Abstract
This chapter covers the digestive ontogeny of T. ovatus from hatch to 32-day post-hatch (DPH). The development of the digestive system in T. ovatus can be divided into three stages: stage I starting from hatching and ending at the onset of exogenous feeding (3 DPH), stage II starting from first feeding and ending at the formation of gastric glands in the fish stomach (15 DPH), and stage III starting from the appearance of gastric glands and continuing onward. The specific activities of lipase, trypsin, and amylase in fish increased sharply from the exogenous feeding to 5–7 DPH. The pepsin activity was detected on 15 DPH, and the specific activities increased with fish age. The dynamics of enzyme activity reflected the structural development in the fish digestive system. After the formation of gastric glands in the stomach, the enzyme activities became stable. Depending on the development of the digestive system, the larvae of T. ovatus can begin weaning at 15 DPH. This chapter updates the improved understanding of the ontogeny of T. ovatus during the larval phase and provides the protocol of feeding and weaning for this economically important fish in aquaculture.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
3.1 Introduction
Upon hatching, the digestive system of marine fish larvae is immature and undergoes major changes before fully having the function of food digestion (Cahu and Zambonino-Infante 2001; Hu et al. 2018; Ma et al. 2012). In spite of advances in larval fish culturing technology, most of marine fish hatcheries still depend on live feed such as rotifers Brachionus spp. and Artemia sp. as feed for early larvae of fish (Hamlin and Kling 2001; Sorgeloos et al. 2001; Hu et al. 2018). In practice, live feed supply is usually required beyond metamorphosis until larvae are weaned onto formulated diet (Alves et al. 2006; Ma et al. 2014; Cui et al. 2017). Nevertheless, the long-term use of live feed is costly and may lead to malnutrition due to unbalanced nutrition, and live feed does not meet the nutritional requirement for larval fish (Le Ruyet et al. 1993; Baskerville-Bridges and Kling 2000; Callan et al. 2003). Thus, the weaning of fish larvae at their early stage is essential.
Weaning with artificial feed instead of live feed is a gradual process during the larval stage. In aquaculture practice, most temperate marine fish, such as Lutjanus erythopterus and Seriola lalandi, usually begin weaning after metamorphosis (Cui et al. 2017; Ma et al. 2014). Marine fish can make weaning easier to manage and more effective if compound diet is introduced early (Baskerville-Bridges and Kling 2000; Hart and Purser 1996). It is likely to have a negative impact on the growth and survival of fish if artificial feed is introduced too early because larvae of fish have no ability to digest artificial feed (Andrade et al. 2012; Cahu and Zambonino-Infante 2001; Ma et al. 2014). The co-feeding protocol for fish larvae with both live and artificial feed has been developed in finfish hatchery to improve poor digestion of artificial feed at the early stage of development. The proportion of live feed is gradually reduced during the weaning stage, and the co-feeding strategy allows the fish larvae to receive artificial feed earlier in terms of nutrition (Rosenlund et al. 1997; Engrola et al. 2009b). The growth, survival, and the quality of marine fish species can be significantly improved by co-feeding such as Sciaenops ocellatus (Lazo et al. 2000), Rhombosolea tapirina (Hart and Purser 1996), and Solea senegalensis (Engrola et al. 2009a).
The response of fish to nutrient supply can be assessed by RNA/DNA ratio that has been used as an indicator for somatic tissue growth (Bailey et al. 1995; Buckley et al. 1999; Gwak et al. 2003). Since the amount of DNA per cell is almost constant, cross-species fish growth can be measured by the RNA/DNA ratio as a consistent measure (Pilar Olivar et al. 2009; Gwak and Tanaka 2001), whereas the quantity of RNA reflects the amount of protein synthesis in cells (Höök et al. 2008; Tanaka et al. 2008). Because the nutritional condition is related to feeding success and food supply (Tanaka et al. 2008), fish with an adequate nutrition supply may have a higher RNA/DNA ratio than malnourished fish (Boyd and Tucker 1992; Gronkjer et al. 1997).
Although fish can consume artificial diets in their early period of development, this does not guarantee the success of the artificial diet digestion and absorption, because the gut of fish may be filled with artificial diet and death (Cahu and Zambonino-Infante 2001). Therefore, introduction time of artificial diet of fish larvae should be determined according to the development of digestive system (Cahu and Zambonino-Infante 2001). In some studies, the histological structure degrades when fish larvae are malnourished (Yufera et al. 1993; Chen et al. 2007). Midgut cells’ height is a histological indicator of fish nutrient supply as evidenced by the early development of Seriola lalandi (Chen et al. 2007) and Theragra chalcogramma (Theilacker and Watanabe 1989).
The Trachinotus ovatus belongs to the Carangidae family and is extensive cultured in the Asia-Pacific region. We examined the development of the digestive system in previous studies; in particular, attention is paid to pepsin secretion and the appearance of gastric glands in the stomach (Ma et al. 2013). In this chapter, we discuss the suitable time of weaning for T. ovatus, aiming to improve fish survival and establish a cost-effective feed regime for the fish that are commercially important.
3.2 Weaning Scheme Design
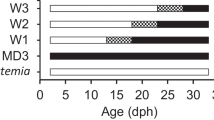
On 12 DPH, the fish larvae were randomly divided into 12, 300-L experimental tanks (20 fish L−1) for early weaning trial, which began on 13 DPH. The daily water exchange rate was 300% volume per day. Put an air stone in each tank to keep adequate dissolved oxygen and uniform the distribution of microalgae, rotifers, and Artemia nauplii. The weaning stage lasted for 10 days, including 5 days of co-feeding and 5 days of live feed combined with the introduction of artificial feed. Only feed the fish larvae with artificial diet after completion of weaning (Fig. 3.1).
Four co-feeding and weaning treatments to wean pompano T. ovatus larvae from 13 DPH to 32 DPH with separate commencing time by 3 days. W13: 13–22 DPH, W16: 16–25 DPH, W19: 19–26 DPH, and W22: 22–33 DPH (Ma et al. 2015)
Weaning treatments were composed of the same feeding protocol but began at four start time schemes after hatch: (1) start at 13 DPH and end at 22 DPH (W13), (2) start at 16 DPH and end at 25 DPH (W16), (3) start at 19 DPH and end at 28 DPH (W19), and (4) start at 22 DPH and end at 31 DPH (W22 as control); each group had three replicates. Each group has weaning interval for 3 days (i.e., 13, 16, 19, and 22 DPH, Fig. 3.1).
3.3 Growth, Survival, and Variation of RNA/DNA Ratios
The standard length (SL) of T. ovatus larvae on 1 DPH was 3.38 ± 0.17 mm (mean ± SD). When the weaning experiment was 13 DPH, the average SL of fish was 5.14 ± 0.44 mm. In the end, fish of W22 and W19 treatments increased significantly compared to fish in the W13 and W16 treatments (P < 0.05).
The specific growth rates of fish larvae of W13 and W16 treatments decreased significantly compared to fish in the W19 and W22 treatments, which were 7.32% day−1 and 7.82% day−1, respectively (P < 0.05, Fig. 3.2). No significant difference of final survival rate of fish was found in W22 and W19 treatments, which were 85.17% and 86.5%, respectively (P > 0.05). No significant differences of final rates of fish were found in W13 and W16 treatments (P > 0.05), but it significantly decreased compared to fish in the W19 and W22 treatments (P < 0.05, Fig. 3.2).
Weaning time can significantly affect the growth of marine fish larvae (Curnow et al. 2006; Engrola et al. 2007). Inappropriate weaning time may lead to starvation of fish because of poor food digestion and absorption (Hu et al. 2018; Ma et al. 2012, 2014). There is no adequate nutrient supply during weaning period, and fish will use the energy stored in the body to maintain basic metabolism and to distribute less energy to growth, causing slow growth during the weaning period as reported in sand bass Paralabrax maculatofasciatus (Civera-Cerecedo et al. 2008) and Senegalese sole (Engrola et al. 2007). In T. ovatus, weaning time significantly affects the growth of fish larvae. The specific growth rates of fish of W19 and W22 groups were significantly higher than those of W13 and W16 groups. As we suggested in a previous study, T. ovatus is a fish with rapid development (Ma et al. 2013), and a functional digestive system appears at about 15 DPH. Higher fish growth rates were found in the W19 and W22 groups and may be associated with the function of the digestive system development.
During the ontogenetic development, the time of weaning regulated the RNA/DNA ratio (P < 0.05, Fig. 3.3). RNA/DNA ratio of fish of the W13 group at the end of weaning revealed a downregulating trend, and the ratio decreased from 7.4 to 5.6. A significant increase of the RNA/DNA ratios of fish after weaning was found in W16, W19, and W22 groups (P < 0.05, Fig. 3.3), and there was no significant difference in RNA/DNA ratios among these groups (P > 0.05, Fig. 3.3) though the mean variation of RNA/DNA ratios increased with the starting time of co-feeding and weaning (Ma et al. 2015).
The RNA/DNA ratios are used to understand growth pattern of many fish species during ontogenetic development (Pepin et al. 1999; Gwak et al. 2003; Höök et al. 2008). The RNA/DNA ratio of larval fish can be used to evaluate the fish nutritional condition because it is associated with food availability (Esteves et al. 2000; Diaz et al. 2011). The RNA content of larval fish such as turbot Scophthalmus maximus and herring Clupea harengus decreased when first feeding was not properly conducted (Clemmesen 1987). Previous studies have confirmed that the RNA/DNA ratio can be used to assess diet adequacy (Ben Khemis et al. 2000; Mendoza et al. 2008). In T. ovatus, weaning time can influence the change of the RNA/DNA ratio. When weaning was completed, the RNA/DNA ratio of fish of the W13 group indicated a reducing trend, which may suggest that weaning time has a negative impact on nutritional condition and growth of fish.
However, the positive variation of RNA/DNA ratios of fish was found in W16, W19, and W22 groups, which may indicate that fish adapt for artificial diet during weaning.
3.4 Height of Midgut Epithelial Cells
The epithelial cell height in the midgut of fish larvae is an excellent histological indicator to assess their nutritional condition (Gwak et al. 1999) because the form of enterocyte cells in the fish intestine can be changed by starvation (Domeneghini et al. 2002). The histological changes of fish larvae starvation vary with fish species and duration (Theilacker and Porter 1995; Gisbert et al. 2004; Ma et al. 2012). In larval yellowtail kingfish Seriola lalandi, the response of the midgut epithelium height to starvation occurred only before 33 DPH, and subsequently, there was no significant difference between normal fed and starved fish (Chen et al. 2007). In T. ovatus, the starting time of weaning significantly affected the intestinal epithelium, especially in the W13 treatment (Fig. 3.4). The height of epithelial cells of midgut of T. ovatus was decreased compared to the fish in the control group, from 15 DPH (W22), which is similar to the reports of Hamza et al. (2007) and Ostaszewska et al. (2005) where artificial diet is used to feed fish larvae. Starvation may cause a decrease of epithelial cell heights owing to incapability to digest artificial feed during the period of early weaning. However, the use of artificial diet results in low cell height because of intestinal epithelium damage (Hamza et al. 2007).
3.5 Survival and Jaw Malformation
The co-feeding of live feed and artificial diet has been suggested as an effective program to improve the survival of larvae fish during weaning (Engrola et al. 2009a; Nhu et al. 2010; Clay et al. 2011). For example, whether artificial diet or Artemia, the alligator Atractosteus spatula’s survival rate is about 60% (Mendoza et al. 2008). However, the alligator’s survival reached 95% when the alligator larvae were co-fed with 20% Artemia and 80% of artificial diet (Mendoza et al. 2002). In T. ovatus, co-feeding and weaning of fish larvae were successfully conducted. The survival rate of fish of W19 and W22 groups was higher (>85%) at the end of the experiment.
Nutrition is a significant factor affecting skeleton deformity (Cahu et al. 2003; Cobcroft et al. 2004; Sandel et al. 2010). During the period of co-feeding and early weaning, any inappropriate feeding protocol may lead to malnutrition of fish larvae.
The co-feeding time and early weaning time of marine fish larvae can affect the quality of fish such as skeletal malformation (Baskerville-Bridges and Kling 2000; Hamlin and Kling 2001). For example, compared with longer co-feeding and weaning time, shorter co-feeding and the time of weaning can improve spinal malformation incidence in southern flounder Paralichthys lethostigma (Faulk and Holt 2009). Similarly, during the weaning of pikeperch Sander lucioperca, a high malformation rate was found in the earlier weaning treatment (Kestemont et al. 2007). In this study, weaning time had no significant effects on jaw malformation (P > 0.05, Fig. 3.5). The jaw malformation rates were 15.54%, 11.78%, 14.00%, and 11.54% in the W13, W16, W19, and W22 treatments, respectively. The time of co-feeding and weaning cannot significantly affect the jaw malformation of larval T. ovatus. The existing evidence indicates that any of the current co-feeding and weaning regimes in this study can supply adequate nutrient to T. ovatus larvae during their development.
The jaw malformation of T. ovatus larvae of different weaning groups. Different letters represent significant difference (P < 0.05). Abbreviations refer to Fig. 3.1
3.6 Conclusion
The T. ovatus larvae can be weaned from live feed to artificial diet after 16 DPH without affecting the fish growth and survival or increasing jaw deformity. However, the introduction of artificial diet before 16 DPH may affect the fish growth, survival, and nutritional condition. Although some T. ovatus larvae could be weaned on 13 DPH, we suggested the optimal time of weaning for T. ovatus larvae should be from 16 to 22 DPH.
References
Alves JTT, Cerqueira VR, Brown JA (2006) Early weaning of fat snook (Centropomus parallelus Poey 1864) larvae. Aquaculture 253:334–342
Andrade CAP, Nascimento F, Conceição L, Linares F, Lacuisse M, Dinis MT (2012) Red porgy, Pagrus pagrus, larvae performance and nutritional condition in response to different weaning regimes. J World Aquacult Soc 43:321–334
Bailey KM, Canino MF, Napp JN, Spring SM, Brown AL (1995) Contrasting years of prey levels, feeding conditions and mortality of larval walleye pollock Theragra chalcogramma in the western gulf of Alaska. Mar Ecol Prog Ser 119:11–23
Baskerville-Bridges B, Kling LJ (2000) Early weaning of Atlantic cod (Gadus morhua) larvae onto a microparticulate diet. Aquaculture 189:109–117
Ben Khemis I, Noue JDL, Audet C (2000) Feeding larvae of winter flounder Pseudopleuronectes americanus (Walbaum) with live prey or microencapsulate diet: linear growth and protein, RNA and DNA content. Aquac Res 31:377–386
Boyd CE, Tucker CS (1992) Water quality and pond soil analysis for aquaculture. Alabama Agricultural Experiment Station, Auburn University, Alabama, p 183
Buckley LJ, Caldarone EM, Ong TL (1999) RNA-DNA ratio and other nucleic acid-based indicators for growth and condition of marine fishes. Hydrobiologia 401:265–277
Cahu C, Zambonino-Infante J (2001) Substitution of live food by formulated diets in marine fish larvae. Aquaculture 200:161–180
Cahu C, Zambonino Infante J, Takeuchi T (2003) Nutritional components affecting skeletal development in fish larvae. Aquaculture 227:245–258
Callan C, Jordaan A, Kling LJ (2003) Reducing Artemia use in the culture of Atlantic cod (Gadus morhua). Aquaculture 219:585–595
Chen BN, Qin JG, Carragher JF, Clarke SM, Kumar MS, Hutchinson WG (2007) Deleterious effects of food restrictions in yellowtail kingfish Seriola lalandi during early development. Aquaculture 271:326–335
Civera-Cerecedo R, Alvarez-Gonzalez CA, Garica-Gomez RE, Carrasco-Chavez V, Ortiz-Galindo JL, Rosales-Velazquez MO, Alamo TG, Moyano-Lopez FJ (2008) Effect of microparticulate diets on growth and survival of spotted sand bass larvae, Paralabrax maculatofasciatus, at two early weaning times. J World Aquacult Soc 39:22–36
Clay TA, Suchy MD, Ferrara AM, Fontenot QC (2011) Early growth and survival of larval alligator gar, Atractosteus spatula, reared on artifical floating feed with or without a live Artemia spp. supplement. J World Aquacult Soc 42:412–416
Clemmesen C (1987) Laboratory studies on RNA/DNA ratios of starved and fed herring (Clupea harengus) and turbot (Scophthalmus maximus) larvae. ICES J Mar Sci 43:122–128
Cobcroft JM, Pankhurst PM, Poortenaar C, Hickman B, Tait M (2004) Jaw malformation in cultured yellowtail kingfish (Seriola lalandi) larvae. N Z J Mar Freshw Res 38:67–71
Cui K, Cheng D, Ma Z, Qin JG, Jiang S, Sun D, Ma S (2017) Ontogenetic development of digestive enzymes in larval and juvenile crimson snapper Lutjanus erythopterus (Bloch 1790). Aquac Res 48(8):4533–4544
Curnow J, King J, Bosmans J, Kolkovski S (2006) The effect of reduced Artemia and rotifer use facilitated by a new microdiet in the rearing of barramundi Lates calcarifer (BLOCH) larvae. Aquaculture 257:204–213
Diaz MV, Pajaro M, Olivar MP, Martos P, Macchi GJ (2011) Nutritional condition of Argentine anchovy Engraulis anchoita larvae in connection with nursery ground properities. Fish Res 109:330–341
Domeneghini C, Radaelli G, Bosi G, Arrighi A, Di Giancamillo A, Pazzaglia M, Mascarello F (2002) Morphological and histochemical differences in the structure of the alimentary canal in feeding and runt (feed deprived) white sturgeons (Acipenser transmontanus). J Appl Ichthyol 18:341–346
Engrola S, Conceicao LEC, Dias L, Pereira R, Ribeiro L, Dinis MT (2007) Improving weaning strategies for Senegalese sole: effect of body weight and digestive capacity. Aquac Res 38:696–707
Engrola S, Figueira L, Conceição LEC, Gavaia PJ, Ribeiro L, Dinis MT (2009a) Co-feeding in Senegalese sole larvae with inert diet from mouth opening promotes growth at weaning. Aquaculture 288:264–272
Engrola S, Mai M, Dinis MT, Conceição LEC (2009b) Co-feeding of inert diet from mouth opening does not impair protein utilization by Senegalese sole (Solea senegalensis) larvae. Aquaculture 287:185–190
Esteves E, Pina T, Alexandra Chicharo M, Pedro Andrade J (2000) The distribution of estuarine fish larvae: nutrition condition and cooccurrence with predators and prey. Acta Oecol 21:161–173
Faulk CK, Holt GJ (2009) Early weaning of southern flounder, Paralichthys lethostigma, larvae and ontogeny of selected digestive enzymes. Aquaculture 296:213–218
Gisbert E, Conklin DB, Piedrahita RH (2004) Effects of delayed first feeding on the nutritional condition and mortality of California halibut larvae. J Fish Biol 64:116–132
Gronkjer P, Clemmesen C, John MST (1997) Nutritional condition and vertical distribution of Baltic cod larvae. J Fish Biol 51:352–369
Gwak WS, Tanaka M (2001) Developmental change in RNA:DNA ratios of fed and starved laboratory-reared Japanese flounder larvae and juveniles, and its application to assessment of nutritional condition for wild fish. J Fish Biol 59:902–915
Gwak WS, Seikai T, Tanaka M (1999) Evaluation of starvation status of laboratory-reared Japanese flounder Paralichthys olivaceus larvae and juveniles based on morphological and histological characteristics. Fish Sci 65:339–346
Gwak WS, Tsusaki T, Tanaka M (2003) Nutritional condition, as evaluated by RNA/DNA ratios, of hatchery-reared Japanese flounder from hatch to release. Aquaculture 219:503–514
Hamlin HJ, Kling LJ (2001) The culture and early weaning of larval haddock (Melanogrammus aeglefinus) using a microparticulate diet. Aquaculture 201:61–72
Hamza N, Mhetli M, Kestemont P (2007) Effects of weaning age and diets on ontogeny of digestive activities and structures of pikeperch (Sander lucioperca) larvae. Fish Physiol Biochem 33:121–133
Hart PR, Purser GJ (1996) Weaning of hatchery-reared greenback flounder (Rhombosolea tapirina Gunther) from live to artificial diet: effect of age and duration of the changeover period. Aquaculture 145:171–181
Höök TO, Gorokhova E, Hansson S (2008) RNA:DNA ratios of Baltic Sea herring larvae and copepods in embayment and open sea habitats. Estuar Coast Shelf Sci 76:29–35
Hu J, Liu Y, Ma Z, Qin JG (2018) Feeding and development of warm water marine fish larvae in early life. In: Yufera M (ed) Emerging issues in fish larvae research. Springer International Publishing AG, Cham, pp 275–276
Kestemont P, Xueliang X, Hamza N, Maboudou J, Imorou Toko I (2007) Effect of weaning age and diet on pikeperch larviculture. Aquaculture 264:197–204
Lazo JP, Dinis MT, Holt GJ, Faulk C, Arnold CR (2000) Co-feeding microparticulate diets with algae: toward eliminating the need of zooplankton at first feeding in larval red drum (Sciaenops ocellatus). Aquaculture 188:339–351
Le Ruyet JP, Alexandre JC, Thebaud L, Mugnier C (1993) Marine fish larvae feeding: formulated diets or live prey? J World Aquacult Soc 24:211–224
Ma Z, Qin JG, Nie Z (2012) Morphological changes of marine fish larvae and their nutrition need. In: Pourali K, Raad VN (eds) Larvae: morphology, biology and life cycle. Nova Science Publishers, Inc., New York, pp 1–20
Ma Z, Guo H, Zheng P, Wang L, Jiang S, Qin JG, Zhang D (2013) Ontogenetic development of the digestive system in golden pompano, Trachinotus ovatus (Linnaeus 1758). Fish Physiol Biochem 40(4):1157–1167
Ma Z, Qin JG, Hutchinson W, Chen BN, Song L (2014) Responses of digestive enzymes and body lipids to weaning times in yellowtail kingfish Seriola lalandi (Valenciennes, 1833) larvae. Aquac Res 45(6):973–982
Ma Z, Zheng P, Guo H, Zhang N, Wang L, Jiang S, Qin JG, Zhang D (2015) Effect of weaning time on the performance of Trachinotus ovatus (Linnaeus 1758) larvae. Aquac Nutr 21:670–678
Mendoza R, Aguilera C, Montemayor J, Revol A, Holt J (2002) Studies on the physiology of Atractosteus spatula larval development and its implications to early weaning on to artificial diets. In: Cruz-Suarez LE et al (eds) Avances en Nutrition Acuicola VI, Memorias del VI Simposium International de Nutricion Acuicola. Cancun, Quintana Roo, Mexico, pp 197–226
Mendoza R, Aguilera C, Carreon L, Montemayor J, Gonzalez M (2008) Weaning of alligator gar (Atractosteus spatula) larvae to artificial diets. Aquac Nutr 14:223–231
Nhu VC, Dierchens K, Nguyen HT, Hoang TMT, Le TL, Tran MT, Nys C, Sorgeloos P (2010) Effect of early co-feeding and different weaning diets on the performance of cobia (Rachycentron canadum) larvae and juveniles. Aquaculture 305:52–58
Ostaszewska T, Dabrowski K, Palacios ME, Olejniczak M, Wieczorek M (2005) Growth and morphological changes in the digestive tract of rainbow trout (Oncorhynchus mykiss) and pacu (Piaractus mesopotamicus) due to casein replacement with soybean proteins. Aquaculture 245:273–286
Pepin P, Evans GT, Shears TH (1999) Patterns of RNA/DNA ratios in larval fish and their relationship to survival in the field. ICES J Mar Sci 56:697–706
Pilar Olivar M, Diaz MV, Chicharo MA (2009) Tissue effect on RNA:DNA ratios of marine fish larvae. Sci Mar 73:171–182
Rosenlund G, Stoss J, Talbot C (1997) Co-feeding marine fish larvae with inert and live diets. Aquaculture 155:183–191
Sandel E, Nixon O, Lutzky S, Ginsbourg B, Tandler A, Uni Z, Koven W (2010) The effect of dietary phosphatidylcholine/phosphatidylinositol ratio on malformation in larvae and juvenile gilthead sea bream (Sparus aurata). Aquaculture 304:42–48
Sorgeloos P, Dhert P, Candreva P (2001) Use of the brine shrimp, Artemia spp., in marine fish larviculture. Aquaculture 200:147–159
Tanaka Y, Satoh K, Yamada H, Takebe T, Nikaido H, Shiozawa S (2008) Assessment of the nutritional status of field-caught larval Pacific bluefin tuna by RNA/DNA ratio based on a starvation experiment of hatchery-reared fish. J Exp Mar Biol Ecol 354:56–64
Theilacker GH, Porter SM (1995) Condition of larval walleye pollock, Theragra chalcogramma, in the western Gulf of Alaska assessed with histological and shrinkage indices. Fish Bull 93:333–344
Theilacker GH, Watanabe Y (1989) Midgut cell height defines nutritional status of laboratory raised larval northern anchovy, Engraulis mordax. Fish Bull 87:457–469
Yufera M, Pascual E, Polo A, Sarasquete MC (1993) Effect of starvation on the feeding ability of gilthead seabream (Sparus aurata L.) larvae at first feeding. J Exp Mar Biol Ecol 169:259–272
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2022 China Agriculture Press
About this chapter
Cite this chapter
Fang, W., Ma, Z., Qin, J.G. (2022). Weaning Regimes for Golden Pompano Trachinotus ovatus Larvae. In: Ma, Z., Yu, G., Qin, J.G. (eds) Ontogenetic development of pompano Trachinotus ovatus. Springer, Singapore. https://doi.org/10.1007/978-981-19-1712-7_3
Download citation
DOI: https://doi.org/10.1007/978-981-19-1712-7_3
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-19-1711-0
Online ISBN: 978-981-19-1712-7
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)