Opinion statement
Research into novel therapies for gynecologic cancers is underfunded, and as a result, we are still playing catchup with other solid tumors in the realm of immune checkpoint inhibition. This is despite the fact that two of the most common gynecologic cancers in the USA have strong biologic rationales for response to these agents. Work is now underway to demonstrate safe and effective therapies for our patients. As we better understand the immune system, and more specifically the tumor microenvironment, we will be able to achieve complete responses. The immune system can learn, adapt, and provide ongoing surveillance; if only we could mimic its abilities.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Lynch syndrome and gynecologic cancers
Lynch syndrome (LS), also known as hereditary non-polyposis colorectal cancer (HNPCC), is caused by inherited defects in DNA mismatch repair (MMR) proteins. Common genes with mutations include MLH1, MSH2, and MSH6 [1]. The LS spectrum also includes, among others, endometrial and ovarian carcinomas [2, 3]. Women with LS have a greater than 40% lifetime risk of endometrial cancer and a 10–12% lifetime risk of ovarian cancer [4]. Tumors with deficient mismatch repair (dMMR) have been identified as excellent targets for immunotherapy, particularly checkpoint inhibitors [5].
Immune checkpoint inhibitors
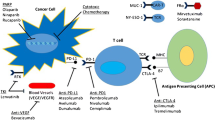
Immune checkpoint proteins act as coinhibitory regulators of T cell activation [6]. To date, the checkpoint proteins that are best understood are as follows: cytotoxic T lymphocyte antigen 4 (CTLA-4), programmed cell death 1 (PD-1), and programmed cell death ligand 1 (PD-L1) [7]. Monoclonal blocking antibodies to these cellular targets have been developed and tested for clinical utility. Antibodies to CTLA-4 include ipilimumab and tremelimumab; antibodies to PD-1 include nivolumab and pembrolizumab; and antibodies to PD-L1 include atezolizumab, avelumab, and durvalumab. At the core of checkpoint inhibition therapy is the concept of tumor immunogenicity, or the ability of a tumor to be recognized by the immune system [8]. High expression of PD-L1 by tumor cells promotes immunosuppression by inhibiting cytotoxic T cell activity in the tumor microenvironment. High expression of PD-L1 on cancer cells has been associated with poor clinical outcomes [9]. Although some studies have suggested that cells expressing high levels of PD-L1 may be more likely to benefit from checkpoint blockade, clinical responses have not directly correlated to levels of PD-L1 expression.
In the last 3 years, the clinical application of immunotherapies in oncology has gained considerable attention with the promise of addressing lingering treatment gaps. To date, immunotherapy with checkpoint inhibitors has demonstrated modest but promising progress in patients with advanced and recurrent gynecologic cancers.
Endometrial cancer
Endometrial carcinoma will affect an estimated 61,880 women in the USA in 2019, and just over 12,000 women will die from the disease [10]. The relatively favorable death to case ratio of endometrial cancer is due to the fact that most women are diagnosed at an early stage when surgical removal of the uterus effectively cures their cancer [11]. Unfortunately, up to 30% of cases each year present with regional or distant metastases and an associated decrease in estimated survival (69% and 16.9% 5-year survival, respectively) despite the use of multimodal therapies. Most advanced and recurrent endometrial cancers are incurable and embody a population of patients for whom we lack meaningful treatments [12].
The traditional classification of endometrial cancer into type I cancers that are estrogen dependent with a relatively favorable prognosis versus type II cancers that are poorly differentiated with a worse prognosis dates back to the 1980s [13]. More recently, The Cancer Genome Atlas project has identified four genetically distinct groups of endometrial cancer: the ultra-mutated DNA polymerase epsilon (POLE) group, the hyper-mutated microsatellite instable (MSI) group, the copy number low (microsatellite stable (MSS)) group, and the copy number high (serous-like) group of cancers [14•]. Each group demonstrated a different prognosis, with the ultra-mutated POLE cancers having the most favorable survival profile, and the serous-like copy number high cancers the poorest survival estimates. The high frequency of genetic mutations in the POLE and MSI cancers is associated with the creation of neoantigens [15]. Consequently, these tumors are thought to be more immunogenic and are often found associated with tumor-infiltrating lymphocytes (TILs) [16•]. Additionally, these tumors express high levels of both PD-1 and PD-L1 which downregulate the potential cytotoxic effect of activated TILs [6, 17]. The resulting tumor microenvironment is characterized by paralysis of the immune response and thus may be uniquely suited to treatment with immune checkpoint inhibition [18].
Clinical experience with checkpoint inhibitors in endometrial cancer
Targeting PD-1
The first signal that checkpoint inhibitors may have a role in advanced endometrial cancers came in a landmark study of the anti-PD-1, pembrolizumab, by Le et al. [16]. This study enrolled both MMR-deficient (dMMR) and MMR-proficient (pMMR) colon cancers as well as other solid tumors with MMR deficiency. Among the dMMR colon cancers, the objective response rate (ORR) to pembrolizumab was 40% compared with 11% in the pMMR colon cancers. In the group of dMMR non-colon cancers, the ORR was 71% (this group included two endometrial cancers). This was followed by the phase I KEYNOTE-028 trial, also investigating pembrolizumab, with a larger group of unselected (in terms of MMR) endometrial cancer patients (n = 24) [19]. The ORR was 13%, and the toxicity profile was as expected for a checkpoint inhibitor: fatigue, anorexia, pruritis, and pyrexia. In an updated analysis by Le et al., response to pembrolizumab across a variety of cancer types was most closely correlated with mismatch repair deficiency (dMMR) [20••]. Tumors that were MMR deficient demonstrated an impressive ORR of 53% across multiple histologies with 21% achieving a complete response. Following the publication of this study, the FDA approved pembrolizumab for cancers with deficient mismatch repair, regardless of tissue of origin [21]. Another anti-PD-1, nivolumab, has been reported to have dramatic results in two patients with advanced endometrial cancers that were heavily pre-treated and refractive to both surgery and radiotherapy [22]. Notably, one patient’s tumor was an ultra-mutated POLE type and the second a hyper-mutated MSI type—giving further weight to the idea that a high mutational burden represents a phenotype (possible biomarker) that is potentially the best clinical scenario for immune checkpoint inhibition.
Recently, Fuh and colleagues presented phase I translational data on the use of pembrolizumab in the neoadjuvant (pre-surgery) setting, followed by standard of care therapy, and pembrolizumab maintenance [23]. They reported 6 of 8 patients completed protocol-directed therapy, with one patient experiencing a rapid progression of their cancer and the other worsening of their other comorbid conditions. Other safety data were pending at the time of the report. Another phase I trial investigating the use of pembrolizumab prior to surgery is ongoing [24]. Given the emerging data that patients whose tumors are MMR deficient or have POLE mutations have an improved response to checkpoint inhibitors, a phase II trial is investigating the use of pembrolizumab as a single agent in this selected population [25]. Makker et al. reported their experience with the combination of lenvatinib (a multi-kinase inhibitor of vascular endothelial growth factor receptors 1–3) with pembrolizumab in patients with advanced endometrial cancers in a phase II trial [26]. They enrolled 53 patients and observed an ORR in 21 (39.6%, 95% CI 26.5–54.0) with 16/53 (30%) having serious adverse events, including one death from intracranial hemorrhage. These data have supported the initiation of the phase III, KEYNOTE-775 trial comparing the combination of pembrolizumab and lenvatinib to physician’s choice of cytotoxic chemotherapy (paclitaxel or doxorubicin) [27]. Finally, the NRG will investigate pembrolizumab in combination with paclitaxel and carboplatin followed by pembrolizumab maintenance compared with paclitaxel and carboplatin with placebo followed by placebo maintenance in advanced and recurrent endometrial cancers [28]. This trial is pending activation.
Targeting PD-L1
PD-L1 expression has been seen in about 70% of clear cell endometrial cancers and in some undifferentiated endometrial cancers, making this an important target in tumors that are known to be resistant to standard therapies [29, 30]. Current anti-PD-L1 agents under investigation include avelumab, atezolizumab, and durvalumab. There are several trials underway investigating these agents alone or in combination with either additional checkpoint inhibitors or cytotoxic chemotherapy. Antill et al. recently reported the phase II PHAEDRA trial evaluating durvalumab in advanced endometrial cancers stratified by MMR status [31]. They enrolled 71 patients (35 dMMR, 36 pMMR) and noted that the ORR was 40% (14/35, 95% CI 26–56) for dMMR tumors compared with 3% (1/36, 95% CI 1–14) for pMMR tumors. Among the dMMR responders, there were four complete responses and 10 partial responses with 7 others demonstrating stable disease for 16 weeks. Response was noted regardless of prior therapy. Immune-related adverse events (irAE) occurred in 14/71 (20%) of patients with hyperthyroidism, hypothyroidism, pneumonitis, and hepatitis seen. A two-stage, phase II study evaluating avelumab recently reported preliminary results [32]. Patients with any histology of recurrent or persistent endometrial cancer with any prior therapy were included, and then divided into two groups by immunohistochemical (IHC) staining: MSI/POLE and MSS. Thirty-one patients were evaluated (15 MSI and 16 MSS). Among the MSS patients, only one achieved objective response giving an ORR of 6.25% (95% CI 0.16–30.2). The MSI/POLE cohort had four responders giving an ORR of 26.7% (95% CI 7.8–55.1). Additionally, 6 patients remained progression free at 6 months (PFS6) giving a rate 40% (95% CI 16.3–66.7). The rate of grade 3 toxicity was 19%, and there were no grade 4/5 events. Importantly, 5 of the 6 patients who were progression free at 6 months had three or more prior lines of therapy and their tumors were IHC negative for PD-L1. Lastly, a phase III trial of atezolizumab in combination with paclitaxel and carboplatin followed by maintenance atezolizumab compared with paclitaxel and carboplatin with placebo followed by placebo maintenance in advanced and recurrent endometrial cancers has been announced and is open to enrollment (NCT 03603184) [33].
Targeting CTLA-4
Data on the use of anti-CTLA-4 agents (ipilimumab and tremelimumab) in endometrial cancers are limited. Recent reports point to their use in combination with other checkpoint inhibitors. Rubinstein et al. have presented an interim analysis of their randomized phase II trial of durvalumab as a single agent compared with the combination of durvalumab and tremelimumab in recurrent or persistent endometrial cancers [34]. The authors noted modest activity in this setting with an ORR of 14.8% (95% CI 6.6–100) for durvalumab and 11.1% (95% CI 4.2–100) for the combination. Serious adverse events were more common with doublet therapy (44.4% vs 11.1%). Of note, these preliminary data suggested that response to these agents did not correlate with microsatellite status. Finally, a recent case report highlights our limited understanding of the ideal patients for immunotherapy. The authors presented a patient with advanced endometrial cancer whose tumor was noted to be pMMR as well as PD-L1 negative by IHC [35]. She was treated with the combination of nivolumab plus ipilimumab and demonstrated a partial response that both continues to improve and has now been sustained over 12 months. This report is notable in three ways. First, the continuation and duration of response is impressive. Second, the combination of agents may point to a potential synergism between targeting CTLA-4 and PD-1, as they act in different phases of the immune response [7, 36]. Third, by our current understanding, this patient does not fit the profile of a candidate for checkpoint inhibition with intact mismatch repair and little to no expression of PD-L1. This suggests other factors are key in determining response to immune inhibition and that perhaps studies should offer broader eligibility.
Emerging understanding of the endometrial cancer microenvironment
As noted previously, there are several candidate biomarkers for response to immune checkpoint inhibition in endometrial cancers: mismatch repair status, POLE mutation status, tumor mutational burden, and PD-1/PD-L1 expression [37,38,39]. T cell immunoglobulin and mucin domain (TIM-3) has recently been proposed as another tumor-associated biomarker [40]. While the status of each of these has been used to characterize endometrial cancers, they may not provide information regarding the tumor-associated immune cells or other effectors in the microenvironment such as tumor-infiltrating lymphocytes (TILs) and tumor-associated macrophages (TAMs). Tumors with deficient mismatch repair have been found to have significantly higher levels of TILs compared with those with intact mismatch repair [39]. Additionally, the microenvironment of dMMR tumors is suggestive of an overall T cell–inflamed phenotype with high levels of CD8+ cytotoxic lymphocytes [41]. These observations may further improve our understanding of why dMMR tumors have consistently demonstrated better responses to checkpoint inhibition. Antomarchi et al. recently characterized the tumor microenvironment of grades 1–3 endometrial cancers compared with both normal endometrial samples as well as endometrial hyperplasia [36]. The authors demonstrated that the tumor microenvironment for grade 1 tumors had a far more tumoricidal phenotype compared with a more tumor-tolerant phenotype in grades 2–3 cancers. These findings have implications for the use of combination checkpoint inhibitors, such as targeting both CTLA-4 as well as PD-1 and PD-L1.
Future directions
Prospective molecular profiling of endometrial cancers with triage to adjuvant therapies has been proposed and has been shown to be feasible [42]. Currently, The Post-Operative Radiation Therapy in Endometrial Carcinoma (PORTEC)-4a trial is enrolling in Europe (NCT 03469674). This protocol includes high-intermediate risk endometrial cancers which are then randomized to either a protocol-directed therapy or standard vaginal brachytherapy. Those randomized to protocol-directed therapy are classified into favorable, intermediate, or unfavorable risk profiles based on both clinical and molecular features. The favorable risk group will not receive adjuvant therapy and will be observed, the intermediate risk group will receive vaginal brachytherapy, and the unfavorable risk group will receive pelvic radiotherapy. Immune therapies are not part of this protocol; however, the molecular classifiers in use are now very familiar: POLE, MSI, p53, among others. Additionally, there is an emerging understanding between the use of radiation and the immune system, particularly in endometrial cancer [43,44,45]. This will undoubtedly be an active area of future research.
Holistic characterization of both the tumor as well as the immune microenvironment may provide the roadmap to the most effective therapies for our patients. The molecular classification of endometrial cancers by TCGA has fundamentally shifted our understanding yet has not been applied in everyday clinical decision making given the resource intensive nature of the analysis. The Proactive Molecular Risk Classifier for Endometrial Cancer (ProMisE) has been proposed as an alternate and more practical way of classifying endometrial cancers [46, 47]. The four groups of endometrial cancers in ProMisE are POLE, MSI, p53 mutant, and p53 wildtype. Talhouk et al. recently suggested that the immune response, rather than molecular subtype alone, may best predict response to checkpoint inhibition [48••]. Specifically, they demonstrated that there are subpopulations of pMMR and p53 mutant tumors with a notable number of TILs that signal a recognition by the immune system—thus marking them as potential responders to checkpoint inhibition. Additionally, they noted some dMMR and POLE tumors with low numbers of TILs, demonstrating that molecular subtype does not always correlate with immune response. In an accompanying editorial, Mullen and Mutch propose that adding an assessment of TILs to the ProMisE classifications would yield a more comprehensive evaluation for an individual patient’s response to checkpoint inhibition [49]. Table 1 summarizes the two molecular classification systems, along with tumor and microenvironmental features that may help guide immunotherapy.
Ovarian cancer
Ovarian carcinoma remains one of the leading causes of cancer death in women and is the most lethal gynecologic cancer. In 2019, there will be an estimated 22,530 new ovarian cancers and 13,980 deaths in the USA [10]. One of the reasons for the high mortality rate is that despite the favorable response to first line therapy (over 80% of patients will be in remission following surgery and chemotherapy), recurrences are very high and associated with a poor prognosis. The majority of women with ovarian cancer will be diagnosed with advanced (stage III–IV) disease which is associated with a poor 5-year overall survival rate despite maximum surgical effort and multiagent cytotoxic therapy [50].
Due to the challenges of ovarian cancer treatment and the recent success in treatment of other cancers, there is great interest in immunotherapy as an alternative to traditional chemotherapy for ovarian cancer. Of the immunotherapies utilized in the treatment of cancers, immune checkpoint inhibitors have been the most commonly investigated.
Clinical experience with checkpoint inhibitors in ovarian cancer
Targeting PD-1 and PD-L1
Hamanishi et al. reported results from a phase II trial investigating nivolumab in the setting of platinum-resistant ovarian cancer [51•]. In this study 20 women were evaluated, and the ORR was 15% (95% CI, 3.2–37.9) and the disease control rate was 45% (95% CI, 23.1–68.5). There were two patients (10%) who had a complete response. Notably, the rate of tumor PD-L1 expression (which was evaluated in all tumors) was not significantly correlated with response to treatment to nivolumab. The same group recently evaluated the correlation between response to treatment with nivolumab and the presence of gene fusions contained in somatic mutations or transcriptomic alignments [52]. They found a strong correlation between clinical response to nivolumab and the number of fusion genes in ovarian cancer. They concluded that passenger fusion genes may be a possible predictive biomarker for response to anti-PD-1 therapy in ovarian cancer.
In the recently reported KEYNOTE-028 trial, 26 patients with advanced, metastatic ovarian cancers whose tumors were PD-L1 positive were treated with pembrolizumab [53]. The ORR was 11.5% (95% CI, 2.4–30.2), and the median progression-free (PFS) and overall survival (OS) were 1.9 months (95% CI, 1.8–3.5) and 13.8 months (6.7–18.8), respectively. The rate of treatment-related adverse events (TRAEs) was 27%. Notably, the dose of pembrolizumab used in this study was weight based and differs from the recommended fixed dose used in currently enrolling studies. This may account for the higher rate of adverse events observed. In a remarkable case report, Bellone et al. described a complete clinical response to pembrolizumab in a heavily pretreated patient with advanced ovarian cancer [54]. The patient’s molecular profile was notable for an aberrant PD-L1 protein expression.
In the phase Ib JAVELIN trial, 125 patients were evaluated as an expansion cohort with the objective to assess the efficacy and safety of avelumab, an antibody targeting PD-L1 [55]. An objective response occurred in 12 patients (9.6%, 95% CI 5.1–16.2), including one complete response. The 1-year PFS rate was 10.2% (95% CI, 5.4–16.7), and median OS was 11.2 months (95% CI, 8.7–15.4). TRAEs were seen in 20% of patients. Importantly, two patients with clear cell ovarian cancer, a histologic subtype that responds poorly to standard chemotherapy, demonstrated a response to avelumab.
Checkpoint inhibitors and chemotherapy
There have been several reported trials (with additional trials underway) evaluating immune checkpoint inhibitors in combination with chemotherapy, radiotherapy, and/or other targeted therapies. The rationale for combination with chemotherapy is related to its potential to activate the immune system by causing tumor cell death and thereby releasing neoantigens [56]. Also, the expression of PD-L1 can be induced by chemotherapy, priming the tumor microenvironment for checkpoint inhibition [57]. Building on the results noted above for avelumab, the interim results for the phase III JAVELIN Ovarian 200 trial have been presented [58]. In this study patients with platinum-resistant or refractory ovarian cancer were randomized to one of three arms: avelumab alone, avelumab in combination with pegylated liposomal doxorubicin (PLD), or PLD alone. Although clinical activity was demonstrated with the combination of avelumab plus PLD, PFS and OS were not significantly improved compared with PLD alone: ORR 3.7% (95% CI 1.5–7.5) for avelumab, 13.3% (95% CI 8.8–19.0) for avelumab plus PLD, and 4.2% (95% CI 1.8–8.1) for PLD. However, in a planned sub-group analysis, patients with PD-L1-positive cancers did derive benefit from avelumab plus PLD: ORR 18.5% (95% CI 11.1–27.9) in the PD-L1-positive group compared with 3.4% (95% CI 0.4–11.9) for the PD-L1-negative group. Rates of grade 3 or higher TRAEs were high with 49.7%, 68.7%, and 59.3% of patients in each group, respectively. Liao et al. reported their interim results from a phase I/II trial evaluating pembrolizumab with low-dose carboplatin in patients with recurrent platinum-resistant ovarian, fallopian tube, and primary peritoneal cancer [59]. The combination was well tolerated and showed activity in heavily pretreated platinum-resistant advanced ovarian cancers: ORR was 13.0% (95% CI, 2.7–33.6), median PFS was 4.6 months (95% CI, 2.7–6.2). The most common TRAEs were lymphopenia (18%) and anemia (9%). Finally, the international IMagyn050/GOG 3015/ENGOT-OV39 trial recently completed accrual [60]. This phase III study enrolled patients with advanced ovarian cancers both in a neoadjuvant and adjuvant setting, randomizing them to atezolizumab versus placebo along with paclitaxel, carboplatin, and bevacizumab. Results are forthcoming.
Checkpoint inhibitors and PARP inhibitors
The combination of PARP and checkpoint inhibitors has been an area of study in ovarian cancer in part because tumors with homologous recombination deficiencies (HRD) have been shown to have increased expression of PD-1 [61]. PD-1 expression was recently noted in 30% of a cohort of patients with ovarian cancer and BRCA mutations, with a higher rate in BRCA1 carriers compared with BRCA2 carriers (50% vs 13%) [62]. PD-L1 expression was also observed in 67% of patients in the same cohort. Similarly, BRCA1 carriers also had higher rates of expression compared with BRCA2 carriers (93% vs 44%).
Clinical experience combining PARP and immune checkpoint inhibitors has been promising. The TOPACIO/KEYNOTE-162 trial investigated the combination of niraparib with pembrolizumab [63]. The ORR was 18% (90% CI, 11–29) with a disease control rate of 65% (90% CI, 54–75) including 3 (5%) confirmed complete responses. Importantly, response rates were similar across subgroups: sensitivity to platinum-based chemotherapy, prior bevacizumab, or tumor BRCA or HRD biomarker status. Lastly, the ATHENA trial is an on-going, phase III double-blind trial randomizing patients with advanced ovarian cancer following primary therapy (including both neoadjuvant and adjuvant settings) to one of four arms: rucaparib plus nivolumab, rucaparib plus placebo, nivolumab plus placebo, or placebo only [64].
Conclusions
Lynch syndrome–associated gynecologic cancers are clear candidates for immune checkpoint inhibitor therapy. Our emerging understanding of the costimulatory and coinhibitory signals of the local tumor microenvironment will continue to shape treatment options for our patients. Additional work seeking effective biomarkers for response as well as new classification systems of disease will allow us to bring these powerful therapies to the right patients at the right time.
References and Recommended Reading
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Lynch HT, Lynch J. Lynch syndrome: genetics, natural history, genetic counseling, and prevention. J Clin Oncol. 2000;18(21 Suppl):19S–31S.
Lynch HT, Casey MJ, Lynch J, White TE, Godwin AK. Genetics and ovarian carcinoma. Semin Oncol. 1998;25(3):265–80.
Barrow E, Robinson L, Alduaij W, Shenton A, Clancy T, Lalloo F, et al. Cumulative lifetime incidence of extracolonic cancers in Lynch syndrome: a report of 121 families with proven mutations. Clin Genet. 2009;75(2):141–9.
Schmeler KM, Soliman PT, Sun CC, Slomovitz BM, Gershenson DM, Lu KH. Endometrial cancer in young, normal-weight women. Gynecol Oncol. 2005;99(2):388–92.
Dudley JC, Lin MT, Le DT, Eshleman JR. Microsatellite instability as a biomarker for PD-1 blockade. Clin Cancer Res. 2016;22(4):813–20.
Boussiotis VA. Molecular and biochemical aspects of the PD-1 checkpoint pathway. N Engl J Med. 2016;375(18):1767–78.
Iwai Y, Hamanishi J, Chamoto K, Honjo T. Cancer immunotherapies targeting the PD-1 signaling pathway. J Biomed Sci. 2017;24(1):26.
Pietzner K, Nasser S, Alavi S, Darb-Esfahani S, Passler M, Muallem MZ, et al. Checkpoint-inhibition in ovarian cancer: rising star or just a dream? J Gynecol Oncol. 2018;29(6):e93.
Hamanishi J, Mandai M, Matsumura N, Abiko K, Baba T, Konishi I. PD-1/PD-L1 blockade in cancer treatment: perspectives and issues. Int J Clin Oncol. 2016;21(3):462–73.
Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019. CA Cancer J Clin. 2019;69(1):7–34.
Cronin KA, Lake AJ, Scott S, Sherman RL, Noone AM, Howlader N, et al. Annual report to the nation on the status of cancer, part I: national cancer statistics. Cancer. 2018;124(13):2785–800.
McMeekin DS, Filiaci VL, Thigpen JT, Gallion HH, Fleming GF, Rodgers WH, et al. The relationship between histology and outcome in advanced and recurrent endometrial cancer patients participating in first-line chemotherapy trials: a gynecologic oncology group study. Gynecol Oncol. 2007;106(1):16–22.
Bokhman JV. Two pathogenetic types of endometrial carcinoma. Gynecol Oncol. 1983;15(1):10–7.
• Cancer Genome Atlas Research N, Kandoth C, Schultz N, Cherniack AD, Akbani R, Liu Y, et al. Integrated genomic characterization of endometrial carcinoma. Nature. 2013;497(7447):67–73 Landmark paper that shifted our understanding of how to classify endometrial cancers.
Zou W, Wolchok JD, Chen L. PD-L1 (B7-H1) and PD-1 pathway blockade for cancer therapy: mechanisms, response biomarkers, and combinations. Sci Transl Med. 2016;8(328):328rv4.
• Le DT, Uram JN, Wang H, Bartlett BR, Kemberling H, Eyring AD, et al. PD-1 blockade in tumors with mismatch-repair deficiency. N Engl J Med. 2015;372(26):2509–20 Demonstrated the potential of checkpoint inhibitors in dMMR tumors, including gynecologic cancers.
Herzog TJ, Arguello D, Reddy SK, Gatalica Z. PD-1, PD-L1 expression in 1599 gynecological cancers: implications for immunotherapy. Gynecol Oncol. 2015;137:204–5.
Eggink FA, Van Gool IC, Leary A, Pollock PM, Crosbie EJ, Mileshkin L, et al. Immunological profiling of molecularly classified high-risk endometrial cancers identifies POLE-mutant and microsatellite unstable carcinomas as candidates for checkpoint inhibition. Oncoimmunology. 2017;6(2):e1264565.
Ott PA, Bang YJ, Berton-Rigaud D, Elez E, Pishvaian MJ, Rugo HS, et al. Safety and antitumor activity of pembrolizumab in advanced programmed death ligand 1-positive endometrial cancer: results from the KEYNOTE-028 study. J Clin Oncol. 2017;35(22):2535–41.
•• Le DT, Durham JN, Smith KN, Wang H, Bartlett BR, Aulakh LK, et al. Mismatch repair deficiency predicts response of solid tumors to PD-1 blockade. Science. 2017;357(6349):409–13 Established checkpoint inhibitors as a therapeutic option for dMMR tumors, regardless of tissue of origin.
Lemery S, Keegan P, Pazdur R. First FDA approval agnostic of Cancer site - when a biomarker defines the indication. N Engl J Med. 2017;377(15):1409–12.
Santin AD, Bellone S, Buza N, Choi J, Schwartz PE, Schlessinger J, et al. Regression of chemotherapy-resistant polymerase epsilon (POLE) ultra-mutated and MSH6 hyper-mutated endometrial tumors with nivolumab. Clin Cancer Res. 2016;22(23):5682–7.
Fuh K, Lomonosova E, Guo L, Fantl W, Pachynski R, Malkova O, et al. Identifying a potential biomarker for anti-PD-1 immunotherapy in patients with advanced stage, surgically-resectable endometrial cancer. ASCO Annual Meeting; June 1, 2019; Chicago, IL. J Clin Oncol. 2019;37(suppl; abstr 5590).
Secord AA. A study of pembrolizumab on the tumoral immunoprofile of gynecologic cancers. NCT02728830. ClinicalTrials.gov: NCI; [Available from: https://clinicaltrials.gov/ct2/show/NCT02728830. Accessed 14 June 2019.
Santin AD. Pembrolizumab in ultramutated and hypermutated endometrial cancer. NCT02899793. ClinicalTrials.gov: NCI; [Available from: https://clinicaltrials.gov/ct2/show/NCT02899793?term=02899793&rank=1. Accessed 14 June 2019.
Makker V, Rasco D, Vogelzang NJ, Brose MS, Cohn AL, Mier J, et al. Lenvatinib plus pembrolizumab in patients with advanced endometrial cancer: an interim analysis of a multicentre, open-label, single-arm, phase 2 trial. Lancet Oncol. 2019;20(5):711–8.
Makker V, Herraez A, Aghajanian C, Fujiwara K, Pignata S, Penson RT, et al. A phase 3 trial evaluating efficacy and safety of lenvatinib in combination with pembrolizumab in patients with advanced endometrial cancer. ASCO Annual Meeting; June 1, 2019; Chicago, IL. J Clin Oncol. 2019;37(suppl; abstr TPS5607).
Eskander RN, NRG-Oncology. Testing the addition of the immunotherapy drug pembrolizumab to the usual chemotherapy treatment (paclitaxel and carboplatin) in stage iii-iv or recurrent endometrial cancer, NCT03914612. clinicaltrials.gov: NCI; [Available from: https://clinicaltrials.gov/ct2/show/NCT03914612?term=03914612&rank=1. Accessed 14 June 2019.
Willis BC, Sloan EA, Atkins KA, Stoler MH, Mills AM. Mismatch repair status and PD-L1 expression in clear cell carcinomas of the ovary and endometrium. Mod Pathol. 2017;30(11):1622–32.
Al-Hussaini M, Lataifeh I, Jaradat I, Abdeen G, Otay L, Badran O, et al. Undifferentiated endometrial carcinoma, an immunohistochemical study including PD-L1 testing of a series of cases from a single cancer center. Int J Gynecol Pathol. 2018;37(6):564–74.
Antill Y, Kok P, Robledo K, Barnes E, Friedlander M, Baron-Hay S, et al. Activity of durvalumab in advanced endometrial cancer (AEC) according to mismatch repair (MMR) status: The phase II PHAEDRA trial (ANZGOG1601). ASCO Annual Meeting; June 3, 2019; Chicago, IL. J Clin Oncol. 2019;37(suppl; abstr 5501).
Konstantinopoulos PA, Liu JF, Luo W, Krasner CN, Ishizuka J, Gockley A, et al. Phase 2, two-group, two-stage study of avelumab in patients (pts) with microsatellite stable (MSS), microsatellite instable (MSI), and polymerase epsilon (POLE) mutated recurrent/persistent endometrial cancer (EC). ASCO Annual Meeting; June 3, 2019; Chicago, IL. J Clin Oncol. 2019;37(suppl;abstr 5502).
Colombo N, Barretina-Ginesta M, Beale P, Harano K, Hudson E, Marme F, et al. AtEnd/ENGOT-en7: A multicenter phase III double-blind randomized controlled trial of atezolizumab in combination with paclitaxel and carboplatin in women with advanced/recurrent endometrial cancer. . ASCO Annual Meeting; June 1, 2019; Chicago, IL. J Clin Oncol. 2019;37(suppl; abst TPS5608).
Rubinstein M, Caird I, Zhou Q, Iasonos A, Friedman C, Cadoo K, et al. A phase II trial of durvalumab with or without tremelimumab in patients with persistent or recurrent endometrial carcinoma and endometrial carcinosarcoma. . ASCO Annual Meeting June 1, 2019; Chicago, IL J Clin Oncol. 2019;37(suppl; abstr 5582).
Oh MS, Chae YK. Deep and durable response with combination CTLA-4 and PD-1 blockade in mismatch repair (MMR)-proficient endometrial cancer. J Immunother. 2019;42(2):51–4.
Antomarchi J, Ambrosetti D, Cohen C, Delotte J, Chevallier A, Karimdjee-Soilihi B, Ngo-Mai M, Schmid-Alliana A, Schmid-Antomarchi H. Immunosuppressive tumor microenvironment status and histological grading of endometrial carcinoma. Cancer Microenviron. 2019.
Winer I, Jones NL, Xiu J, Ellerbrock A, Brown J, Herzog T. Mutational burden, tumor immune checkpoint expression, and microsatellite instability in gynecologic malignancies: implications for immune therapy. Gynecol Oncol. 2018;149:45.
Jones NL, Xiu J, Herzog T, Winer I. Immune checkpoint expression, microsatellite instability, and mutational burden: identifying immune biomarker phenotypes in uterine cancer. Gynecol Oncol. 2018;149:44.
Yamashita H, Nakayama K, Ishikawa M, Nakamura K, Ishibashi T, Sanuki K, et al. Microsatellite instability is a biomarker for immune checkpoint inhibitors in endometrial cancer. Oncotarget. 2018;9(5):5652–64.
Moore M, Ring KL, Mills AM. TIM-3 in endometrial carcinomas: an immunotherapeutic target expressed by mismatch repair-deficient and intact cancers. Mod Pathol. 2019;32:1168–79.
Asaka S, Yen TT, Wang TL, Shih IM, Gaillard S. T cell-inflamed phenotype and increased Foxp3 expression in infiltrating T-cells of mismatch-repair deficient endometrial cancers. Mod Pathol. 2019;32(4):576–84.
Wortman BG, Bosse T, Nout RA, Lutgens LCHW, van der Steen-Banasik EM, Westerveld H, et al. Molecular-integrated risk profile to determine adjuvant radiotherapy in endometrial cancer: evaluation of the pilot phase of the PORTEC-4a trial. Gynecol Oncol. 2018;151(1):69–75.
Lee L, Matulonis U. Immunotherapy and radiation combinatorial trials in gynecologic cancer: a potential synergy? Gynecol Oncol. 2019.
Son CH, Fleming GF, Moroney JW. Potential role of radiation therapy in augmenting the activity of immunotherapy for gynecologic cancers. Cancer Manag Res. 2017;9:553–63.
Reijnen C, Küsters-Vandevelde HVN, Prinsen CF, Massuger LFAG, Snijders MPML, Kommoss S, et al. Mismatch repair deficiency as a predictive marker for response to adjuvant radiotherapy in endometrial cancer. Gynecol Oncol. 2019.
Talhouk A, McConechy MK, Leung S, Yang W, Lum A, Senz J, et al. Confirmation of ProMisE: a simple, genomics-based clinical classifier for endometrial cancer. Cancer. 2017;123(5):802–13.
Britton H, Huang L, Lum A, Leung S, Shum K, Kale M, et al. Molecular classification defines outcomes and opportunities in young women with endometrial carcinoma. Gynecol Oncol. 2019;153(3):487–95.
•• Talhouk A, Derocher H, Schmidt P, Leung S, Milne K, Gilks CB, et al. Molecular subtype not immune response drives outcomes in endometrial carcinoma. Clin Cancer Res. 2019;25(8):2537–48 A remarkable paper that further demonstrated the utility of the ProMisE classification of endometrial cancers, as well as the significance of TILs in the microenvironment.
Mullen MM, Mutch DG. Endometrial tumor immune response: predictive biomarker of response to immunotherapy. Clin Cancer Res. 2019;25(8):2366–8.
Bookman MA, Brady MF, McGuire WP, Harper PG, Alberts DS, Friedlander M, et al. Evaluation of new platinum-based treatment regimens in advanced-stage ovarian cancer: a phase III trial of the gynecologic cancer InterGroup. J Clin Oncol. 2009;27:1419–25.
• Hamanishi J, Mandai M, Ikeda T, Minami M, Kawaguchi A, Murayama T, et al. Safety and antitumor activity of anti-PD-1 antibody, nivolumab, in patients with platinum-resistant ovarian cancer. J Clin Oncol. 2015;33(34):4015–22 Demonstrated the use of checkpoint inhibitors in ovarian cancer.
Hamanishi J, Murakami R, Baba T, Yamaguchi K, Abiko K, Mandai M. Passenger fusion genes are correlated to antitumor effect of anti-PD-1 antibody nivolumab for ovarian cancer. 2019 SGO annual meeting; march 2019; Honolulu, HI.
Varga A, Piha-Paul S, Ott PA, Mehnert JM, Berton-Rigaud D, Morosky A, et al. Pembrolizumab in patients with programmed death ligand 1-positive advanced ovarian cancer: analysis of KEYNOTE-028. Gynecol Oncol. 2019;152(2):243–50.
Bellone S, Buza N, Choi J, Zammataro L, Gay L, Elvin J, et al. Exceptional response to pembrolizumab in a metastatic, chemotherapy/radiation-resistant ovarian cancer patient harboring a PD-L1-genetic rearrangement. Clin Cancer Res. 2018;24(14):3282–91.
Disis ML, Taylor MH, Kelly K, Beck JT, Gordon M, Moore KM, et al. Efficacy and safety of avelumab for patients with recurrent or refractory ovarian cancer: phase 1b results from the JAVELIN solid tumor trial. JAMA Oncol. 2019;5:393–401.
Emens LA, Kok M, Ojalvo LS. Targeting the programmed cell death-1 pathway in breast and ovarian cancer. Curr Opin Obstet Gynecol. 2016;28(2):142–7.
Zhang G, Liu C, Bai H, Cao G, Cui R, Zhang Z. Combinatorial therapy of immune checkpoint and cancer pathways provides a novel perspective on ovarian cancer treatment. Oncol Lett. 2019;17(3):2583–91.
Pujade-Lauraine E, Fujiwarab K, Ledermann JA, Oza A, Kristeleit R, Ray-Coquard I, et al. Avelumab alone or in combination with pegylated liposomal doxorubicin versus pegylated liposomal doxorubicin alone in platinum-resistant or refractory epithelial ovarian cancer: primary and biomarker analysis of the phase III JAVELIN Ovarian 200 trial. 2019 SGO Annumal meeting; march 17, 2019; Honolulu, HI.
Liao JB, Gwin W, Urban R, Hitchcock-Bernhardt K, Coveler A, Higgins D, et al. Pembrolizumab with low dose carboplatin for recurrent platinum resistant ovarian, fallopian tube, and primary peritoneal cancer-interim results. 2019 ASCO annual meeting; Chicago, IL. J Clin Oncol. 2019;37(suppl; abstr 5519).
Moore KN, Pignata S. Trials in progress: IMagyn050/GOG 3015/ENGOT-OV39. A phase III, multicenter, randomized study of atezolizumab versus placebo administered in combination with paclitaxel, carboplatin, and bevacizumab to patients with newly-diagnosed stage III or stage IV ovarian, fallopian tube, or primary peritoneal cancer. Int J Gynecol Cancer. 2019.
Liu YL, Zamarin D. Combination immune checkpoint blockade strategies to maximize immune response in gynecological cancers. Curr Oncol Rep. 2018;20(12):94.
Penn C, Lester J, Bohrer K, Moon C, Yearley J, Karlan BY, et al. PD-1/PD-L1 expression in BRCA1/2 mutated ovarian cancers. 2019 SGO annual meeting march 2019; Honolulu, HI.
Konstantinopoulos PA, Waggoner S, Vidal GA, Mita M, Moroney JW, Holloway R, et al. Single-arm phases 1 and 2 trial of niraparib in combination with pembrolizumab in patients with recurrent platinum-resistant ovarian carcinoma. JAMA Oncol. 2019;5:1141.
Monk BJ, Coleman RL, Fujiwara K, Oza AM, Westin SN, O’Malley DM, et al. ATHENA (GOG-3020/ENGOT-ov45): a randomised, double-blind, placebo-controlled, phase 3 study of rucaparib + nivolumab following front-line platinum-based chemotherapy in ovarian cancer. 2018 International Gynecologic Cancer Society Biennial Meeting; Kyoto, Japan.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare they have no conflict of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Gynecologic Cancers
Rights and permissions
About this article
Cite this article
Ferriss, J.S., Williams-Brown, M.Y. Immunotherapy: Checkpoint Inhibitors in Lynch-Associated Gynecologic Cancers. Curr. Treat. Options in Oncol. 20, 75 (2019). https://doi.org/10.1007/s11864-019-0676-8
Published:
DOI: https://doi.org/10.1007/s11864-019-0676-8