Abstract
The physiological status of euryhaline teleost is regulated by environmental salinity through different mechanisms. This chapter discusses the salinity to the juvenile golden pompano Trachinotus ovatus (Linnaeus 1758) rearing performance impact.
Rearing salinity significantly affected fish growth and the RNA/DNA ratio. When the salinity was 34‰, the fish growth rate and RNA/DNA ratio were higher. The effect of salinity on pepsin activity was not significant. However, rearing salinity had a significant effect on α-amylase activity. The α-amylase activity of fish reared at the salinity of 10‰ was significantly lower than fish at the salinity of 34‰. Raising salinity has significant effects on FCR of juvenile golden pompano. The FCR of fish cultured at the salinity of 10‰ was five times higher than the FCR of fish reared at 34‰. The GPX activity was highest when the salinity was 26‰ and lowest when the salinity was 34‰. The activities of SOD of fish reared at 18‰ and 34‰ were significantly higher than those reared at 10‰ and 26‰. The lowest activity of Na+K+-ATPase was obtained in fish at 34‰, while the highest activity of Na+K+-ATPase was obtained when fish at 18‰. Juvenile golden pompano can be reared above 26‰ without affecting fish performance, and the salinity <18‰ is not suitable for the growth of juvenile golden pompano.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Salinity
- Rearing performance
- Digestive enzyme activity
- Antioxidant enzyme
- Na+K+-ATPase
- Trachinotus ovatus
6.1 Introduction
Salinity is the most important environmental factor affecting aquatic habitats, and it has been involved in many studies regarding on its impact on fish growth performance (Rubio et al. 2005). Previous studies have suggested that environmental salinity can change physiological activities such as feed intake (Rubio et al. 2005), metabolic rate (Dutil et al. 1997), activity of enzyme (Moutou et al. 2004), and feed conversion rate (Alava 1998), which are closely linked to the fish growth. In practice, the growth performance of fish is better under moderate salinity conditions, but the underlying mechanisms are still controversial (Moutou et al. 2004; Baeuf and Payan 2001).
The enzyme analysis of digestive has been considered a reliable method to understand the digestive process and nutrition condition of fish (Ueberschär 1988; Ma et al. 2014). Previous studies have demonstrated that changes of salinity can alter the enzyme activities of digestive in species such as Salmo gairdnerii (Colin et al. 1985), Sparus sarba (Kelly et al. 1999), Centropomus parallelus (Tsuzuki et al. 2007), and Sparus aurata (Moutou et al. 2004). Such variation of digestive enzyme activities can significantly affect the growth of fish (Tsuzuki et al. 2007). Since proteinases can catalyze the hydrolytic degradation of proteins, it plays a crucial role in living organism’s growth and survival (Klomklao 2008). Alpha-amylase is an important enzyme for carbohydrate digestion and is involved in carbohydrate metabolism of energy supply (Papoutsoglou and Lyndon 2003). As fish require more metabolic energy for osmoregulation, a higher α-amylase activity may indicate energy spending in the process of osmoregulatory. The α-amylase and pepsin activities have been used to explore the influence of salinity digestibility to fish (Yan and Wu 2010).
Although ambient salinity can affect fish physiological condition via different mechanisms, these underlying mechanisms are not well understood (Arnason et al. 2013). When ambient salinity is approaching the physiological tolerance limit, fish may be stressed, and the system of immune defense may be compromised (Harris and Bird 2000). The relationship between salinity variation and fish immune defense has been paid much attention (Zhang et al. 2011; Choi et al. 2013; Arnason et al. 2013).
Scavenging enzymes such as superoxide dismutase (SOD), catalase (CAT), and glutathione peroxidase (GPX) are the main components of physiological antioxidant protection of marine fish and play an important role in the immune defense system of marine fish (Winston and Di Giulio 1991; Halliwell and Gutteridge 1996). Within the physical process, SOD promotes the dismutation of two O2− molecules to H2O2 and O2, and CAT and GPX convert H2O2 to H2O. The inadequate antioxidant defenses to combat reactive oxygen species can lead to oxidative stress (Martinez-Alvarez et al. 2002). Nevertheless, knowledge about the response of antioxidant enzymes to salinity of marine fish is still limited.
Golden pompano Trachinotus ovatus has been identified as a good aquaculture candidate species due to its fast growth, high flesh quality, and suitability for cage farming. In South China, most golden pompano farming is carried on small farms in marine and brackish environments using discontinuous and non-quantified methods. During the rearing period of golden pompano, salinity variations are often associated with low growth, disease outbreak, and massive mortality. In this chapter, the effects of environmental salinities (10‰, 18‰, 26‰, and 34‰) on juvenile golden pompano (wet weight 3.24 ± 0.14 g) during the grow-out phase are discussed, aiming to increase the production efficiency of commercial farming of golden pompano.
6.2 Growth and Survival of Golden Pompano Under Different Salinity
Fish adaptations to salinity vary among pompano species. For instance, the recommended low salinity range is 15–25‰ for T. blochii (Kalidas et al. 2012), 12–19‰ for T. carolinus (Moe et al. 1968), and 10–20‰ for T. marginatus (Costa et al. 2008). In golden pompano, juveniles showed a reasonable survival rate at 18‰, 26‰, and 34‰, suggesting a good adaption of this species within this salinity range. A previous study suggests that fish adaption to ambient salinity changes is life stage-dependent (Aliume et al. 1997) with some metabolic restraints (Peters et al. 1998; Rocha et al. 2007). Although some marine fish species can tolerate a wide range of salinity gradient changes, the consumption of metabolic energy during osmotic regulation is unavoidable (Woo and Kelly 1995; Moser and Miller 1994; Tseng and Hwang 2008). Even in species with lower metabolic rates, osmoregulation seems to consume a high proportion of the available energy, ranging from 20% to 50% of the total energetic expenditure (Baeuf and Payan 2001).
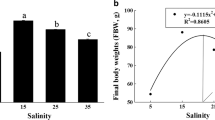
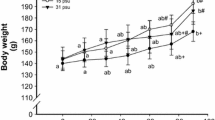
Maximum growth would occur in an isosmotic environment (10 ± 2‰) because of low osmoregulatory energy demand (Brett 1979), but optimal salinity for fish growth is species-specific. For example, the optimal growth salinity is 55‰ for Chanos chanos (Swanson 1998) and 14‰ for Gadus morhua (Lambert et al. 1994). In contrast, the growth of Acanthopagrus butcheri is not significantly affected by the rearing salinity from 0‰ to 12‰ (Partridge and Jenkins 2002), and salinity in the range of 5–35‰ has no effect on the growth of Centropomus parallelus (Tsuzuki et al. 2007). In golden pompano, the growth of juvenile fish was sensitive to the rearing salinity, and the highest growth rate was recorded in fish cultured at 34‰ (Table 6.1). The lowest growth rate was observed in fish cultured at the salinity of 10‰. These results indicate that the growth of juvenile golden pompano is reduced at lower salinity. The RNA/DNA ratio is used as an indicator of the fish’s growth potential when sufficient food is provided to young fish under laboratory conditions (Tanaka et al. 2007). In juvenile golden pompano, culture salinity had a significant effect on the RNA/DNA ratio (Table 6.1). Since the diet, food availability, feeding scheme, and environmental conditions were the same across treatments, the salinity should cause the RNA/DNA ratios change. Higher RNA/DNA ratio is under the condition of high salinity farmed and higher RNA/DNA ratio, and high specific growth rate is the same.
6.3 Digestive Enzyme Activities of Golden Pompano Under Different Salinity
The alternation of ambient salinities can lead to the changes of digestive enzyme activities (Moutou et al. 2004; Woo and Kelly 1995). This effect may further affect the digestion and absorption of dietary protein (Tsuzuki et al. 2007). Previous studies have also evaluated the relationship between growth rate and digestive enzyme activities of fish at different salinity, and a correlation is shown between growth and target digestive enzymes. Previous studies have evaluated the fish growth rate under different salinity and the relationship between the activity of digestive enzymes and indicated the growth and the correlation between target enzymes (Moutou et al. 2004; Woo and Kelly 1995). In larval golden pompano, the activities of amylase in fish at 26‰ and 34‰ salinities were higher than those at 10‰ and 18‰ salinities, and also the growth rate of fish at 34‰ was higher than fish at 10‰. But the existing literature does not support that amylase activity corresponds to fish growth. Therefore, it may be worth further investigating the relationship between amylase activity and fish growth.
The FCR of cultured fish is different under different environmental salinity, and the response of feed conversion ratio to salinity is species-specific (Partridge and Jenkins 2002). For example, when Gadus morhua are reared at salinities of 7‰, 14‰, and 28‰, the best FCR was obtained at 14‰ (Lambert et al. 1994), but better FCR can be achieved when fish were reared at 24‰ in Acanthopagrus butcheri (Partridge and Jenkins 2002). However, compared with the treatment groups with salinity of 8‰, 18‰, and 38‰, Carassius auratus reared at salinity of 28‰ could obtain the best FCR (Klaoudatos and Conides 1996). In juvenile golden pompano, the FCR of fish increase with the increase of ambient salinity, and the optimal FCR was observed when fish group is reared at 34‰ (Table 6.1). Coincidently, higher amylase activity was also found when fish were reared at 34‰. This could indicate that the digestibility is increased when fish are cultured at 34‰.
6.4 Antioxidant Enzyme and Na+K+-ATPase Activities of Golden Pompano Under Different Salinity
Ambient salinity can change fish metabolism and result in different survival rates. The alternation of antioxidant enzyme activities in fish may be caused by a hypo-osmotic shock (Roche and Boge 1996). In juvenile golden pompano, the GPX activity in fish liver gradually increased, when the ambient salinity was between 10‰ and 28‰, while the CAT activity of the liver presented a gradually declining trend. Similar results have also been reported by Wilhelm Filho et al. (1993) and Martinez-Alvarez et al. (2002). The activity of SOD of fish at 28‰ salinity was significantly lower compared to 34‰ salinity (Fig. 6.1). Furthermore, when fish were reared in the salinity of 28‰, the highest GPX activity and the lowest CAT activity were also observed, and the final survival rate of fish at 28‰ was significantly higher than in other treatments. This may indicate that the salinity of 28‰ is more suitable for the juvenile golden pompano’s basal metabolism.
The GSH, SOD, CAT, and Na+K+-ATPase activities of juvenile golden pompano cultured at 10‰, 18‰, 26‰, and 34‰ salinities. Different letters represent significant difference (P < 0.05) (Ma et al. 2016b)
The Na+-K+-ATPase (NKA) actively transports Na+ out and K+ in animal cells among the transporters that modulate ion fluxes (Post and Jolly 1957), and NKA generally involved in the maintenance of an internal hypo-osmotic state during changes in environmental salinity. NKA activity in the osmoregulatory organ is accompanied by the change of ambient salinity (Hirose et al. 2003; Burg et al. 2007; Marshall 2002). In juvenile golden pompano, after 30 days of the study, the NKA activity of fish was corresponding to the rearing salinity. Compared to the control group, fish reared at the salinities of 18‰ and 10‰ showed higher activity of NKA (Fig. 6.1). This result is consistent with the previous research results (Madsen et al. 1996; McCormick 1995; Morgan et al. 1997). Under low salinity treatment, NKA activity was higher, and SGR was lower, indicating that low salinity of 10–18‰ was not suitable for the physiology of juvenile pompano.
6.5 Conclusion
Ambient salinity has significant effects on fish growth and RNA/DNA ratio. When the salinity was 34‰, the growth rate and RNA/DNA ratio of fish were higher. The FCR of fish cultured at the salinity of 10‰ was five times higher than the FCR of fish reared at 34‰. The activities of NKA and antioxidant enzymes corresponded with fish survival. Fish have a higher survival rate when salinity is 26‰. Juvenile golden pompano can be raised above 26‰ without affecting the performance of fish, while salinity <18‰ is not suitable for the juvenile golden pompano growth.
References
Alava VR (1998) Effect of salinity, dietary lipid source and level on growth of milkfish (Chanos chanos) fry. Aquaculture 167:229–236
Aliume C, Zerbi A, Miller JM (1997) Nursery habitat and diet of juvenile Centropomus undecimalis species in Puerto Rico estuaries. Gulf Mexico Sci 15:77–87
Arnason T, Magnadottr B, Bjornsson B, Steinarsson A, Bjornsson BT (2013) Effects of salinity and temperature on growth, plasma ions, cortisol and immune parameters of juvenile Atlantic cod (Gadus morhua). Aquaculture 380–383:70–79
Baeuf G, Payan P (2001) How should salinity influence fish growth? Comp Biochem Physiol C Toxicol Pharmacol 130:411–423
Brett JR (1979) Environmental factors and growth. In: Hoar WS et al (eds) Fish physiology. Academic, New York, pp 599–675
Burg MB, Ferraris JD, Dmitrieva NI (2007) Cellular response to hyperosmotic stresses. Physiol Rev 87:1441–1474
Choi K, Cope WG, Harms CA, Law JM (2013) Rapid decreases in salinity, but not increases, lead to immune dysregulation in Nile tilapia, Oreochromis niloticus (L.). J Fish Dis 36:389–399
Colin DA, Nonnotte G, Leray C, Nonnotte L (1985) Na transport and enzyme activities in the intestine of the freshwater and seawater adapted trout (Salmo gairdnerii R.). Comp Biochem Physiol A Comp Physiol 81:695–698
Costa LF, Miranda-Filho KC, Severo MP, Sampaio LA (2008) Tolerance of juvenile pompano Trachinotus marginatus to acute ammonia and nitrite exposure at different salinity levels. Aquaculture 285:270–272
Dutil JD, Lambert Y, Boucher E (1997) Does higher growth rate in Atlantic cod (Gadus morhua) at low salinity result from lower standard metabolic rate or increased protein digestibility? Can J Fish Aquat Sci 54:99–103
Halliwell B, Gutteridge JMC (1996) Lipid peroxidation: a radical chain reaction. In: Halliwell B, Gutteridge JMC (eds) Free radicals in biology and medicine. Clarendon Press, Oxford, pp 188–266
Harris I, Bird DJ (2000) Modulation of the fish immune system by hormones. Vet Immunol Immunopathol 77:163–176
Hirose S, Kaneko T, Naito N, Takei Y (2003) Molecular biology of major components of chloride cells. Comp Biochem Physiol B Biochem Mol Biol 136:593–620
Kalidas C, Sakthivel M, Tamilmani G, Pamesh Kumar P, Abdul AK, Jayakumar R, Balamurugan, Ramkumar, Jothi P, Gopakumar G (2012) Survival and growth of juvenile silver pompano Trachinotus blochii (Lacepede, 1801) at different salinities in tropical conditions. Indian J Fish 59:95–98
Kelly SP, Chow INK, Woo NYS (1999) Effect of prolactin and growth hormone on strategies of hypoosmotic adaption in a marine teleost Sparus sarba. Gen Comp Endocrinol 113:9–22
Klaoudatos SD, Conides AJ (1996) Growth, food conversion, maintenance and long-term survival of gilthead sea bream, Sparus auratus L., juveniles after abrupt transfer to low salinity. Aquac Res 27:765–774
Klomklao S (2008) Digestive proteinases from marine organisms and their applications. Aerosp Sci Technol 30:37–46
Lambert Y, Dutil JD, Munro J (1994) Effects of intermediate and low salinity conditions on growth rate and food conversion of Atlantic cod (Gadus morhua). Can J Fish Aquat Sci 51:1569–1576
Ma Z, Guo H, Zheng P, Wang L, Jiang S, Qin JG, Zhang D (2014) Ontogenetic development of digestive functionality in golden pompano Trachinotus ovatus (Linnaeus 1758). Fish Physiol Biochem 40:1157–1167
Ma Z, Guo H, Zheng P, Wang L, Jiang S, Zhang D, Qin JG (2016a) Effect of salinity on the rearing performance of juvenile golden pompano Trachinotus ovatus (Linnaeus 1758). Aquac Res 47(6):1761–1769
Ma Z, Zheng P, Guo H, Jiang S, Qin JG, Zhang D (2016b) Salinity regulates antioxidant enzyme and Na+K+-ATPase activities of juvenile golden pompano Trachinotus ovatus (Linnaeus 1758). Aquac Res 47(5):1481–1487
Madsen SS, Larsen BK, Jensen FB (1996) Effects of freshwater to seawater transfer on osmoregulation, acid-base balance and respiration in river migrating whitefish (Coregonmus lavaretus). J Comp Physiol B 166:101–109
Marshall WS (2002) Na+, Cl−, Ca2+ and Zn2+ transport by fish gills: retrospective review and prospective synthesis. J Exp Zool 293:264–283
Martinez-Alvarez RM, Hidalgo MC, Domezain A, Morales AE, Garcia-Gallego M, Sanz A (2002) Physiological changes of sturgeon Acipenser naccarii caused by increasing environmental salinity. J Exp Biol 205:3699–3706
McCormick SD (1995) Hormonal control of gill Na+, K+-ATPase and chloride cell function. In: Wood CM, Shuttleworth TJ (eds) Cellular and molecular approaches to fish ionic regulation. Academic, San Diego, pp 285–315
Moe MAJ, Lewis RH, Ingle RM (1968) Pompano mariculture: preliminary data and basic consideration. State of Florida Board of Conservation Technical Series 55:65
Morgan JD, Sakamoto T, Grau EG, Iwama GK (1997) Physiological and respiratory responses of the Mozambique tilapia (Oreochromis mossambicus) to salinity acclimation. Comp Biochem Physiol A Physiol 117:391–398
Moser ML, Miller JM (1994) Effects of salinity fluctuation on routine metabolism of juvenile spot, Leistomus xanthurus. J Fish Biol 45:335–340
Moutou KA, Panagiotaki P, Mamuris Z (2004) Effects of salinity on digestive protease activity in the euryhaline sparid Sparus aurata L.: a preliminary study. Aquac Res 35:912–914
Papoutsoglou ES, Lyndon AR (2003) Distribution of α-amylase along the alimentary tract of two Mediterranean fish species, the parrotifish Sparisoma cretense L. and the stargazer, Uranoscopus scaber L. Mediterr Mar Sci 4:115–124
Partridge GJ, Jenkins GI (2002) The effect of salinity on growth and survival of juvenile black bream (Acanthopagrus butcheri). Aquaculture 210:219–230
Peters KM, Matheson RE Jr, Taylor RG (1998) Reproduction and early life history of common snook, Centropomus undecimalis (Bloch), in Florida. Bull Mar Sci 62:509–529
Post RL, Jolly PC (1957) The linkage of sodium, potassium, and ammonium active transport across the human erythrocyte membrane. BBA-Biomembranes 25:118–128
Rocha AJS, Gomes V, Ngan PV, Passos MJACR, Furia RR (2007) Effects of anionic surfactant and salinity on the bioenergetics of juveniles of Centropomus parallelus (Poey). Ecotox Environ Safe 68:397–404
Roche H, Boge G (1996) Fish blood parameters as a potential toll for identification of stress caused by environmental factors and chemical intoxication. Mar Environ Res 41:27–43
Rubio VC, Sanchez-Vazquez FJ, Madrid JA (2005) Effects of salinity on food intake and macronutrient selection in European sea bass. Physiol Behav 85:333–339
Swanson C (1998) Interactive effects of salinity on metabolic rate, activity, growth and osmoregulation in the euryhaline milkfish (Chanos chanos). J Exp Biol 201:3355–3366
Tanaka Y, Gwak WS, Tanaka M, Sawada Y, Okada T, Miyashita S, Kumai H (2007) Ontogenetic changes in RNA, DNA and protein contents of laboratory-reared Pacific bluefin tuna Thunnus orientalis. Fish Sci 73:378–384
Tseng YC, Hwang PP (2008) Some insights into energy metabolism for osmoregulation in fish. Comp Biochem Physiol C Toxicol Pharmacol 148:419–429
Tsuzuki MY, Sugai JK, Maciel JC, Francisco CJ, Cerqueira VR (2007) Survival, growth and digestive enzyme activity of juveniles of the fat snook (Centropomus parallelus) reared at different salinities. Aquaculture 271:319–325
Ueberschär B (1988) Determination of the nutritional condition of individual marine fish larvae by analyzing their proteolytic enzyme activities with a highly sensitive fluorescence technique. Meeresforsch 32:144–154
Wilhelm Filho D, Giulivi C, Boveris A (1993) Antioxidant defences in marine fish-I. Teleosts. Comp Biochem Physiol C Pharmacol Toxicol Endocrinol 106:409–413
Winston GW, Di Giulio RT (1991) Prooxidant and antioxidant mechanisms in aquatic organisms. Aquat Toxicol 19:137–161
Woo NYS, Kelly SP (1995) Effects of salinity and nutritional status on growth and metabolism of Sparus sarba in a closed seawater system. Aquaculture 135:229–238
Yan M, Wu X (2010) Effects of different salinities on digesitve enzyme activities of the pufferfish Takifugu ocellatus. Chin J Anim Nutr 22:797–803
Zhang Y, Mai K, Ma H, Ai Q, Zhang W, Xu W (2011) Rearing in intermediate salinity enhances immunity and disease-resistance of turbot (Scophthalmus maximus L.). Acta Oceanol Sin 30:122–128
Author information
Authors and Affiliations
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2022 China Agriculture Press
About this chapter
Cite this chapter
Zhou, S., Han, M., Yang, R., Hu, J. (2022). Physical Responses of Golden Pompano Trachinotus ovatus to Rearing Salinity. In: Ma, Z., Yu, G., Qin, J.G. (eds) Ontogenetic development of pompano Trachinotus ovatus. Springer, Singapore. https://doi.org/10.1007/978-981-19-1712-7_6
Download citation
DOI: https://doi.org/10.1007/978-981-19-1712-7_6
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-19-1711-0
Online ISBN: 978-981-19-1712-7
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)