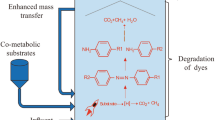
Abstract
Dyes are synthetic organic chemicals that are widely employed in a variety of sectors, including textiles, leather, plastics, food, pharmaceuticals, and paints. Untreated industrial effluents have accumulated resistant azo dyes, which have had a negative influence on the ecology. Discharging textile effluents in a water body has aesthetic, environmental, and toxicological issues that should be considered. Biological treatment, particularly anaerobic treatment, is usually considered to be one of the most effective ways for eliminating the majority of contaminants from complex or high-strength organic wastewater. The purpose of this book chapter is to describe the effect of biological treatment on dye removal, taking into account both anaerobic and aerobic treatments separately as well as a hybrid approach.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Anaerobic process
- Dye removal
- Hybrid dye removal method
- Aerobic process
- Azo dyes
- Synthetic dyes
- Wastewater
- Biodegradation
- Colour removal
- Industrial waste treatment
1 Introduction
The textile business is both one of the largest and one of the most polluting industries in the world. Textile manufacture entails a number of procedures, the majority of which produce highly polluted wastes [21]. One of the most important steps in the textile industries is dyeing. About 15% of colours used in the dyeing process are expected to be released in wastewater [40]. Among the many dye classes, azo dyes are the most popular, accounting for 70% of colours used in textile wastewater [110]. Under anaerobic conditions, spontaneous reduction of the azo bond or photocatalytic degradation may occur, resulting in extremely carcinogenic and mutagenic colourless aromatic amines [3]. The remaining dyes (10–15%) have a considerable impact on sunlight penetration, changing the natural water body [85]. The presence of various amounts of dyes with high nitrogen and organic content causes eutrophication, which causes the aquatic ecology to be disrupted [62].
Different treatment techniques for removing dyes and their related metabolites from textile effluents have been proposed such as adsorption [108], coagulation [29], electrocoagulation [6], filtering [50], chemical processes such as oxidation (Fenton's oxidation) [28], ozonation [96], and biological methods [12]. The main disadvantages of these technologies are their high prices and the vast amount of sludge produced. The elimination of the principal contaminants from complicated or high-strength organic wastewater is typically thought to be most effective via biological treatment, either aerobic or anaerobic [79].
Biological approaches are the most basic, natural, efficient, and cost-effective. Using biological techniques large amounts of effluents can be treated without causing significant environmental harm. Activated sludge and microaerophilic modes are accessible in a variety of bioreactors that can function in anaerobic, anoxic, and aerobic settings [8, 63]. Although some attempts to decolorize dyes under aerobic circumstances have proven successful, most azo dyes are still thought to be non-biodegradable [89]. Various studies found that under aerobic circumstances, dyestuff did not have substantial biodegradation rates. Because most aerobic bacteria are incapable of destroying dye molecules, the traditional activated sludge technique is ineffective in the treatment of textile wastewater in terms of colour. Under anaerobic conditions, however, in contrast to aerobic treatment, reductive cleavage of azo bonds is common. The development of high-rate bioreactors, which achieve a high reaction rate per unit reactor volume by retaining the biomass in the reactor for long periods of time, is critical for the successful application of anaerobic technology for the treatment of industrial wastewaters [7].
In this chapter, we will be discussing the importance of dye removal strategies, different aspects of using anaerobic method as an efficient dye removal process and how it is better than the present technologies and how it can be made more efficient.
2 Classification of Dyes
Dyes are categorised in a number of ways. The classification of a single parameter is exceedingly complicated and useless from a practical view. Each class of dye is distinguished by its source of material, chemical structure, chemical characteristics, and mode of interaction with substrate molecules [39]. Figure 1 classifies the dyes on the basis of source of material, ionic nature and chemical structure.
Broad classification of dyes [66]
2.1 Classification Based on the Source of Material
2.1.1 Natural Dyes
Natural dyes are primarily derived from plants, animals, and minerals. The majority of natural dyes are extracted from plant materials i.e., leaves, stem, roots, flower and seeds. Natural dyes are biodegradable, non-toxic, and have a greater environmental compatibility than synthetic dyes [54]. The most of natural dyes have a negative charge, and positive charge dyes are very rare. Natural dyes are categorised based on their chemical constitution and colour (Fig. 2).
Classification of natural dyes based on chemical structure and colour [101]
2.1.2 Synthetic Dyes
Synthetic dyes are man-made dyes generated from organic and inorganic chemicals, and they are usually created from petroleum by-products or earth minerals as synthetic resources. Synthetic dyes are used in textile dyeing, pharmaceutical, food, paper printing, cosmetic, colour photography, and leather industries [17, 53]. The ease of application, low cost, and wide range of pigments rendered these dyes popular. Synthetic dyes, on the other hand, can be toxic, mutagenic, carcinogenic, and clastogenic to living organisms, resulting in huge contamination of water and soil [4]. Synthetic dyes are divided into different classes as following.
2.1.3 Direct Dyes
Direct dyes are anionic dyes that are water soluble. These dyes are referred to as direct dyes since they do not require any sort of fixing. Protein fibres and synthetic fibres such as nylon and rayon are dyed with direct dyes and they bind to the fibre due to Van der Waals forces. These dyes are applied in an electrolyte and ionic salts under aqueous bath [84]. Direct dyes are azo dyes having multiple azo bonds, as well as stilbene, phthalocyanine, and oxazine compounds. Cotton, leather, paper, wool, silk, and nylon are all dyed with direct dyes.
2.1.4 Basic Dyes
Basic dyes are cationic water-soluble dyes that are commonly used on acrylic fibres [39]. They have cationic functional groups i.e. –NR3 + or = NR2 +. In 1856, H. W. Perkin developed mauveine guilty basic dye, the world's first synthetic dye. Acetic acid is commonly added to the dyebath to enhance dye uptake onto the fibre, and the dyes connect to the fibres by forming ionic bonds with the anionic groups of the fibres. Paper is dyed with the basic dyes as well.
2.1.5 Acidic Dyes
Acid dyes are water-soluble anionic dyes that are used to colour fabrics such as silk, nylon, wool, and modified acrylic fibres in neutral to acid dyebaths. At least half of the dye's attachment to the fibre is linked to salt formation between anionic groups in the dyes and cationic groups in the fibre [61] (Ansari and Seyghali 2013). This category includes the majority of synthetic food colours.
2.1.6 Reactive Dyes
Reactive dyes are most commonly used on cellulosic fibres, and they make a covalent link with the right textile functionality. Certain functional groups in the dye molecule can react with the –OH, –SH, and –NH2 groups found in textile fibres [73]. Reactive dyes have around 29 percent of the global textile dyes market. Their emergence to the market in the mid-1950s gave rise to a new class of cellulose dyes to replace sulphur, vats, azoics.
2.1.7 Vat Dyes
Cotton and other cellulosic materials are dyed with Vat Dyes, which are soluble leuco salts. They are also employed in the production of rayon and wool dyes. The main chemical classes of these water-insoluble colours are anthraquinon (including polycyclic quinones) and indigoids [39].
2.1.8 Disperse Dyes
Disperse dyes are water insoluble dyes that were originally developed to colour cellulose acetate. The dyes are finely ground and marketed as a spray-dried and marketed as a powder in the presence of a dispersing agent [39]. They are most generally used to colour polyester, although they can also cellulose triacetate, colour nylon and acrylic fibres. The small particle size allows for a wide surface area, which aids in dissolving and fibre uptake and the dispersion agent employed during the grinding process might have a big impact on the dyeing rate.
2.1.9 Sulphur Dyes
Sulphur dyes are water insoluble in nature and used to colour cotton [13]. Dyeing is done by submerging the cloth in an organic compound solution, which interrelates with the sulphide source to produce dark colours that stick to the fabric [66].
2.1.10 Azoic Dyes
Azoic dye contains a functional group R–N = N–R’, with R and R’ typically being aryl groups. Azoic dyeing is a process that involves applying an insoluble azo dye directly to or within the fibre. This is accomplished by combining diazoic and coupling components in a fibre. However, because the chemicals employed are harmful, this procedure is not employed when colouring cottons.
2.2 Classification Based on Ionic Nature
Dyes are categorized as cationic (all basic dyes), anionic (direct, acid, and reactive dyes), or non-ionic (all those other dyes) based on particle charge upon dispersion in aqueous application medium (dispersed dyes).
2.2.1 Cationic Dyes
Cationic dyes are positively charged, water soluble, and produce colourful cations when mixed in solution. Cationic dyes are also known as basic dyes and are used in the dyeing of acrylic, silk, nylon, silk, and wool. This set of dyes encompasses a wide range of chemical structures, most of which are based on substituted aromatic groups [24]. These dyes act as a toxin and has been linked to skin irritation, allergic dermatitis, mutations, and cancer [25]. Cationic functionality can be found in a variety of dyes, most notably methane dyes and cationic azo dyes, as well as anthraquinone, di- and tri-arylcarbenium, phthalocyanine dyes, and a variety of polycarbocyclic and solvent colours. Anthraquinone dyes are costly and weak, whereas azo dyes are strong and cost effective.
2.2.2 Anionic Dyes
Anionic dyes encompass a wide range of compounds from many dye classes that have structural variances (e.g., azoic, triphenylmethan, anthraquinone, and nitro dyes), but all include water-solubilizing, ionic substituents as a common property. These can be used to dye protein fabrics like wool, silk, and nylon. To improve solubility, acid dyes have a complicated structure with big aromatic molecules, a sulfonyl group, and an amino group [86].
2.3 Classification Based on Chemical Structures
2.3.1 Azo Dyes
Azo dyes are the most vital group among the half of all marketable dyes [39, 90]. Azo dyes have been explored extensively than any other class of dyes, and currently a large amount of data is available. Azo dyes contain one nitrogen-nitrogen (N = N) double bond, but they can have a variety of structures [35]. Diazonium salts, phenols or aromatic/naphthyl amines are used to prepare azo dyes by the coupling reactions. These dyes are the most widely used in the textile, leather, pharmaceutical, paper, plastic, and printing industries, owing to their moderately simple synthesis.
2.3.2 Anthraquinone Dyes
Anthraquinone dyes are the second most common type of dye after azo dyes. This class have anthraquinone chromophore groups and are widely utilised in the textile industry. Anthraquinone dyes have an intricate and stable structure than azo dyes, and they are more hazardous to microorganisms and human cells [70]. Anthraquinone dyes exhibited a number of advantages, including higher illumination and virtuous fastness [34]. Anthraquinones are essential natural compounds found in bacteria, fungi, lichens, and plants, unlike azo dyes, which have no natural analogues [43]. The nucleus of substituted anthraquinones and quinizarin (1,4-dihydroxyanthraquinone) is usually prepared by synthesis of the nucleus using phthalic anhydride and a benzene derivative. Most anthraquinone dyes exhibit prominent absorption bands and large molar extinction coefficients in the long-wavelength range. Anthraquinone dyes have notable photo stability, as a result, they have been employed in dye-sensitized solar cells [55].
2.3.3 Nitro and Nitroso Dyes
Nitro and nitroso dyes are of modest commercial value currently, but their minuscule molecular structures are intriguing. Nitro or nitroso groups are conjugated with an electron donating group through an aromatic system. Nitrous acid reacts with phenols and naphthols to generate hydroxynitroso compounds. The nitroso compounds do not dye themselves, but they can produce metal complexes that are either pigments or acid dyes [34].
2.3.4 Triarylmethane Dyes
Triarylmethane dyes are prepared by the condensation of ketone, aldehydes, acid anhydride or acid chlorides with aromatic amines or phenols (naphthols). Triarylmethane dyes are employed as textile dyes for silk, cotton, wool, and as well as in the manufacture of inks, dyeing of paper, colourants in foods, pharmaceuticals and cosmetics. They are also used as biological stains, and as anti-infective, antibacterial, and anthelmintic agents [22, 57, 64]. They have also been used to clean blood in vitro of flagellate parasites like Trypanosoma cruzi, as well as dye-assisted laser inactivation of enzymes [80].
2.3.5 Indigoid Dyes
Indigoid dyes are the one of the oldest organic dyes belongs to vat dyes. These are blue-coloured chemical compound contains carbonyl groups. Indigo dye has been used for textile dyeing for over 5000 years, when it was initially derived from plants. Indigo is nearly entirely used to colour denim pants and jackets. People like it because of its blue colour and the fact that it fades over time to reveal softer blue colours. In the late nineteenth century these dyes were derived from natural sources, but it became one of the first natural chemicals to be synthesised with the development of the modern chemical industry. Dyers favoured synthetically made indigo over indigo from plants because it was of higher quality. Almost all imported indigo is now synthetically generated, and most branded commercial manufacturing is based on versions of the Pflegers technique [32].
3 Common Treatment Strategies in Dye Removal Process
There are three most common treatment strategies of textile effluent physical methods (membrane filtration processes and sorption techniques), chemical methods (coagulation or flocculation and conventional oxidation processes), and biological methods [83, 87].
3.1 Physical Methods
These treatment methods are mostly used for small-scale dye removal. Physical methods for dye removal are costly and influenced by other effluent components and also have disposal problems. Major physical treatment processes are given below.
3.1.1 Adsorption
The adsorption technique is the most popular physical method. Despite a lower utilisation rate, this procedure has recently acquired interest because to its high dye removal effectiveness when compared to traditional procedures. Physical adsorption is caused by weak bonding interactions between the adsorbate and the adsorbent, such as hydrogen bonding, dipole–dipole, and van der wall interactions [38]. Due to its high adsorption property activated carbon is the best-known adsorbent that can reduce COD and 92% of color from the textile wastewater [30]. Other adsorbents that can be used to remove colours from wastewater include rice husks, sawdust, pine sawdust, alkaline white mud, bauxite, silica, and chitin, [111]. Some disadvantages of this procedure include the need for high-cost chemicals for precipitation and pH changes, as well as problems with disposal and dewatering of generated sludge following treatment.
3.1.2 Membrane Filtration
Another major approach for extracting colours from an aqueous solution is membrane filtration. The dye liquid is filtered through a micropore membrane of a specific size. The saturate can be reused after these membrane filters separate the dye from the effluent [10]. However, it acts as a physical barrier for the filtration of dyes, chemicals, and processed water for reuse and does not degrade or decolorize the dye [59]. After primary and secondary treatment of dye effluents, it is utilised as a principal post-treatment technique such as reverse osmosis, ultrafiltration, nanofiltration, and microfiltration. Solutes can be retained by membranes with varying pore sizes according to their different molecular weight cut-offs (MWCO) and are classified as: reverse osmosis (<1000 MWCO), nanofiltration (500–15,000 MWCO), and ultrafiltration membranes (1000–100,000 MWCO). The method effectively eliminates all types of dyes, although it generates a lot of sludge. High costs of labor and membrane replacement, since membranes are prone to clogging and fouling are major drawbacks of this method.
3.1.3 Irradiation
Irradiation is a useful approach for degrading dyes that are resistant to chemical oxidation /reduction. The amount of radiation and the availability of oxygen both influence the rate of deterioration [106]. For organic molecules to be successfully broken down by radiation, dissolved oxygen is required. It is an effective technique for the removal of reactive, acid, and disperse dyes as well as toxic organic compounds. The photocatalytic oxidation processes such as UV/H2O2, UV/TiO2, UV/Fenton, and UV/O3 form free radicals due to UV irradiations [45]. The method's main shortcomings are such high chemical cost and low UV light penetration in highly coloured effluent [113].
3.1.4 Coagulation/Flocculation
The use of a chemical reagent, a coagulant, disrupts the electrostatic connections that exist between the molecules of reactive hydrolyzed dyes (or auxiliaries) and water [5]. Coagulation is employed in conjunction with flocculation or sedimentation, and its effectiveness is determined by the medium's pH and the type of flocculant utilised. [5]. The usage of a high amount of chemicals and the formation of a large amount of sludge are the main disadvantages of this method [74].
3.2 Chemical Methods
3.2.1 Fenton’s Oxidation Method
The Fenton oxidation process generates radicals (-OH-) from Fenton's reagent (Fe2+/H2O2) when the Fe2+ ion is oxidised by H2O2 [98]. Both soluble and insoluble dyes can be efficiently removed from effluent. The procedure is generally inexpensive and effective in treating wastewater, resistant to biological treatment. It also has a high COD removal efficiency [88]. The main disadvantage of Fenton's approach is that it is difficult to treat effluents with an alkaline pH, as well as the precipitation of ferric ion salts, which results in a huge volume of sludge and the formation of radicals, which slows down the reaction rate [1].
3.2.2 Ozonation
Ozone was first used in the early 1970s because of its high instability, which made it a good oxidising agent (oxidation potential, 2.07) when compared to chlorine (1.36), and H202 (1.78). Ozonation is a chemical process in which ozone due to its oxidizing property decolorizes the dye at faster rates. In industrial processes, ozone breaks the conjugated bonds of chromophore groups in dye structure resulting in their decolorization with no sludge generation of toxic metabolites formation. Ozonation decolorizes water-soluble dyestuffs at a high rate as compared to non-soluble dyestuffs. One of the advantages is that it is applied in its gaseous state, so it does not affect the overall volume of wastewater. The disadvantage of the ozonation method is the short half-life (20 min) of ozone. Dye materials, pH, temperature, and the presence of effluent salts all affect the half-life. In addition, the cost of operation is higher than with electrochemical treatment. Another drawback of the ozonation process is the formation of secondary products such as aldehydes and dicarboxylic acids which are known to be more toxic than parent molecules [72].
3.2.3 Electrochemical Destruction
In the mid-1990s, the electrochemical destruction technique was developed. An electrochemical reduction/oxidation, electrocoagulation, and electroflotation reaction occur when an electric current is applied to the effluent using the electrodes (anode and cathode). Electrochemical oxidation of pre-treated textile effluent using boron-doped diamond (BDD) anode system resulting in 80% reduction in the COD after 12 h. A novel bioelectrochemical system (BES) based on electrodes; dissolved oxygen and bacterial biofilm formed on electrode had been reported by [109] for treatment of Methyl Orange (MO) that leads to the complete decolorization of the dye (0.15 mM) after 5.5 h. The main advantage of this method is no or little consumption of chemicals and no sludge formation. The major drawback of this technique is high electricity cost, foaming, the short life span of electrodes, poor decolorization of some dyes, and generation of unwanted products [16, 77].
3.2.4 Photochemical Oxidation
This method degrades dye molecules to CO2 and H2O by UV treatment in the presence of H2O2. High concentrations of hydroxyl radicals are produced during the reaction which results in the degradation of the dye molecules. These hydroxyl radicals broke down the chromophore group of unsaturated dye molecules. Advantages of this method are no sludge formation, a great reduction in foul odors. However, the dye removal rate is influenced by the intensity of the UV radiation, pH, dye structure, and the dye bath composition [27, 94].
3.3 Biological Process of Dye Removal
Dyes, organic pollutants, are well known for their harmful effects on human health and the environment. They are used in a wide variety of industries such as textile, pharmaceutics, cosmetics, food, leather, printer ink, or leather producing industries. The effluents released from these industries contain a wide variety of dyes [31]. Due to complex chemical structure and high molecular weight, the biodegradability of these stable dyes is more difficult [42, 104] and hence, direct release of such potent pollutants causes serious environmental impacts [31]. The discharge of these dyes has persistent colour and a high BOD (Biological Oxygen Demand) load. The toxic carcinogenic dyes obstruct the sunlight penetration into the water, inhibiting photosynthesis and growth of aquatic biota, and interferes with gas solubility [9]. Hence, dye removal from industrial effluent is a necessary process to meet environmental regulations. There are three different methods of dye removal—physical, chemical, and biological method. Physicochemical methods (adsorption, chemical and electrochemical oxidation, ion exchange, ozonation, coagulation/flocculation, membrane process, irradiation, sonication, and filtration with coagulation) are easy to use and some are cost-effective but there are some drawbacks associated with these methods [76]. High electricity consumption [18, 19], non-reusable by-products and sludge, multistage processing with long retention time, use of costly chemicals for pH modification (precipitation and coagulation), and dewatering are some of the prominent challenges associated with the physicochemical methods [49, 100].
Biological methods involve the conversion of synthetic dyes into less toxic inorganic compounds with the help of microorganisms (Table 1). These methods are considered to be cost-efficient, environmentally friendly, and results in reduced sludge production when compared with other techniques. The process uses aerobic or anaerobic organisms such as bacteria, fungi, algae and plants, and the enzymatic system.
3.3.1 Biological Removal of Dye Using Bacteria:
Bacteria can degrade organic pollutants by using them as an energy source and can also oxidize sulphur containing dyes into sulphuric acid. Azo dyes are most commonly treated with the two-stage bacterial process [56]. The first stage involves direct or indirect reduction of azo bond anaerobically and to facilitate this reaction auxiliary substrate must be present to generate reduction equivalents. The breakdown is brought by the azoreductase enzyme resulting in the colourless solution of aromatic amines that can be a mutagenic, toxic and potent carcinogen. The metabolites so formed are further catabolized by the aerobic method in the second stage.
3.3.2 Biological Removal of Dye Using Fungi:
Ligninolytic fungi has shown the most promising results of effluent treatment. It produces laccase, lignin peroxidase, and manganese peroxidase enzymes that are proven to oxidize soluble, insoluble, phenolic, and non-phenolic dyes. Lignin peroxidase oxidizes aromatic ring to cationic radical. Manganese peroxidase oxidizes Mn (II) to Mn (III) which further oxidize phenolic compounds to phenoxyl radicals and Laccase also oxidizes phenols to phenoxyl radicals. Fungal cells are first immobilized either by entrapment (microorganisms are captured in porous or fibrous material or are restrained in a solid or porous matrix-like natural polymeric gels and synthetic polymeric gels [47] or attachment (adherence to the surface like polyurethane foam or nylon foam or to other organisms). When immobilized fungi along with the effluent are kept in the bioreactor, decolourization takes place due to enzymatic action [56].
3.3.3 Biological Removal of Dye Using Algae:
Algae are potent biosorbents as they are having high surface area and high binding ability. They are found in both marine and freshwater. The decolourization by algae takes place by three different mechanisms—utilizing chromophores for harvesting algal biomass, carbon dioxide, and water, converting chromophore to non-chromophoric material, and finally, the resulting product is attached to algal biomass. It has been reported that algae may produce azoreductase dye enzyme against azo dyes resulting in decolourization [12]. The product obtained by enzymatic action is further degraded into organic compounds or carbon dioxide. Some algae can even use azo dyes as the sole source of carbon and nitrogen [9]. Dead algal biomass is more efficient than living algal biomass because of the nutritional requirement of living cells and hence, algal biomass can be stored and use for longer periods.
3.3.4 Biological Removal of Dye Using Plants:
Plants can also be used for the bioremoval of dyes from industrial effluents. Plants are autotrophic systems having large biomass and having little nutrient cost. They are accepted because of their easy handling, environmental sustainability, and aesthetic demand [36, 48]. Hydroponic system, plant tissue culture (hairy root, whole plant, callus, cell suspension), purified plant enzymes, and synergistic action of plants and microbes have been implemented/ explored for the biodegradation of dyes.
4 Importance of Anaerobic Process in Dye Removal
Due to their environmental safety and low input requirements, anaerobic treatment has been deemed the most promising technology for wastewater treatment in dyeing industry [107]. Various anaerobic treatment methods in the degradation of a wide range of synthetic dyes have been proved efficient many times [20]. Anaerobic decolourization uses a hydrogen-based oxidation–reduction reaction rather than free molecular oxygen in an aerobic environment to decolorize azo and other water-soluble pigments [83]. Anaerobic reduction of azo dyes can be a cost-effective and efficient way to remove colour from textile wastewater [26]. Chemical reduction by sulphide is partially responsible for the anaerobic transformations of Acid Orange 7, according to certain experiments. The experimental results were mathematically evaluated, and it was discovered that autocatalysis was crucial, as 1-amino-2-naphthol hastened the chemical reduction of the azo link. Under anaerobic conditions, decolourization of reactive water-soluble azo dyes was performed using glucose as a carbon source [14]. The addition of tapioca starch improved the efficacy of colour removal from synthetic blue effluent. Under anaerobic circumstances, methanogenic granular sludge was used to decrease and decolourize Mordant Orange 1 and Azodisalicylate [81]. In a traditional sewage treatment system, Reactive Red 141 was similarly decolorized under anaerobic surroundings. The chemical identification of dye degradation products revealed that decolourization occurred via a reduction mechanism.
Tartrazine, a synthetic dye, was revealed to be easily decolorized in an anaerobic baffled reactor (Bell et al. 2000; Plumb et al. 2001). In anoxic sediment–water systems, Disperse Blue 79 was likewise decreased, with the principal breakdown products being N, Ndisubstituted 1,4 azobenzene and 3-bromo-6-nitro-1,2-diaminobenzene (Weber and Adams 1995). The impact of various modern technologies on the decomposition rate of dyes, as well as the effect of the presence of other chemicals in the media, has received a lot of attention. The creation of high-rate systems, in which hydraulic retention times are uncoupled from solids retention times, has recently been discovered to facilitate the removal of dyes from textile industry wastewaters (Lier van et al. 2001). Another work (Zee van der et al. 2001) demonstrated the viability of using anaerobic granular sludge for total decolourization of 20 azo dyes. It was also shown that using the redox mediator anthraquinone-2,6-disulfonic acid greatly accelerates up the breakdown of azo dyes (Zee van der et al. 2001b). Under anaerobic conditions, the effect of salts (nitrate and sulphate) on the breakdown rate of the azo dye Reactive Red 141 was investigated. The findings revealed that nitrate slows the commencement of breakdown, whereas sulphate had no effect on the biodegradation process (Carliell et al. 1998).
Several high-rate anaerobic reactors, such as the upflow anaerobic sludge blanket (UASB) and the anaerobic baffled reactor (ABR), have been used in textile wastewater treatment in various investigations (Sen et al. 2003). The ability of a thermophilic UASB anaerobic system to discolour synthetic textile dye wastewater as a unit operation clearly shows that it has a considerable advantage over a comparable mesophilic system and can efficiently decolourize such wastewater with a greater efficiency (Willetts et al. 2000). Under anaerobic conditions, using a sequencing batch biofilter, discolouration of commercially relevant azo dyes Orange II, Black 2 HN exhibited > 99 percent colour removal up to a dye concentration of 400 mg/l for both colours [67]. The anaerobic filter and the UASB reactor outperformed the other reactor types evaluated in terms of colour removal efficiency.
5 Integration of Aerobic/Anaerobic Systems to Work as a Hybrid System
The co-existence of anaerobic and aerobic microbes in a single biofilm is the foundation of this system (Zitomer et al. 1998). An integrated anaerobic–aerobic system can be maintained by supplying oxygen to an oxygen-tolerant anaerobic consortium (Tan et al. 1999). Initially, to remove certain dyes such as Mordant Yellow 10 and 4-Phenylazophenol, Expanded Granular Sludge Bed (EGSB), which is an integrated method with oxygenation of recovered effluent was used (Tan et al. 1999; Tan et al. 2001). Reactive Red 5 was removed using a baffled reactor with anaerobic and aerobic compartments, demonstrating excellent colour removal efficiency (Gottlieb et al. 2002). Van der Zee and Villaverdee (2005) in their review article mentioned the degradation of biological azo dye in two stages where the first step is the reductive cleavage of the azo bond, leading to colour reduction which is more efficient in the anaerobic digestion process but also results in the production of aromatic amines which can, in turn, have a high toxicity. It is more appropriate to have a subsequent degradation of the produced aromatic amines, in aerobic environments, which are a very efficient method for the removal of aromatic amines. Due to limitations of aerobic digestion leading to low colour removal and anaerobic digestion resulting in the generation of aromatic amines and limitation of its mineralization, many authors like [23] and [60] have suggested using both the aerobic—anaerobic process for treating effluents with different types of azo dyes.
Despite the effectiveness of anaerobic reduction in the removal of azo dyes the intermediate products such as carcinogenic aromatic amines need to be degraded by an aerobic process. In a study where this type of hybrid system was involved, decolourization rates were found to be 20%, 72%, and 78% for Acid Yellow 17, Basic Blue 3, and Basic Red 2, respectively [2]. This method has successfully used for the degradation of bisazo vinylsulphonyl, anthraquinone vinylsulphonyl and anthraquinone monochlootriazine reactive dyes (Panswad and Luangdilok, 2000). A study has also shown the removal of Basic Red Dye using a sequential anaerobic/aerobic filter system [11]. In a similar study, further two-stage anaerobic/aerobic system has shown the successful decomposition of sulfonated azo dyes such as Acid Orange 10, Acid Red 14, and Acid Red 18 (FitzGerald and Bishop, 1995). In a study by [91], it was further demonstrated that an anaerobic/aerobic treatment is highly useful for the breakage of the azo bond in various azo dyes. The investigation comprising of the removal of reactive diazo Remazol Black B dye by aerobic and anoxic along with anaerobic/aerobic sequencing batch reactor (SBR) activated sludge processes showed that longer anoxic and anaerobic period-promoted decolourization [75].
5.1 Typical Effluent Treatment Process
The typical effluent treatment process can be categorized into the primary, secondary, and tertiary treatment processes. Since most of the dye using industries like textile, paper printing, leather, plastic, cosmetics, etc. consume large quantities of water at every stage of their process, wastewater coming from such industries is the main effluent laden with dye and other wastes. An overview provided by [95] of typical primary, secondary and tertiary treatment technologies. The typical primary treatment process consists of screening, sedimentation, homogenization, neutralization, mechanical flocculation, and or chemical coagulation. Secondary treatment can include any or combination of aerobic and or anaerobic treatment which can be sequential, integrated or staggered, aerated lagoons, activated sludge process, trickling filtration, oxidation ditch, and pond. Tertiary treatment which is also considered as the end to pipe or final treatment stage includes technologies individually or in combination like membrane filtration (modified cellulose membrane), adsorption (biochar, agricultural modified waste, etc.) oxidation technique, electrolytic precipitation, foam fractionation, electrochemical processes, ion exchange method, photocatalytic degradation and or thermal evaporation. Below we have tried to list some of the key novel technologies which can be used for the removal of dye residues or by-products generated because of its treatment.
5.2 Hybrid Model (Anaerobic–Aerobic Digestion) with Photocatalysis
[44] demonstrated the use of photocatalysis (fixed titanium dioxide) for the treatment of azo, anthraquinone, and phthalocyanine textile dyes. Photocatalysis was applied on treated product generated post-biological (anaerobic–aerobic) treatment of azo dyes. The product generated post photocatalysis showed non-toxicity to methanogenic bacteria.
5.3 Hybrid Model with Partial Ozonisation
[52] combined biological and chemical treatment into a sequential batch reactor (SBR) for the treatment of residual dyehouse liquors, which has a high concentration of reactive azo dye and recalcitrant compounds. SBR had anaerobic and aerobic phases where water-soluble reactive dyes were reductively cleaved and decolorized by a facultative anaerobic bacterial mixed culture under anaerobic conditions. Further mineralization of the cleaved products produced during anaerobic degradation was subjected to the mixed bacterial population in aerobic conditions. For mineralization of recalcitrant products present initial, ozone was used for partial oxidation in the aerobic phase. Ozone supported mineralization by increasing selectivity of reaction and better biological mineralization. At the end of the process, complete decolourization was achieved along with overall 90% degradation of dissolved organic carbon (DOC).
5.4 Adsorption with Anaerobic Digestion
[37] demonstrated a combination of the adsorption process with anaerobic biological treatment. Anaerobic treatment is the most used method to treat complex wastes present in textile effluent. Sawdust was used as adsorption material for sludge generated post anaerobic treatment. This resulted in ease of sludge collection and disposal.
When compared to other sequential systems, the advantage of these systems is the small-scale requirement. One thing to keep in mind is that these systems were largely used in lab settings using synthetic colours. To determine the usefulness of such a system in an industrial textile mill, a full-scale test should be conducted.
6 Conclusion
Dye decolourization and degradation is now one of the most common problems in textile plants as a result of the current stringent standards. The aesthetic and environmental difficulties of discharging textile effluents in a water body should be considered while designing an effluent treatment facility. The activated sludge technique is the most widely utilized treatment in textile wastewater treatment plants around the world. According to published research, such a technique is unable to entirely remove colour from effluent. Several applications are using lengthy course of actions to increase the bio-adsorption of dyes into bio-sludge using just aerobic processes. This type of process operation may meet local rules for effluent needs in some countries; however, it is ineffective for dye degradation since dyes remain in the sludge. Anaerobic reduction, particularly with azo dyes, can be a cost-effective and efficient way to remove colour from textile effluent. Another significant benefit is that the biogas produced by anaerobic degradation can be used in a cogeneration system to generate both heat and energy. The best treatment plant structure generally incorporates spatially consecutive anaerobic and aerobic treatments. The tertiary treatment procedure should be focused on for effective removal of dye residues remaining after secondary treatment, as this is where the main load of leftover residues like cleaved products post dye degradation needs to be addressed. However, it can be concluded that a combination of different technologies like hybrid model involving anaerobic–aerobic treatment and or usage of adsorption, filtration, ozonation, photocatalysis, etc. needs to be selected appropriately to cater in their removal and thus no toxic by-products are left to enter the environment which can further cause damage.
References
Abou-Gamra ZM (2014) Kinetic and thermodynamic study for fenton-like oxidation of amaranth red dye. Advances in chemical engineering and science (2014)
An H, Qian Y, Gu X, Tang WZ (1996) Biological treatment of dye wastewaters using an anaerobic-oxic system. Chemosphere 33(12):2533–2542
Ajaz M, Shakeel S, Rehman A (2020) Microbial use for azo dye degradation—a strategy for dye bioremediation. Int Microbiol 23(2):149–159. https://doi.org/10.1007/s10123-019-00103-2
Ali H (2010) Biodegradation of synthetic dyes - a review. Water Air Soil Pollut 213(1–4):251–273. https://doi.org/10.1007/s11270-010-0382-4
Allegre C, Maisseu M, Charbit F, Moulin P (2004) Coagulation–flocculation–decantation of dye house effluents: concentrated effluents. J Hazard Mater 116(1–2):57–64
Amani-Ghadim AR, Aber S, Olad A, Ashassi-Sorkhabi H (2013) Optimization of electrocoagulation process for removal of an azo dye using response surface methodology and investigation on the occurrence of destructive side reactions. Chem Eng Process 64:68–78. https://doi.org/10.1016/j.cep.2012.10.012
Ayed L, Ksibi IE, Charef A, Mzoughi RE (2021) Hybrid coagulation-flocculation and anaerobic-aerobic biological treatment for industrial textile wastewater: pilot case study. J Textile Inst 112(2):200–206
Baêta BEL, Lima DRS, Queiroz Silva S, Aquino SF (2016) Influence of the applied organic load (OLR) on textile wastewater treatment using submerged anaerobic membrane bioreactors (SAMBR) in the presence of redox mediator and powdered activated carbon (PAC). Braz J Chem Eng 33(4):817–825. https://doi.org/10.1590/0104-6632.20160334s20150031
Banat IM, Nigam P, Singh D, Marchant R (1996) Microbial decolorization of textile-dyecontaining effluents: a review. Biores Technol 58(3):217–227
Barredo-Damas S, Alcaina-Miranda MI, Iborra-Clar MI, Mendoza-Roca JA (2012) Application of tubular ceramic ultrafiltration membranes for the treatment of integrated textile wastewaters. Chem Eng J 192:211–218
Basibuyuk MESUT, Forster CF (1997) The use of sequential anaerobic/aerobic processes for the biotreatment of a simulated dyeing wastewater. Environ Technol 18(8):843–848
Bhatia D, Sharma NR, Singh J, Kanwar RS (2017) Biological methods for textile dye removal from wastewater: a review. Crit Rev Environ Sci Technol 47(19):1836–1876. https://doi.org/10.1080/10643389.2017.1393263
Božič M, Kokol V (2008) Ecological alternatives to the reduction and oxidation processes in dyeing with vat and sulphur dyes. Dyes Pigm 76(2):299–309. https://doi.org/10.1016/j.dyepig.2006.05.041
Carliell CM, Barclay SJ, Buckley CA (1996) Treatment of exhausted reactive dyebath effluent using anaerobic digestion: laboratory and full-scale trials. Water SA 22(3):225–233
Chinwetkitvanich S, Tuntoolvest M, Panswad T (2000) Anaerobic decolorization of reactive dyebath effluents by a two-stage UASB system with tapioca as a co-substrate. Water Res 34(8):2223–2232
Cui L, Wu J, Li J, Ge Y, Ju H (2014) Electrochemical detection of Cu2+ through Ag nanoparticle assembly regulated by copper-catalyzed oxidation of cysteamine. Biosens Bioelectron 55:272–277
Couto SR (2009) Dye removal by immobilised fungi. Biotechnol Adv 27(3):227–235
Demirbas A (2009) Agricultural based activated carbons for the removal of dyes from aqueous solutions: a review. J Hazard Mater 167(1–3):1–9
De Gisi S, Lofrano G, Grassi M, Notarnicola M (2016) Characteristics and adsorption capacities of low-cost sorbents for wastewater treatment: a review. Sustain Mater Technol 9:10–40
Delee W, O’Neill C, Hawkes FR, Pinheiro HM (1998) Anaerobic treatment of textile effluents: a review. J Chem Technol Biotechnol: Int Res Process, Environ Clean Technol 73(4):323–335
dos Santos AB, Cervantes FJ, van Lier JB (2007) Review paper on current technologies for decolourisation of textile wastewaters: perspectives for anaerobic biotechnology. Biores Technol 98(12):2369–2385. https://doi.org/10.1016/j.biortech.2006.11.013
Duxbury DF (1993) The photochemistry and photophysics of triphenylmethane dyes in solid and liquid media. Chem Rev 93(1):381–433
Koupaie EH, Moghaddam MR, Hashemi SH (2011) Post-treatment of anaerobically degraded azo dye acid red 18 using aerobic moving bed biofilm process: enhanced removal of aromatic amines. J Hazard Mater 195:147–154
Eren E, Afsin B (2008) Investigation of a basic dye adsorption from aqueous solution onto raw and pre-treated bentonite surfaces. Dyes Pigm 76(1):220–225. https://doi.org/10.1016/j.dyepig.2006.08.019
Eren E (2009) Investigation of a basic dye removal from aqueous solution onto chemically modified Unye bentonite. J Hazard Mater 166(1):88–93. https://doi.org/10.1016/j.jhazmat.2008.11.011
Erkurt HA (2010) Biodegradation of azo dyes. Springer
Forgacs E, Cserhati T, Oros G (2004) Removal of synthetic dyes from wastewaters: a review. Environ Int 30(7):953–971
Fernandes NC, Brito LB, Costa GG, Taveira SF, Cunha-Filho MSS, Oliveira GAR, Marreto RN (2018) Removal of azo dye using Fenton and Fenton-like processes: evaluation of process factors by Box-Behnken design and ecotoxicity tests. Chem Biol Interact 291:47–54. https://doi.org/10.1016/j.cbi.2018.06.003
Gaydardzhiev S, Karthikeyan J, Ay P (2006) Colour removal from model solutions by coagulation - surface charge and floc characterisation aspects. Environ Technol 27(2):193–199. https://doi.org/10.1080/09593332708618633
Ghasemian E, Palizban Z (2016) Comparisons of azo dye adsorptions onto activated carbon and silicon carbide nanoparticles loaded on activated carbon. Int J Environ Sci Technol 13(2):501–512
Gholami M, Nasseri S, Alizadehfard MR, Mesdaghinia A (2003) Textile dye removal by membrane technology and biological oxidation. Water Quality Res J 38(2):379–391
Głowacki ED, Voss G, Leonat L, Irimia-Vladu M, Bauer S, Sarıçiftçi NS (2012) Indigo and Tyrian purple—from ancient natural dyes to modern organic semiconductors. Isr J Chem 52:1–12
Gottlieb A, Shaw C, Smith A, Wheatley A, Forsythe S (2003) The toxicity of textile reactive azo dyes after hydrolysis and decolourisation. J Biotechnol 101(1):49–56
Gregory P (1990) Classification of dyes by chemical structure. In: Waring DR, Hallas G (eds) The chemistry and application of dyes. Plenum Press, New York
Growther L, Meenakshi M (2011) Biotechnological approaches to combat textile effluents. ChemInform 42(29)
Ghodake GS, Talke AA, Jadhav JP, Govindwar SP (2009) Potential of Brassica juncea in order to treat textile—effluent—contaminated sites. Int J Phytorem 11(4):297–312
Gobinath R, Saravanan SP, Kovendiran S, Hema S, Anas SM, Thangaraj M, Sangeetha M (2015) Treatment of textile wastewater by adsorption process combined with anaerobic digestion. Int J Appl Eng Res 10(53):101–106
Guo X, Yao Y, Yin G, Kang Y, Luo Y, Zhuo L (2008) Preparation of decolorizing ceramsites for printing and dyeing wastewater with acid and base treated clay. Appl Clay Sci 40(1–4):20–26
Gupta VK, Suhas (2009) Application of low-cost adsorbents for dye removal - a review. J Environ Manage 90(8):2313–2342. https://doi.org/10.1016/j.jenvman.2008.11.017
Hai FI, Yamamoto K, Fukushi K (2007) Hybrid treatment systems for dye wastewater. Crit Rev Environ Sci Technol 37(4):315–377. https://doi.org/10.1080/10643380601174723
Haapala R, Linko S (1993) Production of phanerochaete chrysosporium lignin peroxidase under various culture conditions. Appl Microbiol Biotechnol 40(4):494–498
Hao OJ, Kim H, Chiang P-C (2000) Decolorization of wastewater. Crit Rev Environ Sci Technol 30(4):449–505
Han YH, Van der Robert H, Verpoorte R (2001) Biosynthesis of anthraquinones in cell cultures of the Rubiaceae. Plant Cell Tiss Org 67:201–220
Harrelkas F, Paulo A, Alves MM, El Khadir L, Zahraa O, Pons MN, Van Der Zee FP (2008) Photocatalytic and combined anaerobic–photocatalytic treatment of textile dyes. Chemosphere 72(11):1816–1822
Isaev AB, Aliev ZM, Adamadzieva NK, Alieva NA, Magomedova GA (2009) The photocatalytic oxidation of azo dyes on Fe2 O3 nanoparticles under oxygen pressure. Nanotechnol Russ 4(7):475–479
Jiang H, Bishop PL (1994) Aerobic biodegradation of azo dyes in biofilms. Water Sci Technol 29(10–11):525
Katzbauer B, Narodoslawsky M, Moser A Classification system for immobilization techniques.“ Bioprocess Engineering 12, no. 4 (1995): 173–179.
Kagalkar AN, Jagtap UB, Jadhav JP, Bapat VA, Govindwar SP (2009) Biotechnological strategies for phytoremediation of the sulfonated azo dye Direct Red 5B using Blumea malcolmii hook. Biores Technol 100(18):4104–4110
Kim S-H, Kim T-W, Cho D-L, Lee D-H, Kim J-C, Moon H (2002) Application of characterization procedure in water and wastewater treatment by adsorption. Korean J Chem Eng 19(5):895–902
Konsowa AH, Abd El-Rahman HB, Moustafa MA (2011) Removal of azo dye acid orange 7 using aerobic membrane bioreactor. Alex Eng J 50(1):117–125. https://doi.org/10.1016/j.aej.2011.01.014
Khandare RV, Govindwar SP (2015) Phytoremediation of textile dyes and effluents: current scenario and future prospects. Biotechnol Adv 33(8):1697–1714
Krull R, Hemmi M, Otto P, Hempel DC (1998) Combined biological and chemical treatment of highly concentrated residual dyehouse liquors. Water Sci Technol 38(4–5):339–346
Kuhad RC, Sood N, Tripathi KK, Singh A, Ward OP (2004) Developments in microbial methods for the treatment of dye effluents. Adv Appl Microbiol 56:185–213. https://doi.org/10.1016/S0065-2164(04)56006-9
Kusumawati N, Santoso AB, Sianita MM, Muslim S (2017) Extraction, characterization, and application of natural dyes from the fresh mangosteen (Garcinia mangostana L.) peel. Int J Adv Sci, Eng Inf Technol 7(3):878–884. https://doi.org/10.18517/ijaseit.7.3.1014
Li C, Yang X, Chen R, Pan J, Tian H, Zhu H, Wang X, Hagfeldt A, Sun L (2007) Anthraquinone dyes as photosensitizers for dye-sensitized solar cells. Sol Energ Mat Sol C 91:1863–1871
Libra JA, Borchert M, Vigelahn L, Storm T (2004) Two stage biological treatment of a diazo reactive textile dye and the fate of the dye metabolites. Chemosphere 56(2):167–180
Lillie RD, Conn HJ (1991) HJ Conn's biological stains. Sigma Chemical Co
Luangdilok W, Panswad T (2000) Effect of chemical structures of reactive dyes on color removal by an anaerobic-aerobic process. Water Sci Technol 42(3–4):377–382
Lu Y, Shi W, Qin J, Lin B (2010) Fabrication and characterization of paper-based microfluidics prepared in nitrocellulose membrane by wax printing. Anal Chem 82(1):329–335
Silva MER, Firmino PIM, Sousa MR, Dos Santos AB (2012) Sequential anaerobic/ aerobic treatment of dye-containing wastewaters: colour and cod removals, and ecotoxicity tests. Appl Biochem Biotechnol 166:1057–1069
Mani S, Bharagava RN (2010) 3 Textile industry wastewater environmental and health hazards and treatment approaches, pp 47–69
Meerbergen K, Willems KA, Dewil R, Van Impe J, Appels L, Lievens B (2018) Isolation and screening of bacterial isolates from wastewater treatment plants to decolorize azo dyes. J Biosci Bioeng 125(4):448–456. https://doi.org/10.1016/j.jbiosc.2017.11.008
Melgoza AM, Cruz A, Buitrón G (2004) Anaerobic/aerobic treatment of colorants present in textile effluents. Water Sci Technol 50(2):149–155. https://doi.org/10.2166/wst.2004.0111
Marmion DM (1991) Handbook of US colorants: foods, drugs, cosmetics, and medical devices. Wiley
Mitrović J, Radović M, Bojić D, Anđelković T, Purenović M, Bojić A (2012) Decolorization of textile azo dye reactive orange 16 with UV/H2O2 process. J Serb Chem Soc 77(4):465–481
Mani S, Bharagava RN (2018) Textile industry wastewater: environmental and health hazards and treatment approaches. In: Recent advances in environmental management, pp 47–69. CRC Press
Manu B, Chaudhari S (2002) Anaerobic decolorisation of simulated textile wastewater containing azo dyes. Biores Technol 82(3):225–231
Miao Y, Ouyang L, Zhou S, Xu L, Yang Z, Xiao M, Ouyang R (2014) Electrocatalysis and electroanalysis of nickel, its oxides, hydroxides and oxyhydroxides toward small molecules. Biosens Bioelectron 53:428–439
Nakamura Y, Sawada T, Sungusia MG, Kobayashi F, Kuwahara M, Ito H (1997) Lignin peroxidase production by Phanerochaete chrysosporium immobilized on polyurethane foam. J Chem Eng Jpn 30(1):1–6
Novotný Č, Dias N, Kapanen A et al (2006) Comparative use of bacterial, algal, and protozoan tests to study the toxicity of azo- and anthraquinone dyes. Chemosphere 63(9):1436–1442
Omar HH (2008) Algal decolorization and degradation of monoazo and diazo dyes. Pak J Biol Sci 11(10):1310–1316
Patil NN, Shukla SR (2015) Decolorization of reactive blue 171 dye using ozonation and UV/H2O2 and elucidation of the degradation mechanism. Environ Prog Sustain Energy 34(6):1652–1661
Panda R, Panda H, Panda A (2009) Reactive dyes. Colourage 56(6):44–49. https://doi.org/10.1201/b21336-17
Pandey R, Patel S, Pandit P, Nachimuthu S, Jose S (2018) Colouration of textiles using roasted peanut skin-an agro processing residue. J Clean Prod 172:1319–1326
Panswad T, Techovanich A, Anotai J (2001) Comparison of dye wastewater treatment by normal and anoxic+ anaerobic/aerobic SBR activated sludge processes. Water Sci Technol 43(2):355–362
Piaskowski K, Świderska-Dąbrowska R, Zarzycki PK (2018) Dye removal from water and wastewater using various physical, chemical, and biological processes. J AOAC Int 101(5):1371–1384
Pelegrini R, Peralta-Zamora P, de Andrade AR, Reyes J, Durán N (1999) Electrochemically assisted photocatalytic degradation of reactive dyes. Appl Catal B 22(2):83–90
Purkait MK, DasGupta S, De S (2005) Adsorption of eosin dye on activated carbon and its surfactant based desorption. J Environ Manage 76(2):135–142. https://doi.org/10.1016/j.jenvman.2005.01.012
Rai HS, Bhattacharyya MS, Singh J, Bansal TK, Vats P, Banerjee UC (2005) Removal of dyes from the effluent of textile and dyestuff manufacturing industry: a review of emerging techniques with reference to biological treatment. Crit Rev Environ Sci Technol 35(3):219–238. https://doi.org/10.1080/10643380590917932
Ramirez LE, Lages-Silva E, Pianetti GM, Rabelo RMC, Bordin JO, Moraes-Souza H (1995) Prevention of transfusion-associated Chagas’ disease by sterilization of Trypanosoma cruzi-infected blood with gentian violet, ascorbic acid, and light. Transfusion 35(3):226–230
Razo-Flores E, Luijten M, Donlon B, Lettinga G, Field J (1997) Biodegradation of selected azo dyes under methanogenic conditions. Water Sci Technol 36(6–7):65–72
Rodríguez Couto S (2009) Dye removal by immobilised fungi. Biotechnol Adv 27(3):227–235. https://doi.org/10.1016/j.biotechadv.2008.12.001
Robinson T, Marchant R, Nigam P (2001) Remediation of dyes in textile effluent: a critical review on current treatment technologies with a proposed alternative. Biores Technol 77:247–255
Royer B, Cardoso NF, Lima EC, Vaghetti JCP, Simon NM, Calvete T, Veses RC (2009) Applications of Brazilian pine-fruit shell in natural and carbonized forms as adsorbents to removal of methylene blue from aqueous solutions-Kinetic and equilibrium study. J Hazard Mater 164(2–3):1213–1222. https://doi.org/10.1016/j.jhazmat.2008.09.028
Salem SS, Mohamed AA, Gl-Gamal MS, Talat M, Fouda A (2019) Biological decolorization and degradation of azo dyes from textile wastewater effluent by Aspergillus niger. Egyptian J Chem 62(10):1799–1813. https://doi.org/10.21608/EJCHEM.2019.11720.1747
Salleh MAM, Mahmoud DK, Karim WAWA, Idris A (2011) Cationic and anionic dye adsorption by agricultural solid wastes: a comprehensive review. Desalination 280(1–3):1–13. https://doi.org/10.1016/j.desal.2011.07.019
Saratale RG, Saratale GD, Chang JS, Govindwar SP (2011) Bacterial decolorization and degradation of azo dyes: a review. J Taiwan Inst Chem Eng 42(1):138–157
Saratale RG, Sivapathan S, Saratale GD, Banu JR, Kim DS (2019) Hydroxamic acid mediated heterogeneous Fenton-like catalysts for the efficient removal of Acid Red 88, textile wastewater and their phytotoxicity studies. Ecotoxicol Environ Saf 167:385–395
Sarkar S, Banerjee A, Halder U, Biswas R, Bandopadhyay R (2017) Degradation of synthetic azo dyes of textile industry: a sustainable approach using microbial enzymes. Water Conserv Sci Eng 2(4):121–131. https://doi.org/10.1007/s41101-017-0031-5
Shah M (2014) Effective treatment systems for azo dye degradation: a joint venture between physico-chemical and microbiological process. Int J Environ Bioremed Biodegradat 2(5):231–242. https://doi.org/10.12691/ijebb-2-5-4
Seshadri S, Bishop PL, Agha AM (1994) Anaerobic/aerobic treatment of selected azo dyes in wastewater. Waste Manage 14(2):127–137
Şen S, Demirer GN (2003) Anaerobic treatment of real textile wastewater with a fluidized bed reactor. Water Res 37(8):1868–1878
Singh PK, Singh RL (2017) Bio-removal of azo dyes: a review. Int J Appl Sci Biotechnol 5(2):108–126 (2017)
Slokar YM, Le Marechal AM (1998) Methods of decoloration of textile wastewaters. Dyes Pigm 37(4):335–356
Srebrenkoska V, Zhezhova S, Risteski S, Golomeova S (2014) Methods for waste waters treatment in textile industry. In: International scientific conference UNITECH, pp 248 – 252
Soares OSGP, Órfão JJM, Portela D, Vieira A, Pereira MFR (2006) Ozonation of textile effluents and dye solutions under continuous operation: Influence of operating parameters. J Hazard Mater 137(3):1664–1673. https://doi.org/10.1016/j.jhazmat.2006.05.006
Tan NC, Borger A, Slenders P, Svitelskaya A, Lettinga G, Field JA (2000) Degradation of azo dye Mordant Yellow 10 in a sequential anaerobic and bioaugmented aerobic bioreactor. Water Sci Technol 42(5–6):337–344
Tantak NP, Chaudhari S (2006) Degradation of azo dyes by sequential Fenton’s oxidation and aerobic biological treatment. J Hazard Mater 136(3):698–705
Thirumagal J, Panneerselvam A (2016) Isolation of azoreductase enzyme in its various forms from Chlorella Pyrenoidosa and its immobilization efficiency for treatment of water. Int J Sci Res 5:2133–2138
Ukiwe LN, Ibeneme SI, Duru CE, Okolue BN, Onyedika GO, Nweze CA (2014) Chemical and electro-coagulation techniques in coagulation-flocculation in water and wastewater treatment-a review. J Adv Chem 9(3):2321–807
Vankar PS (2000) Chemistry of natural dyes. Resonance 5(10):73–80. https://doi.org/10.1007/bf02836844
Van der Zee FP, Lettinga G, Field JA (2000) The role of (auto) catalysis in the mechanism of an anaerobic azo reduction. Water Sci Technol 42(5–6):301–308
Van der Zee FP, Villaverde S (2005) Combined anaerobic–aerobic treatment of azo dyes—a short review of bioreactor studies. Water Res 39(8):1425–1440
Wang S, Li H (2007) Kinetic modeling and mechanism of dye adsorption on unburned carbon. Dyes Pigm 72(3):308–314
Willetts JRM, Ashbolt NJ (2000) Understanding anaerobic decolourisation of textile dye wastewater: mechanism and kinetics. Water Sci Technol 42(1–2):409–415
Wojnarovits L, Takacs E (2008) Irradiation treatment of Azo dye containing wastewater: an overview. Radiat Phys Chem 77(3):225–244
Xu H, Yang B, Liu Y, Li F, Shen C, Ma C, Sand W (2018) Recent advances in anaerobic biological processes for textile printing and dyeing wastewater treatment: a mini-review. World J Microbiol Biotechnol 34(11):1-9
Yagub MT, Sen TK, Afroze S, Ang HM (2014) Dye and its removal from aqueous solution by adsorption: a review. Adv Coll Interface Sci 209:172–184. https://doi.org/10.1016/j.cis.2014.04.002
Yang Q, Wang J, Wang H, Chen X, Ren S, Li X, Li X (2012) Evolution of the microbial community in a full-scale printing and dyeing wastewater treatment system. Bioresour Technol 117:155–163
Yurtsever A, Sahinkaya E, Aktaş Ö, Uçar D, Çinar Ö, Wang Z (2015) Performances of anaerobic and aerobic membrane bioreactors for the treatment of synthetic textile wastewater. Biores Technol 192:564–573. https://doi.org/10.1016/j.biortech.2015.06.024
Zazouli MA, Balarak D, Mahdavi Y (2013) Application of Azolla for 2-chlorophenol and 4-chrorophenol removal from aqueous solutions. Iranian J Health Sci 1(2):43–55
Zitomer DH, Speece RE (1993) Sequential environments for enhanced biotransformation of aqueous contaminants. Environ Sci Technol 27(2):226–244
Zyoud A, Zu’bi A, Helal MH, Park D, Campet G, Hilal HS (2015) Optimizing photo-mineralization of aqueous methyl orange by nano-ZnO catalyst under simulated natural conditions. J Environ Health Sci Eng 13(1):1–10
Author information
Authors and Affiliations
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2022 The Author(s), under exclusive license to Springer Nature Singapore Pte Ltd.
About this chapter
Cite this chapter
Vashisht, A. et al. (2022). Anaerobic Processes in Dye Removal. In: Khadir, A., Muthu, S.S. (eds) Biological Approaches in Dye-Containing Wastewater. Sustainable Textiles: Production, Processing, Manufacturing & Chemistry. Springer, Singapore. https://doi.org/10.1007/978-981-19-0545-2_4
Download citation
DOI: https://doi.org/10.1007/978-981-19-0545-2_4
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-19-0544-5
Online ISBN: 978-981-19-0545-2
eBook Packages: Chemistry and Materials ScienceChemistry and Material Science (R0)