Abstract
The cylindrical lithium-ion cells are being considered as one of the preferred energy storage systems in Electric Vehicles (EV). However, they have operational challenges involving temperature that greatly affects its life and performance. If the cell operating temperature exceeds the threshold limit, decomposition of the battery active material may occur which can trigger thermal runaway leading to the explosion in certain conditions. Therefore, a battery thermal model is essential to analyze the thermal response of the cell to design an efficient and effective battery thermal management system. This paper presents a simplified unsteady one-dimensional radial analytical thermal model to predict the temperature profile and heat generation of an isolated cylindrical cell under natural and forced convection. The model treats the cell as homogeneous body with uniform heat generation throughout the cell and the thermo-physical properties of the cell are assumed to be independent of temperature. The prediction of the model on the effect of forced convection cooling on the surface temperature of the cell for different heat transfer coefficient values is quite interesting. It is seen that surface temperature is under 30 °C for heat transfer co-efficient of 100 W/m2 °C. The core and surface temperature non-uniformity across the radius of the cell is nearly 2 °C for different state of charge (SOC).
Access provided by Autonomous University of Puebla. Download conference paper PDF
Similar content being viewed by others
Keywords
1 Introduction
The fast-depleting fossil fuel resources and the environmental concerns has motivated the need to explore environment friendly and viable alternative source of energy for transportation demand and other applications across the world. Also, the pollutants released from Internal Combustion (IC) engines working on fossil fuels is causing severe air pollution resulting in global warming and depletion of ozone layer. Kumar et al. [1] carried out a detail analysis of effects and causes of vehicular emissions and its impact on global climate change and environment. Thus, Electric Vehicles (EV) are being developed as one of the alternate modes of mobility to IC engine powered vehicles to reduce the vehicular emissions, greenhouse gases and safeguard the environment for future generations. Honda, one of the leading automobile manufacturers claims to electrify two thirds of its global automobile’s unit sales in 2030 with zero emission as EV does not have tailpipe emission or fuel evaporation [2]. The EV needs a portable source of energy to drive and power its powertrain. Thus, one of the requirements of EV is the Energy Storage System (ESS). The cost and performance of EV is dependent on the type of onboard ESS. ESS includes batteries, fuel cells and ultra-capacitors which supplies power to drive the EV. The secondary (rechargeable) batteries used to power EV come under the category of Electrochemical Storage Systems (EcSS) which is classified as another type of ESS. In EcSS energy is transformed from electrical to chemical and vice-versa through chemical reactions when connected with external electrical circuit. Rechargeable batteries store energy during charging phase and release energy during discharge phase [3, 4].
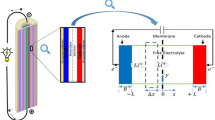
Lead-acid, Nickel-Cadmium, Nickel—Metal Hydride, Lithium-Ion, Lithium-Polymer are the different battery technologies available to meet the power requirements of EV. The choice of a battery technology for a given EV depends on number of factors viz. battery chemistry, stability, reliability, and its operating life, besides, performance of EV, range and cost. The different battery technologies and their specifications with potential applications are tabulated in Table 1. Lithium-ion batteries are preferred to power EV as it has high energy density, lower self-discharge rates, higher efficiency, and longer lifespan compared to other types of battery technologies [5]. In Li-ion batteries, the energy release and storage occur, when the lithium ions move from positive to negative electrode, back and forth via the electrolyte. Lithium cell, like general battery, consists of electrodes, electrolyte and separator as shown in Fig. 1. The lithium is initially present in positive electrode (anode) in the form of metal oxides, moves back and forth due to chemical reactions during charging and discharging cycles. Generally, lithiated metal oxides or phosphates are used as anode materials and graphite/silicon or lithiated titanium oxides materials as cathode. The Li-ion batteries can be of various types depending on the chemistry of materials used for electrodes and electrolytes. The performance of li-ion cells is influenced predominantly by different cell chemistries. Apart from the battery chemistries, li-ion batteries also come in various form factor such as prismatic cell, cylindrical, button and pouch cells. Prismatic and cylindrical li-ion cells are preferably used in current EV owing to ease of manufacturing, packaging, and maintenance. A few of the most common li-ion battery chemistry used in EV are listed in Table 1.
Lithium-ion battery chemistry: a Discharging and charging b Sectional view of cylindrical battery [6]
The performance of a lithium-ion battery is greatly influenced by operating temperature. The safe operating temperature of a typical li-ion cells/module/packs ranges from 15 °C to 35 °C [7]. Operating the battery outside the permissible range of temperature results in battery degradation, reduced life, low EV range and in extreme cases, can lead to thermal runaway and explosion of battery pack. Thermal modelling of li-ion batteries is essential in the design of battery pack to ensure it is operating in safe working limits. Li-ion cells generate heat due to complex chemical reactions occuring inside the cell during charge and discharge cycles. EV used for transportation necessitates batteries to be charged and discharged at very high C-rates which leads to enormous heat generation rate. The generated heat must be dissipated to provide safe working environment for the li-ion cells. Therefore, thermal modelling of li-ion cells is essential to get an insight into the heat generation of the cell at various C-rates which may provide inputs to model the cooling requirements of the battery in thermal management system.
Li-ion cells exhibit a large anisotropic behavior in thermal conductivity (k) of the cell. The in-plane thermal conductivity is substantial in magnitude than the through-plane conductivity resulting in increased thermal resistance in the through-plane direction. In cylindrical battery cells (rolled stack layers), thermal conductivity is higher in axial direction (10–50 W/mK) and very low in radial direction (0.3–0.5 W/mK). Thus, the heat dissipation in cylindrical cells along the radial direction is minimal resulting in increased cell temperature [8, 9]. In the present work, a simplified unsteady one-dimensional radial analytical thermal model is developed to predict temperature of the li-ion cell. The battery cell is considered as homogeneous body in the thermal model with uniform heat generation throughout the cell and the thermo-physical properties of the cell are assumed to be independent of temperature. This approach in modelling is adopted to reduce the complexity besides achieving satisfactory prediction (Table 2).
2 Thermal Modeling
2.1 Theoretical Background
Thermal models predict the temperature profile of the cells during charge and discharge cycles. Few of the thermal models reported in the literature are simplified lumped parameter model, pseudo electrochemical-thermal model, equivalent circuit model, one-dimensional (1D), two-dimensional (2D), three-dimensional (3D) complex numerical (FEA/CFD) models [11]. All these models require heat generation rates and thermo-physical properties of the cells as the input for modeling. A lumped parameter model considers entire cell at uniform temperature with negligible temperature gradient within the cell. The temperature prediction of the cell by the thermal model is sensitive to its complexity involving various heat generation parameters of the cell. The source of heat generation in a li-ion cell is represented by the following equation [12].
where \(\dot{Q}\) is the heat generated/consumed, I is the current, V voltage, U is equilibrium potential, T is temperature, \(\Delta H\) the variation of enthalpy of a chemical reaction i, \({r}_{i}\) the rate of reaction, \({\overline{H} }_{j}\) the partial molar enthalpy of species j, \({C}_{j}\) its concentration, t the time and v the volume. The term ‘avg’ represents properties being evaluated at volume averaged concentration. The first term on the right side of Eq. (1) is the heat generated due to joule heating (irreversible resistive dissipation heat) and is always positive. The second term is reversible entropic potential and can be positive or negative depending on whether the cell is charging or discharging. The third term denotes the heat of chemical reaction of species. The last term of equation represents the heat of mixing occurring due to change in species concentration gradients of the cell. As the contribution of third and fourth term is minimal compared to the first two terms, and can be ignored with minimal errors in the temperature profile [12].
Consequently, the equation (1) reduces to simplest form [13] and is expressed as follows.
where the term I (V-U) is heat generation due to joule heating and \(-IT\frac{\partial U}{\partial T}\) is the entropy change heat source term.
The irreversible heat generation due to joule heating can be expanded as
Where ‘R’ is the internal resistance of the cell.
And the reversible heat generation due to entropic coefficient is expressed as
where ‘\(\Delta S\)’ is the entropy change, n charge number (n = 1 for li-ion battery) and ‘F’ Faraday constant.
2.2 Model Description
The energy balance equation in radial direction is expressed as [11]
The following boundary conditions at the cell centre and cell surface with initial conditions are applied.
Here, \({\dot{Q}}_{gen}\) is the heat generation per unit volume, \(\alpha\) thermal diffusivity, k thermal conductivity, h heat transfer coefficient. Some part of the heat generated remains inside the cell as sensible heat and remaining part gets conducted through the cell layers to the cell surface and then it is dissipated to the surroundings by convective heat transfer. Mahamud and park [9] reported that the heat transfer in spirally wounded cylindrical cells, in the radial direction is very much lower than the axial direction due to the interfaces between the electrode layers. In the present model, 1D heat transfer with internal heat generation in the cylindrical cell is restricted to radial heat transfer only.
3 Results and Discussion
3.1 Validation of One-Dimensional Radial Analytical Model
The simplified one-dimensional radial battery thermal model is simulated using MATLAB©. The input parameters used in the model is tabulated in Table 3. The temperature profile of the cylindrical li-ion cell is obtained by simulating the model at constant current discharge rates of 0.5C, 1C and 1.5C. The thermal model solves the energy balance equation (5) for the given ambient conditions and input parameters to obtain temperature points within the cell along the radial direction for different state of charge (SOC). The density of the cell is estimated using the mass and volume data of the cylindrical cell. The heat generated at the battery core is conducted through the spirally wound battery layers to the surface of the cell which is then dissipated to the surroundings by natural convection as depicted in Fig. 2. The validation of model prediction considers both convection and radiation at the battery surface and thus equivalent heat transfer coefficient is utilized in the simulation. The comparison of average volumetric heat generation at 0.5C, 1C and 1.5C with the literature is presented in Table 4. The proposed model presents the temperature distribution in the cell at the battery core and the battery surface. The surface temperature curves are validated with results published in [14]. It is observed in Fig. 3 that the prediction agrees well with the experimental data for 50% of the total time with some deviation thereafter. The deviations observed in the predictions are attributed to the following.
Comparison of thermal model simulation and experimental data [14] for temperature rise at 0.5C, 1C and 1.5C discharge rates
-
The experimental data from the referred literature were obtained from three-dimensional model
-
The model discussed in present work assumes uniform heat generation which predicts a nearly linear trend of temperature with C-rates.
-
Averaged value of reversible heat source is considered due to unavailability of the data in the literature.
3.2 Effect of Forced Convection on the Temperature Profile
The temperature variation in the li-ion cells depends upon the rate at which the heat is dissipated from the battery to the surroundings. A forced convective heat transfer is effective in dissipating heat compared to natural convection. Most of the battery thermal management systems employ either liquid or air based forced cooling system to dissipate the heat to the surroundings. The cell thermal model is simulated at the maximum discharge current of 2C under the forced convection by using the heat transfer coefficient of 25, 50, 75 and 100 W/m2 C. It can be observed from the Fig. 4 that the rise in cell surface temperature saturates quite early for heat transfer coefficient higher than 75 W/m2 C For 100 W/m2 C, the cell surface temperature is contained under 30 °C for forced convection cooling. Any further increase in the heat transfer coefficient may not improve the cooling effectively. This is because the heat flow from the core of the cell to the ambient encounters two resistances in series, viz. conduction and convection resistance. Although convection resistance reduces by increasing heat transfer coefficient, conductance resistance is responsible for the thermal response of the cell. Figure 5 shows the temperature variation across the radius of the cell from cell center to surface for different state of charge (SOC) at heat transfer coefficient of 75 and 100 W/m2 °C. A temperature non-uniformity exists between the cell core and surface with maximum temperature gradient of ~3 °C for a given SOC. It is inferred that decreasing the convection resistance only helps in reducing the cell surface temperature but does not have any effect on temperature gradient inside the cell.
4 Conclusions
This paper presents a one-dimensional radial thermal model to predict the temperature profile and heat generation of the cylindrical lithium-ion cell. The prediction of the model agrees satisfactorily with the experimental data. The model also predicts the effect of forced convection cooling on the surface temperature of the cell for different heat transfer coefficient values. Surface temperature of the cell remains nearly constant after 50% of discharge time for high heat transfer coefficient values attributing to zero heat dissipation from the cell after 50% SOC. It is also observed that the cell surface temperature is contained within the threshold limits for heat transfer co-efficient of 100 W/m2 °C. The core and surface temperature non-uniformity across the radius of the cell is found to be around ~3 °C for a given SOC. Temperature gradient between the core and the surface of the cell remains almost constant with any heat transfer coefficient values higher than 50 W/m2 ºC.
Abbreviations
- Cp:
-
specific heat capacity [J kg−1 K−1]
- D:
-
diameter of cylindrical cell [m]
- \(\frac{\partial U}{\partial T}\) :
-
entropic coefficient [mV K−1]
- h:
-
convective heat transfer coefficient [W m−2 K−1]
- H:
-
enthalpy of reaction [kJ kg−1]
- I:
-
cell current [A]
- k:
-
thermal conductivity [W m−1 K−1]
- L:
-
length [m]
- \(Q\) :
-
heat generation [W]
- \({\dot{Q}}_{gen}\) :
-
volumetric heat generation [Wm−3]
- R:
-
cell radius [mm]
- Re:
-
internal cell resistance [mΩ]rradial dimension [mm]
- SOC:
-
state of charge [%]
- T:
-
temperature [K]
- t:
-
time [s]
- V:
-
open circuit voltage [V]
- U:
-
cell potential [V]
- α:
-
thermal diffusivity [m2 s−1]
- ρ:
-
density [kg m-3]
- a:
-
ambient condition
- avg:
-
area averaged
- c:
-
core
- irr:
-
irreversible
- o:
-
initial condition
- rev:
-
reversible
- s:
-
surface condition
References
Gireesh Kumar P, Lekhana P, Tejaswi M, Chandrakala S (2020) Effects of vehicular emissions on the urban environment- a state of the art. Materials Today Proceedings
Honda Global Homepage (2021). https://global.honda/innovation/technology/automobile/electric-vehicles.html. Accessed May 24, 2021
Linden, Reddy D (2002) TB Handbook of Batteries. 3rd edn. McGraw-Hill: New York, NY, USA
Ibrahim H, Ilinca A, Perron J (2008) Energy storage systems—characteristics and comparisons. Renew Sustain Energy Rev 12(5):1221–1250
Manzetti S, Mariasiu F (2015) Electric vehicle battery technologies: from present state to future systems. Renew Sustain Energy Rev 51:1004–1012
Hannan MA, Hoque MM, Mohamed A, Ayob A (2017) Review of energy storage systems for electric vehicle applications: issues and challenges. Renew Sustain Energy Rev 69:771–789
An Z, Jia L, Ding Y, Dang C, Li X (2017) A review on lithium-ion power battery thermal management technologies and thermal safety. J Therm Sci 26(5):391–412
Drake SJ, Wetz DA, Ostanek JK, Miller SP, Heinzel JM, Jain A (2014) Measurement of anisotropic thermophysical properties of cylindrical Li-ion cells. J Power Sources 252:298–304
Mahamud R, Park C (2013) Spatial-resolution, lumped-capacitance thermal model for cylindrical Li-ion batteries under high Biot number conditions. Appl Math Model 37(5):2787–2801
Stan A, Świerczyński M, Stroe D, Teodorescu R, Andreasen SJ (2014) Lithium ion battery chemistries from renewable energy storage to automotive and back-up power applications—an overview. Int Conf Optim Elect Elect Equip (OPTIM) 2014:713–720
Al Hallaj S, Maleki H, Hong JS, Selman JR (1999) Thermal modeling and design considerations of lithium-ion batteries. J Power Sourc 83(1–2):1–8
Forgez C, Vinh Do D, Friedrich G, Morcrette M, Delacourt C (2010) Thermal modeling of a cylindrical LiFePO4/graphite lithium-ion battery. J Power Sourc
Bernardi D, Pawlikowski E, Newman J (1970) A General Energy Balance for Battery Systems 132(1)
Paccha-Herrera E, Calderón-Muñoz WR, Orchard M, Jaramillo F, Medjaher K (2020) Thermal modeling approaches for a licoo2 lithium-ion battery—a comparative study with experimental validation. Batteries 6(3):1–23
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2022 The Author(s), under exclusive license to Springer Nature Singapore Pte Ltd.
About this paper
Cite this paper
Bhat, P., Varpe, M.K. (2022). A Simplified Thermal Model to Predict Temperature Profile and Heat Generation of Cylindrical Lithium-Ion Cells. In: Krishna, V., Seetharamu, K.N., Joshi, Y.K. (eds) Recent Advances in Hybrid and Electric Automotive Technologies. Lecture Notes in Mechanical Engineering. Springer, Singapore. https://doi.org/10.1007/978-981-19-2091-2_2
Download citation
DOI: https://doi.org/10.1007/978-981-19-2091-2_2
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-19-2093-6
Online ISBN: 978-981-19-2091-2
eBook Packages: EngineeringEngineering (R0)