Abstract
Purpose
Correcting intestinal microecological imbalance has become one of the core strategies to treat chronic diseases. Some traditional microecology-based therapies targeting intestine, such as prebiotic therapy, probiotic therapy and fecal microbiota transplantation therapy, have been used in the prevention and treatment of clinical chronic diseases, which still facing low safety and poor controllability problems. The development of synthetic biology technology has promoted the development of intestinal microecology-based therapeutics for chronic diseases, which exhibiting higher robustness and controllability, and become an important part of the next generation of microecological therapy. The purpose of this review is to summarize the application of synthetic biology in intestinal microecology-based therapeutics for chronic diseases.
Methods
The available literatures were searched to find out experimental studies and relevant review articles on the application of synthetic biology in intestinal microecology-based therapeutics for chronic diseases from year 1990 to 2023.
Results
Evidence proposed that synthetic biology has been applied in the intestinal microecology-based therapeutics for chronic diseases, covering metabolic diseases (e.g. diabetes, obesity, nonalcoholic fatty liver disease and phenylketonuria), digestive diseases (e.g. inflammatory bowel disease and colorectal cancer), and neurodegenerative diseases (e.g. Alzheimer’s disease and Parkinson’s disease).
Conclusion
This review summarizes the application of synthetic biology in intestinal microecology-based therapeutics for major chronic diseases and discusses the opportunities and challenges in the above process, providing clinical possibilities of synthetic biology technology applied in microecological therapies.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Major chronic diseases, including metabolic diseases, digestive diseases and neurodegenerative diseases, bring about serious negative effects on human health, further influence the quality of life. In the past decade, microecological therapies targeting unbalanced intestinal flora have attracted wide attention in the biomedical research community. Common microecological therapies mainly include prebiotics therapy, probiotics therapy and fecal microbiota transplantation (FMT) therapy. Prebiotics are a type of substrate that can be selectively utilized by host microorganisms and beneficial to host [1]. They can enrich specific microorganisms, such as Bifidobacterium, and produce metabolic end products to improve the body health [2]. Probiotics is a kind of microorganism that can bring health benefits to the host when given in sufficient [3]. Its main products, probiotic dietary supplements and over-the-counter drugs, have been used to reduce symptoms of gastrointestinal dysfunction, improve immune system function and regulate energy metabolism [4,5,6,7]. However, until now, the effectiveness of prebiotics and probiotics therapies has controversy in many application scenarios [8, 9]. Wilson et al. found that prebiotics, such as β-galactose oligosaccharide and guar gum, could not significantly improve the symptoms of irritable bowel syndrome and other functional bowel patients [10]. Costeloe et al. found that Bifidobacterium brig-001 had no significant effect in preventing necrotizing enterocolitis and late-onset septicemia in very premature infants [11]. In addition, recent experimental results also showed that rhamnose-Lactobacillus GG and other probiotics had no valid evidence in alleviating acute gastroenteritis caused by common pathogens such as rotavirus [12] and preventing ventilator-associated pneumonia in critically ill patients [13]. FMT refers to transplantation of functional flora, obtained from healthy people feces, into the gastrointestinal tract of patients, for the reconstruction of new intestinal flora to achieve the purpose of treating diseases [14]. In 2013, FMT was first written into the treatment guidelines for recurrent or refractory Clostridium difficile infection [15]. In the past ten years, FMT has achieved outstanding curative effect in the treatment of CDI. However, as an immature clinical treatment, the safety and reproducibility of FMT need to be further verified [16].
By virtue of synthetic biology technology, engineered probiotics expressing enzymes, cytokines, antimicrobial peptides, hormones, antibodies and other specific products under the stimulation of physiological or pathological signals in the intestine to improve the intestinal microenvironment may be a promising development direction for the treatment of chronic diseases [17]. Compared with traditional drug therapy, engineered probiotics have higher flexibility, specificity, predictability and controllability. At present, Lactobacillus, especially Lactococcus lactis, are often used as expression hosts to deliver therapeutic drugs due to their high safety, which has a wide range of clinical application value. In addition, Bacillus and Escherichia coli are also common chassis microorganisms in the process of recombinant living biological drugs research [18, 19]. In recent years, with the development of synthetic biology, the genetic toolbox of microbial in vivo therapy has been expanded.
In this paper, engineered probiotics targeting metabolic diseases, digestive diseases and neurodegenerative diseases are reviewed and explored. The application and regulatory role of engineered probiotics in these major chronic diseases, and the opportunities and challenges of using engineered probiotics to treat major chronic diseases are discussed.
Engineered probiotics and metabolic diseases
Metabolic diseases refer to a class of diseases caused by abnormal metabolism of glucose, protein, lipid and other nutrients in the body, including obesity, diabetes and non-alcoholic fatty liver disease, etc. [20]. In recent years, with changes in dietary habits and lifestyle, the incidence of metabolic diseases has been increasing year by year [21]. More and more studies have shown that metabolic diseases are related to intestinal microecological disorders. Many studies have demonstrated the efficacy of engineered probiotics in the treatment of metabolic diseases.
Engineered probiotics and diabetes
Diabetes is a type of metabolic diseases with hyperglycemia as the common phenotype, including type 1 diabetes (T1DM) and type 2 diabetes (T2DM) [22]. In addition to lifestyle and dietary habits, there is increasing evidence that intestinal flora plays an important role in the development of diabetes [23].
Glucagon-like peptide-1 (GLP-1), a short incretin primarily produced by intestinal L-cells, reduces glucagon secretion, induces glucose-dependent insulin secretion, then decreases blood glucose [24]. In GLP-1 application, the short half-life is a major barrier [25]. In Zucker Diabetic Fatty (ZDF) rats, strain Lactococcus lactis transformed with the plasmid encoding GLP-1 resulting in a significant increase in insulin and decrease in blood glucose compared to the control [26]. The wide type GLP-1 was optimized with 8th serine substitution, which both were transformed into L. lactis. In db/db mice, the optimized L. lactis strain could significantly improve random glycemic control and reduce systemic inflammation [27]. Exendin-4 (Exd4) is a GLP-1 receptor agonist. Exd4 secreted by recombinant L. lactis enhanced glucose-dependent insulin synthesis and activated PI3-K/AKT signaling pathway [28].
Cytokines play a key role in connecting the risk factors that contribute to T1DM development and trigger the destructive action of T cells in pancreatic islets [29]. Recombinant L. lactis strain, controlling secretion of anti-inflammatory cytokine interleukin-10 (IL-10) and T1DM autoantigen GAD65370-57, could improve nonobese diabetes (NOD) by releasing proinsulin in the intestine of mice [30]. Combined expressed cytokines IL-4 and IL-10 in strain L. lactis MG1363 FnBPA+ was shown that the recombinant bacterial could reduce pancreatic islets-destruction and prevent hyperglycemia [31].
In addition, recombinant L. lactis expressing staphylococcal nuclease (SNase) was constructed to delay the onset of T1DM by exerting protective effects on pancreatic islets and relieving inflammation of the small intestine in NOD mice [32]; recombinant L. lactis expressing HSP65-6P277, mucosal administration of fusion protein of HSP65 with tandem repeats of P277, resulted in decreased blood glucose levels and reduced islet inflammation in NOD mice [33]; L. lactis secreting Lactobacillus sakei originated L-arabinose isomerase (L-AI) in fusion with the signal peptide usp45 had the ability to produce D-tagatose in vivo, showing good hypoglycemic effects on diabetic mice [34].
Engineered probiotics and obesity
Obesity is a chronic metabolic disease caused by multiple factors, which resulting in excessive accumulation of fat in the body for the harm of health [35]. Many studies have shown that the occurrence of obesity is closely related to the imbalance of intestinal flora. Further studies have shown that unbalanced intestinal flora can accelerate the formation of obesity by affecting the body’s energy intake and metabolism and stimulating pro-inflammatory response [36]. It may be a new approach to prevent and manage obesity to develop therapeutic methods for various factors affecting metabolism starting from intestinal microecology.
GLP-1 also can delay gastric emptying and control appetite [37]. Escherichia coli Nissle 1917 (EcN) secreting modified GLP-1 had potential beneficial effects on obesity, which may be related to the regulating of neuropeptide expression in the hypothalamus [38]. Engineered MG1363-pMG36e-GLP-1, constitutively secreted GLP-1, could improve obesity by increasing diversity of intestinal microbial and promoting oxidation of fatty acid probably [39].
In addition to GLP-1, the researchers also focused on oxyntomodulin (OXM), uncarboxylated or low-carboxylated OC (ucOC), fibroblast growth factor 21 (FGF21) and other hormone drugs for the treatment of obesity. A novel oral drug delivery system using Bifidobacterium as a carrier for the delivery of OXM and its analogue had been developed [40]. In overweight mice, strain B. longum transformed with OXM could reduce body weight, food intake, and level of plasma lipid. Strain Lactococcus lactis was engineered to produce and secrete recombinant mouse ucOC in the presence of the inducer nisin, which could treat obesity by triggering GLP-1 secretion in the small intestine [41]. Cao et al. synthesized L. lactis NZ3900/PNZ8149 system and realized the expression of human FGF21, which significantly reduced the body weight of mice and improved the overall homeostasis [42]. N-acyl phosphatidylethanolamines (NAPEs), precursors of N-acylethanolamides, are synthesized in the small intestine in response to reduce food intake and obesity [43]. Davies and his team have demonstrated that using Arabidopsis originated NAPEs synthase to modify EcN can significantly reduce body weight, hepatic triglycerides, fatty acid synthesis genes, and increase fatty acid oxidation genes in mice [44, 45]. In summary, the use of recombinant probiotics for in-situ delivery of anti-obesity drugs into the gut microbiota can potentially serve as an adjuvant therapy to retard development of obesity.
Engineered probiotics and NAFLD
Nonalcoholic fatty liver disease (NAFLD) has type 2 diabetes mellitus, dyslipidemia, and metabolic syndrome risk factors, led to lipid deposition, in more serious cases, even inflammation and fibrosis in the liver. Ling Zhi 8 (LZ8) is an immunomodulatory protein, possessing a broad range of pharmacological effects [46]. The influence of recombinant L. lactis expressing LZ8 was evaluated in a cholesterol-fed rabbit model. The recombinant L. lactis could inhibit IL-1 expression, decrease fat droplet deposits and inflammatory cells infiltration, and improve liver function [47].
Engineered probiotics and phenylketonuria
Phenylketonuria (PKU) is a metabolic disease caused by biallelic mutations in the PAH gene that result in an inability to convert phenylalanine (Phe) to tyrosine, elevated blood Phe levels and severe neurological complications if untreated [48]. Expressing L-amino acid deaminase (LAAD) and phenylalanine ammonia lyase (PAL) based on ECN, designated as SYNB1618, catalyzed the deamination of Phe to the non-toxic product trans-cinnamate. Administration of SYNB1618 to the Pahenu2/enu2 PKU mouse model and healthy Cynomolgus monkeys, SYNB1618 reduced blood Phe concentration, independent of dietary protein intake [49]. Lately, SYNB1618 was studied in healthy volunteers and patients with PKU adult, which alleviated the symptoms of PKU with safety and tolerability [50]. Then, to optimize whole cell PAL activity, mutant PAL libraries was screened and used in the construction of SYNB1934, which demonstrated a better performance in PKU adult compared to SYNB1618 [51].
Intestinal microecology-based therapeutics targeting metabolic diseases are summarized in Table 1.
Engineered probiotics and digestive diseases
Digestive diseases are a group of chronic intestinal diseases that are frequent and common, covering inflammatory bowel disease (IBD) and colorectal cancer. Although the specific pathogenesis remains unclear, studies have shown that unbalanced intestinal flora is an important cause of digestive diseases [53, 54]. Engineered probiotic therapies targeting unbalanced gut flora show great potential in the prevention and treatment of digestive diseases.
Engineered probiotics and IBD
IBD, covering ulcerative colitis (UC) and Crohn’s disease (CD), is a chronic intermittent disorder characterized by epithelial barrier damage and intestinal inflammation [55]. The intestinal delivery of therapeutic agents targeting inflammatory factors is recognized as a latent effective therapy for the treatment of IBD. Secreting system of L. lactis expressing IL-10 [52] and tumor necrosis factor α (TNFα) [56] were constructed, respectively. Daily oral administration resulted in local release of IL-10 and anti-TNF-α nanoantibodies in the colon, avoided side effects caused by systemic administration, and effectively alleviated colon inflammation levels in mice. Through a combination of directed evolution and synthetic gene circuits, engineered yeast for the treatment of IBD was introduced. The engineered yeast, expressing a human P2Y2 purinergic receptor, could activate the expression and secretion of adenosine triphosphate diphosphatase, and then provide local inflammation treatment [57]. 3-hydroxybutyrate (3HB) was also synthesized in EcN, which realized the treatment of mice colitis by regulating the structure of intestinal flora and increasing the content of short-chain fatty acids [58]. In addition, recombinant probiotics can also treat IBD by delivering protein drugs, such as insulin-like growth factor, transforming growth factor, schistosome immunoregulatory protein Sj16, superoxide dismutase, cystatin, etc. [59,60,61].
The damaged intestinal mucosal barrier is also a potential target for IBD treatment. Trefoil factors (TFFs) and epidermal growth factor (EGF) are two important mucosal interventions that promote cell migration and repair mucosal wounds in a relatively short time. However, when administered orally, TFF and EGF are easily degraded by digestive enzymes and adhere to small intestinal mucus, resulting in decreased bioavailability. It had been proved that recombinant probiotics producing TFF [62, 63] and EGF [64] generated from EcN could promote wound healing of intestinal mucosa and play a protective role in colitis.
Engineered probiotics and CRC
Colorectal cancer (CRC) originates in the colon or rectum [65]. Myrosinase was expressed in EcN, which successfully converted glucosinolate to raphanin with anticancer activity, inhibiting the proliferation of more than 95% cancer cells [66]. A new anticancer drug p8, isolated from Lactobacillus rhamnosus, was introduced into Pediococcus pentosaceus to construct a bacterial drug delivery system for CRC treatment. The results showed that the system had positive therapeutic effect in both CRC xenotransplantation and azoxymethane/DSS induced models [67]. Recombinant EcN, which converted glucose into anticancer drug 5-aminolevulinic acid (5-ALA), developed a new treatment for CRC in combination with photothermal therapy technology [68]. In conclusion, compared with traditional therapies, engineered probiotics have more innate advantages in CRC treatment, which can recognize cancer cells more intelligently and release ideal therapeutic components inside tumor tissues.
Intestinal microecology-based therapeutics targeting digestive diseases are summarized in Table 2.
Engineered probiotics and neurodegenerative diseases
Neurodegenerative diseases are a group of complex psychological syndromes, mainly manifested as cognitive or behavioral disorders of different degrees [69]. At present, the pathogenesis of neurological diseases is not completely clear. Gut flora is considered as a potential target for treating neurological diseases. They are linked to the brain via a bidirectional gut-brain axis that influences brain development and regulates host behavior.
Engineered probiotics and AD
Alzheimer’s disease (AD) is a central nervous system disease characterized by progressive cognitive dysfunction and memory impairment [70]. Engineering probiotics that release brain beneficial substances in the intestine may be used as direct or auxiliary therapy for AD, becoming a new choice for clinical treatment of brain diseases. In addition to anti-sugar and fat reduction effects, GLP-1 can prevent the toxic effect of β-amyloid peptide by binding to receptors on the dendritic membrane or cell membrane of nerve cells, thereby preventing or reversing the neurodegenerative process. Engineered probiotic L. lactis cremoris with high GLP-1 expression had improved spatial learning and memory impairments, reduced inflammation in the brain and reduced beta-amyloid buildup in the hippocampus [71]. The improvement of these indexes was mainly related to the growth inhibition of enterococcus and other pathogens, and the destruction of phosphorylation of MAPKs and PI3K/Akt pathway [72]. Another study used L. lactis strain carrying pExu plasmid encoding the human p62 protein for the treatment of AD. By introducing pExu plasmid, memory was ameliorated, the ubiquitin-proteasome system was modulated, amyloid peptides level was reduced levels, and neuronal oxidative and inflammatory processes were diminished [73]. In conclusion, engineered probiotics have shown a broad prospect in the prevention and treatment of AD, but so far this kind of research has mostly stayed at the cellular level, so it is necessary to further carry out in vitro and in vivo verification and mechanism exploration for engineered probiotics.
Engineered probiotics and PD
Similarly with AD, Parkinson’s disease (PD) are also neurodegenerative diseases characterized by progressive degeneration of the central nervous system, and few medications are available to halt the progression of PD [74]. Via the probiotics effects of L. lactis MG1363, engineered strain MG1363-pMG36e-GLP-1 was constructed which continuously express GLP-1, and reduced motor dysfunction in PD mice [71, 75]. The possible mechanism targeted at the TLR4/NF-κB signaling pathway, which activating the Keap1/Nrf2/GPX4 signaling pathway to suppress ferroptosis by up-regulating FSP1 and down-regulating ACSL4. High-throughput sequencing results showed that recombinant strain reduced the abundance of Proteus and Enterococcus pathogens, and increased the abundance of Akkermansia, Akkermansia muciniphila, Sutterella and Oscillospira probiotics at the genus level.
Intestinal microecology-based therapeutics targeting neurodegenerative diseases are summarized in Table 3.
Discussion
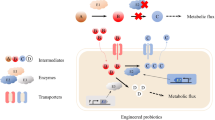
The human gut microbiota is a key target for the prevention and treatment of major chronic diseases. Many studies have emphasized the important role of prebiotics, probiotics and FMT therapies targeting intestinal microorganisms in the prevention and treatment of major chronic diseases. However, due to the low safety and poor controllability problems, the therapeutic effects of these traditional microecology-based treatments are not always reliable. As an alternative, engineered probiotics developed by synthetic biology technology show higher robustness and controllability, and has become an important component of the next generation of microecology-based treatments. To date, utilization of synthetic biology in intestinal microecology-based therapeutics has been applied in the treatment of metabolic diseases (e.g. diabetes, obesity, nonalcoholic fatty liver disease and phenylketonuria), digestive diseases (e.g. inflammatory bowel disease and colorectal cancer), and neurodegenerative diseases (e.g. Alzheimer’s disease and Parkinson’s disease). Brief schematic diagram is depicted in Fig. 1.
In addition, other chronic diseases need to pursue for intervention strategy which suitable for the application of synthetic biology. For example, depression, as one common cause of suicide, has no direct and effective treatment currently [76]. Studies have shown a significant negative correlation between gamma-aminobutyric acid (GABA) and depression [77]. A recombinant E. coli, synthesizing GABA by colocating glutamate synthase, glutamate decarboxylase and GABA transporter, is constructed [78], which may be as a potential therapeutics for the treatment of depression.
Except for application of synthetic biology in production of corresponding medicaments directly, the utilization of synthetic biology can promote the colonization and growth of engineered probiotics in the gut, which may enhance the therapeutic effect of engineered probiotics. By using synthetic biology to display ligands on the surface of probiotics and express bio-membrane formation or adhesin [79] can improve the adhesiveness of engineered probiotics on intestinal mucosa and improve the intestinal colonization of engineered probiotics, thereby protecting engineered probiotics from stomach acid and improving their robustness. Combinatorial engineering probiotics simultaneously producing corresponding medicaments and protecting factors may grow up a new strategy for intestinal microecology-based therapeutics for chronic diseases.
At present, using synthetic biology, the customized transformation of single bacteria has been preliminarily realized. However, considering the problems such as low load, weak anti-interference ability and limited execution of complex function of single engineered probiotics, artificial intestinal flora is expected to become a new generation of microecological therapy. The application of synthetic biology to the construction of artificial intestinal flora has a unique advantage, which can modify microorganisms according to the intestinal microecological environment of different individuals to build synthetic intestinal flora, and detect a variety of disease markers to develop precise diagnostic products, so as to carry out personalized treatment and maintain intestinal health without causing damage to other cells or tissues. But in fact, until now, it is just our beautiful vision. In practice, the research of synthetic microflora targeting intestinal microecology, then for the treatment of chronic diseases still faces a series of opportunities and challenges.
Although synthetic biology plays a unique role in the application of intestinal microecology-based therapeutics for major chronic diseases, there are still many problems to be solved. How to improve production of corresponding medicaments, how to ensure the safety of engineered probiotics, and how to standardize the construction strategy have not been systematically proved. The development of CRISPR-Cas [80] and Mobile-CRISPRi [81] and other tools may provide effective methods for the metabolic engineering to engineer probiotics to increase production. Furthermore, this kind of research only remains at the application stage of how to use synthetic biology in intestinal microecology-based therapeutics in cellular level and model mice, which has not been really used in the clinical treatment of major chronic diseases. In the future, it is necessary to deepen the crossover of synthetic biology, microecology and other disciplines, make full use of the advantages of each discipline to further develop the construction of probiotics biosynthesizing corresponding medicaments, and finally design a universal method to help the development of human health.
Conclusion
Correcting intestinal microecological imbalance has become one of the core strategies to treat chronic diseases. Some traditional microecology-based therapies targeting intestine, such as prebiotic therapy, probiotic therapy and fecal microbiota transplantation therapy, have been used in the prevention and treatment of clinical chronic diseases, which still facing low safety and poor controllability problems. The development of synthetic biology technology has promoted the development of intestinal microecology-based therapeutics for chronic diseases, which exhibiting higher robustness and controllability, and become an important part of the next generation of microecological therapy. Evidence proposed that synthetic biology has been applied in the intestinal microecology-based therapeutics for chronic diseases, covering metabolic diseases (e.g. diabetes, obesity, nonalcoholic fatty liver disease and phenylketonuria), digestive diseases (e.g. inflammatory bowel disease and colorectal cancer), and neurodegenerative diseases (e.g. Alzheimer’s disease and Parkinson’s disease). This review summarizes the application of synthetic biology to construct engineered probiotics for the improvement of intestinal microecology to treat major chronic diseases and discusses the opportunities and challenges in the above process, providing clinical possibilities of engineered probiotics applied in microecological therapies.
Abbreviations
- 3HB:
-
3-hydroxybutyrate
- 5-ALA:
-
5-aminolevulinic acid
- AD:
-
Alzheimer’s disease
- CD:
-
Crohn’s disease
- CRC:
-
Colorectal cancer
- DPP4:
-
Dipeptidylpeptidase-4
- DSS:
-
Dextran sulfate sodium
- EcN:
-
Escherichia coli Nissle 1917
- EGF:
-
Epidermal growth factor
- FMT:
-
Fecal microbiota transplantation
- GABA:
-
Gamma-aminobutyric acid
- GLP-1:
-
Glucagon-like peptide-1
- IBD:
-
Inflammatory bowel disease
- IL-10:
-
Interleukin-10
- LAAD:
-
L-amino acid deaminase
- L. lactis :
-
Lactococcus lactis
- MPTP:
-
1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine
- NOD:
-
Nonobese diabetes
- PAL:
-
Phenylalanine ammonia lyase
- PD:
-
Parkinson’s disease
- PKU:
-
Phenylketonuria
- T1DM:
-
Type 1 diabetes
- T2DM:
-
Type 2 diabetes
- TFFs:
-
Trefoil factors
- UC:
-
Ulcerative colitis
References
Gibson GR, Hutkins R, Sanders ME, Prescott SL, Reimer RA, Salminen SJ, et al. Expert consensus document: the International Scientific Association for Probiotics and Prebiotics (ISAPP) consensus statement on the definition and scope of prebiotics. Nat reviews Gastroenterol Hepatol. 2017;14(8):491–502. https://doi.org/10.1038/nrgastro.2017.75.
Wilson B, Rossi M, Kanno T, Parkes GC, Anderson S, Mason AJ, et al. beta-galactooligosaccharide in Conjunction with Low FODMAP Diet improves irritable bowel syndrome symptoms but reduces fecal bifidobacteria. Am J Gastroenterol. 2020;115(6):906–15. https://doi.org/10.14309/ajg.0000000000000641.
Lilly DM, Stillwell RH. Probiotics: growth-promoting factors produced by microorganisms. Science. 1965;147(3659):747–8. https://doi.org/10.1126/science.147.3659.747.
Sanders ME, Merenstein DJ, Reid G, Gibson GR, Rastall RA. Probiotics and prebiotics in intestinal health and disease: from biology to the clinic. Nat reviews Gastroenterol Hepatol. 2019;16(10):605–16. https://doi.org/10.1038/s41575-019-0173-3.
Martin R, Miquel S, Benevides L, Bridonneau C, Robert V, Hudault S, et al. Functional characterization of Novel Faecalibacterium prausnitzii strains isolated from healthy volunteers: a Step Forward in the Use of F. prausnitzii as a Next-Generation Probiotic. Front Microbiol. 2017;81226. https://doi.org/10.3389/fmicb.2017.01226.
Henn MR, O’Brien EJ, Diao L, Feagan BG, Sandborn WJ, Huttenhower C, et al. A phase 1b safety study of SER-287, a spore-based Microbiome Therapeutic, for active mild to Moderate Ulcerative Colitis. Gastroenterology. 2021;160(1):115–27. https://doi.org/10.1053/j.gastro.2020.07.048.
Perraudeau F, McMurdie P, Bullard J, Cheng A, Cutcliffe C, Deo A, et al. Improvements to postprandial glucose control in subjects with type 2 diabetes: a multicenter, double blind, randomized placebo-controlled trial of a novel probiotic formulation. BMJ open diabetes research & care. 2020;8(1). https://doi.org/10.1136/bmjdrc-2020-001319.
Maldonado-Gomez MX, Martinez I, Bottacini F, O’Callaghan A, Ventura M, van Sinderen D, et al. Stable engraftment of Bifidobacterium longum AH1206 in the human gut depends on individualized features of the Resident Microbiome. Cell Host Microbe. 2016;20(4):515–26. https://doi.org/10.1016/j.chom.2016.09.001.
Marx W, Scholey A, Firth J, D’Cunha NM, Lane M, Hockey M et al. Prebiotics, probiotics, fermented foods and cognitive outcomes: a meta-analysis of randomized controlled trials. Neuroscience and biobehavioral reviews. 2020, 118:472–84https://doi.org/10.1016/j.neubiorev.2020.07.036.
Wilson B, Rossi M, Dimidi E, Whelan K. Prebiotics in irritable bowel syndrome and other functional bowel disorders in adults: a systematic review and meta-analysis of randomized controlled trials. Am J Clin Nutr 2019, 109(4):1098–111https://doi.org/10.1093/ajcn/nqy376.
Costeloe K, Hardy P, Juszczak E, Wilks M, Millar MR. Bifidobacterium breve BBG-001 in very preterm infants: a randomised controlled phase 3 trial. Lancet. 2016;387(10019):649–60. https://doi.org/10.1016/S0140-6736(15)01027-2.
Freedman SB, Finkelstein Y, Pang XL, Chui L, Tarr PI, VanBuren JM et al. Pathogen-Specific Effects of Probiotics in Children with Acute Gastroenteritis seeking Emergency Care: a Randomized Trial. Clinical infectious diseases: an official publication of the infectious Diseases Society of America. 2022, 75(1):55–64. https://doi.org/10.1093/cid/ciab876.
Johnstone J, Meade M, Lauzier F, Marshall J, Duan E, Dionne J, et al. Effect of Probiotics on Incident Ventilator-Associated Pneumonia in critically ill patients: a Randomized Clinical Trial. JAMA. 2021;326(11):1024–33. https://doi.org/10.1001/jama.2021.13355.
Zhang F, Cui B, He X, Nie Y, Wu K, Fan D. Microbiota transplantation: concept, methodology and strategy for its modernization. Protein Cell. 2018;9(5):462–73. https://doi.org/10.1007/s13238-018-0541-8.
Mullish BH, Quraishi MN, Segal JP, McCune VL, Baxter M, Marsden GL, et al. The use of faecal microbiota transplant as treatment for recurrent or refractory Clostridium difficile infection and other potential indications: joint british society of gastroenterology (BSG) and Healthcare infection Society (HIS) guidelines. Gut. 2018;67(11):1920–41. https://doi.org/10.1136/gutjnl-2018-316818.
DeFilipp Z, Bloom PP, Torres Soto M, Mansour MK, Sater MRA, Huntley MH et al. Drug-resistant E. coli Bacteremia transmitted by fecal microbiota transplant. New Engl J Med 2019, 381(21):2043–50.https://doi.org/10.1056/NEJMoa1910437.
Riglar DT, Silver PA. Engineering bacteria for diagnostic and therapeutic applications. Nat Rev Microbiol. 2018;16(4):214–25. https://doi.org/10.1038/nrmicro.2017.172.
McCarty NS, Ledesma-Amaro R. Synthetic Biology tools to engineer Microbial Communities for Biotechnology. Trends Biotechnol. 2019;37(2):181–97. https://doi.org/10.1016/j.tibtech.2018.11.002.
Pedrolli DB, Ribeiro NV, Squizato PN, de Jesus VN, Cozetto DA. Engineering Microbial living therapeutics: the Synthetic Biology Toolbox. Trends Biotechnol. 2019;37(1):100–15. https://doi.org/10.1016/j.tibtech.2018.09.005.
Cani PD. Microbiota and metabolites in metabolic diseases. Nat reviews Endocrinol. 2019;15(2):69–70. https://doi.org/10.1038/s41574-018-0143-9.
He Y, Wu W, Wu S, Zheng HM, Li P, Sheng HF et al. Linking gut microbiota, metabolic syndrome and economic status based on a population-level analysis. Microbiome 2018, 6(1):172https://doi.org/10.1186/s40168-018-0557-6.
Guthrie RA, Guthrie DW. Pathophysiology of diabetes mellitus. Critical care nursing quarterly. 2004, 27(2):113–25https://doi.org/10.1097/00002727-200404000-00003.
Paun A, Danska JS. Modulation of type 1 and type 2 diabetes risk by the intestinal microbiome. Pediatr Diabetes. 2016;17(7):469–77. https://doi.org/10.1111/pedi.12424.
Farilla L, Bulotta A, Hirshberg B, Li Calzi S, Khoury N, Noushmehr H et al. Glucagon-like peptide 1 inhibits cell apoptosis and improves glucose responsiveness of freshly isolated human islets. Endocrinol 2003, 144(12):5149–58https://doi.org/10.1210/en.2003-0323.
Lee YS, Jun HS. Anti-diabetic actions of glucagon-like peptide-1 on pancreatic beta-cells. Metab Clin Exp. 2014;63(1):9–19. https://doi.org/10.1016/j.metabol.2013.09.010.
Agarwal P, Khatri P, Billack B, Low WK, Shao J. Oral delivery of glucagon like peptide-1 by a recombinant Lactococcus lactis. Pharm Res. 2014;31(12):3404–14. https://doi.org/10.1007/s11095-014-1430-3.
Zhang H, Dong M, Yuan S, Jin W. Oral glucagon-like peptide 1 analogue ameliorates glucose intolerance in db/db mice. Biotechnol Lett. 2022;44(10):1149–62. https://doi.org/10.1007/s10529-022-03288-1.
Zeng Z, Yu R, Zuo F, Zhang B, Ma H, Chen S. Recombinant Lactococcus lactis expressing bioactive exendin-4 to promote insulin secretion and beta-cell proliferation in vitro. Appl Microbiol Biotechnol 2017, 101(19):7177–86https://doi.org/10.1007/s00253-017-8410-6.
Vaarala O, Atkinson MA, Neu J. The “perfect storm” for type 1 diabetes: the complex interplay between intestinal microbiota, gut permeability, and mucosal immunity. Diabetes 2008, 57(10):2555–62https://doi.org/10.2337/db08-0331.
Robert S, Gysemans C, Takiishi T, Korf H, Spagnuolo I, Sebastiani G, et al. Oral delivery of glutamic acid decarboxylase (GAD)-65 and IL10 by Lactococcus lactis reverses diabetes in recent-onset NOD mice. Diabetes. 2014;63(8):2876–87. https://doi.org/10.2337/db13-1236.
Preisser TM, da Cunha VP, Santana MP, Pereira VB, Cara DC, Souza BM, et al. Recombinant Lactococcus lactis carrying IL-4 and IL-10 coding vectors protects against type 1 diabetes in NOD mice and attenuates Insulitis in the STZ-Induced Model. J Diabetes Res. 2021. https://doi.org/10.1155/2021/6697319.
Lang J, Wang X, Liu K, He D, Niu P, Cao R et al. Oral delivery of staphylococcal nuclease by Lactococcus lactis prevents type 1 diabetes mellitus in NOD mice. Appl Microbiol Biotechnol 2017, 101(20):7653–62https://doi.org/10.1007/s00253-017-8480-5.
Ma Y, Liu J, Hou J, Dong Y, Lu Y, Jin L, et al. Oral administration of recombinant Lactococcus lactis expressing HSP65 and tandemly repeated P277 reduces the incidence of type I diabetes in non-obese diabetic mice. PLoS ONE. 2014;9(8):e105701. https://doi.org/10.1371/journal.pone.0105701.
Rhimi M, Bermudez-Humaran LG, Huang Y, Boudebbouze S, Gaci N, Garnier A, et al. The secreted L-arabinose isomerase displays anti-hyperglycemic effects in mice. Microb Cell Fact. 2015;14:204. https://doi.org/10.1186/s12934-015-0391-5.
Caballero B. Humans against obesity: who Will Win? Adv Nutr. 2019, 10(suppl_1):S4-https://doi.org/10.1093/advances/nmy055.
Le Chatelier E, Nielsen T, Qin J, Prifti E, Hildebrand F, Falony G et al. Richness of human gut microbiome correlates with metabolic markers. Nat 2013, 500(7464):541–6https://doi.org/10.1038/nature12506.
Hira T, Pinyo J, Hara H. What is GLP-1 really doing in obesity? Trends in endocrinology and metabolism: TEM. 2020, 31(2):71–80https://doi.org/10.1016/j.tem.2019.09.003.
Ma J, Li C, Wang J, Gu J. Genetically Engineered Escherichia coli Nissle 1917 secreting GLP-1 Analog exhibits potential antiobesity effect in High-Fat Diet-Induced obesity mice. Obes (Silver Spring). 2020;28(2):315–22. https://doi.org/10.1002/oby.22700.
Wang L, Chen T, Wang H, Wu X, Cao Q, Wen K et al. Engineered Bacteria of MG1363-pMG36e-GLP-1 attenuated Obesity-Induced by High Fat Diet in mice. Frontiers in cellular and infection microbiology. 2021, 11:595575https://doi.org/10.3389/fcimb.2021.595575.
Long RT, Zeng WS, Chen LY, Guo J, Lin YZ, Huang QS, et al. Bifidobacterium as an oral delivery carrier of oxyntomodulin for obesity therapy: inhibitory effects on food intake and body weight in overweight mice. Int J Obes (Lond). 2010;34(4):712–9. https://doi.org/10.1038/ijo.2009.277.
Namai F, Shigemori S, Sudo K, Sato T, Yamamoto Y, Nigar S, et al. Recombinant mouse osteocalcin secreted by Lactococcus lactis promotes Glucagon-Like Peptide-1 induction in STC-1 cells. Curr Microbiol. 2018;75(1):92–8. https://doi.org/10.1007/s00284-017-1354-3.
Cao WY, Dong M, Hu ZY, Wu J, Li YC, Xu HD. Recombinant Lactococcus lactis NZ3900 expressing bioactive human FGF21 reduced body weight of Db/Db mice through the activity of brown adipose tissue. Beneficial microbes. 2020;11(1):67–78. https://doi.org/10.3920/BM2019.0093.
Fu J, Astarita G, Gaetani S, Kim J, Cravatt BF, Mackie K, et al. Food intake regulates oleoylethanolamide formation and degradation in the proximal small intestine. J Biol Chem. 2007;282(2):1518–28. https://doi.org/10.1074/jbc.M607809200.
Dosoky NS, Guo L, Chen Z, Feigley AV, Davies SS. Dietary fatty acids control the species of N-Acyl-phosphatidylethanolamines synthesized by therapeutically modified Bacteria in the intestinal tract. ACS Infect Dis 2018, 4(1):3–13https://doi.org/10.1021/acsinfecdis.7b00127.
May-Zhang LS, Chen Z, Dosoky NS, Yancey PG, Boyd KL, Hasty AH, et al. Administration of N-Acyl-phosphatidylethanolamine expressing Bacteria to low density lipoprotein Receptor(-/-) mice improves indices of Cardiometabolic Disease. Sci Rep. 2019;9(1):420. https://doi.org/10.1038/s41598-018-37373-1.
Hsu HA, Wu CY, Chu JS, Lin LH, Lu CA, Ou KL. Effect of recombinant human bone morphogenetic protein-2 and Ling Zhi-8 on osteogenesis: a comparative study using a rabbit sinus model. Journal of oral and maxillofacial surgery: official journal of the American Association of Oral and Maxillofacial Surgeons. 2014, 72(9):1703 ehttps://doi.org/10.1016/j.joms.2014.02.037.
Lee MF, Chiang CH, Lin SJ, Song PP, Liu HC, Wu TJ et al. Recombinant Lactococcus lactis expressing Ling Zhi 8 protein ameliorates nonalcoholic fatty liver and early atherogenesis in Cholesterol-Fed rabbits. BioMed research international. 2020, 2020:3495682https://doi.org/10.1155/2020/3495682.
de Groot MJ, Hoeksma M, Blau N, Reijngoud DJ, van Spronsen FJ. Pathogenesis of cognitive dysfunction in phenylketonuria: review of hypotheses. Mol Genet Metab. 2010;99(Suppl 1):86–9. https://doi.org/10.1016/j.ymgme.2009.10.016.
Isabella VM, Ha BN, Castillo MJ, Lubkowicz DJ, Rowe SE, Millet YA et al. Development of a synthetic live bacterial therapeutic for the human metabolic disease phenylketonuria. Nat Biotechnol 2018, 36(9):857–64https://doi.org/10.1038/nbt.4222.
Puurunen MK, Vockley J, Searle SL, Sacharow SJ, Phillips JA 3rd, Denney WS, et al. Safety and pharmacodynamics of an engineered E. coli Nissle for the treatment of phenylketonuria: a first-in-human phase 1/2a study. Nat metabolism. 2021;3(8):1125–32. https://doi.org/10.1038/s42255-021-00430-7.
Adolfsen KJ, Callihan I, Monahan CE, Greisen PJ, Spoonamore J, Momin M, et al. Improvement of a synthetic live bacterial therapeutic for phenylketonuria with biosensor-enabled enzyme engineering. Nat Commun. 2021;12(1):6215. https://doi.org/10.1038/s41467-021-26524-0.
Steidler L, Hans W, Schotte L, Neirynck S, Obermeier F, Falk W, et al. Treatment of murine colitis by Lactococcus lactis secreting interleukin-10. Science. 2000;289(5483):1352–5. https://doi.org/10.1126/science.289.5483.1352.
Sommer F, Ruhlemann MC, Bang C, Hoppner M, Rehman A, Kaleta C, et al. Microbiomarkers in inflammatory bowel diseases: caveats come with caviar. Gut. 2017;66(10):1734–8. https://doi.org/10.1136/gutjnl-2016-313678.
Tilg H, Adolph TE, Gerner RR, Moschen AR. The intestinal microbiota in Colorectal Cancer. Cancer cell. 2018, 33(6):954–64https://doi.org/10.1016/j.ccell.2018.03.004.
Sun Y, Duan B, Chen H, Xu X. A Novel Strategy for treating inflammatory Bowel Disease by Targeting Delivery of Methotrexate through Glucan particles. Adv Healthc Mater 2020, 9(6):e1901805.https://doi.org/10.1002/adhm.201901805.
Vandenbroucke K, de Haard H, Beirnaert E, Dreier T, Lauwereys M, Huyck L, et al. Orally administered L. lactis secreting an anti-TNF nanobody demonstrate efficacy in chronic colitis. Mucosal Immunol. 2010;3(1):49–56. https://doi.org/10.1038/mi.2009.116.
Scott BM, Gutierrez-Vazquez C, Sanmarco LM, da Silva Pereira JA, Li Z, Plasencia A et al. Self-tunable engineered yeast probiotics for the treatment of inflammatory bowel disease. Nat Med 2021, 27(7):1212–22https://doi.org/10.1038/s41591-021-01390-x.
Yan X, Liu XY, Zhang D, Zhang YD, Li ZH, Liu X, et al. Construction of a sustainable 3-hydroxybutyrate-producing probiotic Escherichia coli for treatment of colitis. Cell Mol Immunol. 2021;18(10):2344–57. https://doi.org/10.1038/s41423-021-00760-2.
Mays ZJ, Nair NU. Synthetic biology in probiotic lactic acid bacteria: at the frontier of living therapeutics. Current opinion in biotechnology. 2018, 53:224–31https://doi.org/10.1016/j.copbio.2018.01.028.
Dou J, Bennett MR. Synthetic Biology and the gut Microbiome. Biotechnol J. 2018;13(5):e1700159. https://doi.org/10.1002/biot.201700159.
Wang L, Liao Y, Yang R, Zhu Z, Zhang L, Wu Z, et al. An engineered probiotic secreting Sj16 ameliorates colitis via Ruminococcaceae/butyrate/retinoic acid axis. Bioeng translational Med. 2021;6(3):e10219. https://doi.org/10.1002/btm2.10219.
Praveschotinunt P, Duraj-Thatte AM, Gelfat I, Bahl F, Chou DB, Joshi NS. Engineered E. coli Nissle 1917 for the delivery of matrix-tethered therapeutic domains to the gut. Nat Commun 2019, 10(1):5580https://doi.org/10.1038/s41467-019-13336-6.
Yan Z, Yin M, Chen J, Li X. Assembly and substrate recognition of curli biogenesis system. Nat Commun. 2020;11(1):241. https://doi.org/10.1038/s41467-019-14145-7.
Yu M, Kim J, Ahn JH, Moon Y. Nononcogenic restoration of the intestinal barrier by E. coli-delivered human EGF. JCI insight. 2019;4(16). https://doi.org/10.1172/jci.insight.125166.
Nadeem MS, Kumar V, Al-Abbasi FA, Kamal MA, Anwar F. Risk of colorectal cancer in inflammatory bowel diseases. Seminars in cancer biology 2020, 64:51–60https://doi.org/10.1016/j.semcancer.2019.05.001.
Ho CL, Tan HQ, Chua KJ, Kang A, Lim KH, Ling KL, et al. Engineered commensal microbes for diet-mediated colorectal-cancer chemoprevention. Nat biomedical Eng. 2018;2(1):27–37. https://doi.org/10.1038/s41551-017-0181-y.
An BC, Ryu Y, Yoon YS, Choi O, Park HJ, Kim TY et al. Colorectal Cancer therapy using a Pediococcus pentosaceus SL4 Drug Delivery System secreting lactic acid Bacteria-derived protein p8. Molecules and cells. 2019, 42(11):755–62https://doi.org/10.14348/molcells.2019.0064.
Chen J, Li X, Liu Y, Su T, Lin C, Shao L, et al. Engineering a probiotic strain of Escherichia coli to induce the regression of colorectal cancer through production of 5-aminolevulinic acid. Microb Biotechnol. 2021;14(5):2130–9. https://doi.org/10.1111/1751-7915.13894.
Gmitrowicz A, Kucharska A. [Developmental disorders in the fourth edition of the american classification: diagnostic and statistical manual of mental disorders (DSM IV -- optional book)]. Psychiatr Pol. 1994;28(5):509–21.
Den H, Dong X, Chen M, Zou Z. Efficacy of probiotics on cognition, and biomarkers of inflammation and oxidative stress in adults with Alzheimer’s disease or mild cognitive impairment - a meta-analysis of randomized controlled trials. Aging 2020, 12(4):4010–39https://doi.org/10.18632/aging.102810.
Fang X, Zhou X, Miao Y, Han Y, Wei J, Chen T. Therapeutic effect of GLP-1 engineered strain on mice model of Alzheimer’s disease and Parkinson’s disease. AMB Express. 2020;10(1):80. https://doi.org/10.1186/s13568-020-01014-6.
Chen T, Tian P, Huang Z, Zhao X, Wang H, Xia C et al. Engineered commensal bacteria prevent systemic inflammation-induced memory impairment and amyloidogenesis via producing GLP-1. Applied microbiology and biotechnology. 2018, 102(17):7565–75https://doi.org/10.1007/s00253-018-9155-6.
Cecarini V, Bonfili L, Gogoi O, Lawrence S, Venanzi FM, Azevedo V et al. Neuroprotective effects of p62(SQSTM1)-engineered lactic acid bacteria in Alzheimer’s disease: a pre-clinical study. Aging 2020, 12(16):15995–6020https://doi.org/10.18632/aging.103900.
Gitler AD, Dhillon P, Shorter J. Neurodegenerative disease: models, mechanisms, and a new hope. Dis Models Mech. 2017;10(5):499–502. https://doi.org/10.1242/dmm.030205.
Yue M, Wei J, Chen W, Hong D, Chen T, Fang X. Neurotrophic Role of the Next-Generation Probiotic Strain L. lactis MG1363-pMG36e-GLP-1 on Parkinson’s Disease via Inhibiting Ferroptosis. Nutrients. 2022;14(22). https://doi.org/10.3390/nu14224886.
Smith K. Mental health: a world of depression. Nature. 2014;515(7526):181. https://doi.org/10.1038/515180a.
Strandwitz P, Kim KH, Terekhova D, Liu JK, Sharma A, Levering J, et al. GABA-modulating bacteria of the human gut microbiota. Nat Microbiol. 2019;4(3):396–403. https://doi.org/10.1038/s41564-018-0307-3.
Dung Pham V, Somasundaram S, Lee SH, Park SJ, Hong SH. Efficient production of gamma-aminobutyric acid using Escherichia coli by co-localization of glutamate synthase, glutamate decarboxylase, and GABA transporter. J Ind Microbiol Biotechnol. 2016;43(1):79–86. https://doi.org/10.1007/s10295-015-1712-8.
Mays ZJS, Chappell TC, Nair NU. Quantifying and Engineering Mucus Adhesion of Probiotics. ACS synthetic biology. 2020, 9(2):356–367.https://doi.org/10.1021/acssynbio.9b00356.
Goh YJ, Barrangou R. Harnessing CRISPR-Cas systems for precision engineering of designer probiotic lactobacilli. Current opinion in biotechnology. 2019, 56:163–71https://doi.org/10.1016/j.copbio.2018.11.009.
Peters JM, Koo BM, Patino R, Heussler GE, Hearne CC, Qu J, et al. Enabling genetic analysis of diverse bacteria with Mobile-CRISPRi. Nat Microbiol. 2019;4(2):244–50. https://doi.org/10.1038/s41564-018-0327-z.
Acknowledgements
This work is supported by the Natural Science Foundation of Shanxi Province [No. 202203021211244, 202203021212343], the Cultivation Project of Science and Technology Innovation of Shanxi University of Chinese Medicine [No. 2023PY-TH-10], to whom the authors are grateful.
Funding
This work is supported by the Natural Science Foundation of Shanxi Province [No. 202203021211244, 202203021212343], the Cultivation Project of Science and Technology Innovation of Shanxi University of Chinese Medicine [No. 2023PY-TH-10].
Author information
Authors and Affiliations
Contributions
Data collection and writing manuscript: LYY, JQG, BYZ. Content development, editing and formatting: YLN, LPL. Overall correction and supervision: LYY.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Guo, J., Zhou, B., Niu, Y. et al. Engineered probiotics introduced to improve intestinal microecology for the treatment of chronic diseases: present state and perspectives. J Diabetes Metab Disord 22, 1029–1038 (2023). https://doi.org/10.1007/s40200-023-01279-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40200-023-01279-1