Abstract
Society faces eco-environmental challenges when it comes to managing industrial wastewaters. In particular, textile effluents are one of the main threats to living being due to toxic dyes. Technologies used to remove dye compounds include physicochemical and membrane filtration processes. However, since current technologies have several limitations, including high investment and energy demand, researchers have investigated cheaper and eco-friendly alternatives. Among them, anaerobic processes have been an effective method to decolourise dye-containing effluents. In that direction, the up-flow anaerobic sludge blanket (UASB) technology stands out in terms of high cost-effectiveness. The authors reviewed the published literature on UASB reactors in dye compounds removal. Mechanisms, merits, demerits, and technical aspects of UASB reactors are introduced. Challenges and opportunities are discussed. The major points are (1) mechanisms of dye removal in UASB reactors comprise mainly dye adsorption onto sludge granules and azo bond cleavage (biodegradation), (2) dye structure and concentration, external organic carbon source, redox mediators, and bioreactor operating conditions play a key role in the treatment performance, and (3) UASB technology exhibits high decolourisation rates. Removal efficiencies of chemical oxygen demand and colour lie within the range of 60–85% and 75–96%, respectively. However, anaerobic treatments may not be able to mineralise by-products of anaerobic metabolisms. Consequently, post-treatment of the anaerobically treated effluent is required, and 4) the energy production during the decolourisation process in UASB reactors is estimated at 22 kWh per m3 of treated wastewater. Bio-energy recovery can promote wastewater valorisation and decrease the economic burdens of dye-containing effluents treatment. Future studies should focus on optimising influence parameters of full-scale UASB reactors and biogas recovery from dye-containing wastewater treatment.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Anaerobic process
- Biodegradation
- Bio-energy
- Decolourisation
- Dye
- Granular sludge
- Industrial wastewater
- Methanogens
- Resource recovery
- UASB
1 Introduction
The ecological and social impacts caused by dye compounds from dyeing, pharmaceutical, pesticides, cosmetics, and food industries have been among the most significant environmental sanitation challenges. In particular, textile industries used about 50% of the total dyes produced and consume a considerable quantity of water; hence, it is considered one of the largest activities responsible for aquatic pollution [86]. Due to the presence of chemicals and non-biodegradable substances, textile effluents have genotoxicity, mutagenicity, and carcinogenicity potentials, which brings attention to public health security and safety of terrestrial environments [37].
Several methods for decolourisation of textile wastewaters have been reported in the literature, such as coagulation-flocculation [95], chemical oxidation [1], adsorption [58], and membrane-based technologies [34]. However, physical–chemical processes are associated with high installation and operational costs, high chemical demands, and the generation of polluted sludge and membrane concentrates [78]. Furthermore, it should be noted that the change in toxicity during the treatment by chemical oxidation methods and the possible generation of by-products also represents a significant drawback [47], which has pushed dye industries to investigate cheaper and eco-friendly options for full-scale applications.
Biological techniques stand out in terms of simplicity and high cost-effectiveness. Based on oxygen requirement, biological methods are classified into aerobic and anaerobic. In aerobic processes, microorganisms use oxygen as an oxidising agent to mineralise pollutants, while anaerobic biotransformation consists of removing contaminants in the absence of oxygen. In an anaerobic environment, sulphate, nitrate, and carbon dioxide act as oxidising agents [2]. The anaerobic process is a reliable and cost-effective method for textile effluent treatment due to its several advantages, such as low energy and chemical demand and low polluted sludge generation [32, 46, 70]. Besides, energy demand increase has motivated studies in the field of anaerobic technology. During anaerobic metabolism, biogas—a high calorific energy source—is produced and can be converted to thermal and/or electrical power [101].
Among the various anaerobic bioreactors, up-flow anaerobic sludge blanket (UASB) technology, developed by Lettinga et al. [59] in the late 1970s, has been applied to treat a broad of industrial effluents and has achieved maturity in the treatment of domestic wastewater [19]. UASB reactors can be (1) applied for high-strength organic wastewater [91], (2) employed on small and large scales [67], and (3) used for recovery resource proposes [75]. Concerning treatment efficiency, they have high pollutants removal rates and the capacity to withstand organic shock loads [45].
In light of these facts, the present chapter provides an overview of the application of UASB reactors in dye wastewaters treatment. Mechanisms of dye removal and technical aspects of UASB reactors are discussed. Besides, the authors investigate the factors that determine dye removal in UASB reactors. In the end, challenges and opportunities are summarised.
2 UASB Reactors
2.1 Bioreactor Concept
The UASB reactors have been applied in wastewater treatment systems due to their reliability, simplicity, and high cost-effectiveness. UASB reactors have a significant position in sewage treatment plants in emerging economies such as Brazil and Mexico and have successfully been used in distilleries, dairy industries, slaughterhouses, and chemical companies for industrial effluents [67]. Data analysis of different industrial anaerobic treatment plants showed that UASB reactors are the most predominant system. Of 1,215 surveyed facilities installed in 65 nations, UASB reactors were used in 682 of them (56%) [38].
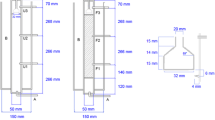
The UASB reactor comprises a rectangular or cylindrical unmixed tank and a three-phase (gas–liquid-solid) separator located on top of it. The typical height-diameter ratio of UASB reactors ranges from 0.2 to 0.5 [62]. Inside the bioreactor, wastewater flows upward, crossing a blanket of active biomass, with good organics biotransformation and settling ability [63]. Biogas produced at the bottom of the reactor and the influent flow cause natural turbulence, keeping efficient contact between active biomass (granular sludge) and wastewater (influent). Biomass concentration in the bioreactor can reach 80 g L−1 [91]. The three-phase separator allows the physical removal of suspended solids and guarantees high sludge retention time (SRT), operating the system without the necessity of support media to prevent biomass washout [89]. Figure 1 shows a schematic diagram of the UASB reactor.
The development of a dense granular sludge bed in the UASB reactors was decisive to the success of this technology. In fact, their high treatment performance is attributed to the formation of a dense sludge bed in the bottom of the bioreactor [49]. The granular biomass is an immobilised microbial aggregate with a highly compact structure and huge specific surface area, positive to adsorb and biotransform the pollutants. In contrast, it usually takes 2–8 months to develop anaerobic granular sludge, which requires a long bioreactor start-up period, one of UASB technology's main bottlenecks [61]. Despite several studies having been performed, the formation of anaerobic granules is not comprehensively known. Hulshoff Pol et al. [48] reviewed theories on sludge granulation in UASB reactors. In sum, they found that inert support particles jointly with operational conditions play a pivotal role in forming granular sludge.
Design and operational parameters strongly influence biodegradation performance and sludge settling ability of UASB reactors. Several factors, including effluent characteristics, temperature regime, up-flow velocity, organic loading rate (OLR), and hydraulic retention time (HRT), play a vital role in the UASB biodecolourisation. For instance, HRTs ranging from 3 to 10 h appeared optimal, resulting in chemical oxygen demand (COD) removal efficiency in the range of 60 to 85% at temperatures higher than 20 ºC [45]. OLR is also a key parameter. Industrial wastewaters are commonly operated with OLR ranging from 4 to 15 kg COD m3 d−1 [91]. Additionally, the up-flow velocity of the liquid is responsible for maintaining mixing and guarantees efficient contact between sludge and influent. In full-scale bioreactors treating high-strength wastewaters, the up-flow velocity is around 2.0 m h−1, while settling velocities range from 20 to 80 m h−1, ensuring high SRT in the treatment system [18].
Souza [88] introduced the main design features of UASB reactors, while [25] described the effects of process parameters on bioreactors’ operational performance. Readers are guided to these contributions for background information. UASB reactors have several advantages, including (1) low investment and operating costs, (2) low footprint, (3) high organic matter removal efficiency, (4) the ability to withstand organic shock loads, and (5) biogas production, which can be recovered as energy input. On the other hand, some issues need to be addressed, such as long bioreactor start-up, acidification by accumulating organic acids, inhibition risks due to toxic substances, insufficient pathogens/nutrients removals, which require effluent post-treatment, and sludge management. The pros and cons of UASB’s technological applications are summarised in Table 1.
2.2 Mechanisms of Dye Compounds Removal in UASB Reactors
Dye removal under anaerobic conditions is a reduction process in which literature primarily covers the biochemistry of azo dyes. Azo dyes account for more than half of dyes produced worldwide [31]. The main mechanism of their degradation in anaerobic conditions comprises azo bond (–N = N–) cleavage via extracellular azoreductase enzyme, which involves a transfer of four electrons (reducing equivalents). The decolourisation process occurs through two stages at the –N = N– linkage. Azo dyes act as electron acceptors. In the first stage, intermediates hydrazo are formed. Afterwards, hydrazo undergoes reductive cleavage leading to the formation of aromatic amines—uncoloured by-products, as shown in Eq. 1 [86].
where R1 and R2 are aryls or heteroaryl groups.
However, produced aromatic amines are, in general, anaerobically recalcitrant and have higher toxicity than dye precursors [39]. Consequently, anaerobically treated effluent needs further treatment. Many scholars propose to use hybrid anaerobic–aerobic systems to complete the dye removal effectively [5, 15, 51 56, 69]. Under low oxygen concentration, some facultative aerobes can consume oxygen and introduce hydroxyl groups into polyaromatic compounds, which facilitates subsequent biodegradation pathways [40]. Therefore, the aerobic process acts as a polishing step completing the mineralisation of intermediates of the anaerobic biotransformation [74].
In addition, the adsorption of dyes in the sludge granules can be significant for the decolourisation process in UASB reactors. Haider et al. [46] operated a UASB reactor in the intermittent regime (OLR of 2 kg COD m−3 d−1, HRT 24 h). They observed that the non-feeding period of run contributed more for total COD removal than continuous runs, concluding that physical dye entrapment onto biomass granules was preponderant. Indeed, kinetic studies show that the dye removal mechanism in UASB reactors is first abiotic (adsorption) and then biotic (biodegradation) [43]. Therefore, the adsorption mechanism by sludge granules makes an important contribution during decolourisation processes in UASB reactors.
The anaerobic process is divided into four steps: hydrolysis, acidogenesis, acetogenesis, and methanogenesis. In the three former steps named acid fermentation, organic macromolecules are hydrolysed and metabolised by fermentative bacterias and converted to carbon dioxide, hydrogen, and acetic acid. In the last step, acetic acid, carbon dioxide, and hydrogen are converted to carbon dioxide and methane by methanogenic archaeans [8]. The sequential stages of the anaerobic process are shown in Fig. 2.
As stated above, anaerobic decolourisation is a reductive process of azo bond cleavage via extracellular azoreductase enzyme, which involves a transfer of four electrons (reducing equivalents). The reducing equivalents (i.e., electron donors) are formed during the conversion of the organic matter through different stages of anaerobic metabolism. H2, CO2, ethanol, and formate are effective electron donors. The syntrophic relationship among microorganisms plays a pivotal role in the anaerobic process, and poor electron transfer inter-species can hamper the treatment performance. It is important to note that biodecolourisation under anaerobic conditions requires additional organic carbon sources since dye-reducing microbial consortia cannot use dye as the growth substrate [27, 30, 81]. Fermentative bacteria and hydrogenotrophic methanogens are the main ones responsible for dye reduction. Methanosarcina archaea, Clostridium, Enterococcus, Pseudomonas, Bacillus, Aeromonas, Enterococcus, Desulfovibrio, and Desulfomicrobium bacteria are reported to be effective in the anaerobic biodecolourisation [82, 85, 105].
2.3 Influence Factors of UASB Reactors in Dye Removal
Dye structure and concentration, electron donors and redox mediators, pH, temperature regime, hydraulic retention time (HRT), and organic loading rate (OLR) are the main influence parameters governing dyes removal in UASB reactors [81, 102] (Fig. 3). It is consensus that monitoring the anaerobic process to ensure the balance among these influencing parameters is pivotal for a stable reactor operation. Thus, the present section describes the main influence parameters during the decolourisation process in UASB reactors.
2.3.1 Dye Structure and Concentration
Dye compounds are heterogeneous chemicals of high molecular weight, complex structures, low biodegradability, and high toxicity. Literature is available stating that high dye concentration leads to poor biodecolourisation efficiencies due to the inhibition of the dye-reducing anaerobes by dye toxicity or blocking azoreductase enzymes [83, 106]. Dai et al. [24] showed that a high azo dye concentration (>450 mg L−1) could decrease the granular sludge porosity and strength, reduce its settling ability, and inhibit methanogenic activity.
Decolourisation of textile effluents was studied in a UASB reactor at 25, 50, 100, 150, and 300 mg dye L−1 [87]. Colour removal decreased for the increase of dye concentration. Decolourisation was 94% at 150 mg dye L−1 and 89% at 300 mg dye L−1. Similar findings have been reported by Murali et al. [66].
Furthermore, high dye dosage is usually associated with high salinity, reducing microbial activity, especially methanogens [100]. Excess salts adversely affect granulation and UASB stability. Wang et al. [97] demonstrated that anaerobic granules could tolerate salts concentration up to 10 g L−1. High salinity conditions decreased biomasses size and hydrophobicity, which hinders biodegradation and sludge settling ability. Sulfuric acid is commonly added to adjust the pH to overcome the salinity of textile effluents. On the other hand, Amaral et al. [5] reported that sulphate dosage higher than 300 mg L−1 could also inhibit anaerobic metabolism. In anaerobic conditions, sulphates and dye molecules compete to become the final electron acceptor of the reducing equivalents. As a result, sulphates obstruct the electron transfer to dye compounds, reducing the biodecolourisation efficiency [30]. Despite that, this mechanism is not fully understood, and hence, further studies are required.
Additionally, dye structure variability could also be a significant obstacle to the overall mineralisation of the molecules by microorganisms. Due to the many functional groups of dye compounds, steric hindrance can hamper enzymatic activity. Even minor structural differences can affect biodecolourisation [65]. Chinwetkitvanich [20] studied the anaerobic removal of four different dyes. Decolourisation efficiencies of anthraquinone monochlorotriazinyl, anthraquinone vinylsulphonyl, and bis azo vinylsulphonyl were 66, 64, and 63%, respectively. Moreover, the author stated that different chemical structures imply different removal mechanisms. For example, anthraquinone dye was mainly removed through adsorption into sludge flocs, while biodegradation via azo bond cleavage was prominent in azo dye decolourisation.
2.3.2 Electron Donors and Redox Mediators
The rate of anaerobically dye removal depends on the dye structure and concentration, electron donors (i.e., organic carbon sources), and redox mediators. Redox mediators are essential in the anaerobic decolourisation, accelerating the electron transfer from organic matter to dye compounds; thus, they accelerate the biotransformation kinetic [35]. Riboflavin and sulfonated compounds such as anthraquinone sulfonate and disulfonated anthraquinone are usually employed as redox mediators [16, 29]. Martins et al. [64] investigated the effect of riboflavin and different carbon sources on removing azo dye named Remazol Golden Yellow. Decolourisation without riboflavin was about 30.7% at 25ºC during 24 h. The addition of soluble riboflavin (0.0175 mg L−1) led to increased biodecolourisation of more than 50% during 48 h. Similar results were obtained using yeast extract (500 mg L−1) as a carbon source and anthraquinone‐2,6‐disulfonate as a redox mediator during the anaerobic treatment in UASB reactors [9, 28].
2.3.3 pH
The pH is related to establishing a micro-environment that affects the rate of microbial growth and enzymatic activity; therefore, it strongly influences dye removal efficiency [99]. In anaerobic processes, a very acidic or alkaline environment inhibits the activity of methanogens and the growth of acid-producing bacteria by increasing non-ionic organic acid, which can reduce biodecolourisation efficiency. Literature shows that anaerobes can decolourise at a wide range of pH from 5.0 to 10.0 [17, 44]. On the other hand, methanogens grow efficiently in the pH range of 6.0–8.0 and are very sensitive to pH fluctuation [42]. Chen et al. [17] have observed 97% decolourisation of azo dye Direct Black G at pH 8.0,79% decolourisation at pH 11.0; and 81% decolourisation at pH 4.0 after 48 h of incubation.
2.3.4 Temperature
Temperature significantly affects the dye-reducing microbial consortia, especially methanogens, therefore for stable bioreactor operation, maintenance of specific temperature is pivotal. Generally, an efficient anaerobic process occurs in mesophilic (35–40 °C) and thermophilic (50–65 °C) regimes. Many studies have reported that temperature is an important control parameter in anaerobic treatment and strongly influences the microbial community structure and the effluent treatability [29, 72, 79]. Typically, dye removal is fast at the thermophilic range. However, sky-high temperatures can reduce microbiota diversity and, as a consequence, decrease the biodecolourisation efficiency [12]. A study demonstrated that the optimum temperature range for biodecolourisation ranges from 30 to 55 °C, and exceeding this scope could harm the syntrophic relationship among anaerobic microorganisms [28].
2.3.5 Organic Loading Rate
Organic loading rate (OLR) can be defined as the concentration of dye or chemical oxygen demand (COD) entering the bioreactor in continuous mode per day. Many researchers have investigated the optimal OLR and predicted its maximum tolerable value. For example, COD removal efficiency in dye wastewater treatment was 61% at OLR of 2.40 kg COD m−3 d−1, which decreased to 37% when the OLR was increased to 22.5 kg COD m−3 d−1 in an anaerobic reactor [54]. In another biodecolourisation study, OLR ranging from 1.03 to 6.65 kg COD m−3 d−1 was selected as the optimum value. This range provided colour removal efficiencies from 92 to 95% (Işık and Sponza, 2004a). In contrast, Amaral et al. [5] reported that at OLRs of 1.84, 2.42, and 2.70 kg COD m−3 d−1, decolourizing rates were only 30%, 37%, and 52%, respectively.
It is important to note that at the start-up stage of the UASB reactor, adding a massive amount of dye can temporarily inhibit microbial activities because the anaerobes are not fully acclimatised. Furthermore, high OLR can affect methanogens metabolism and inhibit methane production due to the acidification of the medium [60]. For example, [54] obtained methane production efficiencies of 75% at OLR of 2.4 kg COD m−3 d−1 and 38% when OLR was increased to 22.5 kg COD m−3 d−1. It is helpful to mention that thermophilic conditions and effluent recirculation are the potential parameters that minimise the adverse effect of high OLR in anaerobic reactors [77].
2.3.6 Hydraulic Retention Time
Hydraulic retention time (HRT) represents the time that the affluent spend in the bioreactor and is closely associated with the bioreactor's robustness and treatment effectiveness. HRT is linked to microbial growth and dependent on temperature regime, organic loads, and dye structure. Decreasing the HRT can lead to misdeveloping granular sludge and/or acidification, whereas a longer than optimal HRT results in low utilisation of reactor components and biomass washout [25].
In a recent study, the colour removals decrease from 98 to 94% when the HRT decreased from 12 to 3 h [41]. In contrast, Amaral et al. [4] found that increasing the HRT parameter did not improve azo dye removal under anaerobic conditions. Decolourisation efficiency was 67% at an HRT of 16 h and 55% at 96 h. For the treatment of dye-containing wastewaters in UASB reactor, researchers have reported successful operation at 5–20 h of HRT [50, 53, 73]. However, it should be mentioned that differences can be observed depending on several factors, such as operating procedures and wastewater composition.
3 Decolourisation Performance of UASB Reactors
3.1 Dye Removal Efficiency
Coloured effluents contain persistent pollutants, which can exhibit mutagenic, carcinogenic, and toxic effects. Therefore, dye wastewaters need to be adequately treated before being discharged in watercourses [57]. UASB technology has been proven to be a promising method to remove dyes from effluents. Considering the chapter topic, the performance of UASB technology for azo dye removal is the most general information available. As previously discussed, the removal process is achieved through adsorption into granular sludges and anaerobic biodegradation via azo bond cleavage. Accordingly, the authors focused on azo dye biodegradation studies. Table 2 displays COD/colour removals and operational conditions of UASB reactors treating coloured wastewaters.
As shown in the table above, several studies have been conducted to evaluate dye removal in UASB reactors. The treatment efficiency is primarily assessed in terms of organic matter reduction and decolourisation. For instance, Cui et al. [23] used a UASB reactor for azo dye Alizarin Yellow R removal. The UASB reactor was operated under a batch condition, 25 ± 2ºC, OLR of 100 g dye m−3 d−1, and HRT ranging from 8 to 12 h. Colour and COD removal efficiencies of 96% and 54% were recorded at a dye concentration of 50 mg L−1 and HRT of 12 h per batch. The authors also investigated the OLR regime during the UASB treatment. They ranged the OLR from 100 to 800 g dye m−3 d−1. As discussed earlier, high dye loading rates can lead to poor decolourisation performance due to the inhibition of the anaerobic microbiota by dye toxicity. The results showed that the colour removal efficiency decreased to 63% at OLR of 800 g dye m−3 d−1. However, the inhibition was reversible, and decolourisation efficiency was recovered to 92% when OLR was decreased to 600 g dye m−3 d−1.
In a recent study, the effects of the intermittent operation on the biodegradation of dye compounds were tested using a laboratory UASB reactor [46]. The feeding period of 12 h and non-feeding period of 12 h provided decolourisation of 71% and COD removal efficiency of 58% (at HRT of 24 h and OLR of 2 kg COD m−3 d−1). During the non-feeding period, anaerobes resisted dye toxicity and handled operational changes in temperature, HRT, and OLR. The authors concluded that the discontinuous operation could be used as a strategy to improve the stability of decolourisation systems [46].
Firmino et al. [36] evaluated the efficacy of the UASB reactor for Direct Red 28 dye removal. The best system performance was at an HRT of 24 h, where 57.1% of colour and 60.3% of COD removals efficiency (on average) were registered. Işik and Sponza [53] studied Direct Red 28 azo dye mineralisation in a UASB reactor operated at continuous mode. Colour disappeared within a few minutes after entering into the UASB reactor due to the adsorption by anaerobic granules. Decolourisation efficiency remained at 99% during 103 d of operation.
UASB reactors are efficient for dye removal and exhibit a very high decolourisation efficiency (up to 99%). However, anaerobic treatment may not mineralise by-products of anaerobic metabolisms, such as polyaromatic amines and recalcitrant substances. Consequently, post-treatment of the anaerobically treated effluent is needed to achieve the required regulatory disposal standards. In this sense, anaerobic–aerobic combined systems have been proposed to remove aromatic by-products and recalcitrant COD efficiently. Gadow and Li [41] combined continuous UASB and aerobic processes to remove azo dye 2-Naphthol Red from industrial textile wastewater. The system achieved 98.9% and 98.4% of COD and colour removal at optimum conditions (OLR of 12.97 g COD m−3 d−1; HRT of 6 h).
In previous research, activated sludge and shallow polishing ponds were used as polishing steps in combined anaerobic–aerobic systems to remove azo dye Yellow Gold Remazol (50 mg L−1) [11]. Despite the low colour and COD removal efficiencies (around 20%), these aerobic processes produced effluents free of toxicity to bioluminescent Vibrio fisheri bacteria. In other studies, Ferraz et al. [33], Amaral et al. [4, 5] investigated submerged aerated biofilters as a polishing step for UASB effluents in order to remove anaerobic metabolites. The researchers confirmed the effectiveness of the aerobic process for the oxidation of aromatic amines, obtaining a COD removal efficiency of more the 50%. Furthermore, Ferraz et al. [33] obtained treated effluents with non-toxicity to bioorganism Daphnia magna.
In another interesting work, Carvalho et al. [15] proposed a microaerated UASB reactor to remove Direct Black 22 azo dye. The UASB reactor was aerated in the upper part (0.18 ± 0.05 mg O2 L−1) to mineralise amines generated in the anaerobic process. COD and colour removal ranged from 59 to 78%. Treated effluent of microaerated reactor was 16-fold less toxic when compared to conventional UASB, confirming the effectiveness of microaeration method for removal of anaerobic intermediates. Similar results were reported under aeration condition of 1.0 mL air min−1 treating azo dye Reactive Red 2 (50 mg L−1) [26].
Based on the reviewed literature, removal efficiencies of COD and colour in UASB reactors lie within the range of 60–85% and 75–96%, respectively. Typically, high efficiencies are obtained at operational conditions of 30–40 ºC, TRH of 20–30 h, and OLR of 2–15 kg COD m−3 d−1. Nevertheless, it must be emphasised that despite the excellent UASB descolourising efficiency reported in published studies, its treatment performance is case-specific and depending on wastewater composition and operational conditions. Furthermore, the available literature is mainly based on laboratory investigations and, therefore, more research is needed to scale-up and evaluate UASB techno-economic feasibility in field applications.
3.2 Bio-Energy Production
Anaerobic technology has the potential to degrade dye pollutants while at the same time providing a huge potential source of clean energy. Dye-containing wastewaters are loaded with organic chemicals, and in UASB reactors, the organic load is biotransformed and converted to biogas. Depending on the wastewater characteristic, biogas composition typically lies within the ranges CH4 = 50–70%, CO2 = 30–50%, and H2 = 1–5%, along with traces of water vapour, N2, H2S, ammonia, and siloxanes [7]. Hence, it has a high calorific value and can produce thermal and/or electrical energy. Biogas also can be processed to produce biomethane—a direct substitute for natural gas [103].
Katal et al. [56] operated a lab-scale UASB reactor to treat textile effluent and determined the biogas production yield. At HRT of 50 h, maximum biogas productivity of 36 L d−1 with a biomethane content of 79% was obtained. In other bench studies, biomethane rates from 0.36 to 2.7 L d−1 were archived [50,51,52]. As previously stated, removal efficiencies of COD and colour in UASB reactors treating dye effluents lie within the range of 60–85% and 75–96%, respectively. On the other hand, based on literature references, biomethane production can reach up to 0.30 m3 CH4 per kg of COD removed [41, 105]. Ranges of COD/colour removal and CH4 yield are shown in Fig. 4, which is constructed based on the data compiled in Table 2 and Ref. [41, 105].
From the characterisation of dye-containing effluents, a COD average of 7.3 kg m−3 was found by Santos et al. [30]. This value multiplied by biomethane yield (0.30 m3 CH4 kg CODremoved) means CH4 productivity of 2.19 m3 per m3 of wastewater. Assuming a conversion factor of 10 kWh m−3 of CH4 [96], UASB reactors could reach up to 21.90 kWh of bio-energy recovery per m3 of treated effluent. Considering that large scale dyers discharge from 70 to 400 m3 d−1 of wastewater [84], UASB reactors could lead to an annual energy saving of 1,878,472.50 kWh at the treatment facility as a result of the electric energy production surplus, corresponding to a saving of US$ 184,090.30 year−1. Thus, biogas recovery from UASB reactors can play a strategic role in promoting wastewater valorisation and decreasing textile wastewater treatment's economic burdens.
4 Challenges and Opportunities
Despite the merits of UASB reactors, four issues can be identified as limitations for their implementation in the treatment of colourised effluents. First, the development of granular sludge is a time-consuming process, which requires a long bioreactor start-up period. Furthermore, great efforts are still needed to elucidate granulation mechanisms and ensure process robustness. Second, as discussed in Sect. 2.3, various parameters influence the treatment performance, and hence, an imbalance among them can result in poor decolourising efficiency. Besides, anaerobic microorganisms are susceptible to inhibitory effects by high salinity, sulphate, and dye dosage, for example. These issues, if not adequately monitored and controlled, lead to process deterioration. Third, anaerobic processes may not be able to mineralise by-products of anaerobic metabolisms such as toxic aromatic amines. Therefore, post-treatment of the anaerobically treated effluent is required. As previously discussed, aerobic systems have been coupled to UASB reactors for efficient dye removal. However, studies assessing the treatment performance in pilot and full-scale are scarce, as well as biotoxicity assays to evaluate the quality of treated water. Last, the management of sludge containing dye compounds is a big challenge that must be addressed. Excess sludge from UASB reactors requires treatment in the form of dewatering, drying, stabilisation, disinfection, and disposal [22]. The polluted sludge contains toxic chemicals, and therefore its proper management must be guaranteed. Within a circular economy context, efforts have been made to recover add-value products from sludges (e.g., dyes, energy, salts, metals, and nutrients) [14]. For example, Yildirir and Ballice [104] treated textile biological sludges via hydrothermal gasification to produce fuel gas with a high calorific value.
It is beyond the scope of the present chapter to consider recovered add-value products from dye industry wastes in any detail. Currently, this topic has been investigated even more, and a comprehensive review of resource recovery of coloured effluents was recently published by Varjani et al. [92]. Indeed, the development of sustainable and cost-effective methods for resource recovery and their assessment based on a life-cycle perspective is a promising field of research [55].
Wastewater treatment plants (WWTPs) generally have a high energy demand [21]. UASB technology offers opportunities for renewable energy production and reduction of fossil fuel consumption. In fact, the hierarchy structure for wastewater management (Fig. 5) should be implemented to ensure efficient treatment, wastewater valorisation, and the transition of WWTPs to sustainable facilities. In this respect, bio-energy recovery can play a strategic role. It should also be underlined that anaerobic treatments are less energy-intensive and produce lower excess sludge when compared with aerobic processes [3]. Furthermore, among the positive impacts of biogas recovery via UASB technology stand out the mitigation of greenhouse gas emissions such as methane and carbon dioxide, which can foster the carbon neutrality of WWTPs in the middle and long term [98].
5 Conclusions
The present chapter reviewed the published literature about UASB reactors in dye removal. UASB technology exhibits high decolourisation rates with COD and colour removal efficiencies within the range of 60–85% and 75–96%, respectively. However, the available literature is mainly based on laboratory investigations and, therefore, more research is needed to scale up and evaluate UASB techno-economic feasibility in field applications. Four major challenges have been identified for UASB reactors implementation in dye wastewater treatment: (1) long start-up period, (2) inhibitory effects by high dye dosage, salinity, and sulphate, (3) treated effluent needs post-treatment due to the ineffectiveness of UASB reactors in mineralise by-products of anaerobic metabolism, and (4) sludge management. It should be emphasised that this is not an exhaustive overview of UASB reactors in the decolourisation process since unravelling all the detailed mechanisms of dye removal, UASB optimisation strategies, and research opportunities are hard to achieve in a single chapter. The following aspects should be addressed in further studies: (1) strategies to reduce the reactor start-up, (2) mechanisms of sludge granulation, influence factors, and granulation control, (3) optimising of influence parameters of UASB reactors treating real textile wastewater, (4) developing combined systems to boost the treatment performance, (5) biotoxicity assays of treated effluent, and (6) techno-economic assessment of biogas recovery during the treatment of real dye-containing effluents.
6 Conflicts of Interest
The authors declare no conflict of interest.
References
Al-Kdasi A, Idris A, Saed K, Guan CT (2004) Treatment of textile wastewater by advanced oxidation processes–a review. Global NEST J 6(1):222–230
Ali H (2010) Biodegradation of synthetic dyes—a review. Water Air Soil Pollut 213(1–4):251–273. https://doi.org/10.1007/s11270-010-0382-4
AlSayed A, Soliman M, Eldyasti A (2020) Anaerobic-based water resources recovery facilities: a review. Energies 13(14):3662. https://doi.org/10.3390/en13143662
Amaral FM, Florêncio L, Kato MT, Santa-Cruz PA, Gavazza S (2017) Hydraulic retention time influence on azo dye and sulfate removal during the sequential anaerobic–aerobic treatment of real textile wastewater. Water Sci Technol 76(12):3319–3327. https://doi.org/10.2166/wst.2017.378
Amaral FM, Kato MT, Florêncio L, Gavazza S (2014) Color, organic matter and sulfate removal from textile effluents by anaerobic and aerobic processes. Biores Technol 163:364–369. https://doi.org/10.1016/j.biortech.2014.04.026
An H, Qian Y, Gu X, Tang WZ (1996) Biological treatment of dye wastewaters using an anaerobic-oxic system. Chemosphere 33(12):2533–2542. https://doi.org/10.1016/S0045-6535(96)00349-9
Angelidaki I, Treu L, Tsapekos P, Luo G, Campanaro S, Wenzel H, Kougias PG (2018) Biogas upgrading and utilization: current status and perspectives. Biotechnol Adv 36(2):452–466. https://doi.org/10.1016/j.biotechadv.2018.01.011
Anukam A, Mohammadi A, Naqvi M, Granström K (2019) A review of the chemistry of anaerobic digestion: methods of accelerating and optimizing process efficiency. Processes 7(8):504. https://doi.org/10.3390/pr7080504
Baêta BEL, Aquino SF, Silva SQ, Rabelo CA (2012) Anaerobic degradation of azo dye Drimaren blue HFRL in UASB reactor in the presence of yeast extract a source of carbon and redox mediator. Biodegradation 23(2):199–208. https://doi.org/10.1007/s10532-011-9499-4
Bahia M, Borges TA, Passos F, de Aquino SF, de Silva Q (2020) Evaluation of a combined system based on an Upflow Anaerobic Sludge Blanket Reactor (UASB) and Shallow Polishing Pond (SPP) for textile effluent treatment Braz Arch Biol Technol 63:1–8. https://doi.org/10.1590/1678-4324-2020180130
Bahia M, Passos F, Adarme OFH, Aquino SF, Silva SQ (2018) Anaerobic-aerobic combined system for the biological treatment of Azo Dye solution using Residual Yeast. Water Environ Res 90(8):729–737. https://doi.org/10.2175/106143017X15131012153167
Boonyakamol A, Imai T, Chairattanamanokorn P, Higuchi T, Sekine M, Ukita M (2009) Reactive Blue 4 Decolorization under Mesophilic and Thermophilic Anaerobic Treatments. Appl Biochem Biotechnol 152(3):405–417. https://doi.org/10.1007/s12010-008-8237-9
Brás R, Gomes A, Ferra MIA, Pinheiro HM, Gonçalves IC (2005) Monoazo and diazo dye decolourisation studies in a methanogenic UASB reactor. J Biotechnol 115(1):57–66. https://doi.org/10.1016/j.jbiotec.2004.08.001
Bratina B, Šorgo A, Kramberger J, Ajdnik U, Zemljič LF, Ekart J, Šafarič R (2016) From municipal/industrial wastewater sludge and FOG to fertilizer: a proposal for economic sustainable sludge management. J Environ Manag 183:1009–1025. https://doi.org/10.1016/j.jenvman.2016.09.063
Carvalho JRS, Amaral FM, Florencio L, Kato MT, Delforno TP, Gavazza S (2020) Microaerated UASB reactor treating textile wastewater: the core microbiome and removal of azo dye Direct Black 22. Chemosphere 242:125157. https://doi.org/10.1016/j.chemosphere.2019.125157
Cervantes FJ, Garcia-Espinosa A, Moreno-Reynosa MA, Rangel-Mendez JR (2010) Immobilized redox mediators on anion exchange resins and their role on the reductive decolorization of azo dyes. Environ Sci Technol 44(5):1747–1753. https://doi.org/10.1021/es9027919
Chen Y, Feng L, Li H, Wang Y, Chen G, Zhang Q (2018) Biodegradation and detoxification of Direct Black G textile dye by a newly isolated thermophilic microflora. Biores Technol 250:650–657. https://doi.org/10.1016/j.biortech.2017.11.092
de Chernicharo CAL (2007) Biological wastewater treatment series: anaerobic reactors, vol. 4. IWA Publishing, London, UK, p. 184. http://iwaponline.com/ebooks/book-pdf/1100/wio9781780402116.pdf
Chernicharo CAL, Almeida PGS, Lobato LCS, Couto TC, Borges JM, Lacerda YS (2009) Experience with the design and start up of two full-scale UASB plants in Brazil: enhancements and drawbacks. Water Sci Technol J Int Assoc Water Pollut Res 60(2):507–515. https://doi.org/10.2166/wst.2009.383
Chinwetkitvanich S (2000) Anaerobic decolorization of reactive dyebath effluents by a two-stage UASB system with tapioca as a co-substrate. Water Res 34(8):2223–2232. https://doi.org/10.1016/S0043-1354(99)00403-0
Chrispim MC, Scholz M, Nolasco MA (2021) Biogas recovery for sustainable cities: a critical review of enhancement techniques and key local conditions for implementation. Sustain Cities Soc 72:103033. https://doi.org/10.1016/j.scs.2021.103033
Cieślik BM, Namieśnik J, Konieczka P (2015) Review of sewage sludge management: standards, regulations and analytical methods. J Clean Prod 90:1–15. https://doi.org/10.1016/j.jclepro.2014.11.031
Cui M-H, Cui D, Liang B, Sangeetha T, Wang A-J, Cheng H-Y (2016) Decolorization enhancement by optimizing azo dye loading rate in an anaerobic reactor. RSC Adv 6(55):49995–50001. https://doi.org/10.1039/C6RA04665G
Dai R, Chen X, Luo Y, Ma P, Ni S, Xiang X, Li G (2016) Inhibitory effect and mechanism of azo dyes on anaerobic methanogenic wastewater treatment: can redox mediator remediate the inhibition? Water Res 104:408–417. https://doi.org/10.1016/j.watres.2016.08.046
Daud MK, Rizvi H, Akram MF, Ali S, Rizwan M, Nafees M, Jin ZS (2018) Review of upflow anaerobic sludge blanket reactor technology: effect of different parameters and developments for domestic wastewater treatment. J Chem 2018:1–13. https://doi.org/10.1155/2018/1596319
de Barros AN, da Silva MER, Firmino PIM, de Vasconcelos EAF, dos Santos AB (2018) Impact of microaeration and the redox mediator anthraquinone-2, 6-disulfonate on azo dye reduction and by-products degradation. Clean: Soil, Air, Water 46(8):1700518. https://doi.org/10.1002/clen.201700518
Demirel B, Scherer P (2008) The roles of acetotrophic and hydrogenotrophic methanogens during anaerobic conversion of biomass to methane: a review. Rev Environ Sci Bio/Technol 7(2):173–190. https://doi.org/10.1007/s11157-008-9131-1
dos Santos AB, Traverse J, Cervantes FJ, van Lier JB (2005) Enhancing the electron transfer capacity and subsequent color removal in bioreactors by applying thermophilic anaerobic treatment and redox mediators. Biotechnol Bioeng 89(1):42–52. https://doi.org/10.1002/bit.20308
dos Santos AB, Bisschops IAE, Cervantes FJ, van Lier JB (2004) Effect of different redox mediators during thermophilic azo dye reduction by anaerobic granular sludge and comparative study between mesophilic (30ºC) and thermophilic (55ºC) treatments for decolourisation of textile wastewaters. Chemosphere 55(9):1149–1157. https://doi.org/10.1016/j.chemosphere.2004.01.031
dos Santos AB, Cervantes FJ, van Lier JB (2007) Review paper on current technologies for decolourisation of textile wastewaters: perspectives for anaerobic biotechnology. Biores Technol 98(12):2369–2385. https://doi.org/10.1016/j.biortech.2006.11.013
Farooqi IH, Basheer F, Tiwari P (2017) Biodegradation of methylene blue dye by sequential treatment using anaerobic hybrid reactor and submerged aerobic fixed film bioreactor. J Inst Eng (India) Ser A 98(4):397–403. https://doi.org/10.1007/s40030-017-0251-x
Fazal S, Huang S, Zhang Y, Ullah Z, Ali A, Xu H (2019) Biological treatment of red bronze dye through anaerobic process. Arab J Geosci 12(13):415. https://doi.org/10.1007/s12517-019-4572-0
Ferraz ADN, Kato MT, Florencio L, Gavazza S (2011) Textile effluent treatment in a UASB reactor followed by submerged aerated biofiltration. Water Sci Technol 64(8):1581–1589. https://doi.org/10.2166/wst.2011.674
Fersi C, Gzara L, Dhahbi M (2005) Treatment of textile effluents by membrane technologies. Desalination 185(1–3):399–409. https://doi.org/10.1016/j.desal.2005.03.087
Field JA, Cervantes FJ, van der Zee FP, Lettinga G (2000) Role of quinones in the biodegradation of priority pollutants: a review. Water Sci Technol 42(5–6):215–222. https://doi.org/10.2166/wst.2000.0516
Firmino PIM, da Silva MER, Cervantes FJ, dos Santos AB (2010) Colour removal of dyes from synthetic and real textile wastewaters in one- and two-stage anaerobic systems. Biores Technol 101(20):7773–7779. https://doi.org/10.1016/j.biortech.2010.05.050
Florêncio TM, de Godoi LA, Rocha VC, Oliveira JMS, Motteran F, Gavazza S, Vicentine KFD, Damianovic MHRZ (2021) Anaerobic structured-bed reactor for azo dye decolorization in the presence of sulfate ions. J Chem Technol Biotechnol 96(6):1700–1708. https://doi.org/10.1002/jctb.6695
Frankin RJ (2001) Full-scale experiences with anaerobic treatment of industrial wastewater. Water Sci Technol J Int Assoc Water Pollut Res 44(8):1–6. https://doi.org/10.2166/wst.2001.0451
Frijters CTMJ, Vos RH, Scheffer G, Mulder R (2006) Decolorizing and detoxifying textile wastewater, containing both soluble and insoluble dyes, in a full scale combined anaerobic/aerobic system. Water Res 40(6):1249–1257. https://doi.org/10.1016/j.watres.2006.01.013
Fuchs G (2008) Anaerobic metabolism of aromatic compounds. Ann N Y Acad Sci 1125(1):82–99. https://doi.org/10.1196/annals.1419.010
Gadow SI, Li Y-Y (2020) Development of an integrated anaerobic/aerobic bioreactor for biodegradation of recalcitrant azo dye and bioenergy recovery: HRT effects and functional resilience. Bioresour Technol Rep 9:100388. https://doi.org/10.1016/j.biteb.2020.100388
Garcia J-L, Patel BKC, Ollivier B (2000) Taxonomic, phylogenetic, and ecological diversity of methanogenic archaea. Anaerobe 6(4):205–226. https://doi.org/10.1006/anae.2000.0345
Gonzalez-Gutierrez LV, Escamilla-Silva EM (2009) Reactive red azo dye degradation in a UASB bioreactor: mechanism and kinetics. Eng Life Sci 9(4):311–316. https://doi.org/10.1002/elsc.200900036
Guan Z-B, Zhang N, Song C-M, Zhou W, Zhou L-X, Zhao H, Xu C-W, Cai Y-J, Liao X-R (2014) Molecular cloning, characterization, and dye-decolorizing ability of a temperature- and pH-stable laccase from Bacillus subtilis X1. Appl Biochem Biotechnol 172(3):1147–1157. https://doi.org/10.1007/s12010-013-0614-3
van Haadel A, van der Lubbe J (2019) Chapter 4: UASB reactor design guidelines. In: van Haandel A, van der Lubbe J (eds) Anaerobic sewage treatment: optimization of process and physical design of anaerobic and complementary processes. IWA Publishing, pp. 133–192. https://doi.org/10.2166/9781780409627
Haider A, Khan SJ, Nawaz MS, Saleem MU (2018) Effect of intermittent operation of lab-scale upflow anaerobic sludge blanket (UASB) reactor on textile wastewater treatment. Desalin Water Treat 136:120–130. https://doi.org/10.5004/dwt.2018.23231
Holkar CR, Jadhav AJ, Pinjari DV, Mahamuni NM, Pandit AB (2016) A critical review on textile wastewater treatments: possible approaches. J Environ Manage 182:351–366. https://doi.org/10.1016/j.jenvman.2016.07.090
Hulshoff Pol LW, de Castro Lopes SI, Lettinga G, Lens PNL (2004) Anaerobic sludge granulation. Water Res 38(6):1376–1389. https://doi.org/10.1016/j.watres.2003.12.002
Hulshoff Pol LW, de Zeeuw WJ, Velzeboer CTM, Lettinga G (1983) Granulation in UASB-reactors. Water Sci Technol 15(8–9):291–304. https://doi.org/10.2166/wst.1983.0172
Işik M (2004) Efficiency of simulated textile wastewater decolorization process based on the methanogenic activity of upflow anaerobic sludge blanket reactor in salt inhibition condition. Enzyme Microb Technol 35(5):399–404. https://doi.org/10.1016/j.enzmictec.2004.04.018
Işık M, Sponza DT (2004) Decolorization of azo dyes under batch anaerobic and sequential anaerobic/aerobic conditions. J Environ Sci Health, Part A 39(4):1107–1127. https://doi.org/10.1081/ESE-120028417
Işik M, Sponza DT (2004) Anaerobic/aerobic sequential treatment of a cotton textile mill wastewater. J Chem Technol Biotechnol 79(11):1268–1274. https://doi.org/10.1002/jctb.1122
Işik M, Sponza DT (2005) Effects of alkalinity and co-substrate on the performance of an upflow anaerobic sludge blanket (UASB) reactor through decolorization of Congo Red azo dye. Biores Technol 96(5):633–643. https://doi.org/10.1016/j.biortech.2004.06.004
Karatas M, Dursun S, Argun ME (2010) The decolorization of azo dye Reactive Black 5 in a sequential anaerobic-aerobic system. Ekoloji 19(74):15–23
Karimi Estahbanati MR, Kumar S, Khajvand M, Drogui P, Tyagi RD (2021) Environmental impacts of recovery of resources from industrial wastewater. In: Biomass, biofuels, biochemicals, pp 121–162. https://doi.org/10.1016/B978-0-12-821878-5.00003-9
Katal R, Zare H, Rastegar SO, Mavaddat P, Darzi GN (2014) Removal of dye and chemical oxygen demand (COD) reduction from textile industrial wastewater using hybrid bioreactors. Environ Eng Manag J 13(1):43–50. https://doi.org/10.30638/eemj.2014.007
Kishor R, Purchase D, Saratale GD, Saratale RG, Ferreira LFR, Bilal M, Chandra R, Bharagava RN (2021) Ecotoxicological and health concerns of persistent coloring pollutants of textile industry wastewater and treatment approaches for environmental safety. J Environ Chem Eng 9(2):105012. https://doi.org/10.1016/j.jece.2020.105012
Koumanova B, Allen SJ (2005) Decolourisation of water/wastewater using adsorption (review). J Univ Chem Technol Metall 40:175–192
Lettinga G, van Velsen AFM, Hobma SW, de Zeeuw W, Klapwijk A (1980) Use of the upflow sludge blanket (USB) reactor concept for biological wastewater treatment, especially for anaerobic treatment. Biotechnol Bioeng 22(4):699–734. https://doi.org/10.1002/bit.260220402
Liu T, Schnürer A, Björkmalm J, Willquist K, Kreuger E (2020) Diversity and abundance of microbial communities in UASB reactors during methane production from hydrolyzed wheat straw and lucerne. Microorganisms 8(9):1394. https://doi.org/10.3390/microorganisms8091394
Liu Y, Xu H-L, Yang S-F, Tay J-H (2003) Mechanisms and models for anaerobic granulation in upflow anaerobic sludge blanket reactor. Water Res 37(3):661–673. https://doi.org/10.1016/s0043-1354(02)00351-2
Mainardis M, Buttazzoni M, Goi D (2020) Up-flow anaerobic sludge blanket (Uasb) technology for energy recovery: a review on state-of-the-art and recent technological advances. Bioengineering 7(2). https://doi.org/10.3390/bioengineering7020043
Mao C, Feng Y, Wang X, Ren G (2015) Review on research achievements of biogas from anaerobic digestion. Renew Sustain Energy Rev 45:540–555. https://doi.org/10.1016/j.rser.2015.02.032
Martins LR, Baêta BEL, Gurgel LVA, de Aquino SF, Gil LF (2015) Application of cellulose-immobilized riboflavin as a redox mediator for anaerobic degradation of a model azo dye Remazol Golden Yellow RNL. Ind Crops Prod 65(1):454–462. https://doi.org/10.1016/j.indcrop.2014.10.059
Methneni N, Morales-González JA, Jaziri A, Mansour HB, Fernandez-Serrano M (2021) Persistent organic and inorganic pollutants in the effluents from the textile dyeing industries: ecotoxicology appraisal via a battery of biotests. Environ Res 196:110956. https://doi.org/10.1016/j.envres.2021.110956
Murali V, Ong S-A, Ho L-N, Wong Y-S (2013) Decolorization of methyl orange using upflow anaerobic sludge blanket (UASB) reactor—an investigation of co-substrate and dye degradation kinetics. Desalin Water Treat 51(40–42):7621–7630. https://doi.org/10.1080/19443994.2013.782255
Noyola A, Padilla-Rivera A, Morgan-Sagastume JM, Güereca LP, Hernández-Padilla F (2012) Typology of municipal wastewater treatment technologies in Latin America. Clean: Soil, Air, Water 40(9):926–932. https://doi.org/10.1002/clen.201100707
O’Flaherty V, Collins G, Mahony T (2006) The microbiology and biochemistry of anaerobic bioreactors with relevance to domestic sewage treatment. Rev Environ Sci Bio/Technol 5(1):39–55. https://doi.org/10.1007/s11157-005-5478-8
Oh Y-K, Kim Y-J, Ahn Y, Song S-K, Park S (2004) Color removal of real textile wastewater by sequential anaerobic and aerobic reactors. Biotechnol Bioprocess Eng 9(5):419–422. https://doi.org/10.1007/BF02933068
Oktem YA, Palabiyik BB, Selcuk H (2019) Removal of colour and organic matter from textile wastewaters using two anaerobic processes. Desalin Water Treat 172:229–234. https://doi.org/10.5004/dwt.2019.24756
Ong S-A, Toorisaka E, Hirata M, Hano T (2005) Decolorization of azo dye (Orange II) in a sequential UASB–SBR system. Sep Purif Technol 42(3):297–302. https://doi.org/10.1016/j.seppur.2004.09.004
Panswad T, Luangdilok W (2000) Decolorization of reactive dyes with different molecular structures under different environmental conditions. Water Res 34(17):4177–4184. https://doi.org/10.1016/S0043-1354(00)00200-1
Pereira RA, Salvador AF, Dias P, Pereira MFR, Alves MM, Pereira L (2016) Perspectives on carbon materials as powerful catalysts in continuous anaerobic bioreactors. Water Res 101:441–447. https://doi.org/10.1016/j.watres.2016.06.004
Popli S, Patel UD (2015) Destruction of azo dyes by anaerobic–aerobic sequential biological treatment: a review. Int J Environ Sci Technol 12(1):405–420. https://doi.org/10.1007/s13762-014-0499-x
Rosa AP, Chernicharo CAL, Lobato LCS, Silva RV, Padilha RF, Borges JM (2018) Assessing the potential of renewable energy sources (biogas and sludge) in a full-scale UASB-based treatment plant. Renew Energy 124:21–26. https://doi.org/10.1016/j.renene.2017.09.025
Rozzi A, Remigi E (2004) Methods of assessing microbial activity and inhibition under anaerobic conditions: a literature review. Rev Environ Sci Bio/Technol 3(2):93–115. https://doi.org/10.1007/s11157-004-5762-z
Ryue J, Lin L, Kakar FL, Elbeshbishy E, Al-Mamun A, Dhar BR (2020) A critical review of conventional and emerging methods for improving process stability in thermophilic anaerobic digestion. Energy Sustain Dev 54:72–84. https://doi.org/10.1016/j.esd.2019.11.001
Samsami S, Mohamadi M, Sarrafzadeh M-H, Rene ER, Firoozbahr M (2020) Recent advances in the treatment of dye-containing wastewater from textile industries: Overview and perspectives. Process Saf Environ Prot 143:138–163. https://doi.org/10.1016/j.psep.2020.05.034
Samuchiwal S, Gola D, Malik A (2021) Decolourization of textile effluent using native microbial consortium enriched from textile industry effluent. J Hazard Mater 402:123835. https://doi.org/10.1016/j.jhazmat.2020.123835
Santos EMA, Nascimento ATP, do Paulino TRS, BarrosoBCS, Aguiar CR.(2016) Reator anaeróbio tipo UASB conjugado com processo Fenton para remoção de cor e demanda química de oxigênio de água residuária sintética de indústria têxtil. Engenharia Sanitaria e Ambiental 22(2):285–292. https://doi.org/10.1590/s1413-41522016148154
Saratale RG, Saratale GD, Chang JS, Govindwar SP (2011) Bacterial decolorization and degradation of azo dyes: a review. J Taiwan Inst Chem Eng 42(1):138–157. https://doi.org/10.1016/j.jtice.2010.06.006
Sarayu K, Sandhya S (2012) Current technologies for biological treatment of textile wastewater–a review. Appl Biochem Biotechnol 167(3):645–661. https://doi.org/10.1007/s12010-012-9716-6
Seyedi ZS, Zahraei Z, Jookar Kashi F (2020) Decolorization of reactive black 5 and reactive red 152 Azo dyes by new Haloalkaliphilic bacteria isolated from the textile wastewater. Curr Microbiol 77(9):2084–2092. https://doi.org/10.1007/s00284-020-02039-7
Shuchista D, Ashraful I (2015) A review on textile wastewater characterization in Bangladesh. Resour Environ 5(1):15–44. https://doi.org/10.5923/j.re.20150501.03
Silva SQ, Silva DC, Lanna MCS, Baeta BEL, Aquino SF (2014) Microbial dynamics during azo dye degradation in a UASB reactor supplied with yeast extract. Braz J Microbiol 45(4):1153–1160. https://doi.org/10.1590/S1517-83822014000400005
Singh K, Arora S (2011) Removal of synthetic textile dyes from wastewaters: a critical review on present treatment technologies. Crit Rev Environ Sci Technol 41(9):807–878. https://doi.org/10.1080/10643380903218376
Somasiri W, Ruan W, Xiufen L, Jian C (2006) Decolourization of textile wastewater containing acid dyes in UASB reactor system under mixed anaerobic granular sludge. Elec J Env Agricult Food Chem Title 5(1):1224–1234
Souza ME (1986) Criteria for the utilization, design and operation of UASB reactors. Water Sci Technol 18(12):55–69. https://doi.org/10.2166/wst.1986.0163
Speece RE (1983) Anaerobic biotechnology for industrial wastewater treatment. Environ Sci Technol 17(9):416A-427A. https://doi.org/10.1021/es00115a001
Tawfik A, Zaki D, Zahran M (2014) Use of sequential UASB/DHS processes for the decolorization of reactive dyes wastewater. Sustain Environ Res 24(2):129–138
van Lier JB, van der Zee FP, Frijters CTMJ, Ersahin ME (2015) Celebrating 40 years anaerobic sludge bed reactors for industrial wastewater treatment. Rev Environ Sci Bio/Technol 14(4):681–702. https://doi.org/10.1007/s11157-015-9375-5
Varjani S, Rakholiya P, Shindhal T, Shah AV, Ngo HH (2021) Trends in dye industry effluent treatment and recovery of value added products. J Water Process Eng 39:101734. https://doi.org/10.1016/j.jwpe.2020.101734
Venkatesh S, Venkatesh K, Quaff AR (2017) Dye decomposition by combined ozonation and anaerobic treatment: Cost effective technology. J Appl Res Technol 15(4):340–345. https://doi.org/10.1016/j.jart.2017.02.006
Verma AK, Bhunia P, Dash RR (2016) Performance of UASB reactor treating synthetic textile wastewater: effect of physicochemical pretreatment. Desalin Water Treat 57(18):8050–8060. https://doi.org/10.1080/19443994.2015.1017739
Verma AK, Dash RR, Bhunia P (2012) A review on chemical coagulation/flocculation technologies for removal of colour from textile wastewaters. J Environ Manage 93(1):154–168. https://doi.org/10.1016/j.jenvman.2011.09.012
Volschan Junior I, de Almeida R, Cammarota MC (2021) A review of sludge pretreatment methods and co-digestion to boost biogas production and energy self-sufficiency in wastewater treatment plants. J Water Process Eng 40:101857. https://doi.org/10.1016/j.jwpe.2020.101857
Wang W, Wu B, Pan S, Yang K, Hu Z, Yuan S (2017) Performance robustness of the UASB reactors treating saline phenolic wastewater and analysis of microbial community structure. J Hazard Mater 331:21–27. https://doi.org/10.1016/j.jhazmat.2017.02.025
Wei J, Hao X, van Loosdrecht MCM, Li J (2018) Feasibility analysis of anaerobic digestion of excess sludge enhanced by iron: a review. Renew Sustain Energy Rev 89:16–26. https://doi.org/10.1016/j.rser.2018.02.042
Wuhrmann K, Mechsner K, Kappeler T (1980) Investigation on rate-determining factors in the microbial reduction of azo dyes. Eur J Appl Microbiol Biotechnol 9(4):325–338. https://doi.org/10.1007/BF00508109
Xu H, Yang B, Liu Y, Li F, Shen C, Ma C, Tian Q, Song X, Sand W (2018) Recent advances in anaerobic biological processes for textile printing and dyeing wastewater treatment: a mini-review. World J Microbiol Biotechnol 34(11):165. https://doi.org/10.1007/s11274-018-2548-y
Yadvika S, Sreekrishnan TR, Kohli S, Rana V (2004) Enhancement of biogas production from solid substrates using different techniques-a review. Biores Technol 95(1):1–10. https://doi.org/10.1016/j.biortech.2004.02.010
Yasar A, Tabinda AB (2010) Anaerobic treatment of industrial wastewater by UASB reactor integrated with chemical oxidation processes; an overview. Pol J Environ Stud 19(5):1051–1061
Yentekakis IV, Goula G (2017) Biogas management: advanced utilization for production of renewable energy and added-value chemicals. Front Environ Sci 5:7. https://doi.org/10.3389/fenvs.2017.00007
Yildirir E, Ballice L (2019) Supercritical water gasification of wet sludge from biological treatment of textile and leather industrial wastewater. J Supercrit Fluids 146:100–106. https://doi.org/10.1016/j.supflu.2019.01.012
Zhang W, Liu F, Wang D, Jin Y (2018) Impact of reactor configuration on treatment performance and microbial diversity in treating high-strength dyeing wastewater: anaerobic flat-sheet ceramic membrane bioreactor versus upflow anaerobic sludge blanket reactor. Biores Technol 269:269–275. https://doi.org/10.1016/j.biortech.2018.08.126
Zin KM, Effendi Halmi MI, Abd Gani SS, Zaidan UH, Samsuri AW, Abd Shukor MY (2020) Microbial decolorization of triazo dye, direct blue 71: an optimization approach using Response Surface Methodology (RSM) and Artificial Neural Network (ANN). Biomed Res Int 2020:1–16. https://doi.org/10.1155/2020/2734135
Acknowledgements
Ronei de Almeida acknowledges the financial support received from Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) (Grant Number 165018/2018-6).
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2022 The Author(s), under exclusive license to Springer Nature Singapore Pte Ltd.
About this chapter
Cite this chapter
de Almeida, R., de Souza Guimarães, C. (2022). Up-Flow Anaerobic Sludge Blanket Reactors in Dye Removal: Mechanisms, Influence Factors, and Performance. In: Khadir, A., Muthu, S.S. (eds) Biological Approaches in Dye-Containing Wastewater. Sustainable Textiles: Production, Processing, Manufacturing & Chemistry. Springer, Singapore. https://doi.org/10.1007/978-981-19-0545-2_7
Download citation
DOI: https://doi.org/10.1007/978-981-19-0545-2_7
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-19-0544-5
Online ISBN: 978-981-19-0545-2
eBook Packages: Chemistry and Materials ScienceChemistry and Material Science (R0)