Abstract
Decompressive Craniectomy (DC) surgery is recommended to treat patients who suffered from large ischaemic cerebral infarction. Although DC surgery has been proven to reduce intracranial pressure (ICP) within the skull, too large of DC opening may contribute to risk of tissue injury. Computational studies nowadays are very useful in predicting and decision making, especially in clinical studies. Therefore, a simulation was performed using mathematical modeling on an idealized 3D brain model to evaluate the outcome of different skull opening sizes in DC towards treating the brain tissue swelling in ischaemic stroke. The model is simulated based on poroelastic theory and capillary filtration. Our results show that larger craniectomy size may reduce the midline shift of the ventricle due to swelling tissue. Nevertheless, the bulging of swollen tissue out from the skull opening causes a little amount of stress applied at the edges of the opening. This modelling work may be used for further research in further research in evaluating the suitable craniectomy size for DC.
Access provided by Autonomous University of Puebla. Download conference paper PDF
Similar content being viewed by others
Keywords
1 Introduction
Large hemispheric infarction may contribute to the elevation of intracranial pressure (ICP) which can result in brain herniation. The failure of medical treatment to relieve the increasing of ICP in brain has led to the seeking of alternative treatments. Therefore, decompressive craniectomy (DC) surgery may be an effective treatments in treating patients with large brain infarction after ischaemic stroke by creating an additional space to allow for the swelling tissue to extend outward from the skull by removing a portion of bone flap of the skull [1].
In this surgery, the skull opening size is one of the important parameters before the removal process. Large skull opening has always been recommended to reduce the brain swelling. However, too large bone flap removed causes higher risk for complications to occur after the surgery such as infection and hydrocephalus [2]. The minimum diameter of 12 cm for skull opening has been extensively used to minimize ICP level in the brain [3]. The results shown in this article is that 57% of patients who undergo the surgery with skull opening less than 12 cm are found dead after the treatment due to haematoma evacuation and decompression [4].
Computational studies nowadays have been proven to be useful in predicting the clinical outcome and decision making for clinical practice [5]. Hence, in this article, we will evaluate the effect of different sizes of skull opening in minimizing the outcome for swelling of brain tissue due to prolonged ischaemic period by developing the dealized model of 3D brain and application of mathematical modeling based on theory of poroelastic and filtration of capillary previously developed in [6]. Four different of skull opening sizes are used to evaluate their outcome in DC surgery for brain tissue swelling in ischaemic stroke and its complications. This mathematical modeling concept hopefully can be used as prediction strategy into the application of DC for the treatment of ischaemic stroke.
2 Methodology
2.1 Mathematical Model
The swelling of brain tissue formed due to prolonged ischaemic period has been developed by applying filtration of capillary model and equation of poroelastic [6]. The tissue of brain is assumed as an isotropic poroelastic material, which contains of a solid tissue matrix permeated by interstitial fluid and the process of swelling takes place with little amount of strain. The poroelastic model has following equation:
where the term \(\sigma_{ij}\) is the tissue stress total, \(P_{{\text{w}}}\) is the pressure of interstitial water, \(\alpha_{w}\) is the Biot parameter of water. This equation defined relationship between the stress of brain tissue and ICP. The equation of interstitial fluid pressure distribution of is as follow:
where \(Q_{w}\) known as the relative compressibility of water, t is the time while \(k_{w}\) is water permeability in porous tissue. Meanwhile, net flow of water into the tissue of brain from space of capillary due to capillary filtration is defined as term of \(S_{{{\text{b}} \to {\text{w }}}}\). the filtration of capillary is assumed to take place after ischaemic stroke, in which cause by break down of a specialized layer called blood brain barrier (BBB). As a result, particles such as tiny proteins and ions to travel across this layer and accumulate in the extracellular space of brain tissue. The accumulations may cause the water to enter the extracellular space of brain tissue from reperfused blood, which cause swelling of tissue.
\(\sigma_{{{\text{ij}}}}\), total stress is linearly correlated to the strain, \(\varepsilon_{{{\text{ij}}}}\), as given by:
G is the shear modulus, meanwhile υ is the Poisson’s ratio of brain tissue and it has been shown that they play significant roles in the progression of brain swelling [7]. The strain and the displacement of tissue, \(u_{i}\), are then related, in which associated to the following relationship:
Meanwhile, \(S_{b \to w} { }\) term is given below:
where \(\overline{n}_{b}\) is blood fraction baseline volume, \(L_{p}\) is capillary hydraulic permeability, \(R_{c}\) is capillary radius baseline value, σ is the coefficient of reflection, \({\Pi }_{b}\) known as pressure of osmotic in the capillary meanwhile \(P_{b}\) is presumed as a constant, which known to be pressure of blood. Finally, f term indicates as capillaries ratio that maintain open after process of swelling and ischaemia–reperfusion at exact point in space and time.
2.2 Idealized 3D Brain Model
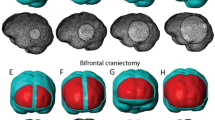
The idealized of brain geometry model, shown in Fig. 1, is developed based on spherical shape suggested by [8]. The brain model is developed by a half sphere with a hole of half spherical at the center, which indicating the lateral ventricles. Another sphere is constructed within the geometry of half spherical to indicate the infarct of ischaemic. Then, 3D brain model is layered by another half spherical with an opening to represent the skull geometry with DC surgery.
The radius of brain, ventricle and infarct are set to be 80 mm, 24 mm and 14 mm respectively. Meanwhile, to evaluate the opening skull size outcome on DC surgery, the opening skull sizes are varied of 10, 15, 25 and 30 mm.
2.3 Numerical Procedure
The idealized 3D brain model is simulated using finite element software named COMSOL Multiphysic 5.3a. In this simulation, the skull has Young’s modulus and Poisson’s ratio, described based on Table 1. Meanwhile, the tissue of brain has separate material properties, in which also listed in Table 1 [6].
Boundary Condition. To allow for the outcome of brain tissue swelling on the ventricle movement, the ventricle, \(R_{\nu }\), has been set to be moved freely by setting the total stress on the tissue, \(\sigma_{ij}\) on ventricle as follows:
Initial Conditions. Initially, the pressure of interstitial fluid, \(P_{w}\) and the displacement of tissue \(u\) are set at baseline \(\overline{P}\) and zero, as follow:
Mesh Element Size. 10-node tetrahedral elements are used to mesh all geometries. Meanwhile, number of elements are varied in within 16,000–19,000. The ischaemic infarct has finer meshes than rest of geometry.
3 Results and Discussion
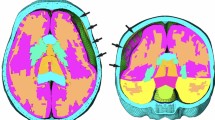
A simulation of an idealized 3D brain model was performed to evaluate the outcome of DC surgery using different size of skull opening in reducing the midline shift after 5 h of ischaemia–reperfusion. For a simple visualization, the simulation results displayed below based on part of brain that been sliced half through infarcts and skull opening, in which take part at the brain geometry centre.
Figure 2 shows the displacement of tissue of brain for infarct radius of 14 mm with different skull opening sizes. Midline shift that takes place in the middle of ventricle is also known as the brain herniation. In clinical study, brain herniation is used as indicator to predict the severity of brain tissue swelling in stroke [9, 10]. Based on Fig. 2, skull opening size of 30 mm experiences the least herniation compared to the skull opening of 10, 15 and 25 mm of skull opening size. This is because the skull opening causes the reduction of ventricle compression by allowing the brain tissue to swell towards the opening. Even though DC surgery has been proven to reduce the elevation of ICP [11] in brain as well as to reduce herniation, this surgery may also contribute some complications that still need to be considered.
Further, Fig. 3 shows that the skull opening size of 10 mm experience major stress in between the opening edges and brain tissue. This is because, small skull opening size increases the compression at the opening edges and also due to high brain tissue strain to bulge out from the skull. This may increase the risk of brain tissue damaged and infections [12].
Meanwhile, the largest skull opening size of 30 mm shows a much higher stress in between the edges of DC opening. Although the larger size of opening reduce the herniation, it may increase the risk of hydrocephalus development [1]. The more the swollen tissue bulged out from the skull, the higher the stress at the area of contact in between the edges of the opening and brain tissue [11]. Too large portion of skull being removed may result in an increase of rate of infections. The DC treatment for brain tissue swelling is still incomplete understood in reducing the severity of the swelling. Based on the findings obtained through this simulation, the larger craniectomy size may improve outcome, however it may also contribute to other complications such as risk of tissue damage, which is observed by the high tissue stress at the opening.
4 Conclusion
In conclusion, the mathematical model developed in this paper can assist the clinicians to obtain related surgical decision before performing the DC surgery on patients with brain tissue swelling due to ischaemic stroke. In our simulation the craniectomy model is developed to show how craniectomy size in DC may affect the brain herniation and complications that may occur after surgery as the treatment for swelling of brain tissue after ischaemia–reperfusion injury in stroke. Further investigation on the effect of ICP during DC surgery can be done to further understanding the efficacy of this treatment.
References
Kolias AG, Kirkpatrick PJ, Hutchinson PJ (2013) Decompressive craniectomy: past, present and future. Nat Publ Gr., no. Dc, pp 1–11
Weickenmeier J, Butler CAM, Young PG, Goriely A, Kuhl E (2017) The mechanics of decompressive craniectomy: personalized simulations. Comput Methods Appl Mech Eng 314:180–195
Wagner S, Schnippering H, Aschoff A, Koziol JA, Schwab S, Steiner T (2001) Suboptimum hemicraniectomy as a cause of additional cerebral lesions in patients with malignant infarction of the middle cerebral artery. J Neurosurg 94(5):693–696
Tagliaferri F et al (2012) Decompressive craniectomies, facts and fiction: a retrospective analysis of 526 cases. Acta Neurochir (Wien) 154(5):919–926
Fletcher TL, Wirthl B, Kolias AG, Adams H, Hutchinson PJ, Sutcliffe MP (2016) Modelling of brain deformation after decompressive craniectomy. Ann Bioemdical Eng 44(12):3495–3509
Mohamed Mokhtarudin MJ, Payne SJ (2015) Mathematical model of the effect of ischemia-reperfusion on brain capillary collapse and tissue swelling. Math Biosci 263:111–120
Mohamed Mokhtarudin MJ, Shabudin A, Payne SJ (2019) Effects of brain tissue mechanical and fluid transport properties during ischaemic brain oedema: a poroelastic finite element analysis. In: 2018 IEEE-EMBS Conference on Biomedical Engineering and Sciences (IECBES), pp 1–6
Hakim S, Venegas JG, Burton JD (1976) The physics of the cranial cavity, hydrocephalus and normal pressure hydrocephalus: mechanical interpretation and mathematical model. Surg Neurol 5(3):187–210
Steiner T, Ringleb P, Hacke W (2001) Treatment options for large hemispheric stroke. Neurology 57(5 SUPPL. 2):61–68
Ryu JH et al (2013) Induced and sustained hypernatremia for the prevention and treatment of cerebral Edema following brain injury. Neurocrit Care 19(2):222–231
Nadzri AN, Mokhtarudin MJ, Naim WN, Payne SJ. Simulation of decompressive craniectomy for ischaemic stroke treament: a conceptual modeling study. In: 2020 IEEE-EMBS Conference on Biomedical Engineering and Sciences (IECBES), pp 303–308
Von Holst H, Li X, Kleiven S (2012) Increased strain levels and water content in brain tissue after decompressive craniotomy. Acta Neurochir (Wien) 154(9):1583–1593
Acknowledgements
This research was supported by Fundamental Research Grant Scheme FRGS/1/2018/TK03/UMP/02/15 (RDU190132) and the Universiti Malaysia Pahang Postgraduate Research Grant Scheme (PGRS2003173). Wan Naimah Wan Ab Naim, is a recipient of the Universiti Malaysia Pahang Post-Doctoral Research Scheme.
Author information
Authors and Affiliations
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2022 The Author(s), under exclusive license to Springer Nature Singapore Pte Ltd.
About this paper
Cite this paper
Nadzri, A.N., Mohamed Mokhtarudin, M.J., Wan Ab Naim, W.N., Payne, S. (2022). Simulation of Craniectomy Size in Decompressive Craniectomy for Ischaemic Stroke. In: Abdul Sani, A.S., et al. Enabling Industry 4.0 through Advances in Manufacturing and Materials. Lecture Notes in Mechanical Engineering. Springer, Singapore. https://doi.org/10.1007/978-981-19-2890-1_56
Download citation
DOI: https://doi.org/10.1007/978-981-19-2890-1_56
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-19-2889-5
Online ISBN: 978-981-19-2890-1
eBook Packages: Chemistry and Materials ScienceChemistry and Material Science (R0)