Abstract
The self-setting apatite-based bone cement, prepared using the solubility phase diagram of calcium phosphates, consists of metastable calcium phosphates and can be transformed to the low-crystallinity apatite with bio-affinity to natural bones. Various drugs were kneaded into the cement to create an artificial bone cement with sustained drug release capability and the drug release from the cement matrices can be controlled based on the parameters of the Higuchi equation, such as drug concentration, porosity, granular diameter, and diffusion coefficient. Results from in vitro and in vivo drug release experiments suggested that in vivo drug release from the apatite cement was dependent on calcium concentration in the body fluids. The results indicated that anti-osteoporosis drug release from apatite bone cement could be controlled based on the severity of the disease in osteoporotic rats. In addition, the amount of diseased bone in the rat increased with the apatite bone cement. Furthermore, anti-osteoporotic drugs were added to the apatite-collagen composite cement for the manufacture of three-dimensional perforated macro-porous bone cell scaffold. The collagen-HAp cement containing DNA complex was administered, and the growth of cancer cells was effectively suppressed by the continuous DNA release of the injected collagen-HAp cement containing DNA complex. This highly functional artificial bone was shown to be suitable for bone regeneration.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Apatite bone cement
- Bioaffinity
- Higuchi equation
- Osteoporosis responsive drug release
- Apatite-collagen composite cement
- Three-dimensionally perforated macro-porous bone cell scaffold
8.1 Introduction
Bone-related diseases, such as osteoporosis, often involve bone fracture accidents and result in a bedridden state, which greatly reduces the patient’s quality of life. Artificial bones and hip joints have been used as medical devices to restore the functionality of osteoporosis patients and improve their quality of life [1]. Artificial bones are made of metal (titanium and stainless steel), ceramics (zirconia, alumina, and calcium phosphate), or polymers (poly-ether-ether-keton and polyethylene terephthalate) [2, 3].
Hydroxyapatite (HAp), which has good compatibility with hard tissues in the body, has been used for artificial bones, artificial joints, and dental implants [4, 5]. However, time is required for the stabilization of HAp implants after surgical implantation. Clinically, it is desirable to develop an artificial bone system that allows a faster bone cell proliferation and has high cohesiveness with natural bones.
An implantable drug delivery system using a biodegradable polymer material such as gelatin has been developed as a novel drug delivery system to deliver loaded pharmaceuticals to natural hard tissue and promote bone formation [6,7,8]. However, when using these implants in patients with bone defects, it is difficult to obtain good clinical scores. Therefore, artificial bone systems have been developed in which antibiotics and anticancer agents are embedded in synthetic porous HAp beads and are composed of biocompatible inorganic materials that fill bone defects and control drug release [9, 10]. However, it is not easy to control the amount of drug being loaded and sintering at high temperatures eliminates the possibility of using the artificial bone cement for sustained drug release applications as the drug is only physical adsorbed on surface of the micro-pores.
On the other hand, when using self-setting HAp bone cement [11] that is transformed to stable crystalline HAp after kneading, as the bone cement material, the drug can be easily sustained released for long-term effects by embedding the drug powder in the cement. Intelligent drug delivery systems (DDS) have been developed that release various drugs in response to the degree of pathological condition by making use of the drug release control technology and biomimetic technology based on apatite-related bone cement. In this study, the development process of these DDS has been described.
8.2 Self-hardening Mechanism of the Setting HAp-Related Bone Cement
Brown and Chou [11] prepared a self-setting HAp-related bone cement based on the solubility phase diagram of calcium phosphates. The metastable calcium phosphates in the cement rapidly underwent crystal transition to form stable HAp by kneading with a phosphoric acid solution. The cement rapidly self-set to HAp with low crystallinity as observed by powder X-ray diffraction analysis. Self-setting HAp bone cement was developed using this technique, and it has been used clinically and has been confirmed as an excellent biocompatible artificial bone material. The artificial bone cement was used as a base material to control drug delivery function by incorporating various drug powder into the self-setting HAp cement. Since the drug-containing cement was self-hardened and transformed to low-crystalline carbonate HAp with high biocompatibility, the drug powder was distributed in the interconnected pores that exists within the cement. Therefore, after the cement is set within the human body, drug powder is dissolved and slowly released from the artificial bone cement throughout micro-pores of the HAp cement matrices.
8.3 Diffusion Theory of Controlled Drug Release from Self-setting HAp-Related Bone Cement, and Therapeutical Applications of These Cement Systems
The first application of self-setting HAp-related bone cement was to treat infections caused by methicillin-resistant Staphylococcus aureus, often occurring after surgery to attach an artificial bone. Traditionally, antibacterial agents either in the form of an injection or in the tablet form is administered after the surgical implantation of artificial bone in an effort to prevent bacterial infections after surgery. However, the disadvantage of this approach is the fast dissolution of normal drug powder, rapid drug diffusion from the implanted site, the body metabolizes the medication, and eventually gets broken down absorbed by the body. Therefore, a biocompatible HAp cement was used as the base cement to prevent postoperative infections for an extended period, and consequently, a HAp bone cement containing the antibiotic cephalexin (CEX) was synthesized [12].
Figure 8.1 shows the in vitro drug release profile of the HAp bone cement in a phosphate buffer at 37 °C. It indicated that the HAp cement had CEX sustained-release characteristics for an extended period of 1 week or more, and the rate of drug release increased depending on the drug content. The drug filled in the hardened cement matrix was released by diffusion through the micro-pores. Higuchi reported that the drug release rate from the cement matrices with micro-pores at time t is dependent on the porosity of the cement, drug content, drug solubility, surface area of the cement, and tortuosity of the pores, and was expressed by the following equation [13]:
where Mt is the drug release amount at time t; A is the surface area of the cement; t is time; Di is the drug diffusion constant; τ is the tortuosity; ε is the porosity; Cd is the drug concentration; M0 is the total amount of drug; and Cs is the drug solubility.
The CEX release profiles of the HAp bone cements were applied to the Higuchi equation, as shown in Fig. 8.2 [12]. Since good linear relationships were observed, the drug diffusion rate in the micro-pores in the cements was the rate-determining step of the drug release rate, and it indicated that the drug content affected the release of the drug from the cement [14]. Based on this result, when considering the Higuchi equation (8.1), it was possible to control the drug release rate without restraints from artificial bone cement by controlling the geometrical elements of the cement and the elements related to the drug diffusion characteristics.
Second, based on the Higuchi equation (8.1), the drug release from the HAp bone cement should be controlled by changing the cement surface area based on the particle size. As application to treat rheumatic bone deformity, the anti-inflammatory drug release from the cements containing indomethacin (IMC) with various granular size and drug concentration of the bone cement granules were tested [15]. The HAp cement granules with diameters of 2, 4, and 15 mm were prepared and their drug release rate tested, as shown in Fig. 8.3. The drug release rate of the granules increased with decrease in granular size of the cement, implying that their release rates were dependent on the size and drug concentrations. However, when the amounts of drugs released were corrected to release amount per surface area, the initial drug release profiles from the granular cements of all sizes overlapped to produce nearly identical drug release profiles, as shown in Fig. 8.4. This was an evidence that the drug release from the HAp cement with various size granules followed the Higuchi equation. The result also suggested that it was possible to control drug release by changing the granular size of the cements.
In addition, as an application to correct bone defects after bone cancer resection surgery, controlling the release rate of an anticancer drug, 6 mercaptopurine (6-MP), from the sustained-release bone cement was examined by changing the cement powder and kneading liquid ratio [16]. Based on the Higuchi equation (8.1), the geometric structural factors of the micro-pores, such as the porosity (ε) and the tortuosity (τ), in the self-setting HAp cement can control drug release, and hence, the pore parameters of the cements were varied by changing the powder-liquid ratio of the cement. Figure 8.5 shows the effect of change in the powder-liquid ratio of the HAp cement containing 6-MP on the release rate. The symbols and dotted lines represent the measured values and simulated values, respectively, based on the Higuchi equation (8.1). The 6-MP release from the HAp cement increased with increasing in the powder-liquid ratio, and the measured values were fitted with Higuchi equation (8.1). The result indicated that the drug release parameters, the porosity (ε) and the porosity (τ), in the Higuchi equation were dependent on the powder-liquid ratio. Thus, it was concluded that the anticancer drug release rate from the HAp bone cement could be freely controlled by varying the powder/liquid ratio.
HAp is known to have high adsorption on proteins and peptides and has been utilized as a material in separation column for liquid chromatography owing to this property [17]. Therefore, the physicochemical properties, including the adsorption property of the applied drug to HAp, significantly affects the drug release property from HAp bone cement. Thus, the interaction between HAp and highly therapeutic polypeptide-based drugs, such as calcitonin (a therapeutic agent for osteoporosis), bone growth factors, and anticancer agents were investigated.
In this study, insulin, bovine serum albumin (BSA), and the tripeptide drug cephalexin were applied to self-setting HAp cement as model peptide drugs with different molecular weights [18], and the drug release rates from the pores of HAp bone cement were compared. The in vitro BSA release from the HAp bone cement was very slow, since BSA has a greater molecular weight and a high affinity for apatite crystals. In contrast, the drug release of short peptide, cephalexin, was much faster than that of BSA or Insulin.
Figure 8.6 shows the relationship between the drug release rate and the molecular weight of various polypeptide drugs from the HAp bone cement matrices. The relationship revealed a decrease in the drug release rate as the molecular weight increases, and the result supports the theoretical relationship between diffusion rate constant and molecular weight. Therefore, it is considered that the drug release rate from the HAp bone cement decreases as the molecular weight increases. Based on this finding, it was shown that the polypeptide-based drug can be released in a sustained manner from the apatite cement DDS for an extended period of several months.
8.4 Effect of Drug Loading Geometrical Structure on Controlled Drug Release from Self-setting HAp-Related Bone Cement
Figure 8.7 shows application models of the homogeneous drug loaded-HAp bone cement and heterogeneous drug loaded-HAp bone cement. As described in the previous sections, the drug powder was distributed homogeneously within the bone cement base, and the drug release followed the Higuchi equation. The homogeneous drug loaded system was a clinically feasible application method, and had an advantage that safety at the time is ensured during surgery. In contrast, heterogeneous drug-loaded cement was prepared by designing a drug storage compartment inside the cement and the outer surface of the compartment encapsulated by the cement to maintain porosity and tortuosity of the cement pores by increasing or decreasing the cement kneading solution [19]. The device could control drug release from bone cement by a release mechanism according to the Fick's equation. The heterogeneous drug filling system allowed for much longer drug release than the homogenous system.
Figure 8.8 shows the drug release profile from HAp bone cement filled with aspirin, which is an anti-inflammatory drug, in a heterogeneous drug loading system. Since the HAp bone cement of the release control layer was prepared by changing the amount of seed crystal and kneading liquid, the porosity and tortuosity of micro-pores in the molded cement were changed in the formulation. The drug release rate of the prepared HAp bone cement system varied significantly depending on the formulation (amount of seed crystal and kneading liquid) of the release control cement layer. Accurate drug release behavior could be controlled over a long period by filling the bone defect with aspirin-containing cement and encapsulating the outside with apatite cement.
8.5 Osteoporosis-Responsive Drug Delivery System Based on Self-setting HAp Bone Cement
By applying the basic concept of drug release kinetics, the relationship between the physicochemical properties and drug release properties of the artificial bones made from self-setting HAp bone cement were theoretically elucidated in the above section. However, in the process of developing HAp bone cement containing anti-inflammatory drug indomethacin [20], in order to clarify interaction between HAp cement matrices and bone cells, the in vitro and the in vivo drug release profiles were directly compared, but those were not matched well in long-term drug release period. The ionic concentrations of calcium and phosphoric acid in the serum of healthy humans are high and supersaturated with respect to HAp contained in bone. Therefore, it has been reported that low crystalline HAp, similar to natural bone, precipitates in simulated body fluid (SBF) containing calcium, phosphoric acid, etc. in the same concentrations as in healthy human body fluid [21]. In contrast, the phosphate buffer solution used the in vitro experiments in above section was an unsaturated solution because it did not contain calcium ion and was similar to those observed in osteoporotic condition, so the HAp cement matrices were dissolved and disintegrated from the cement surface. To explain quantitatively the gap phenomenon of drug release of HAp bone cement containing the anti-inflammatory drug indomethacin between in vitro and in vivo experiments, drug kinetics method was applied to the plasma drug concentration profiles [20]. The in vivo drug release rate from the HAp bone cement implanted in normal rats was determined based on the plasma drug concentration profiles using the deconvolution method. After implanting the bone cement into the living body, rapid in vivo drug release in the deconvoluted drug release profiles was confirmed during the initial stages; however, it slowed down significantly after 1 week when compared to the in vitro drug release in phosphate buffer after 1 week [20]. Figure 8.9 shows the relationship between the in vitro (in phosphate buffer) and in vivo drug release profiles [20]. In initial drug release stage within a week, the drug released amount of the in vitro drug release was almost the same as that of the in vivo release. However, the drug release profile for a prolonged period, i.e. 1 week or longer, revealed that the in vitro release tend to be significantly higher than that of the in vivo release [20].
Relationship between in vitro and in vivo drug release from the HAp bone cement containing indomethacin. In vitro test was performed in 0.1 M phosphate buffer at pH 6.8, while the in vivo test was performed in healthy rats. ο: 1% drug-loaded cement; •: 2% drug-loaded cement; △: 5% drug-loaded cement
Based on the findings of the in vitro and in vivo drug release from various HAp bone cements, the authors attempted to design an intelligent HAp bone cement that had drug release responsive to osteoporosis pathology. In vitro release test of anti-osteoporosis drug estrogen from the HAp bone cement DDS in SBF with various calcium concentrations showed that the drug release in SBF containing a high concentration of calcium (10 mg/100 mL Ca+) was suppressed by precipitated HAp-like crystals on the surface of the cement (Fig. 8.10) [21]. In contrast, in the SBF solution containing no calcium (0 mg/100 mL Ca+), it was observed that the HAp dissolved and disintegrated on the cement surface, and the volume of pores present within the increased, which accelerated drug release. From these findings, it was confirmed that the plasma calcium concentration-dependent drug release control mechanism of HAp bone cement in rats with osteoporosis was consistent with the results of the in vitro physicochemical experiments.
To assess the therapeutic application for the treatment of osteoporosis, an in vivo anti-osteoporosis drug release test of HAp bone cement DDS was conducted using three groups of rats: rats with osteoporosis pathology, rats recovering from pathology, and healthy rats. As shown in Fig. 8.11, the plasma concentration of calcium in osteoporotic pathological rats was 5 mg/100 mL, and that of healthy rats was 10 mg/100 mL. The plasma concentration of calcium in the recovery model rats changed from 5 mg/100 mL at initial stage to 10 mg/100 mL after 14 days.
Figure 8.12 shows the graph of plasma drug concentration after implanting the HAp bone cements containing anti-osteoporosis drug estradiol in these three groups of rats, including rats with osteoporosis [22]. In vivo estradiol release in osteoporotic rats was significantly accelerated, whereas normal rats were significantly suppressed. In addition, the plasma drug concentration in the recovery rat model was equivalent to that in osteoporotic rats at the initial stage. However, the plasma drug concentration was suppressed to the same level as that in normal rats after 2 weeks. Based on these findings, it was confirmed that the drug release from HAp bone cement DDS was dependent on the plasma concentration of calcium [22].
Results of an in vivo study showed that HAp bone cement was dissolved or disintegrated by the acid secreted by the activated osteoclasts cells under the condition of progressive osteoporosis and decrease in the plasma concentration of calcium, and the drug release was accolated from the cement. Subsequently, it was shown that when the pathological condition improved and plasma concentration of calcium increased, osteoblasts were activated on the surface of bone cement to form new bone and drug release was suppressed. To verify the therapeutic effect of the HAp bone cement DDS containing estradiol, the bone mineral density of the lumbar vertebrae of the osteoporotic rats was examined 22 days after implanting DDS cement. Figure 8.13 shows the radiograms of osteoporotic rats treated with the DDS cement and/or fed a calcium diet, and the result of the bone mineral density evaluated from the radiogram. The bone mineral density of the rat groups treated with both the DDS cement and the calcium diet was significantly higher than that of the other groups, and the implantation of the DDS cement to the osteoporotic rats resulted in higher therapeutic effect to improve the bone mineral density.
8.6 Drug Delivery System for Hard Tissues Based on Self-setting Collagen-HAp Composite Cement
In the biological hard tissue, dense bones and shells of marine creatures are functionally arranged by a complex of HAp and collagen to form a light, strong and supple geometric structure. In addition, the space between bones includes osteoblasts, osteoclasts, and hematopoietic cells, and has various physiological functions. To obtain a bio-absorbable and high-strength bone material that mimics biological hard tissue, an attempt was made to synthesize an organic–inorganic composite material using HAp and collagen. The HAp cement and insoluble type I collagen were mechanochemically treated in a centrifugal ball mill to obtain self-setting collagen HAp bone cement.
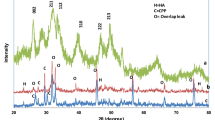
As shown in Fig. 8.14, the main part of the HAp bone cement was transformed into HAp; however, its X-ray diffraction profile showed some small diffraction peaks attributable to original tetra-calcium phosphate, suggesting that the transformation was not perfect [23]. In contrast, X-ray diffraction profile of the collagen HAp bone cement indicated that the cement was almost 100% transformed into HAp. These results suggest the following: the collagen-HAp bone cement, as an organic–inorganic composite, was a functional material that had the ability to self-set and harden when kneaded with phosphoric acid and undergoes biodegradation after implantation in a living body [23]. The collagen-HAp bone cement was transformed on the collagen fiber to HAp with low crystallinity and was similar to natural bone (Fig. 8.14). In addition, the biodegradation rate of the hardened cement matrices could be controlled by changing the amount of collagen to 5–20%. These results showed that the collagen-HAp composite bone cement, which was capable of controlling biodegradation rate and drug delivery rate, could be prepared by mechanochemical treatment [23, 24].
The artificial bone implant for osteoporosis treatment that had a bone proliferative effect was designed based on the collagen-HAp composite bone cement containing phytonadione (VK2) [23], which acted on osteoblasts and had a bone growth effect. After implantation of the bone cement DDS containing VK2 in the back of osteoporotic rats, the mineral mass of the cement devices was measured by radiography. It was observed that the mass of the cement with VK2 was significantly higher than that without (Figs. 8.15 and 8.16). After 22 days of implantation, the bone cements were removed from the osteoporotic rats and stained. Figure 8.17 shows the microphotographs of the tissue cross sections of the implanted bone cement DDS after 22 days. Collagen and new bone were observed inside the collagen-HAp bone cements, with and without VK2. This indicated that bone remodeling had occurred within the implant. However, the implanted cement with VK2 showed a tendency to increase mineral mass, whereas the cement without VK2 showed a tendency to decrease mineral mass. In the former, the bone grew in the cement, but in the latter, the cement was phagocytosed from outside by bone cells.
8.7 Bone Cell Scaffold with Biodegradable Collagen-HAp Composite Cement with Inter-connective-Macro-pores
Conventional bone implant without micro-pore structure, made from sintered HAp, is commonly used as a filler for bone defects in patients. However, the binding of the HAp implants to bone is not sufficient. Therefore, the HAp implant with macro-pores similar to spongy bone was developed as shown in Fig. 8.18. However, their therapeutic score was limited since the pore shape of the implant was similar to an inkbottle, and it was not easy for bone cells and blood vessels to penetrate the implant. In contrast, the biodegradable biomaterial developed and described in the previous section could freely regulate drug release, was an intelligent material to control bone growth. Therefore, it was possible to prepare the material having a function closer to that of living bone using collagen-HAp composite material. Additionally, the preparation of a novel functional material (including living bone cells) having a bone formation function closer to living bone was attempted.
To develop a new artificial bone that solves these problems, biomaterials containing three-dimensional nano-pore-structures with inter-connective-macro-pores were designed, as shown in Fig. 8.18. These biomaterials allow the penetration of bone cells, supporting their activities, and enabling the formation of capillaries that promote nutritional supply to the cells. Three-dimensional cement with macro-pores was prepared using self-setting collagen/HAp cement, and their cross-sections imaged using X-ray CT, as shown in Fig. 8.18 [25]. The collagen-HAp cement having inter-connective-macro-pores and HAp cement without macro-pores were implanted in healthy rats and the in vivo absorption rates were measured and compared using X-ray CT (Fig. 8.19). The collagen-HAp cement with inter-connective-macro-pores was rapidly invaded by osteoclast cells and biodegraded and absorbed by remodeling, as shown in Fig. 8.19 [23, 25]. However, in other cases, bone cells invaded the macro-pores and showed biocompatibility, but their implants were not absorbed. The collagen-HAp cement with macro-pores was shown to be remodeled rapidly by bone cells. The result indicated that if bone growth factors could be released in a sustained manner from the collagen-HAp cement with inter-connective macro-pores bone structure, the cell invasion into the artificial bone could be accelerated and osteoblasts could be activated, thereby enhancing the function of bone formation, and thus it could be used as an ideal cell scaffold. Therefore, to design a biomimetic artificial bone close to the biological hard tissue, it is necessary to equip a drug release control capability from the collagen-HAp cement structure. To control drug release from the collagen- HAp cement with inter-connective macro-pores, the cement with various number of the macro-pores was designed and prepared.
Figure 8.20 shows the effect of the number of macro-pores on the drug release from the collagen-HAp device containing indomethacin [26]. All drug release profiles showed straight lines, with a lag time on the Higuchi plot, and the drug release rates increased as the number of macro-pores increased. It was thus confirmed that the drug release accelerated because the cement surface area increased as the number of macro-pores increased. Therefore, it was shown that by controlling the geometric structure of the HAp cement device, the drug release rate could be appropriately controlled, and cell activity could be activated. Artificial bone implants with inter-connected macro-pores made by the collagen-HAp composite cement containing VK2 (PC) [24] were designed similar to natural hard tissues and were implanted in osteoporotic rats. Figure 8.21 shows the implant mass profiles of the PC, collagen-HAp cement implant (NC) and HAp cement implant (NN), which were evaluated based on their respective radiograms. In the initial 30 days, the implant mass of the PC increased due to the invasion of bone cells into the macro pores, and then the total implant mass decreased after 30 days due to the phagocytosis from the outside of the device. In the NC, after the slight increase in mass due to bone cell invasion, the implant mass decreased. In NN, no increase in mass was observed, and the implant mass gradually decreased due to external phagocytosis.
After the animal experiments, the X-ray diffraction profiles, thermal analytical curves, and infrared absorption spectra of the removed implanted artificial bone were measured. The mass of the implants increased and/or decreased due to new bone-like substance formation by osteoblast-like cells and/or absorption by the osteoclast-like-cells during bone remodeling [27]. As shown here, an artificial bone with inter-connective-macro-pore structure that mimicked the geometric structure of the natural bone was prepared with collagen HAp cement that mimicked the molecular level structure of the bone, and their biocompatibility and bone formation were demonstrated in actual osteoporotic rats. These results indicate that the development of a biomimic artificial bone is possible using such bone cement.
8.8 Gene Delivery System Consisting of Collagen-HAp Based Cement with a Cell-Activity-Dependent Drug Release Mechanism
Various viral vectors have been investigated in gene therapy with high gene expression efficiency. However, problems with safety, such as immunogenicity, have been encountered [28]. Therefore, liposomes and micelles have been widely studied as safe non-viral vectors, instead of viral vectors. Among these, DNA/polycation complexes, using a cationic component that electrostatically adheres to DNA, have been developed with high efficacy and safety [29]. The DNA/polycation complexes have already achieved extremely high gene expression efficiency in some experiments using cultured cells [30]. It was found that by adding hyaluronic acid to the DNA/polycation complex, a ternary complex with excellent dispersibility could be obtained. Furthermore, by concentrating the ternary DNA complex prepared at a low concentration by freeze-drying, a DNA complex nanoparticle concentrated having a diameter of about 70 nm could be obtained [30].
Injectable collagen HAp cement with good biocompatibility has been developed for controlled drug release rate by adjusting the crystallinity and additives [31]. To develop a gene delivery system that could control gene release over a long period of time, stable hyaluronic acid-coated DNA-cation polymer complex nanoparticles [30] were encapsulated in self-setting collagen-HAp cement. The collagen-HAp composite cement, containing the ternary DNA complexes, was cultured with osteoclast-like model cells (MCL-6 cells) and osteoblast-like model cells (B16 melanoma) of the osteoclast model, followed by the measurement of the device weight change and DNA release amount.
Figure 8.22 shows the effect of the type of bone cells on the DNA release pattern from the collagen-HAp composite cement containing the ternary DNA complexes [31]. When the collagen-HAp device containing DNA was cultured with osteoclast-like model cells, it showed significantly higher DNA concentrations than when cultured with osteoblasts model cells. At this stage, based on the result regarding the behavior of osteocyte-like-cells under a microscope, it was observed that the model cells aggregated and adhered on the cement surface and dissolved the surface.
In addition, the HAp cement containing DNA and the collagen-HAp composite cement containing DNA were injected into mice transplanted with solid cancer cells to examine the growth of cancer cells. The mass change profiles of the injected cement device in the mice were measured with an X-ray CT device [31]. Figure 8.23 shows the mass change profile of the cement devices implanted in mice [31]. The biodegradation rate of the HAp cement containing DNA was extremely slow, and even after 12 weeks, the cement mass was 95% or more, resulting in almost no biodegradation. In contrast, the collagen-HAp cement containing DNA showed a cement mass of 60% at 4 weeks and 30% or less at 12 weeks, indicating that it was gradually biodegraded.
Figure 8.24 shows the therapeutic effect after injecting the HAp cement containing DNA complex and the collagen-HAp cement containing DNA complex into mice transplanted with solid cancer cells in the abdomen [31]. When the DNA complex was injected alone, cancer cells proliferated 1 week later. After injecting the HAp cement containing DNA complex, cancer cells proliferated after 3 weeks. In contrast, when the collagen-HAp cement containing DNA complex was injected, the cancer cells hardly proliferated during the 100-day test period. Therefore, it was considered that the growth of cancer cells was effectively suppressed by the continuous release of the injected collagen-HAp cement containing DNA complex at an appropriate DNA concentration.

Reprint with permission [31]
Therapeutic effect of collagen-HAp bone cement containing DNA after implantation in cancer-bearing rat.
8.9 Concluding Remarks
Various bone metabolism-stimulating compounds can be applied to HAp-based artificial bone to control drug release from the device. By activating biological bone metabolism, these devices could contribute to the regeneration of hard tissue that is difficult to regenerate using other means. It was confirmed that the drug released from the HAp cement matrix was dependent on the concentration of calcium in the biological fluid, and it was possible to construct an osteoporotic responsive drug release control system. By controlling the release of various anti-osteoporotic drugs from these devices, it is possible to maintain the homeostasis in the living hard tissue. An artificial bone with inter-connected macro-pores was prepared from collagen HAp composite cement, with high functionality, such as biodegradable character and three-dimensional geometric structure that could retain bone cells. Addition of the controlled release of bone growth factor VK2 to the three-dimensional geometrically designed artificial bones allowed controlled bone formation and bone remodeling, and thereby contribute to hard tissue regenerative medicine. The release of DNA from functional artificial bones could effectively suppress solid cancers in cancer-bearing rats and develop into new biomaterials that could contribute to bone regenerative medicine.
References
Pharmaceuticals and Medical Device Agency (2017) Surgical simplex® Radiopaque bone cement. http://www.info.pmda.go.jp/downfiles/md/PDF/730093/730093_15700BZY01342000_A_C3_04.pdf. Accessed 14 Apr 2017
Webster TJ, Patel AA, Rahaman MN et al (2012) Anti-infective and osteointegration properties of silicon nitride, poly(ether ether ketone), and titanium implants. Acta Biomater 8:4447–4454
Niwa S (2004) Current status and future prospects of biomaterials for artificial bones. Mater Jpn 43:186–192
The Ceramic Society of Japan (2008) Hydroxyapatite artificial bone replacement material. http://www.ceramic.or.jp/museum/contents/pdf/seitai01.pdf. Accessed 24 Apr 2017
Aizawa M, Furuzono T (2012) Recent advance, artificial materials (inorganic materials). Jinkou Zouki 43:207–211
Elzoghby AO (2013) Gelatin-based nanoparticles as drug and gene delivery systems: reviewing three decades of research. J Control Release 172:1075–1091
Kumari A, Yadav SK, Yadav SC (2010) Biodegradable polymeric nanoparticles based drug delivery systems. Colloids Surf B Biointerfaces 75:1–18
Tabata Y (2007) Development of functional drug carriers to realize advanced medical therapy. Yakugaku Zasshi 127:825–837
Chou J, Valenzuela S, Green DW et al (2014) Antibiotic delivery potential of nano- and micro-porous marine structure-derived β-tricalcium phosphate spheres for medical applications. Nanomedicine 9:1131–1139
Yamamura K, Chou G, Iwata H (1991) The development of antibiotics loaded hydroxyapatite beads. Drug Deliv Syst 6:103–108
Brown WE, Chou LC (1986) Combinations of sparingly soluble calcium phosphates in slurries and pastes as mineralizers and cements. US Patent 4,612,053A, 16 Sept 1986
Yu D, Wong J, Matsuda Y et al (1992) Self-setting hydroxyapatite cement: a novel skeletal drug-delivery system for antibiotics. J Pharm Sci 81:529–531
Higuchi T (1963) Mechanism of sustained-action medication. Theoretical analysis of rate of release of solid drugs dispersed in solid matrices. J Pharm Sci 52:1145–1149
Otsuka M, Matsuda Y, Suwa Y et al (1994) A novel skeletal drug delivery system using self-setting calcium phosphate cement. 2. Physicochemical properties and drug release rate of the cement-containing indomethacin. J Pharm Sci 83:611–615
Otsuka M, Nakahigashi Y, Matsuda Y et al (1998) Effect of geometrical cement size on in vitro and in vivo indomethacin release from self-setting apatite cement. J Control Release 52:281–289
Otsuka M, Matsuda Y, Fox JL et al (1995) A novel skeletal drug delivery system using self-setting calcium phosphate cement. 9: effects of the mixing solution volume on anticancer drug release from homogeneous drug-loaded cement. J Pharm Sci 84:733–736
Kawasaki T, Takahashi S, Ikeda K (1985) Hydroxyapatite high-performance liquid chromatography: column performance for proteins. Eur J Biochem 152:361–371
Otsuka M, Matsuda Y, Suwa Y et al (1994) A novel skeletal drug-delivery system using self-setting calcium phosphate cement. 3. Physicochemical properties and drug-release rate of bovine insulin and bovine albumin. J Pharm Sci 83:255–258
Otsuka M, Matsuda Y, Suwa Y et al (1994) A novel skeletal drug-delivery system using self-setting calcium phosphate cement. 4. Effects of the mixing solution volume on the drug-release rate of heterogeneous aspirin-loaded cement. J Pharm Sci 83:259–263
Otsuka M, Nakahigashi Y, Matsuda Y et al (1997) A novel skeletal drug delivery system using self-setting calcium phosphate cement VIII: the relationship between in vitro and in vivo drug release from indomethacin-containing cement. J Control Release 43:115–122
Kim HM, Himeno T, Kokubo T et al (2005) Process and kinetics of bonelike apatite formation on sintered hydroxyapatite in a simulated body fluid. Biomaterials 26:4366–4373
Otsuka M, Yoneoka K, Matsuda Y et al (1999) Effect of plasma-calcium-level-responsive oestradiol release from apatitic bone cement on bone mineral density in ovariectomized rats. J Pharm Pharmacol 51:475–481
Otsuka M, Kuninaga T, Otsuka K et al (2006) Effect of nanostructure on biodegradation behaviors of self-setting apatite/collagen composite cements containing vitamin K2 in rats. J Biomed Mater Res B Appl Biomater 79:176–184
Otsuka M, Nakagawa H, Otsuka K et al (2013) Effect of geometrical structure on the in vivo quality change of a three-dimensionally perforated porous bone cell scaffold made of apatite/collagen composite. J Biomed Mater Res B Appl Biomater 101:338–345
Hamada H, Ohshima H, Ito A et al (2010) Effect of geometrical structure on the biodegradation of a three-dimensionally perforated porous apatite/collagen composite bone cell scaffold. Biol Pharm Bull 33:1228–1232
Otsuka M, Nakagawa H, Ito A et al (2010) Effect of geometrical structure on drug release rate of a three-dimensionally perforated porous apatite/collagen composite cement. J Pharm Sci 99:286–292
Ohshima H, Otsuka M (2016) Stimulus-responsive intelligent drug delivery system based on hydroxyapatite-related materials. In: Ohshima H (ed) Encyclopedia of biocolloid and biointerface science. Wiley, New York, pp 403–411
Taira K, Kataoka K, Niidome T (eds) (2005) Non-viral gene therapy: gene design and delivery. Springer, Tokyo
Morille M, Passirani C, Vonarbourg A et al (2008) Progress in developing cationic vectors for non-viral systemic gene therapy against cancer. Biomaterials 29:3477–3496
Ito T, Otsuka M, Koyama Y (2008) Preparation of fine DNA particles and high-level tumor-targeted in vivo gene expression after intravenous injection. Mol Ther 16:S366. https://doi.org/10.1016/S1525-0016(16)40381-3
Ito T, Koyama Y, Otsuka M (2012) DNA complex-releasing system by injectable self-setting apatite cement. J Gene Med 14:251–261
Acknowledgements
The author would like to thank Professor William I. Higuchi of the University of Utah for his scientific advice and encouragement. This research received partial financial support from the Creating Happiness Incubation Musashino University, Research Center for Biomedical Engineering (No. 2042) and from the Japan Agency for Medical Research and Development (No. JP19mk0101105h0102).
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2022 The Author(s), under exclusive license to Springer Nature Singapore Pte Ltd.
About this chapter
Cite this chapter
Otsuka, M. (2022). Intelligent Drug Delivery System for Artificial Bone Cement Based on Hydroxyapatite-Related Organic/Inorganic Composite Materials. In: Choi, A.H., Ben-Nissan, B. (eds) Innovative Bioceramics in Translational Medicine I. Springer Series in Biomaterials Science and Engineering, vol 17. Springer, Singapore. https://doi.org/10.1007/978-981-16-7435-8_8
Download citation
DOI: https://doi.org/10.1007/978-981-16-7435-8_8
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-16-7434-1
Online ISBN: 978-981-16-7435-8
eBook Packages: Chemistry and Materials ScienceChemistry and Material Science (R0)