Abstract
This chapter briefly explains the anaerobic digestion system to optimize biogas production and integrate digestion stabilization in rural semi-lot digesters, using agro-industrial waste mixtures to establish C/N ratios; with and without the inclusion of methanogenic inoculants. A second topic refers to obtaining second generation bioethanol from lignocellulosic wastes. General concepts of biogas and bioethanol production, related parameters and typical production schemes are described. On the other hand, it will focus on the application of fermentative processes as an activity of three different bacterial communities in biogas production and on induction systems using microorganisms to obtain bioethanol. The symbiosis between microorganisms will be presented, in the sense that in fermentation processes, metabolic actions of several microorganisms act together. In the final part, some practical applications related to the installation and start up of rural biodigesters are presented. In the case of lignocellulosic materials, pre-treatments, and processes for obtaining second generation bioethanol will be presented, as well as the current market trend. Similarly, it seeks to show the benefits for users, society and the environment, in the sectors of energy production, transformation of organic waste into high quality fertilizers, improvement of hygienic conditions, reduction or elimination of wood consumption and environmental advantages.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
1 Biogas Production
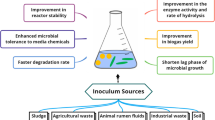
Biogas is understood as the combination of gases originating from the decomposition of organic matter (fermentation process) by microorganisms under anaerobic conditions (Kapoor et al. 2020). This process is called anaerobic digestion or biomethanization and the oxidizing bacteria: methanobacteria (Röske et al. 2014). The proportion of mixed gases depends on the digested substrate, being generally formed by 50–70% of CH4, 30–40% of CO2, and small proportions of H2S, N2, H2, and others. These methane agents can be found in cow manure, in wastewater and in the sheep rumen (Budiyono et al. 2013; Kivaisi and Eliapenda 1994; Taherzadeh and Karimi 2008).
1.1 Operation Process of a Biogas Production Plant
A brief description of the operation process and general functioning of a biogas production plant is presented (Atelge et al. 2020; Lee et al. 2017; Taherzadeh and Karimi 2008; Wardani et al. 2020). Prior to the entry of the raw material into the plant for the biogas production process, the following steps must be followed:
-
Step 1: Elimination of contaminating components
The organic fuel to be fed to the plant must be analyzed for contaminants. The organic material (OM) used in anaerobic digestion (AD) should be biodegradable material such as food waste, vegetable waste, market waste, crop residues, sludge and sewage, animal excrement from pigs and cows, etc. (Daza Serna et al. 2016).
-
Step 2: Treatment of organic material
Once screened, the fuel must be treated to ensure a smooth consistency; this is because many AD units are fed by continuous flow reactor to achieve a better cost-benefit ratio. Once screened and treated as needed, the fuel is fed to a digestion unit for further decomposition by microorganisms (Aristizábal-Marulanda et al. 2020).
-
Step 3: At the Biogas production plant. The digestion units are expressed as a function of the temperature range in which the microorganisms grow (mesophilic or thermophilic) (Bouallagui et al. 2003, 2005).
Step 4: The different stages of decomposition. The decomposition of OM begins and involves four stages of chemical processes that are responsible for converting matter into usable biogas. DA is carried out in four steps, which occur partly simultaneously (Al-Zuahiri et al. 2015).
-
Hydrolysis: The first step is hydrolysis, and it is the slowest step, where bacteria convert the most complex organic materials such as carbohydrates and proteins (long chain chemicals), into sugars and amino acids (individual molecules) (Dererie et al. 2011).
The waste is inside the sealed module and is sprayed with partially degassed percolate. The percolate is drained with fatty acids and pumped into the tank for gasification.
-
Acidogenesis: The second step is acidogenesis, microorganisms break down individual molecules of sugars and amino acids into ethanol and fatty acids, in addition to producing CO2 and H2S as byproducts (Ylitervo et al. 2013).
-
Acetogenesis: The third step is acetogenesis, in this third stage, ethanol and fatty acids are converted into H2, CO2 and CH3COOH (Aristizábal-Marulanda et al. 2020).
-
Methanogenesis: The last step is methanogenesis where methanogenic bacteria convert H2 and CH3COOH into CH4 gas and CO2 (de la Torre et al. 2019).
The gas mixture also contains H2S, N2, O2 and H2. As a percentage by volume, methane (Biogas) is approximately 60%, while carbon dioxide is 40%. Sulfhydric acid is usually less than 2% (Ituen et al. 2009).
-
1.2 Feeding Materials
Biogas plants can work with different renewable raw materials. With the help of a loader, the solid raw material can be poured into a cement or metal tank, which allows it to be filled approximately once a day. The raw materials are energy-rich and due to their high degree of reduction are suitable for use in biogas plants. The storage container can be equipped with a hydraulic unloader that continuously feeds the raw material through a conveyor belt. A scale under the conveyor registers the weight of the raw material.
Liquid manure is the most important basic substrate that some biogas plants can use. After storage, it is pumped through pipes directly to the mixing pump installed next to the conveyor belt. At the same time, the feedstock falls from the conveyor belt into the separators, which are equipped with two mixing rollers. In this way, the raw material is mixed just before the fermentation process (Fig. 1) (Leite et al. 2015). One or more fermentation tanks can be fed with fresh substrate according to the design requirements, even if they are not close by (Janke et al. 2015). Liquid waste from the food industry can be another substrate used, however, since the availability of this waste varies considerably, a storage tank must be installed for this purpose. Integrating the entire system serves to reduce odors and prevent epidemics.
The liquid waste is then heated to 240 °C in a tubular heat exchanger using a countercurrent process. After heating for 1 h, the hydrogenation of the substrates is completed, so that they can be poured into the fermenter, where the biogas is formed (Mirahmadi et al. 2010). The substrates are continuously agitated, to avoid the formation of layers of material on the top or bottom. A hot water heater brings the substrate to a temperature between 35 and 55 °C, to accelerate the formation of biogas (CH4). The substrate remains in the fermenter for a period of 30 days before moving to fermenter for another 30 days to complete the biogas formation process (Kotarska et al. 2019). When the fermentation is complete, the thin liquid substrate (digestate) is pumped into reinforced concrete tanks where it is stored until it can be taken to the field to be used as fertilizer.
1.3 Gas Produced
The controlled fermentation of biomass in biogas plants produces a gas that can be used to generate thermal-electric energy due to its high percentage of methane. The raw materials (substrates) used in biogas plants are products of agricultural liquid manure, agro-industrial waste, and liquid waste from the food industry (Kapoor et al. 2020).
If the fermenters are regularly filled with biomass, heated and shaken, the biogas is formed in a matter of days. The formation of gas is a complex and delicate process, the fats or organic carbohydrates contained in the substrates are what feed the fermenter to be digested by different types of bacteria (Wardani et al. 2020). This is the starting point to produce biogas, the contents are continuously mixed, the biogas rises slowly to the top of the digester. It consists of approximately 50–70% methane (green color), carbon dioxide, water vapor, H2 and H2S (Hagman et al. 2018). Because water vapor and H2S are problematic, for the further utilization of the biogas, it is necessary to treat the biogas. First the gas is released from the water, the condensed water is collected and pumped to another location. It is expected that the biogas will be accompanied by an aggressive hydrogen sulphide gas stream and will now be directed to a biological desulphurisation plant. By introducing air into the container, certain bacterial cultures to establish colonies on a metal chain placed inside the container (Moraes et al. 2015a, b).
There they break down the hydrogen sulfide into harmless sulfur, thus almost infertilizing the biogas. Finally, the biogas is fed to a compressor where it is brought to a pressure of 70 mbar required to burn it in order to completely condense any remaining water vapor and free the biogas from any suspended matter. The biogas is subjected to a washing and drying process, which is carried out with a steam at the freezing point, cooling the biogas to a temperature below 5 °C (Ben Yahmed et al. 2016).
In order to control the purification of the gas, it is constantly tested with an online measurement system which records the amounts of CH4, H2S, CO2 and all this will guarantee a high degree of efficiency and safety. In case of an overproduction of biogas, it is necessary to operate a burner, to burn the methane preventing it from escaping into the atmosphere. Up to 30% of the waste heat from the water used for cooling the machines in the plant is used for heat exchange in the fermenter so that no additional heat is required (Dererie et al. 2011; Goshadrou et al. 2013; Ituen et al. 2009; Kotarska et al. 2019; Moshi et al. 2015). Once the production of CH4 is stable and of high quality, it can be used in a gas engine to produce electricity, or the gas can be arranged for distribution. The electrical power generated by the generator in the case of using the biogas for electricity generation is converted to the main voltage level in the transformer, then the electricity can be fed into the grid.
2 Instrumentation and Control
There is a general agreement that the usual control parameters in biogas production plants in anaerobic digestion systems (e.g. pH control), are not sufficient or their measurement is not reliable. So far, none of the usual control loop systems provide a direct link to the biochemistry related to the microbiology of the anaerobic digestion system. Therefore, the design of a successful control strategy that is more closely related to the biochemistry of the digester with an analytical approach and evaluation of variables is necessary. Obtaining a model that relates all the biochemical and microbiological variables related to the anaerobic digestion process would provide the opportunity to tune controllers into control loops that can be implemented at the industrial level (Jiménez-Castro et al. 2020).
Like all living things, methanogens need an environment to live and thrive. Since in the process of acidogenesis acid (acidogenic bacteria) is produced, leading to a decrease in the pH in the digestion tank (Larsson et al. 2015), it is crucial to constantly measure the pH throughout the process to ensure the continuous well-being of the microorganisms and thus the production of methane (Mao et al. 2015).
A biogas production plant with an annual production capacity of 15,000 tons per year requires between 3 and 5 h of work per day. To reduce the amount of work, to a minimum, the use of effective measurement systems and process control technology is recommended. Thanks to a secure data exchange, it is possible for a person outside the plant to monitor and control the unit. For example, agitators can be started or stopped, container levels can be checked, or solids supply equipment can be monitored from remote locations (Mussoline et al. 2012; Shafiei et al. 2011).
As in other chemical processes, if the temperature is controlled during the digestion process, and is maintained at the top of the given specific range, the reaction rate will be at its highest value producing more gas in the same amount of time (Barta et al. 2010). While higher temperatures produce higher gas yields, they are also more difficult and expensive to maintain, making temperature a vital aspect to measure throughout the entire anaerobic digestion process (Holm-Nielsen et al. 2009).
It is vital to constantly measure biogas emissions (methane, carbon dioxide, small amounts of siloxanes, hydrogen sulfide, ammonia, hydrocarbons, and water), as production levels are good indications of production abnormalities, biomass quantities and microorganism welfare (Mladenović et al. 2018). The gases emitted should indicate the amount of biomass that remains to be decomposed. If a batch system is used, the measurement of gas emissions will provide a schedule for the complete digestion of the biomass and indicate when new biomass should be added to the digester. If a continuous system is used, the measurement of biogas production will indicate how efficiently the entire process is running (Sommersacher et al. 2013).
Monitoring for trace gases and volatile fatty acids can provide some measure of the metabolic state of an anaerobic system. In contrast to liquid phase sampling, gas analysis is susceptible to real time data acquisition. Monitoring both hydrogen and CO together, and volatile fatty acids, can provide significant insight into the metabolic state of the digestion process and has the potential to indicate process disturbances in real time. The entire process can be controlled locally through a PLC (programmable logic controller) system (Jouzani 2018; Shafiei 2018; Smajevic et al. 2012) or with a SCADA (supervisory control and data acquisition) system with support available through the internet (Werther et al. 2000).
3 Conversion of Organic Waste into Biogas and Fertilizer
The following materials are generally considered organic and can be processed in a digester:
Animal Manure
-
Food waste
-
Fats and oils
-
Industrial organic waste
-
Biosolids
Below is a summary of the process that must be performed on organic waste before it is sent to a biogas production plant:
-
Collect organic waste
-
Processing waste with standard machinery
-
Previous classification
-
Separate plastics and large impurities from the organic fraction
-
Make a good mix, first of the green structural material
-
Then several pre-selected waste loads
-
Finally, thick structural material
-
Load the mixture into the process module
-
The module is closed and sealed
If the plant accepts more than one agricultural raw material, it is called a co-digestion plant. The organic compounds that feed the anaerobic digestion process are composed of carbon (C), nitrogen (N), oxygen (O). The microorganisms use these organic compounds as a substrate for growth and combine them with water (H2O) to form carbon dioxide (CO2) and methane (CH4). The actual decomposition of the organic compounds into methane is not done by one microorganism alone but occurs in three stages through the teamwork of several microorganisms. The first microorganisms convert the organic compounds into an intermediate substance that other microorganisms can convert into organic acids. Anaerobic methanogenic (methane producing) bacteria convert the organic acids into methane (Akbulut 2012; Ren et al. 2006).
4 Anaerobic Digestion (AD)
Anaerobic digestion is sometimes referred to as methanogenesis. Composting is a treatment option for organic solid waste and is a process of controlled aerobic (in the presence of oxygen) degradation. The anaerobic digestion process is used in the treatment of domestic and industrial wastewater. Within this process primary (solid) and secondary (liquid) organic waste can be anaerobically digested. In simple terms AD is the decomposition of organic materials into gases (CH4 and CO2), with some water as a by-product (Cakir and Stenstrom 2005; Holm-Nielsen et al. 2009; Khalid et al. 2011; Marin et al. 2010).
Anaerobic digestion is the group of processes where microorganisms degrade biodegradable organic matter in the absence of oxygen. This can occur in swamps or even in the stomach of ruminants. Its importance in solid waste management lies in the fact that microorganisms can produce biogas, which is a mixture of methane and carbon dioxide. In practice, what is interesting in this process is the production of methane, because it is flammable and can be used for energy generation (heat and electricity) and on the other hand, a nutritious digestate (wet mixture that is generally separated into a solid and a liquid and is rich in nutrients) is obtained that can be reused to apply to crops as fertilizer. The interesting thing is to use this process applying engineering and design of control systems, in a reactor(digester) without air influence, to produce biogas (by means of anaerobic bacteria) and digestate (Izumi et al. 2010; Kabouris et al. 2009; Kivaisi and Eliapenda 1994; Linke 2006).
4.1 Benefits of Anaerobic Digestion (AD)
Firstly, this is a renewable energy source, which reduces greenhouse gas emissions. On the other hand, subway reactors can be designed and built, requiring little space, eliminating the use of wood, reducing the volume of solid waste, avoiding disposal costs and finally, recovering value from solid waste, biogas and nutrients (Karagiannidis and Perkoulidis 2009; Kim et al. 2006).
4.2 Disadvantages of Anaerobic Digestion (AD)
The DA process is more sensitive, less stable and slower, and no energy is generated inside the reactor, because the energy is contained in the produced methane, which means that there is no heat generation. DA is a more technically complex process requiring higher levels of skill and investment. Finally, obtaining models that represent the dynamics of the process and can be used for control purposes is not an easy task (Ariunbaatar et al. 2015; Banks et al. 2011; Forster-Carneiro et al. 2008a, b).
4.3 Practical Parameters and Operational Conditions of Anaerobic Digestión
It is possible to start with the raw materials (input materials), but a distinction must be made between the solid content and the water content. The dry matter is also called total solids (TS), some of them are biodegradable and others not. The relevant fraction is the biodegradable organic fraction and is called volatile solids (SV: biodegradable fraction of total solids). The levels of total solids and volatile solids in solid waste differ depending on the type of waste. For example, for vegetable waste, the total solids content is between 5 and 20% of the organic solid waste, while the amount of volatile solids is between 76 and 90% of the total solids (Bouallagui et al. 2005; Chen et al. 2008; Gómez et al. 2006; Sawayama et al. 1997).
Depending on the type of solid waste, different amounts of methane produced can be expected, for example, lignin-rich organic waste has a methane yield of 200 L/kg of SV, while fruit and vegetable waste has a yield of 420 L/kg of SV. Lignin does not degrade under anaerobic conditions, so anaerobic digestion is not suitable for treating garden waste or wood waste (Bouallagui et al. 2009).
An important parameter in anaerobic digestion is the organic load rate (OCR), which quantifies the amount of substrate per reactor volume and time. Organic loading rate = mass of volatile solids, SV/reactor volume and time (kg SV/m3dia). A good daily loading rate for unshaken reactors is TCO ≤ 2, while with a shaken reactor this can be higher, and can be increased to a loading rate of 8, i.e. <2 kg SV/m3dia without shaking and 4–8 kg SV/m3dia with shaking. The pH range for anaerobic digestion is between 6.5 and 7.5. There is a risk of acidification of the reactor by acidogenic bacteria when the OCT is very high. However, in the acidic phases, the pH is low, while in the methanogenic phase it is a little higher. Methanogenic bacteria are rather sensitive to these parameters and will therefore be inhibited. In order to react to this situation the organic load rate must be reduced or lime or sodium hydroxide can be added to increase the pH level (Moraes et al. 2015b; Zuo et al. 2012).
Another factor that influences the process of anaerobic digestion is temperature. Temperatures below 15 °C are not ideal (not much activity occurs) and the organisms decrease their activity. Subterranean buildings or installations can buffer this temperature variation, but the anaerobic process is more comfortable in two temperature zones (ideal range): the mesophilic temperature between 30 and 40 °C and the thermophilic temperature between 45 and 60 °C. Operations in the mesophilic range are more stable and can tolerate large changes in parameters and consume less energy, however, they are slower in degradation and need more time. Thermophilic organisms are faster but the system is more sensitive to changes. Mesophilic units operate between 20 and 45 °C, with an optimal temperature of 37 °C. These are more common, because they are cheaper to build and maintain due to their lower operating temperature (Bouallagui et al. 2009; Forster-Carneiro et al. 2008b; Linke 2006; Wardani et al. 2020).
The hydraulic retention time (HRT) is another parameter that influences the anaerobic digestion process, and corresponds to the amount of time the input material remains in the reactor. An ideal time is between 10 and 40 days, the lower values correspond to the higher temperatures in the thermophilic range, because the process is faster (HRT = reactor volume/input volume per day) (Khalid et al. 2011).
Here is a confrontation with an optimization process. If for already defined input materials the volume of the reactor is small, then the HRT is low, which means that little biogas will be obtained, since there is little time for the process. If the reactor volume is large, then the HRT increases and you will have a higher biogas production, but at the cost of having a large reactor (more space and higher investment costs). Another parameter is the C/N (Carbon/Nitrogen) ratio, ideally a value between 16 and 25. A higher value implies limited nitrogen supply, which means food for the bacteria and therefore less biogas production. A lower value of the C/N ratio can cause an accumulation of ammonia which can inhibit the anaerobic process (Bouallagui et al. 2003; Mel et al. 2015; Westerholm et al. 2012).
The last parameter corresponds to the size of the particles in the input material. The smaller the size the better, sizes smaller than 5 cm are ideal. What this does is increase the surface area of the material and allow microorganisms to degrade the material faster. For operations this means that generally the input material is fragmented using a crusher to get smaller particles. All anaerobic digestion systems adhere to the same basic principles, whether the raw material is food waste, animal manure or sludge from a wastewater treatment plant. Systems may have some differences in design, but the process is basically the same (Jeihanipour et al. 2013; Salehian and Karimi 2012).
4.4 Use of the Products of Anaerobic Digestión
The main products of anaerobic digestion are biogas and digestate. The substrate chain corresponds to different organic wastes, the collection and transport and the pre-treatment stage. Using an appropriate digester and with good operational conditions, the reactor will produce a good amount of biogas, which is saturated with water vapour and in the first instance the water vapour that moves through the pipes will condense and the humidity will be collected as water in the pipes and must be removed (process called dewatering).
Water can be removed at the lowest point in the pipes, if it is not removed it can block the pipes making the flow of biogas difficult. Because of this a siphon (water trap) should be installed at the lowest point in the gas pipes (Cesaro and Belgiorno 2015a).
There are different types of siphons that can be installed, Fig. 2 shows a simple elbow used to drain condensate into a biogas line. There are different types of traps that can be installed, they can be automatic or manual. Both have a T-shaped connection where the condensate can flow. The automatic trap empties when it is full, using a U-shaped drain, while the manual trap empties with the opening of a manual valve. The biogas produced in the reactor varies during the day according to the feeding models and according to the changes in ambient temperature (Jeihanipour et al. 2010).
Biogas production also continues during the night when less gas is used and therefore there are periods when the gas must be stored. This can be done in the same digester at the top of the fixed digesters, or in bags or balloons. Biogas can also be stored at high or medium pressure in gas tanks, through the use of compressors, requiring the use of energy, in three steps: conditioning, cleaning of the biogas and compression. In its uncompressed form, the energy content of one cubic meter of biogas corresponds to about half a liter of diesel. As a general rule, about 10 kg of organic solid waste (in wet weight) is needed to produce one cubic meter of biogas (Casaretto et al. 2019).
As an example, the calorific value of diesel is 12 kWh/kg, it is needed to burn half a liter (0.5 kg) of diesel to generate the same amount of energy as one cubic meter of biogas (6 kWh/m3). This means that burning half a liter of diesel generates the same energy as burning 1 m3 of biogas (Silva et al. 2019).
For all its uses in machinery, engines or generators, the biogas needs to be conditioned or cleaned. The objective is to remove water vapor, and hydrogen sulfide which is very corrosive, and carbon dioxide which has no energy value. The removal of hydrogen sulfide is called desulfurization and can be done with ferrous oxide, which then transforms the hydrogen sulfide into iron sulfide. This can also be done with water purifiers, which at the same time can remove carbon dioxide.
If you think about the usefulness and use of biogas, the simplest way is to use the biogas near the anaerobic digestion facility for direct combustion, without the need for conditioning. The household ovens for cooking only need 200–450 L/h of biogas. Often when the direct use of biogas is not obvious, methane is introduced into the gas generators to produce electricity. However, with transformation losses only about 30% of the energy can be retained as electricity, the rest is lost as heat.
In addition to the gas, another production from anaerobic digestion is the digestate. In wet digestion systems, these are a kind of nutritive sludge with nitrogen, phosphorus, potassium and traces of other elements. Fifty percent of this nitrogen is available as ammonia that can be directly assimilated by plants and in general terms is a good fertilizer that can be used in agriculture. But if the operation is in the mesophilic range, you will not have high temperatures and if you have pathogenic substances these will not be sanitized. If it is not possible to use these digests in agriculture, then they must be treated before disposal, because they still have a high organic load and based on the regulations they are not allowed to be disposed of in surface waters (Atelge et al. 2020).
In a biogas system it is necessary to regularly check for leaks, using for example a pressure test, to see if the gas pipes are in good condition. The pipes also need to be checked and released if necessary. The siphons must be emptied and the sludge level in the outer compartment must be also regularly observed. The digester should not be overloaded, as this can lead to a process of acidification. From time to time the sludge must be removed from the digester (every 5–10 years), most of it will be found gravel and sand that accumulate at the bottom of the digester.
To undertake anaerobic digestion projects, it is necessary to have access to a good source of organic waste that is well sorted and accessible. Similarly, local skills and experience in the construction and operation of digesters need to be considered. Then, another requirement is the need and demand for gas or a small network that can be powered (institutional-state environment that allows power to be fed into the electricity grid). Likewise, the opportunity to compete with other energy sources is needed. Finally, there is a demand for digestate for use in agriculture.
4.5 Anaerobic Digestion Technologies and Practical Operation
There is a classification of the different types of anaerobic digestion technologies. A distinction can be made between dry and wet systems, between a batch and a continuous system, between thermophilic and mesophilic operating systems, and between single-stage and multi-stage technologies. Wet reactors (operating with a low total ST solid content of 16% or less), continuous (the system is fed at a regular interval and at the same time an equivalent volume enters, an equivalent volume leaves the reactor), mesophilic and single-stage will be discussed (Bouallagui et al. 2003; Wardani et al. 2020).
Figure 3 shows a fixed dome reactor, which is a watertight gas structure built of bricks and covered with plaster and is often installed underground. It has an inlet pipe through which solid waste enters the digester, a digester with a volume of sludge and a space at the top to store the biogas produced. There is a compensation chamber (outlet) and a drainage system. The gas pipe is located in the highest part, with a valve to open or close the biogas passage. There are several designs of fixed dome digesters and sizes may vary. This is the most common type of reactor in developing countries (Bouallagui et al. 2009).
When the reactor is first started up, it is important to inoculate it with metonogenic bacteria. The easiest way to do this is to add cattle manure and water in a ratio of 1:1, or somewhat easier to use the digestate from another reactor that is already in operation. As a rule, about 10% of the reactor volume is needed with livestock manure to start the process, although more than 10% is good or even better (Bouallagui et al. 2009).
Operation
The solid waste is mixed with water and introduced into the reactor and mixed with the material that was already inside the reactor, being subsequently degraded. The biogas is generated in the sludge by means of anaerobic digestion, the gas bubbles are moved to the top of the reactor, where they accumulate and begin to generate pressure. If the gas outlet valve is closed, the gas pressure will continue to increase and push the sludge down into the reactor and up into the dewatering chamber, where it is recovered as Biol (Yin et al. 2008).
When the gas is used, the pressure of the gas inside the digester decreases and the level of the sludge will be equalized generating a new balance.
Advantages
Long life, no moving components, space-saving subway construction, low construction cost, easy to operate.
Disadvantages
Need for specific technical skills (specifically at the construction stage), special sealing is required to ensure hermiticity of the structure, fluctuation of gas pressure depending on the volume of gas stored and its use.
It has similar characteristics to the previous reactor (inlet, digester to accumulate the sludge, outlet and drainage), the gas container is not a fixed unit, but a mobile unit, floating on a water jacket or in some cases floating directly on the sludge. When the gas pressure increases, the gas collection vessel (dome) moves upwards, and when the gas is used the weight of the hood pushes it back down. The hood is made of metal (painted to protect it from corrosion), fiberglass, plastic reinforced or galvanized sheet metal. In this design there is a guide rod, to ensure its stabilization while moving up and down. There is also a dividing wall that helps prevent circulation of the sludge from the inlet to the outlet directly. The digester is often positioned underground, while the gas container is located above ground (Duan et al. 2014; Khan et al. 2009).
Advantages
Simple and easy to operate, the gas volume is directly visible (it is possible to see if the system is working or not), it has a constant gas pressure, or it is possible to increase it by adding some kind of weight to the gas tank or container (dome), relatively easy to build.
Disadvantages
High material costs for the steel bell, and the steel parts are susceptible to corrosion, There are regular maintenance costs that increase when performing preventive maintenance against corrosion.
They are common in Latin American regions. They are long horizontal plastic and rubber tubes or balls (Fig. 4). The inlet and outlet lines are attached directly to the ball’s shell. These balls need some kind of protection from the top (to avoid damage to its structure), as well as from the bottom, which is usually a compacted filling. A biogas accumulation system can be installed in the upper part of the biodigester.
Advantages
Low construction cost, very simple to install, easy to build, easy to transport, higher digester temperatures in hot climates, easy emptying and maintenance.
Disadvantages
Relatively short service life (5–10 years, depending on the material used for construction), susceptible to mechanical damage.
5 Practical Applications in Biogas Production
Figure 5 shows a tubular type biodigester, 10 m long by one meter in diameter, located in the municipality of “Anzoategui” (Department of Tolima-Colombia)—Vereda Berdun high part and fed with pig excrement (28 pigs). The biogas is used for cooking food. The equipment used for measuring the parameters is a Multitec 540, reporting volume concentrations of 46.2%v for methane (CH4) and 0.2 for hydrogen sulfide, %v (H2S) (Mladenović et al. 2009; Xiang et al. 2018).
Biogas treated to meet pipeline quality standards can be distributed through the natural gas pipeline and used in homes and businesses, or it can be cleaned to produce compressed natural gas (CNG) or liquefied natural gas (LNG), which can be used to fuel automobiles. The digestate can be applied directly to the soil and incorporated into soils to improve soil characteristics and facilitate plant growth. It can also be processed into products to recover nitrogen and phosphorus and create concentrated nutrient products, such as ammonium magnesium phosphate and ammonium phosphate fertilizers (Ferella 2020).
If there are many animals on a farm, the resulting manure and wastewater can have significant environmental impacts if they are allowed to simply run on the land and into storm drains and surface waters. The waste depletes oxygen from the water as it degrades, which is harmful to aquatic life. Containment of animal waste is often required to protect water quality. Anaerobic digestion reduces the volume of this waste, produces methane and provides a byproduct that can be used as fertilizer (Ozgen et al. 2021).
6 Bioethanol Production
Bioethanol is a clear liquid that looks like water, it is mixed with gasoline (fuel additive) to produce a cleaner fuel that improves oxygen. Bioethanol is a chemical compound that can be used in different applications, such as alcoholic beverages, chemicals, pharmaceuticals and biofuels (Piccolo and Bezzo 2009).
6.1 Process Details for Bioethanol Production
Below is a diagram of the ethanol fermentation process.
The raw material is sent to the production plant and the handling of the material must be done, then comes the pre-treatment process, followed by the hydrolysis or fermentation process (Fig. 6) and then goes to the distillation process and finally through the evaporation process the ethanol is separated (Aruwajoye et al. 2020; Schläfle et al. 2017). The wastewater that comes out of the pre-treatment process can be used to produce biogas, which can be used to operate a boiler and the steam can be used for distillation purposes or to produce electrical energy. The following is the main equation in the alcohol fermentation process in a plant:
Sucrose reacts with water to produce ethanol and carbon dioxide, with the help of a yeast that acts as a catalyst in this process. The process can be given in feed-batch mode, where the diammonium phosphate (essential nutrient for the yeast), is fed intermittently into the reactor during the process. The stages of the process correspond to mixing, fermentation, and purification. The input consists of sugar syrup, yeast and nutrient which is fed intermittently into the reactor. The output consists of carbon dioxide, yeast, and ethanol (Balat 2011; Boluda-Aguilar et al. 2010; Rodríguez et al. 2010).
There are many crucial steps in the design of a bioreactor, first the type of reactor (e.g. stirred tank in batch feed), second the size and arrangement (a typical size for an industrial fermenter is between 50 and 100 m3). The bioreactors use an agitation system that includes shock absorbers (buffers) and impellers. Using the mass flow rate and density the working volume can be found. Smaller fermenters are more economical, the minimum number of parallel fermenters is 4, to ensure optimum process efficiency (Plácido et al. 2013; Zuo et al. 2012).
Proceso de operación de una planta de producción de bioetanol a partir de materia prima lignocelulósica:
Any sugar fermentable by a yeast can act as a raw material for alcohol fermentation. The ethanol manufacturing process depends mainly on the raw material. The most used steps in the production of bioethanol from raw materials containing lignocellulose are:
-
Pretreatment of cellulose and hemicellulose to make it more accessible in the subsequent steps.
-
Acid or enzymatic hydrolysis of polysaccharides in simple sugars (hexose and pentoses) to ethanol.
-
Separation and concentration of ethanol.
6.2 Fermentation Process of Lignocellulosic Hydrolysates
Different microorganisms (e.g. Saccharomyces serevisiae) are used for glucose to ethanol fermentation. This microorganism is able to metabolize mono- and disaccharides (glucose, fructose, maltose and sucrose), but not pentoxes (xylose and arabinose), moreover it is not able to directly assimilate cellulose and hemicellulose (Lee et al. 2017; Oyeleke and Jibrin 2009).
There are pentose-fermenting microorganisms (Pichia Stipitis, Pachysolen Tannophilus and Candida Shehatae), however, they have a production rate of ethanol at least five times lower than the production of ethanol from glucose by S. cerevisiae, and on the other hand the tolerance to oxygen and ethanol is 2–4 times lower.
Zymobacter palmae Gram-negative strain is an anaerobic with potential to metabolize hexose, di- and trisaccharides bound to α and sugar alcohols (fructose, galactose, glucose, mannose, maltose, melibiose, sucrose, raffinose, mannitol and sorbitol). This strain produces approximately two mole of ethanol per mole of glucose without accumulation of byproducts and shows a productivity like Z. mobilis which is used to produce glucose alcohol in conjunction with the enzyme pyruvate decarboxylase and alcohol dehydrogenase (Dias et al. 2013; Nair et al. 2017).
The filamentous fungus Fusarium oxysporum is known for its ability to produce ethanol, but the conversion rate is low and produces significant amounts of acetic acid as a byproduct. In a bioethanol production plant, hydrolysis and fermentation can operate separately (HFS) or saccharification and fermentation can operate simultaneously (SFS) (Zuo et al. 2012).
HFS hydrolyzes the lignocellulosic raw material pre-treated to glucose and then ferments it to alcohol using different bioreactors for each process. After pre-treatment of the lignocellulosic raw materials, the solid phase is separated from the liquid phase, which contains mainly sugars (pentoses and some hexose). After pre-treatment with diluted acid, the residual solid phase contains mainly lignin and cellulose. The cellulose is hydrolyzed by the addition of cellulolytic enzymes. Here both hydrolysis and fermentation take place at their optimum temperatures (50 °C for hydrolysis and 28–32 °C for yeast fermentation) (Carrillo-Nieves et al. 2019; Jafari et al. 2011; Moraes et al. 2015a).
Special microorganisms perform the fermentation of hexose and pentoses (present in the liquid phase) separately, because microorganisms that use pentoses metabolize pentoses and hexose more slowly than microorganisms that only assimilate hexose and are more sensitive to ethanol and inhibitors. The accumulation of released sugars (glucose, cellobiose) during enzymatic hydrolysis inhibits cellulase activity, with the effect of cellobiose being greater than that of glucose (Branco et al. 2019; Mirahmadi et al. 2010; Rastogi and Shrivastava 2017).
During SFS, hydrolysis and fermentation take place in a single bioreactor, with the sugars released in the enzymatic hydrolysis being used immediately by the microorganism. Relatively low concentrations of sugar will occur in the culture medium, and consequently the inhibition of the cellulase enzyme by the released sugars is reduced. The optimal temperature is 38 °C, with a compromise between optimal hydrolysis (45–50 °C) and fermentation temperatures (30 °C). Improvements can be achieved by selecting improved enzymes and yeast strains. In this technology T. reesei and S. cerevisiae are most frequently used (Bernier-Oviedo et al. 2018).
FSS Advantages
increased hydrolysis rate by reducing cellulase inhibition by released sugars, lower enzyme demand, higher bioethanol yield, lower sterile condition requirement, shorter bioprocess time, reduced process, and separation equipment cost.
Disadvantages of SFS
Incompatible hydrolysis and fermentation temperatures, microbial tolerance to ethanol, and enzyme inhibition by etanol.
Improvements can be made in order to optimize the process of obtaining bioethanol. For example, the inclusion of pentose fermentation in the FSS is an integration alternative; this process is called simultaneous saccharification and co-fermentation (SCFS). The two producing microorganisms must be compatible in terms of optimal pH and temperature. The development of microbial strains capable of growing at elevated temperatures can significantly improve techno-economic indicators. By co-fermentation of pentoses and hexose in a bioreactor, capital costs are reduced, as well as the possibility of contamination (Lynd 1996).
A second integration approach for the conversion of lignocellulose-containing feedstocks into bioethanol is consolidated bioprocessing (CBP). The production of cellulase and fermentation requires only one microorganism. Therefore, cellulase production, cellulose hydrolysis and fermentation are performed in one step. BPC involves four biological reactions in a single step: enzyme production (cellulases and hemicellulases), hydrolysis of carbohydrates into sugars, fermentation of hexose (glucose, mannose, and galactose) and fermentation of pentoses (xylose and arabinose). CBP has the following advantages compared to other techniques for bioethanol production: enzymatic and fermentation systems are fully compatible and therefore bioethanol production costs are reduced, and no capital and operating investments are required (Aden and Foust 2009; von Sivers and Zacchi 1996).
6.3 Feeding Materials
The different types of biomass have potential as a raw material for bioethanol production. Due to their chemical composition (source of carbohydrates), they mostly form three groups: raw materials containing sugar (sugar beet, sugar cane, molasses, whey, sweet sorghum). Raw materials containing starch (corn, wheat, yucca). Lignocellulosic biomass (straw, agricultural residues, crop residues and wood) (Chovau et al. 2013; Hamelinck et al. 2005).
Raw materials containing sugar and cotton (first generation), compete with their use as food or fodder, which influences their supply. Therefore, lignocellulosic biomass (second generation), represents an alternative raw material for bioethanol production due to its low cost, availability, high distribution and is not competitive with food crops. Microalgae can accumulate starch as a reserve polysaccharide that can be used for bioethanol production (third generation), after the pre-treatment process, the residual biomass can be used as fertilizer. In the alcoholic fermentation process, there are three types of raw material (Azaizeh et al. 2020; Kumari and Singh 2020).
6.4 Raw Materials Containing Sugar (Sucrose)
Saccharin materials (mostly comprise sugar materials: they need little or no pre-treatment other than dilution (sugar cane, beet sugar, molasses, and fruit juices). Sugar cane and beet are the most important sugar producing plants in the world, they can be easily hydrolyzed by the invertase enzyme that is synthesized by most Saccharomyces species. Pretreatment for bioethanol production is not necessary, which makes this bioprocess more feasible than using starchy raw materials (Lu et al. 2011).
Sugar cultures only need a milling process for the extraction of sugars from the fermentation medium, and here bioethanol can be produced directly from juice or molasses. Total residual sugars in molasses can amount to 50–60%(m/V), of which approximately 60% corresponds to sucrose, making this substrate suitable for bioethanol production on an industrial scale. Sugarcane is less expensive than other raw materials used for bioethanol production (rapid processing and greater productivity) (Aden and Foust 2009; Jeihanipour et al. 2010).
The beet is used for the production of sugar, obtaining a fine and thick raw juice, as an intermediate product formed during the processing of sugar beet, as well as crystalline sugar of high purity, which could be converted into bioethanol and/or bio-based products. Another sugar-containing material that can be used for bioethanol production is whey, a by-product of cheese making, which contains about 4.9% (m/V) lactose. Due to the relatively low sugar content a moderate size bioethanol plant requires a considerable volume of whey.
6.5 Raw Materials Containing Starch
Materials with starch, need treatment to decompose starch into glucose (corn, malt, barley, rice, oats, rye). Grain crops (corn, barley, wheat, or sorghum), and root/tubular crops (cassava, potato), contain large amounts of starch. Pretreatment of cassava tubers for bioethanol production includes the following operations: cleaning, peeling, chopping, and drying. Dry cassava chips are used for bioethanol production. The crucial enzyme for the hydrolysis of starch is α-amylase active at α −1.4, but not in links α −1.6 in amylopectin (Budiyono et al. 2013; Cesaro and Belgiorno 2015b; Ferreira et al. 2018; Safarian and Unnthorsson 2018; Sarkar et al. 2012).
To produce bioethanol from starch-containing raw materials, it is necessary to perform starch hydrolysis (mainly through α-amylase and glucoamylase), into glucose syrup, which can be converted into ethanol by Saccharomyces cerevisiae yeast. This step is an additional cost compared to the production of bioethanol from raw materials containing sugars. Bacillus licheniformis bacteria and genetically modified strains of the bacteria Escherichia coli and Bacilus subtilis produce α-amylase. Aspergilus niger and Rhizopus sp. produce glucoamylases. Under anaerobic conditions the Saccharomyces cerevisiae yeast metabolizes glucose into ethanol (Branco et al. 2019; Carrillo-Nieves et al. 2019; Rastogi and Shrivastava 2017).
The maximum conversion efficiency of glucose to ethanol is 51% by mass. Since yeast also uses glucose for cell growth and synthesis of other metabolic products, the maximum conversion efficiency is reduced. In practice, 40–48% by mass of glucose is converted to ethanol.
6.6 Raw Materials Containing Lignocellulose
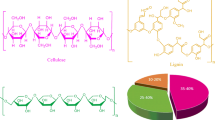
Cellulosic material, requires pre-treatment, to decompose cellulose into glucose (wood, residual sulfite liquor, cane bagasse). First the lignin is removed, then the cellulose is hydrolyzed, and glucose is produced, which is finally fermented and converted into bioethanol. Lignocellulosic biomass is renewable and not competitive with food crops, and a considerable reduction in greenhouse gas emissions is achieved. It can be obtained from different residues or harvested directly from the forest and its price can be lower than that of previous raw materials. They form six main groups: crop residues (sweet sorghum cane and bagasse, corn residues, different types of straw, rice husks), hard wood, soft wood (pine), cellulose waste (paper waste and recycled paper sludge), herbaceous biomass (grasses), municipal solid waste (Jafari et al. 2011; Moraes et al. 2015a; Zuo et al. 2012).
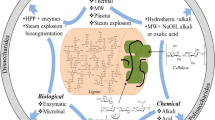
Lignocellulosic biomass contains 43% cellulose, 27% lignin, 20% hemicellulose and 10% other components. The heterogeneous structure of this biomass requires more complex chemical processes than uniform and consistent raw materials. Pretreatment and Hydrolysis: Hydrolysis of lignocellulosic biomass into monomeric sugars is necessary before microorganisms can metabolize them. This process is performed by acids, alkaline substances, or enzymes. The alkaline pre-treatment stage is necessary to obtain the conditions for efficient enzymatic hydrolysis (Chovau et al. 2013).
Pretreatment methods are divided into four groups: physical, chemical, physicochemical and biological. In pretreatment, reduction of the degree of polymerization and of the crystallinity index, breaking of lignin-carbohydrate bonds, removal of lignin and hemicelluloses and increase in the porosity of the material must occur to ensure effective enzymatic hydrolysis of the lignocellulosic biomass. The choice of pretreatment depends on the nature of the raw material and the formation of by-products during the selected pretreatment. The harsh conditions used during pretreatments lead to the synthesis of toxic compounds, such as furans (2-furaldehyde: furfural) and 5-hydroxymethylfurfural, carboxylic acids (acetic, formic and levulinic acids) and phenolic compounds (aldehydes, ketones) (Dias et al. 2013; Lee et al. 2017; Nair et al. 2017; Oyeleke and Jibrin 2009).
Because these compounds are potential inhibitors of yeast, the following methods can be used to reduce their impact on process performance: removal of inibitors by solvent extraction, ion exchange, use of zeolites, use of highly tolerant inhibitor yeast strains, selection of an effective pre-treatment method that causes minimal degradation of sugars and subsequent inhibitor formation. Most detoxification methods only partially remove inhibitors, but they also contribute to sugar loss, which also increases the costs of the final process (Balan et al. 2013).
6.7 Conversion of Organic Waste into Bioetanol
The following is a brief description of the different processes that take place during the conversion of organic waste to bioethanol.
6.7.1 Pre-treatment Methods for Starches
-
Acid Hydrolysis: Starches can be chemically treated by hydrolyzing in the presence of acid to obtain a saccharified starch.
$$ \mathrm{Starch}\ \mathrm{material}\overset{{\mathrm{H}}_2{\mathrm{SO}}_4}{\to}\mathrm{Saccharified}\ \mathrm{starch} $$In the acid hydrolysis of starch, high pressures and high temperatures are used, resulting in the formation of brown compounds due to the polymerization of sugars.
-
Enzymatic hydrolysis: amylolytic enzymes are used for the conversion of starches to sugars.
$$ \mathrm{Starch}\ \mathrm{material}\underset{\mathrm{Amylolytic}\ \mathrm{enzymes}}{\overset{\mathrm{Sprouted}\ \mathrm{barley}\ \mathrm{grains}}{\to }}\mathrm{Fermentable}\ \mathrm{Sugar} $$Cellulose can be degraded to sugars using concentrated HCL.
$$ \left(\mathrm{a}\right)\kern1em \mathrm{cellulose}\underset{\mathrm{concentrated}\ \mathrm{HCL}}{\overset{30-35{}^{\circ}\mathrm{C}}{\to }}\mathrm{Sugars}\ \left(\mathrm{Glucose}\right)\&\mathrm{Brownian}\ \mathrm{Reactions} $$$$ \left(\mathrm{b}\right)\kern1em \mathrm{cellulose}\underset{\mathrm{HCL}\ \mathrm{diluted}}{\overset{150-160{}^{\circ}\mathrm{C}}{\to }}\mathrm{Sugars}\ \left(\mathrm{Glucose}\right)\&\mathrm{Brownian}\ \mathrm{Reactions} $$
7 Fermentation Process
It is a biological process that converts sugars such as glucose, fructose and sucrose into cellular energy, producing ethanol and carbon dioxide as products, also known as alcoholic fermentation process. Molasses is a raw material that contains 50% of sugars and is suitable for the process of bioethanol fermentation and in the presence of enzymes is converted to glucose and fructose. Glucose passes through a pathway that produces pyruvic acid, and this when subjected to a decarboxylation reaction, produces acetaldehyde (Branco et al. 2019; Kotarska et al. 2019).
Yeast carries out this conversion in the absence of oxygen, so this is considered an anaerobic process, here the sugar is converted to bioethanol (Taherzadeh and Karimi 2008). The alcohol is named to indicate the source of the raw material from which it is made or to indicate the general purpose for which it is to be used. Grain alcohol (made from grains such as corn, wheat, and rice), molasses alcohol (produced from sugar cane molasses), industrial alcohol (used for industrial purposes), and fuel alcohol (used in combination with gasoline or other motor fuel) (Karimi et al. 2013).
7.1 Microorganisms Used in Alcohol Fermentation
The microorganisms used in the alcoholic fermentation process are the yeast, widely used in the industry to ferment the sugars present in the raw materials to form carbon dioxide and ethanol and the bacteria (Zymomonas mobilis) (Batista Meneses et al. 2020). Examples of yeasts are Saccharomyces cerevisiae (has a hard cell wall and is easy to multiply) and Saccharomyces carlsbergensis. Microorganisms are used to produce the product of interest, and in this way only the organisms of interest need to be present in the space that produces the product (the bioreactor), however, the organisms are present everywhere for example in the space where the bioreactor is installed, for this reason the surroundings must remain clean and only the microorganisms of interest are added to the bioreactor and this is done to multiply and produce the product of interest (Zhang et al. 2010).
In the surroundings there may be organisms with concentrations of 104 cells/ml in the air, there may be many types of organisms and it is not desired that they be present in the bioreactor which is designed to produce a particular product (bioethanol). What happens is that, if other microorganisms arrive, they may grow much faster than the organisms of interest, and they will compete for the food that is available in the bioreactor, and this results in a failure called bioreactor contamination (Koike et al. 2009).
All microorganisms should be removed from a bioreactor, for example, using very high temperatures (120 °C), for about 15 min. Liquid chemicals or vapors can also be used to sterilize the spaces. The characteristics of alcoholic fermentation yeast are
-
The organism can tolerate high concentrations of sugar and alcohol.
-
High sugar fermentation power: higher fermentation rate, the characteristics of the organism can be determined with respect to Ks and μmax: (kinetic parameters of the Monod equation: maximum growth rate, see application example 1). Alcohol is the product associated with growth, which means that the rate of alcohol formation is proportional to the rate of cell mass formation.
-
High biochemical stability: this means that a uniform alcohol concentration is achieved, which is highly desirable on an industrial level.
-
Tolerate a wide pH range with optimal acid, which makes its fermentation less susceptible to infection than bacteria
-
Can grow under non-sterile conditions (this is the main advantage of the alcohol fermentation process).
In the mechanisms of the alcoholic fermentation process, two theories have been established
-
Neuberg Theory (mainly theoretical bases)
There are glucose molecules (1 mol of glucose), then hydrate the methyl glyoxal, this is a pathway to pyruvic acid (2 moles), after a dexcarboxylation reaction is produced acetaldehyde and finally by hydrogenation takes place the reduction and alcohol is produced. This is one way in which alcohol is produced. Another way is where glycerol can be produced, and another way is where acetic acid can be produced, and ethanol is produced (Li et al. 2009).
Stoichiometry of conversion:
From the stoichiometric equation, 1 mole of glucose can produce 2 moles of ethanol and two moles of pure carbon dioxide (Balat 2011; Hoekman et al. 2011). Then 100 g of glucose, produce 51.1 g of ethyl alcohol. At industrial level, this fementation is carried out in closed vessels so that carbon dioxide can be collected, and at high pressure it can be converted into dry ice, which can be sent to cold producing industries. The theoretical yield of ethanol is about 51.1 % g/g. A typical analysis of the fermentation product is presented in the following Table 1.
7.2 Instrumentation and Control
In the industrial field, the liquid gas chromatograph corresponds to an equipment of daily use by the operators, as well as equipment for titration, pH monitoring and microscopes to monitor the yeast. During distillation, the alcohol is sampled and tested for purity. In the control room the operator can control the whole plant from the computer, he can start different processes: the pre-treatment process, the hydrolysis, the fermentation process, and the distillation process. It is possible to control at what temperature the process needs to run, how many gallons per minute it needs to run, or change the flow rates, the pH, and in general any variable that needs to be changed from the computer. An operator must be in the control room 24 h a day, 7 days a week (Zhang et al. 2010).
In the case of a Feed Batch Fermenter, the objective is to control the flow, the level, the temperature (at 30 °C), the pressure (from 1 to 1.1 bar), and finally the pH (from 4 to 5). The control strategies can be ratio control for nutrient flow and feed back control for the other variables of interest. There are four main objectives that need to be controlled: the control of the feed liquid entering the top using Feedback control, control of the flow of carbon dioxide and ethanol entering the bottom using Feedback control, control of the level of the liquid at the bottom to prevent it from overflowing and finally control of the pressure at 1 bar using Feedback control (Oberoi et al. 2011). For process control with centrifuge, the objective is to control the flow and level in the equipment. The flow can be controlled by adjusting the inlet current, while the level can be controlled by adjusting the outlet current. The objective of the control and instrumentation of a distillation column is to maintain the level in the column, the temperature (80 °C) in the column, to maintain the pressure at 6.1 bar in the column, and to maintain the external reflux ratio and the level in the reflux balloon.
7.3 Fermentation Process of Alcohol from Broken Rice
The raw material used is broken rice, which is sent to the milling machine, mixed, liquefied (liquefaction), heated to gelatinize the starch, then the enzyme is added in the saccharomization tank. The saccharified material, rich in sugar, is placed in the fermenter where some yeast and nutrients are added and where it undergoes the fermentation process and finally is sent to the distillation column for distillation (Mussoline et al. 2012).
The output of the distillation column is sent to the rectification column where the alcohol is obtained, and the water is removed from the bottom. The bottom distillate (stillage) coming from the distillation column is sent to a stillage separator, then to a mixer and finally to a drying unit where a dry green solid distillate is obtained which can be used by adding other nutrients such as good fodder (Daza Serna et al. 2016).
7.4 Fermentation Process of Alcohol from Rice Husks
The use of biofuels generates a new energy alternative and a solution to the dependence on fossil fuels, opening new possibilities for the controlled use of agricultural residues in favour of the environment. In recent years, the way to degrade and use biomasses, in this case rice husks, has been studied. The rice husk is suitable for the production of bioethanol, since it is composed of lignin (20–25%), cellulose (35–40%) and hemicellulose (15–20%), so it is considered a suitable substrate for the production of this, given its availability and its relatively low cost (Pellera et al. 2012).
Worldwide, the largest producers of bioethanol are the United States and Brazil; the Latin American countries that are producing bioethanol are, of course, Brazil, Argentina and Colombia. This relatively new fuel market offers great advantages, since the demand for the product is high, since the country is using a 10% bioethanol blend and the rest in gasoline, while in countries like Brazil, the blend is up to 27.5%. Figure 2 shows a block diagram representing the process flow corresponding to the production of bioethanol from rice husks, and Fig. 7 represents a process flow diagram to produce bioethanol from rice husks (Jouzani 2018).
7.4.1 Process Description
The process begins with the reception of the raw material that arrives in trucks, which are weighed in scales, then the rice husk is lifted by an ejector to the storage hoppers, to be processed the next day (Fig. 7). From the storage hopper it passes through pipes to the inside of the plant, where it arrives to a conveyor belt, which as it moves the husk is sprayed with water to wash the raw material. It is then taken to a hammer crusher, whose objective is to reduce the size of the particle to increase the exposure area and achieve better results in prehydrolysis and hydrolysis. Once in the Pre Hydrolyzer, the raw material has gained a percentage of water by washing, there is added H2SO4 diluted in a percentage of 7.65% and water. The pre-treatment process is handled at 30 °C and 1 atm. At the time of leaving the stream has already decomposed some of the cellulose, hemicellulose and lignin (Jouzani 2018).
The filtrate obtained enters a chromatographic separation column, which is packed with a calcium-based ion exchange resin. In this equipment, the separation is performed because the resin that is positively charged retains the SO4− ions from the sulfuric acid, letting the sugars pass through, therefore, these are part of the first current that leaves the column, and the acid is retained in the column for a while, when all the sugars have already passed through it and the column is saturated with SO4 ions; distilled water is pumped and the acid begins to leave it, thus recovering more than 90% of the acid used (Taherzadeh and Karimi 2008).
The stream of sugars is sent to the centrifuge where water and lime are added to neutralize the remaining acid in this stream, this produces a quantity of gypsum. The acid that is recovered when the distilled water is pumped into the column is transported to an evaporator where the concentration is raised again and then recirculated back to the pre-hydrolysis and hydrolysis stages. The stream of sugars coming from the centrifuge enters the fermenter, where the fermentation is carried out with the help of the enzyme, Zymomonas mobilis; which are the bacteria in charge of the transformation of sugars into alcohol, in an efficient way (Zuo et al. 2012). After the fermentation is finished, the system is depressurized in such a way that the CO2 is eliminated. The outlet stream from the fermenter is passed through another centrifuge, where the ethanol inoculum is removed, and then transported to a flash separator in order to obtain the ethanol vapors that are then condensed and discharged into the distillate stream.
Before the flash separator there is a heat exchanger that takes the alcohol-water solution to the necessary temperature at the entrance to the separator so that the desired concentration is obtained at the exit. Finally, from the condensate equipment, the ethanol is passed to a storage tank, and from there it is loaded into the tank cars that will distribute the final product (Carrillo-Nieves et al. 2019).
7.5 Benefits of Fermentation and the Use of Ethanol
-
A complete combustion is carried out (it burns more efficiently). Most of the time the ethanol is burned completely, to produce carbon dioxide and water. Ethanol has a lower ignition temperature than petroleum. A 10% ethanol-gasoline blend burns more quickly which produces less engine stress and less fuel consumption, while reducing carbon monoxide emissions by up to 30%. In the case of a hydrocarbon reacting with oxygen, in an incomplete combustion, solid carbon like graphite will be produced, and this deteriorates the spark plugs of the cars.
-
Ethanol has a higher flammability range (it ignites and burns more easily).
-
It produces less toxic emissions
-
The by-products of oil combustion are greenhouse gases, which deplete the ozone layer. In the case of bioethanol use, ozone depletion is reduced.
-
It is a renewable source, it is derived from plant material, these raw materials can grow rapidly to support the demand.
-
It has positive implications for sustainable development.
-
It is a neutral greenhouse.
-
The carbon dioxide emitted is recycled back into the crop through absorption and photosynthesis
-
7.6 Disadvantages of Fermentation and the Use of Etanol
-
If more than 15% ethanol is used in the mixture, some modifications need to be made to the machine, because bioethanol is corrosive and also absorbs water, and gets dirty easily, and will circulate through the parts of the machine such as the carburetor and the fuel injection system.
-
Contaminants must be filtered out (a molecular sieve can be used) or they will cause corrosion or damage to the machine.
-
Bioethanol has a lower heat of combustion than petroleum, which means that the energy released per gram of ethanol is lower than that of octane. This means that if you have two cars, one with an ethanol-gasoline blend and the other with only high-octane gasoline, and both are supplied with the same volume of fuel, the high-octane will go further.
-
Large areas of land (farmland) are needed to cultivate and produce bioethanol. This land will be available for fuel production rather than food production and in most places, there is a shortage of dry land, water and fertilizer.
-
Large energy inputs are needed to produce bioethanol. A considerable amount of energy is used in the production of fertilizers, that is, for the crops that produce the bioethanol, as well as for the distillation or refining of the ethanol.
-
This process will only be effective if more energy is saved than is spent on the manufacture and use of bioethanol. This is the point where the industry is stuck, because if you put more energy into the input and you don’t get more energy out, it would be worthless and very expensive to do the whole process.
-
It is difficult to eliminate residual smelly fermentation liquors after the removal of the alcohol.
7.7 Practical Parameters and Operational Conditions of Fermentation
The factors that affect the alcohol fermentation process are:
-
The efficiency of the pre-treatment.
-
The optimal concentration of sugars: the cost of recovery or purification depends on the concentration of the product in the fermentation broth, if this is high the cost of recovery will be lower, if the concentration of ethanol in the fermentation broth is increased, more sugar will have to be used and a higher concentration of sugar is used, some kind of osmotic pressure is caused.
-
Optimal temperature and pH: Yeast fermentation usually takes place at an acidic pH: 4.5–5 and at a temperature close to room temperature (30 °C).
-
The addition of nutrients: it is necessary to add some magnesium, nitrogen, and phosphorus.
-
The use of a vigorous yeast strain: a highly productive strain
-
The maintenance of anaerobic conditions during fermentation: it allows the yeast to convert the sugars into alcohol. One thing to note is that the process of alcohol fermentation usually takes place in an open bed, in an acidic environment where fewer organisms can grow. Yeast can grow under aerobic conditions and produce cell mass and under anaerobic conditions produce alcohol. Initially, whatever dissolved oxygen will be used for the growth and metabolism of the yeast cell as soon as it is consumed the anaerobic condition prevails and the sugar is converted into alcohol.
-
Finally, prompt distillation of the fermented product.
8 Fermentation Technologies and Practical Operation
The reactor is the heart of the bioethanol production process. Let us look at some types of reactors used in bioethanol production. The reactors can operate in three different ways, the first is a batch operation, the second is a semi-batch operation and the last is a continuous form (Cardona Alzate and Sánchez Toro 2006).
8.1 Agitated Tank Reactor (ATR)
It is a vessel, a reactor widely used at industrial level and consists of a cylindrical tank and can be small (1 or 2 L/reactor), medium (15 L) or very large occupying large spaces in the industry (Fig. 8). All bioreactors have an agitator to shake the reactor contents and keep the cells in suspension (Faraco and Hadar 2011; Jafari et al. 2011).
This reactor has several measuring instruments (temperature, pH, dissolved oxygen meters) and is highly controlled. The factories that produce the products (bioethanol) are the cells (culture) in the bioreactor, in this case that manufacture bioethanol, everything necessary is done by these microscopic cells, whose size ranges between 2 and 5 microns, although there may be larger (Mtui and Nakamura 2005).
Batch Operation or Process
When the agitated tank is operated in batch mode, what is done is that everything is dumped into the bioreactor (the medium, the cells, the inoculum), and then the reaction takes place, and we wait until the bioproducts are formed and at the end of time everything is dumped out of the bioreactor and further processing takes place (downstream process steps to separate and purify the product). Many processes in the industry are carried out in batch mode, as it is very simple to operate bioreactors in batch compared to other types of bioreactors (Mahmoodi et al. 2018).
Continuous Operation or Process
Here there is a continuous input of the substrate (nutrient broth), it is not necessary that it contains cells. There is a continuous output of the bioreactor, with the product produced (Bioetanol). The times are adjusted so that the product is produced properly, and this operation is called continuous operation. During the process there is cell growth, product formation, and nutrient flow, all happening simultaneously within the reactor (van Dyk et al. 2013).
Semi-Batch Operation or Process (Fed-Batch)
Between the Batch operation and the continuous operation, there is something called semi-batch operation. There could be an intermittent input, and an intermittent output. You probably start with a batch, and for some well-known reasons, you add substrate may be in intervals (not continuously), this is a Fed-batch feed. You can remove some part of the reaction broth intermittently, or you can add or remove it inermittently, this kind of operation is called a Fed-batch operation (Safarian and Unnthorsson 2018). There are several reasons to operate in Fed-batch mode, for example, some products that are made by these cells may be toxic to the cells themselves, as is the case with bioethanol. Ethanol above 18% is toxic to cells, so it cannot be allowed to continue to grow. There are other products that can be toxic at much lower concentrations, and to manage the toxicity, a portion of the culture is removed, and fresh culture is added so that the concentration of the toxic product is maintained (Casaretto et al. 2019).
8.2 Air-Lift Bioreactor
The main difference with the previous one is that in this bioreactor there is no agitator, although it is necessary to keep the culture medium of the bioreactor in agitation, only this way the bioreactor will work properly. The agitation and mixing nature is caused by air pumped by a compressor (Liguori and Faraco 2016).
The air is lifted through the internal cylinder located inside the reactor and carries the cells along (the air keeps the cells in suspension) and is then recirculated.
8.3 Packed Bed Biorreactor
This bioreactor has a stationary bed (site where biorreactions take place and where microorganisms convert the reactant into the desired product) that is packaged and typically operated continuously. This type of reactor is more used in water treatment, where the bed can be sand through which the water to be treated flows and at the exit clean water is obtained. The fluidized bed is a variant of the packed bed, but the bed is not stationary, but is moving, is jumping (Kumari and Singh 2018).
9 Practical Applications in Bioethanol Production
Application example: A distillery industry produces 100 m3 of rectified alcohol (containing 92% ethanol %v/v) in a chemostat from cane molasses (containing 50% sugar %w/w) using S. Cerevisiae. Find the bioreactor volume and the amount of cane molasses required per day. The characteristics of the yeast are the following (under anaerobic condition) (Fig. 9).
Solution:
-
For a sterile environment: X0 = 0
-
Concentration of biomass in stationary state:
That is, it must be found under steady state conditions, with which dilution rate D, the maximum cell mass production will be achieved.
That is, it must be found under steady state conditions, with which dilution rate D, the maximum cell mass production will be achieved.
Then the maximum cell growth will give the maximum amount of product formation (Bioethanol). It must be found under what circumstances the maximum amount of cell mass is produced.
-
Then, the Dmax value is calculated,
$$ {\displaystyle \begin{array}{c}{D}_{max}={\mu}_{max}\left(1-\sqrt{\frac{K_S}{K_S+{S}_0}}\right)\\ {}\kern8.5em =0.05\left(1-\sqrt{\frac{2}{2+350}}\right)=0.0462\ {\mathrm{h}}^{-1}\end{array}} $$ -
Substrate concentration in steady state
$$ {\displaystyle \begin{array}{c}\begin{array}{c}\mathrm{S}=\frac{{\mathrm{K}}_{\mathrm{S}}\ast {\mathrm{D}}_{\mathrm{max}}}{\upmu_{\mathrm{max}}-{\mathrm{D}}_{\mathrm{max}}}\\ {}=\frac{2\ast 0.0462}{0.05-0.0462}\end{array}\\ {}=24.31\ \mathrm{g}/\mathrm{L}\end{array}} $$ -
Biomass concentration in steady state
$$ {\displaystyle \begin{array}{c}\begin{array}{c}X={Y}_{\raisebox{1ex}{$X$}\!\left/ \!\raisebox{-1ex}{$S$}\right.}\left({S}_0-S\right)\\ {}\kern2.5em =0.05\left(350-24.31\right)\end{array}\\ {}=16.28\ \mathrm{g}/\mathrm{L}\end{array}} $$$$ {\displaystyle \begin{array}{l}\mathbf{Calculation}\ \mathbf{basis}:100\ {\mathrm{m}}^3=92\ {\mathrm{m}}^3\mathrm{of}\ \mathrm{ethanol}\ \mathrm{production}\ \mathrm{per}\ \mathrm{day}\ \left(92\%\mathrm{v}/\mathrm{v}\right)\\ {}\kern19.5em =92,000\ \mathrm{L}\ \mathrm{of}\ \mathrm{ethanol}/\mathrm{day}\end{array}} $$ -
Ethanol Density, ρ = 780 g/L
-
Calculate the amount of ethanol that is produced
$$ {\displaystyle \begin{array}{l}{\mathrm{m}}_{\mathrm{et}}={\uprho}_{\mathrm{et}\ast \mathrm{V}=780\ \mathrm{g}/\mathrm{L}\ast 92,000\ \mathrm{L}=71.76\times {10}^6}\mathrm{g}\\ {}\kern1.6em =71.76\times {10}^3\mathrm{kg}\ \mathrm{de}\ \mathrm{etanol}\ \mathrm{por}\ \mathrm{dia}\end{array}} $$ -
Substrate required (sugar required per day)
$$ {\displaystyle \begin{array}{c}\begin{array}{c}\begin{array}{c}{Y}_{\raisebox{1ex}{$p$}\!\left/ \!\raisebox{-1ex}{$S$}\right.}=\frac{p}{S}\\ {}S\kern1em =\frac{p}{Y_{\raisebox{1ex}{$p$}\!\left/ \!\raisebox{-1ex}{$S$}\right.}}=\frac{71.76\times {10}^3\mathrm{kg}\ \mathrm{etanol}/\mathrm{day}}{0.5}=143.52\times {10}^3\ \mathrm{kg}\ \mathrm{sustrato}/\mathrm{day}\end{array}\\ {}\kern10.75em \end{array}\\ {}\kern13.25em \end{array}} $$ -
Volumetric feed flow rate, F
$$ F=\frac{S}{S_0}=\frac{143.52\times {10}^3\ \mathrm{kg}\ \mathrm{sustrato}/\mathrm{day}}{350\ \mathrm{g}/\mathrm{L}}=\frac{143.52\times {10}^3\ \mathrm{kg}\ \mathrm{sustrato}/\mathrm{day}}{350\ \mathrm{kg}/{\mathrm{m}}^3}=410.057\frac{{\mathrm{m}}^3}{\mathrm{day}}=17.08\frac{{\mathrm{m}}^3}{\mathrm{h}} $$ -
Time required by the continuous stirred tank reactor, CSTR
$$ {\tau}_{CSTR}=\frac{S_0-S}{-{r}_s} $$$$ {\displaystyle \begin{array}{c}\begin{array}{c}\begin{array}{c}\begin{array}{c}\begin{array}{c}-{r}_s=-\frac{ds}{dt}=-\frac{ds}{dX}\frac{dX}{dt}\\ {}\end{array}\\ {}-{r}_s=\frac{1}{Y_{\raisebox{1ex}{$X$}\!\left/ \!\raisebox{-1ex}{$S$}\right.}}\mu X=\frac{1}{Y_{\raisebox{1ex}{$X$}\!\left/ \!\raisebox{-1ex}{$S$}\right.}}{D}_{max}X=\frac{1}{0.05}\times 0.0462\times 16.28\ \mathrm{g}/\mathrm{Lh}=15.04\ \mathrm{g}/\mathrm{Lh}\end{array}\\ {}\kern3.5em \end{array}\\ {}\kern9.75em \end{array}\\ {}\kern3em \end{array}} $$ -
Calculate τCSTR
$$ {\displaystyle \begin{array}{c}\begin{array}{c}{\tau}_{CSTR}=\frac{S_0-S}{-{r}_s}=\frac{V}{F}=\frac{\left(350-24.31\right)}{15.04}\ \mathrm{h}=21.65\ \mathrm{h}\\ {}\kern4.5em \end{array}\\ {}\kern1.25em \end{array}} $$ -
Calculate the volume of the reactor, V
$$ \kern0.5em {\displaystyle \begin{array}{c}\begin{array}{c}V=F\times {\tau}_{CSTR}=17.08\frac{{\mathrm{m}}^3}{\mathrm{h}}\times 21.65\ \mathrm{h}=369.78\ {\mathrm{m}}^3\\ {}\kern6.25em \end{array}\\ {}\kern3.5em \end{array}} $$ -
Cane molasses required
Substrate required/day = 143.52 × 103 kg Substrate/day, Sugar content of cane molasses 50% w/w, Cane molasses required = 287.04 × 103 kg/day. This is the amount of molasses required to operate the plant and obtain 100 m3 of rectified ethanol. Most industries use CSTR (Continuous Stirred Tank Reactor) because they have the highest productivity.
10 Economic Overview of Bioethanol Production
The sustainability of the ethanol and biogas economy is based on four fundamental pillars: government participation and policies, corporate social responsibility, venture capital investment and energy conversion costs (Quintero et al. 2012).
Government Participation
Governments’ concern about energy dependence on hydrocarbons has forced them to generate policies to encourage and support projects that seek to minimize the dependency ratio. Throughout the world, various strategies have been developed by governments to promote the development of the bioethanol industry, policies that have had variable effects but that, in one way or another, contribute to energizing this economic sector. Brazil, the second largest producer of bioethanol in the world after the United States, has doubled its agricultural frontier for the production of sugarcane for bioethanol and has almost tripled its domestic consumption of fuel alcohol in the last decade (Arora et al. 2019) (Fig. 10).
In the case of Brazil, an outstanding national program was developed that involved the local sugar production industry, research institutions, the automotive industry and the government with policies to encourage investment in infrastructure and regulations for the use and guarantee prices of bioethanol, guaranteeing demand by establishing minimum quantities of fuel mixture, a situation that links the demand for fuel alcohol to the consumption and expenditure of motor fuels (Piccolo and Bezzo 2009). This situation is thought to be favorable, in which the demand for biofuels is linked to the consumption of fuels by the automotive industry, and generates a great deal of uncertainty for this agroindustry when the world is undergoing global dynamics of reduction in the use of traditional fossil fuels or static demand effects resulting from dramatic changes in consumption, such as those experienced as of the second quarter of 2020 in reference to the global pandemic that affected many human activities (Balan et al. 2013; Sari and Ernawati 2019).
In the case of the United States, the world’s leading bioethanol producer, its promotion policies include the fact that 95% of the gasoline consumed in its territory contains between 10 and 15% bioethanol, a production based on its large corn agro-industry, subsidizing bioethanol by between 10.6 and 15.9 US$/L, a condition that alters competitiveness in favor of national production, although it is estimated that the subsidies reach the surplus production and reach the export of bioethanol, affecting the stability of the domestic industry in other countries such as Colombia (Chovau et al. 2013).
Notes: A more detailed breakdown of fuel ethanol production by country for 2000–2012 can be viewed at the U.S. Energy Information Administration’s (EIA) International Energy Statistics: Biofuels Production (Sunarno et al. 2020).
Geopolitical
The energy sources available in each country will determine the autonomy and development of the industries that depend on energy sources to move their gears; the privileged standard of living of a good part of the societies of industrialized countries is based on the wealth of the subsoil and its hydrocarbons originated and treasured during thousands of years in it. For any state to talk about geopolitics will have to see as a prominent issue the production or supply of oil, that is why being a non-renewable resource or in some cases not available in their territories for exploitation (Quintero et al. 2008; Yu and Tao 2008). The search for independence from fossil fuels or to diminish the importance of suppliers is a state policy that has generated the development of the ethanol and biogas agro-industry, especially in Western countries such as the United States, Brazil and, incipiently, in the European Union, which have sought to reduce dependence on oil from the East and natural gas from Russia (Georgieva and Ahring 2007; Novo et al. 2012).
The importance of the issue is very visible when, for example, the U.S. Congress passed laws based on the average yield of gasoline, which should be 35 miles per gallon, an increase in the use of ethanol to 36 billion gallons per year by 2022, prohibiting the use of fuels that pollute more than synthetic fossil fuel (Quintero et al. 2012).
Corporate Social Responsibility (CSR)
CSR is a modern way of doing business and organizations have identified this strategy to create and preserve market value and investment. Society is rewarding the efforts of corporations that demonstrate a focus of responsibility in social and environmental issues, in this direction the negotiation of energy and environmentally friendly fuels have a general acceptance from consumers and the state, that is, the profitability of these initiatives are marked by a consumer demand and subsidies, preferential tax rates that states create to encourage and energize these economic sectors (Barrera et al. 2016).
Risk Capitals
The concept of risk capital is used about investments made in companies with cutting-edge or innovative concepts or proposals, which are going to enter markets with the uncertainty of the success of their business models. According to The Economist Newspaper Limited magazine, green energies, where ethanol and biogas production is located, have become an outstanding reference for the interest of large risk capitals and research institutions; ethanol being one of the green energies of greatest interest for risk capitalists (Brown 2015).
10.1 Used Raw Materials
According to the FAO, it is expected that the ethanol industry will make use of between 15 and 20% of the world’s corn and sugarcane production by 2026. The growth and dedication of sugarcane is also projected to contribute 35% to the world’s ethanol production by 2026, while corn will remain the main source of extraction, with 58% of its use in obtaining bioethanol (Eggeman and Elander 2005; Kabir et al. 2015).
Other alternative biomasses for obtaining bioethanol such as vegetable residues, fibers and cellulose materials are estimated to grow by 8% as contributors of bioethanol sources in world trade, a figure that is linked to the reduction of extraction costs from these sources which, although they do not impact food security, constitute a different technological challenge (Dabwan et al. 2020; Padi and Chimphango 2020).
In the European Union it is estimated that the production of Bioethanol will stabilize at 7.3 billion liters, mainly obtained from wheat and sugar beet. In the United States, it is estimated that by 2020 there will be a demand for 61.6 billion liters of bioethanol, obtained mainly from corn. As for Asia, China is expected to reach a production of 1.8 billion liters of bioethanol produced mainly from corn, cassava, and soybeans (Lohrasbi et al. 2010; Padi and Chimphango 2020).
10.2 How Bioethanol Will Be Used
The growth of this agroindustry has been linked to the demand for gasoline and automotive fuels, so that the use and growth of its market will depend on the dynamics of fossil fuels. Until this condition is broken, and massive propulsion technologies are developed using only bioethanol, the bioethanol agroindustry will be dependent on the good for which it was developed (Chiaramonti et al. 2012; Chiranjeevi et al. 2018; Prasara-A and Gheewala 2017).
Figure 11 shows the ethanol production and consumption trend from 2000 to 2019. To the extent that less transport fuel is used, the need for bioethanol will also decrease; this situation is expected to be very strong in the European Union, Canada and Japan, which are increasingly generating policies to discourage the use of fossil fuels and create transport systems that use emission-free energy (He et al. 2014).
The demand for bioethanol will grow mainly due to consumption in developing countries such as Brazil, China, India and Thailand. Brazil is called upon to be the main driver of demand with an increase of 6 billion liters, which represents 35% of the global increase in bioethanol use. Due to the fuel ethanol blending policies of some provinces in China, demand is expected to increase by 1 billion liters, as well as Thailand where ethanol’s share in fuel will generate an additional demand of 0.7 billion liters (Cara et al. 2007; Yu et al. 2018).
The bioethanol production process is being developed as a state policy strategy, as mentioned above, so that demand is met internally in most cases, which means that international trade in this biofuel has very little growth, with projections of stagnation after 2020 and setbacks after 2026 (Kumar and Sharma 2017; Martínez-Patiño et al. 2017; Solarte-Toro et al. 2018, 2019; Sritrakul et al. 2017).
10.3 Costs Associated with Bioethanol Production
The production of ethanol has become a survival strategy for the sugar industry that has been affected by the drop in sugar prices as a result of regulations and restrictions on food as well as cultural changes in consumption that have caused the demand for sugar as a sweetener to stop growing. The ethanol agro-industry is an integral part of the business strategy of the old sugar mills that took refuge in the renewable energy business and through technological reconversion are saving this agro-industrial line for the time being (Alvira et al. 2010; Chiranjeevi et al. 2018; Náthia-Neves et al. 2018).
Parity prices to define sugar or bioethanol production in this market are hardly stable, going through relevant variations over time due to climatic aspects, various production subsidy policies, tariff barriers to bioethanol or sugar imports make, for example, domestic prices higher than international reference prices and therefore parity in the definition of production is indifferent in many sugar producing countries (Manzanares et al. 2017).
Installation scales for ethanol production generate direct production costs that include such aspects as necessary investments in the distillery and its fermentation, distillation and auxiliary systems, including biomass treatment and conditioning for processing, and will vary depending on the scale of the assembly and to a very small extent on the source of the ethanol (Yu et al. 2018).
Ethanol from sugar cane has a production cost in the range of 0.27 to 0.51 USD/L. The production costs of ethanol from molasses indicate a production cost in the range of 0.43–0.64 USD/L. These costs may differ depending on the valorization of molasses as a raw material, since it is an important factor in the decision of sugar mills on the use and disposal of this co-product. By August 2020, the market reference prices for Bioethanol are Brazil*: 0.50, United States*: 0.52, Thailand*: 0.58, France*: 0.81, Sweden*: 1.43, Spain*: 1.71. Therefore, there is a margin for the agroindustrial business that will vary according to the local conditions of taxes, incentive policies and controlled demand (Sarto et al. 2019; Solarte-Toro et al. 2019; Sritrakul et al. 2017; Taherdanak et al. 2016; Venturin et al. 2018).
References
Aden A, Foust T (2009) Technoeconomic analysis of the dilute sulfuric acid and enzymatic hydrolysis process for the conversion of corn stover to ethanol. Cellulose 16:535–545. https://doi.org/10.1007/s10570-009-9327-8
Akbulut A (2012) Techno-economic analysis of electricity and heat generation from farm-scale biogas plant: Çiçekdaĝi{dotless} case study. Energy 44:381–390. https://doi.org/10.1016/j.energy.2012.06.017
Alvira P, Tomás-Pejó E, Ballesteros M, Negro MJ (2010) Pretreatment technologies for an efficient bioethanol production process based on enzymatic hydrolysis: a review. Bioresour Technol 101:4851–4861. https://doi.org/10.1016/j.biortech.2009.11.093
Al-Zuahiri F, Pirozzi D, Ausiello A, Florio C, Turco M, Micoli L, Zuccaro G, Toscano G (2015) Biogas production from solid state anaerobic digestion for municipal solid waste. Chem Eng Trans 43:2407–2412. https://doi.org/10.3303/CET1543402
Aristizábal-Marulanda V, Solarte-Toro JC, Cardona Alzate CA (2020) Study of biorefineries based on experimental data: production of bioethanol, biogas, syngas, and electricity using coffee-cut stems as raw material. Environ Sci Pollut Res. https://doi.org/10.1007/s11356-020-09804-y
Ariunbaatar J, Scotto Di Perta E, Panico A, Frunzo L, Esposito G, Lens PNL, Pirozzi F (2015) Effect of ammoniacal nitrogen on one-stage and two-stage anaerobic digestion of food waste. Waste Manag 38:388–398. https://doi.org/10.1016/j.wasman.2014.12.001
Arora R, Sharma NK, Kumar S, Sani RK (2019) Chapter 9—Lignocellulosic ethanol: feedstocks and bioprocessing. In: Ray RC, Ramachandran S (eds) Bioethanol production from food crops. Academic Press, pp 165–185. https://doi.org/10.1016/B978-0-12-813766-6.00009-6
Aruwajoye GS, Faloye FD, Kana EG (2020) Process optimisation of enzymatic saccharification of soaking assisted and thermal pretreated cassava peels waste for bioethanol production. Waste Biomass Valori 11:2409–2420. https://doi.org/10.1007/s12649-018-00562-0
Atelge MR, Krisa D, Kumar G, Eskicioglu C, Nguyen DD, Chang SW, Atabani AE, Al-Muhtaseb AH, Unalan S (2020) Biogas production from organic waste: recent Progress and perspectives. Waste Biomass Valori 11:1019–1040. https://doi.org/10.1007/s12649-018-00546-0
Azaizeh H, Abu Tayeh HN, Gerchman Y (2020) Valorisation of olive oil industry solid waste and production of ethanol and high value-added biomolecules. In: Biovalorisation of wastes to renewable chemicals and biofuels. Elsevier, Cambridge, MA, pp 27–40. https://doi.org/10.1016/b978-0-12-817951-2.00002-x
Balan V, Chiaramonti D, Kumar S (2013) Review of US and EU initiatives toward development, demonstration, and commercialization of lignocellulosic biofuels. Biofuels Bioprod Biorefin. https://doi.org/10.1002/bbb.1436
Balat M (2011) Production of bioethanol from lignocellulosic materials via the biochemical pathway: a review. Energy Convers Manag 52:858–875. https://doi.org/10.1016/j.enconman.2010.08.013
Banks CJ, Chesshire M, Heaven S, Arnold R (2011) Anaerobic digestion of source-segregated domestic food waste: performance assessment by mass and energy balance. Bioresour Technol 102:612–620. https://doi.org/10.1016/j.biortech.2010.08.005
Barrera I, Amezcua-Allieri MA, Estupiñan L, Martínez T, Aburto J (2016) Technical and economical evaluation of bioethanol production from lignocellulosic residues in Mexico: Case of sugarcane and blue agave bagasses. Chem Eng Res Des 107:91–101. https://doi.org/10.1016/j.cherd.2015.10.015
Barta Z, Reczey K, Zacchi G (2010) Techno-economic evaluation of stillage treatment with anaerobic digestion in a softwood-to-ethanol process. Biotechnol Biofuels 3. https://doi.org/10.1186/1754-6834-3-21
Batista Meneses D, Montes de Oca-Vásquez G, Vega-Baudrit JR, Rojas-Álvarez M, Corrales-Castillo J, Murillo-Araya LC (2020) Pretreatment methods of lignocellulosic wastes into value-added products: recent advances and possibilities. Biomass Convers Biorefin. https://doi.org/10.1007/s13399-020-00722-0
Ben Yahmed N, Jmel MA, Ben Alaya M, Bouallagui H, Marzouki MN, Smaali I (2016) A biorefinery concept using the green macroalgae Chaetomorpha linum for the coproduction of bioethanol and biogas. Energy Convers Manag 119:257–265. https://doi.org/10.1016/j.enconman.2016.04.046
Bernier-Oviedo DJ, Rincón-Moreno JA, Solanilla-Duqué JF, Muñoz-Hernández JA, Váquiro-Herrera HA (2018) Comparison of two pretreatments methods to produce second-generation bioethanol resulting from sugarcane bagasse. Ind Crop Prod 122:414–421. https://doi.org/10.1016/j.indcrop.2018.06.012
Boluda-Aguilar M, García-Vidal L, González-Castañeda FDP, López-Gómez A (2010) Mandarin peel wastes pretreatment with steam explosion for bioethanol production. Bioresour Technol 101:3506–3513. https://doi.org/10.1016/j.biortech.2009.12.063
Bouallagui H, Ben Cheikh R, Marouani L, Hamdi M (2003) Mesophilic biogas production from fruit and vegetable waste in a tubular digester. Bioresour Technol 86:85–89. https://doi.org/10.1016/S0960-8524(02)00097-4
Bouallagui H, Touhami Y, Ben Cheikh R, Hamdi M (2005) Bioreactor performance in anaerobic digestion of fruit and vegetable wastes. Process Biochem 40:989–995. https://doi.org/10.1016/j.procbio.2004.03.007
Bouallagui H, Rachdi B, Gannoun H, Hamdi M (2009) Mesophilic and thermophilic anaerobic co-digestion of abattoir wastewater and fruit and vegetable waste in anaerobic sequencing batch reactors. Biodegradation 20:401–409. https://doi.org/10.1007/s10532-008-9231-1
Branco RHR, Serafim LS, Xavier AMRB (2019) Second generation bioethanol production: On the use of pulp and paper industry wastes as feedstock. Fermentation. https://doi.org/10.3390/fermentation5010004
Brown TR (2015) A techno-economic review of thermochemical cellulosic biofuel pathways. Bioresour Technol. https://doi.org/10.1016/j.biortech.2014.09.053
Budiyono B, Syaichurrozi I, Sumardiono S (2013) Biogas production from bioethanol waste: the effect of pH and urea addition to biogas production rate. Waste Technol 1:1–5. https://doi.org/10.12777/wastech.1.1.2013.1-5
Cakir FY, Stenstrom MK (2005) Greenhouse gas production: a comparison between aerobic and anaerobic wastewater treatment technology. Water Res 39:4197–4203. https://doi.org/10.1016/j.watres.2005.07.042
Cara C, Romero I, Oliva JM, Sáez F, Castro E (2007) Liquid hot water pretreatment of olive tree pruning residues. Appl Biochem Biotechnol:379–394. https://doi.org/10.1007/s12010-007-9066-y
Cardona Alzate CA, Sánchez Toro OJ (2006) Energy consumption analysis of integrated flowsheets for production of fuel ethanol from lignocellulosic biomass. Energy 31:2447–2459. https://doi.org/10.1016/j.energy.2005.10.020
Carrillo-Nieves D, Rostro Alanís MJ, de la Cruz Quiroz R, Ruiz HA, Iqbal HMN, Parra-Saldívar R (2019) Current status and future trends of bioethanol production from agro-industrial wastes in Mexico. Renew Sust Energ Rev 102:63–74. https://doi.org/10.1016/j.rser.2018.11.031
Casaretto R, Agdal EBL, Cayenne A, Chaturvedi T, Holm-Nielsen JB (2019) Low-temperature pretreatment of lignocellulosic biomass for enhanced biogas production. Chem Eng Technol 42:2565–2573. https://doi.org/10.1002/ceat.201900348
Cesaro A, Belgiorno V (2015a) Combined biogas and bioethanol production: opportunities and challenges for industrial application. mdpi.com, vol 8, pp 8121–8144. https://doi.org/10.3390/en8088121
Cesaro A, Belgiorno V (2015b) Combined biogas and bioethanol production: opportunities and challenges for industrial application. Energies 8:8121–8144. https://doi.org/10.3390/en8088121
Chen Y, Cheng JJ, Creamer KS (2008) Inhibition of anaerobic digestion process: a review. Bioresour Technol 99:4044–4064. https://doi.org/10.1016/j.biortech.2007.01.057
Chiaramonti D, Prussi M, Ferrero S, Oriani L, Ottonello P, Torre P, Cherchi F (2012) Review of pretreatment processes for lignocellulosic ethanol production, and development of an innovative method. Biomass Bioenergy 46:25–35. https://doi.org/10.1016/j.biombioe.2012.04.020
Chiranjeevi T, Mattam AJ, Vishwakarma KK, Uma A, Peddy VCR, Gandham S, Ravindra Velankar H (2018) Assisted single-step Acid pretreatment process for enhanced delignification of rice straw for bioethanol production. ACS Sustain Chem Eng 6:8762–8774. https://doi.org/10.1021/acssuschemeng.8b01113
Chovau S, Degrauwe D, van der Bruggen B (2013) Critical analysis of techno-economic estimates for the production cost of lignocellulosic bio-ethanol. Renew Sust Energ Rev. https://doi.org/10.1016/j.rser.2013.05.064
Dabwan YN, Pei G, Gao G, Feng J, Li J (2020) A novel integrated solar tri-generation system for cooling, freshwater and electricity production purpose: Energy, economic and environmental performance analysis. Sol Energy 198:139–150. https://doi.org/10.1016/j.solener.2020.01.043
Daza Serna LV, Solarte Toro JC, Serna Loaiza S, Chacón Perez Y, Cardona Alzate CA (2016) Agricultural waste management through energy producing biorefineries: the Colombian case. Waste Biomass Valori 7:789–798. https://doi.org/10.1007/s12649-016-9576-3
de la Torre I, Martin-Dominguez V, Acedos MG, Esteban J, Santos VE, Ladero M (2019) Utilisation/upgrading of orange peel waste from a biological biorefinery perspective. Appl Microbiol Biotechnol 103:5975–5991. https://doi.org/10.1007/s00253-019-09929-2
Dererie DY, Trobro S, Momeni MH, Hansson H, Blomqvist J, Passoth V, Schnürer A, Sandgren M, Ståhlberg J (2011) Improved bio-energy yields via sequential ethanol fermentation and biogas digestion of steam exploded oat straw. Bioresour Technol 102:4449–4455. https://doi.org/10.1016/j.biortech.2010.12.096
Dias MOS, Junqueira TL, Rossell CEV, MacIel Filho R, Bonomi A (2013) Evaluation of process conFiguretions for second generation integrated with first generation bioethanol production from sugarcane. Fuel Process Technol 109:84–89. https://doi.org/10.1016/j.fuproc.2012.09.041
Duan F, Zhang JP, Chyang CS, Wang YJ, Tso J (2014) Combustion of crushed and pelletized peanut shells in a pilot-scale fluidized-bed combustor with flue gas recirculation. Fuel Process Technol 128:28–35. https://doi.org/10.1016/j.fuproc.2014.06.022
Eggeman T, Elander RT (2005) Process and economic analysis of pretreatment technologies. Bioresour Technol 96:2019–2025. https://doi.org/10.1016/j.biortech.2005.01.017
Faraco V, Hadar Y (2011) The potential of lignocellulosic ethanol production in the Mediterranean Basin. Renew Sust Energ Rev 15:252–266. https://doi.org/10.1016/j.rser.2010.09.050
Ferella F (2020) A review on management and recycling of spent selective catalytic reduction catalysts. J Clean Prod. https://doi.org/10.1016/j.jclepro.2019.118990
Ferreira JA, Brancoli P, Agnihotri S, Bolton K, Taherzadeh MJ (2018) A review of integration strategies of lignocelluloses and other wastes in 1st generation bioethanol processes. Process Biochem 75:173–186. https://doi.org/10.1016/j.procbio.2018.09.006
Forster-Carneiro T, Pérez M, Romero LI (2008a) Influence of total solid and inoculum contents on performance of anaerobic reactors treating food waste. Bioresour Technol 99:6994–7002. https://doi.org/10.1016/j.biortech.2008.01.018
Forster-Carneiro T, Pérez M, Romero LI (2008b) Thermophilic anaerobic digestion of source-sorted organic fraction of municipal solid waste. Bioresour Technol 99:6763–6770. https://doi.org/10.1016/j.biortech.2008.01.052
Georgieva TI, Ahring BK (2007) Potential of agroindustrial waste from olive oil industry for fuel ethanol production. Biotechnol J 2:1547–1555. https://doi.org/10.1002/biot.200700128
Gómez X, Cuetos MJ, Cara J, Morán A, García AI (2006) Anaerobic co-digestion of primary sludge and the fruit and vegetable fraction of the municipal solid wastes. Conditions for mixing and evaluation of the organic loading rate. Renew Energy 31:2017–2024. https://doi.org/10.1016/j.renene.2005.09.029
Goshadrou A, Karimi K, Taherzadeh MJ (2013) Ethanol and biogas production from birch by NMMO pretreatment. Biomass Bioenergy 49:95–101. https://doi.org/10.1016/j.biombioe.2012.12.013
Hagman L, Blumenthal A, Eklund M, Svensson N (2018) The role of biogas solutions in sustainable biorefineries. J Clean Prod 172:3982–3989. https://doi.org/10.1016/j.jclepro.2017.03.180
Hamelinck CN, van Hooijdonk G, Faaij APC (2005) Ethanol from lignocellulosic biomass: techno-economic performance in short-, middle- and long-term. Biomass Bioenergy 28:384–410. https://doi.org/10.1016/j.biombioe.2004.09.002
He Y, Zhang L, Zhang J, Bao J (2014) Helically agitated mixing in dry dilute acid pretreatment enhances the bioconversion of corn stover into ethanol. Biotechnol Biofuels 7. https://doi.org/10.1186/1754-6834-7-1
Hoekman SK, Broch A, Robbins C (2011) Hydrothermal carbonization (HTC) of lignocellulosic biomass. Energy Fuels 25:1802–1810. https://doi.org/10.1021/ef101745n
Holm-Nielsen JB, al Seadi T, Oleskowicz-Popiel P (2009) The future of anaerobic digestion and biogas utilization. Bioresour Technol 100:5478–5484. https://doi.org/10.1016/j.biortech.2008.12.046
Ituen EE, John NM, Bassey BE (2009) Biogas production from organic waste in Akwa Ibom State of Nigeria. Appropriate Technologies for Environmental Protection in the Developing World—Selected Papers from ERTEP 2007, pp 93–99. https://doi.org/10.1007/978-1-4020-9139-1_11
Izumi K, Okishio Y, Nagao N, Niwa C, Yamamoto S, Toda T (2010) Effects of particle size on anaerobic digestion of food waste. Int Biodeterior Biodegrad 64:601–608. https://doi.org/10.1016/j.ibiod.2010.06.013
Jafari V, Labafzadeh SR, Jeihanipour A, Karimi K, Taherzadeh MJ (2011) Construction and demolition lignocellulosic wastes to bioethanol. Renew Energy 36:2771–2775. https://doi.org/10.1016/j.renene.2011.04.028
Janke L, Leite A, Nikolausz M, Schmidt T, Liebetrau J, Nelles M, Stinner W (2015) Biogas production from sugarcane waste: assessment on kinetic challenges for process designing. Int J Mol Sci 16:20685–20703. https://doi.org/10.3390/ijms160920685
Jeihanipour A, Karimi K, Niklasson C, Taherzadeh MJ (2010) A novel process for ethanol or biogas production from cellulose in blended-fibers waste textiles. Waste Manag 30:2504–2509. https://doi.org/10.1016/j.wasman.2010.06.026
Jeihanipour A, Aslanzadeh S, Rajendran K, Balasubramanian G, Taherzadeh MJ (2013) High-rate biogas production from waste textiles using a two-stage process. Renew Energy 52:128–135. https://doi.org/10.1016/j.renene.2012.10.042
Jiménez-Castro MP, Buller LS, Sganzerla WG, Forster-Carneiro T (2020) Bioenergy production from orange industrial waste: a case study. Biofuels Bioprod Biorefin. https://doi.org/10.1002/bbb.2128
Jouzani GS (2018) Metadata of the chapter that will be visualized in SpringerLink, pp 333–349. https://doi.org/10.1007/978-3-319-77335-3
Kabir MM, Rajendran K, Taherzadeh MJ, Sárvári Horváth I (2015) Experimental and economical evaluation of bioconversion of forest residues to biogas using organosolv pretreatment. Bioresour Technol 178:201–208. https://doi.org/10.1016/j.biortech.2014.07.064
Kabouris JC, Tezel U, Pavlostathis SG, Engelmann M, Dulaney J, Gillette RA, Todd AC (2009) Methane recovery from the anaerobic codigestion of municipal sludge and FOG. Bioresour Technol 100:3701–3705. https://doi.org/10.1016/j.biortech.2009.02.024
Kapoor R, Ghosh P, Kumar M, Sengupta S, Gupta A, Kumar SS, Vijay V, Kumar V, Kumar Vijay V, Pant D (2020) Valorization of agricultural waste for biogas based circular economy in India: a research outlook. Bioresour Technol. https://doi.org/10.1016/j.biortech.2020.123036
Karagiannidis A, Perkoulidis G (2009) A multi-criteria ranking of different technologies for the anaerobic digestion for energy recovery of the organic fraction of municipal solid wastes. Bioresour Technol 100:2355–2360. https://doi.org/10.1016/j.biortech.2008.11.033
Karimi K, Shafiei M, Kumar R (2013) Progress in physical and chemical pretreatment of lignocellulosic biomass. In: Biofuel technologies: recent developments. Springer-Verlag, Berlin, pp 53–96. https://doi.org/10.1007/978-3-642-34519-7_3
Khalid A, Arshad M, Anjum M, Mahmood T, Dawson L (2011) The anaerobic digestion of solid organic waste. Waste Manag 31:1737–1744. https://doi.org/10.1016/j.wasman.2011.03.021
Khan AA, de Jong W, Jansens PJ, Spliethoff H (2009) Biomass combustion in fluidized bed boilers: potential problems and remedies. Fuel Process Technol 90:21–50. https://doi.org/10.1016/j.fuproc.2008.07.012
Kim JK, Oh BR, Chun YN, Kim SW (2006) Effects of temperature and hydraulic retention time on anaerobic digestion of food waste. J Biosci Bioeng 102:328–332. https://doi.org/10.1263/jbb.102.328
Kivaisi AK, Eliapenda S (1994) Pretreatment of bagasse and coconut fibres for enhanced anaerobic degradation by rumen microorganisms. Renew Energy 5:791–795. https://doi.org/10.1016/0960-1481(94)90089-2
Koike Y, An MZ, Tang YQ, Syo T, Osaka N, Morimura S, Kida K (2009) Production of fuel ethanol and methane from garbage by high-efficiency two-stage fermentation process. J Biosci Bioeng 108:508–512. https://doi.org/10.1016/j.jbiosc.2009.06.007
Kotarska K, Dziemianowicz W, Swierczyńska A (2019) Study on the sequential combination of bioethanol and biogas production from corn straw. Molecules 24. https://doi.org/10.3390/molecules24244558
Kumar AK, Sharma S (2017) Recent updates on different methods of pretreatment of lignocellulosic feedstocks: a review. Bioresour Bioprocess. https://doi.org/10.1186/s40643-017-0137-9
Kumari D, Singh R (2018) Pretreatment of lignocellulosic wastes for biofuel production: a critical review. Renew Sust Energ Rev 90:877–891. https://doi.org/10.1016/j.rser.2018.03.111
Kumari D, Singh R (2020) Ultrasonic assisted petha waste water pretreatment of rice straw for optimum production of methane and ethanol using mixed microbial culture. Renew Energy 145:682–690. https://doi.org/10.1016/j.renene.2019.06.082
Larsson M, Jansson M, Grönkvist S, Alvfors P (2015) Techno-economic assessment of anaerobic digestion in a typical Kraft pulp mill to produce biomethane for the road transport sector. J Clean Prod 104:460–467. https://doi.org/10.1016/j.jclepro.2015.05.054
Lee K, Chantrasakdakul P, Kim D, Kim JS, Park KY (2017) Biogas productivity of algal residues from bioethanol production. J Mater Cycles Waste Manag 19:235–240. https://doi.org/10.1007/s10163-015-0413-8
Leite AF, Janke L, Harms H, Zang JW, Fonseca-Zang WA, Stinner W, Nikolausz M (2015) Assessment of the variations in characteristics and methane potential of major waste products from the Brazilian bioethanol industry along an operating season. Energy Fuels 29:4022–4029. https://doi.org/10.1021/ef502807s
Li H, Kim NJ, Jiang M, Kang JW, Chang HN (2009) Simultaneous saccharification and fermentation of lignocellulosic residues pretreated with phosphoric acid-acetone for bioethanol production. Bioresour Technol 100:3245–3251. https://doi.org/10.1016/j.biortech.2009.01.021
Liguori R, Faraco V (2016) Biological processes for advancing lignocellulosic waste biorefinery by advocating circular economy. Bioresour Technol 215:13–20. https://doi.org/10.1016/j.biortech.2016.04.054
Linke B (2006) Kinetic study of thermophilic anaerobic digestion of solid wastes from potato processing. Biomass Bioenergy 30:892–896. https://doi.org/10.1016/j.biombioe.2006.02.001
Lohrasbi M, Pourbafrani M, Niklasson C, Taherzadeh MJ (2010) Process design and economic analysis of a citrus waste biorefinery with biofuels and limonene as products. Bioresour Technol 101:7382–7388. https://doi.org/10.1016/j.biortech.2010.04.078
Lu W-J, Wang H-T, Nie Y-F, Wang Z-C, Huang D-Y, Qiu X-Y, Chen J-C (2011) Effect of inoculating flower stalks and vegetable waste with ligno-cellulolytic microorganisms on the composting process. J Environ Sci Health B 39(5–6):871–887. https://doi.org/10.1081/LESB-200030896
Lynd LR (1996) Overview and evaluation of fuel ethanol from cellulosic biomass: technology, economics, the environment, and policy. Annu Rev Energy Environ. https://doi.org/10.1146/annurev.energy.21.1.403
Mahmoodi P, Karimi K, Taherzadeh MJ (2018) Efficient conversion of municipal solid waste to biofuel by simultaneous dilute-acid hydrolysis of starch and pretreatment of lignocelluloses. Energy Convers Manag 166:569–578. https://doi.org/10.1016/j.enconman.2018.04.067
Manzanares P, Ruiz E, Ballesteros M, Negro MJ, Gallego FJ, López-Linares JC, Castro E (2017) Residual biomass potential in olive tree cultivation and olive oil industry in Spain: valorization proposal in a biorefinery context. Span J Agric Res 15. https://doi.org/10.5424/sjar/2017153-10868
Mao C, Feng Y, Wang X, Ren G (2015) Review on research achievements of biogas from anaerobic digestion. Renew Sust Energ Rev. https://doi.org/10.1016/j.rser.2015.02.032
Marin J, Kennedy KJ, Eskicioglu C (2010) Effect of microwave irradiation on anaerobic degradability of model kitchen waste. Waste Manag 30:1772–1779. https://doi.org/10.1016/j.wasman.2010.01.033
Martínez-Patiño JC, Ruiz E, Romero I, Cara C, López-Linares JC, Castro E (2017) Combined acid/alkaline-peroxide pretreatment of olive tree biomass for bioethanol production. Bioresour Technol 239:326–335. https://doi.org/10.1016/j.biortech.2017.04.102
Mel M, Yong ASH, Avicenna, Ihsan SI, Setyobudi RH (2015) Simulation study for economic analysis of biogas production from agricultural biomass. In: Energy procedia. Elsevier, pp 204–214. https://doi.org/10.1016/j.egypro.2015.01.026
Mirahmadi K, Kabir MM, Jeihanipour A, Karimi K, Taherzadeh MJ (2010) Alkaline pretreatment of spruce and birch to improve bioethanol and biogas production. Bioresources 5:928–938
Mladenović R, Dakić D, Erić A, Mladenović M, Paprika M, Repić B (2009) The boiler concept for combustion of large soya straw bales. Energy 34:715–723. https://doi.org/10.1016/j.energy.2009.02.003
Mladenović M, Paprika M, Marinković A (2018) Denitrification techniques for biomass combustion. Renew Sust Energ Rev. https://doi.org/10.1016/j.rser.2017.10.054
Moraes BS, Triolo JM, Lecona VP, Zaiat M, Sommer SG (2015a) Biogas production within the bioethanol production chain: Use of co-substrates for anaerobic digestion of sugar beet vinasse. Bioresour Technol 190:227–234. https://doi.org/10.1016/j.biortech.2015.04.089
Moraes BS, Zaiat M, Bonomi A (2015b) Anaerobic digestion of vinasse from sugarcane ethanol production in Brazil: challenges and perspectives. Renew Sust Energ Rev 44:888–903. https://doi.org/10.1016/j.rser.2015.01.023
Moshi AP, Temu SG, Nges IA, Malmo G, Hosea KMM, Elisante E, Mattiasson B (2015) Combined production of bioethanol and biogas from peels of wild cassava Manihot glaziovii. Chem Eng J 279:297–306. https://doi.org/10.1016/j.cej.2015.05.006
Mtui G, Nakamura Y (2005) Bioconversion of lignocellulosic waste from selected dumping sites in Dar es Salaam, Tanzania. Biodegradation 16:493–499. https://doi.org/10.1007/s10532-004-5826-3
Mussoline W, Esposito G, Lens P, Garuti G, Giordano A (2012) Design considerations for a farm-scale biogas plant based on pilot-scale anaerobic digesters loaded with rice straw and piggery wastewater. Biomass Bioenergy 46:469–478. https://doi.org/10.1016/j.biombioe.2012.07.013
Nair RB, Lennartsson PR, Taherzadeh MJ (2017) Bioethanol production from agricultural and municipal wastes. In: Current developments in biotechnology and bioengineering: solid waste management. Elsevier, San Diego, pp 157–190. https://doi.org/10.1016/B978-0-444-63664-5.00008-3
Náthia-Neves G, de Neves TA, Berni M, Dragone G, Mussatto SI, Forster-Carneiro T (2018) Start-up phase of a two-stage anaerobic co-digestion process: Hydrogen and methane production from food waste and vinasse from ethanol industry. Biofuel Res J 5:813–820. https://doi.org/10.18331/BRJ2018.5.2.5
Novo A, Jansen K, Slingerland M (2012) The sugarcane-biofuel expansion and dairy farmers’ responses in Brazil. J Rural Stud 28:640–649. https://doi.org/10.1016/j.jrurstud.2012.07.004
Oberoi HS, Vadlani PV, Saida L, Bansal S, Hughes JD (2011) Ethanol production from banana peels using statistically optimized simultaneous saccharification and fermentation process. Waste Manag 31:1576–1584. https://doi.org/10.1016/j.wasman.2011.02.007
Oyeleke S, Jibrin MN (2009) Production of bioethanol from guinea cornhusk and millet husk. Afr J Microbiol Res 3:147–152
Ozgen S, Cernuschi S, Caserini S (2021) An overview of nitrogen oxides emissions from biomass combustion for domestic heat production. Renew Sust Energ Rev 135. https://doi.org/10.1016/j.rser.2020.110113
Padi RK, Chimphango A (2020) Feasibility of commercial waste biorefineries for cassava starch industries: techno-economic assessment. Bioresour Technol 297. https://doi.org/10.1016/j.biortech.2019.122461
Pellera FM, Giannis A, Kalderis D, Anastasiadou K, Stegmann R, Wang JY, Gidarakos E (2012) Adsorption of Cu(II) ions from aqueous solutions on biochars prepared from agricultural by-products. J Environ Manag 96:35–42. https://doi.org/10.1016/j.jenvman.2011.10.010
Piccolo C, Bezzo F (2009) A techno-economic comparison between two technologies for bioethanol production from lignocellulose. Biomass Bioenergy 33:478–491. https://doi.org/10.1016/j.biombioe.2008.08.008
Plácido J, Imam T, Capareda S (2013) Evaluation of ligninolytic enzymes, ultrasonication and liquid hot water as pretreatments for bioethanol production from cotton gin trash. Bioresour Technol 139:203–208. https://doi.org/10.1016/j.biortech.2013.04.012
Prasara-A J, Gheewala SH (2017) Sustainable utilization of rice husk ash from power plants: a review. J Clean Prod 167:1020–1028. https://doi.org/10.1016/j.jclepro.2016.11.042
Quintero JA, Montoya MI, Sánchez OJ, Giraldo OH, Cardona CA (2008) Fuel ethanol production from sugarcane and corn: comparative analysis for a Colombian case. Energy 33:385–399. https://doi.org/10.1016/j.energy.2007.10.001
Quintero M, Castro L, Ortiz C, Guzmán C, Escalante H (2012) Bioresource Technology Enhancement of starting up anaerobic digestion of lignocellulosic substrate: fique’ s bagasse as an example. Bioresour Technol 108:8–13. https://doi.org/10.1016/j.biortech.2011.12.052
Rastogi M, Shrivastava S (2017) Recent advances in second generation bioethanol production: An insight to pretreatment, saccharification and fermentation processes. Renew Sust Energ Rev 80:330–340. https://doi.org/10.1016/j.rser.2017.05.225
Ren LH, Nie YF, Liu JG, Jin YY, Sun L (2006) Impact of hydrothermal process on the nutrient ingredients of restaurant garbage. J Environ Sci (China) 18:1012–1019. https://doi.org/10.1016/S1001-0742(06)60031-4
Rodríguez LA, Toro ME, Vazquez F, Correa-Daneri ML, Gouiric SC, Vallejo MD (2010) Bioethanol production from grape and sugar beet pomaces by solid-state fermentation. Int J Hydrog Energy 35:5914–5917. https://doi.org/10.1016/j.ijhydene.2009.12.112
Röske I, Sabra W, Nacke H, Daniel R, Zeng AP, Antranikian G, Sahm K (2014) Microbial community composition and dynamics in high-temperature biogas reactors using industrial bioethanol waste as substrate. Appl Microbiol Biotechnol 98:9095–9106. https://doi.org/10.1007/s00253-014-5906-1
Safarian S, Unnthorsson R (2018) An assessment of the sustainability of lignocellulosic bioethanol production from wastes in Iceland. Energies 11. https://doi.org/10.3390/en11061493
Salehian P, Karimi K (2012) Alkali pretreatment for improvement of biogas and ethanol production from different waste parts of pine tree. Ind Eng Chem Res 52:972–978. https://doi.org/10.1021/ie302805c
Sari NK, Ernawati D (2019) Process fermentation of filtrate bamboo with saccharomyces cerevisiae and zymomonas mobilis. J Phys Conf Ser 1295:012033. https://doi.org/10.1088/1742-6596/1295/1/012033
Sarkar N, Ghosh SK, Bannerjee S, Aikat K (2012) Bioethanol production from agricultural wastes: an overview. Renew Energy 37:19–27. https://doi.org/10.1016/j.renene.2011.06.045
Sarto S, Hildayati R, Syaichurrozi I (2019) Effect of chemical pretreatment using sulfuric acid on biogas production from water hyacinth and kinetics. Renew Energy 132:335–350. https://doi.org/10.1016/j.renene.2018.07.121
Sawayama S, Inoue S, Minowa T, Tsukahara K, Ogi T (1997) Thermochemical liquidization and anaerobic treatment of kitchen garbage. J Ferment Bioeng 83:451–455. https://doi.org/10.1016/S0922-338X(97)82999-6
Schläfle S, Senn T, Gschwind P, Kohlus R (2017) Feasibility and energetic evaluation of air stripping for bioethanol production. Bioresour Technol 231:109–115. https://doi.org/10.1016/j.biortech.2017.02.001
Shafiei M (2018) Techno-economic aspects of biogas plants. Springer, Cham, pp 333–353. https://doi.org/10.1007/978-3-319-77335-3_13
Shafiei M, Karimi K, Taherzadeh MJ (2011) Techno-economical study of ethanol and biogas from spruce wood by NMMO-pretreatment and rapid fermentation and digestion. Bioresour Technol 102:7879–7886. https://doi.org/10.1016/j.biortech.2011.05.071
Silva AFV, Santos LA, Valença RB, Porto TS, da Motta Sobrinho MA, Gomes GJC, Jucá JFT, Santos AFMS (2019) Cellulase production to obtain biogas from passion fruit (Passiflora edulis) peel waste hydrolysate. J Environ Chem Eng 7. https://doi.org/10.1016/j.jece.2019.103510
Smajevic I, Kazagic A, Music M, Becic K, Hasanbegovic I, Sokolovic S, Delihasanovic N, Skopljak A, Hodzic N (2012) Co-firing bosnian coals with woody biomass: Experimental studies on a laboratory-scale furnace and 110 MW e power unit. Therm Sci 16:789–804. https://doi.org/10.2298/TSCI120120122S
Solarte-Toro JC, Romero-García JM, López-Linares JC, Ramos ER, Castro E, Alzate CAC (2018) Simulation approach through the biorefinery concept of the antioxidants, lignin and ethanol production using olive leaves as raw material. Chem Eng Trans 70:925–930. https://doi.org/10.3303/CET1870155
Solarte-Toro JC, Romero-García JM, Susmozas A, Ruiz E, Castro E, Cardona-Alzate CA (2019) Techno-economic feasibility of bioethanol production via biorefinery of olive tree prunings (OTP): optimization of the pretreatment stage. Holzforschung 73:3–13. https://doi.org/10.1515/hf-2018-0096
Sommersacher P, Brunner T, Obernberger I, Kienzl N, Kanzian W (2013) Application of novel and advanced fuel characterization tools for the combustion related characterization of different wood/kaolin and straw/kaolin mixtures. Energy Fuels 27:5192–5206. https://doi.org/10.1021/ef400400n
Sritrakul N, Nitisinprasert S, Keawsompong S (2017) Evaluation of dilute acid pretreatment for bioethanol fermentation from sugarcane bagasse pith. Agric Nat Resour 51:512–519. https://doi.org/10.1016/j.anres.2017.12.006
Sunarno JN, Prasertsan P, Duangsuwan W, Cheirsilp B, Sangkharak K (2020) Improve biotransformation of crude glycerol to ethanol of Enterobacter aerogenes by two-stage redox potential fed-batch process under microaerobic environment. Biomass Bioenergy 134. https://doi.org/10.1016/j.biombioe.2020.105503
Taherdanak M, Zilouei H, Karimi K (2016) The influence of dilute sulfuric acid pretreatment on biogas production form wheat plant. Int J Green Energy 13:1129–1134. https://doi.org/10.1080/15435075.2016.1175356
Taherzadeh M, Karimi K (2008) Pretreatment of lignocellulosic wastes to improve ethanol and biogas production: a review. Int J Mol Sci 9:1621–1651. https://doi.org/10.3390/ijms9091621
van Dyk JS, Gama R, Morrison D, Swart S, Pletschke BI (2013) Food processing waste: Problems, current management and prospects for utilisation of the lignocellulose component through enzyme synergistic degradation. Renew Sust Energ Rev 26:521–531. https://doi.org/10.1016/j.rser.2013.06.016
Venturin B, Frumi Camargo A, Scapini T, Mulinari J, Bonatto C, Bazoti S, Pereira Siqueira D, Maria Colla L, Alves SL, Paulo Bender J, Steinmetz LR, Kunz A, Fongaro G, Treichel H (2018) Effect of pretreatments on corn stalk chemical properties for biogas production purposes. Bioresour Technol 266:116–124. https://doi.org/10.1016/j.biortech.2018.06.069
von Sivers M, Zacchi G (1996) Ethanol from lignocellulosics: a review of the economy. Bioresour Technol 56:131–140. https://doi.org/10.1016/0960-8524(96)00018-1
Wardani NA, Afiqah N, Azis MM, Budhijanto W (2020) Comparison of biogas productivity in thermophilic and mesophilic anaerobic digestion of bioethanol liquid waste. In: IOP conference series: earth and environmental science. Institute of Physics Publishing. https://doi.org/10.1088/1755-1315/448/1/012002
Werther J, Saenger M, Hartge EU, Ogada T, Siagi Z (2000) Combustion of agricultural residues. Prog Energy Combust Sci. https://doi.org/10.1016/S0360-1285(99)00005-2
Westerholm M, Hansson M, Schnürer A (2012) Improved biogas production from whole stillage by co-digestion with cattle manure. Bioresour Technol 114:314–319. https://doi.org/10.1016/j.biortech.2012.03.005
Xiang X, Gong G, Shi Y, Cai Y, Wang C (2018) Thermodynamic modeling and analysis of a serial composite process for biomass and coal co-gasification. Renew Sust Energ Rev. https://doi.org/10.1016/j.rser.2017.10.008
Yin C, Rosendahl LA, Kær SK (2008) Grate-firing of biomass for heat and power production. Prog Energy Combust Sci. https://doi.org/10.1016/j.pecs.2008.05.002
Ylitervo P, Akinbomi J, Taherzadeh MJ (2013) Membrane bioreactors potential for ethanol and biogas production: A review. Environ Technol 34:1711–1723. https://doi.org/10.1080/09593330.2013.813559
Yu S, Tao J (2008) Life cycle simulation-based economic and risk assessment of biomass-based fuel ethanol (BFE) projects in different feedstock planting areas. Energy 33:375–384. https://doi.org/10.1016/j.energy.2007.10.009
Yu Z, Du Y, Shang X, Zheng Y, Zhou J (2018) Enhancing fermentable sugar yield from cassava residue using a two-step dilute ultra-low acid pretreatment process. Ind Crop Prod 124:555–562. https://doi.org/10.1016/j.indcrop.2018.08.029
Zhang CM, Mao ZG, Wang X, Zhang JH, Sun FB, Tang L, Zhang HJ (2010) Effective ethanol production by reutilizing waste distillage anaerobic digestion effluent in an integrated fermentation process coupled with both ethanol and methane fermentations. Bioprocess Biosyst Eng 33:1067–1075. https://doi.org/10.1007/s00449-010-0432-8
Zuo Z, Tian S, Chen Z, Li J, Yang X (2012) Soaking pretreatment of corn stover for bioethanol production followed by anaerobic digestion process. Appl Biochem Biotechnol 167:2088–2102. https://doi.org/10.1007/s12010-012-9751-3
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2023 The Author(s), under exclusive license to Springer Nature Singapore Pte Ltd.
About this chapter
Cite this chapter
Muñoz-Henández, J.A., Sánchez-Jiménez, C.A., Roa-Acosta, D.F., Bravo-Gómez, J.E., Solanilla-Duque, J.F., Muñoz-Henández, H. (2023). Waste Processes to Obtain Biogas and Bioethanol. In: Aguilar, C.N., Abdulhameed, S., Rodriguez-Herrera, R., Sugathan, S. (eds) Microbial Biodiversity, Biotechnology and Ecosystem Sustainability. Springer, Singapore. https://doi.org/10.1007/978-981-19-4336-2_21
Download citation
DOI: https://doi.org/10.1007/978-981-19-4336-2_21
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-19-4335-5
Online ISBN: 978-981-19-4336-2
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)