Abstract
Fluid management in the brain-injured neurological patient revolves around the primary aim of maintenance of adequate cerebral blood flow and prevention of intracranial hypertension. The type and tonicity of the maintenance fluid have a tremendous impact on the development of secondary brain injuries and hence outcome in these patients. Historically, 0.9% sodium chloride has been the most frequently administered intravenous fluid in neurosurgical practice. However with a growing body of evidence raising fundamental concerns regarding the hyperchloremia caused by 0.9% saline, novel balanced intravenous solutions have emerged as an attractive alternative in clinical neurosurgery. These “balanced” solutions derive their denomination from being buffered with precursors of bicarbonate and hence more closely mimic plasma electrolyte composition, particularly with regard to their chloride content. With desirable features of preservation of acid-base and electrolyte balance and preservation of renal function, balanced solutions are now seen to have potential to be an ideal or at least a better fluid choice than 0.9% saline in neurosurgery.
This chapter offers a comprehensive and updated review on balanced intravenous solutions, firstly, by providing a physiological background of balanced solutions; secondly, by summarizing their potential pathophysiologic effects; and lastly, by presenting the clinical evidence available at the present time to support or refute their use in specific neurosurgical scenarios.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
FormalPara Practice Points/Clinical Pearls-
1.
Fluid management in both routine neurosurgery and neurocritical care should be targeted at euvolemia using isotonic fluids.
-
2.
Balanced fluid prevents hyperchloremia and better preserves acid-base milieu, electrolyte balance, and renal function as compared to saline solutions, with preservation of intracranial pressure.
-
3.
Larger studies are required to investigate the effects of balanced solutions on brain swelling and neurological recovery in specific neuropathological disorders.
Introduction
Fluid management in brain-injured patients has several distinct requisites not seen with other non-neurological patients:
-
1.
Tonicity of the transfused intravenous fluid has a significant impact on the cerebral dynamics.
-
2.
Fluid-induced/fluid-aggravated cerebral edema produces an antagonistic volume-pressure intracranial relationship, resulting in impairment of both cerebral blood flow and oxygenation.
-
3.
Cerebral perfusion pressure goal-directed fluid therapy is intrinsically challenging due to the need for sophisticated CBF and cerebral oxygenation monitoring.
However, the above goal of maintenance of cerebral oxygenation and adequate CPP is met by unique challenges for anesthesiologists and intensivists involved in fluid management of the neurosurgical patient [1]. While on one hand brain-injured patients are often on diuretics for prevention or treatment of cerebral edema and/or intracranial hypertension, they may also require infusion of large quantities of intravenous fluids to correct preoperative dehydration and/or to maintain perioperative hemodynamics as mandated for the management of vasospasm or intraoperative blood loss [1]. The above concerns make appropriate type and composition of the perioperative intravenous fluid an important determinant in the outcome.
While searching for the “ideal” intravenous solution for the brain injured, and with increasing evidence that the commonly used crystalloid solution, 0.9% NaCl, may be harmful [2], novel crystalloid solutions are finding a foothold in neurosurgical practice, namely, intravenous “balanced solutions” [2, 3]. At the present time, no conclusive human data exists, concerning the impact of different compositions of intravenous fluids and balanced solutions in particular, on the injured brain to make firm recommendations.
This chapter will address some of the physical determinants of water movement between the intravascular space and the central nervous system (CNS) and the current evidence surrounding balanced salt solutions in detail. These factors would help make some reasonable recommendations regarding balanced fluid therapy in the neurological patient.
Water Movement Across the Blood-Brain Barrier: Determinants and Physiopathology
The three properties of the blood that are amenable to manipulation with intravenous fluids include [2, 3]:
-
1.
Osmotic pressure (determined by concentrations of small and large molecules)
-
2.
Colloid oncotic pressure (COP; determined by large molecules alone)
-
3.
Hematocrit
-
1.
Osmotic pressure: This is the hydrostatic force that equalizes the concentration of water on both sides of an impermeable membrane. Osmolarity describes the molar number of osmotically active particles per liter of solution. In practice, the osmolarity of a solution can be “calculated” by adding up the mEq concentrations of the various ions in the intravenous fluid. Osmolality describes the molar number of osmotically active particles per kilogram of solvent. This value is directly “measured” by determining either the freezing point or the vapor pressure of the solution. For most dilute salt solutions, osmolality is equal to or slightly less than osmolarity.
-
2.
Colloid oncotic pressure: COP is the osmotic pressure generated by large molecules (e.g., albumin, hetastarch).
-
3.
Hematocrit: Fluid infusion is often accompanied by a reduction in hematocrit. In the normal brain, this hemodilution and decreased arterial oxygen content is often compensated by an increase in cerebral blood flow (CBF) [4,5,6,7]. However, it is important to realize that in the backdrop of brain injury, the normal CBF responses to hemodilution and hypoxia are attenuated, and both changes may contribute to secondary tissue damage [4,5,6,7]. A hematocrit level of 30–33% provides the ideal combination of viscosity and O2 carrying capacity, and may improve neurological outcome [1, 4]. However, marked hemodilution (HCT < 30%) exacerbates neurologic injury [6].
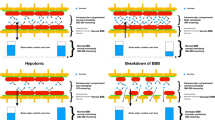
The fluid movement across the BBB is determined by the “total” osmotic gradient, generated both by large molecules and small ions (Fig. 1). Since there are very few protein (large) molecules, their effect on serum osmolality is minimal. Also this has an attenuated effect on the alterations in colloid oncotic pressure. These differences explain the peripheral edema due to dilutional reduction of COP and not cerebral edema with the administration of large volumes of isotonic crystalloids [2, 8, 9]. When plasma osmolality decreases, the osmotic gradient drives water into the brain tissue. Even small changes in plasma osmolality (<5%) increase brain water content and ICP [2, 10, 11]. The above scenario describes the situation in conditions of normal BBB (Fig. 1). After a brain lesion, according to the severity of the damage (head trauma, tumor, seizure, abscess, or other damage), there can be varying degrees of loss of BBB integrity, which can respond differently to the osmotic/oncotic changes. With complete breakdown of the BBB, no osmotic gradient can be established [1, 15, 16]. It is possible that with a less severe injury to the BBB, the barrier may function similarly to the peripheral tissue [11]. Finally, there is usually a significant portion of the brain where the BBB is normal. The presence of a functionally intact BBB is essential if osmotherapy is to be successful [10,11,12].
Implications in Designing an Intravenous Fluid for Neurosurgical Population
-
First is the requisite for osmolarity (hence, the need for electrolytes present in the solution) to be as close as possible to plasma osmolarity. There is definite evidence of increased cerebral edema and intracranial hypertension, consequent to infusion of hypoosmotic solutions in the already injured brain.
-
Second is the need, in specific clinical scenarios, for the presence of oncotic pressure in the intravenous fluid. This is imperative to contain or restrict the infused volume within the intravascular compartment.
Intravenous “Balanced” Solutions
Definition
The term “balanced fluids” encompasses intravenous solutions having an electrolyte composition equal to or closest to the plasma composition and also displaying electrical neutrality (total free cations = total free anions) [11,12,13,14,15]. These concepts have further been expanded to the manufacture of “balanced colloid solutions” in which the colloid molecules are incorporated into “balanced” solvents.
Historically, 0.9% sodium chloride has been the most frequently administered intravenous fluid in neurosurgical practice. However, the perioperative use of these saline solutions is seen to be associated with hyperchloremia with its attendant ill effects. With the aim to avoid these deleterious effects of supraphysiological concentrations of chloride as in 0.9% NaCl, more recently, researchers have broadened the term “balanced” solution to also indicate intravenous solutions with low chloride content as compared to plasma [11].
Balanced Fluids and Acid-Base Balance
Bearing the above assumptions in mind, there are two important aspects which need to be considered while designing an ideal “balanced fluid.”
Firstly, most of the available intravenous solutions (excepting 0.9% NaCl and pure dextrose-containing solutions) have organic anions added to them (e.g., acetate, lactate, malate, gluconate, etc.), as precursors of HCO3 in order to balance the total cations (Table 1) [11, 12]. This is necessary as the process of including HCO3 directly to the intravenous fluid is complex and often unrealistic.
Secondly, to meet the goals of maintaining electrical neutrality and to avoid both hypotonicity and a high strong ion difference (SID, hence maintaining the Na+-Cl– difference between 24 and 30 mEq L−1), balanced salt solutions have been developed with a relatively high concentration of chloride (Table 1).
Conversely, with the mounting evidence of the deleterious renal effects of supraphysiologic Cl–-containing solutions, balanced fluids have been expected to have lower chloride content as compared to plasma.
Composition of Balanced Fluids: Qualitative and Quantitative
It would be easy to understand from the previous section that two classifications of intravenous “balanced” solutions are available:
-
(1)
Balanced solutions with minimal effect on acid-base equilibrium, with a SID value close to 24–29 mEq L−1, e.g., lactated Ringer’s, acetated Ringer’s, Sterofundin ISO, Hartmann’s solution, Tetraspan, and Hextend
-
(2)
Balanced solutions with a Cl– content equal or lower than 110 mEq L−1, e.g., lactated Ringer’s, acetated Ringer’s, Hartmann’s solution, Plasma-Lyte, Elo-Mel Isoton, Isoplex, and Gelaspan (Table 1).
Thus, it is apparent that an “ideal” balanced solution meeting all these three goals of acid-base neutrality, iso-osmolarity, and physiological chloride concentrations cannot be realized in a single intravenous formulation or rather has not been designed so far. All the available balanced solutions fit into just one of the categories described above (i.e., have an effect on acid-base equilibrium with normal chloride content or vice versa) or when fitting into both present with obvious limitations of hypotonicity, as in Hartmann’s solution, acetated Ringer’s, and lactated Ringer’s [12, 15].
Electrolyte Content of Balanced Solutions and the Neurological Patient
Apart from Na + and Cl-, the other important aspect related to “balanced” solutions and bearing relevance in the neurosurgical patient concerns the content of specific electrolytes, in particular of magnesium, calcium, and potassium.
Magnesium and Cerebral Vasospasm
Magnesium, one of the most ubiquitous cations in the body, has been shown to cross the blood-brain barrier in humans and animals [16,17,18,19]. In the context of cerebral vasospasm, its role as a physiologic calcium antagonist and hence a potent vasodilator has been the subject of most interest, although some neuroprotective effects have also been documented [16, 17]. One extensively studied therapy in vasospasm is magnesium (Mg) both as a competitive antagonist of calcium at the N-methyl-D-aspartate (NMDA) receptor and a noncompetitive antagonist of both IP3 and voltage-gated calcium channels, leading to smooth muscle relaxation.
Hypomagnesemia, with plasma levels less than 1.5 mg dL−1, is a relatively common finding in critically ill neurological patients admitted to ICU. Though the evidence is sparse, low magnesium levels are associated with poor neurological outcome in patients with TBI, seizures, stroke, and cerebral vasospasm [17,18,19].
Hence, when dealing with fluid replacement, it may be prudent to employ intravenous fluids containing magnesium, in order to prevent hypomagnesemia. With this rationale, the newer generation of “balanced” fluids (Plasma-Lyte, Sterofundin ISO) has been developed with the inclusion of magnesium, as compared to the older generation (lactated Ringer’s, or Hartmann’s solution) (see Table 1). Though there are no studies investigating the effects of balanced solutions on the incidence of hypomagnesemia, it would be reasonable to favor the use of these novel balanced solutions, in neurological situations warranting large volume replacements.
Calcium and Neurological Outcome
Hypocalcemia is the commonest electrolyte abnormality observed in acutely ill patients [24] with well-documented deleterious consequences like alterations in muscle contractility, peripheral and central nervous system function, and cardiac dysrhythmias. Hypocalcemia has been implicated as a prognostic factor in mortality and morbidity in patients with stroke and in moderate and severe traumatic brain injury [24].
Though it seems an attractive option to include Ca2+ in an ideal “balanced” solution, especially in the face of large volume replacements, it is offset by the risk of precipitation as Ca2+-citrate or Ca2+-carbonate when infused through a common vascular access. This limitation underlies the rationale for development of Ca2+-free intravenous balanced solutions (Table 1).
Potassium Content
Hypokalemia is a life-threatening electrolyte abnormality often observed in the critically ill and emergent neurosurgical patient [23]. All the available intravenous balanced fluids present a concentration of K+ within normal ranges. This feature however should not be erroneously considered a reason for preferring the use of 0.9% NaCl as the only intravenous solution potentially applicable in the case of the neurological patients with acute or chronic renal failure.
Balanced Solutions Versus 0.9% Normal Saline in the Neurological Patient-Clinical Evidence
Although owing to its osmotic benefits, 0.9% sodium chloride (saline) has been the most popular intravenous fluid in neurological practice [20, 21, 25, 26], there is perpetuating evidence of the supraphysiological chloride content in normal saline to cause hyperchloremia [22, 23], acidosis [26, 27], renal vasoconstriction [28], acute renal injury [29, 36], hypotension [30], inflammation, and death [29,30,31].
Chloride-mediated renal vasoconstriction [29, 30] and decreased renal perfusion have been implicated to be mechanisms behind the renal dysfunction caused by 0.9% sodium chloride with higher rates of acute kidney injury [29], renal replacement therapy [35], and death [33,34,35] with saline than with balanced crystalloids, although results have been inconsistent [35]. Recently, several human studies reported that metabolic acidosis, vasoconstriction, and AKI are less pronounced when using a balanced salt solution, which has a physiologic level of chloride and a neutral pH, compared to 0.9% saline [34, 35] in the critically ill patient. However, BC also have potential side effects such as hyperlactacidemia [14] and may exacerbate alkalosis. This is because all BC are relatively alkaline compared to NS [37]. A recent meta-analysis of fluid resuscitation with high versus low chloride content solutions in the perioperative or critical care settings observed fewer days on mechanical ventilation with the use of balanced crystalloids [32].
On the question of neurological effects of normal saline versus balanced crystalloid, such as lactated Ringer’s solution or Plasma-Lyte A, there are still no high-quality data in neurocritically ill patients. Albeit few, all the prospective randomized trials that have been conducted in the neurosurgical population have indicated that balanced intravenous fluids provide significantly better control over acid-base balance, sodium, and chloride levels, without increasing intracranial pressure when used as intraoperative fluid maintenance and replacement during elective neurosurgery and in the brain-injured patient [38,39,40,41]. In these balanced solutions, the use of malate and acetate allows for the reduction of chloride concentration while ensuring isotonicity. Though the isotonic balanced solutions have a lower osmolarity than normal saline, they have no adverse effects on intracranial pressure due to their lower chloremia-induced neuronal chloride ion efflux, thus limiting brain swelling despite decreased osmolarity compared with 0.9%.
One study has shown that combination of a balanced crystalloid with colloid results in lower serum chloride concentrations and maintenance of acid-base balance compared to unbalanced crystalloid in combination with an unbalanced colloid [38].
At the present time, balanced fluids can at best be said to have the potential to be a better alternative to 0.9% normal saline in the neurosurgical patient, but barring high-quality evidence, firm recommendations cannot be made.
Balanced Fluids in Specific Neurological Scenarios
Traumatic Brain Injury
In TBI, a blunt or penetrating injury incites mechanical and autodigestive destruction of the normally tightly intact endothelium of the blood-brain barrier. This allows uncontrolled movement of fluid and serum proteins into the brain parenchyma, eventually leading to vasogenic cerebral edema and increased ICP. Intracranial hypertension (ICH) is the primary cause of secondary brain insult and death after brain injury [39]. These patients are prone to ion homeostasis disruption, plausibly due to hormonal dysfunction such as diabetes insipidus and cerebral salt wasting syndrome or through alterations of chloride-dependent channels such as the NKCC1 transporter [39, 41, 42].
Thus, administration of hypo-osmolar solutions should be avoided in brain-injured patients [39, 43]. Hyposmolarity and hyperchloremia with its attendant acidosis are touted to be the two major modifiable implicates of cerebral edema after brain injury.
Chloride channels regulate cell edema [39, 42], and dyschloremia contributes to brain swelling. With the recognition that chloride-rich fluids are the primary cause of hyperchloremic acidosis in critically ill neurological patients [2], a general chloride-restrictive strategy can be recommended for decreased incidence of renal failure and improved neuronal homeostasis.
Balanced solutions have been inconclusively proven to decrease the incidence of hyperchloremic acidosis, in patients with severe brain injury as compared with saline solutions. This feature along with the isotonicity of the balanced solutions may authorize their utilization in the neuro-ICU, but few data are available to make firm recommendations. The Neuro-Intensive Care and Emergency Medicine (NICEM) Section of the European Society of Intensive Care Medicine consensus document stated that HES is not recommended in the context of brain injury [44]. The effect of albumin or artificial colloids in isotonic fluids on outcome in traumatic brain injury has not been investigated.
Subarachnoid Hemorrhage (SAH)
Secondary brain injuries related to cerebral vasospasm and consecutive ischemic brain injury and intracerebral edema are the main contributors to mortality and morbidity in these patients. Hyponatremia and hypovolemia secondary to cerebral salt wasting have been identified as the two most important culprits for these cerebral events.
Traditionally, patients with SAH have been managed with normal saline for baseline and substitution requirements. If hyponatremia is more severe or significant cerebral edema exists, the use of mild hypertonic fluids (1.25 or 1.5% saline) and strict avoidance of free water administration are usually successful in reversing the hyponatremia.
The guidelines of the American Heart Association [45] recommend that volume contraction be treated with isotonic fluids (Class IIa, Level of Evidence B) and that “administration of large volumes of hypotonic fluids should generally be avoided after SAH (Class I, Level of Evidence B).” Similarly, guidelines of the Neurocritical Care Society for the management of patients with SAH recommend maintaining euvolemia and avoiding both prophylactic hypervolemia therapy and fluid restriction to treat hyponatremia [46] (high-quality evidence; strong recommendation). Neither set of guidelines addresses the composition of baseline fluid administration in patients without oral intake.
In SAH, standard fluid management with saline may have alternatives with more balanced solutions resulting in more stable electrolytes, less fluid intake, and less activation of the pituitary axis stress hormones (cortisol, TSH) [40].
A randomized, double-blind trial demonstrates a greater incidence of hyperchloremia, hyperosmolality, and positive fluid balance with 0.9% saline in early SAH, with balanced solutions not causing frequent hyponatremia or hypoosmolality [40]. Awaiting the results of few ongoing studies [47] and need for larger studies, no appropriate conclusions can be drawn on the beneficial effects of balanced crystalloids in SAH.
Ischemic Injury
The 2018 AHA/ASA guidelines for the early management of patients with acute ischemic stroke [48] stress upon the use of isotonic fluids rather than hypotonic fluids (might exacerbate ischemic brain edema). AHA/ASA recommendations for the management of cerebral and cerebellar infarction with swelling make similar recommendations advocating the use of isotonic fluids [49]. Balanced intravenous fluids being isotonic seem to be an attractive option in such scenarios.
Elective Supratentorial Craniotomy for Brain Tumors
The existing evidence, albeit sparse, indicates more stable electrolytes, particularly chloride, magnesium, calcium, and phosphate levels as well as a preserved acid-base balance with balanced fluids especially during prolonged elective supratentorial craniotomy, all distinct advantages over 0.9% NS. Day et al. published a study comparing normal saline with Plasmalyte as intraoperative maintenance fluid during craniotomy for excision of brain tumors [53]. Like the findings of the earlier studies, they find that the acid-base status and renal profile were better with Plasmalyte. Two novel findings of this study were the preservation of brain relaxation and a significantly lower level of neutrophil gelatinase-associated lipocalin (NGAL), a biomarker of kidney injury with the use of Plasmalyte for resuscitation.
Perceived advantages of balanced crystalloid solutions over non-buffered solutions are reflected in the British Consensus Guidelines on Intravenous Fluid Therapy for Adult Surgical Patients (GIFTASUP) [55] that recommend the use of balanced solutions for crystalloid fluid resuscitation or replacement.Evidence level 1b1–6.
Major Spine Surgery
The combined physiologic effects of prone positioning and the risk of substantial blood loss pose patients undergoing multilevel spine surgery at high risk for intraoperative hemodynamic instability. Emerging evidence suggest that balanced crystalloids and third-generation colloids in balanced salt solution are potentially safer with no significant effect on coagulation when compared to crystalloids [56, 57].
Dose of Balanced Intravenous Fluids for Perioperative Replacement/Resuscitation: How Much Is “Balanced?”
As a general ordinance, intraoperative balanced fluids when used should be administered at a rate sufficient to replace the urinary and insensible losses. As a rough guide, 1 L of isotonic crystalloid and 1 L of hetastarch result in 250 ml and 750 ml of the intravascular volume expansion, respectively. Current data indicates that as long as normal serum osmolality and oncotic pressure are maintained and cerebral hydrostatic pressures are not markedly increased (e.g., due to true volume overload and elevated right heart pressures), volume replacement will not have any effect on cerebral edema and ICP.
In the critically ill neurological patient, the goal of fluid administration is to maintain near normal serum osmolality by repeated monitoring of this parameter. Any reduction in osmolality should be strictly avoided. When feasible, fluid management based on volumetric hemodynamic monitoring like CVP or pulmonary artery occlusion pressure (PAOP)-directed management and transpulmonary thermodilution (TPT) techniques [54] could provide real-time and more accurate guides to fluid management in the critically ill neurological patient.
Lactated Ringer’s when administered in small volumes (not strictly isoosmotic, measured osmolality 252–255 mOsm/kg) are not likely to be detrimental, and can be used safely. However, when the clinical situation mandates large volume replacements (blood loss or other source of volume loss), a change to a more isotonic balanced fluid is advisable. With large volume loss, a combination of isotonic crystalloids and colloids may be the most prudent choice. The combined use of these fluids can avoid reductions both in serum osmolality and COP. Even when administered in a balanced substrate, hetastarch should be used with caution due to coagulation factor VIII depletion when administered at volumes >1000 mL [52]. The newer formulation, pentastarch with minimal effects on coagulation, presents as a better alternative [51]. Future investigations to determine the end point of fluid resuscitation should focus on the parameters of cerebral perfusion and oxygenation where direct effects of systemic fluid management on the brain are examined, such as PBrO2 [50].
The Clinical Equipoise
The SPLIT trial, the first large randomized controlled trial prospectively comparing the effects of a balanced solution (Plasma-Lyte 148) with those of 0.9% NaCl in critically ill patients, showed, unexpectedly, precise equipoise between the two treatments, although presenting important limitations [14]. However, the small number of neurological patients included in this study makes it difficult to draw appropriate conclusions in the neurocritically ill.
Conclusion
Indubitably, intravenous balanced fluids are gaining a foothold in clinical neurosurgery owing to their multitude of physiologically relevant advantages. However, the actual translation of such physiological effects into clinically relevant outcomes is still unclear, especially in the high-risk neurosurgical population (sepsis, trauma, AKI) exposed to larger amounts of such fluids.
Moreover, the “ideal” intravenous balanced solution incorporating all the defined characteristics (electrolyte content equal to plasma with maintenance of acid-base equilibrium) is yet to be made available.
Awaiting multicentric high-quality research on the potential mechanisms underlying their clinical effects and patient-centered clinical outcome in neurosurgery, such fluids continue to be considered as “drugs” and must be used with consideration to physiological rationale, clinical supporting evidence, and awareness of the adverse effects.
References
Tommasino C, Picozzi V. Volume and electrolyte management. Best Pract Res Clin Anaesthesiol. 2007;21:497–516.
Martorano P, Candela C, Colonna R, Agrò FE. Fluid management in neurosurgery. In: Agrò FE, editor. Body fluid management. Milano: Springer; 2013. https://doi.org/10.1007/978-88-470-2661-2_14.
Boer C, Bossers SM, Koning NJ. Choice of fluid type: physiological concepts and perioperative indications. Br J Anaesth. 2018;120(2):384–96. https://doi.org/10.1016/j.bja.2017.10.022. Epub 2017 Dec 1
Zornow MH, Todd MM, More SS. The acute cerebral effects of changes in plasma osmolality and oncotic pressure. Anesthesiology. 1987;67:936–41.
Paulson OB, Strandgaard S, Edvinsson L. Cerebral autoregulation. Cerebrovasc Brain Metab Rev. 1990;2:161–92.
Harrison MJG. Influence of haematocrit in the cerebral circulation. Cerebrovasc Brain Metabol Rev. 1989;1:55–67.
Hudak ML, Koehler RC, Rosenberg AA, et al. Effect of hematocrit on cerebral blood flow. Am J Phys. 1986;251:H63–70.
Tu KY, Heros RC, Karacostas D, et al. Isovolemic hemodilution in experimental focal cerebral ischemia. Part 2: effects on regional cerebral blood flow and size of infarction. J Neurosurg. 1988;69:82–91.
Reasoner DK, Ryu KH, Hindman BJ, et al. Marked hemodilution increases neurologic injury after focal cerebral ischemia in rabbits. AnesthAnalg. 1996;82:61–7.
Hindman BJ, Funatsu N, Cheng DCH, et al. Differential effect of oncotic pressure on cerebral and extracerebral water content during cardiopulmonary bypass in rabbits. Anesthesiology. 1990;73:951–7.
Langer T, Santini A, Scotti E, Van Regenmortel N, Malbrain MLNG, Caironi P. Intravenous balanced solutions: from physiology to clinical evidence. Anaesthesiol Intensive Therapy. 2015;47(1):s78–88. https://doi.org/10.5603/AIT.a2015.0079.
Van der Jagt M. Fluid management of the neurological patient: a concise review. Crit Care. 2016;20:126. https://doi.org/10.1186/s13054-016-1309-2.
Yunos NM, Bellomo R, Hegarty C, et al. Association between a chloride- -liberal vs. Chloride-restrictive intravenous fluid administration strategy and kidney injury in critically ill adults. JAMA. 2012;308:1566–72. https://doi.org/10.1001/jama.2012.13356.
Young P, Bailey M, Beasley R, et al. Effect of a buffered crystalloid solution vs. Saline on acute kidney injury among patients in the intensive care unit: the SPLIT Randomized Clinical Trial. JAMA. 2015;314:1701–10. https://doi.org/10.1001/jama.2015.12334.
Carlesso E, Maiocchi G, Tallarini F, et al. The rule regulating pH changes during crystalloid infusion. Intensive Care Med. 2011;37:461–8. https://doi.org/10.1007/s00134-010-2095-y.
Odom MJ, Zuckerman SL, Mocco J. The role of magnesium in the management of cerebral vasospasm. Neurol Res Int. 2013;2013:943914. https://doi.org/10.1155/2013/943914.
Nayak R, Attry S, Ghosh SN. Serum magnesium as a marker of neurological outcome in severe traumatic brain injury patients. Asian J Neurosurg. 2018;13(3):685–8. https://doi.org/10.4103/ajns.AJNS_232_16.
Alves SC, Tomasi CD, Constantino L, et al. Hypomagnesemia as a risk factor for the non-recovery of the renal function in critically ill patients with acute kidney injury. Nephrol Dial Transplant. 2013;28:910–6. https://doi.org/10.1093/ndt/gfs268.
Soliman HM, Mercan D, Lobo SS, et al. Development of ionized hypomagnesemia is associated with higher mortality rates. Crit Care Med. 2003;31:1082–7.
Gattinoni L, Carlesso E, Cadringher P, Caironi P. Strong ion difference in urine: new perspectives in acid-base assessment. Crit Care. 2006;10:137.
Reid F, Lobo DN, Williams RN, et al. (Ab)normal saline and physiological Hartmann’s solution: a randomized double-blind crossover study. ClinSci (Lond). 2003;104:17–24.
Lee JW. Fluid and electrolyte disturbances in critically ill patients. Electrolyte Blood Press. 2010;8:72–81. https://doi.org/10.5049/EBP.2010.8.2.72.
Finfer S, Liu B, Taylor C, et al. Resuscitation fluid use in critically ill adults: an international cross-sectional study in 391 intensive care units. Crit Care. 2010;14:R185.
Manuel VR, Martin SA, Juan SR, Fernando MA, Frerk M, Thomas K, Christian H. Hypocalcemia as a prognostic factor in mortality and morbidity in moderate and severe traumatic brain injury. Asian J Neurosurg. 2015;10(3):190–4. https://doi.org/10.4103/1793-5482.161171.
Awad S, Allison SP, Lobo DN. The history of 0.9% saline. Clin Nutr. 2008;27:179–88.
Kellum JA, Bellomo R, Kramer DJ, Pinsky MR. Etiology of metabolic acidosis during saline resuscitation in endotoxemia. Shock. 1998;9:364–8.
Wilcox CS. Regulation of renal blood flow by plasma chloride. J Clin Invest. 1983;71:726–35.
Zhou F, Peng Z-Y, Bishop JV, Cove ME, Singbartl K, Kellum JA. Effects of fluid resuscitation with 0.9% saline versus a balanced electrolyte solution on acute kidney injury in a rat model of sepsis. Crit Care Med. 2014;42(4):e270–8.
Chowdhury AH, Cox EF, Francis ST, Lobo DN. A randomized, controlled, double-blind crossover study on the effects of 2-L infusions of 0.9% saline and Plasma-Lyte 148 on renal blood flow velocity and renal cortical tissue perfusion in healthy volunteers. Ann Surg. 2012;256:18–24.
Kellum JA, Song M, Venkataraman R. Effects of hyperchloremic acidosis on arterial pressure and circulating inflammatory molecules in experimental sepsis. Chest. 2004;125:243–8.
Kellum JA. Fluid resuscitation and hyperchloremic acidosis in experimental sepsis: improved short-term survival and acid-base balance with Hextend compared with saline. Crit Care Med. 2002;30:300–5.
Krajewski ML, Raghunathan K, Paluszkiewicz SM, Schermer CR, Shaw AD. Meta-analysis of high- versus low-chloride content in perioperative and critical care fluid resuscitation. Br J Surg. 2015;102:24–36.
Raghunathan K, Shaw A, Nathanson B, et al. Association between the choice of IV crystalloid and in-hospital mortality among critically ill adults with sepsis. Crit Care Med. 2014;42:1585–91.
Hammond DA, Lam SW, Rech MA, Smith MN, Westrick J, Trivedi AP, Balk RA. Balanced crystalloids versus saline in critically ill adults: a systematic review and meta-analysis. Ann Pharmacother. 2020;54(1):5–13. https://doi.org/10.1177/1060028019866420. Epub 2019 Jul 31
Shaw A. Models of preventable disease: contrast-induced nephropathy and cardiac surgery-associated acute kidney injury. Contrib Nephrol. 2011;174:156–62.
Palevsky PM, Molitoris BA, Okusa MD, et al. Design of clinical trials in acute kidney injury: report from an NIDDK workshop on trial methodology. Clin J Am SocNephrol. 2012;7:844–50.
Hafizah M, Liu CY, Ooi JS. Normal saline versus balanced-salt solution as intravenous fluid therapy during neurosurgery: effects on acid-base balance and electrolytes. J Neurosurg Sci. 2017;61(3):263–70. https://doi.org/10.23736/S0390-5616.16.03221-5. Epub 2015 Apr 9
Roquilly A, Loutrel O, Cinotti R, et al. Balanced versus chloride-rich solutions for fluid resuscitation in brain-injured patients: a randomised double-blind pilot study. Crit Care. 2013;17(2):R77. https://doi.org/10.1186/cc12686.
Semler MW, Wanderer JP, Ehrenfeld JM, et al. Balanced crystalloids versus saline in the intensive care unit: the SALT randomized trial. Am J Respir Crit Care Med. 2017;195:1362–72.
Lehmann L, Bendel S, Uehlinger DE, et al. Randomized, double-blind trial of the effect of fluid composition on electrolyte, acid–base, and fluid homeostasis in patients early after subarachnoid hemorrhage. Neurocrit Care. 2013;18:5–12. https://doi.org/10.1007/s12028-012-9764-3.
Audibert G, Hoche J, Baumann A, Mertes PM. Water and electrolytes disorders after brain injury: mechanism and treatment [in French]. Ann Fr Anesth Reanim. 2012;17:e109–15. https://doi.org/10.1016/j.annfar.2012.04.014.
Jayakumar AR, Norenberg MD. The Na-K-Cl co-transporter in astrocyte swelling. Metab Brain Dis. 2010;17:31–8. https://doi.org/10.1007/s11011-010-9180-3.
Carney N, Totten AM, O'Reilly C, Ullman JS, Hawryluk GW, Bell MJ, Bratton SL, Chesnut R, Harris OA, Kissoon N, Rubiano AM, Shutter L, Tasker RC, Vavilala MS, Wilberger J, Wright DW, Ghajar J. Guidelines for the management of severe traumatic brain injury, Fourth Edition. Neurosurgery. 2017;80(1):6–15. https://doi.org/10.1227/NEU.0000000000001432.
Reinhart K, Perner A, Sprung CL, Jaeschke R, Schortgen F, Groeneveld ABJ, Beale R, Hartog CS. European Society of Intensive Care Medicine: consensus statement of the ESICM task force on colloid volume therapy in critically ill patients. Intensive Care Med. 2012;38:368–83.
Bederson JB, Connolly ES Jr, Batjer HH, American Heart Association, et al. Guidelines for the management of aneurysmal subarachnoid hemorrhage: a statement for healthcare professionals from a special writing group of the Stroke Council, American Heart Association. Stroke. 2009;40:994–1025.
Diringer M, Bleck T, Claude Hemphill J, et al. Critical care management of patients following aneurysmal subarachnoid hemorrhage: recommendations from the Neurocritical Care Society’s multidisciplinary consensus conference. Neurocrit Care. 2011;15:211–40.
Crystalloid fluid choice and neurological outcome in patients after subarachnoid haemorrhage (crystallbrain):ongoing trial.
Powers WJ, Rabinstein AA, Ackerson T, Adeoye OM, Bambakidis NC, Becker K, Biller J, Brown M, Demaerschalk BM, Hoh B, Jauch EC, Kidwell CS, Leslie-Mazwi TM, Ovbiagele B, Scott PA, Sheth KN, Southerland AM, Summers DV, Tirschwell DL, American Heart Association Stroke Council. Guidelines for the early management of patients with acute ischemic stroke: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2018;49(3):e46–e110. https://doi.org/10.1161/STR.0000000000000158. Epub 2018 Jan 24. Erratum in: Stroke. 2018 Mar;49(3):e138. Erratum in: Stroke. 2018 Apr 18
Wijdicks EF, Sheth KN, Carter BS, Greer DM, Kasner SE, Kimberly WT, Schwab S, Smith EE, Tamargo RJ, Wintermark M, American Heart Association Stroke Council. Recommendations for the management of cerebral and cerebellar infarction with swelling: a statement for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2014;45(4):1222–38. https://doi.org/10.1161/01.str.0000441965.15164.d6. Epub 2014 Jan 30
Kurtz P, Helbok R, Ko SB, Claassen J, Schmidt JM, Fernandez L, Stuart RM, Connolly ES, Badjatia N, Mayer SA, Lee K. Fluid responsiveness and brain tissue oxygen augmentation after subarachnoid hemorrhage. Neurocrit Care. 2014 Apr;20(2):247–54. https://doi.org/10.1007/s12028-013-9910-6.
Strauss RG, Stansfield C, Henriksen RA, Villhauer PJ. Pentastarch may cause fewer effects on coagulation than hetastarch. Transfusion. 1988;28(3):257–60. https://doi.org/10.1046/j.1537-2995.1988.28388219155.x.
Cully MD, Larson CP Jr, Silverberg GD. Hetastarch coagulopathy in a neurosurgical patient. Anesthesiology. 1987 May;66(5):706–7. https://doi.org/10.1097/00000542-198705000-00030.
Dey A, Adinarayanan S, Bidkar PU, Bangera RK, Balasubramaniyan V. Comparison of normal saline and balanced crystalloid (plasmalyte) in patients undergoing elective craniotomy for supratentorial brain tumors: a randomized controlled trial. Neurol India. 2018;66:1338–44.
Taccone FS, Citerio G. Participants in the international multi-disciplinary consensus conference on multimodality monitoring. Advanced monitoring of systemic hemodynamics in critically ill patients with acute brain injury. Neurocrit Care. 2014;21(Suppl 2):S38–63. https://doi.org/10.1007/s12028-014-0033-5.
Soni N. British consensus guidelines on intravenous fluid therapy for adult surgical patients (GIFTASUP): Cassandra's view. Anaesthesia. 2009;64(3):235–8. https://doi.org/10.1111/j.1365-2044.2009.05886_1.x.
Ongaigui C, Fiorda-Diaz J, Dada O, Mavarez-Martinez A, Echeverria-Villalobos M, Bergese SD. Intraoperative fluid management in patients undergoing spine surgery: a narrative review. Front Surg. 2020;7:45. https://doi.org/10.3389/fsurg.2020.00045.
Jang MS, Han JH, Lee S, Kim SE. Postoperative blood loss and coagulation changes after balanced 6% hydroxyethyl starch 130/0.4 administration during spine surgery: a retrospective study. Clin Spine Surg. 2019 Mar;32(2):E65–70. https://doi.org/10.1097/BSD.0000000000000727.
Author information
Authors and Affiliations
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2022 The Author(s), under exclusive license to Springer Nature Singapore Pte Ltd.
About this chapter
Cite this chapter
Moningi, S., Padhy, S. (2022). Balanced Fluids. In: Prabhakar, H., S Tandon, M., Kapoor, I., Mahajan, C. (eds) Transfusion Practice in Clinical Neurosciences. Springer, Singapore. https://doi.org/10.1007/978-981-19-0954-2_6
Download citation
DOI: https://doi.org/10.1007/978-981-19-0954-2_6
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-19-0953-5
Online ISBN: 978-981-19-0954-2
eBook Packages: MedicineMedicine (R0)