Abstract
A series of studies over the past 32 years has revealed many remarkable parameters of the life history of Parastrachia japonensis Scott (Heteroptera: Parastrachiidae), the parental shield bug. This chapter briefly reviews the information that had been derived by earlier researchers; discusses the morphology and worldwide distribution of the bug, as well as the specific habitat and resource that it utilizes at our field site in Japan; and presents the overall life cycle of P. japonensis. P. japonensis inhabits secondary forests in regions from eastern China to southern Japan. It feeds solely on drupes of its host tree, the grey twig tree, Schoepfia jasminodora (Schoepfiaceae), an ephemeral, unpredictable, and scarce resource. Early studies suggested that females care for young, and perhaps provision nests with drupes. There were about 59 subpopulations at the field site in Japan, Mt. Hinokuma, in Kanzaki Town, Saga, Japan, and there is a host tree within 10 m of every subpopulation of bugs. Subpopulations range from just a few dozen to around 10,000 individuals, and subpopulation size is correlated with the size of the host tree. Not every host tree has a subpopulation of P. japonensis, and the combination of ecological features that must coincide for a subpopulation to exist was clarified. The life history can last 1–2 years. The reproductive period (mating, oviposition, egg, and nymph guarding and progressive provisioning) extends from May through mid-July; the remainder of the year is spent in a long reproductive diapause which includes hibernation below ground from December through February. The bug’s utilization of a toxic resource is also discussed.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Distribution
- Geographic distribution
- Habitat
- Host tree
- Life cycle
- Local distribution
- Morphology
- Parastrachia japonensis
- Parastrachia nagaensis
- Phylogeny
- Resource
1.1 Introduction
Many early studies on the life history of Parastrachia japonensis (Scott) documented findings that suggested the bug had a very interesting life history and unusual behaviors (Esaki 1930a, b; Hiura 1952; Miyamoto 1954, 1956, 1965, 1966; Nakao 1956; Tachikawa 1980, 1984; Gyôtoku and Tachikawa 1980; Tachikawa and Gyotoku 1981; Tachikawa and Schaefer 1985; Schaefer et al. 1988). Most of the studies were done in Kurume City and Fukuoka Prefecture, Japan, and they all indicated a tantalizing need for more in-depth field studies on this fascinating bug. This chapter begins with a review of the historical studies and then describes the morphology and classification of P. japonensis and its elusive sister species, P. nagaensis. We then introduce the phylogenetic status of the genus based on morphologic, molecular, and behavioral data. The global distribution of the two sister species is described, followed by detailed descriptions of the local distribution of P. japonensis at Mt. Hinokuma Park, in Kanzaki town, Saga Prefecture, Japan, where most of the recent studies have taken place. We delve into the particular features of the local habitat that support subpopulations of the bug and the distribution in the local habitat of the sole resource it feeds on, drupes of the grey twig tree, Schoepfia jasminodora (Schoepfiaceae), its phenology, and its toxic nature. Finally, we describe the details of the life cycle that have been filled in over the years.
1.2 Historical Perspective of Studies on the Life History of P. japonensis
Prior to the beginning of L.F.’s graduate studies, there had been no intensive, long-term studies done on this fascinating bug. Several authors had reported on its existence, but for the most part, the early literature on Parastrachia japonensis Scott (Heteroptera : Parastrachiidae) primarily represented findings from short-term studies and, more often, snapshot impressions of the bug’s life history . Many observations were anecdotal, made in isolation of a larger life history context, while others involved data collected several times over a few hours of observations during different seasons. Clearly all of the studies contributed to our understanding of this fascinating species; however, because of the irregular and short-term nature of these early studies, several of the findings incorrectly represented the bug’s behaviors. In a book on subsocial heteropterans of Japan , Tachikawa (1991) reported findings of the earliest studies on P. japonensis, and we summarize some of that information here.
The earliest reports on P. japonensis (Esaki 1930a, b) are based on observations made at Koura Mountain in Kurume City, on Kyushu Island in Japan . Esaki observed the bugs, which he identified as a genus in a subfamily of the predatory Pentatomidae , on Camellia japonica and Lyonia ovalifolia and incorrectly assumed that was their food source. Hiura (1952) made ten observations of a large aggregation at the same site from April to July and estimated the life history sequence without actually observing all the stages, erroneously stating that mating occurred in July. He reported the habitat as a fairly dark, densely forested area of primarily broadleaf evergreen trees such as Castanopsis and Camellia, which is consistent with other reports. Hiura observed a large aggregation of post-hibernation adults comprising more than 4000 individuals on leaves of ferns and other ground cover. Because of evidence of penetration by the proboscis on the back of leaves, he also incorrectly concluded that the evergreen trees were the food source of P. japonensis. He observed that, while they are attracted to and fly to lights at night, the aggregations are primarily sedate, with individuals displaying very little movement. Hiura also was the first to describe the mechanism for the stridulatory chattering sound emitted by P. japonensis adults.
In July of 1953, Miyamoto (1954) observed third and fourth instar nymphs feeding as aggregations on apparently rotting unidentified drupes and also observed five incidents of cannibalism . Miyamoto (1956) reported that he had been unable to observe mating and oviposition under rearing conditions in the laboratory, but on June 8–9, he collected an egg mass under leaf litter in the field, where he assumed the female had deposited it. The eggs were similar in appearance to those of familiar cydnids.
In 1956, Nakao reported a hibernating aggregation under the leaf litter and concluded that the tendency to aggregate after hibernation was just a continuation of the overwintering structure.
Oviposition was finally observed by Miyamoto (1965) in early June; he noted that females guarded a round egg mass and that nymphs remained in the leaf litter on the ground and tended to gather on still unidentified drupes. He later observed that oviposition occurred during the rainy season, which begins in June and goes on until mid- to late July, under the leaf litter (Miyamoto 1966). He noted that the pattern of oviposition of a round egg mass and guarding of the egg mass by females resembled the ecology of Adomerus triguttulus (Heteroptera: Cydnidae). Finally, Miyamoto (1966) also observed that while eggs were very inconspicuous under the leaf litter, the bright red color of nymphs as they roam on the forest floor while foraging rendered them highly conspicuous.
Tachikawa carried out a series of field and laboratory observational studies between 1978 and 1982 and presented his findings at several Japanese conferences and at an International Entomology Congress in 1984 (Tachikawa 1984). Gyôtoku and Tachikawa (1980) and Tachikawa and Schaefer (1985) described more details of the life history of P. japonensis that had been gleaned through 1982, and the earlier report finally correctly identified the sole food of this bug as drupes of the gray twig tree, Schoepfia jasminodora Sieb. & Zucc. Thereafter follows a blank in the scientific literature, though the studies continued and were presented at several conferences in Japan , until several papers between 1985 and 1991 that described the general biology and detailed anatomy and also attempted to sort out the confusing taxonomy (Tachikawa and Schaefer 1985; Schaefer et al. 1988; Tachikawa 1991). The following information, based on field and laboratory observations from June 1981 to July 1982 and summarized in Tachikawa (1991), essentially represents what was known at the time our research began in 1989.
P. japonensis is a monophagous insect that feeds on the endosperm of Schoepfia jasminodora Sieb. & Zucc. (Schoepfiaceae Rosidae: Santalales), a host plant that invariably occurs wherever the bug is found and one that requires a stable secondary forest. The bug has one generation per year, and its life cycle coincides with the phenology of the host tree . Adults are strongly gregarious; they remain aggregated on plants and trees in the understory and herbaceous layer that are sheltered from direct sunlight ; the aggregations remain largely sedentary. Throughout most of the year, the bugs don’t eat, and the ovaries remain undeveloped. In fact, males and females apparently remain in a state of reproductive diapause throughout much of the year. When an aggregation is disturbed, individuals crawl into and over each other and make a chattering sound ; they also release a noxious odor. Individuals fall from the tree to the ground and feign death (thanatosis); they later climb back onto the plants and reform into aggregations.
When S. jasminodora is fruiting, a portion of the males and females mate near the aggregations; females leave the aggregations and relocate to the S. jasminodora tree. Mated females feed on the drupes to develop their eggs. Mothers climb down the host tree and excavate a shallow burrow under the leaf litter where they deposit about 60–100 eggs in a round mass. Moreover, Tachikawa (1991) observed females dragging drupes under the leaf litter, and suggested this might be for young, but never actually observed provisioning .
1.3 General Morphology
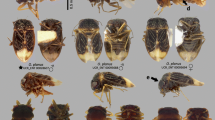
P. japonensis is a large bug of 16–19 mm in length (Fig. 1.1). The coloration comprises five black patches on a red background: large black diamond-shaped patches on the forewings, black patches on the bottom of the hindwings forming a diamond when closed, a triangular black patch on the pronotum , and a horizontal rectangular black patch on the dorsal thorax (Tachikawa 1991). The morphological peculiarity of this species placed it in the Cydnidae ; however, it was suggested that this genus should actually be assigned to a separate subfamily, Parastrachiinae (Schaefer et al. 1988) (it has since been elevated to family level, Parastrachiidae; Sweet and Schaefer 2002).
1.4 Classification
1.4.1 Phylogenetic Status Based on Morphology
There are only two species within the genus Parastrachia, P. japonensis Scott (Scott 1880), and P. nagaensis Distant (Distant 1908). Although distinct morphological features clearly indicated that the genus belonged in the Pentatomoidea, its classification beyond that had a very ambiguous beginning. The history of its earliest classification is detailed in Schaefer et al. (1988) and Tachikawa (1991). The genus had been variously assigned to the Pentatomidae : Asopinae or Pentatominae , Cydnidae : Sehirinae , Tessaratomidae , and there was also a suggestion of Urostylidae . Schaefer et al. (1988) concluded that none of the arguments for those groupings were convincing enough to merit the argued placement and, while recognizing that it was still not a perfect fit, assigned Parastrachia to a new monogeneric subfamily, Parastrachiinae , within the Cydnidae. Although there is considerable discrepancy between Parastrachia and other members of the Cydnidae with regard to size and color, with the cydnids being small and mostly drab, dark coloring, compared to the much larger and aposematically colored Parastrachia, similarities in the pattern of oviposition , the shape of the eggs, and the maternal parental care behaviors suggest that these behaviors arose in a common ancestor of Parastrachia and Cydnidae (Schaefer et al. 1988). Sweet and Schaefer (2002) describe 13 morphological features they considered sufficient to exclude Parastrachia from Pentatomidae and Tessaratomidae. In addition, the difference in the eggs (round, cohesive egg mass in the former and barrel-shaped or round eggs deposited individually or in a monolayer on a substrate in the latter two families) also deviated from those families.
Sweet and Schaefer (2002) also described 12 morphological features that excluded Parastrachia from the Cydnidae , including the striking aposematic color of the former, compared to the dark color of the latter. The authors concluded that most of the morphological features described for Parastrachia that excluded them from the other families were likely plesiomorphic. Finally, they described 13 largely apomorphic morphological features, some unique, that unite Parastrachia and ultimately concluded the most reasonable course, one that helped maintain the integrity of the Cydnidae, was to elevate the genus to family status (Parastrachiidae, Oshanin 1922) (Sweet and Schaefer 2002).
Parastrachia japonensis and P. nagaensis can be distinguished by genitalia and some color or somatic features (Schaefer et al. 1991), though these features are less reliable. Differences in the male aedeagus , particularly the vesicular process , the proximal conjunctival appendages , and the conjunctiva’s midventral sclerotization, are notable (Schaefer et al. 1991). On the other hand, similarities in the aedeagus between the two species , such as the large vesicular process, the two pairs of well-sclerotized laterodorsal conjunctival appendages, and the small gonoporal process, unite them (Schaefer et al. 1988, 1991).
1.4.2 Phylogenetic Status Based on Morphology and Molecular Data
Grazia et al. (2008) carried out a phylogenetic analysis of the Pentatomoidea using both morphological and DNA sequence data. A character matrix employing 57 morphological features, particularly similarities in the spermatheca (Pluot-Sigwalt and Lis 2008) and pretarsal structures (Lis 2010), supported the monophyly of Parastrachia + the six species in the African genus Dismegistus with Sehirinae within the Cydnidae , considered to be the closest relatives to both genera. A POY of a 92-taxon dataset using 1:2 and 2:2 indel/transition-transversion cost ratios also supported the monophyly. The findings suggested that Parastrachia + Dismegistus formed a monophyletic group , which placed Dismegistus within the Parastrachiidae (Pluot-Sigwalt and Lis 2008; Lis 2010). However, two Bayesian analysis trees of combined 28S + 18S rDNA datasets using three different substitution models did not support the monophyly of these two genera (Lis et al. 2017). Rather, in that study Parastrachia japonensis was never identified as a clade with Dismegistus. Instead, Parastrachia japonensis formed a sister taxon to Ochetostethomorpha secunda in the Cydnidae subfamily Sehirinae, with both species forming a sister clade to the monophyletic group consisting of the four remaining species of Sehirinae examined. In their analyses, Dismegistus always branched off as an outgroup to the Sehirinae + Parastrachia (Lis et al. 2017). Most recently, Pluot-Sigwalt and Lis (2018) reported that uradenia , paired exocrine glands located ventrally in the abdomen and uniting several superfamilies in the infraorder Pentatomomorpha, were observed in male Dismegistus. However, these glands, which until this report were considered to be absent in the Pentatomoidea , did not occur in either males or females of Parastrachia (Pluot-Sigwalt and Lis 2018), again muddying the waters regarding the monophyletic status of these two genera. To be sure, the rounded body shape of Dismegistus is more similar to Thyreocoridae , Thaumastellidae , or Pentatomidae than Parastrachiidae. It does appear that the puzzling relationship between Dismegistus, Parastrachia, and the other Sehirinae is not quite resolved, as yet.
1.4.3 Phylogenetic Status Based on Unique Behavioral Features
Sadly, there is virtually no information available on the biology of Dismegistus , so it is impossible at this time to say whether in the context of its biology it belongs in the group comprising the five Sehirinae species that share several features related to parental care with Parastrachia japonensis. All six species progressively provision seeds to their nests, and the five species that are not Parastrachia are all in the Cydnidae subfamily Sehirinae: Sehirus cinctus Palisot (Sites and McPherson 1982; Kight 1997; Agrawal et al. 2001, 2005; Kolliker et al. 2006); Adomerus triguttulus Motschulsky (Nakahira 1992, 1994; Kudo and Nakahira 2005; Nakahira and Kudo 2008; Nakahira et al. 2013); Canthophorus niveimarginatus Scott (Filippi et al. 2009); Adomerus variegatus Signoret (Mukai et al. 2010); and Adomerus rotundus Hsaio (Mukai et al. 2012; Inadomi et al. 2014).
Cervantes et al. (2013) provided compelling evidence that females of yet another cydnid, Melanaethus crenatus Signoret, also progressively provision young. Interestingly, this species belongs to the subfamily Cydninae and, thus, represents the only non-Sehirinae cydnid to progressively provision its nest . We explore the variation in egg production and parental care among these different species in a later chapter.
1.5 Geographic Distribution of P. japonensis and P. nagaensis
The most recent and detailed report of the distribution of P. japonensis (Fig. 1.2; Zhu et al. 2013) shows a continuous distribution extending from Guangxi in the Hengduan region of southeastern China , apparently the southernmost limit to its range , north to western Mt. Qinling . Moving east, in South Korea , it has been recorded in Cheju Island . In Japan , P. japonensis occurs in Okinawa , throughout Kyushu , north to Yamaguchi Prefecture in southern Honshu , which is apparently the northern limit to its range, and Ehime Prefecture in western Shikoku , the eastern limit of its range . The long-term studies reported here took place at Mt. Hinokuma Park , in Kanzaki City , Saga Prefecture , Japan. Thus, the distribution of P. japonensis ranges in latitude from 23° 43′ 29.28″ N to 33° 36′ 9″ N and in longitude from 107°37′05″ E to 132° 47′ 8.88″ E (Table 1.1).
Geographic distribution of Parastrachia. Low to high altitudes are shown as a gradient from white to black. The Chinese province names in the text were simplified on the map (NM, Inner Mongolia; HLJ, Heilongjiang; SC, Sichuan; GZ, Guizhou; GX, Guangxi ; YN, Yunnan ). Numbers beside black dots indicate the P. nagaensis records (1, Vietnam , New country record; 2, India ; 3, Ya’an , China ; 4, Yunnan, China; 5, Mt. Qingcheng , China; 6, Laos). Adapted from Zhu et al. (2013). Gengping Zhu, Guoqing Liu, Wenjun Bu, Jerzy A. Lis, Geographic distribution and niche divergence of two stinkbugs, Parastrachia japonensis and Parastrachia nagaensis , Journal of Insect Science, Volume 13, Issue 1, 2013, 102, https://doi.org/10.1673/031.013.10201
Parastrachia nagaensis occurs further west and south, compared to P. japonensis, and tends to occur at higher altitudes (Fig. 1.2). The westernmost record of P. nagaensis is from the Naga Region of Assam , India . Moving east from there, it has been recorded at several sites in China , from Yunnan north to Ya’an and Mt. Qingcheng . P. nagaensis has also been recorded in Vietnam , the easternmost record, and Laos, the southernmost and most recent record. The distribution of P. nagaensis thus ranges from a latitude of 20° 13′ 12″ N to 30° 53′ 60″ N and a longitude of 94° 33′ 36″ E to 106° 17′ 60″ E (Schaefer et al. 1988; Schaefer and Kikuhara 2007; Zhu et al. 2013).
The host tree of P. japonensis was identified by Gyotoku & Tachikawa (1980) as the Jasminodora grey twig ( Schoepfia jasminodora : Schoepfiaceae ). Several related species of Schoepfia occur throughout the range of the two Parastrachia species; however, the food resource of P. nagaensis has not yet been identified. It has been suggested by Schaefer et al. (1988, 1991) that P. japonensis is the plesiomorphic species that more closely resembles the ancestor of the two species and spread to the east and that P. nagaensis diverged to the west and likely uses another Schoepfia species as its host tree. In support of this hypothesis, Zhu et al. (2013) have described distinct niche differences between the two species, likely related, in part, to differences in altitude. Moreover, some samples of Parastrachia collected in the overlap region have features in between the easternmost populations of P. japonensis and P. nagaensis but were mostly designated as P. japonensis (Zhu et al. 2013). These findings and data from various niche divergence models described in that report also support the divergence hypothesis of Schaefer et al. (1988, 1991).
1.6 General Characteristics of the Habitat
Early descriptions of the habitat of P. japonensis (Miyamoto 1954, 1956; Hiura 1977; Gyôtoku and Tachikawa 1980; Tachikawa and Schaefer 1985; Tachikawa 1991) are very consistent with the field site at Mt. Hinokuma Park in Kanzaki City , Saga Prefecture , Japan , where we carried out studies for nearly 30 years (Fig. 1.3a-e). Like the sites identified in those earlier studies, Hinokuma is a dense secondary laurel forest on a small mountain encompassing a series of valleys and hilly areas throughout. Vegetation includes a variety of young broadleaf evergreen trees such as Eurya japonica Thunb. and Castanopsis and ferns such as Woodwardia and Gleichenia . Several species of tall deciduous trees, including Ilex chinensis , Toxicodendron, several species of Quercus, Viburnum japonicum Thunb., Rhus sylvestris Sieb. & Zucc., and Laurus camphora L., form a dense canopy that essentially blocks the sun , creating a fairly dark habitat. Host trees, Schoepfia jasminodora Sieb. & Zucc., in various stages of maturity, are scattered throughout the site (Tsukamoto and Tojo 1992; Filippi-Tsukamoto et al. 1995b).
Mt. Hinokuma , Kanzaki City , Saga , Japan . Clockwise from top left: Mountain range including Mt. Hinokuma viewed from the north. Arrow indicates Mt. Hinokuma; Mt. Hinokuma viewed from the west; access point to initial field site; Pond II, along the trail at the field site; Mt. Hinokuma viewed from the south where we approached the main access point to our field sites
1.7 Climate in Southern Japan
We present here a detailed description of the climate throughout the year because the constraints imposed on P. japonensis by the extreme climatic conditions it has experienced over evolutionary time have contributed greatly to molding the life history of this bug. The climate in Southern Japan is mild temperate, with distinct and dramatic seasonal features throughout the year. The winter, from December through February, is cold, damp, and gray, with heavy clouds; temperatures range from about 4–13° C (Japan Meteorological Agency n.d.) and reach freezing. The spring, from March through May, brings many days of fine weather with temperatures ranging from about 12 to 24 °C. The latter half of spring is fairly sunny.
There is a relentless rainy season throughout most of June and July, which coincides with the reproductive phase of P. japonensis’ life history . Temperatures during the rainy season range from 20° to 30 °C. The rain can be quite heavy, resulting in intense flooding and landslides in the early days of our studies that often created a river of rainwater flowing down the path we used to get to the local field site. At that stage in our research, instead of a maintained park, as its name implied, Mt. Hinokuma was essentially a hilly forest in a natural state of secondary succession. As such, when we first began our studies, the path leading up the small mountain had not been maintained, and for the entire duration of our first field season, because the torrential rains kept the vegetation growing at a feverish rate, we literally had to fight our way through the brush with a sickle every day to get to our site.
The rainy season ends abruptly in mid-July. There will be rain one day, and then it literally ends the next day; the sky dramatically turns a deep cerulean blue and is decorated with huge billowing white clouds. Prior to this day, the persistent rain makes for uncomfortably chilly and wet days, but once the rains stop, the days immediately become unbearably hot! The temperatures are not particularly high for summer, ranging from 24 to 33 °C, but the humidity , often 100%, and only going down to 70% indoors with the air conditioner blasting, renders it uncomfortably hot even in the shade. The intense heat continues through August and much of September, when the next dramatic shift in weather, the typhoon season, arrives.
Typhoon season, from late August through the months of September and October, can be severe and typically brings with it lots of rain, which again leads to significant flooding and landslides. Temperatures during the typhoon season range from 15 to 30 °C. One of the severest typhoon seasons occurred in the fall of 1989, just after our first field season. Very bad flooding then caused a major landslide at our field site, tragically burying a subpopulation of about 8000 bugs and rearranging the landscape of the mountain. It was a terrible loss, but following that devastating event, the town invested in considerable infrastructure work, building and maintaining a real path up the mountain, which made our work a bit easier, and a true park area at the base of the mountain. November makes up the rest of the fall, with temperatures ranging from 10 to 18 °C; it is another gray, chilly month.
Thus, the life history of P. japonensis has adapted to frequent major flooding; extended periods of intense heat and no rain, though it is very humid then; and frequent severe damage to the habitat when typhoons occur.
1.8 Local Field Site at Mt. Hinokuma Park
The field site features several inherent dangers that include giant hornets (because of recent introduction , now known in North America as murder hornets) and pit vipers, so it was a rule that no one was allowed to go to the field site alone. Alas, L.F. found that rule hard to abide by and in fact never enjoyed field visits so much as when alone. There is a different level of attention one pays to one’s surroundings when alone, free of any conversation and distraction. On one such solitary visit, L.F. noticed something peculiar. We had noted that most of the nests made by females were not within the area where drupes from the host tree were available. Moreover, they were not randomly scattered about but clustered in one particular area (see Fig. 4.1; Tsukamoto and Tojo 1992). This seemed counterintuitive. Why wouldn’t the females nest in the middle of the fallen drupe area, where investment in foraging would be at a minimum and independent older nymphs could more easily access food on their own? In the quiet of the forest, L.F. looked around the area for clues. While there was leaf litter throughout the site, it was admittedly sparse under the host tree. That was a clue. However, it did not explain the clustering of nests in one place, while leaf litter was scattered throughout much of the site. It was immediately apparent that most nests were confined to the area of the field site that was closest to the host tree but also were still within the area covered by the crown of a tall Ilex chinensis tree. It suddenly made sense. That tree had leaves on it in March, dead, brown leaves, but they were still attached to the tree. The leaves fell in early spring and so were not as degraded as the leaf litter from most of the deciduous trees that had dropped to the ground in the fall. This tree and other deciduous trees that drop their leaves in the spring apparently represent an important feature of the local habitat because of the high-quality, airy leaf litter they provide during the nesting season.
Survey site at Mt. Hinokuma Park. Small and large arrows represent host trees of 50–69 cm and 70–129 cm circumference, respectively. Only trees with a circumference larger than 50 cm are shown (91/342 trees). Small and large arrows represent subpopulations of ≤500 or 500–10,000 individuals, respectively. The size of the survey site was approximately 10 ha. Adapted from Filippi-Tsukamoto et al. (1995b)
1.9 Local Habitat Required to Support a Subpopulation of P. japonensis
We were also aware that, despite there being many S. jasminodora trees scattered throughout the forested mountain, many of which produced abundant drupes, we did not find a subpopulation of P. japonensis associated with every host tree . What was it about the locations of these seemingly fine host trees that was not conducive to supporting a subpopulation of bugs? To answer this question, and to assess the number and size of subpopulations inhabiting the area, we undertook an exhaustive study from April 4 to 29 of 1994 to identify all subpopulations at the larger, more inclusive, field site (Fig. 1.4) and clarify the ecological conditions that favored establishment of a subpopulation of P. japonensis. We surveyed and mapped a roughly rectangular area that comprised approximately ten hectares and included two small ponds. The survey was done during the flowering season of the host tree. We assumed that, as a minimum requirement, only those trees that were mature enough to flower could produce drupes and therefore support a subpopulation of bugs, so trees that were too young to be flowering were excluded from the survey. We measured the circumference of each tree at its base, noted the presence or absence of any subpopulations associated with each tree, and counted or estimated, when very large, the number of individuals in each subpopulation (Fig. 1.4; Filippi-Tsukamoto et al. 1995a).
Size distribution of host trees at Hinokuma. HT host tree ; SP subpopulation. Filippi-Tsukamoto et al. (1995b)
We also assessed in a 10 meter radius around each host tree the quality of six habitat attributes we considered relevant to the life history of P. japonensis. The attributes assessed were sunlight on the host tree, degree of slope , quality of surrounding bush for aggregating, intensity of sunlight on that surrounding bush, quantity of leaf litter , and quality of leaf litter.
Finally, to assess the correlation between tree size and abundance of drupes produced, we set a triangular seed trap (43 cm2) made of black netting and supported with three garden poles under three trees for each of three different size categories: small (30–40 cm circumference), medium (45–55 cm circumference), and large (70–95 cm circumference). The bottom of the nets of the nine seed traps were set about 50 cm from the ground, midway between the trunk and the edge of the crown. Drupes were collected weekly from May 20 to September 21, 1994 and counted.
1.10 Distribution of Host Trees and P. japonensis and Abundance of Drupes
As indicated in the map (Fig. 1.4; Filippi-Tsukamoto et al. 1995a), the survey site , and indeed all of Hinokuma Mountain, is mostly sloping terrain, gently sloping in places, but steep in others. In addition to the two ponds indicated in the map, several small streams also flowed through the site. Most of Hinokuma Mountain was a fairly early stage secondary forest at the start of our studies, but even early on, the survey site included some older areas, as well. Earlier stage areas were characterized by having denser understory growth, while later stage areas had taller deciduous trees that formed denser canopies, less sunlight , and less understory growth. In the approximately 10-hectare site surveyed, flowering host trees ranged from 15 to 129 cm in diameter, and we identified 342 S. jasminodora trees of flowering maturity. About 60% (207) of the host trees were associated with a subpopulation of P. japonensis (Fig. 1.5; Filippi-Tsukamoto et al. 1995b), and no subpopulation occurred where the largest host tree was smaller than 30 cm diameter; thus we concluded that 30 cm was the minimum size necessary to sustain even a small subpopulation. Nearly 40% of all host trees had a diameter smaller than 30 cm. On average, 55% of trees with a diameter smaller than 50 cm were associated with a subpopulation of P. japonensis, while an average of 72% of trees larger than 50 cm were associated with a subpopulation. Consistent with this finding, large trees produced nearly 4 times as many drupes (350) as medium trees (84) and over 18 times as many drupes as small trees (19) and were clearly able to support more bugs. Host trees tended to occur in clusters, typically, but not always, with one older tree, likely the parent, and several younger ones nearby. We identified 59 subpopulations of P. japonensis, and at least one host tree larger than 30 cm in diameter was invariably located within 10 m of each subpopulation. The subpopulations ranged in size from just 15 individuals to about 10,000, but the vast majority of subpopulations contained between 100 and 500 individuals (Fig. 1.6; Filippi-Tsukamoto et al. 1995b). Not surprisingly, subpopulation size was positively correlated with the cumulative diameter of the flowering host trees within 10 m of the subpopulation, and that correlation was particularly strong with subpopulations greater than 1000 individuals (Fig. 1.7; Filippi-Tsukamoto et al. 1995b).
Distribution of P. japonensis subpopulation size at Hinokuma. Adapted from Filippi-Tsukamoto et al. (1995b)
Correlation between cumulative host tree diameter and P. japonensis subpopulation size. P < 0.01. Adapted from Filippi-Tsukamoto et al. (1995b)
1.11 Non-food Resource Qualities Essential to the Microhabitat
Clearly, access to the single food resource is of utmost importance in determining whether P. japonensis will be found in a given area, but when food is available, what other features of the microhabitat are critical? We assigned a value of 1–5 for each parameter assessed based on its suitability to the bug’s life history : 1 was poor, 2 was fair, 3 was adequate/good, 4 was very good, and 5 was excellent. Light intensity on the host tree was considered important because the host tree is a pioneer species and is adapted to flourish, in other words produce more drupes, in direct sunlight . It was considered that a fair degree of slope in the nesting area would be important to keep the nests from becoming flooded during the rainy season . Surrounding vegetation, referred to as bush, comprised of small broadleaf evergreen trees, shrubs, and plants such as fern is essential to support aggregations because P. japonensis does not enter hibernation until mid-December, well after the deciduous trees have dropped their leaves. The intensity of light on the aggregation bush should be extremely low because the bugs only aggregate in well-shaded and dark locations. As with other insects, this preference for darkness is likely related to conservation of moisture . The quantity of leaf litter should be fairly dense because females nest under the leaf litter. Finally, the quality of leaf litter was considered high if it was primarily composed of the freshly fallen leaf litter that females apparently prefer for nesting.
The biggest contradictory feature of the bug’s habitat is the need of the host tree to have access to direct sunlight , but for the bugs’ aggregations to have good shade. The host tree, being a pioneer species, apparently emerges as one of the first trees in the secondary forest. It grows slowly but flourishes in the open areas exposed to full sunlight, flowering densely and producing abundant drupes. However, S. jasminodora never grows very tall, reaching heights of only about 30 feet, and other deciduous trees, Quercus, Ilex, Viburnum, Rhus, and Laurus, gradually overtake it. These trees eventually form a tall and thick canopy over S. jasminodora, blocking much of S. jasminodora’s access to sunlight, and we have noted a marked decrease in drupe production of host trees cast in the deep shade of older parts of the forest. The main pollinator of S. jasminodora at Mt. Hinokuma is the honeybee, which relies on sunlight to detect the flowers it collects pollen from. We suspect that the darkened habitat interferes with the honeybee’s ability to find the flowers, thus leading to reduced pollination and drupe production. At any rate, it remains a conundrum that the only food source P. jasminodora utilizes requires a condition that is in direct conflict with a key environmental criterion of the bugs, deep shade. Reflecting this conundrum, we found that the optimal level of sunlight on the host tree for predicting whether a subpopulation would be found was a compromise, the middle level, which was apparently bright enough for the tree to produce an adequate supply of drupes, yet not too bright for the bugs (Fig. 1.8; Filippi-Tsukamoto et al. 1995b). Thirty three percent more of the host trees in this category were associated with subpopulations, than those without subpopulations, but for light intensities greater or less, the numbers with and without subpopulations were identical.
Assessment of six relevant environmental parameters around host trees with and without subpopulations of P. japonensis. Assessments were determined based on the condition of the parameter that was deemed to be most appropriate for the life history of P. japonensis using a scale of 1–5, where 1 was the poorest condition and 5 was the best condition. Adapted from Filippi-Tsukamoto et al. (1995b)
For each of the other five parameters assessed, more subpopulations were invariably associated with conditions that were assessed as 3 (good/adequate) and above. Of course, it would be rare to find excellent quality for all parameters at any given locale, and the data indicate that sites with a minimum requirement of good/adequate or better for each of the criteria were much more likely to be associated with a subpopulation of P. japonensis than those with parameters of lower quality.
1.12 Do P. japonensis Discriminate Habitat Based on Collective Optimality of Essential Environmental Features?
We were interested in understanding whether P. japonensis only chose habitats where all of the criteria were optimal for their life history . We considered an optimal habitat to be one that was assessed as level three or above for all attributes except light intensity on the host tree . Light intensity on the host tree was not included in this because that attribute showed no difference between presence and absence of a subpopulation (Fig. 1.8), likely because most trees were inhabiting the optimal area to begin with. In fact, a significant proportion of P. japonensis subpopulations (60%) were associated with host trees found in a habitat where all of the other five environmental parameters met optimal levels, and a significant portion of host trees with optimal conditions (69%) were associated with a subpopulation (Table 1.2; \( \mathcal{X} \) 2 = 11.72, p = 0.0006).
1.12.1 Most Determinant Environmental Features
Finally, we were curious to know which of the environmental parameters had the greatest impact on determining subpopulation size. Because we anticipated that total resource available to a given subpopulation would be an important determinant, for this assessment, we added a seventh environmental factor , cumulative host tree size at a given subpopulation, and carried out a multivariate regression analysis. Subpopulation size and cumulative host tree size were log transformed for normalization. The stepwise method (Table 1.3) revealed that Cumulative Host Tree size (CumHT), Leaf litter Quality (LQlt), and, surprisingly, Slope (Slp) were the three variables that best explained the variation in subpopulation size (SP size) using this multiple regression model:
Food resource availability, as represented by cumulative host tree size, was, predictably, determined to be the single most important of the three variables in determining subpopulation size. The multiple correlation coefficient of the model was significant (p = 0.0006), and the model accounted for 27% of the total variance in subpopulation size. Slope was likely important to prevent flooding of the nest.
1.13 Phenology of the Host Tree and Instability of the Resource
Like P. japonensis, Schoepfia jasminodora Sieb. & Zucc. (Schoepfiaceae ), or gray twig, is the single-genus, single-species representative of its family, Schoepfiaceae, in Japan . It is a flowering deciduous tree of medium height, bearing fragile branches and very thin and delicate leaves (Fig. 1.9a). In fact, the thinner branches are so fragile that they break off under minimal pressure, and new growth continually pops out where the branches have broken off giving the tree a very disorderly appearance. For this reason, the common name for S. jasminodora in Japan is “tattered tree .” The leaves are a very lovely Kelly green, and the branches and leaves become quite dense. At the field site in Saga , Japan, S. jasminodora leaves emerge in early April, and flowers begin to bloom in mid-April. The delicate, clustered, bell-shaped flowers, 5–7 mm in length, are cream to pale yellow, sometimes with pale green borders (Fig. 1.9b). In early May, tiny green drupes begin to form on the tree. The oval drupes mature in a staggered fashion over the course of about a month as the color changes from green and yellow to pink, then deep red, and finally purple, reaching a final length of about 5–9 mm (Fig. 1.9c). Probably owing to the fragile nature of the tree, many drupes fall to the ground before they are ripe; color does not necessarily coincide with the degree of endosperm development , and drupes of any color might have a well-developed endosperm or very little endosperm, the part of the drupe that P. japonensis feeds on. Drupes fall to the ground for about 1 month, coinciding with the early reproductive phase of the bug (Tachikawa and Schaefer 1985). The delicate leaves begin to turn brown in late August, well before other deciduous trees, and fall to the ground in September/October. Because the leaves are so thin , they deteriorate very quickly once they fall from the tree.
1.13.1 Variation in Drupe Quality
The habitat study was done fairly early on in our research, but considering the decline in population size that has happened at the original site where early studies took place at the later succession stage Koura Mountain in Kurume City, Fukuoka (LF personal observation), and what we have seen over the years since this study began, we have come to understand a good bit more about habitat suitability for P. japonensis. Firstly, the host tree clearly becomes a poorer producer of the valued drupes as the canopy around it grows denser, blocking the sunlight . An otherwise healthy and large tree will produce relatively few drupes under such conditions, so a once excellent site with abundant drupes and a large subpopulation of P. japonensis will gradually become poor, and the subpopulation will disperse and/or die off. Secondly, we quickly came to realize that the resource is not only ephemeral but the abundance of drupes produced and the proportion of drupes with adequate/good endosperm fluctuate dramatically from year to year for a given host tree, even when the tree is located in a suitably sunny site. Over 4 years, three of them consecutive (1995, 1997, 1998, and 1999), the total number of drupes and the proportion of adequate drupes collected in seed traps varied considerably (36, 26, 14, 167 adequate drupes, respectively; Fig. 1.10; Filippi et al. 2002). In fact, over 10 years of observations , only 1 year had a high proportion of adequate drupes (Filippi et al. 2002). Thus, the availability of the food resource is generally poor, ranging from typically scarce to rarely abundant, and, as such, constitutes an unpredictable and scarce resource.
Total yearly number of drupes collected in seed traps over 4 years. Good drupes have >80% endosperm. Adapted from Filippi et al. (2002)
1.14 Toxic Drupes and Toxic Bugs
On a positive note, despite the scarce, ephemeral nature of their food resource , the bugs have little competition for the endosperm of the precious drupes that they are wholly dependent on because they are toxic . We had long suspected that the very delicious looking deep red drupes were toxic because we never saw any birds or other vertebrates eating them. One very enthusiastic graduate student, and a coauthor of this book, Mantaro Hironaka, keen to verify whether the drupes were, indeed, toxic decided to taste one. He immediately spit it out and described the taste as nasty and strongly astringent. To our great surprise, and well above and beyond the call of duty, he then very bravely put a bug in his mouth and chewed it! The bug was spat out as quickly as the drupe and apparently had the same nasty, astringent taste. A rather primitive and possibly dangerous experiment, but the results were clear. The bug tastes nasty, probably from the toxins in the drupe that it feeds on, and the red and black color warns all would-be color-sighted visual predators that they attack the bug at their own peril.
In 2004, Professor Tojo decided a more scientific approach to the matter of plant and insect toxins was in order. He contacted a colleague at Kyoto University, Professor Ritsuo Nishida, who specializes in insect toxins. The findings of the studies supported Mantaro’s anecdotal bioassay. Schoepfia jasminodora drupes contain a large quantity of toxic lipids that the plant no doubt uses for defense. Acetylenic triglycerides such as 9, 11, 13-octadecatriynoic acid and 9, 11-octadecadiynoic acid occur in the drupes along with other typical fatty acids. When orally administered to mice, synthetic esters of the fatty acids were lethal (R. Nishida, unpublished data). The bug sequesters these triglycerides, making it a very unpalatable prey, which explains the beautiful aposematic coloration of P. japonensis (Kamata et al., 2004, 2005). When hungry quails were given the opportunity to feed on the bugs and nothing else, they ate them reluctantly but always vomited afterwards. When given a choice between P. japonensis and the southern green stink bug, Nezara viridula , which is certainly an unappetizing prey item in its own right, the quails always chose the southern green stink bug (S.N., unpublished data). In thousands of hours of observations in the field, with the exception of one unlucky frog who leaped in and stole a bug we had just marked with nail polish, we have never seen birds or any other vertebrate actively feeding on P. japonensis. Ants eat the legs and wings of adults but leave the toxin-laden body behind. However, to our great dismay, they eagerly and voraciously feed on eggs and early instar nymphs , which frustratingly tended to wreak havoc on our sample sizes. Ground beetles (Carabidae) are also major egg and nymph predators in the nesting area. We once saw a large unidentified centipede feeding on a female bug and one male hanging fly actually presenting a female P. japonensis to its mate as a nuptial gift (L.F., unpublished finding). We did not wait to see if the toxic gift proved to be her last meal. These invertebrate predators might not benefit from the aposematic coloration, but vertebrate predators surely would.
Thus, P. japonensis uses the toxic triglycerides as both an energy source and a defense mechanism (Kamata et al. 2004, 2005). Clearly, one of the very useful evolutionary innovations that has emerged in P. japonensis is the ability to process and utilize these toxins effectively, enabling the bugs to acquire a scarce but rich resource free of competition . Other herbivores such as moth larvae will eat the outer fleshy part of the drupe, but, to the best of our knowledge, the drupes are apparently unappetizing to every other organism, save P. japonensis, who has managed to make the best of a rather poor situation.
1.15 Life Cycle of Parastrachia japonensis
The life cycle of P. japonensis takes either 1 or 2 years to complete (Fig. 1.11). After adults emerge from their subterranean hibernation in early March, they reform aggregations on broadleaf evergreens , such as Eurya japonica and ferns. At this point, the reproductive organs of both males and females are undeveloped (Tsukamoto 1991; Tsukamoto et al. 1994). We discovered early on that only a portion of the bugs in the aggregations become reproductively active during the first post-hibernation spring; the rest, comprising 5–95% of the subpopulation, remarkably, remain in reproductive diapause until the following spring, when they mate (Tsukamoto et al. 1994). Mating behavior is described in detail in Chap. 3. Briefly, the male testes mature over about 5 weeks and contain abundant sperm by mid-April. Female ovaries develop minimally over the same period. Males become active before females and fly about the aggregations seeking a mate. Females do not fly during mating , apparently conserving their energy for the herculean task of provisioning later on. Males mate with many females over a 2-week period, burning up their remaining fat stores, and then die without ever having fed since they were fifth instars the previous summer (Tsukamoto et al. 1994). Inseminated females relocate to a host tree where they feed on a succession of as yet fairly small and immature drupes to develop their eggs (Gyôtoku and Tachikawa 1980; Tachikawa and Schaefer 1985; Tsukamoto and Tojo 1992; Tsukamoto et al. 1994; Filippi-Tsukamoto et al. 1995a; Filippi et al. 2001). Eggs are deposited in early June, after which mothers guard the egg mass until hatching. After hatch , females split their time between guarding and provisioning nymphs throughout June, and then they begin to die off. Nymphs become independent from late June to mid-July. They go through five molts before adults eclose from late July to mid-August. The new adults join the aggregations of adults that remained in reproductive diapause . For the remainder of the summer, the estivating aggregations move down into crevasses around the tree roots on particularly hot and dry days and back up the aggregation trees/vegetation again on less stressful days. No more feeding occurs, though they do take water from the veins of leaves. The bugs remain aggregated until December, when they burrow in holes in the ground that were made by other animals such as snakes and moles. They remain hibernating in aggregations until late February, early March when they emerge, and the cycle begins again.
Life cycle of Parastrachia japonensis. Adapted from Filippi et al. (2001)
References
Agrawal, A., E.D. Brodie, and J. Brown. 2001. Parent-offspring coadaptation and the dual genetic control of maternal care. Science 292: 1710–1712.
Agrawal, A., N. Combs, and E.D. Brodie III. 2005. Insights into the costs of complex maternal care behavior in the burrower bug (Sehirus cinctus). Behavioral Ecology and Sociobiology 57: 566–574.
Cervantes, L., C. Mayorga, and M. Lopez Ortega. 2013. Description of immature stages of Melanaethus crenatus (Hemiptera: Heteroptera: Cydnidae: Cydninae: Geotomini) with notes on oviposition, seed-carrying and feeding behaviors. Florida Entomologist 96: 1434–1441.
Distant, W.L. 1908. Rhynchota, vol. IV. Fauna of British India, including Ceylon and Burma. London: Taylor and Francis.
Esaki, T. 1930a. On the taxonomic position and habits of the unusual stink bug, Parastrachia japonensis (Scott). Journal of Animal Science 42 (503): 334–335. (In Japanese).
———. 1930b. On the systematic position of the pentatomid genera Parastrachia Distant, and Eumenotes Westwood. Annals and Magazine of Natural History 10 (5): 627–631.
Filippi-Tsukamoto, L., S. Nomakuchi, K. Kuki, and S. Tojo. 1995a. Adaptiveness of parental care in Parastrachia japonensis (Hemiptera: Cydnidae). Annals of the Entomological Society of America 88: 374–383.
Filippi-Tsukamoto, L., S. Nomakuchi, and S. Tojo. 1995b. Habitat selection, distribution, and abundance of Parastrachia japonensis (Hemiptera: Cydnidae) and its host tree. Annals of the Entomological Society of America 88: 456–464.
Filippi, L., M. Hironaka, and S. Nomakuchi. 2001. Review of the ecological parameters and implications of subsociality in a shield bug, Parastrachia japonensis (Hemiptera: Cydnidae), a semelparous species that feeds on a poor resource. Population Ecology 43: 41–50.
———. 2002. Risk-sensitive decisions during nesting may increase maternal provisioning capacity in the subsocial shield bug, Parastrachia japonensis. Ecological Entomology 27: 152–162.
Filippi, L., N. Baba, I. Kyoichi, M. Hironaka, and S. Nomakuchi. 2009. Pre- and post-hatch trophic egg production in the subsocial burrower. Naturwissenschaften 96: 201–211.
Grazia, J., R.T. Schuh, and W.C. Wheeler. 2008. Phylogenetic relationships of family groups in Pentatomoidea based on morphology and DNA sequences (Insecta: Heteroptera). Cladistics 24: 932–976. https://doi.org/10.1111/j.1096-0031.2008.00224.x.
Gyôtoku, N., and S. Tachikawa. 1980. Life history of Parastrachia japonensis (Scott) (Cydnidae: Sehirinae) Rostria, 33: 359–368. (In Japanese).
Hiura, I. 1952. On the stinkbug Parastrachia japonensis. Nymph 1: 1–7. (in Japanese).
———. 1977. Aggregation in Heteroptera. Iden. 31: 29–36. (In Japanese).
Inadomi, K., M. Wakiyama, M. Hironaka, H. Mukai, L. Filippi, and S. Nomakuchi. 2014. Postovipositional maternal care in the burrower bug, Adomerus rotundus (Hemiptera: Cydnidae). The Canadian Entomologist 146: 211–218. https://doi.org/10.4039/tce.2013.69.
Japan Meteorological Agency. n.d.. https://www.data.jma.go.jp/gmd/cpd/longfcst/en/tourist/file/Northern_Kyushu.html. Accessed 3 April 2020.
Kamata, A., Y. Takeuchi, R. Nishida, Y. Kuwabara, M. Hironaka, and S. Tojo. 2004. Host specific triglycerides contained in the body of Parastrachia japonensis. 48th Annual conference of the Japanese Society of Applied Entomology and Zoology.
———. 2005. Sequestration of acetylenic triglycerides from host drupes in an aposematic stink bug, Parastrachia japonensis. The 3rd Asia-Pacific Conference on Chemical Ecology.
Kight, S.L. 1997. Factors influencing maternal behaviour in a burrower bug, Sehirus cinctus (Heteroptera: Cydnidae). Animal Behaviour 53: 105–115.
Kolliker, M., J.P. Chuckalovcak, K.F. Haynes, and E.D. Brodie. 2006. Maternal food provisioning in relation to condition-dependent offspring odours in burrower bugs (Sehirus cinctus). Proceedings of the Royal Society of London B 273: 1523–1528.
Kudo, S., and T. Nakahira. 2005. Trophic-egg production in a sub-social bug: Adaptive plasticity in response to resource conditions. Oikos 111: 459–464. https://doi.org/10.1111/j.1600-0706.2005.14173.x.
Lis, J.A. 2010. Pretarsal structures in the family Parastrachiidae (Hemiptera: Heteroptera: Pentatomoidea). Zootaxa 2693: 60–62.
Lis, J.A., D.J. Ziaja, B. Lis, and P. Gradowska. 2017. Non-monophyly of the “cydnoid” complex within Pentatomoidea (Hemiptera: Heteroptera) revealed by Bayesian phylogenetic analysis of nuclear rDNA sequences. Arthropod Systematics and Phylogeny 73: 481–496.
Miyamoto, S. 1954. Food habits of the stink bug, Parastrachia japonensis. Pulex 3: 10. (In Japanese).
———. 1956. Discovery of the eggs of the red stink bug. Konchu 24: 232. (In Japanese).
———. 1965. The red stinkbug. Primary Color Insect Great Encyclopedia, III. Hokuryukan, Tokyo. (in Japanese).
———. 1966. The red stink bug. Nature of Kitakyushu. Osaka, Japan: Rokugatsusha. (In Japanese).
Mukai, H., M. Hironaka, N. Baba, T. Yanagi, K. Inadomi, L. Filippi, and S. Nomakuchi. 2010. Maternal- care behaviour in Adomerus variegatus (Hemiptera: Cydnidae). The Canadian Entomologist 142: 52–56.
Mukai, H., M. Hironaka, S. Tojo, and S. Nomakuchi. 2012. Maternal vibration induces synchronous hatching in a subsocial burrower bug. Animal Behaviour 84: 1443–1448.
Nakahira, T. 1992. Reproductive history and parental behaviour in the cydnid bug Adomerus triguttulus. M.Sc. thesis, Department of Agriculture, Hokkaido University, Sapporo, Japan.
———. 1994. Production of trophic eggs in the subsocial burrower bug, Adomerus triguttulus. Naturwissenschaften 81: 413–414.
Nakahira, T., and S. Kudo. 2008. Maternal care in the burrower bug, Adomerus triguttulus: Defensive behavior. Journal of Insect Behavior 21: 306–316. https://doi.org/10.1007/s10905-008-9129-0.
Nakahira, T., K.D. Tanaka, and S. Kudo. 2013. Maternal provisioning and possible joint breeding in the burrower bug, Adomerus triguttulus (Heteroptera: Cydnidae). Entomological Science 16: 151–161.
Nakao, S. 1956. The winter life of stink bugs. New Insects 9 (2): 7–10.
Pluot-Sigwalt, D., and J.A. Lis. 2008. Morphology of the spermatheca in the Cydnidae (Hemiptera: Heteroptera): Bearing of its diversity on classification and phylogeny. European Journal of Entomology 105: 279–312.
———. 2018. Presence of uradenia in male adults of the genus Dismegistus (Hemiptera: Heteroptera: Parastrachiidae). Acta Entomologica 58: 187–193.
Schaefer, C.W., W.R. Dolling, and S. Tachikawa. 1988. The shieldbug genus Parastrachia and its position within the Pentatomoidea (Insecta: Hemiptera). Zoological Journal of the Linnean Society 93: 283–311.
Schaefer, C.W., L.Y. Zheng, and S. Tachikawa. 1991. A review of Parastrachia (Hemiptera: Cydnidae: Parastrachiinae). Oriental Insects 25: 131–144.
Schaefer, C.W., and Y. Kikuhara. 2007. Parastrachia nagaensis (Distant) (Hemiptera: Parastrachiidae) from Laos. Oriental Insects 41: 459–462.
Scott, I. 1880. On a collection of Hemiptera from Japan. Transactions of the Entomological Society of London 1880 (4): 305–317.
Sites, R.W., and J.E. McPherson. 1982. Life history and laboratory rearing of Sehirus cinctus (Hemiptera: Cydnidae), with descriptions of immature stages. Annals of the Entomological Society of America 75: 210–215.
Sweet, M.H., and C.W. Schaefer. 2002. Parastrachiinae (Hemiptera: Cydnidae) raised to family level. Annals of the Entomological Society of America 95: 441–448.
Tachikawa, S., and C.W. Schaefer. 1985. The biology of Parastrachia japonensis (Hemiptera: Pentatomoidea: ?-idae). Annals of the Entomological Society of America. 78 (3): 387–397. https://doi.org/10.1093/aesa/78.3.387.
Tachikawa, S. 1980. Parental care of Pentatomidae (Heteroptera) in Japan. 16th International Congress of Entomology, Kyoto (abstract 436).
———. 1984. Life history of a monophagous fruit-bug, Parastrachia japonensis (Cydnidae: Sehirinae). 17th International Congress of Entomology (Hamburg). Abstract 312.
———. 1991. Subsocial terrestrial heteropterans of Japan: Parent-offspring relationships in stink bugs. Tokyo, Japan: Tokyo Agricultural University Press.
Tachikawa, S., and N. Gyotoku. 1981. The habitat and distribution of Parastrachia japonensis. The 41st Annual Meeting of the Japan Entomological Society. Abstract 59.
Tsukamoto, L. 1991. Life history and reproductive ecology of a stink bug, Parastrachia japonensis Scott (Hemiptera: Cydnidae). Master’s thesis, Saga University, Saga, Japan.
Tsukamoto, L., and S. Tojo. 1992. A report of progressive provisioning in a stink bug, Parastrachia japonensis (Hemiptera: Cydnidae). Journal of Ethology 10: 21–29.
Tsukamoto, L., K. Kuki, and S. Tojo. 1994. Mating tactics and constraints in the gregarious shield bug, Parastrachia japonensis (Hemiptera: Cydnidae). Annals of the Entomological Society of America. 87: 962–971.
Yang, W. 1934. Notes on Chinese Asopinae (Heteroptera, Pentatomidae) Sinensia (Academica Sinica) 5: 92–120.
Zhu, G., G. Liu, W. Bu, and J.A. Lis. 2013. Geographic distribution and niche divergence of two stinkbugs, Parastrachia japonensis and Parastrachia nagaensis. Journal of Insect Science 13: 102. https://doi.org/10.1673/031.013.10201.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2022 Springer Nature Singapore Pte Ltd.
About this chapter
Cite this chapter
Tachikawa, S., Nomakuchi, S., Filippi, L. (2022). General Biology of Parastrachia japonensis. In: Filippi, L., Nomakuchi, S. (eds) The Life History of the Parental Shield Bug, Parastrachia japonensis. Entomology Monographs. Springer, Singapore. https://doi.org/10.1007/978-981-19-3018-8_1
Download citation
DOI: https://doi.org/10.1007/978-981-19-3018-8_1
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-19-3017-1
Online ISBN: 978-981-19-3018-8
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)