Abstract
The intent of this research is to reveal the ramification of Na2O content on the mechanical strength of ground granulated blast furnace slag (GGBS) incorporated fly ash (FA)-based geopolymer paste. The different percentage of incorporation of GGBS was 10, 20, 30, 40, and 50% by weight of FA which was done. In the manufacturing of geopolymer paste, FA and GGBS were taken as source materials while alkaline activators comprise of sodium hydroxide (NaOH) and sodium silicate (Na2SiO3). The percentage of Na2O was taken 6, 7, and 8% (by weight of source materials) and that of SiO2 was fixed at 7%. The performance was figured out on the behalf of residual strength at the predetermined periods of time. XRD was also accompanied to examine the changes in mineralogy. The results reveal that incorporation of GGBS in FA-based geopolymer paste significantly improves its performance.
Access provided by Autonomous University of Puebla. Download conference paper PDF
Similar content being viewed by others
Keywords
1 Introduction
The growing appeal of infrastructural improvement within growing international locations contributes to more concrete consumption. A hefty quantity of standard Portland Cement (OPC) is used as the main binder cloth to meet the insistence for the manufacture of concrete. But the processing of OPC calls for a large quantity of herbal sources, as well as a large system of energy, and additionally releases more quantity of carbon dioxide within the surroundings.
As a result, environmental degradation and the protection of natural resources have become critical issues that need to be sorted out in order to promote sustainability within the construction industry. Therefore, the use of additional building materials in concrete has an excellent momentum to protect the environment. GGBS is used as an auxiliary for cement, and the replacement share may be up to 85 percent depending upon the applications. Usually, 50% is employed in most applications. Geopolymers mainly comprise of aluminosilicate source materials like FA, blast furnace slag, etc., hardened by activating solution. Mixture of X-hydroxide and X-silicate (where X-sodium, potassium, barium, etc.) is treated as activating solution. Several researches have been conducted on various source materials and activating solution. The mechanism for geopolymers may be a chemical action that involves a chemical change of alumina-silicate materials within the presence of alkaline medium, which ends to the formation of three-dimensional compound chain [1,2,3,4,5]. The results reveal that geopolymers are resistant to acids, sulfate, and elevated temperatures [6, 7]. Nath and Sarkar investigated the effect of incorporation of different percentage of GGBS (0%, 10%, and 20%) on workability and mechanical potency of FA-based geopolymer concrete. They concluded that strength is increased significantly but the workability was decreased with higher percentage of GGBS [8]. He et al. compared the strength parameter of metakaolin and red mud-based geopolymers. The results indicate the superiority of metakaolin over red mud [9]. Nath and Kumar studied about FA, GGBS, and granulated corex slag geopolymer of 6M sodium hydroxide solution. They quoted that performancewise, granulated corex slag has an edge over granulated furnace slag [10]. Salih et al. reported that higher degree of geopolymerization takes place when blast furnace slag with higher percentage of calcium and aluminum is added to geopolymer [11]. Mehta and Siddique presented their experimental results on performance of partially replaced FA by ordinary Portland cement (0%, 10%, 20%, and 30%) as source material in geopolymer concrete. The results denote increase in compressive strength and decrease in porosity, sorptivity, and chloride permeability [12]. Aiken et al. compared the performance of Portland cement and geopolymer system to silage effluent attack and concluded that geopolymer has better resistance [13]. Tamburini et al. provide thorough data of chemical and physical characterization of geopolymer which shows the stable nature of geopolymers toward leaching, freeze-thaw effect, and elevated temperature treatments [14]. Rashad et al. investigated the performance of GGBS activated by two concentrations (1% and 3% by weight) by sodium sulfate. The early strength examination was done on 3 days and 7 days along with 28 days. The specimens were also exposed to high temperatures ranging from 200 to 800 °C. The results indicate that sodium sulfate is an efficient activator and shows better chemical stability at elevated temperatures [15]. Soustos et al. studied the curing temperature, alkaline activators dosage, properties (physical and chemical) of FA, and amount of GGBS incorporation on FA reactivity as a source material in geopolymer concrete. The results indicate that curing temperature, alkaline activators dosage, amount of GGBS incorporated play a very important role in strength development of geopolymer [16]. Mehta and Siddique studied about various industrial waste (bottom ash, FA, metakaolin, blast furnance slag, etc.) and concise their review that geopolymers can be synthesized by these industrial waste [17]. Kumar and Singh studied the behavior of potassium feldspar mixed FA-based geopolymer paste at preeminent temperature [18].
From the study of literature, it is concluded that synthesis of geopolymers is done by source materials which mainly comprises of aluminosilicate source materials and alkaline activating solution. The strength of geopolymer is imparted due to the concentration of alkali solution which depends on molarity of sodium hydroxide. As the molarity increase, the compressive strength and durability increase. Higher molarity corresponds to higher Na2O content in the solution, but the amount of Na2O present in sodium silicate is not taken in the consideration. As sodium hydroxide contains Na2O in some percentage, sodium silicate also contains Na2O in some percentage. Therefore, the current research and experiments were carried out to estimate the consequence of Na2O content on the incorporation of five different percentages (10%, 20%, 30%, 40%, and 50% by weight) of blast furnace slag in alkali-activated FA-based geopolymer paste. From the best of researcher’s knowledge from previous literature reviews, it can be concluded that there is lack of work on the effect of Na2O content. Therefore, the enactment of specimens was assessed in terms of compressive strength after incorporation of GGBS in FA geopolymer paste.
2 Experimental Procedure
2.1 Source Materials
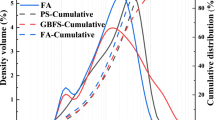
In the current experimental program, fly ash (FA) was supplied by Marshall Corporation, Kolkata, India, and GGBS was purchased from Owndust India. The chemical composition of ash and blast furnace slag is shown in Table 1. Figure 1 shows the mineralogical composition of GGBS and FA, respectively. More than 75% of particles were finer than 45 microns of FA.
2.2 Alkaline Solution
Sodium silicate solution (SiO2 = 26.5%, Na2O = 8%, and 65.5% water) with bulk density of 1410 kg/m3 and silicate modulus of 3.30 (approx.) and sodium hydroxide pellets (98% purity) of laboratory grade were acquired from Sharma Bros, Imphal, India. The percentage of Na2O = 7%, 8%, and 9% along with SiO2 = 8% by weight of source material was fixed in activating solution, prepared one day before the fabrication of geopolymer paste. Water from a pond nearby the NIT campus was used in the study [18]. Figure 1 presents X-ray diffractogram (XRD) for mineralogical composition of fly ash and GGBS. In the FA, the existence of quartz (Q), mullite (M), and hematite (H) is detected while in GGBS shows its amorphous nature.
2.3 Preparation of Specimens
The calculated measure of sodium hydroxide pellets was mixed directly with sodium silicate solution to produce a homogeneous geopolymer paste. So, we got three alkaline solutions with 7, 8 and 8% Na2O content, keeping 8% SiO2 constant. The water-to-source material ratio was 0.33. The mixture of sodium hydroxide and sodium silicate is an exothermic reaction; therefore, the alkaline solution obtained was kept overnight at room temperature to produce geopolymeric paste. Aluminosilicate source material and alkaline activator solution have been mixed in the Hobart mixer for 5 min. The paste was of gray color, sticky in nature and of normal workability. The mixture was poured into cube molds of 50 × 50 × 50 mm and vibrated on a vibration table for 10 minutes to expel the air. The specimens were demolded after 24 hours of casting and kept at room temperature till the predetermined time for testing.
2.4 Compressive Strength
The compressive strength testing was done on UTM at 7 days, 28 days, and 56 days. Specimens were casted in cube molds of dimensions 50 × 50 × 50 mm. Cubes were demolded after 24 hours of casting and kept at room temperature for testing till 7 days, 28 days, and 56 days. Compressive strength test was performed as per guidelines of IS-4031 (part6)-1988.
3 Results and Discussion
3.1 Residual Compressive Strength
Figures 2, 3, and 4 represent the effect of Na2O content on the initial compressive strength of FA geopolymer paste specimens with 10%, 20%, 30%, 40%, and 50% GGBS replacement at time intervals of 7 days, 28 days, and 56 days. Samples GPC6, GPC7, and GPC8 represent geopolymer paste having 6, 7, and 8% Na2O, respectively, with SiO2 percentage fixed at 7%. All the geopolymer specimens were cured at ambient temperature. The experimental data shows that majority of strength is achieved in early days after casting the geopolymer paste specimens. The data of experiments reveal that both Na2O content and incorporation of GGBS affect the compressive strength of geopolymer paste. The geopolymer specimen’s strength increases in due course of time which exhibits that continuity of geopolymerization reaction. However, previous studies show that without heat curing fly FA of heat curing GGBS is added and it is observed that the setting time is significantly reduced along with the increase in compressive strength. Mainly two factors are responsible for crediting compressive strength to FA geopolymer paste, which is (a) Na2O content and (b) GGBS.
Na2O content facilitates the formation of sodium aluminate silicate hydrate gel (N-A-S-H) which leads to polycondensation to form a 3D network of aluminosilicate structure. Higher the Na2O content, higher dissolution of Si and Al ions takes place. It forms a dense network and reduces pore volume [19]. A stable network imparts greater strength to geopolymer.
Addition of GGBS plays two roles in the mechanism. Primarily, it reduces the need for heat curing required for geopolymerization reaction and helps in its casting at ambient temperature. Secondly, it imparts the strength. From the experiments, replacement of FA with ground GGBS increases the compressive strength of geopolymer. The same phenomenon was also observed in previous literature. The reason for proving the strength to geopolymer can be due to two reasons: firstly, due to the higher content of calcium which results in formation of C-S-H gel; secondly, the requirement of water to C-S-H gel which causes the deficiency of water, resulting in the rise in alkalinity of the medium. Higher alkalinity results in higher dissolution of Si and Al ions from source materials and forms a dense network which imparts the strength [10, 19, 21, 22].
3.2 XRD
Figure 5 shows the diffraction spectra of geopolymer specimens having 40% GGBS and 60% FA. Diffraction pattern in a, b, c shows the specimens of 6%, 7%, and 8% of Na2O, respectively, with SiO2 7% at 56 days. Comparing Figs. 1 and 5, we observe a change hump shape which refers to the formation of new amorphous materials when geopolymerization takes place. Within Fig 2a, b, c, it is seen that that due to alkali activation of source materials, the intensity of SiO2 (quartz) varies. As the percentage of Na2O increases, more dissolution of ions takes place which leads to strength in specimens. Due to this, SiO2 peaks show a lower level of intensity with increase in Na2O content. The same results were detected in previous studies [23, 24]. This may demonstrate the arrangement of a higher measure of geopolymerized gel with a higher level of crystallinity as the dose of GGBS in the fastener expanded. It might well explain the expanding pattern in compressive quality outcomes saw at 10% to half GGBS substitution [25].
4 Conclusion
This paper shows the effect of Na2O content on compressive strength of specimens casted with incorporation of different percentages of GGBS in FA geopolymer paste.
-
It reveals that both Na2O and GGBS promote the performance of geopolymers. Increase in Na2O content favors the geopolymerization, and GGBS reduces the need of heat curing required for geopolymerization reaction.
References
Author F, Hardjito D, Wallah SE, Sumajouw DMJ, Rangan BV (2004) Brief review of development of geopolymer concrete. Invited Paper, George Hoff symposium, American Concrete Institute
Van Deventer JSJ, Provis JL, Duxson P (2012) Technical and commercial progress in the adoption of geopolymer cement. Miner Eng 29:89–104
Duxson JL, Fernández-Jiménez P, Provis A et al (2007) Geopolymer technology: the currentstate of the art. J Mater Sci 42:2917–2933. https://doi.org/10.1007/s10853-006-0637-z
Hardjito D, Wallah SE, Sumajouw DMJ, Rangan BV (2004) On the development of fly ash-based geopolymer concrete. ACI Mater J 101:467–472. https://doi.org/10.14359/13485
Bakharev T (2005) Durability of geopolymer materials in sodium and magnesium sulfate solu- tions. Cem Concr Res 35:1233–1246. https://doi.org/10.1016/j.cemconres.2004.09.002
Tempest B, Sanusi O, Gergely J, Ogunro V, Weggel D (2009) In world of coal ash (WOCA) conference, pp 1–17. http://www.flyash.info/2009/045-tempest2009.pdf
Assi LN, (Eddie) Deaver E, ElBatanouny MK, Ziehld P (2016) Investigation of early compressive strength of fly ash-based geopolymer concrete. Constr Build Mater 112:807–815. https://doi.org/10.1016/j.conbuildmat.2016.03.008
Nath P, Sarker PK (2014) Effect of GGBFS on setting, workability and early strength properties of fly ash geopolymer concrete cured in ambient condition. Constr Build Mater 66:163–171. https://doi.org/10.1016/j.conbuildmat.2014.05.080
He J, Zhang J, Yu Y, Zhang G (2012) The strength and microstructure of two geopolymers derived from metakaolin and red mud-fly ash admixture: A comparative study. Constr Build Mater 30:80–91. https://doi.org/10.1016/j.conbuildmat.2011.12.011
Nath SK, Kumar S (2013) Influence of iron making slags on strength and microstructure of fly ash geopolymer. Constr Build Mater 38:924–930. https://doi.org/10.1016/j.conbuildmat.2012.09.070
Salih MA, Farzadnia N, Abang Ali AA, Demirboga R (2015) Development of high strength alkali activated binder using palm oil fuel ash and GGBS at ambient temperature. Constr Build Mater 93:289–300
Mehta A, Siddique R (2017) Properties of low-calcium fly ash based geopolymer concrete incorporating OPC as partial replacement of fly ash. Constr Build Mater 150:792–807. https://doi.org/10.1016/j.conbuildmat.2017.06.067
Aiken TA, Sha W, Kwasny J, Soutsos MN (2017) Resistance of geopolymer and Portland cement based systems to silage effluent attack. Cem Concr Res 92:56–65. https://doi.org/10.1016/j.cemconres.2016.11.015
Tamburini S et al (2017) Geopolymer matrix for fibre reinforced composites aimed at strengthening masonry structures. Constr Build Mater 141:542–552. https://doi.org/10.1016/j.conbuildmat.2017.03.017
Rashad AM, Bai Y, Basheer PAM, Collier NC, Milestone NB (2012) Chemical and mechanical stability of sodium sulfate activated slag after exposure to elevated temperature. Cem Concr Res 42:333–343. https://doi.org/10.1016/j.cemconres.2011.10.007
Soutsos M, Boyle AP, Vinai R, Hadjierakleous A, Barnett SJ (2016) Factors influencing the compressive strength of fly ash based geopolymers. Constr Build Mater 110:355–368. https://doi.org/10.1016/j.conbuildmat.2015.11.045
Mehta A, Siddique R (2016) An overview of geopolymers derived from industrial byproducts. Constr Build Mater 127:183–198. https://doi.org/10.1016/j.conbuildmat.2016.09.136
Kumar R, Mayengbam SS (2021) Enhancement of the thermal durability of fly ash-based geopolymer paste by incorporating potassium feldspar. J Inst Eng India Ser A. https://doi.org/10.1007/s40030-020-00498-6
Li Z, Liu S (2007) Influence of Slag as Additive on Compressive Strength of Fly Ash-Based Geopolymer. J Mater Civ Eng 19:470–474
Puligilla S, Mondal P (2013) Role of slag in microstructural development and hardening of fly ash-slag geopolymer. Cem Concr Res 43:70–80. https://doi.org/10.1016/j.cemconres.2012.10.004
Kumar S, Kumar R, Mehrotra SP (2010) Influence of granulated blast furnace slag on the reaction, structure and properties of fly ash based geopolymer. J Mater Sci 45:607–615. https://doi.org/10.1007/s10853-009-3934-5
Khater HM (2012) Effect of Calcium on Geopolymerization of Aluminosilicate wastes. J Mater Civil Eng 24:92–101. https://doi.org/10.1061/(ASCE)MT.1943-5533.0000352
Zhang L, Ahmari S, Zhang J (2011) Synthesis and characterization of fly ash modified mine tailings-based geopolymers. Constr Build Mater 25:3773–3781. https://doi.org/10.1016/j.conbuildmat.2011.04.005
Boonserm K, Sata V, Pimraksa K, Chindaprasirt P (2012) Improved geopolymerization of bottom ash by incorporating fly ash and using waste gypsum as additive. Cem Concr Compos 34:819–824. https://doi.org/10.1016/j.cemconcomp.2012.04.001
Salih MA, Farzadnia N, Abang Ali AA, Demirboga R (2015) Development of high strength alkali activated binder using palm oil fuel ash and GGBS at ambient tempera ture. Constr Build Mater 93:289–300. https://doi.org/10.1016/j.conbuildmat.2015.05.119
Author information
Authors and Affiliations
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2022 The Author(s), under exclusive license to Springer Nature Singapore Pte Ltd.
About this paper
Cite this paper
Kumar, R., Singh, M.S. (2022). Effect of Na2O Content on Ground Granulated Blast Furnace Slag Incorporated Fly Ash-Based Geopolymer Pastes. In: Verma, P., Samuel, O.D., Verma, T.N., Dwivedi, G. (eds) Advancement in Materials, Manufacturing and Energy Engineering, Vol. II. Lecture Notes in Mechanical Engineering. Springer, Singapore. https://doi.org/10.1007/978-981-16-8341-1_42
Download citation
DOI: https://doi.org/10.1007/978-981-16-8341-1_42
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-16-8340-4
Online ISBN: 978-981-16-8341-1
eBook Packages: EngineeringEngineering (R0)