Abstract
Microbes play several vital physiological and metabolic functions in human body. It has been observed that alteration in human gut microbiota has resulted in various chronic and acute metabolic diseases such as obesity, hypertension, neurogenic diseases (Parkinson’s and Alzheimer), diabetes, etc. Hence, re-establishment of microbial population, with the help of commensal probiotic bacteria, to improve the gut dysbiosis, has always been the topic of interest. Currently, with the growing knowledge of synthetic biology, genetic engineering, metabolic engineering, and other advanced tools, researchers are attempting to design recombinant probiotic strains, which are capable of carrying therapeutic molecules to the target site. These designer probiotics will enhance the efficacy of the carried molecule without showing any side effects. However, currently, the consumer acceptance of such “Designer Probiotics” is very low. The current chapter envisages a brief introduction about designer probiotics, their developmental strategies, applications of designer probiotics in regulating metabolic diseases, and the challenges in the path of their development discussing examples of few designer probiotic strains. Overall, this chapter intends to provide insight towards the development of designer probiotics to improve the human health.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Phenylketonuria
- Patho-biotechnology
- Receptor mimicking
- Synthetic oligosaccharides
- Anti-microbial peptide
12.1 Introduction
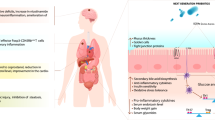
In human body ecosystem, microbes are found most abundantly in the gastrointestinal (GI) tract, and these gut microbiota plays a significant beneficial role in human life by participating in various physiological functions advantageous to the host (Tlaskalova-Hogenova et al. 2004; Raghuwanshi et al. 2015; Kristensen et al. 2016). Here, the body works as a host, which provides a suitable condition for growth, while the commensal microbes perform their counterparts by preventing pathogens (Hand 2016), increasing host-immunity (Round and Mazmanian 2009; Patel and DuPont 2015; Macpherson et al. 2017; Raghuwanshi et al. 2018), enhancing the stimulus for GI-hormones (Saulnier et al. 2013), and controlling brain behavior (De Palma et al. 2014; Steenbergen et al. 2015; Kristensen et al. 2016; De Palma et al. 2017). The uniqueness of these gut microbiota is their capability evolve naturally and inhabiting every potential tissue.
Though the normal gut microbiota are essential for several vital processes, however, occasionally they fails, which can be the result of gut microbiota manipulation through hygiene, lifestyle changes, and diet, for example, diet can cause an impact to promote phylogenetic variations in the microbiota (Graf et al. 2015). Moreover, physical activity is also known to affect the gut-microbiome diversity as it is evident that athletes have a more diverse gut microbiome than non-athletes (Clarke et al. 2014). Besides these passive factors, the active manipulators of gut microbiota are antibiotics. The use of antibiotics has been linked to dysbiosis (Langdon et al. 2016), leading to low diversity and evenness among gut microbes (Dethlefsen and Relman 2010; Francino 2016). Moreover, presence and expression of microbial genes are altered following antibiotic therapy, which also lead to detrimental functions of microbiota (Reijnders et al. 2016).
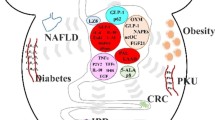
All these factors for gut dysbiosis including overly use of antibiotics, passive lifestyle, use of pesticides in farms, etc., may lead to antibiotic resistance against pathogens, increase obesity epidemic, inflammation, resistance to insulin, diabetic condition, heart diseases (CVDs), brain-related disorders, dyslipidemia, patho-physiological conditions such as allergy, intestinal inflammatory diseases, and even cancers (Amaral et al. 2012; Benbouziane et al. 2013; Andreu and Torrent 2015; Aydin et al. 2015). However, enhancing the functional repertoire of probiotics, the commensal organisms that can be harnessed for therapeutic benefit, is a promising approach to combat these issues. Probiotics can affect the host either directly or through their products, or even can influence the activity of resident bacteria in the host (Scott et al. 2015). The first health benefit report of probiotics was discussed by Russian scientist, Eli Metchnikoff (1907), according to whom “the dependence of the intestinal microbes on the food makes it possible to adopt measures to modify the flora in our bodies and to replace the harmful microbes with useful microbes.” Later, WHO and FAO defined the probiotics as “live microorganisms which when administered in adequate amounts confer a health benefit on the host” (WHO/FAO 2006; Hill et al. 2014). There are several reports of beneficial impacts of probiotic organisms in metabolic disorders, inflammatory disorders, CNS related disorders, pathogen infections, dyslipidemia, and regulating mucosal immune response (Fig. 12.1) (Miettinen et al. 1996; Kwon et al. 2010; Yin 2010; Chen et al. 2011; Yan and Polk 2011; Klaenhammer et al. 2012; Asemi et al. 2013; Kim et al. 2013; Plaza-Diaz 2014; Reichold et al. 2014; Savcheniuk et al. 2014; Wang et al. 2014; Kasińska and Drzewoski 2015; Di Cerbo et al. 2016; Kobyliak et al. 2016; Nazemian et al. 2016; Wallace and Milev 2017).
12.2 Why Designer Probiotics?
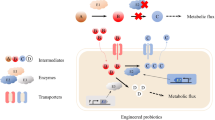
The probiotics are categorized into mono-strain or multi-strain probiotics and it has been well documented that multi-strain probiotics poses significant positive effects due to symbiosis among the strains used in formulation (Timmerman et al. 2004). A list of few multispecies probiotic consortium and their positive impacts are shown in Table 12.1. However, recognition of the importance of microorganism–receptor interactions in the pathogenesis of disease and limited probiotic strain diversity was the main drawback (Marotz and Zarrinpar 2016). To combat such issues, researchers used synthetic biology and genetic engineering approaches to develop recombinant probiotic strains, commonly called as “Designer Probiotics.” Moreover, it was also postulated that genetic engineering of target specific probiotic strains or development of probiotics as a vehicle to carry vaccine and drug molecules is a promising approach (Braat 2006; Paton 2012; Kumar 2016; Maxmen 2017). Such designer probiotics as vaccine vehicle also offer an advantage of no possibility of reversion to a virulent phenotype, which always remains a threat with the attenuated pathogenic strains (Seegers 2002).
Moreover, according to researchers (Paton et al. 2006; Sleator and Hill 2008) this approach has several other advantages as well.
-
(i)
Oral administration of probiotic,
-
(ii)
Well characterized receptors recognized by enteric pathogens/toxins,
-
(iii)
Inhibition of pathogen adherence leading to lower infection,
-
(iv)
Sequestration of a toxin by the improved host immune system which prevent clinical symptoms,
-
(v)
It does not apply a selective pressure on the pathogen, so development of resistance against it is very unlikely.
12.3 Strategies of Developing Designer Probiotics
Among various strategies to develop designer probiotics, few main approaches are as below:
12.3.1 Patho-Biotechnology Based Designer Probiotics
Patho-biotechnology describes the concept of using a pathogenic organism for beneficial use in the biotechnological application. This stands for the use of their ability to adapt against stress, their strong host invasion system and virulence related abilities to other different areas. Recently, it has been suggested to use them in the field of development of probiotics as food supplements (Sleator and Hill 2006). In addition, patho-biotechnology can also improve strain’s resistance during manufacturing process and storage period for improved probiotic response. The most common example is the use of Listeria monocytogenes, L. salivarius, and Bifidobacterium breve for the development of targeted designer probiotics. For instance, the betaine transporter gene (betL) from L. monocytogene resulted in reducing the stress and improved the survival rate of probiotics (Hoffmann et al. 2013; Sleator et al. 2003a).
12.3.2 Receptor-Mimicking Based Designer Probiotics
In this approach, the probiotics are developed by engineering the expression of host-receptor-mimics on the surface of a commensal bacterium. Paton and group developed a designer probiotics for the prevention of gastrointestinal infections using a strategy involving the expression of host cell receptor-mimics on the surface of probiotic strains (Paton et al. 2006; Sleator and Hill 2008). Expression of two galactosyl-transferase genes (lgtC and lgtE) from Neisseria gonorrhoeae into Escherichia coli strain generated a lipopolysaccharide terminating in Gal(α1, 4)Gal(β1, 4)Glc, which mimics Shiga-toxin (stx) receptor and was found effective against shigatoxigenic E. coli (STEC) (Paton et al. 2000). Using a similar strategy to that mentioned above, an E. coli strain was engineered to produce a chimeric LPS receptor mimic capable of binding a heat-labile enterotoxin (Paton et al. 2005). Similarly, a probiotic strain with an altered LPS was designed by Focareta et al. (2006). This altered LPS receptor terminates in a structure that mimics the GM1 ganglioside terminus, which is the binding receptor for cholera toxin (Focareta et al. 2006). Similar approaches have also been used to develop probiotics for enterotoxigenic E. coli (ETEC) (Paton et al. 2005).
12.3.3 Synthetic Oligosaccharide-Based Designer Probiotics
In this approach, oligosaccharides in specific conformation or in multivalent interaction correspond to a given receptor epitope to inhibit the ligand binding (Zopf and Roth 1996; Mulvey et al. 2001). Using this strategy, a probiotic (“Synsorb-pk”) comprising silica particles linked to synthetic Gal(α1,4)Gal(β1,4)Glc oligosaccharide was developed for severe gastroenteritis, which can progress to hemolytic uremic syndrome (HUS) (Paton and Paton 1998). Similarly, probiotic (Synsorb 90) displaying a Gal(α1,3)Gal(β1,4)GlcNAc epitope was developed against Clostridium difficile (Heerze et al. 1994). Merritt et al. (2002) developed a probiotic for cholera toxin consisting of a pentacyclic core, each displaying m-nitrophenyl-α-d-galactoside, which showed enhanced binding to the toxin. “SUPERTWIGS” having dendrimers with multiple tri-saccharides was also developed using similar strategy against an O157:H7 STEC (Nishikawa et al. 2002; Nishikawa et al. 2005; Watanabe et al. 2004).
12.3.4 Anti-Microbial Peptides Based Designer Probiotics
Probiotics produced from commensal bacteria are capable of expressing antagonism against pathogenic microorganisms. Expression of anti-microbial peptides with potential to overcome the antibiotic resistance among pathogens resulted in combined benefits of anti-microbial peptides as well as of probiotics (Proctor 2011; Reid et al. 2015). This can be achieved by cloning and expression of anti-microbial peptide specific genes in the probiotic bacteria.
12.4 Designer Probiotic Strains
Most of the probiotics as well as probiotic consortium are developed from Bifidobacterium, Lactobacillus species, and other lactic acid bacteria (LAB) or specific yeast strains (Saccharomyces cerevisiae, S. boulardii, Kluyveromyces lactis, and Pichia pastoris) (Govender et al. 2013). While on the other hand, when we consider designer probiotics, there are some specific promising bacterial species that are currently under consideration.
12.4.1 Faecalibacterium Prausnitzii
F. prausnitzii is a bacterium of Clostridium cluster IV (Martín et al. 2017), and its anti-inflammatory response proposed its usage as targeted anti-inflammatory drugs for Crohn’s disease (Quévrain et al. 2016). In addition, it can induce the Clostridium-specific IL-10-secreting regulatory T cell subset, and reduce IL-12 and IFNg production, to maintain the gut barrier immune function (Quévrain et al. 2016). Also it is reported to reduce the severity of trinitrobenzene sulfonic acid (TNBS) colitis and correct the associated dysbiosis (Sokol et al. 2008). These studies suggest that F. prausnitzii can be regarded as a potential probiotic candidate for chronic gut inflammation and Crohn’s disease (Sokol et al. 2008; Martín et al. 2017).
12.4.2 Akkermansia Muciniphila
Schneeberger et al. (2015) reported the impact of A. muciniphila in lipid metabolism, inflammatory markers in adipose tissue, regulation of glucose level, resistance to insulin, and occurrence of plasma. This led to investigate the role of this bacterium in adipose tissue homeostasis and metabolism. A study conducted by Dao et al. (2016) suggested the A. muciniphila is associated with body fat distribution and glucose homeostasis. While the effect of A. muciniphila in reversing the atherosclerotic lesson and improving the metabolic endotoxemia-induced inflammation and controlling the dysbiosis has also been reported (Li et al. 2016). All these prospects put A. muciniphila in the category of potential designer probiotics (Dao et al. 2016).
12.4.3 Bacteroides Fragilis
Bacteroides species are gram −ve, obligate anaerobe, and non-spore forming commensal bacteria, which constitutes approximately 25% of our gut microbiota (Wexler 2007). They passed from mother to infant during vaginal delivery and thus are considered as gut’s primary colonizers. Among various Bacteroides species, B. fragilis is the most common and this produces an immunomodulatory molecule, polysaccharide A (PSA), which play a vital role in homeostasis and development of the host immune system and preserves the balance between T cell types (Troy and Kasper 2010; Round et al. 2011).
12.4.4 Bacteroides Uniformis
Oral administration of B. uniformis has shown considerable improvement in lipid profile, glucose insulin and leptin levels, TNF-a production, and phagocytosis in mice studies (Gauffin Cano et al. 2012). Moreover, it has also been reported that their administration can improve immunological dysfunction and metabolic disorder related to gut dysbiosis (Gauffin Cano et al. 2012; Yang et al. 2016).
12.4.5 Eubacterium Hallii
E. hallii is an anaerobic bacterium that resides in our gut and affects the gut metabolism (Engels et al. 2016). It is a natural butyrate producer and is supposed to lower mucosal inflammation and oxidative status, enhance the host–gut microbiota homeostasis, strengthen the gut barrier, increase energy metabolism, improve insulin sensitivity, and act as energy (Canani et al. 2011; Engels et al. 2016). Moreover, it has also been regarded safe as its high dose did not cause any negative impact (Udayappan et al. 2016).
12.4.6 Clostridium Cluster Members
Patients suffering from inflammatory bowel disease (IBD) have reduced number of bacterium related to Clostridium spp. clusters IV and XIVa, which are supposed to be exceptional Tregs (Regulated T cells) inducer in the colon (Atarashi et al. 2011). These Tregs are also considered as potential therapeutic agents for allergies and IBD (Atarashi et al. 2011). Later, Atarashi et al. (2013) again reported that these Clostridia clusters XIVa, IV, and XVIII were also playing role in Treg cell differentiation and accumulation (Atarashi et al. 2013). Moreover, these Clostridium clusters were also reported to produce short chain fatty acids (SCFAs) to improve the gut dysbiosis conditions (Atarashi et al. 2013).
12.4.7 Listeria Monocytogenes
It is a pathogenic bacterium, which as auxotrophic mutant or after selective elimination of virulence genes can be used as novel vaccine and drug delivery vehicles (Zhao et al. 2005). Also these bacterium can be used to incorporate stress tolerant genes into non-pathogenic probiotic strains (Sleator and Hill 2006). Such genes can support the survival of probiotic strain in stressful conditions such as gastric juice, bile juice, low pH, etc. (Mattila-Sandholm et al. 2002; Sleator and Hill 2007a). L. monocytogenes serves as an ideal candidate for this concept as its genome has been fully sequenced and can be manipulated to resisting numerous stresses and also eliciting a strong host immune response in probiotics (Glaser et al. 2001; Hamon et al. 2006; Gray et al. 2006; Lecuit 2005). L. monocytogenes has three solute uptake genes betL, gbu, and opuC (Wemekamp-Kamphuis et al. 2002). Among them, betL is reported to be a betaine transporter gene (Sleator et al. 1999; Sleator et al. 2000; Hoffmann et al. 2013), whose cloning has contributed to probiotic survival under a variety of stresses (Sheehan et al. 2006; Sleator et al. 2003a).
12.4.8 Bifidobacterium Breve
B. breve UCC2003 strains expressing betL were shown to exhibit significantly increased tolerance to simulated gastric juice (pH 2.5) as well as osmotic stress. Moreover, the heterologous expression of the BilE system from L. monocytogenes would increase bile tolerance and subsequent gastrointestinal persistence of the probiotic strains (Sheehan et al. 2007). Interestingly, B. breve UCC2003 expressing betL gene has improved survival rate even in gastric juice (Sheehan et al. 2007), which may be attributed to the fact that improving compatible solutes accumulation leads to more physiologically robust probiotic strains (Sleator et al. 2003b).
12.5 Applications of Designer Probiotics in Metabolic Disorders
Enzymes are the crucial factors in cellular metabolism and their deficiency can result in metabolic disorder. Manipulating the gut ecosystem by designer probiotics expressing therapeutic biomolecules such as enzymes might serve as alternative approaches against metabolic disorders (Singh et al. 2017; Isabella et al. 2018; Kurtz et al. 2019).
12.5.1 Diabetes
Diabetes is a condition of hypo-secretion of insulin resulted in higher blood glucose levels in the body, which may subsequently create several acute health concerns such as cardiovascular disease and Alzheimer, etc. (Li et al. 2015). Diabetes are of 2 types, type 1 is related to the impaired cells in the pancreas and type 2 occurs due to insulin resistance (Klöppel et al. 1985). Compared to conventional insulin injection, use of probiotics capable of secreting pro-insulin or cytokines would be a better alternative because of less pain and negligible side effects (Vinay et al. 2005; Van Belle et al. 2011; Bluestone et al. 2010). Recently, several attempts have been made to engineer probiotics carrying interleukins (ILs), pro-insulin, glucagon like proteins (GLPs), and other therapeutic protein, which have shown proven records in combating diabetes in mouse models. Liu (2016) developed a probiotic strain of Lactococcus lactis expressing HSP65-6IA2P2 protein (pro-insulin autoantigen), which showed significant improvement in diabetes mellitus conditions among diabetic mice (Liu 2016). Oral administration of IL-10, and this designer probiotics with anti-CD3 showed stability against diabetes in 59% of tested mice (Liu 2016). Similarly, engineered L. lactis NZ9000 expressing fusion protein HSP65-6P277 was found effective against diabetes (Ma et al. 2014). Moreover, L. lactis harboring auto-antigen GAD65370-575 and IL-10 brought about stabilization in pancreatic inflammation (Robert et al. 2014). While designer probiotic strain of Lactobacillus casei induces SP (Usp45)-INS-specific antibodies, which improve the levels of IL-4 and protect them from pancreas injury (Schwenger et al. 2015). GLP-1 (1–37) fused with USP45-LEISS secretion marker and polyhistidine tag, expressed in Lactobacillus gasseri ATCC 33323 showed improved insulin secretion by converting rat cells into insulin-secreting cells (Duan et al. 2015). In another report, Lactobacillus paracasei expressing exendin-4 peptide also enhanced insulin secretion (Zheng et al. 2017).
12.5.2 Phenylketonuria (PKU)
Phenylketonuria (PKU) another metabolic disorder is caused by defect in the phenylalanine hydroxylase (PAH) that prevents the action of phenylalanine ammonia lyase (PAL), which breakdown the phenylalanine into ammonia and cinnamic acid (Wang et al. 2005). The rise in level of phenylalanine results in acute health concerns resulting in reduced intellectual ability, seizures, etc. (Mitchell et al. 2011). Researchers are working for developing probiotic strains expressing PAL gene to be a potential solution for PKU. Overexpressing the PAL gene from Rhodosporidium toruloides in E. coli significantly reduced the phenylalanine level in mouse model (Sarkissian et al. 1999). Similar results were obtained when Lactobacillus reuteri having PAL gene from Anabaena variabilis was administered (Durrer et al. 2017). Recently, E. coli Nissle 1917 was genetically engineered to overexpress PAL and L-amino acid deaminase to convert phenylalanine into phenylpyruvate, which substantially lowered the level of phenylalanine in blood (Isabella et al. 2018).
12.5.3 Hyperammonemia
Hyperammonemia is caused due to an enzymatic defect in metabolizing free ammonia to urea resulting in increased ammonia level in blood (Leonard and Morris 2002). This condition can be reversed using lactulose or antibiotics; however, the use of antibiotics may have many side effects (Auron and Brophy 2012). Recently probiotic strain SYNB1020 was developed from E. coli Nissle 1917 by deleting thyA and argR genes (−ve regulators for arginine translocation) and incorporating an argA215 gene (N-acetyl glutamate) to convert ammonia into L-arginine. This designer probiotics on administration resulted in 50% improvement in the survival rate of hyperammonemia suffering mice model (Kurtz et al. 2019).
12.5.4 Parkinson’s Disease
Parkinson’s disease is linked with the low level of dopamine, a neurotransmitter, in the brain cells. The medicine, which is used for the treatment of Parkinson’s disease, is Levodopa. This levodopa is converted to dopamine on decarboxylation mediated by an enzyme acid decarboxylase (AADC) (Bergmann et al. 1974). However, besides brain, this levodopa can also be decarboxylated in gut causing side effects and reduced its bioavailability. Recently, pyridoxal-5-phosphate dependent tyrosine decarboxylase from Enterococcus faecalis was reported to decarboxylate the L-dopa, while Eggerthella lenta was found to have dopamine dehydroxylase (Dadh) gene for conversion of L-Dopa to m-tyramine in gut (Rekdal et al. 2019). They also observed that in dopamine dehydrogenase enzyme the variants have arginine at 506th position could metabolize L-Dopa while the variants with Serine at 506th position could not. This indicated that an SNP mutation in enzyme can predict the L-Dopa metabolism in complex gut microbiota (Rekdal et al. 2019).
12.5.5 Alzheimer’s Disease
Alzheimer’s disease is a chronic neurodegenerative condition caused by abnormal accumulation of amyloid and tau protein in and around the brain cell, which decreased the level of acetylcholine and resulted in memory loss and cognitive loss (Selkoe and Hardy 2016). Recently, its progression was found to be linked with dysbiosis in gut microbiota, which caused accumulation of amino acids. This amino acid accumulation further activates the M1 microglia that is responsible for cognitive loss (Wang et al. 2019). For the reversal of this condition, a drug molecule (GV-971), a mixture of acidic linear oligosaccharides with varied degree of polymerization, was used to improve cognition by re-establishing the gut flora (Wang et al. 2019). This suggests that designer probiotic strains linked to these oligosaccharide molecule will be a potential candidate to improve this metabolic disease.
12.5.6 Obesity
Obesity is now considered a worldwide pandemic, which is associated with alteration in gut flora (Robert et al. 2014). Researchers have attempted to engineer probiotics having potential to deliver therapeutic molecules such as leptins and N-acylphosphatidyl-ethanolamines (NAPEs) to reduce the obesity (Steidler et al. 2000). Stritzker and Szalay (2013) reported reduction in the level of obesity in mice administered with E. coli expressing NAPEs. Similarly, E. coli Nissle 1917 expressing NAPEs was found to reduce obesity in mice (Chen et al. 2014), while those expressing pyrroloquinoline quinone and fructose dehydrogenase facilitate in treating fructose induced hepatic steatosis in mice (Somabhai et al. 2016).
12.5.7 Angiotensin Level Linked Hypertension
Angiotensin is a hormone, which functions as vaso-contractor, i.e., it narrows the blood vessels, which resulted in hypertension (high blood pressure conditions), however if the receptors for angiotensin are blocked, this condition can be reversed. In this aspect, angiotensin-converting enzyme inhibitory peptides (ACEIPs) are reported to reduce the blood pressure by relaxing the blood vessels. Yang et al. (2015) engineered a probiotic strain Lactobacillus plantarum NC8 expressing ACEIPs, which on administration to mice was observed to decrease the angiotensin levels and subsequently reduced the blood pressure.
12.6 Challenges in Development of Designer Probiotics
The use of designer probiotics has the potential to significantly impact the mortality/morbidity rates. However, being genetically engineered, these designer probiotics contain additional genetic elements for inducing antigenicity, immunomodulation, and effect on normal metabolic pathways, and hence safety of bioengineered probiotics is an important issue (Singh et al. 2016). Besides the issue of biological containment, the stress due to change in water activity and temperature are other challenges for probiotics (Sleator and Hill 2007b), Also, the viability percentage of the bacterial strain during product manufacturing and storage processes is also a matter of concern (El Hage et al. 2017).
In addition, the probiotics have also to face the regulatory issues, as worldwide they are also classified into different categories depending on their use in a particular condition. For instance, in the USA, as a dietary supplement, probiotics are considered as “food” and should be regulated by the Dietary Supplement Health and Education Act (DSHEA); and in case of therapeutic purpose, the probiotic comes under drug category and hence it must be approved by the FDA. On the other hand, in Japan probiotics are categorized as foods as well as drugs. Since, the classification and definition of probiotics by different regulatory bodies vary across world; the regulatory status of these probiotics is still unknown (El Hage et al. 2017).
12.7 Conclusions
Development of antibiotic resistance in pathogenic bacteria and increase in metabolism related diseases have led an urge to search for alternative cost-effective approaches to conventional antibiotic prescription. In this aspect, probiotics have shown proven records for health benefits by preventing illness, increasing immunity and maintaining the gut microbiome. Moreover, with the growing understanding about the synthetic biology and genetic engineering of microbes, researchers are now inclining towards development of recombinant “designer probiotics” to be used as drug delivery system, gene therapy vectors, invaders to pathogenic microbes and also career of therapeutic proteins in a more target specific manner. These designer probiotics comprising an anti-microbial, anti-inflammatory, and immunomodulatory repertoire not only help the host against infectious diseases but also their target specific nature reduce the production level and subsequently the cost. Few designer probiotics strains and their role in prevention and treatment of human diseases are listed in Table 12.2.
Though this concept is gaining interest and popularity in view of long-term protection against chronic diseases, however, the issue of biological containment and consumer acceptance of recombinant probiotics is still a significant roadblock. In conclusion, we are optimistic that utilizing the advancement in technologies such as synthetic biology, genetic engineering, and patho-biotechnology, research in this area will continue to generate stable recombinant designer probiotics with strongly regulated gene expression and clearly demonstrable medical benefits. Moreover, with the use of rigorous biological containment strategies, detailed risk analysis, scientific evidences, and consumer education, these designer probiotics will attain broader acceptance in the near future.
References
Amaral AC, Silva ON, Mundim NC et al (2012) Predicting antimicrobial peptides from eukaryotic genomes: in silico strategies to develop antibiotics. Peptides 37:301–308
Andreu D, Torrent M (2015) Prediction of bioactive peptides using artificial neural networks. Methods Mol Biol 1260:101–118
Asemi Z, Zare Z, Shakeri H et al (2013) Effect of multispecies probiotic supplements on metabolic profiles, Hs-CRP, and oxidative stress in patients with Type 2 diabetes. Ann Nutr Metab 63:1–9
Atarashi K, Tanoue T, Shima T et al (2011) Induction of colonic regulatory T cells by indigenous Clostridium species. Science 331:337–341
Atarashi K, Tanoue T, Oshima K et al (2013) Treg induction by a rationally selected mixture of Clostridia strains from the human microbiota. Nature 500:232–236
Auron A, Brophy PD (2012) Hyperammonemia in review: pathophysiology, diagnosis, and treatment. Pediatr Nephrol 27:207–222
Aydin A, Ahmed K, Zaman I et al (2015) Recurrent urinary tract infections in women. Int Urogynecol J 26:795–804
Benbouziane B, Ribelles P, Aubry C et al (2013) Development of a stress-inducible controlled expression (SICE) system in Lactococcus lactis for the production and delivery of therapeutic molecules at mucosal surfaces. J Biotechnol 168:120–129
Bergmann S, Curzon G, Friedel J et al (1974) The absorption and metabolism of a standard oral dose of levodopa in patients with Parkinsonism. Br J Clin Pharmacol 1:417
Besseling-van der Vaart I, Heath MD, Guagnini F, Kramer MF (2016) In vitro evidence for efficacy in food intolerance for the multispecies probiotic formulation EcologicR tolerance (Syngut™). Benef Microbes 7:111–118
Bluestone JA, Herold K, Eisenbarth G (2010) Genetics, pathogenesis and clinical interventions in type 1 diabetes. Nature 464:1293
Braat H (2006) A phase I trial with transgenic bacteria expressing interleukin-10 in Crohn’s disease. Clin Gastroenterol Hepatol 4:754–759
Canani RB, Costanzo MD, Leone L et al (2011) Potential beneficial effects of butyrate in intestinal and extraintestinal diseases. World J Gastroenterol 17:1519–1528
Chen J, Wang R, Li X-F, Wang R-L (2011) Bifidobacterium adolescentis supplementation ameliorates visceral fat accumulation and insulin sensitivity in an experimental model of the metabolic syndrome. Br J Nutr 107:1429–1434
Chen Z, Guo L, Zhang Y et al (2014) Incorporation of therapeutically modified bacteria into gut microbiota inhibits obesity. J Clin Invest 124:3391–3406
Clarke SF, Murphy EF, O’Sullivan O et al (2014) Exercise and associated dietary extremes impact on gut microbial diversity. Gut 63:1913–1920
Dao MC, Everard A, Aron-Wisnewsky J et al (2016) Akkermansia muciniphila and improved metabolic health during a dietary intervention in obesity: relationship with gut microbiome richness and ecology. Gut 65:426–436
De Palma G, Collin SM, Bercik P (2014) The microbiota-gut-brain axis in functional gastrointestinal disorders. Gut Microb 5:419
De Palma G, Lynch MDJ, Lu J et al (2017) Transplantation of fecal microbiota from patients with irritable bowel syndrome alters gut function and behavior in recipient mice. Sci Transl Med 9:6397
Dethlefsen L, Relman DA (2010) Incomplete recovery and individualized responses of the human distal gut microbiota to repeated antibiotic perturbation. Proc Natl Acad Sci U S A 108:4554–4561
Di Cerbo A, Palmieri B, Aponte M et al (2016) Mechanisms and therapeutic effectiveness of lactobacilli. J Clin Pathol 69:187–203
Duan FF, Liu JH, March JC (2015) Engineered commensal bacteria reprogram intestinal cells into glucose-responsive insulin-secreting cells for the treatment of diabetes. Diabetes 64:1794–1803
Durrer KE, Allen MS, Von Herbing IH (2017) Genetically engineered probiotic for the treatment of phenylketonuria (PKU); assessment of a novel treatment in vitro and in the PAHenu2 mouse model of PKU. PLoS One 12:e0176286
El Hage R, Hernandez-Sanabria E, Van de Wiele T (2017) Emerging trends in “smart probiotics”: functional consideration for the development of novel health and industrial applications. Front Microbiol 8:1889
Engels C, Ruscheweyh H-J, Beerenwinkel N et al (2016) The common gut microbe Eubacterium hallii also contributes to intestinal propionate formation. Front Microbiol 7:713
Focareta A, Paton JC, Morona R et al (2006) A recombinant probiotic for treatment and prevention of cholera. Gastroenterology 130:1688–1695
Francino MP (2016) Antibiotics and the human gut microbiome: dysbiosis and accumulation of resistances. Front Microbiol 6:1543
Gauffin Cano P, Santacruz A, Moya Á, Sanz Y (2012) Bacteroides uniformis CECT 7771 ameliorates metabolic and immunological dysfunction in mice with high-fat-diet induced obesity. PLoS One 7:e41079
Glaser P, Frangeul L, Buchrieser C et al (2001) Comparative genomics of Listeria species. Science 2001(294):849–852
Govender M, Choonara YE, Kumar P et al (2013) A review of the advancements in probiotic delivery: conventional vs non-conventional formulations for intestinal flora supplementation. AAPS Pharmsci Tech 15:29–43
Graf D, Di Cagno R, Fåk F et al (2015) Contribution of diet to the composition of the human gut microbiota. Microb Ecol Health Dis 26:26164
Gray MJ, Freitag NE, Boor KJ (2006) How the bacterial pathogen listeria monocytogenes mediates the switch from environmental Dr Jekyll to pathogenic Mr Hyde. Infect Immun 74:2505–2512
Hamon M, Bierne H, Cossart P (2006) Listeria monocytogenes: a multifaceted model. Nat Rev Microbiol 4:423–434
Hand TW (2016) The role of the microbiota in shaping infectious immunity. Trends Immunol 37:647–658
Heerze LD, Kelm MA, Talbot JA, Armstrong GD (1994) Oligosaccharide sequences attached to an inert support (SYNSORB) as potential therapy for antibiotic associated diarrhea and pseudomembranous colitis. J Infect Dis 169:1291–1296
Hill C, Guarner F, Reid G et al (2014) Expert consensus document: the international scientific association for probiotics and prebiotics consensus statement on the scope and appropriate use of the term probiotic. Nat Rev Gastroenterol Hepatol 11:506
Hoffmann RF, McLernon S, Feeney A et al (2013) A single point mutation in the listerial betL σ(a)-dependent promoter leads to improved osmo- and chill-tolerance and a morphological shift at elevated osmolarity. Bioengineered 4:401–407
Isabella VM, Ha BN, Castillo MJ et al (2018) Development of a synthetic live bacterial therapeutic for the human metabolic disease phenylketonuria. Nat Biotechnol 36:857–864
Jafarnejad S, Saremi S, Jafarnejad F, Arab A (2016) Effects of a multispecies probiotic mixture on glycemic control and inflammatory status in women with gestational diabetes: a randomized controlled clinical trial. J Nutr Metab 2016:5190846
Kajander K, Myllyluoma E, Rajilic-Stojanovic M et al (2008) Clinical trial: multispecies probiotic supplementation alleviates the symptoms of irritable bowel syndrome and stabilizes intestinal microbiota. Aliment Pharmacol Ther 27:48–57
Kasińska MA, Drzewoski J (2015) Effectiveness of probiotics in Type 2 diabetes: a meta-analysis. Pol Arch Intern Med 125:803–813
Kim S-W, Park K-Y, Kim B et al (2013) Lactobacillus rhamnosus GG improves insulin sensitivity and reduces adiposity in highfat diet-fed mice through enhancement of adiponectin production. Biochem Biophys Res Commun 431:258–263
Klaenhammer TR, Kleerebezem M, Kopp MV, Rescigno M (2012) The impact of probiotics and prebiotics on the immune system. Nat Rev Immunol 12:728–734
Klöppel G, Löhr M, Habich K et al (1985) Islet pathology and the pathogenesis of type 1 and type 2 diabetes mellitus revisited. Surv Synth Pathol Res 4:110–125
Kobyliak N, Conte C, Cammarota G et al (2016) Probiotics in prevention and treatment of obesity: a critical view. Nutr Metab 13:14
Koning CJ, Jonkers DM, Stobberingh EE et al (2008) The effect of a multispecies probiotic on the intestinal microbiota and bowel movements in healthy volunteers taking the antibiotic amoxicillin. Am J Gastroenterol 103:178–189
Kristensen NB, Bryrup T, Allin KH et al (2016) Alterations in fecal microbiota composition by probiotic supplementation in healthy adults: a systematic review of randomized controlled trials. Genome Med 8:52
Kumar M (2016) Bioengineered probiotics as a new hope for health and diseases: potential and prospects: an overview. Future Microbiol 11:585–600
Kurtz CB, Millet YA, Puurunen MK et al (2019) An engineered E coli Nissle improves hyperammonemia and survival in mice and shows dose-dependent exposure in healthy humans. Sci Transl Med 11:eaau7975
Kwon H-K, Lee C-G, So J-S et al (2010) Generation of regulatory dendritic cells and CD4CFoxp3C T cells by probiotics administration suppresses immune disorders. Proc Natl Acad Sci U S A 107:2159–2164
Langdon A, Crook N, Dantas G (2016) The effects of antibiotics on the microbiome throughout development and alternative approaches for therapeutic modulation. Genome Med 8:39
Lecuit M (2005) Understanding how Listeria monocytogenes targets and crosses host barriers. Clin Microbiol Infect 11:430–436
Leonard JV, Morris AAM (2002) Urea cycle disorders. Semin Neonatol Sn 7:27–35
Li X, Song D, Leng SX (2015) Link between type 2 diabetes and Alzheimer’s disease: from epidemiology to mechanism and treatment. Clin Interv Aging 10:549
Li J, Lin S, Vanhoutte PM et al (2016) Akkermansia muciniphila protects against atherosclerosis by preventing metabolic endotoxemia-induced inflammation in apoe−/−mice clinical. Circulation 133:2434–2446
Liu KF (2016) Oral administration of Lactococcus lactis expressing heat shock protein 65 and tandemly repeated IA2P2 prevents type 1 diabetes in NOD mice. Immunol Lett 174:28–36
Ma Y, Liu J, Hou J et al (2014) Oral administration of recombinant Lactococcus lactis expressing HSP65 and tandemly repeated P277 reduces the incidence of type I diabetes in non-obese diabetic mice. PLoS One 9:e105701
Macpherson AJ, de Agüero MG, Ganal-Vonarburg SC (2017) How nutrition and the maternal microbiota shape the neonatal immune system. Nat Rev Immunol 17:508–517
Marotz CA, Zarrinpar A (2016) Treating obesity and metabolic syndrome with fecal microbiota transplantation. Yale J Biol Med 89:383–388
Martín R, Miquel S, Benevides L et al (2017) Functional characterization of novel Faecalibacterium prausnitzii strains isolated from healthy volunteers: a step forward in the use of F prausnitzii as a next-generation probiotic. Front Microbiol 8:1226
Mattila-Sandholm T, Myllärinen P, Crittenden R et al (2002) Technological challenges for future probiotic foods. Int Dairy J 12:173–182
Maxmen A (2017) Living therapeutics: scientists genetically modify bacteria to deliver drugs. Nat Med 23:5–7
Merritt EA et al (2002) Characterization and crystal structure of a high-affinity pentavalent receptor binding inhibitor for cholera toxin and E coli heat labile enterotoxin. J Am Chem Soc 124:8818–8824
Metchnikoff E (1907) Lactic acid as inhibiting intestinal putrefaction. The prolongation of life: optimistic studies. W Heinemann, London, pp 161–183
Miettinen M, Vuopio-Varkila J, Varkila K (1996) Production of human tumor necrosis factor alpha, interleukin-6: and interleukin-10 is induced by lactic acid bacteria. Infect Immun 64:5403–5405
van Minnen LP, Timmerman HM, Lutgendorff F et al (2007) Modification of intestinal flora with multispecies probiotics reduces bacterial translocation and improves clinical course in a rat model of acute pancreatitis. Surgery 141:470–480
Mitchell JJ, Trakadis YJ, Scriver CR (2011) Phenylalanine hydroxylase deficiency. Genetics Med 13:697
Mulvey G, Kitov PI, Marcato P et al (2001) Glycan mimicry as a basis for antiinfective drugs. Biochimie 83:841–847
Nazemian V, Shadnoush M, Manaheji H, Zaringhalam J (2016) Probiotics and inflammatory pain: a literature review study Middle East. J Rehabil Health Stud 3:e36087
Nishikawa K et al (2002) A therapeutic agent with oriented carbohydrates for treatment of infections by Shiga toxin-producing Escherichia coli O157:H7. Proc Natl Acad Sci U S A 99:7669–7674
Nishikawa K et al (2005) Identification of the optimal structure required for a Shiga toxin neutralizer with oriented carbohydrates to function in the circulation. J Infect Dis 191:2097–2105
Patel R, DuPont HL (2015) New approaches for bacteriotherapy: prebiotics, new-generation probiotics, and synbiotics. Clin Infect Dis 60:S108–S121
Paton AW (2012) Bioengineered microbes in disease therapy. Trends Mol Med 18:417–425
Paton AW, Morona R, Paton JC (2000) A new biological agent for treatment of Shiga toxigenic Escherichia coli infections and dysentery in humans. Nat Med 6:265–270
Paton AW, Jennings MP, Morona R et al (2005) Recombinant probiotics for treatment and prevention of enterotoxigenic Escherichia coli diarrhea. Gastroenterology 128:1219–1228
Paton AW, Morona R, Paton JC (2006) Designer probiotics for prevention of enteric infections. Nat Rev Microbiol 4:193–200
Paton JC, Paton AW (1998) Pathogenesis and diagnosis of Shiga toxin-producing Escherichia coli infections. Clin Microbiol Rev 11(3):450–479
Plaza-Diaz J (2014) Modulation of immunity and inflammatory gene expression in the gut, in inflammatory diseases of the gut and in the liver by probiotics. World J Gastroenterol 20:15,632
Proctor LM (2011) The human microbiome project in 2011 and beyond. Cell Host Microbe 10:287–291
Quévrain E, Maubert MA, Michon C et al (2016) Identification of an anti-inflammatory protein from Faecalibacterium prausnitzii, a commensal bacterium deficient in Crohn’s disease. Gut 65:415–425
Raghuwanshi S, Misra S, Sharma S et al (2015) Indian perspective for probiotics: a review. Indian J Dairy Sci 68(3):195–205
Raghuwanshi S, Misra S, Sharma S et al (2018) Probiotics: nutritional therapeutic tool. J Probiotics Health 6(1):1–8
Reichold A, Brenner SA, Spruss A et al (2014) Bifidobacterium adolescentis protects from the development of nonalcoholic steatohepatitis in a mouse model. J Nutr Biochem 25:118–125
Reid G, Brigidi P, Burton JP et al (2015) Microbes central to human reproduction. Am J Reprod Immunol 73:1–11
Reijnders D, Goossens GH, Hermes GD et al (2016) Effects of gut microbiota manipulation by antibiotics on host metabolism in obese humans: a randomized double-blind placebo controlled trial. Cell Metab 24:63–74
Rekdal VM, Bess EN, Bisanz JE et al (2019) Discovery and inhibition of an interspecies gut bacterial pathway for levodopa metabolism. Science 364:eaau6323
Robert S, Gysemans C, Takiishi T et al (2014) Oral delivery of glutamic acid decarboxylase (GAD)-65 and IL10 by Lactococcus lactis reverses diabetes in recent-onset NOD mice. Diabetes 63:2876–2887
Round JL, Mazmanian SK (2009) The gut microbiota shapes intestinal immune responses during health and disease. Nat Rev Immunol 9:600
Round JL, Lee SM, Li J et al (2011) The toll like receptor 2 pathway establishes colonization by a commensal of the human microbiota. Science 332:974–977
Sarkissian CN, Shao Z, Blain F et al (1999) A different approach to treatment of phenylketonuria: phenylalanine degradation with recombinant phenylalanine ammonia lyase. Proc Natl Acad Sci 96:2339–2344
Saulnier DM, Ringel Y, Heyman MB et al (2013) The intestinal microbiome, probiotics and prebiotics in neurogastroenterology. Gut Microbes 4:17–27
Savcheniuk O, Kobyliak N, Kondro M et al (2014) Short-term periodic consumption of multiprobiotic from childhood improves insulin sensitivity, prevents development of nonalcoholic fatty liver disease and adiposity in adult rats with glutamate-induced obesity. BMC Complement Altern Med 14:247
Schneeberger M, Everard A, Gómez-Valadés AG et al (2015) Akkermansia muciniphila inversely correlates with the onset of inflammation, altered adipose tissue metabolism and metabolic disorders during obesity in mice. Sci Rep 5:16,643
Schwenger EM, Tejani AM, Loewen PS (2015) Probiotics for preventing urinary tract infections in adults and children. Cochrane Database Syst Rev 12:CD008772
Scott KP, Antoine JM, Midtvedt T, van Hemert S (2015) Manipulating the gut microbiota to maintain health and treat disease. Microb Ecol Health Dis 26:25877
Seegers JF (2002) Lactobacilli as live vaccine delivery vectors: progress and prospects. Trends Biotechnol 20:508–515
Selkoe DJ, Hardy J (2016) The amyloid hypothesis of Alzheimer’s disease at 25 years. EMBO Mol Med 8:595–608
Sheehan VM, Sleator RD, Fitzgerald GF, Hill C (2006) Heterologous expression of BetL, a betaine uptake system, enhances the stress tolerance of Lactobacillus salivarius UCC118. Appl Environ Microbiol 72:2170–2177
Sheehan VM, Sleator RD, Hill C, Fitzgerald GF (2007) Improving gastric transit, gastrointestinal persistence and therapeutic efficacy of the probiotic strain Bifidobacterium breve UCC2003. Microbiology 153:3563–3571
Singh B, Mal G, Bissi L, Marotta F (2016) The holy grail of designer probiotics: the probiotics with multiple health benefits. J Gastrointest Dig Syst 6:2
Singh B, Mal G, Marotta F (2017) Designer probiotics: paving the way to living therapeutics. Trends Biotechnol 35:679–682
Sleator RD, Hill C (2006) Patho-biotechnology: using bad bugs to do good things. Curr Opin Biotechnol 17:211–216
Sleator RD, Hill C (2007a) Patho-biotechnology; using bad bugs to make good bugs better. Sci Prog 90:1–14
Sleator RD, Hill C (2007b) Food reformulations for improved health: a potential risk for microbial food safety? Med Hypotheses 69:1323–1324
Sleator RD, Hill C (2008) New frontiers in probiotic research. Lett Appl Microbiol 46:143–147
Sleator RD, Gahan CG, Abee T, Hill C (1999) Identification and disruption of BetL, a secondary glycine betaine transport system linked to the salt tolerance of Listeria monocytogenes LO28. Appl Environ Microbiol 65:2078–2083
Sleator RD, Gahan CGMB, Hill C (2000) Analysis of the role of betL in contributing to the growth and survival of listeria monocytogenes LO28. Int J Food Microbiol 60:261–268
Sleator RD, Francis GA, O’Beirne D et al (2003a) Betaine and carnitine uptake systems in Listeria monocytogenes affect growth and survival in foods and during infection. J Appl Microbiol 95:839–846
Sleator RD, Wood JM, Hill C (2003b) Transcriptional regulation and posttranslational activity of the betaine transporter BetL in Listeria monocytogenes are controlled by environmental salinity. J Bacteriol 185:7140–7144
Sokol H, Pigneur B, Watterlot L et al (2008) Faecalibacterium prausnitzii is an anti-inflammatory commensal bacterium identified by gut microbiota analysis of Crohn disease patients. Proc Natl Acad Sci U S A 105:16,731–16,736
Somabhai CA, Raghuvanshi R, Nareshkumar G (2016) Genetically engineered Escherichia coli Nissle 1917 synbiotics reduce metabolic effects induced by chronic consumption of dietary fructose. PLoS One 11:e0164860
Steenbergen L, Sellaro R, van Hemert S et al (2015) A randomized controlled trial to test the effect of multispecies probiotics on cognitive reactivity to sad mood. Brain Behav Immun 48:258–264
Steidler L, Hans W, Schotte L et al (2000) Treatment of murine colitis by Lactococcus lactis secreting interleukin-10. Science 289:1352–1355
Stritzker J, Szalay AA (2013) Single-agent combinatorial cancer therapy. Proc Natl Acad Sci U S A 110:8325–8326
Timmerman HM, Koning CJM, Mulder L et al (2004) Monostrain, multistrain and multispecies probiotics—a comparison of functionality and efficacy. Int J Food Microbiol 96:219–233
Tlaskalova-Hogenova H, Stepankova R, Hudcovic T et al (2004) Commensal bacteria (normal microflora), mucosal immunity and chronic inflammatory and autoimmune diseases. Immunol Lett 93:97–108
Troy EB, Kasper DL (2010) Beneficial effects of Bacteroides fragilis polysaccharides on the immune system. Front Biosci 15:25–34
Udayappan S, Manneras-Holm L, Chaplin-Scott A et al (2016) Oral treatment with Eubacterium hallii improves insulin sensitivity in db/db mice. NPJ Biofilms Microbiomes 2:16,009
Van Belle TL, Coppieters KT, Von Herrath MG (2011) Type 1 diabetes: etiology, immunology, and therapeutic strategies. Physiol Rev 91:79–118
Venturi A, Gionchetti P, Rizzello F et al (1999) Impact on the composition of the faecal flora by a new probiotic preparation: preliminary data on maintenance treatment of patients with ulcerative colitis. Aliment Pharmacol Ther 13:1103–1108
Vinay K, Abbas AK, Fauston N (2005) Robbins and Cotran pathologic basis of disease, vol 8. Saunders, Elsevier, pp 208–221
Wallace CJK, Milev R (2017) The effects of probiotics on depressive symptoms in humans: a systematic review. Ann General Psychiatry 16:14
Wang L, Gamez A, Sarkissian CN et al (2005) Structure-based chemical modification strategy for enzyme replacement treatment of phenylketonuria. Mol Genet Metab 86:134–140
Wang J, Tang H, Zhang C et al (2014) Modulation of gut microbiota during probiotic-mediated attenuation of metabolic syndrome in high fat diet-fed mice. ISME J 9:1–15
Wang X, Sun G, Feng T et al (2019) Sodium oligomannate therapeutically remodels gut microbiota and suppresses gut bacterial amino acids-shaped neuroinflammation to inhibit Alzheimer’s disease progression. Cell Res 29:787–803
Watanabe M et al (2004) Oral therapeutic agents with highly clustered globotriose for treatment of Shiga toxigenic Escherichia coli infections. J Infect Dis 189:360–368
Wemekamp-Kamphuis HH, Wouters JA, Sleator RD et al (2002) Multiple deletions of the osmolyte transporters BetL, Gbu, and OpuC of listeria monocytogenes affect virulence and growth at high osmolarity. Appl Environ Microbiol 68:4710–4716
Wexler HM (2007) Bacteroides: the good, the bad, and the nitty-gritty. Clin Microbiol Rev 20:593–621
WHO/FAO (2006) Probiotics in food health and nutritional properties and guidelines for evaluation. Food and Agriculture Organization of the United Nations, Rome
Yan F, Polk DB (2011) Probiotics and immune health. Curr Opin Gastroenterol 27:496–501
Yang G, Jiang Y, Yang W et al (2015) Effective treatment of hypertension by recombinant Lactobacillus plantarum expressing angiotensin converting enzyme inhibitory peptide. Microb Cell Factories 14:202
Yang J-Y, Lee Y-S, Kim Y et al (2016) Gut commensal Bacteroides acidifaciens prevents obesity and improves insulin sensitivity in mice. Mucosal Immunol 10:104–116
Yin Y-N (2010) Effects of four Bifidobacteria on obesity in high-fat diet induced rats. World J Gastroenterol 16:3394
Yoon JS, Sohn W, Lee OY et al (2013) Effect of multispecies probiotics on irritable bowel syndrome: a randomized, double-blind, placebo-controlled trial. J Gastroenterol Hepatol 29:52–59
Zhao X, Li Z, Gu B, Frankel FR (2005) Pathogenicity and immunogenicity of a vaccine strain of Listeria monocytogenes that relies on a suicide plasmid to supply an essential gene product. Infect Immun 73:5789–5798
Zheng JH, Nguyen VH, Jiang SN et al (2017) Two-step enhanced cancer immunotherapy with engineered Salmonella typhimurium secreting heterologous flagellin. Sci Transl Med 2017:9
Zopf D, Roth S (1996) Oligosaccharide anti-infective agents. Lancet 347:1017–1021
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2022 Springer Nature Singapore Pte Ltd.
About this chapter
Cite this chapter
Gupta, R., Raghuwanshi, S. (2022). Designer Probiotics in Metabolic Disorders. In: Chopra, K., Bishnoi, M., Kondepudi, K.K. (eds) Probiotic Research in Therapeutics. Springer, Singapore. https://doi.org/10.1007/978-981-16-8444-9_12
Download citation
DOI: https://doi.org/10.1007/978-981-16-8444-9_12
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-16-8443-2
Online ISBN: 978-981-16-8444-9
eBook Packages: Chemistry and Materials ScienceChemistry and Material Science (R0)