. Subgroup analysis revealed that a 100% RR (10 of 10 patients) was reported in GIS-positive cases (Foundation Medicine tumor analysis), while a 75% RR (6 of 8 patients) was reported in GIS-negative patients [32].
6.9 SMARCA4
SWI/SNF-Related, Matrix-Associated, Actin-Dependent Regulator of Chromatin, Subfamily A, Member 4 (SMARCA4) (also known as BRG1) is a subunit of the SWI/SNF chromatin remodeling complex, which regulates the expression of several genes [33]. Alterations in the SWI/SNF complex, and in particular, the loss of SMARCA4 expression, are well documented in several types of cancers including ovarian cancer. Small cell carcinoma of the ovary, hypercalcemic type (SCCOHT), is a rare, highly aggressive form of ovarian cancer seen primarily in younger patients, and has low survival rates for later-stage disease. Although low TMB with high intratumoral immune cell infiltration of SCCOHT would not predict responsiveness to an immune checkpoint blockade, a combination of PD-1 inhibitors with pembrolizumab have shown substantial and durable responses in selected patients [34].
6.10 Summary
Advances in clinical oncology and novel drug discoveries are playing a major role in personalized medicine in gynecologic cancers. The final goal of treatment for cancer is focused on patient specificity so that effective treatment is given to the right patient. The heterogeneity between gynecologic cancers among patients and within the same patient must be accounted for in personalized medicine. Therefore, genomic analyses with next generation sequencing and/or gene expression profiling using DNA microarrays along with bioinformatics will comprehensively reveal the diversity of the genome, epigenome, and expression profiles of gynecologic cancers that can be treated with immunotherapies. In the real-world clinical practice in medical oncology, we should perform reverse-translational research by using patients’ samples such as tumor biopsies and/or blood samples to find and develop the next biomarkers or immunoreactive factors [35], which are related not only to antitumor effects but also treatment-refractory/resistant factors to prolong the survival of patients with gynecologic cancers.
References
Liu J, Chen Z, Li Y, Zhao W, Wu J, Zhang Z. PD-1/PD-L1 checkpoint inhibitors in tumor immunotherapy. Front Pharmacol. 2021;12:731798. https://doi.org/10.3389/fphar.2021.731798.
Iwai Y, Hamanishi J, Chamoto K, Honjo T. Cancer immunotherapies targeting the PD-1 signaling pathway. J Biomed Sci. 2017;24(1):26. https://doi.org/10.1186/s12929-017-0329-9.
Vesely MD, Zhang T, Chen L. Resistance mechanisms to anti-PD cancer immunotherapy. Annu Rev Immunol. 2022;40:45–74. https://doi.org/10.1146/annurev-immunol-070621-030155.
Twomey JD, Zhang B. Cancer immunotherapy update: FDA-approved checkpoint inhibitors and companion diagnostics. AAPS J. 2021;23(2):39. https://doi.org/10.1208/s12248-021-00574-0.
Marabelle A, Le DT, Ascierto PA, Di Giacomo AM, De Jesus-Acosta A, Delord JP, et al. Efficacy of pembrolizumab in patients with noncolorectal high microsatellite instability/mismatch repair-deficient cancer: results from the phase II KEYNOTE-158 study. J Clin Oncol. 2020;38(1):1–10. https://doi.org/10.1200/JCO.19.02105.
Ishida Y, Agata Y, Shibahara K, Honjo T. Induced expression of PD-1, a novel member of the immunoglobulin gene superfamily, upon programmed cell death. EMBO J. 1992;11(11):3887–95. https://doi.org/10.1002/j.1460-2075.1992.tb05481.x.
Greenwald RJ, Freeman GJ, Sharpe AH. The B7 family revisited. Annu Rev Immunol. 2005;23:515–48. https://doi.org/10.1146/annurev.immunol.23.021704.115611.
Keir ME, Butte MJ, Freeman GJ, Sharpe AH. PD-1 and its ligands in tolerance and immunity. Annu Rev Immunol. 2008;26:677–704. https://doi.org/10.1146/annurev.immunol.26.021607.090331.
Okazaki T, Honjo T. PD-1 and PD-1 ligands: from discovery to clinical application. Int Immunol. 2007;19(7):813–24. https://doi.org/10.1093/intimm/dxm057. Epub 2007 Jul 2
Chung HC, Ros W, Delord JP, Perets R, Italiano A, Shapira-Frommer R, et al. Efficacy and safety of pembrolizumab in previously treated advanced cervical cancer: results from the phase II KEYNOTE-158 study. J Clin Oncol. 2019;37(17):1470–8. https://doi.org/10.1200/JCO.18.01265.
Tewari KS, Monk BJ, Vergote I, Miller A, de Melo AC, Kim HS, et al. EMPOWER-Cervical 1/GOG-3016/ENGOT-cx9: Interim analysis of phase III trial of cemiplimab vs. investigator’s choice chemotherapy in recurrent/metastatic cervical carcinoma. Abstract presented at: ESMO Virtual Plenary, abstract VP4–2021; 2021 May 12; XXXXX, XX.
Goodman AM, Piccioni D, Kato S, Boichard A, Wang HY, Frampton G, et al. Prevalence of PDL1 amplification and preliminary response to immune checkpoint blockade in solid tumors. JAMA Oncol. 2018;4(9):1237–44. https://doi.org/10.1001/jamaoncol.2018.1701.
Colombo N, Dubot C, Lorusso D, Caceres MV, Hasegawa K, Shapira-Frommer R, et al. Pembrolizumab for persistent, recurrent, or metastatic cervical cancer. N Engl J Med. 2021;385(20):1856–67. https://doi.org/10.1056/NEJMoa2112435.
O’Malley DM, Randall LM, Jackson CG, Coleman RL, Hays JL, Moore KN, et al. RaPiDS (GOG-3028): randomized phase II study of balstilimab alone or in combination with zalifrelimab in cervical cancer. Future Oncol. 2021;17(26):3433–43. https://doi.org/10.2217/fon-2021-0529.
Taha T, Reiss A, Amit A, Perets R. Checkpoint inhibitors in gynecological malignancies: are we there yet? BioDrugs. 2020;34(6):749–62. https://doi.org/10.1007/s40259-020-00450-x.
Gubin MM, Zhang X, Schuster H, Caron E, Ward JP, Noguchi T, et al. Checkpoint blockade cancer immunotherapy targets tumour-specific mutant antigens. Nature. 2014;515(7528):577–81. https://doi.org/10.1038/nature13988.
Merelli B, Massi D, Cattaneo L, Mandalà M. Targeting the PD1/PD-L1 axis in melanoma: biological rationale, clinical challenges and opportunities. Crit Rev Oncol Hematol. 2014;89(1):140–65. https://doi.org/10.1016/j.critrevonc.2013.08.002.
Le DT, Durham JN, Smith KN, Wang H, Bartlett BR, Aulakh LK, et al. Mismatch repair deficiency predicts response of solid tumors to PD-1 blockade. Science. 2017;357(6349):409–13. https://doi.org/10.1126/science.aan6733.
Oaknin A, Tinker AV, Gilbert L, Samouëlian V, Mathews C, Brown J, et al. Clinical activity and safety of the anti-programmed death 1 monoclonal antibody dostarlimab for patients with recurrent or advanced mismatch repair-deficient endometrial cancer: a nonrandomized phase 1 clinical trial. JAMA Oncol. 2020;6(11):1766–72. https://doi.org/10.1001/jamaoncol.2020.4515.
Konstantinopoulos PA, Luo W, Liu JF, Gulhan DC, Krasner C, Ishizuka JJ, et al. Phase II study of avelumab in patients with mismatch repair deficient and mismatch repair proficient recurrent/persistent endometrial cancer. J Clin Oncol. 2019;37(30):2786–94. https://doi.org/10.1200/JCO.19.01021.
Antill YC, Kok PS, Robledo K, Barnes E, Friedlander M, Baron-Hay SE, et al. Activity of durvalumab in advanced endometrial cancer (AEC) according to mismatch repair (MMR) status: the phase II PHAEDRA trial (ANZGOG1601). Abstract presented at: 2019 ASCO Annual Meeting, abstract 5501; 2019 May 31-Jun 4; Chicago, IL. https://ascopubs.org/doi/10.1200/JCO.2019.37.15_suppl.5501.
Makker V, Colombo N, Casado Herráez A et al. A multicenter, open-label, randomized, phase III study to compare the efficacy and safety of lenvatinib in combination with pembrolizumab versus treatment of physician’s choice in patients with advanced endometrial cancer. Abstract presented at: Society of Gynecologic Oncology 52nd Annual Meeting on Women's Cancer, abstract 11512; 2021 18–21; Phoenix, AZ.
Lawrence MS, Stojanov P, Polak P, Kryukov GV, Cibulskis K, Sivachenko A, et al. Mutational heterogeneity in cancer and the search for new cancer-associated genes. Nature. 2013;499(7457):214–8. https://doi.org/10.1038/nature12213.
Marabelle A, Fakih M, Lopez J, Shah M, Shapira-Frommer R, Nakagawa K, Chung HC, Kindler HL, Lopez-Martin JA, Miller WH Jr, Italiano A, Kao S, Piha-Paul SA, Delord JP, McWilliams RR, Fabrizio DA, Aurora-Garg D, Xu L, Jin F, Norwood K, Bang YJ. Association of tumour mutational burden with outcomes in patients with advanced solid tumours treated with pembrolizumab: prospective biomarker analysis of the multicohort, open-label, phase 2 KEYNOTE-158 study. Lancet Oncol. 2020;21(10):1353–65. https://doi.org/10.1016/S1470-2045(20)30445-9. Epub 2020 Sep 10. PMID: 32919526. https://www.thelancet.com/journals/lanonc/article/PIIS1470-2045(20)30445-9/fulltext.
Association of tumour mutational burden with outcomes in patients with advanced solid tumours treated with pembrolizumab: prospective biomarker analysis of the multicohort, open-label, phase 2 KEYNOTE-158 study. Lancet Oncol. 2020;21(10):1353–65. https://doi.org/10.1016/S1470-2045(20)30445-9.
Rousseau B, Foote MB, Maron SB, Diplas BH, Lu S, Argilés G, et al. The spectrum of benefit from checkpoint blockade in hypermutated tumors. N Engl J Med. 2021;384(12):1168–70. https://doi.org/10.1056/NEJMc2031965.
McGrail DJ, Pilié PG, Rashid NU, Voorwerk L, Slagter M, Kok M, et al. High tumor mutation burden fails to predict immune checkpoint blockade response across all cancer types. Ann Oncol. 2021 May;32(5):661–72. https://doi.org/10.1016/j.annonc.2021.02.006.
Matulonis UA, Shapira-Frommer R, Santin AD, Lisyanskaya AS, Pignata S, Vergote I, et al. Antitumor activity and safety of pembrolizumab in patients with advanced recurrent ovarian cancer: results from the phase II KEYNOTE-100 study. Ann Oncol. 2019;30(7):1080–7. https://doi.org/10.1093/annonc/mdz135.
Myriad Genetics [Internet]. Salt Lake City: c2022. My Choice® CDx Myriad HRD Companion Diagnostic Test; [cited YYYY MMM DD]. Available from: https://myriad.com/oncology/mychoicecdx/.
Lee EK, Konstantinopoulos PA. Combined PARP and immune checkpoint inhibition in ovarian cancer. Trends Cancer. 2019;5(9):524–8. https://doi.org/10.1016/j.trecan.2019.06.004.
Drew Y, Kaufman B, Banerjee S, Lortholary A, Hong SH, Park YH, et al. Phase II study of olaparib + durvalumab (MEDIOLA): updated results in germline BRCA-mutated platinum-sensitive relapsed (PSR) ovarian cancer (OC). Ann Oncol. 2019;30(Suppl 5):v475–532.
Drew Y, Penson RT, O’Malley DM, Kim J, Zimmerman S, Roxburgh P, et al. 814MO - phase II study of olaparib (O) plus durvalumab (D) and bevacizumab (B) (MEDIOLA): initial results in patients (pts) with non-germline BRCA-mutated (non-gBRCAm) platinum sensitive relapsed (PSR) ovarian cancer (OC). Ann Oncol. 2020;31(Suppl 4):S551–89.
Wilson BG, Roberts CW. SWI/SNF nucleosome remodellers and cancer. Nat Rev Cancer. 2011;11(7):481–92. https://doi.org/10.1038/nrc3068.
Jelinic P, Ricca J, Van Oudenhove E, Olvera N, Merghoub T, Levine DA, Zamarin DJ. Immune-active microenvironment in small cell carcinoma of the ovary, hypercalcemic type: rationale for immune checkpoint blockade. Natl Cancer Inst. 2018;110(7):787–90. https://doi.org/10.1093/jnci/djx277.
Mills AM, Bullock TN, Ring KL. Targeting immune checkpoints in gynecologic cancer: updates & perspectives for pathologists. Mod Pathol. 2022;35(2):142–51. https://doi.org/10.1038/s41379-021-00882-y.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2022 The Author(s), under exclusive license to Springer Nature Singapore Pte Ltd.
About this chapter
Cite this chapter
Hamanishi, J. (2022). Personalized Treatment in Immunotherapy for Gynecologic Cancer. In: Mandai, M. (eds) Personalization in Gynecologic Oncology. Comprehensive Gynecology and Obstetrics. Springer, Singapore. https://doi.org/10.1007/978-981-19-4711-7_6
Download citation
DOI: https://doi.org/10.1007/978-981-19-4711-7_6
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-19-4710-0
Online ISBN: 978-981-19-4711-7
eBook Packages: MedicineMedicine (R0)