Abstract
Hemodynamic monitoring is widely used in critical care; however, the impact of such intervention in patients with acute brain injury (ABI) remains unclear. Using PubMed, a systematic review was performed (1966–August 2013), and 118 studies were included. Data were extracted using the PICO approach. The evidence was classified, and recommendations were developed according to the GRADE system. Electrocardiography and invasive monitoring of arterial blood pressure should be the minimal hemodynamic monitoring required in unstable or at-risk patients in the intensive care unit. Advanced hemodynamic monitoring (i.e., assessment of preload, afterload, cardiac output, and global systemic perfusion) could help establish goals that take into account cerebral blood flow and oxygenation, which vary depending on diagnosis and disease stage. Choice of techniques for assessing preload, afterload, cardiac output, and global systemic perfusion should be guided by specific evidence and local expertise. Hemodynamic monitoring is important and has specific indications among ABI patients. Further data are necessary to understand its potential for therapeutic interventions and prognostication.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The management of patients with acute brain injury (ABI) includes the diagnosis and management of several medical disorders and complications [1]. Cardiovascular impairment is frequent, e.g., after subarachnoid hemorrhage (SAH), and is associated with increased morbidity and mortality [2, 3]. The complex pathophysiology of these cardiovascular alterations has been directly linked to the neurological injury, with data suggesting involvement of hypothalamic stimulation and/or failure of the autonomic system [4–6]. Different therapeutic interventions to improve cerebral perfusion pressure (CPP), such as hypervolemia or induced hypertension, also can result in cardiac arrhythmias, pulmonary edema, or left ventricular dysfunction [7, 8], which may exacerbate brain injury. In this setting, monitoring systemic hemodynamics can play an important role in avoiding such complications and optimizing cerebral blood flow (CBF) and oxygen delivery [9, 10].
Methods
This systematic review was performed according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement [11].
Search Criteria
Studies were considered eligible based on the PICO approach, which includes (a) Patient population, i.e., critically ill patients with at least one of the following ABI: traumatic brain injury (TBI), SAH, intracranial hemorrhage (ICH), ischemic stroke, coma after cardiac arrest (CA), central nervous system infection, encephalitis, seizures; neurosurgery; (b) Intervention provided, i.e., monitoring of systemic hemodynamics; (c) Controls, i.e., patients with ABI without hemodynamic monitoring or patients without ABI but undergoing hemodynamic monitoring or patients with ABI monitored with two different devices; (d) Outcome endpoints, i.e., mortality, survival with intact neurological function, complications (cardiac, pulmonary, infection), or modification in therapy (including intensity or drug choice). For this review, arterial and central venous lines were considered the minimal monitoring needed in patients with ABI, and mean arterial pressure (MAP) and central venous pressure (CVP) monitoring were used as the “control” group when compared to more advanced monitoring. “Systemic hemodynamic” parameters were divided into the following categories: (a) assessment of systolic and diastolic function [left ventricular ejection fraction (LVEF); rate of rise of left ventricular pressure (dP/dt); continuous ejection fraction; global ejection fraction on trans-pulmonary thermodilution (GEF); esophageal Doppler]; (b) measurement of cardiac output (CO) [i.e., PAC; trans-pulmonary thermodilution (TT); pulse wave contour analysis (PWCA); echocardiography, bioimpedence, etc.]; (c) assessment of preload (pulmonary artery occlusion pressure (PAOP); extravascular lung water (EVLW); global end-diastolic volume (GEV); esophageal flow time corrected (FTC-Doppler)]; (d) assessment of afterload [(systemic vascular resistances (SVR); arterial elastance (Ea)]; (e) assessment of fluid responsiveness [systolic pressure variation (SPV); pulse pressure variation (∆PP); stroke volume variation (SVV); passive leg raising (PLR); pleth variability index)]; (f) adequacy of global perfusion [lactate levels; mixed or central venous saturation; venous-arterial difference in carbon dioxide (∆CO2)]. Evaluation of heart-rate variability or other parameters of autonomic function, and studies using echocardiography to diagnose the etiology of ischemic stroke if no specific hemodynamic parameters were reported were not included in this review.
Information Sources and Search Strategy
Using PubMed, a systematic review of English articles was performed from 1966 through August 15, 2013. The search strategy included the terms “brain injury,” “traumatic brain injury,” “subarachnoid hemorrhage,” “stroke,” “intracranial hemorrhage,” “cardiac arrest,” “seizures,” “epilepsy,” “neurosurgery,” “encephalitis,” “meningitis”—used with one of the following: “hemodynamics,” “hemodynamic monitoring,” “cardiac output,” “ventricular function,” “ejection fraction,” “preload,” “extravascular lung water,” “end-diastolic volume,” “filling pressure,” “venous saturation,” “mixed venous saturation,” “central venous saturation,” “venous-arterial carbon dioxide,” “arterio-venous carbon dioxide difference,” “delta CO2,” “CO2 gap,” “venous CO2,” “lactate,” “fluid responsiveness,” “stroke volume variation,” “systolic pressure variation,” “pulse pressure variation,” “passive leg raising,” “pleth variability index,” “afterload,” “vascular resistances,” and “elastance.” Additional references for relevant studies were also searched from review articles (i.e., defined as “other sources”).
Study Selection and Data Collection
One author independently reviewed all citations, abstracts, and full-text articles to select eligible studies. Excluded were (a) review articles; (b) case reports or case-series with ≤5 patients; (c) experimental studies; (d) studies on pediatric ICU populations (<18 years); (e) studies that were not conducted on ICU patients; (f) studies dealing with brain dead patients. Data were abstracted using a predefined abstraction spreadsheet, according to the PICO system. No attempt was made to re-analyze the data and no meta-analysis was performed since there are insufficient randomized (RCT) or case–control studies.
Review End-Points
The end-points of this review (in patients with ABI) were to answer the following questions:
-
1.
What is the proportion of patients who have altered systemic hemodynamics and how many will develop circulatory failure, inadequate perfusion or organ dysfunction?
-
2.
Can monitoring of systemic hemodynamics help understand the mechanisms of circulatory failure, inadequate perfusion, or organ dysfunction?
-
3.
Does hemodynamic monitoring have a specific role in optimizing brain perfusion and oxygenation or brain-specific therapy?
-
4.
What is the impact of systemic hemodynamic monitoring and related therapies on morbidity, mortality, and neurological outcome?
-
5.
How can fluid responsiveness be assessed in ABI patients?
-
6.
What hemodynamic monitoring is indicated in ABI patients, in particular to diagnose and support the management of unstable or at-risk patients?
Grading of Evidence
The quality of evidence was judged based on the Grades of Recommendation, Assessment, Development, and Evaluation (GRADE) system approach [12].
Literature Summary
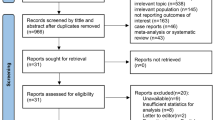
The search retrieved a total of 25,801 citations (Fig. 1), and 118 article met inclusion criteria. The numbers of articles for each disease were 68 for SAH, 12 for TBI, 8 for stroke or ICH, 23 for CA, and 7 for neurosurgery. The search found 4 randomized clinical trials (RCTs), 5 case-controlled studies (all “before-after” studies), 81 prospective/observational studies, and 28 retrospective studies.
Forty-six studies (n = 5,022) included data on cardiac function. Most (38/46) were prospective and focused on SAH; all except three used echocardiography (Table 1). Forty-two studies (n = 4,224) reported data on CO. Most (28/42) were prospective and 27/42 focused on SAH; 3 studies were RCTs (Table 2). Twelve studies (n = 969) reported data on preload assessment. Most were performed in SAH patients (11/12; Tables 3, 4). Five studies (n = 198) reported data on afterload. All evaluated SVR and were mostly performed in SAH patients (4/5). No study evaluated arterial elastance (Table 5). Six studies (n = 250) reported data on fluid responsiveness (Table 6). Twenty studies (n = 3,870) reported data on the adequacy of global perfusion; 3 used ScvO2, 16 measured lactate levels, and only 1 focused on ∆CO2. Most of studies were retrospective (11/20) and evaluated patients after CA (12/20) (Table 6).
What is the Proportion of Patients Who Have Altered Systemic Hemodynamics and How Many Will Develop Circulatory Failure, Inadequate Perfusion or Organ Dysfunction?
Several studies reported altered LVEF after SAH in 2–15 % of patients. [13–15] Similarly, regional wall motion abnormalities (RWMA) were described in 5–45 % of patients [16–19], although RWMA may be present together with normal LVEF [20]. Diastolic dysfunction occurred in 46–89 % patients after SAH [21–23]. Few studies evaluated the incidence of altered CO in patients with ABI. In SAH patients, high CI values were present on admission and progressively diminished on day 5 (from 5.3 ± 0.4 to 3.5 ± 0.2 L/min/m2); higher CI and EVLWI were found in those patients admitted with a poor neurological status (WFNS 4-5) [24]. After ischemic stroke, similar CI values were reported when compared to matched-control subjects; however, patients admitted with poor neurological status had higher CI than others [25]. Finally, low CI was common in the early phase after CA and progressively normalized over time, except in those patients who eventually died in cardiogenic shock [26]. Hypovolemia, suggested by low GEDVI on admission, is frequent after SAH, especially in those patients admitted with a poor neurological status [24].
Can Monitoring of Systemic Hemodynamics Help Understand the Mechanisms of Circulatory Failure, Inadequate Perfusion, or Organ Dysfunction?
Cardiac Function
Among several hypotheses, the main mechanism of cardiac injury following SAH is thought to be associated with sympathetic stimulation and catecholamine release. In one study in 48 SAH patients [27], those with RWMA had significantly higher plasma norepinephrine levels than did those with normal echocardiography (2,098 ± 1,773 vs. 963 ± 839 pg/mL, p = 0.02). Plasma norepinephrine levels also were inversely correlated with LVEF. Multivariate logistic regression analysis revealed that increased plasma norepinephrine levels were predictive of RWMA. Similarly, Sugimoto et al. [28] showed those patients with RWMA on admission had decreased estradiol and elevated norepinephrine levels, and the combination of both significantly increased the risk for RWMA development.
Another important issue was the concomitant presence of altered cardiac function and altered ECG or increased markers of heart injury. SAH patients with cTnI ≥0.3 ng/mL had significantly lower LVEF (52 vs. 63 %, p < 0.001) than others [29]. In addition, 44 % of them had LVEF <50 vs. 5 % in others (p < 0.001). A higher incidence of RWMA among patients with cTnI ≥0.3 ng/mL was found in both early and late phases after SAH [30, 31]. Patients with high cTnI levels also had a higher incidence of diastolic dysfunction [22]. These alterations were associated with normal myocardial perfusion but altered sympathetic innervation [6, 32].
Since pulmonary complications are frequent after ABI, the role of altered cardiac function was evaluated in several studies. Patients who developed pulmonary edema (PE) after SAH had lower global ejection fraction (GEF) than did others [33]. Also, a higher incidence of systolic and diastolic dysfunction was found in patients with PE compared to others after SAH [22, 23]. Naidech et al. [34] reported no association between LV dysfunction and the occurrence of PE, while Junttila et al. [35] showed that the proportion of patients with LVEF <50 % was similar in patients developing neurogenic pulmonary edema (NPE) and those who did not (26 vs. 18 %, p = 0.6). Although patients with NPE often had more a restrictive profile on echography when compared to others (21 vs. 5 %, p = 0.03), filling pressures were similar between groups. Moreover, echocardiographic abnormalities could not predict development of NPE.
Cardiac Output
In patients with SAH, lower CI was reported in patients developing PE compared to others; [36] however, variable hemodynamics, including even high CI after SAH, were found in patients with PE in other studies [37–39].Three studies evaluated changes in CI during the use of therapeutic hypothermia (TH). Cooling resulted in lower heart rate, filling pressure, and CI without deleterious effects on global perfusion in OHCA [40]. TH improved systemic hemodynamics in CA survivors suffering from cardiogenic shock [41]. The use of hypothermia in patients with SAH decreased CI and increased artero-venous jugular difference in oxygen, suggesting a potential role for brain hypoperfusion in this setting [42].
Preload
In three studies, patients with PE or poor oxygenation had a higher ELWI than others [33, 36, 43]. Moreover, PE was associated with higher GEDVI after SAH in another study [33]. However, two other studies reported a poor predictive value of PAOP for PE development [37, 44].
Adequacy of Global Perfusion
Admission lactate levels were significantly higher in patients with shock after CA than the others and were an independent predictor of ICU mortality [45].
Does Hemodynamic Monitoring Have a Specific Role in Optimizing Brain Perfusion and Oxygenation or Brain-Specific Therapy?
Cardiac Output and Preload
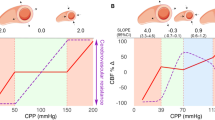
Several studies have shown a relationship between the development of DCI and low CI in SAH patients [33, 44, 46], and different therapeutic interventions have been performed using systemic hemodynamic monitoring in these patients to optimize brain perfusion. Since CBF values are associated with CI [47], the use of a “hyperdynamic” approach (i.e., increase CI optimizing preload and inotropes to increase CBF, Fig. 2) has been observed to be associated with neurological improvement [48–52]. Alternatively, the more traditional “hypertensive” approach (i.e., increase MAP to increase CBF) is used to increase CPP in symptomatic vasospasm after SAH and can improve CBF and brain oxygenation in this setting [53]. Importantly, a “hyperdynamic” approach may still improve neurological status when patients with vasospasm fail to respond to norepinephrine [54].
Afterload
Hadeishi et al. [48] reported that in 8 SAH patients, in whom a PAC was used to optimize therapy for cerebral vasospasm, fluids, and inotropic agents induced an increase of CI (from 3.4 to 4.9 L/min m2) with stable PAOP, while SVR decreased. Patients who develop DCI or symptomatic vasospasm have higher SVRI than others [33, 44]. Finally, the prophylactic use of IABP after SAH was associated with higher SVR than patients treated with a conventional approach but did not affect neurological outcome in this setting [55].
What is the Impact of Systemic Hemodynamic Monitoring and Related Therapies on Morbidity, Mortality, and Neurological Outcome?
Cardiac Function
Several studies have evaluated the impact of hemodynamic alterations on the morbidity and mortality after ABI. Patients with reduced LVEF are more likely to develop ventricular arrhythmias than others (29 vs. 13 %, p = 0.12) [56]. However, there is conflicting data exist on the association between LV dysfunction and survival or neurological outcome after SAH or CA [3, 14, 57–62].
Cardiac Output and Preload
Using a hemodynamic-guided therapy in out-of-hospital cardiac arrest (OHCA), Tagami et al. showed a significant increase of favorable neurological outcome from 0.5 to 3.0 % when compared to an historical cohort, even after adjustment for confounders [63]. The use of such intervention to improve patients’ management was an independent predictor of good outcome. In the sub-group of patients with witnessed ventricular fibrillation, the proportion of patients with good neurological outcome also significantly improved from 7.9 to 26.2 %. In SAH patients, “hyperdynamic” therapy does not always reduce the incidence of TCD-vasospasm or DCI, when compared to norepinephrine [64]. However, the duration of mechanical ventilation (median 8 vs. 19 days, p = 0.01) and ICU stay (11 vs. 21 days, p = 0.01) was less when using dobutamine than norepinephrine. ICU mortality was 18 % for dobutamine and 6 % for norepinephrine (p = 0.33). Kim et al. [65] showed that the occurrence of vasospasm, myocardial infarction, ARDS, and renal failure was similar when using invasive hemodynamic monitoring with PAC in SAH patients compared to non-monitored patients. However, the use of PAC was associated with a reduced incidence of pulmonary edema (6 vs. 14 %, p = 0.003) and sepsis (3 vs. 11 %, p < 0.001). Reduced 6-month mortality was observed using PAC (34 vs. 29 % p = 0.04). Others have observed that the use of TT was associated with significantly less TCD-vasospasm (50 vs. 66 %, p = 0.03), delayed neurological deficit (32 vs. 48 %, p = 0.03), and vasospasm-related infarctions (6 vs. 14 %, p = 0.049) than conventional therapy after SAH [66]. The use of TT also was associated with a reduced number of cardiopulmonary complications (from 12 to 2 %, p = 0.01) and reduced maximal daily fluid intake. Others have observed that the prophylactic use of hypertensive therapy does not reduce the incidence of delayed neurological deficit; instead it increases the occurrence of PE [67].
Adequacy of Global Perfusion
In TBI patients, ScvO2 values are lower in non-survivors (n = 22; 67 ± 12 %) than in survivors (n = 99; 70 ± 9 %, p = 0.04). ScvO2 ≤65 % had a relative risk for increased mortality of 2.3 (95 % CI 1.1–4.8); however, ScvO2 was not an independent predictor of mortality [68]. Gaieski et al. [69] showed that early goal-directed hemodynamic optimization (EGHO) of patients after CA using a target ScvO2 of ≥65 % (Fig. 3) was associated with reduced mortality when compared with historical controls (10/20, 50 % vs.14/18, 78 %; p = 0.15). Similarly, Walters et al. [70] showed better neurological outcome in patients treated with EGHO and TH when compared to historical controls (31 vs. 12 %, p = 0.08).
Optimization of systemic hemodynamics in comatose patients after cardiac arrest. Adapted from [81]
Several studies have described lactate levels in CA patients with varied results. Starodub et al. [71] observed that initial serum lactate (divided in three groups; <5 mmol/L; 5–10 mmol/L; >10 mmol/L) was not associated with mortality. An association with serial lactate levels or lactate clearance and outcome is observed. For example, Kliegel et al. observed that lactate levels were significantly higher in non-survivors than survivors on admission, at 24 and 48 h in patients surviving OHCA [72]. However, lactate levels returned within normal ranges within 24 h in most of patients. A higher proportion of non-survivors had hyperlactatemia (defined as lactate >2 mmol/L) at 48 h than survivors (31 vs. 14 %, p < 0.001), and lactate levels at 48 h were independently associated with mortality. In the same study, similar results were reported when patients were categorized as good (n = 161) or poor (n = 233) neurological outcome. Some studies have shown that, rather than measuring admission lactate levels, the rate of decline in lactate concentration, the so-called “lactate clearance” reflects the improvement of global perfusion during therapy. A higher lactate clearance is independently associated with good outcome in this setting [73].
How Can Fluid Responsiveness be Assessed in ABI Patients?
Berkenstadt et al. [20] studied stroke volume variation in 15 patients undergoing elective neurosurgery; 140 fluid loadings were performed. Half of them were associated with fluid responsiveness (FR). Li et al. [74] examined FR in 48 patients undergoing brain surgery, and SVV was the best predictor of FR (defined as an increase of stroke volume >10 %), while other common hemodynamic variables, including MAP, CVP, and CO, did not discriminate between fluid responders and non-responders. Moretti et al. [75] showed that the inferior vena cava distensibility (dIVC) assessed on echocardiography was a strong predictor of FR in patients with SAH, with an AUC of 0.902 (95 % CI 0.73–0.98). A dIVC >16 % yielded a sensitivity of 71 % and a specificity of 100 % to predict FR. After SAH, changes in GEDVI after fluid loading are associated with changes in SV, while changes in PAOP and CVP were not [66]. The GEDVI had an AUC of 0.73 to predict FR (defined as an increase of stroke volume >10 %) in this setting.
What Hemodynamic Monitoring is Indicated in ABI Patients, in Particular to Diagnose and Support the Management of Unstable or At-Risk Patients?
Eight studies (n = 458) reported data on a specific technique/device to monitor CO and 3 (n = 71) to monitor FR in ABI patients. No study compared different parameters of preload, afterload, or global perfusion in patients with ABI. The main advantages and disadvantages of different monitoring devices are listed in Table 7.
In SAH patients receiving hyperdynamic therapy, the PCWA technique has good accuracy to measure CI when TT was used as reference; [76] the coefficient of agreement was 0.77, and the bias was 0.33 L/min m2 with a percentage of error of 15 %. The incidence of side effects, cerebral infarction, maximal dobutamine doses, and neurological outcome was similar when patients were managed with one of the two techniques. In patients with SAH treated with a hyperdynamic approach, CI measured by TT showed a good correlation (r 2 = 0.78) [66] with the PAC measurements, with a bias 0.05 L/min m2, a precision of 0.11 L/min m2 and a percentage of error of 14 %. In SAH patients undergoing brain surgery, PCWA-measured CI showed a good correlation with TT (r = 0.82); The percentage of error was higher when measurements were collected during mechanical ventilation than on spontaneous breathing [77]. In patients undergoing brain surgery, an important bias on CO measurement was reported (1.7 L/min), with limits of agreement of −2.4 to 5.4 L/min when a PCWA was compared to TT [78]. The percentage of error was 45 %. The bias was larger in those patients receiving norepinephrine and nimodipine, but not in those receiving dobutamine (Table 8). There was a significant negative correlation between SVR and bias. Others have observed a poor agreement between echocardiography, and PAC was reported to measure CO in SAH patients receiving aggressive fluid therapy [79]. After CA, TT, and not PCWA devices, appears to be a reliable technique to assess CO during TH [80, 81]. Finally other studies show that ΔPP or SVV was equivalent to assess FR in ABI patients [75, 82].
Abbreviations
- ABI:
-
Acute brain injury
- CA:
-
Cardiac arrest
- CBF:
-
Cerebral blood flow
- CO:
-
Cardiac output
- CPP:
-
Cerebral perfusion pressure
- CVP:
-
Central venous pressure
- DCI:
-
Delayed cerebral ischemia
- ∆CO2 :
-
Venous-arterial difference in carbon dioxide
- dICV:
-
Inferior vena cava distensibility
- dP/dt:
-
Rate of rise of left ventricular pressure
- Ea:
-
Arterial elastance
- EGHO:
-
Early goal-directed hemodynamic optimization
- EVLW:
-
Extravascular lung water
- FR:
-
Fluid responsiveness
- FTC:
-
Flow time corrected
- GEF:
-
Global ejection fraction
- GEDVI:
-
Global end-diastolic volume index
- ICH:
-
Intracranial hemorrhage
- ICU:
-
Intensive care unit
- LVEF:
-
Left ventricular ejection fraction
- MAP:
-
Mean arterial pressure
- NPE:
-
Neurogenic pulmonary edema
- OHCA:
-
Out-of-hospital cardiac arrest
- PAC:
-
Pulmonary artery catheter
- PAOP:
-
Pulmonary artery occlusion pressure
- PE:
-
Pulmonary edema
- PLR:
-
Passive leg raising
- PWCA:
-
Pulse contour wave analysis
- RCT:
-
Randomized clinical trial
- RWMA:
-
Regional wall motion abnormalities
- SAH:
-
Subarachnoid hemorrhage
- SPV:
-
Systolic pressure variation
- SVR:
-
Systemic vascular resistances
- SVV:
-
Stroke volume variation
- TBI:
-
Traumatic brain injury
- TCD:
-
Transcranial Doppler
- TH:
-
Therapeutic hypothermia
- TT:
-
Trans-pulmonary thermodilution
References
Wartenberg KE, Schmidt JM, Claassen J, Temes RE, Frontera JA, Ostapkovich N, Parra A, Connolly ES, Mayer SA. Impact of medical complications on outcome after subarachnoid hemorrhage. Crit Care Med. 2006;34(3):617–23.
Mascia L, Sakr Y, Pasero D, Payen D, Reinhart K, Vincent JL, Sepsis Occurrence in Acutely Ill Patients (SOAP) Investigators. Extracranial complications in patients with acute brain injury: a post hoc analysis of the SOAP study. Intensive Care Med. 2008;34(4):720–7.
Temes RE, Tessitore E, Schmidt JM, Naidech AM, Fernandez A, Ostapkovich ND, Frontera JA, Wartenberg KE, Di Tullio MR, Badjatia N, Connolly ES, Mayer SA, Parra A. Left ventricular dysfunction and cerebral infarction from vasospasm after subarachnoid hemorrhage. Neurocrit Care. 2010;13(3):359–65.
Doshi R, Neil-Dwyer G. Hypothalamic and myocardial lesions after subarachnoid haemorrhage. J Neurol Neurosurg Psychiatry. 1977;40:821–6.
Goldstein B, Toweill D, Lai S, Sonnenthal K, Kimberly B. Uncoupling of the autonomic and cardiovascular systems in acute brain injury. Am J Physiol. 1998;275(4 Pt 2):R1287–92.
Banki NM, Kopelnik A, Dae MW, Miss J, Tung P, Lawton MT, Drew BJ, Foster E, Smith W, Parmley WW, Zaroff JG. Acute neurocardiogenic injury after subarachnoid hemorrhage. Circulation. 2005;112(21):3314–9.
Contant CF, Valadka AB, Gopinath SP, Hannay HJ, Robertson CS. Adult respiratory distress syndrome: a complication of induced hypertension after severe head injury. J Neurosurg. 2001;95(4):560–8.
Taccone FS, Lubicz B, Piagnerelli M, Van Nuffelen M, Vincent JL, De Backer D. Cardiogenic shock with stunned myocardium during triple-H therapy treated with intra-aortic balloon pump counterpulsation. Neurocrit Care. 2009;10(1):76–82.
Lazaridis C. Advanced hemodynamic monitoring: principles and practice in neurocritical care. Neurocrit Care. 2012;16(1):163–9.
Vincent JL, Rhodes A, Perel A, Martin GS, Della Rocca G, Vallet B, Pinsky MR, Hofer CK, Teboul JL, de Boode WP, Scolletta S, Vieillard-Baron A, De Backer D, Walley KR, Maggiorini M, Singer M. Clinical review: update on hemodynamic monitoring: a consensus of 16. Crit Care. 2011;15(4):229.
Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, Ioannidis JP, Clarke M, Devereaux PJ, Kleijnen J, Moher D. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ. 2009;339:b2700.
Atkins D, Best D, Briss PA, et al. Grading quality of evidence and strength of recommendations. BMJ. 2004;328:1490.
Bulsara KR, McGirt MJ, Liao L, Villavicencio AT, Borel C, Alexander MJ, Friedman AH. Use of the peak troponin value to differentiate myocardial infarction from reversible neurogenic left ventricular dysfunction associated with aneurysmal subarachnoid hemorrhage. J Neurosurg. 2003;98(3):524–8.
Banki N, Kopelnik A, Tung P, Lawton MT, Gress D, Drew B, Dae M, Foster E, Parmley W, Zaroff J. Prospective analysis of prevalence, distribution, and rate of recovery of left ventricular systolic dysfunction in patients with subarachnoid hemorrhage. J Neurosurg. 2006;105(1):15–20.
Jung JH, Min PK, Rim SJ, Ha JW, Chung N, Lee KC. Are electrocardiographic changes in patients with acute subarachnoid hemorrhage associated with Takotsubo cardiomyopathy? Cardiology. 2010;115(2):98–106.
Mayer SA, LiMandri G, Sherman D, Lennihan L, Fink ME, Solomon RA, DiTullio M, Klebanoff LM, Beckford AR, Homma S. Electrocardiographic markers of abnormal left ventricular wall motion in acute subarachnoid hemorrhage. J Neurosurg. 1995;83(5):889–96.
Pollick C, Cujec B, Parker S, Tator C. Left ventricular wall motion abnormalities in subarachnoid hemorrhage: an echocardiographic study. J Am Coll Cardiol. 1988;12(3):600–5.
Davies KR, Gelb AW, Manninen PH, Boughner DR, Bisnaire D. Cardiac function in aneurysmal subarachnoid haemorrhage: a study of electrocardiographic and echocardiographic abnormalities. Br J Anaesth. 1991;67(1):58–63.
Khush K, Kopelnik A, Tung P, Banki N, Dae M, Lawton M, Smith W, Drew B, Foster E, Zaroff J. Age and aneurysm position predict patterns of left ventricular dysfunction after subarachnoid hemorrhage. J Am Soc Echocardiogr. 2005;18(2):168–74.
Berkenstadt H, Margalit N, Hadani M, Friedman Z, Segal E, Villa Y, Perel A. Stroke volume variation as a predictor of fluid responsiveness in patients undergoing brain surgery. Anesth Analg. 2001;92(4):984–9.
Sandvei MS, Amundsen BH, Haugen BO, Støylen A, Slørdahl SA, Vik A. Left ventricular myocardial function during the acute phase of a subarachnoid haemorrhage. Scand Cardiovasc J. 2009;43(2):110–6.
Tanabe M, Crago EA, Suffoletto MS, Hravnak M, Frangiskakis JM, Kassam AB, Horowitz MB, Gorcsan J 3rd. Relation of elevation in cardiac troponin I to clinical severity, cardiac dysfunction, and pulmonary congestion in patients with subarachnoid hemorrhage. Am J Cardiol. 2008;102(11):1545–50.
Kopelnik A, Fisher L, Miss JC, Banki N, Tung P, Lawton MT, Ko N, Smith WS, Drew B, Foster E, Zaroff J. Prevalence and implications of diastolic dysfunction after subarachnoid hemorrhage. Neurocrit Care. 2005;3(2):132–8.
Mutoh T, Kazumata K, Ajiki M, Ushikoshi S, Terasaka S. Goal-directed fluid management by bedside transpulmonary hemodynamic monitoring after subarachnoid hemorrhage. Stroke. 2007;38(12):3218–24.
Treib J, Haass A, Krammer I, Stoll M, Grauer MT, Schimrigk K. Cardiac output in patients with acute stroke. J Neurol. 1996;243(8):575–8.
Laurent I, Monchi M, Chiche JD, Joly LM, Spaulding C, Bourgeois B, Cariou A, Rozenberg A, Carli P, Weber S, Dhainaut JF. Reversible myocardial dysfunction in survivors of out-of-hospital cardiac arrest. J Am Coll Cardiol. 2002;40(12):2110–6.
Sugimoto K, Inamasu J, Kato Y, Yamada Y, Ganaha T, Oheda M, Hattori N, Watanabe E, Ozaki Y, Hirose Y. Association between elevated plasma norepinephrine levels and cardiac wall motion abnormality in poor-grade subarachnoid hemorrhage patients. Neurosurg Rev. 2013;36(2):259–66.
Sugimoto K, Inamasu J, Hirose Y, Kato Y, Ito K, Iwase M, Sugimoto K, Watanabe E, Takahashi A, Ozaki Y. The role of norepinephrine and estradiol in the pathogenesis of cardiac wall motion abnormality associated with subarachnoid hemorrhage. Stroke. 2012;43(7):1897–903.
Naidech AM, Kreiter KT, Janjua N, Ostapkovich ND, Parra A, Commichau C, Fitzsimmons BF, Connolly ES, Mayer SA. Cardiac troponin elevation, cardiovascular morbidity, and outcome after subarachnoid hemorrhage. Circulation. 2005;112(18):2851–6.
Parekh N, Venkatesh B, Cross D, Leditschke A, Atherton J, Miles W, Winning A, Clague A, Rickard C. Cardiac troponin I predicts myocardial dysfunction in aneurysmal subarachnoid hemorrhage. J Am Coll Cardiol. 2000;36(4):1328–35.
Tung P, Kopelnik A, Banki N, Ong K, Ko N, Lawton MT, Gress D, Drew B, Foster E, Parmley W, Zaroff J. Predictors of neurocardiogenic injury after subarachnoid hemorrhage. Stroke. 2004;35(2):548–51.
Abdelmoneim SS, Wijdicks EF, Lee VH, Daugherty WP, Bernier M, Oh JK, Pellikka PA, Mulvagh SL. Real-time myocardial perfusion contrast echocardiography and regional wall motion abnormalities after aneurysmal subarachnoid hemorrhage. Clinical article. J Neurosurg. 2009;111(5):1023–8.
Watanabe A, Tagami T, Yokobori S, Matsumoto G, Igarashi Y, Suzuki G, Onda H, Fuse A, Yokota H. Global end-diastolic volume is associated with the occurrence of delayed cerebral ischemia and pulmonary edema after subarachnoid hemorrhage. Shock. 2012;38(5):480–5.
Naidech AM, Bassin SL, Garg RK, Ault ML, Bendok BR, Batjer HH, Watts CM, Bleck TP. Cardiac troponin I and acute lung injury after subarachnoid hemorrhage. Neurocrit Care. 2009;11(2):177–82.
Junttila E, Ala-Kokko T, Ohtonen P, Vaarala A, Karttunen A, Vuolteenaho O, Salo T, Sutinen M, Karhu T, Herzig KH, Koskenkari J. Neurogenic pulmonary edema in patients with nontraumatic intracerebral hemorrhage: predictors and association with outcome. Anesth Analg. 2013;116(4):855–61.
Sato Y, Isotani E, Kubota Y, Otomo Y, Ohno K. Circulatory characteristics of normovolemia and normotension therapy after subarachnoid hemorrhage, focusing on pulmonary edema. Acta Neurochir (Wien). 2012;154(12):2195–202.
Deehan SC, Grant IS. Haemodynamic changes in neurogenic pulmonary oedema: effect of dobutamine. Intensive Care Med. 1996;22(7):672–6.
Vespa PM, Bleck TP. Neurogenic pulmonary edema and other mechanisms of impaired oxygenation after aneurysmal subarachnoid hemorrhage. Neurocrit Care. 2004;1(2):157–70.
Schulte Esch J, Murday H, Pfeifer G. Haemodynamic changes in patients with severe head injury. Acta Neurochir (Wien). 1980;54(3-4):243–50.
Bergman R, Braber A, Adriaanse MA, van Vugt R, Tjan DH, van Zanten AR. Haemodynamic consequences of mild therapeutic hypothermia after cardiac arrest. Eur J Anaesthesiol. 2010;27(4):383–7.
Zobel C, Adler C, Kranz A, Seck C, Pfister R, Hellmich M, Kochanek M, Reuter H. Mild therapeutic hypothermia in cardiogenic shock syndrome. Crit Care Med. 2012;40(6):1715–23.
Sato K, Sato K, Yoshimoto T. Systemic and cerebral haemodynamics during craniotomy under mild hypothermia in patients with acute subarachnoid haemorrhage. Acta Neurochir (Wien). 2000;142(9):1013–9.
Touho H, Karasawa J, Shishido H, Yamada K, Yamazaki Y. Neurogenic pulmonary edema in the acute stage of hemorrhagic cerebrovascular disease. Neurosurgery. 1989;25(5):762–8.
Mayer SA, Lin J, Homma S, Solomon RA, Lennihan L, Sherman D, Fink ME, Beckford A, Klebanoff LM. Myocardial injury and left ventricular performance after subarachnoid hemorrhage. Stroke. 1999;30(4):780–6.
Lemiale V, Dumas F, Mongardon N, Giovanetti O, Charpentier J, Chiche JD, Carli P, Mira JP, Nolan J, Cariou A. Intensive care unit mortality after cardiac arrest: the relative contribution of shock and brain injury in a large cohort. Intensive Care Med. 2013;39(11):1972–80.
Yousef K, Crago E, Kuo CW, Horowitz M, Hravnak M. Predictors of delayed cerebral ischemia after aneurysmal subarachnoid hemorrhage: a cardiac focus. Neurocrit Care. 2010;13(3):366–72.
Tone O, Tomita H, Tamaki M, Hara M, Inaji M. Correlation between cardiac output and cerebral blood flow following subarachnoid hemorrhage. Keio J Med. 2000;49(Suppl 1):A151–3.
Hadeishi H, Mizuno M, Suzuki A, Yasui N. Hyperdynamic therapy for cerebral vasospasm. Neurol Med Chir (Tokyo). 1990;30(5):317–23.
Tanabe T, Saitoh T, Tachibana S, Takagi H, Yada K. Effect of hyperdynamic therapy on cerebral ischaemia caused by vasospasm associated with subarachnoid haemorrhage. Acta Neurochir (Wien). 1982;63(1–4):291–6.
Otsubo H, Takemae T, Inoue T, Kobayashi S, Sugita K. Normovolaemic induced hypertension therapy for cerebral vasospasm after subarachnoid haemorrhage. Acta Neurochir (Wien). 1990;103(1–2):18–26.
Finn SS, Stephensen SA, Miller CA, Drobnich L, Hunt WE. Observations on the perioperative management of aneurysmal subarachnoid hemorrhage. J Neurosurg. 1986;65(1):48–62.
Mori K, Arai H, Nakajima K, Tajima A, Maeda M. Hemorheological and hemodynamic analysis of hypervolemic hemodilution therapy for cerebral vasospasm after aneurysmal subarachnoid hemorrhage. Stroke. 1995;26(9):1620–6.
Muench E, Horn P, Bauhuf C, Roth H, Philipps M, Hermann P, Quintel M, Schmiedek P, Vajkoczy P. Effects of hypervolemia and hypertension on regional cerebral blood flow, intracranial pressure, and brain tissue oxygenation after subarachnoid hemorrhage. Crit Care Med. 2007;35(8):1844–51.
Levy ML, Rabb CH, Zelman V, Giannotta SL. Cardiac performance enhancement from dobutamine in patients refractory to hypervolemic therapy for cerebral vasospasm. J Neurosurg. 1993;79(4):494–9.
Bulters DO, Birch AA, Hickey E, Tatlow I, Sumner K, Lamb R, Lang D. A randomized controlled trial of prophylactic intra-aortic balloon counterpulsation in high-risk aneurysmal subarachnoid hemorrhage. Stroke. 2013;44(1):224–6.
Frangiskakis JM, Hravnak M, Crago EA, Tanabe M, Kip KE, Gorcsan J 3rd, Horowitz MB, Kassam AB, London B. Ventricular arrhythmia risk after subarachnoid hemorrhage. Neurocrit Care. 2009;10(3):287–94.
Vannemreddy P, Venkatesh P, Dinesh K, Reddy P, Nanda A. Myocardial dysfunction in subarachnoid hemorrhage: prognostication by echo cardiography and cardiac enzymes. A prospective study. Acta Neurochir Suppl. 2010;106:151–4.
Sugimoto K, Watanabe E, Yamada A, Iwase M, Sano H, Hishida H, Ozaki Y. Prognostic implications of left ventricular wall motion abnormalities associated with subarachnoid hemorrhage. Int Heart J. 2008;49(1):75–85.
Chang WT, Ma MH, Chien KL, Huang CH, Tsai MS, Shih FY, Yuan A, Tsai KC, Lin FY, Lee YT, Chen WJ. Postresuscitation myocardial dysfunction: correlated factors and prognostic implications. Intensive Care Med. 2007;33(1):88–95.
Kuwagata Y, Oda J, Ninomiya N, Shiozaki T, Shimazu T, Sugimoto H. Changes in left ventricular performance in patients with severe head injury during and after mild hypothermia. J Trauma. 1999;47(4):666–72.
Urbaniak K, Merchant AI, Amin-Hanjani S, Roitberg B. Cardiac complications after aneurysmal subarachnoid hemorrhage. Surg Neurol. 2007;67(1):21–8.
Yarlagadda S, Rajendran P, Miss JC, Banki NM, Kopelnik A, Wu AH, Ko N, Gelb AW, Lawton MT, Smith WS, Young WL, Zaroff JG. Cardiovascular predictors of in-patient mortality after subarachnoid hemorrhage. Neurocrit Care. 2006;5(2):102–7.
Tagami T, Hirata K, Takeshige T, Matsui J, Takinami M, Satake M, Satake S, Yui T, Itabashi K, Sakata T, Tosa R, Kushimoto S, Yokota H, Hirama H. Implementation of the fifth link of the chain of survival concept for out-of-hospital cardiac arrest. Circulation. 2012;126(5):589–97.
Rondeau N, Cinotti R, Rozec B, Roquilly A, Floch H, Groleau N, Michel P, Asehnoune K, Blanloeil Y. Dobutamine-induced high cardiac index did not prevent vasospasm in subarachnoid hemorrhage patients: a randomized controlled pilot study. Neurocrit Care. 2012;17(2):183–90.
Kim DH, Haney CL, Van Ginhoven G. Reduction of pulmonary edema after SAH with a pulmonary artery catheter-guided hemodynamic management protocol. Neurocrit Care. 2005;3(1):11–5.
Mutoh T, Kazumata K, Ishikawa T, Terasaka S. Performance of bedside transpulmonary thermodilution monitoring for goal-directed hemodynamic management after subarachnoid hemorrhage. Stroke. 2009;40(7):2368–74.
Medlock MD, Dulebohn SC, Elwood PW. Prophylactic hypervolemia without calcium channel blockers in early aneurysm surgery. Neurosurgery. 1992;30(1):12–6.
Di Filippo A, Gonnelli C, Perretta L, Zagli G, Spina R, Chiostri M, Gensini GF, Peris A. Low central venous saturation predicts poor outcome in patients with brain injury after major trauma: a prospective observational study. Scand J Trauma Resusc Emerg Med. 2009;21(17):23.
Gaieski DF, Band RA, Abella BS, Neumar RW, Fuchs BD, Kolansky DM, Merchant RM, Carr BG, Becker LB, Maguire C, Klair A, Hylton J, Goyal M. Early goal-directed hemodynamic optimization combined with therapeutic hypothermia in comatose survivors of out-of-hospital cardiac arrest. Resuscitation. 2009;80(4):418–24.
Walters EL, Morawski K, Dorotta I, Ramsingh D, Lumen K, Bland D, Clem K, Nguyen HB. Implementation of a post-cardiac arrest care bundle including therapeutic hypothermia and hemodynamic optimization in comatose patients with return of spontaneous circulation after out-of-hospital cardiac arrest: a feasibility study. Shock. 2011;35(4):360–6.
Starodub R, Abella BS, Grossestreuer AV, Shofer FS, Perman SM, Leary M, Gaieski DF. Association of serum lactate and survival outcomes in patients undergoing therapeutic hypothermia after cardiac arrest. Resuscitation. 2013;84(8):1078–82.
Kliegel A, Losert H, Sterz F, Holzer M, Zeiner A, Havel C, Laggner AN. Serial lactate determinations for prediction of outcome after cardiac arrest. Medicine (Baltimore). 2004;83(5):274–9.
Donnino MW, Miller J, Goyal N, Loomba M, Sankey SS, Dolcourt B, Sherwin R, Otero R, Wira C. Effective lactate clearance is associated with improved outcome in post-cardiac arrest patients. Resuscitation. 2007;75(2):229–34.
Li J, Ji FH, Yang JP. Evaluation of stroke volume variation obtained by the FloTrac™/Vigileo™ system to guide preoperative fluid therapy in patients undergoing brain surgery. J Int Med Res. 2012;40(3):1175–81.
Moretti R, Pizzi B. Inferior vena cava distensibility as a predictor of fluid responsiveness in patients with subarachnoid hemorrhage. Neurocrit Care. 2010;13(1):3–9.
Mutoh T, Ishikawa T, Kobayashi S, Suzuki A, Yasui N. Performance of Third-generation FloTrac/Vigileo system during hyperdynamic therapy for delayed cerebral ischemia after subarachnoid hemorrhage. Surg Neurol Int. 2012;3:99.
Mutoh T, Ishikawa T, Nishino K, Yasui N. Evaluation of the FloTrac uncalibrated continuous cardiac output system for perioperative hemodynamic monitoring after subarachnoid hemorrhage. J Neurosurg Anesthesiol. 2009;21(3):218–25.
Junttila EK, Koskenkari JK, Ohtonen PP, Ala-Kokko TI. Uncalibrated arterial pressure waveform analysis for cardiac output monitoring is biased by low peripheral resistance in patients with intracranial haemorrhage. Br J Anaesth. 2011;107(4):581–6.
Mayer SA, Sherman D, Fink ME, Homma S, Solomon RA, Lennihan L, Beckford A, Klebanoff LM. Noninvasive monitoring of cardiac output by Doppler echocardiography in patients treated with volume expansion after subarachnoid hemorrhage. Crit Care Med. 1995;23(9):1470–4.
Haenggi M, Barthelmes D, Ulmer H, Takala J, Jakob SM. Comparison of non-calibrated pulse-contour analysis with continuous thermodilution for cardiac output assessment in patients with induced hypothermia after cardiac arrest. Resuscitation. 2011;82(4):423–6.
Tagami T, Kushimoto S, Tosa R, Omura M, Hagiwara J, Hirama H, Yokota H. The precision of PiCCO® measurements in hypothermic post-cardiac arrest patients. Anaesthesia. 2012;67(3):236–43.
Deflandre E, Bonhomme V, Hans P. Delta down compared with delta pulse pressure as an indicator of volaemia during intracranial surgery. Br J Anaesth. 2008;100(2):245–50.
Lee VH, Connolly HM, Fulgham JR, Manno EM, Brown RD Jr, Wijdicks EF. Tako-tsubo cardiomyopathy in aneurysmal subarachnoid hemorrhage: an underappreciated ventricular dysfunction. J Neurosurg. 2006;105(2):264–70.
Jacobshagen C, Pelster T, Pax A, Horn W, Schmidt-Schweda S, Unsöld BW, Seidler T, Wagner S, Hasenfuss G, Maier LS. Effects of mild hypothermia on hemodynamics in cardiac arrest survivors and isolated failing human myocardium. Clin Res Cardiol. 2010;99(5):267–76.
Ruiz-Bailén M, de Aguayo Hoyos E, Ruiz-Navarro S, Díaz-Castellanos MA, Rucabado-Aguilar L, Gómez-Jiménez FJ, Martínez-Escobar S, Moreno RM, Fierro-Rosón J. Reversible myocardial dysfunction after cardiopulmonary resuscitation. Resuscitation. 2005;66(2):175–81.
Miss JC, Kopelnik A, Fisher LA, Tung PP, Banki NM, Lawton MT, Smith WS, Dowd CF, Zaroff JG. Cardiac injury after subarachnoid hemorrhage is independent of the type of aneurysm therapy. Neurosurgery. 2004;55(6):1244–50.
Kono T, Morita H, Kuroiwa T, Onaka H, Takatsuka H, Fujiwara A. Left ventricular wall motion abnormalities in patients with subarachnoid hemorrhage: neurogenic stunned myocardium. J Am Coll Cardiol. 1994;24(3):636–40.
Deibert E, Barzilai B, Braverman AC, Edwards DF, Aiyagari V, Dacey R, Diringer M. Clinical significance of elevated troponin I levels in patients with nontraumatic subarachnoid hemorrhage. J Neurosurg. 2003;98(4):741–6.
Hravnak M, Frangiskakis JM, Crago EA, Chang Y, Tanabe M, Gorcsan J 3rd, Horowitz MB. Elevated cardiac troponin I and relationship to persistence of electrocardiographic and echocardiographic abnormalities after aneurysmal subarachnoid hemorrhage. Stroke. 2009;40(11):3478–84.
Kothavale A, Banki NM, Kopelnik A, Yarlagadda S, Lawton MT, Ko N, Smith WS, Drew B, Foster E, Zaroff JG. Predictors of left ventricular regional wall motion abnormalities after subarachnoid hemorrhage. Neurocrit Care. 2006;4(3):199–205.
Apak I, Iltumur K, Tamam Y, Kaya N. Serum cardiac troponin T levels as an indicator of myocardial injury in ischemic and hemorrhagic stroke patients. Tohoku J Exp Med. 2005;205(2):93–101.
Zaroff JG, Rordorf GA, Ogilvy CS, Picard MH. Regional patterns of left ventricular systolic dysfunction after subarachnoid hemorrhage: evidence for neurally mediated cardiac injury. J Am Soc Echocardiogr. 2000;13(8):774–9.
Tung PP, Olmsted E, Kopelnik A, Banki NM, Drew BJ, Ko N, Lawton MT, Smith W, Foster E, Young WL, Zaroff JG. Plasma B-type natriuretic peptide levels are associated with early cardiac dysfunction after subarachnoid hemorrhage. Stroke. 2005;36(7):1567–9.
Meaudre E, Jego C, Kenane N, Montcriol A, Boret H, Goutorbe P, Habib G, Palmier B. B-type natriuretic peptide release and left ventricular filling pressure assessed by echocardiographic study after subarachnoid hemorrhage: a prospective study in non-cardiac patients. Crit Care. 2009;13(3):R76.
McLaughlin N, Bojanowski MW, Girard F, Denault A. Pulmonary edema and cardiac dysfunction following subarachnoid hemorrhage. Can J Neurol Sci. 2005;32(2):178–85.
Jyotsna M, Prasad V, Indrani G, Trikamji BV. Importance of detection of segmental wall motion abnormalities of left ventricle in nontraumatic subarachnoid hemorrhage: a prospective study. Echocardiography. 2010;27(5):496–500.
Papanikolaou J, Makris D, Karakitsos D, Saranteas T, Karabinis A, Kostopanagiotou G, Zakynthinos E. Cardiac and central vascular functional alterations in the acute phase of aneurysmal subarachnoid hemorrhage. Crit Care Med. 2012;40(1):223–32.
Front D, Frankel A, Israel O, Aharon Y, Satinger A, Linn S. Ejection fraction response of the left ventricle of the heart to acute cerebrovascular accident in patients with coronary artery disease. Stroke. 1986;17(4):613–6.
Khan AH, Bunch TJ, White RD, Packer DL. Prognostic implication of early ejection fraction on long-term mortality and quality of life following out-of-hospital cardiac arrest. Am J Cardiol. 2004;93(8):1027–30.
Rzheutskaya RE. Characteristics of hemodynamic disorders in patients with severe traumatic brain injury. Crit Care Res Pract. 2012;2012:606179.
Tamaki T, Isayama K, Yamamoto Y, Teramoto A. Cardiopulmonary haemodynamic changes after severe head injury. Br J Neurosurg. 2004;18(2):158–63.
Nicholls TP, Shoemaker WC, Wo CC, Gruen JP, Amar A, Dang AB. Survival, hemodynamics, and tissue oxygenation after head trauma. J Am Coll Surg. 2006;202(1):120–30.
Hashimoto T, Young WL, Prohovnik I, Gupta DK, Ostapkovich ND, Ornstein E, Halim AX, Quick CM. Increased cerebral blood flow after brain arteriovenous malformation resection is substantially independent of changes in cardiac output. J Neurosurg Anesthesiol. 2002;14(3):204–8.
Torgersen C, Schmittinger CA, Takala J, Jakob SM, Dünser MW. The association between early hemodynamic variables and outcome in normothermic comatose patients following cardiac arrest. Acta Anaesthesiol Scand. 2010;54(8):1027–35.
Torgersen C, Meichtry J, Schmittinger CA, Bloechlinger S, Jakob SM, Takala J, Dünser MW. Haemodynamic variables and functional outcome in hypothermic patients following out-of-hospital cardiac arrest. Resuscitation. 2013;84(6):798–804.
Yamada R, Katsurada K, Sugimoto T. Haemodynamic defect in patients with severe head injury. Injury. 1975;6(4):351–7.
Chatterjee N, Koshy T, Misra S, Suparna B. Changes in left ventricular preload, afterload, and cardiac output in response to a single dose of mannitol in neurosurgical patients undergoing craniotomy: a transesophageal echocardiographic study. J Neurosurg Anesthesiol. 2012;24(1):25–9.
Stoll M, Treib J, Seltmann A, Anton H, Klaus S. Hemodynamics of stroke patients under therapy with low molecular weight hydroxyethyl starch. Neurol Res. 1998;20(3):231–4.
Mutoh T, Ishikawa T, Suzuki A, Yasui N. Continuous cardiac output and near-infrared spectroscopy monitoring to assist in management of symptomatic cerebral vasospasm after subarachnoid hemorrhage. Neurocrit Care. 2010;13(3):331–8.
Kim DH, Joseph M, Ziadi S, Nates J, Dannenbaum M, Malkoff M. Increases in cardiac output can reverse flow deficits from vasospasm independent of blood pressure: a study using xenon computed tomographic measurement of cerebral blood flow. Neurosurgery. 2003;53(5):1044–51.
Miller JA, Dacey RG Jr, Diringer MN. Safety of hypertensive hypervolemic therapy with phenylephrine in the treatment of delayed ischemic deficits after subarachnoid hemorrhage. Stroke. 1995;26(12):2260–6.
Naidech A, Du Y, Kreiter KT, Parra A, Fitzsimmons BF, Lavine SD, Connolly ES, Mayer SA, Commichau C. Dobutamine versus milrinone after subarachnoid hemorrhage. Neurosurgery. 2005;56(1):21–6.
Vermeij FH, Hasan D, Bijvoet HW, Avezaat CJ. Impact of medical treatment on the outcome of patients after aneurysmal subarachnoid hemorrhage. Stroke. 1998;29(5):924–30.
Lennihan L, Mayer SA, Fink ME, Beckford A, Paik MC, Zhang H, Wu YC, Klebanoff LM, Raps EC, Solomon RA. Effect of hypervolemic therapy on cerebral blood flow after subarachnoid hemorrhage: a randomized controlled trial. Stroke. 2000;31(2):383–91.
Verein M, Valiahmedov A, Churliaev Y, Sitnikov P, Redkokasha L, Lukashev K. Dynamics of extravascular pulmonary water and intracranial pressure in patients with ischemic stroke. Semin Cardiothorac Vasc Anesth. 2010;14(4):226–30.
Karagiannis C, Georgiou M, Kouskouni E, Iacovidou N, Xanthos T. Association of lactate levels with outcome after in-hospital cardiac arrest. Resuscitation. 2012;83(8):e175–6.
Cocchi MN, Miller J, Hunziker S, Carney E, Salciccioli J, Farris S, Joyce N, Zimetbaum P, Howell MD, Donnino MW. The association of lactate and vasopressor need for mortality prediction in survivors of cardiac arrest. Minerva Anestesiol. 2011;77(11):1063–71.
Oddo M, Ribordy V, Feihl F, Rossetti AO, Schaller MD, Chioléro R, Liaudet L. Early predictors of outcome in comatose survivors of ventricular fibrillation and non-ventricular fibrillation cardiac arrest treated with hypothermia: a prospective study. Crit Care Med. 2008;36(8):2296–301.
Shinozaki K, Oda S, Sadahiro T, Nakamura M, Hirayama Y, Watanabe E, Tateishi Y, Nakanishi K, Kitamura N, Sato Y, Hirasawa H. Blood ammonia and lactate levels on hospital arrival as a predictive biomarker in patients with out-of-hospital cardiac arrest. Resuscitation. 2011;82(4):404–9.
Müllner M, Sterz F, Domanovits H, Behringer W, Binder M, Laggner AN. The association between blood lactate concentration on admission, duration of cardiac arrest, and functional neurological recovery in patients resuscitated from ventricular fibrillation. Intensive Care Med. 1997;23(11):1138–43.
Adrie C, Cariou A, Mourvillier B, Laurent I, Dabbane H, Hantala F, Rhaoui A, Thuong M, Monchi M. Predicting survival with good neurological recovery at hospital admission after successful resuscitation of out-of-hospital cardiac arrest: the OHCA score. Eur Heart J. 2006;27(23):2840–5.
Zhao QJ, Zhang XG, Wang LX. Mild hypothermia therapy reduces blood glucose and lactate and improves neurologic outcomes in patients with severe traumatic brain injury. J Crit Care. 2011;26(3):311–5.
Yatsushige H, Takasato Y, Masaoka H, Hayakawa T, Otani N, Yoshino Y, Sumiyoshi K, Sugawara T, Miyawaki H, Aoyagi C, Takeuchi S, Suzuki G. Prognosis for severe traumatic brain injury patients treated with bilateral decompressive craniectomy. Acta Neurochir Suppl. 2010;106:265–70.
Meierhans R, Brandi G, Fasshauer M, Sommerfeld J, Schüpbach R, Béchir M, Stover J. Arterial lactate above 2 mM is associated with increased brain lactate and decreased brain glucose in patients with severe traumatic brain injury. Minerva Anestesiol. 2012;78(2):185–93.
Cureton EL, Kwan RO, Dozier KC, Sadjadi J, Pal JD, Victorino GP. A different view of lactate in trauma patients: protecting the injured brain. J Surg Res. 2010;159(1):468–73.
Brouns R, Sheorajpanday R, Wauters A, De Surgeloose D, Mariën P, De Deyn PP. Evaluation of lactate as a marker of metabolic stress and cause of secondary damage in acute ischemic stroke or TIA. Clin Chim Acta. 2008;397(1–2):27–31.
Jo S, Jeong T, Lee JB, Jin YH, Yoon J, Jun YK, Park B. Initial hyperlactatemia in the ED is associated with poor outcome in patients with ischemic stroke. Am J Emerg Med. 2012;30(3):449–55.
Tsaousi GG, Karakoulas KA, Amaniti EN, Soultati ID, Zouka MD, Vasilakos DG. Correlation of central venous-arterial and mixed venous-arterial carbon dioxide tension gradient with cardiac output during neurosurgical procedures in the sitting position. Eur J Anaesthesiol. 2010;27(10):882–9.
Franchi F, Falciani E, Donadello K, Zacà V, Silvestri R, Taccone FS, Cubattoli L, Mongelli P, Giomarelli P, Scolletta S. Echocardiography and pulse contour analysis to assess cardiac output in trauma patients. Minerva Anestesiol. 2013;79(2):137–46.
Acknowledgments
We would like to thank Christos Lazaridis and Sabino Scoletta for their fruitful comments on this manuscript.
Conflict of interest
Giuseppe Citerio receives speaker honoraria from Codman and has received research funding from Italian Governative agencies (AIFA, Ministero Salute, Regione Lombardia). Fabio Taccone none to declare.
Author information
Authors and Affiliations
Consortia
Corresponding author
Additional information
The Participants in the International Multi-disciplinary Consensus Conference on Multimodality Monitoring (see “Appendix”).
Appendix: Participants in the International Multi-disciplinary Consensus Conference on Multimodality Monitoring
Appendix: Participants in the International Multi-disciplinary Consensus Conference on Multimodality Monitoring
Peter Le Roux, MD, FACS
Brain and Spine Center,
Suite 370, Medical Science Building,
Lankenau Medical Center,
100 East Lancaster Avenue, Wynnewood, PA 19096, USA.
Tel: +1 610 642 3005;
Fax: 610 642 3057
email: lerouxp@mlhs.org
David K Menon MD, PhD, FRCP, FRCA, FFICM, FMedSci
Head, Division of Anaesthesia, University of Cambridge,
Consultant, Neurosciences Critical Care Unit,
Box 93, Addenbrooke’s Hospital,
Cambridge CB2 2QQ, UK
email: dkm13@wbic.cam.ac.uk
Paul Vespa, MD, FCCM, FAAN, FNCS
Professor of Neurology and Neurosurgery,
Director of Neurocritical Care,
David Geffen School of Medicine at UCLA
Los Angeles, CA 90095 USA
email: PVespa@mednet.ucla.edu
Giuseppe Citerio,
Director NeuroIntensive Care Unit,
Department of Anesthesia and Critical Care,
Ospedale San Gerardo, Monza.
Via Pergolesi 33, Monza 20900, Italy
email: g.citerio@hsgerardo.org
Mary Kay Bader RN, MSN, CCNS, FAHA, FNCS
Neuro/Critical Care CNS,
Mission Hospital,
Mission Viejo, CA 92691, USA
email: Marykay.Bader@stjoe.org
Gretchen M. Brophy, PharmD, BCPS, FCCP, FCCM
Professor of Pharmacotherapy & Outcomes Science and Neurosurgery,
Virginia Commonwealth University,
Medical College of Virginia Campus,
410 N. 12th Street,
Richmond, VA 23298-0533, USA
email: gbrophy@vcu.edu
Michael N. Diringer, MD
Professor of Neurology, Neurosurgery & Anesthesiology,
Chief, Neurocritical Care Section,
Washington University,
Department of Neurology, Campus Box 8111,
660 S Euclid Ave,
St Louis, MO 63110, USA
email: diringerm@neuro.wustl.edu
Nino Stocchetti, MD
Professor of Anesthesia and Intensive Care,
Department of physiopathology and transplant,
Milan University,
Director Neuro ICU,
Fondazione IRCCS Cà Granda Ospedale Maggiore Policlinico,
Via F Sforza, 35 20122 Milan, Italy
e-mail: stocchet@policlinico.mi.it
Walter Videtta, MD
ICU Neurocritical Care,
Hospital Nacional ‘Prof. a. Posadas’,
El Palomar - Pcia. de Buenos Aires,
Argentina
email: wvidetta@ar.inter.net
Rocco Armonda, MD
Department of Neurosurgery,
MedStar Georgetown University Hospital,
Medstar Health, 3800 Reservoir Road NW,
Washington, DC 20007, USA
email: Rocco.Armonda@gmail.com
Neeraj Badjatia, MD
Department of Neurology,
University of Maryland Medical Center,
22 S Greene St,
Baltimore, MD 21201, USA
email: nbadjatia@umm.edu
Julian Boesel, MD
Department of Neurology,
Ruprect-Karls University,
Hospital Heidelberg, Im Neuenheimer Feld 400,
D-69120 Heidelberg, Germany
email: Julian.Boesel@med.uni-heidelberg.de
Randal Chesnut, MD, FCCM, FACS
Harborview Medical Center,
University of Washington Mailstop 359766,
325 Ninth Ave,
Seattle, WA 98104-2499, USA
email: chesnutr@u.washington.edu
Sherry Chou, MD, MMSc
Department of Neurology,
Brigham and Women’s Hospital,
75 Francis Street,
Boston, MA 02115, USA
email: schou1@partners.org
Jan Claassen, MD, PhD, FNCS
Assistant Professor of Neurology and Neurosurgery,
Head of Neurocritical Care and Medical Director of the Neurological Intensive Care Unit,
Columbia University College of Physicians & Surgeons,
177 Fort Washington Avenue, Milstein 8 Center room 300,
New York, NY 10032, USA
email: jc1439@cumc.columbia.edu
Marek Czosnyka, PhD
Department of Neurosurgery,
University of Cambridge,
Addenbrooke’s Hospital, Box 167,
Cambridge, CB20QQ, UK
email: mc141@medschl.cam.ac.uk
Michael De Georgia, MD
Professor of Neurology,
Director, Neurocritical Care Center,
Co-Director, Cerebrovascular Center,
University Hospital Case Medical Center,
Case Western Reserve University School of Medicine,
11100 Euclid Avenue,
Cleveland, OH 44106, USA
email: michael.degeorgia@uhhospitals.org
Anthony Figaji, MD, PhD
Head of Pediatric Neurosurgery,
University of Cape Town,
617 Institute for Child Health,
Red Cross Children’s Hospital,
Rondebosch, 7700 Cape Town, South Africa
email: anthony.figaji@uct.ac.za
Jennifer Fugate, DO
Department of Neurology,
Mayo Clinic,
200 First Street SW,
Rochester, MN 55905, USA
email: Fugate.Jennifer@mayo.edu
Raimund Helbok, MD
Department of Neurology, Neurocritical Care Unit,
Innsbruck Medical University,
Anichstr.35, 6020,
Innsbruck, Austria
email: raimund.helbok@uki.at
David Horowitz, MD
Associate Chief Medical Officer,
University of Pennsylvania Health System,
3701 Market Street,
Philadelphia, PA 19104, USA
email: david.horowitz@uphs.upenn.edu
Peter Hutchinson, MD
Professor of Neurosurgery,
NIHR Research Professor,
Department of Clinical Neurosciences,
University of Cambridge,
Box 167 Addenbrooke’s Hospital,
Cambridge, CB2 2QQ, UK
email: pjah2@cam.ac.uk
Monisha Kumar, MD
Department of Neurology,
Perelman School of Medicine, University of Pennsylvania,
3 West Gates,
3400 Spruce Street,
Philadelphia, PA 19104, USA
email: monisha.kumar@uphs.upenn.edu
Molly McNett, RN, PhD
Director, Nursing Research,
The MetroHealth System,
2500 MetroHealth Drive,
Cleveland, OH 44109, USA
email: mmcnett@metrohealth.org
Chad Miller, MD
Division of Cerebrovascular Diseases and Neurocritical Care,
The Ohio State University,
395 W. 12th Ave, 7th Floor,
Columbus, OH 43210, USA
email: ChadM.Miller@osumc.edu
Andrew Naidech, MD, MSPH
Department of Neurology,
Northwestern University Feinberg SOM 710,
N Lake Shore Drive, 11th floor,
Chicago, IL 60611, USA
email: ANaidech@nmff.org
Mauro Oddo, MD
Department of Intensive Care Medicine,
CHUV University Hospital, BH 08-623,
Faculty of Biology and Medicine, University of Lausanne,
1011 Lausanne, Switzerland
email: Mauro.Oddo@chuv.ch
DaiWai Olson, RN, PhD
Associate Professor of Neurology, Neurotherapeutics and Neurosurgery,
University of Texas Southwestern,
5323 Harry Hines Blvd.,
Dallas, TX 75390-8897, USA
email: daiwai.olson@utsouthwestern.edu
Kristine O’Phelan, MD
Director of Neurocritical Care,
Associate Professor, Department of Neurology,
University of Miami, Miller School of Medicine,
JMH, 1611 NW 12th Ave, Suite 405,
Miami, FL 33136, USA
email: kophelan@med.miami.edu
Javier Provencio, MD
Associate Professor of Medicine,
Cerebrovascular Center and Neuroinflammation Research Center,
Lerner College of Medicine,
Cleveland Clinic,
9500 Euclid Ave, NC30,
Cleveland, OH 44195, USA
email: provenj@ccf.org
Corina Puppo, MD
Assistant Professor, Intensive Care Unit,
Hospital de Clinicas, Universidad de la República,
Montevideo,
Uruguay
email: coripuppo@gmail.com
Richard Riker, MD
Critical Care Medicine,
Maine Medical Center,
22 Bramhall Street,
Portland, ME 04102-3175, USA
email: RRiker@cmamaine.com
Claudia Robertson, MD
Department of Neurosurgery,
Medical Director of Center for Neurosurgical Intensive Care,
Ben Taub Hospital,
Baylor College of Medicine,
1504 Taub Loop, Houston, TX 77030, USA
email: claudiar@bcm.tmc.edu
J. Michael Schmidt, PhD, MSc
Director of Neuro-ICU Monitoring and Informatics,
Columbia University College of Physicians and Surgeons,
Milstein Hospital 8 Garden South, Suite 331,
177 Fort Washington Avenue,
New York, NY 10032, USA
email: mjs2134@columbia.edu
Fabio Taccone, MD
Department of Intensive Care, Laboratoire de Recherche Experimentale,
Erasme Hospital,
Route de Lennik, 808,
1070 Brussels, Belgium
email: ftaccone@ulb.ac.be
Rights and permissions
About this article
Cite this article
Taccone, F.S., Citerio, G. & And the Participants in the International Multi-disciplinary Consensus Conference on Multimodality Monitoring. Advanced Monitoring of Systemic Hemodynamics in Critically Ill Patients with Acute Brain Injury. Neurocrit Care 21 (Suppl 2), 38–63 (2014). https://doi.org/10.1007/s12028-014-0033-5
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12028-014-0033-5