Abstract
Agricultural sustainability is predominantly regulated by the functional equilibrium between soil processes and productivity of plants. Growth and productivity of plants frequently depend on their intimate association with the microbial organisms present in the rhizosphere. The present chapter summarizes an updated knowledge on the role of microbial signaling in regulating plant growth and also recommends future research prospects in this area. Plant roots release various organic compounds in its surrounding soil, known as root exudates leading to the induction of the beneficial rhizospheric microbes. Microorganisms also modify the plants’ behavior by producing inter-organismal signaling molecules. Plant growth-promoting rhizobacteria (PGPR) play an important role in the signaling, metabolism, and hormonal homeostasis in plants. They also produce antibiotic compounds that inhibit the growth of poisonous rhizospheric microbes, thereby promoting plant growth. The PGPR also increase the availability and uptake of nutrients and provide resistance to abiotic and biotic stresses leading to agricultural sustainability. Free-living beneficial fungi in the soil microbiome efficiently spread over the rhizosphere and eliminate pathogenic fungal strains by competitive inhibition. They also contribute in the antibiotic production and elicitation of defense responses in plants. Signaling process is executed by other classes of molecules, including N-acyl-l-homoserine lactones (AHLs) and microbial volatile organic compounds (MVOCs). The AHLs and MVOCs play a crucial role in the bacterial downstream signaling, by which the bacterial genes may express. These molecules are also recognized by the plants further contributing in the improvement of plant development and defense mechanisms by upregulating different genes.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
14.1 Introduction
Plants play a pivotal role in maintaining the ecosystem. They have been established to have many evolutionary benefits, which actually help them in acclimatizing with the stressful environments. Additionally, several studies have revealed the plants to possess a complicated nutritional organization (Marschner 1995). Plant-microbe interactions comprise of rhizomicrobiome (microbes associated with root), phyllomicrobiome (microbes associated with aboveground tissues), and endosphere (microbial colonization of internal tissues) (Basu and Kumar 2020a). However, rhizomicrobiome has been extensively studied than the other components.
Root system in plants is the most important morphologically and physiologically plastic structure forming a vigorous assembly after entering inside the soil. In addition to providing anchorage, root has other major contributions directly or indirectly associated with the plant growth and development (Kumar et al. 2022a). Root system also directly perceives various environmental stresses (Kumar et al. 2022b; Mishra et al. 2021). Therefore, development of plants eventually depends upon the environment (Kumar et al. 2021). Root in the soil continuously interacts with the microbial community, supporting in the plant development and immunity. Roots also secrete various chemical components, which play a significant role in regulating the phytomicrobiome.
Soil ecosystem plays a crucial role in the assimilation of the organic compounds in soil, deterioration of living particles, eradication of pathogenic components, and detoxification (Behera and Prasad 2020). In the soil ecosystem, the microorganisms survive with the support of soil carbon, which actually comes from the plants’ rhizosphere. It is the zone of soil surrounding the plant root, which harbors a variety of microbes (Shrivastava et al. 2014). Numerous studies have shown the occurrence of root-microbes and intra-inter-microbial interaction through the soil ecosystem, facilitating the soil fertility, plant growth, root and shoot formation, and development of leaf primordia and flowers (Basu et al. 2020a). However, interactions between the rhizosphere and microbes are still not completely understood.
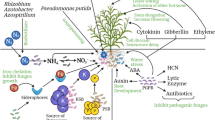
Higher plants interact with their associated microbes in diverse ways affecting each other (Berendsen et al. 2012). Microorganisms modify the plants’ behavior by producing inter-organismal signaling molecules, and plant-synthesized signaling compounds regulate the microbial populations. Thus, plants and microbes alter each other’s behavior for their individual benefit leading to symbiotic association. The signaling in the phytomicrobiome stimulating plant growth has been illustrated in Fig. 14.1.
14.2 Plant Signal: Root Exudates
During the various stages of plant growth, seed germination to flowering the plant interacts with the surrounding soil which has a wide variety of microorganisms. Plant roots release various organic compounds in its surrounding soil, known as root exudates that lead to the improvement of the soil environment (Sarkar et al. 2022). The physical, chemical, and biological nature of the soil changes during the plant growth because of root exudates and rhizodeposition (el Zahar Haichar et al. 2014). This phenomenon of change in nature of surrounding soil is known as the rhizosphere effect. Root exudates are mainly composed of amino acids, organic acids, water-soluble sugars, hormones, vitamins, amino compounds, phenolics, and sugar phosphate esters. It is estimated that around 20–40% of the carbon fixed during photosynthesis is released as exudates from the plant roots (Badri and Vivanco 2009). The nature of the exudates depends on the growth stage of the plant and the environment. Modern ecological theories correlate root exudation for the plants’ benefit for stimulating the beneficial microorganisms, thereby facilitating nutrient acquisition eventually inducing plant growth (Ortiz-Castro et al. 2009).
Based on the chemical nature of the compound, root exudates are categorized into three main classes.
14.2.1 Low-Molecular-Weight Compounds
They contribute to a major portion of root exudates, comprising amino acids, organic acids, phenolics, sugars, vitamins, and several secondary metabolites. These are usually transported through passive transport along the concentration gradient between the root cells and the external (soil) solution.
14.2.2 High-Molecular-Weight Compounds
They include proteins and mucilage.
14.2.3 Volatile Organic Compounds (VOCs)
They include carbon dioxide (CO2), aldehydes, alcohols, and secondary metabolites.
The composition of the root exudates usually varies with plant species, age, and growth stages of plants’ life cycle. Several abiotic factors, including soil type, temperature, light, micro-, and macronutrients also impact the chemical nature and time of exudation (Chai and Schachtman 2021). These factors often increase the rate of the exudation, thereby affecting the membrane integrity. For instance, under nitrogen-deficient conditions, root exudates assist the associations of plants with nitrogen-fixing microbes. Under this circumstance root exudates also inhibit the nitrifying and denitrifying bacteria, thereby reducing soil nitrogen losses. Ramesh et al. (2015) have revealed the anions and γ-aminobutyric acid (GABA) at the root apex to regulate the malate efflux anions channel (ALMT) under abiotic stress. Dong et al. (2004) have found the exudation of organic acids (such as oxalic acid, malic acid, and citric acid) to be predominantly increased in soybean plants under phosphate-deficient and aluminum-contaminated soil conditions. The chemicals present in the root exudates either attract microbes or produce carbon providing nutrition to the microbes.
Ecosystem also influences the nature and signaling cascade of the plant exudates. For instance, microbial infection in the plant root system assists the production of defense-oriented root exudates. The molecular pattern and the concentration of root exudates in soil are complex phenomena, which have not been completely understood so far. These exudates attract soil microbes, thereby establishing the rhizosphere effect. The composition of exudates also affects the microbial populations and activities.
14.3 Rhizosphere
The term “rhizosphere” was coined by a German plant physiologist Lorenz Hiltner in 1904 (Hiltner 1904). Rhizosphere (Greek “rhiza,” root and “sphere,” surrounding area) is defined as the plant root-soil interface or the seed-soil interface (Harley and Russell 1979). It has been described as the area surrounding the plant root (2–80 mm distance from root system), inhabited by distinctive microorganisms inducing the root exudation. It is a region of increased nutrient concentration and biotic activity. Enhanced interactions between the plants and soil microbes can be perceived in this region and, therefore, described as the microbe storehouse. These interactions can be symbiotic (beneficial), pathogenic (harmful), or neutral. The present chapter has discussed the beneficial aspects of the soil microbial interactions and their impact on plant growth and development.
14.3.1 Zones of the Rhizosphere
The rhizosphere is categorized into three zones based on the relative distance from the root (Lynch and Whipps 1991). As the proximity decreases, the influence of the root and its exudates decreases.
14.3.1.1 Endorhizosphere
It is the innermost zone, which includes the cortex and endodermis (internal) where the microbes and cations can occupy the apoplastic space (free space between cells).
14.3.1.2 Rhizoplane
It is the medial zone, which includes the root epidermis and mucilage. It is the root surface directly adjacent to the soil.
14.3.1.3 Exorhizosphere
It is the outermost zone contiguous to the epidermis extending from the rhizoplane out into the bulk soil.
The rhizosphere cannot be defined with a definite area or size due to the diversity and complexity of the plant root system. It is a gradient of physical, chemical, and biological properties changing along the root both radially and longitudinally. The rhizosphere isolated from the bulk soil is termed as edaphosphere (non-rhizosphere), where the rhizosphere effect is negligible or absent. It has 10- to 100-fold less microbial density than the rhizosphere. The rhizosphere is enriched in organic matter and generally more acidic than the edaphosphere. The soil in the rhizosphere is subjected to chemical changes caused by the presence of root exudates and metabolites of microbial degradation.
14.3.2 Rhizosphere Effect and R/E Ratio
The rhizosphere effect indicates enhanced microbial activity in the rhizosphere. It can be quantitatively expressed with R/E ratio, where “R” denotes the amount of root exudates in rhizosphere soil and “E” is the edaphosphere soil content. This ratio is determined from the number of microbes present in the rhizosphere to that of the edaphosphere. The value of R/E ranges within 5–20.
14.3.3 Microbiome in Rhizosphere
Rhizosphere harbors a variety of microbes like bacteria, fungi, actinomycetes, and algae (Campbell and Greaves 1990). Based on the nucleic acid analyses techniques of genomic molecules from soil, the samples have been revealed to exhibit a huge diversity in the rhizospheric microbial population (Basu and Kumar 2021a). Around 98% of the microbes in the soil cannot be cultured, and hence their identification, characterization, and their effect are still not known. Some microbes are in a close association with roots like mycorrhizal fungi. There are a variety of free-living microbes which include filamentous fungi of the genus Trichoderma and a variety of plant growth-promoting rhizobacteria (PGPR) (Prasad et al. 2015). Rhizospheric organisms that have been well studied for their beneficial effects on plant growth and health include the nitrogen-fixing bacteria, mycorrhizal fungi, plant growth-promoting rhizobacteria (PGPR), biocontrol microorganisms, mycoparasitic fungi, and protozoa (Basu and Kumar 2020a, b).
14.3.3.1 Plant Growth-Promoting Rhizobacteria (PGPR)
Plant growth-promoting rhizobacteria (PGPR) are various bacterial members of several taxonomic groups colonizing the rhizosphere. Natural PGPR commonly include various species of Pseudomonas sp. and Bacillus sp. colonizing different plants such as Arabidopsis, barley, and rice. Numerous studies have revealed the PGPR to promote growth and productivity of different agricultural crop species plant (Backer et al. 2018; Tsukanova et al. 2017). They have also been found to confer induced systemic resistance (ISR) and systemic acquired resistance (SAR) consequently reducing phytotoxic microbial populations (Bukhat et al. 2020). PGPR play an important role in the signaling, metabolism, and hormonal homeostasis in plants (Tsukanova et al. 2017). They also produce antibiotic compounds as well as hydrogen cyanide that inhibit the growth of poisonous rhizospheric microbes, thereby promoting plant growth. The PGPR also increase the availability and uptake of nutrients and provide resistance to abiotic and biotic stresses leading to agricultural sustainability.
14.3.3.2 Beneficial Fungi
In the soil microbiome, fungi also play a crucial role in promotion of plant growth. Mycotic populations efficiently spread over the soil and rhizosphere and eliminate the pathogenic fungal strains by competitive inhibition. They also contribute in the antibiotic production and elicitation of defense responses. The beneficial plant fungal populations stabilize the pathogenic microbes by parasitizing their spore (mycoparasitism) and sclerotia, ultimately leading to biocontrol. They also produce chemical compounds and enzymes (e.g., chitinase, glucanase, and protease), which degrade the harmful mycotic populations.
The free-living fungi are beneficial inhabitants of the rhizosphere. Trichoderma sp. belongs to this class and possesses the mycoparasitic capabilities, which is a more predominant attribute of the free-living fungi (Harman et al. 2004). They promote the plant development without showing any detrimental effects on the plants. The root colonization of Trichoderma is associated with the induction of both the local and systemic resistance that is directly influenced by the production of a fungal protein elicitor molecule, designated as small protein1 (Sm1), which lacks toxic activity in plants and microbes (Djonovic et al. 2006). The Sm1 promotes the immunity-related gene expressions in plants. Several studies have shown T. atroviride and T. virens to induce plant growth by producing indole-3-acetic acid (Contreras-Cornejo et al. 2009).
14.4 Microbial Signaling Involved in Plant Growth
Development of plant is driven by multiple factors, including root microbial population as a major contributor. Previous workers have revealed the signaling process to be executed by certain classes of molecules, including phytohormones, N-acyl-l-homoserine lactones (AHLs), and microbial volatile organic compounds (MVOCs) (Mhlongo et al. 2018).
The MVOCs and the AHLs play a crucial role in the bacterial downstream signaling, by which the bacterial genes may express (Ortiz-Castro et al. 2009). The MVOCs are recognized by the plants further contributing in the improvement of plant development and defense mechanisms by upregulating different genes.
In addition, there are many soil bacterial populations that belong to the class of Proteobacteria, which help plants in the uptake of mineral nutrients and nitrogen fixation (Basu and Kumar 2020b). Endophytic microbes help in manipulation of biotic and abiotic stresses in plants (Basu and Kumar 2021a). These evidences establish the existence of a feedback loop system (Mandal et al. 2010).
14.4.1 Phytohormones
Former studies have shown the rhizosphere to potentially contribute the chemical components, which are essential for maintaining the developmental cues in plants (Ortiz-Castro et al. 2009). Phytohormones have been shown to play a crucial role in the plant development, indirectly regulating the nutrient uptake and distribution (Basu et al. 2022). They are produced in root and shoot, but their effectiveness is spatial. Therefore, the shoot and root growth is completely distinct from each other in accordance to the auxin and cytokinin gradient. Fate of root and shoot development has been found to be decided by the auxin and cytokinin produced by the soil microbiota (Su et al. 2011).
Root bacterial and fungal populations produce different phytohormones such as cytokinin, auxin, and ethylene (Table 14.1). Phytohormones auxin and cytokinin possess antagonistic role in development of plants. Conversely, production of these phytohormones occasionally leads to diseased conditions in plants (e.g., infection caused by Agrobacterium tumefaciens or Ustilago maydis). Therefore, the equilibrium of auxin-cytokinin and site of hormone accumulation can determine the beneficial or detrimental role of the microbial interaction.
14.4.1.1 Cytokinin
Cytokinin positively regulates the growth of the whole plant, especially the shoot (Beck 1996). Several studies have revealed the involvement of different gene expressions to stimulate cytokinin production in plants. Signal perception of cytokinins involves three sensor histidine kinases—CRE1/AHK4/WOL, AHK2, and AHK3, which upregulate multiple response regulator expression depending on the concentration of cytokinin (Kakimoto 2003). The cytokinin receptors are also necessary for the viability and normal growth of plants. Furthermore, the cytokinin signaling upregulates the CYCD3 gene that encodes a D-type cyclin leading to the cell cycle progression and cell division in the shoot (Riou-Khamlichi et al. 1999). Additionally, cytokinin gradient has been reported to drive the plant, microbe, and insect interactions, thereby contributing in the plant defense system (Giron et al. 2013).
Several studies have shown the PGPR to produce cytokinin, consequently promoting plant growth and biomass production (Bukhat et al. 2020). Liu et al. (2013) have shown the inoculation of Platycladus orientalis plants with the PGPR Bacillus subtilis AE016877 to stimulate the cytokinin production in shoots. Further, Tahir et al. (2017) have shown the inoculation of tomato plants with PGPR B. subtilis SYST2 to enhance the expression of gene for cytokinin synthesis (SlCKX1) with increased cytokinin level. Another PGPR strain B. megaterium UMCV1 has also been found to promote the growth and biomass of Arabidopsis plants (Lopez-Bucio et al. 2007).
14.4.1.2 Auxin
Auxin concentration along with environmental factors plays an important role in determining the root architecture in plants. The root and shoot meristem size, lateral organ primordial position, and floral morphogenesis are regulated by auxin. It also drives the cell cycle, thereby releasing the bud dormancy in plants (Tsukanova et al. 2017).
Microbial interaction with plant root induces the synthesis of auxin, including indole-3-acetic acid (IAA), indole-3-butyric acid (IBA), phenylacetic acid, 4-chlorindole-3-acetic acid, or their precursors (Spaepen et al. 2007). The IAA, major naturally occurring auxin, reciprocates the signaling during the plant-microbiota interaction. The IAA has been found to be a positive regulator of plant growth. Therefore, IAA overproduction has been suggested to induce the formation of long hypocotyls with increased numbers of lateral roots and root hairs. The amount, localization, and direction of IAA movement in plants have been proved to be affected by the PGPR (Ahmed and Hasnain 2014). Spaepen et al. (2014) have reported the PGPR strain Azospirillum brasilense Sp245 to produce higher concentration of IAA, thereby promoting root growth in A. thaliana. The PGPR strains Aeromonas punctata PNS-1 (Iqbal and Hasnain 2013) and Serratia marcescens 90-166 (Shi et al. 2010) have also been revealed to induce growth of Arabidopsis plants. Singh et al. (2013) have reported the IAA overproducing PGPR strain of Burkholderia cepacia RRE25 to stimulate growth of rice plants.
Several studies have revealed the rhizospheric fungal population to produce IAA leading to enhanced plant growth. This is an interacting system that may have a role in the fungi and plant symbiosis, where the fungi can use IAA and related compounds to interact with plants as part of their colonization strategy, consequently stimulating plant growth. Gravel et al. (2007) have shown the inoculation of tomato plants with T. atroviride to stimulate plant growth and yield by inducing IAA production. Contreras-Cornejo et al. (2009) have reported the plant-beneficial fungi Trichoderma virens to promote lateral root growth with enhanced biomass production in Arabidopsis through the IAA-dependent mechanism. The study has also revealed the mutations in IAA transport or signaling genes (AUX1, AXR1, BIG, and EIR1) to suppress the root growth by diminishing the effects of Trichoderma.
14.4.1.3 Ethylene
Ethylene plays an active role in seed germination, leaf maturation, root elongation, nodulation, root branching, floral initiation, and fruit ripening at low concentrations. However, high concentrations of ethylene have been found to cause phytotoxicity leading to inhibition of root growth, defoliation, and early senescence. Several studies have shown plants to produce 1-aminocyclopropane-1-carboxylate (ACC), the precursor of ethylene under different abiotic and biotic stresses including drought (Basu et al. 2021a), submergence (Basu et al. 2020b; Basu et al. 2021b), and pathogenic infections (Basu and Kumar 2021b).
The PGPR also synthesizes ACC deaminase that helps plants by converting it to α-ketobutyrate and ammonia, thereby regulating the concentration of ACC. Therefore, the PGPR also regulates plant growth under different stress conditions by limiting the toxic level of ethylene. Hall et al. (1996) showed Pseudomonas putida GR12-2 to induce growth in barley, oat, wheat, canola, lettuce, and tomato seedlings. Later Penrose et al. (2001) also showed P. putida GR12-2 to promote plant growth in canola. Mayak et al. (2004) revealed Achromobacter piechaudii ARV8 to stimulate growth of pepper and tomato plants. Different strains of Pseudomonas spp. P. brassicacearum Am3 and P. marginalis Dp have been revealed to promote plant growth in Indian mustard, rape, peas (Safronova et al. 2006), and tomato (Belimov et al. 2007). Naher et al. (2008) showed Corynebacterium sp. Sb26 and Rhizobium sp. Sb16 to induce growth of rice plants. Strain of Azotobacter chroococcum AZO2 has been found to promote growth of sesame plants (Dubey et al. 2012). Belimov et al. (2015) showed strains of Achromobacter xylosoxidans Cm4, Pseudomonas oryzihabitans Ep4, and Variovorax paradoxus 5C-2 to stimulate growth of potato plants.
14.4.2 N-Acyl-L-Homoserine Lactone (AHL)
Bacterial adaptation, proficiency, cellular communication, and reproduction are regulated by an essential strategy, known as quorum sensing (QS) (Basu and Kumar 2021a). N-Acyl homoserine lactones (AHLs) belong to a class of bacterial QS signals from gram-negative bacteria (Hartmann et al. 2021). These compounds enable bacterial cells to regulate gene expression depending on the population density. Accumulation of AHLs confers resistance against phytopathogens (Table 14.2). The AHL-producing bacterial strain Serratia liquefaciens MG1 has been reported to promote plant growth by inducing SAR against phytopathogenic fungus Alternaria alternata in tomato (Schuhegger et al. 2006). Newman et al. (2008) reported the bacteria Bacillus sp. and Pseudomonas sp. to promote SAR in plants against Xylella fastidiosa and Xanthomonas sp. by degrading the diffusible signal factor. Kusari et al. (2014) have shown endophytic bacteria associated with Cannabis sativa plant to regulate phytopathogenic Chromobacterium violaceum by inhibiting QS.
14.4.3 Microbial Volatile Organic Compound (MVOC)
Microbial volatile organic compounds (MVOCs) are variable compounds formed through the bacterial and fungal metabolism (Korpi et al. 2009). The compounds usually have low molecular weight (e.g., hydrocarbon, alcohol, aldehyde, and ketone), distinct odour, low boiling point and high vapor pressure. MVOCs establish communication with plants by functioning as attractants, repellents, or cautioning signals. Lemfack et al. (2014) have reported approximately 350 bacterial and 69 fungal species to produce about 846 diverse MVOCs. Different MVOCs are represented in Fig. 14.2.
The roles of PGPR in stimulating plant growth through the production of diverse MVOCs are well documented (Park et al. 2015). Different species and strains of PGPR, including Bacillus, Pseudomonas, and Serratia, have been revealed to stimulate growth of different plant species. The PGPR or PGPR-derived products frequently require the physical contact with the plants for inducing plant growth. However, different studies have reported about the distant regulation of plant growth without direct interaction with plants, which suggests the possibility of the emission of MVOCs by the PGPR. Different MVOCs stimulating growth of various plant species have been listed in Table 14.3. Ryu et al. (2003) first revealed two MVOCs, 3-hydroxy-2-butanone (acetoin) and 2,3-butanediol produced by PGPR strains B. subtilis GB03 and B. amyloliquefaciens IN937a to induce growth in A. thaliana. Again Zou et al. (2010) showed the PGPR strain B. megaterium XTBG34 to promote the growth of Arabidopsis plants by producing 2-pentylfuran. Park et al. have reported MVOCs (2-methyl-n-1-tridecene, 2-butanone, and 13-tetradecadien-1-ol) produced by the PGPR strain Pseudomonas fluorescens SS101 to stimulate growth of tobacco plants. Xie et al. (2014) have shown the spermidine-producing PGPR strain Bacillus subtilis OKB105 to stimulate growth in tobacco plants. Further, Tahir et al. (2017) have shown another strain of B. subtilis SYST2 to promote growth of tomato plants by producing MVOCs (albuterol and 1,3-propanediole). Kai and Piechulla (2014) showed another PGPR Serratia odorifera to promote the growth of moss Physcomitrella patens by producing CO2.
Fungi being an important associate of the phytomicrobiome release abundant MVOCs playing a key role in plant-microbe interactions. A recent study showed beneficial fungal strain of Trichoderma hamatum FB10 to increase the growth and biomass of cowpea, small millet, maize, green gram, and black gram seedlings by synthesizing bioactive MVOCs (butanoic acid, ethanoic acid, hexadecanoic acid, butyrolactone, and hexadecane) (Baazeem et al. 2021). Another recent study showed the fungal strain of Cladosporium halotolerans NGPF1 to promote growth of tobacco (Nicotiana benthamiana) plants through the production of two MVOCs 2-methyl-butanal and 3-methyl-butanal (Jiang et al. 2021). Further, Paul and Park (2013) showed another strain of Cladosporium cladosporioides CL-1 to stimulate growth and fresh weight of tobacco seedlings by synthesizing MVOCs including dehydroaromadendrene, (−)-trans-caryophyllene, tetrahydro-2,2,5,5-tetramethylfuran, α-pinene, and (+)-sativene. Naznin et al. (2013) have shown the plant growth-promoting fungal strain Phoma sp. GS8-3 to induce the growth of tobacco plants by producing the MVOCs, 2-methyl-propanol and 3-methyl-butanol.
14.5 Future Prospects
The plant rhizosphere harbors a diverse reservoir of culturable microorganisms that can be exploited to benefit mankind. Interactions of plants and rhizospheric organisms influence plants’ root functions, eventually altering their growth and productivity. Therefore, understanding the soil-root and soil-seed interface is essential to manage the microorganisms for sustainable agriculture. Microbial activities and population numbers are often affected by the soil composition, which in turn has an impact on the nematodes and microarthropods that share this environment. Many rhizospheric microbes benefit crop production, reducing the dependence on chemical fertilizers to achieve high productivity. Some microbes also protect plants from the ravages of the severe disease-causing pathogens.
Exploration of rhizosphere may contribute in the enhanced application of plant growth-promoting organisms for sustainable plant growth and usage of the biocontrol agents for suppressing plant diseases and weeds. Rhizospheric organisms can also be used to enhance the formation of stable soil aggregates and as bioremediation agents of contaminated soils. Utilization of the beneficial microorganisms is fully consistent with sustainable agriculture, where the goal of paramount importance is to utilize the natural processes that promote the crops’ output without irreparably damaging the natural resources. Progressive understanding of the ecology and biota in the rhizosphere may help in manipulating this zone of increased nutrients, biotic activity, and interactions to improve plant productivity and environmental quality.
Among the many recent discoveries in rhizosphere research, the ominous is finding that certain potential human pathogenic microorganisms are also successful inhabitants of this nutrient-enriched plant soil environment, and this ecology poses potential public health hazards for both producers and consumers who encounter them. An interesting thought for future exploration is the rhizosphere supports the populations of human health-promoting rhizobacteria (HHPR).
Rhizosphere bioremediation refers to the biodegradation of pollutants by microorganisms in the plant root zone. Plants play an important role to increase both microbial numbers and metabolism in soil, resulting in increased biodegradation activity. Several mechanisms elucidate the enhanced biodegradation in the rhizosphere: the root turnover increases soil organic carbon stimulating microbial activities, thereby metabolizing toxic pollutants; root exudates contain small organic acids, alcohols, and phenolic compounds that favor solubilization and bioavailability of hydrophobic pollutants; root tissues and microorganisms also secrete catabolic enzymes, such as peroxidases and laccases, involved in biodegradation mechanisms; specific compounds released by roots induce microbial enzymes and stimulate biodegradation; roots introduce oxygen in the rhizosphere, which is necessary for oxidative biodegradation by oxygenases.
In the near future, it could be expected that more studies will be conducted on this field, by which plant-microbe interaction will be more understandable. It will further open up new junctions to use microbial strains with a capability to produce the phytohormones for plant improvement under field conditions to sustain the agricultural production.
14.6 Conclusion
Microbes and plants have cohabited and coevolved for millions of years. The complicated interactions of plants with the rhizospheric microbial populations have not been completely understood so far. The microbial populations play a significant role in the nutrient uptake and assimilation. Additionally, they contribute in the plant development and immunity system. The cross talking of both the organisms through vibrant chemical signaling pathways indicates their symbiotic association. The present chapter has explored the potential role of the major signaling molecules in the plant-microbe interactions, which may improve the efficiency of the ecosystem. Therefore, the comprehensive analyses of microbial signaling may be effectively used to pave the way for the agricultural sustainability.
References
Ahmed A, Hasnain S (2014) Auxins as one of the factors of plant growth improvement by plant growth promoting rhizobacteria. Pol J Microbiol 63:261–266
Arkhipova TN, Veselov SU, Melentiev AI, Martynenko EV, Kudoyarova GR (2005) Ability of bacterium Bacillus subtilis to produce cytokinins and to influence the growth and endogenous hormone content of lettuce plants. Plant Soil 272:201–209
Baazeem A, Almanea A, Manikandan P, Alorabi M, Vijayaraghavan P, Abdel-Hadi A (2021) In vitro antibacterial, antifungal, nematocidal and growth promoting activities of Trichoderma hamatum FB10 and its secondary metabolites. J Fungi (Basel) 7(5):331
Backer R, Rokem JS, Ilangumaran G, Lamont J, Praslickova D, Ricci E, Subramanian S, Smith DL (2018) Plant growth-promoting rhizobacteria: context, mechanisms of action, and roadmap to commercialization of biostimulants for sustainable agriculture. Front Plant Sci 9:1473
Badri DV, Vivanco JM (2009) Regulation and function of root exudates. Plant Cell Environ 32:666–681
Basu S, Kumar G (2020a) Stress signalling in the phytomicrobiome: breadth and potential. In: Kumar M, Kumar V, Prasad R (eds) Phyto-microbiome in stress regulation. Environmental and microbial biotechnology, Springer, Singapore, pp 245–268
Basu S, Kumar G (2020b) Nitrogen fixation in a legume-rhizobium symbiosis: the roots of a success story. In: Varma A, Tripathi S, Prasad R (eds) Plant microbe symbiosis. Springer, Singapore, pp 35–53
Basu S, Kumar G (2021a) Plant microbe interaction for changing endophytic colonization to improve plant productivity. In: Verma JP, Macdonald CA, Gupta VK, Podile AR (eds) New and future developments in microbial biotechnology and bioengineering. Elsevier, pp 137–147
Basu S, Kumar G (2021b) Exploring the significant contribution of silicon in regulation of cellular redox homeostasis for conferring stress tolerance in plants. Plant Physiol Biochem 166:393–404
Basu S, Kumar G, Chhabra S, Prasad R (2020a) Role of soil microbes in biogeochemical cycle for enhancing soil fertility. In: Verma JP, Macdonald CA, Gupta VK, Podile AP (eds) New and future developments in microbial biotechnology and bioengineering. Elsevier, Amsterdam, pp 149–157
Basu S, Kumar G, Kumari N, Kumari S, Shekhar S, Kumar S, Rajwanshi R (2020b) Reactive oxygen species and reactive nitrogen species induce lysigenous aerenchyma formation through programmed cell death in rice roots under submergence. Environ Exp Bot 177:104118
Basu S, Kumari S, Kumar A, Shahid R, Kumar S, Kumar G (2021a) Nitro-oxidative stress induces the formation of roots’ cortical aerenchyma in rice under osmotic stress. Physiol Plant 172(2):963–975
Basu S, Kumari S, Kumar P, Kumar G, Rajwanshi R (2021b) Redox imbalance impedes photosynthetic activity in rice by disrupting cellular membrane integrity and induces programmed cell death under submergence. Physiol Plant 172(3):1764–1778
Basu S, Prabhakar AA, Kumari S, Kumar RR, Shekhar S, Prakash K, Singh JP, Singh GP, Prasad R, Kumar G (2022) Micronutrient and redox homeostasis contribute to Moringa oleifera-regulated drought tolerance in wheat. Plant Growth Regul. https://doi.org/10.1007/s10725-022-00795-z
Beck EH (1996) Regulation of shoot/root ratio by cytokinins from roots in Urtica dioica: opinion. Plant Soil 185:1–12
Behera BK, Prasad R (2020) Strategies for soil management. In: Behera BK, Prasad R (eds) Environmental technology and sustainability. Elsevier, pp 143–167
Belimov AA, Dodd IC, Safronova VI, Hontzeas N, Davies WJ (2007) Pseudomonas brassicacearum strain Am3 containing 1-aminocyclopropane-1-carboxylate deaminase can show both pathogenic and growth-promoting properties in its interaction with tomato. J Exp Bot 58(6):1485–1495
Belimov AA, Dodd IC, Safronova VI, Shaposhnikov AI, Azarova TS, Makarova NM, Davies WJ, Tikhonovich IA (2015) Rhizobacteria that produce auxins and contain 1-amino-cyclopropane-1-carboxylic acid deaminase decrease amino acid concentrations in the rhizosphere and improve growth and yield of well-atered and water-limited potato (Solanum tuberosum). Ann Appl Biol 167:11–25
Berendsen RL, Pieterse CMJ, Bakker PAHM (2012) The rhizosphere microbiome and plant health. Trends Plant Sci 8:478–486
Bukhat S, Imran A, Javaid S, Shahid M, Majeed A, Naqqash T (2020) Communication of plants with microbial world: exploring the regulatory networks for PGPR mediated defense signaling. Microbiol Res 238:126486
Campbell R, Greaves MP (1990) Anatomy and community structure of the rhizosphere. In: Lynch JM (ed) The rhizosphere. John Wiley and Sons Ltd, Chichester, pp 11–34
Chai YN, Schachtman DP (2022) Root exudates impact plant performance under abiotic stress. Trends Plant Sci 27(1):80–91. https://doi.org/10.1016/j.tplants.2021.08.003
Chen L, Dodd IC, Theobald JC, Davies WJ, Belimov AA (2013) The rhizobacterium Variovorax paradoxus 5C-2, containing ACC deaminase, promotes growth and development of Arabidopsis thaliana via an ethylene-dependent pathway. J Exp Bot 64:1565–1573
Contreras-Cornejo HA, Macías-Rodriguez LI, Cortes-Penagos C, Lopez-Bucio J (2009) Trichoderma virens, a plant beneficial fungus, enhances biomass production and promotes lateral root growth through an auxin-dependent mechanism in Arabidopsis. Plant Physiol 149:1579–1592
Djonovic S, Pozo MJ, Dangott LJ, Howell CR, Kenerley CM (2006) Sm1, a proteinaceous elicitor secreted by the biocontrol fungus Trichoderma virens induces plant defense responses and systemic resistance. Mol Plant Microbe Interact 19:838–853
Dong D, Peng X, Yan X (2004) Organic acid exudation induced by phosphorus deficiency and/or aluminium toxicity in two contrasting soybean genotypes. Physiol Plant 122:190–199
Dubey RC, Maheshwari DK, Kumar V, Pandey RR (2012) Growth enhancement of Sesamum indicum L. by rhizosphere-competent Azotobacter chroococcum AZO2 and its antagonistic activity against Macrophomina phaseolina. Arch Phytopathol Plant Protect 45:437–454
el Zahar Haichar F, Santaella C, Heulin T, Achouak W (2014) Root exudates mediated interactions belowground. Soil Biol Biochem 77:69–80
Giron D, Frago E, Glevarec G, Pieterse CMJ, Dicke M (2013) Cytokinins as key regulators in plant-microbe-insect interactions: connecting plant growth and defence. Funct Ecol 27(3):599–609
Gravel V, Antoun H, Russell J, Weddell T (2007) Growth stimulation and fruit yield improvement of greenhouse tomato plants by inoculation with Pseudomonas putida or Trichoderma atroviride: possible role of indole acetic acid (IAA). Soil Biol Biochem 39:1968–1977
Großkinsky DK, Tafner R, Moreno MV, Stenglein SA, García de Salamone IE, Nelson LM et al (2016) Cytokinin production by Pseudomonas fluorescens G20-18 determines biocontrol activity against Pseudomonas syringae in Arabidopsis. Sci Rep 6:23310
Hall JA, Peirson D, Ghosh S, Glick BR (1996) Root elongation in various agronomic crops by the plant growth promoting rhizobacterium Pseudomonas putida GR12-2. Isr J Plant Sci 44:37–42
Harley JL, Russell RS (1979) The soil-root interface. Academic, London
Harman GE, Howell CR, Viterbo A, Chet I, Lorito M (2004) Trichoderma species-opportunistic, avirulent plant symbionts. Nat Rev Microbiol 2:43–56
Hartmann A, Klink S, Rothballer M (2021) Importance of N-acyl-homoserine lactone-based quorum sensing and quorum quenching in pathogen control and plant growth promotion. Pathogens 10:1561
Hiltner L (1904) Uber nevere Erfahrungen und Probleme auf dem Gebiet der Boden Bakteriologie und unterbesonderer Beurchsichtigung der Grundungung und Broche. Arbeit Deut Landw Ges Berlin 98:59–78
Iqbal A, Hasnain S (2013) Aeromonas punctata PNS-1: a promising candidate to change the root morphogenesis of A. thaliana in MS and sand system. Acta Physiol Plant 35:657–665
Jiang L, Lee MH, Kim CY, Kim SW, Kim PI, Min SR, Lee J (2021) Plant growth promotion by two volatile organic compounds emitted from the fungus Cladosporium halotolerans NGPF1. Front Plant Sci 12:794349
Kai M, Piechulla B (2014) Impact of volatiles of the rhizobacteria Serratia odorifera on the moss Physcomitrella patens. Plant Signal Behav 5:444–446
Kakimoto T (2003) Perception and signal transduction of cytokinins. Annu Rev Plant Biol 54:605–627
Korpi A, Jarnberg J, Pasanen AL (2009) Microbial volatile organic compounds. Crit Rev Toxicol 39(2):139–193
Kumar A, Basu S, Kumar G (2021) Evaluating the effect of seed-priming for improving arsenic tolerance in rice. J Plant Biochem Biotechnol 31(1):197–201
Kumar A, Basu S, Rishu AK, Kumar G (2022a) Revisiting the mechanisms of arsenic uptake, transport and detoxification in plants. Environ Exp Bot 194:104730
Kumar G, Basu S, Singla-Pareek SL, Pareek A (2022b) Unraveling the contribution of OsSOS2 in conferring salinity and drought tolerance in a high-yielding rice. Physiol Plant 174(1):e13638
Kusari P, Kusari S, Lamshöft M, Sezgin S, Spiteller M, Kayser O (2014) Quorum quenching is an antivirulence strategy employed by endophytic bacteria. Appl Microbiol Biotechnol 98:7173–7183
Lemfack MC, Nickel J, Dunkel M, Preissner R, Piechulla B (2014) mVOC: a database of microbial volatiles. Nucleic Acids Res 42(D1):D744–D748
Li J, Ovakim DH, Charles TC, Glick BR (2000) An ACC deaminase minus mutant of Enterobacter cloacae UW4 no longer promotes root elongation. Curr Microbiol 41:101–105
Liu FC, Xing SJ, Ma HL, Du ZY, Ma BY (2013) Cytokinin-producing, plant growth-promoting rhizobacteria that confer resistance to drought stress in Platycladus orientalis container seedlings. Appl Microb Biotech 97:9155–9164
Lopez-Bucio J, Campos-Cuevas JC, Hernandez-Calderon E, Velasquez-Becerra C, Farias-Rodriguez R, Macias-Rodriguez LI, Valencia-Cantero E (2007) Bacillus megaterium rhizobacteria promote growth and alter root-system architecture through an auxin- and ethylene-independent signaling mechanism in Arabidopsis thaliana. Mol Plant Microbe Interact 20:207–217
Lynch J, Whipps J (1991) Substrate flow in the rhizosphere. In: The rhizosphere and plant growth. Springer, Dordrecht, pp 15–24
Mandal SM, Chakraborty D, Dey S (2010) Phenolic acids act as signaling molecules in plant-microbe symbioses. Plant Signal Behav 5:359–368
Marschner H (1995) Mineral nutrition of higher plants, 2nd edn. Academic, London
Mayak S, Tirosh T, Glick BR (2004) Plant growth promoting bacteria that confer resistance to water stress in tomato and pepper. Plant Sci 166:525–530
Mhlongo MI, Piater LA, Madala NE, Labuschagne N, Dubery IA (2018) The chemistry of plant-microbe interactions in the rhizosphere and the potential for metabolomics to reveal signaling related to defense priming and induced systemic resistance. Front Plant Sci 9:112
Mishra M, Wungrampha S, Kumar G, Singla-Pareek SL, Pareek A (2021) How do rice seedlings of landrace Pokkali survive in saline fields after transplantation? Physiology, biochemistry, and photosynthesis. Photosynth Res 150:117–135
Naher UA, Radziah O, Halimi MS, Shamsuddin ZH, Mohd RI (2008) Effect of inoculation on root exudates carbon sugar and amino acids production of different rice varieties. Res J Microbiol 3:580–587
Naznin HA, Kimura M, Miyazawa M, Hyakumachi M (2013) Analysis of volatile organic compounds emitted by plant growth-promoting fungus Phoma sp. GS8-3 for growth promotion effects on tobacco. Microbes Environ 28:42–49
Newman KL, Chatterjee S, Ho KA, Lindow SE (2008) Virulence of plant pathogenic bacteria attenuated by degradation of fatty acid cell-to-cell signaling factors. Mol Plant Microbe Interact 21:326–334
Ortiz-Castro R, Martinez-Trujillo M, Lopez-Bucio J (2008a) N-acyl-L homoserine lactones: a class of bacterial quorum-sensing signals alters post-embryonic root development in Arabidopsis thaliana. Plant Cell Environ 31:1497–1509
Ortiz-Castro R, Valencia-Cantero E, Lopez-Bucio J (2008b) Plant growth promotion by Bacillus megaterium involves cytokinin signaling. Plant Signal Behav 3:263–265
Ortiz-Castro R, Contreras-Cornejo HA, Macias-Rodriguez L, Lopez-Bucio J (2009) The role of microbial signals in plant growth and development. Plant Signal Behav 4(8):701–712
Park YS, Dutta S, Ann M, Raaijmakers JM, Park K (2015) Promotion of plant growth by Pseudomonas fluorescens strain SS101 via novel volatile organic compounds. Biochem Biophys Res Commun 461(2):361–365
Paul D, Park KS (2013) Identification of volatiles produced by Cladosporium cladosporioides CL-1, a fungal biocontrol agent that promotes plant growth. Sensors (Basel) 13:13969–13977
Penrose DM, Moffatt BA, Glick BR (2001) Determination of 1-aminocyclopropane-1-carboxylic acid (ACC) to assess the effects of ACC deaminase-containing bacteria on roots of canola seedlings. Can J Microbiol 47:77–80
Prasad R, Kumar M, Varma A (2015) Role of PGPR in soil fertility and plant health. In: Egamberdieva D, Shrivastava S, Varma A (eds) Plant growth-promoting rhizobacteria (PGPR) and medicinal plants. Springer International Publishing, Switzerland, pp 247–260
Ramesh SA, Tyerman SD, Xu B, Bose J, Kaur S, Conn V, Domingos P, Ullah S, Wege S, Shabala S, Feijo JA, Ryan PR, Gilliham M (2015) GABA signalling modulates plant growth by directly regulating the activity of plant-specific anion transporters. Nat Commun 6:7879
Riou-Khamlichi C, Huntley R, Jacqmard A, Murray JAH (1999) Cytokinin activation of Arabidopsis cell division through a D-type cyclin. Science 283:1541–1544
Ryu C-M, Farag MA, Hu C-H, Reddy MS, Wei H-X, Pare PW, Kloepper JW (2003) Bacterial volatiles promote growth in Arabidopsis. Proc Natl Acad Sci U S A 100:4927–4932
Safronova VI, Stepanok VV, Engqvist GL, Alekseyev YV, Belimov AA (2006) Root-associated bacteria containing 1-aminocyclopropane-1-carboxylate deaminase improve growth and nutrient uptake by pea genotypes cultivated in cadmium supplemented soil. Biol Fertil Soils 42:267–272
Sarkar S, Basu S, Prasad R, Kumar G (2022) Phytoremediation: mechanistic approach for eliminating heavy metal toxicity from environment. In: Prasad R (ed) Phytoremediation for environmental sustainability. Springer, Singapore, pp 513–543
Schuhegger R, Ihring A, Gantner S, Bahnweg G, Knappe C, Vogg G et al (2006) Induction of systemic resistance in tomato plants by N-acyl-homoserine lactone-producing rhizosphere bacteria. Plant Cell Environ 29:909–918
Shi CL, Park HB, Lee JS, Ryu S, Ryu CM (2010) Inhibition of primary roots and stimulation of lateral root development in A. thaliana by the rhizobacterium Serratia marcescens 90-166 is through both auxin-dependent and -independent signaling pathways. Mol Cells 29:251–258
Shrivastava S, Prasad R, Varma A (2014) Anatomy of root from eyes of a microbiologist. In: Morte A, Varma A (eds) Root Engineering, vol 40. Springer-Verlag, Berlin Heidelberg, pp 3–22
Singh RK, Malik N, Singh S (2013) Improved nutrient use efficiency increases plant growth of rice with the use of IAA-overproducing strains of endophytic Burkholderia cepacia strain RRE25. Microb Ecol 66:375–384
Spaepen S, Vanderleyden J, Remans R (2007) Indole-3-acetic in microbial and microorganism-plant signaling. FEMS Microbiol Rev 31:425–448
Spaepen S, Bossuyt S, Engelen K, Marchal K, Vanderleyden J (2014) Phenotypical and molecular responses of A. thaliana roots as a result of inoculation with the auxin-producing bacterium Azospirillum brasilense. New Phytol 201:850–861
Su HJ, Thseng FM, Chen JS, Ko WH (2011) Production of volatile substances by rhizomorphs of Marasmius crinisequi and its significance in nature. Fungal Divers 49:199–202
Tahir HA, Gu Q, Wu H, Raza W, Hanif A, Wu L, Colman MV, Gao X (2017) Plant growth promotion by volatile organic compounds produced by Bacillus subtilis SYST2. Front Microbiol 8:171
Tsukanova KA, Сhеbоtаr VK, Meyer JJM, Bibikova TN (2017) Effect of plant growth-promoting Rhizobacteria on plant hormone homeostasis. S Afr J Bot 113:91–102
von Rad U, Klein I, Dobrev PI, Kottova J, Zazimalova E, Fekete A et al (2008) Response of Arabidopsis thaliana to N-hexanoyl-DL-homoserine-lactone, a bacterial quorum sensing molecule produced in the rhizosphere. Planta 229:73–85
Xie S, Wu H, Zang H, Wu L, Zhu Q, Gao X (2014) Plant growth promotion by spermidine-producing Bacillus subtilis OKB105. Mol Plant Microbe Interact 27:655–663
Zarkani AA, Stein E, Rohrich CR, Schikora M, Evguenieva-Hackenberg E, Degenkolb T et al (2013) Homoserine lactones influence the reaction of plants to rhizobia. Int J Mol Sci 14:17122–17146
Zou C, Li Z, Yu D (2010) Bacillus megaterium strain XTBG34 promotes plant growth by producing 2-pentylfuran. J Microbiol 48:460–466
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2022 The Author(s), under exclusive license to Springer Nature Singapore Pte Ltd.
About this chapter
Cite this chapter
Basu, S., Priyadarshini, P., Prasad, R., Kumar, G. (2022). Effects of Microbial Signaling in Plant Growth and Development. In: Prasad, R., Zhang, SH. (eds) Beneficial Microorganisms in Agriculture. Environmental and Microbial Biotechnology. Springer, Singapore. https://doi.org/10.1007/978-981-19-0733-3_14
Download citation
DOI: https://doi.org/10.1007/978-981-19-0733-3_14
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-19-0732-6
Online ISBN: 978-981-19-0733-3
eBook Packages: Earth and Environmental ScienceEarth and Environmental Science (R0)