Abstract
Neurodegenerative disorders are progressive conditions of the central nervous system (CNS), resulting in increased morbidity and mortality. The gut microbiome is a microecosystem that consists of billions of bacteria and fungi, most of which are of good benefit to the human body’s internal milieu via regulating the immune system and controlling the neuronal signals intertwining the gut and the nervous system. Differences and deficiencies in the composition of the gut microbiota have been noted to occur in many chronic neurodegenerative CNS disorders like Alzheimer’s disease, Parkinson’s disease, frontotemporal dementia, etc. Multiple studies have been done so as to understand the best composition of the gut bacteria, and studies have also been undertaken to understand how we can tweak the gut by introducing probiotics and prebiotic compounds, which benefits the individual in reducing the chances of acquiring a neurodegenerative illness and may also help to control the progression of the diseases in those already afflicted. There are many ways by which the gut microbiome influences the CNS, including immune and hormonal pathways, short-chain fatty acid metabolism, modulation of the gut–brain axis, etc. An imbalance in the microbiome can have massive consequences for the host, resulting in faulty endocrine, immunological, and neuronal signaling that may accelerate the neurodegenerative process, culminating in debilitating diseases. Nutraceutical therapy using probiotics shows immense hope as prophylactic agents or adjunctive treatment strategy in the neurotherapeutics in this regard as it results in the homeostasis of the gut microbiome, which indirectly affects the CNS, resulting in slowing of the neurodegenerative process.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
1 Introduction
The nervous system forms an important organ system in our body owing to its role as the supreme regulatory center in our body homeostasis. Neurodegenerative disorders are specifically characterized by a long-term, progressive loss of neuronal function attributed to an irreversible localized or generalized loss of neurons and their related tissues and neurotransmitters, thereby culminating in decreased and finally their functions. The commonest neurodegenerative disorders that affect different specific parts of the brain like Alzheimer’s disease (AD), Parkinson’s disease (PD), etc. are associated with long-term adverse outcomes and a huge physical and emotional burden to the patients, their families, and caregivers. Neurological disorders form the second leading group of illnesses with respect to mortalities. Neurological diseases are the second leading cause of illnesses contributing to long-term morbidity, as shown by the disability-adjusted life-years (DALYs) as well as the years lived with disability (YLDs) (Collaborators 2019). Among different neurological illnesses, the incidence of neurodegenerative disorders increased worldwide owing to an increase in the longevity of humanity as one of their attributable factors. The chronicity of many of the neuropathologies, the common progressive deteriorating nature of neurological illnesses, and a lack of curative treatment for most of these neurodegenerative disorders add up to the heavy load of neurological disorders on health care and its research.
Alzheimer’s disease (AD) and Parkinson’s disease (PD) are common neurodegenerative diseases. Other common conditions with neurodegeneration include Huntington’s disease (HD), amyotrophic lateral sclerosis (ALS), frontotemporal dementia (FTD), and spinocerebellar ataxias (SCAs). Neurodegenerative changes are also found in other neurological conditions like multiple sclerosis (MS), cerebrovascular accidents (CVAs), or stroke and traumatic brain injury (TBIs). Longevity, advances in medicine and its worldwide increase in access, environmental factors like pollution, pesticide use, change in dietary practices, etc. are a few attributable factors to the steady rise in numbers of patients with neurodegenerative disorders. Recent researches have been emphasizing the relationship between the gut microbiome and brain-health wherein the results clearly point out the altered gut microbiota dynamically altering the physiological milieu and its homeostasis, thus resulting in the pathological conditions of the brain (Chandra et al. 2020). This also emphasizes the beneficial role of prebiotics and probiotics in the prevention and treatment of not only gut disorders but also the neurodegenerative disorders that are typically characterized by their chronicity and lack of therapeutic agents aimed to prevent onset and progression (Peterson 2020).
The enteric nervous system of our gastrointestinal tract has long been considered the “second brain of the human body” owing to its diverse relation with mood regulation, stress, anxiety, eating patterns, etc. There have been obvious associations between neurodevelopmental disorders and gastrointestinal (GI) issues, as seen in autism, Down’s syndrome, cerebral palsy (CP), etc. Hepatic encephalopathy, Parkinson’s disease, irritable bowel syndrome (IBS), and epilepsy are yet other conditions that connect the digestive system with neurological illnesses (Lynch and Pedersen 2016; Wu et al. 2016).
There is an expansion in the knowledge of the role of the gut microbiome, its qualitative and quantitative variations affecting our neurophysiological well-being, and its disruption contributing to various neuropathologies like neurodegenerative disorders, especially on their onset and progression (Ceppa et al. 2020). The microbial coexistence in the human gut is a symbiotic consortium formed by series of changes that happened during its co-evolution with the host and has time and again proven its beneficial role in normal physiology, including Vitamin K and B synthesis, immunoregulation, and protection from pathogens, digestion, and absorption of nutrients. Our complex human body has as many bacteria as that of our body cells, and the revelation that these microorganisms are metabolically active and can potentially alter our neurological dynamics leads to an uproar in the research interest regarding the gut–microbiota–brain axis and the microbial-derived neurochemicals that are implicated in the biological basis of neurodegenerative disorders.
Diet plays a crucial role in setting up our gut microbiome. Prebiotics are those compounds we eat that stimulate and flourish the growth and activity of the microbial flora. Scientists, medical professionals, and the common folk are aware of the presence of microorganisms that are relevant in health and disease. Microbiologists have isolated thousands of bacteria that have varied responses on the human body, sometimes even resulting in disease and death. In the last 100 years, there has been a revolution in new information acquired, which has advanced our understanding of how gut bacteria positively affects the health of the individual.
1.1 Gut Microbiome and the Pre-, Pro-, and Synbiotics
In the early 1800s, during those dark times when death and disease plagued the whole of Europe, it was noted with interest that people in a remote valley in Bulgaria were pretty much unaffected and were in better health. This was largely postulated due to their consumption of fermented milk and yogurt on a daily basis. In 1905, Dr. Stamen Grigorov, a Bulgarian scientist, found the agent causing this fermentation and named it Lactobacillus bulgaricus. Interested in this development, Dr. Ellie Metchnikoff, a Nobel Prize winning scientist from Russia, later did research on L. bulgaricus and discovered that people in Bulgaria lived much longer than other European countries. He attributed this longevity to Bulgaria’s favorite food—yogurt. Yogurt intake resulted in a favorable health advantage conferred to the individual by the gut bacteria, which modified the gut microbiome into one that was less prone to diseases.
Over the years, scientists have come across and categorized products, bacterial and nonbacterial that enhance the gut health of the individual via indirect means. They are probiotics, prebiotics, and synbiotics.
Probiotics are live microbes that are taken in the belief that they have conferred health benefits to the individual. This is mostly by improving or restoring gut flora resulting in favorable metabolic action.
Prebiotics are compounds that ensure that a milieu in the gut is created as such, which results in a thriving growth of good bacteria and fungi, thereby improving the individuals’ health.
Synbiotics is a term used for an appropriate mixture of pre- and probiotics, thereby aiming at a synergistic combination of the two. This results in much more balanced gut microbiota.
1.2 Gut–Brain Axis
Over the centuries of evolution, over 1000 species of bacteria have colonized 400 m2 of the human gut, and the gist of this complex symbiosis is still not fully studied (Ley et al. 2006). There are 1013 to 1014 bacteria in the colon (Bäckhed et al. 2005), and their species mainly comprise Bacteroidetes, Firmicutes, Proteobacteria, Actinobacteria, and Fusobacteria (Eckburg et al. 2005). Research shows, without doubt, a definite connection between the gut and the brain through complex neurohormonal pathways called the gut–brain axis. The digestive system has about 100 million neurons, almost half of that in the central nervous system. This system of gut neurons is called the enteric nervous system (ENS) and in turn is mainly mediated by vagus nerve, the Xth cranial nerve.
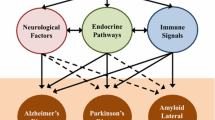
There are three basic means through which the GIT interacts with the central nervous system (CNS), which is via direct neuronal action, chemical neurohormonal mechanisms, and finally the immune system. Multiple studies have pointed out the bidirectional dynamic interaction between the brain and the alimentary canal mediated through conglomerate mechanisms involving the intestinal microflora, autonomic nervous system comprising the parasympathetic and sympathetic neuronal axis, endocrine regulation by neuroendocrinal and enteroendocrine pathways, humoral and cytokine-mediated mechanisms, and via other signaling molecules and neuropeptides (O’Mahony et al. 2015).
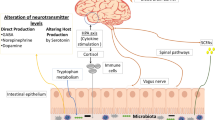
The enteric nervous system (ENS) is quite often considered as our second brain, and many neurotransmitters like serotonin, dopamine, and GABA are produced in the gut. Serotonin or 5-hydroxy tryptamine (5HT) is referred to as a vital neurotransmitter in the brain, and 90% of it is produced in the GIT. Its importance in neuronal development, homeostasis, and sustenance are irrefutable. The neurochemical homeostasis is maintained by a complex interaction between the neurotransmitters like serotonin, dopamine, γ-aminobutyric acid (GABA), and acetylcholine. Currently, there are many medical compounds that target homeostasis between 5HT, DA, GABA, and noradrenaline so as to attain an improvement in neurological status.
Neurodegenerative diseases are a group of incurable and debilitating neurological diseases, resulting in progressive neuronal death and dysfunction. These cause problems in movement and cognition affecting the patient’s ability to move, think, and even breathe. Due to their salutary effects on the gut–brain axis, which has resulted in improved mood, cognition, and general neuronal well-being, pre-, pro-, and synbiotics are being increasingly considered as a therapeutic modality to prevent and treat neurodegenerative disorders.
2 Gut Dysbiosis and the Aging Gut
A vast plethora of bacteria form the gut microbiota that reside within the gastrointestinal tract (GIT). A delicate balance between various microbes is maintained, which is important in the normal functioning of the GIT. There stays a homeostatic balance in maintaining the relative microbial population in the gut and is considered that when this homeostasis is deranged, there occurs an imbalance in the usual feedback loop to CNS or the central nervous system that affects the inflammatory modulation in the brain, an increased oxidative stress, and thus an accelerated neuronal degeneration (Westfall et al. 2017). Dysfunction of the gut microbiome is considered to contribute to depression, anxiety, and neurodegenerative disorders. The gastrointestinal hormones also take part in energy homeostasis mediated via gut-related hormones like insulin and glucagon, taking part primarily in glucose metabolism; leptin, and ghrelin are involved with satiety and hunger, whereas glucagon-like peptide 1 (GLP-1) and glucose-dependent insulinotropic polypeptide (GIP) are involved in growth, repair, and development (Hansotia and Drucker 2005). This highlights the role of insulin, insulin-like peptides, and somatomedins in mitigating neurodegeneration and stresses the GI connection with neurodegenerative disorders.
Aging is associated with alterations in the GI microflora, which culminates in a lack of microbial diversity in the intestines (Hansotia and Drucker 2005). Relative changes in the concentrations of the phyla Bacteroides and Firmicutes are also significant as they form the dominant gut flora (Hansotia and Drucker 2005). Concentrations of pathogenic bacteria would increase at the expense of the symbiotic ones. These changes are known to elicit inflammatory responses that may manifest in sites distant from the GIT, including the CNS.
2.1 Gut Microbiome and Pathophysiology of Neurodegenerative Diseases
Many neurodegenerative disorders occur due to an interaction between genetic predisposition, cellular aging, and environmental toxins. In addition to these, current lifestyle choices, especially dietary habits and sedentary lifestyle, can induce physical and mental stress, thereby worsening oxidative stress and the pathophysiological changes associated with it. Microglial activation is known to be modulated by the gastrointestinal microbiota, which is one of the key factors in the etiopathogenesis of many neurodegenerative disorders. Research suggests that GI milieu modification with short-chain fatty acids (SCFAs) producing bacteria is of beneficial value in improving the gut influence on the brain and neurohormonal and immune activation of the CNS.
Neurodegeneration is the common hallmark in chronically progressive conditions like Alzheimer’s disease (AD) and Parkinson’s disease (PD), which have increased incidence due to longevity. Other neurodegenerative conditions include amyotrophic lateral sclerosis (ALS), frontotemporal dementia (FTD), Huntington’s disease (HD), and spinocerebellar ataxias (SCAs). Though they have varied manifestations, the basic similar pathology in all found is associated with irregularities being noted in increased oxidative stress, protein folding, and reactive oxygen species (ROS) activation, culminating in cellular inflammation, accelerated neuronal aging, and death. The chronicity of many of the neuropathologies, progressively deteriorating the nature of neurodegenerative disorders, and a lack of curative treatment add up to the extensive search and gut microbiome modification, and modulation of the gut–brain axis offers a solid therapeutic potential wherein the early identification and intervention, especially at high-risk cases, can go a long way in prevention and treatment of neurodegenerative diseases (11) (Fig. 5.1).
2.2 Biomolecules in the CNS that Are Targets of Pre/Probiotics
Nonpathogenic gut microbes secrete multiple neurohormonal mediators implicated in neurological homeostasis. Extensive hormonal and immune cascades connecting the GIT and CNS exist, which co-regulate key processes. A well-balanced microbiome may contribute not just toward good GI health but also to neurological well-being. In this chapter, we would like to discuss the factors affecting the gut–brain axis and a few of the many gut microbiome-altering agents, which may in the future find a use for the early prevention and timely intervention in neurodegenerative diseases. An increase in a specific genus of microbes called Proteobacteria spp., a reduction in the numbers of Bifidobacterium spp. and species producing butyrate like Ruminococcus spp., Faecalibacterium spp., etc., and a rise in numbers of the commonly considered pathogenic organisms like Escherichia spp., Enterobacteriaceae spp., Bacteroides, Clostridium difficile, etc. can all lead to an activated immune response (Hansotia and Drucker 2005; Westfall et al. 2017). Hence, it is clearly observed that an abundance in the pathogenic bacteria compared to the symbiotic commensals is commonly found and can also modulate neuroinflammation (Table 5.1).
2.3 Gut-Derived Ferulic Acid
Ferulic acid (FA) is a plant-derived substance found in cereals, vegetables, and fruits. Multiple studies have found this polyphenol, FA, to be a potent inhibitor of ROS pathways and hence has anti-inflammatory properties (Mancuso and Santangelo 2014). FA is well recognized as a free radical scavenger and finds use in various medical conditions, including neurodegenerative conditions. FA can directly stimulate neuronal stem cells, thereby preventing untimely neuronal death, and may also be implicated in neuronal regeneration (Yabe et al. 2010). Current studies have pointed that FA is a key regulator between the activities compassing the commensal gut microbes and the brain (Yu 2011). Apart from the aforementioned natural sources of FA, certain commensal bacteria in the gut, namely L. fermentum and B. animalis, can synthesize FA because they possess the ferulic acid esterase enzyme (Tomaro-Duchesneau et al. 2012). Hence the supplementation of the diet with these probiotics results in achieving a protective level of ferulic acid, thereby protecting against neurological ailments, including chronic debilitating conditions associated with AD, PD, traumatic brain injury, stroke, etc., through its antioxidant and antiapoptotic action (Cheng et al. 2016; Durairajan et al. 2008; Mori et al. 2013; Srinivasan et al. 2007; Yan et al. 2013; Zhang et al. 2015).
2.4 Short-Chain Fatty Acids (SCFAs)
Colonic bacteria produce a good number of major metabolites that exert influence via the gut–brain axis. Soluble fibers such as fructo-oligosaccharides (FOS) and galacto-oligosaccharides (GOS) undergo fermentation, thereby producing a wide variety of various metabolites and have a significant role in the modulation of gut bacteria, thereby exerting an influence on the brain (Kasubuchi et al. 2015). GOS and FOS are prebiotics that provide a favorable milieu for the population of the gut with the beneficial bacteria (den Besten et al. 2013). Other prebiotic SCFAs like acetate (Fushimi et al. 2006), propionate (Todesco et al. 1991), and butyrate (Brahe et al. 2013; Gao et al. 2009) are formed in the gut in ample quantities by fermentation of fiber facilitated by a large number of nonpathogenic bacteria. These commensal bacteria mainly include Bacteroides spp., Bifidobacterium spp., Propionibacterium spp., Eubacterium spp., Lactobacillus spp., Clostridium spp., Roseburia spp., and Prevotella spp. (Tagliabue and Elli 2013; Verbeke et al. 2015; Westfall et al. 2017). The gut microbes belonging to phyla of Firmicutes, particularly of the genera called Roseburia, Eubacterium, etc., and Lachnospiraceae including Clostridia spp. also produce butyrate actively, whereas species like Bifidobacteria generate lactate and acetate (Pérez-Reytor et al. 2021). The role of SCFAs in the well-being of intestinal health has long been known, and further studies have shown light to how they influence the normal physiology of other organ systems.
SCFAs are beneficial to the host by multiple means, including the regulation of metabolic activities. From the gut, it is often transported to the central circulation with specific benefits in lipid, glucose, and cholesterol metabolisms (Pérez-Reytor et al. 2021). They are well-known potent anti-inflammatory agents. SCFAs are considered to affect the synthesis of many neurotransmitters and the expression of receptors to major neurotransmitters like nicotinic and GABA receptors (Lacassagne and Kessler 2000). The SCFAs, propionate, and butyrate are known to be depleted in the vagal nerve (Lal et al. 2001; Sjögren et al. 2002) in the common neurodegenerative disorders like AD and PD (Merrill et al. 2006). Improving SCFA concentration in the gut via means of supplementation of the necessary pre- and probiotics (FOS, GOS, Bifidobacteria, Lactobacilli, etc.) can be a step forward in tackling difficult-to-treat neurodegenerative disorders.
2.5 Microbiota-Modulated Ghrelin
Ghrelin is a peptide molecule produced mainly in the stomach, which stimulates appetite and growth hormone secretion. Ghrelin is an important neurohormone involved in neuromodulation and elicits an important role in metabolism, hunger, energy homeostasis, and neuroinflammation (Merrill et al. 2006). The role of the hunger hormone in neurodegeneration is of great emphasis as it has been shown to exert neuroprotective effects in conditions like Alzheimer’s and Parkinsonism (Merrill et al. 2006). An experimental research study wherein rats were given pre/probiotic agents as nutritional supplementing agents depicts an obvious relationship between the hormone ghrelin and the GI microbiome in rats (Zhang et al. 2009). The ghrelin secreting abilities of gut bacteria give hope that a ghrelin-specific pre/probiotic mixture is of promising therapeutic potential in the future for the treatment of neurodegenerative conditions.
2.6 Serotonin, Tryptophan, and Kynurenine Pathway (KP)
Serotonin is a key neuromodulatory player in the GIT. Its effects include affecting GI secretion, bowel movement or peristalsis, dilation of blood vessels or vasodilation, and perception of pain and nausea. It exerts these multiple effects via a large variety of 5-HT receptors. Tryptophan absorbed from the GIT enters systemic circulation, then crosses the blood–brain barrier (BBB), and then initiates the production of serotonin, thereby demonstrating its action in the CNS. This emphasizes the importance of intestinal metabolism of the amino acid tryptophan and its importance for the serotonin-mediated signaling pathways in the brain (Zhang et al. 2009). It is via the KP that catabolism of tryptophan takes place. Irregularities involving the serotonin and tryptophan metabolism influence the incidence of neurodegenerative diseases like Alzheimer’s disease, memory disturbances, dementia, Huntington’s disease, etc. (Fukumoto et al. 2003; Verbeke et al. 2015).
The type of commensal bacteria in the GI microbiome influences the metabolism of tryptophan and thereby elicits a significant effect of the KP (Reigstad et al. 2015). A few experimental studies done using rats demonstrated that administration of B. infantis reduced 5-HIAA (5-hydroxy indole acetic acid) levels (Desbonnet et al. 2008). 5-HIAA is an important product of serotonin metabolism. It is also regarded as a reliable marker that is found abundant in the frontal cortex along with increased plasma tryptophan levels too (Westfall et al. 2017; Xu et al. 2018; Yahfoufi et al. 2020). When 90% of 5HT is produced in the GIT, the influence of the gut microbiota on its breakdown seems highly relevant. Hence, it stands to reason that administering probiotics as part of the treatment strategy is beneficial in regulating KP dynamics and would be of major benefits when given as part of the neurotherapeutics as prophylaxis and to mitigate the progression in patients with neurodegenerative disorders (Thomas et al. 2012).
3 Neurometabolites and the GIT
Neurometabolites are compounds that include “neurotransmitters that act directly on CNS signaling cascades and through other biochemical effectors that have direct or indirect implications on CNS activities” (Westfall et al. 2017). Deficiencies of neurotransmitters have historically been noted to result in neurodegenerative disorders. Nonpathogenic commensals like Lactobacillus and Bifidobacterium strains produce significant amounts of GABA, which is an inhibitory neurotransmitter. Alterations in neurotransmitter levels are found to be associated with behavioral changes and can also manifest as an increase in dystonia and spontaneous motor activity due to increased levels of neurotransmitters like dopamine (DA), noradrenaline (NA), and serotonin in the striatum of the basal ganglia, which may be beneficial in neurodegenerative movement disorders like PD. Various probiotics have been shown to directly secrete neurotransmitters into the bloodstream, which crosses the BBB and increases the neurotransmitter concentration in the synaptic clefts and vesicles (Barrett et al. 2012).
4 Conclusion
The gut microbiome is a very important part of the gut–brain axis, which modulates the functional interaction between these two very important organ systems. An imbalance in the microbiome can have far-reaching consequences resulting in faulty endocrine, immunological, and neuronal signaling that may accelerate the age-dependent neurodegenerative process, which can culminate in debilitating diseases. Biological therapy using probiotics shows immense potential as therapeutic or prophylactic agents against neurodegenerative disease as it results in the homeostasis of the gut microbiome, which indirectly affects the CNS, resulting in slowing of the neurodegenerative process. However, presently, our knowledge of gut bacteria and the CNS is still in its infancy. Larger studies incorporating subjects suffering from neurodegenerative diseases and the effect of pre/pro/synbiotics in them should be taken up so that new avenues can open up in the treatment of these prognostically bleak diseases.
Abbreviations
- 5-HIAA:
-
5-Hydroxy indole acetic acid
- 5-HT:
-
5-hydroxytryptamine
- Ach:
-
Acetyl choline
- AD:
-
Alzheimer’s disease
- BBB:
-
Blood–brain barrier
- CNS:
-
Central nervous system
- ENS:
-
Enteric nervous system
- GABA:
-
γ-Aminobutyric acid
- GIT:
-
Gastrointestinal tract
- KP :
-
Kynurenine pathways
- PD:
-
Parkinson’s disease
- SCFAs:
-
Short-chain fatty acids
- Spp.:
-
Species
References
Bäckhed F, Ley RE, Sonnenburg JL, Peterson DA, Gordon JI (2005) Host-bacterial mutualism in the human intestine. Science (New York, N.Y.) 307(5717):1915–1920. https://doi.org/10.1126/science.1104816
Barrett E, Ross RP, O’Toole PW, Fitzgerald GF, Stanton C (2012) γ-Aminobutyric acid production by culturable bacteria from the human intestine. J Appl Microbiol 113(2):411–417. https://doi.org/10.1111/j.1365-2672.2012.05344.x
Brahe LK, Astrup A, Larsen LH (2013) Is butyrate the link between diet, intestinal microbiota and obesity-related metabolic diseases? Obes Rev 14(12):950–959. https://doi.org/10.1111/obr.12068
Ceppa FA, Izzo L, Sardelli L, Raimondi I (2020) Human gut-microbiota interaction in neurodegenerative disorders and current engineered tools for its modeling. Front Cell Infect Microbiol 10:297. https://doi.org/10.3389/fcimb.2020.00297
Chandra S, Alam MT, Dey J, Sasidharan BCP, Ray U, Srivastava AK, Gandhi S, Tripathi PP (2020) Healthy gut, healthy brain: the gut microbiome in neurodegenerative disorders. Curr Top Med Chem 20(13):1142–1153. https://doi.org/10.2174/1568026620666200413091101
Cheng C-Y, Tang N-Y, Kao S-T, Hsieh C-L (2016) Ferulic acid administered at various time points protects against cerebral infarction by activating p38 MAPK/p90RSK/CREB/Bcl-2 anti-apoptotic signaling in the subacute phase of cerebral ischemia-reperfusion injury in rats. PLoS One 11(5):e0155748. https://doi.org/10.1371/journal.pone.0155748
den Besten G, van Eunen K, Groen AK, Venema K, Reijngoud D-J, Bakker BM (2013) The role of short-chain fatty acids in the interplay between diet, gut microbiota, and host energy metabolism. J Lipid Res 54(9):2325–2340. https://doi.org/10.1194/jlr.R036012
Desbonnet L, Garrett L, Clarke G, Bienenstock J, Dinan TG (2008) The probiotic Bifidobacteria infantis: an assessment of potential antidepressant properties in the rat. J Psychiatr Res 43(2):164–174. https://doi.org/10.1016/j.jpsychires.2008.03.009
Durairajan SSK, Yuan Q, Xie L, Chan W-S, Kum W-F, Koo I, Liu C, Song Y, Huang J-D, Klein WL, Li M (2008) Salvianolic acid B inhibits Abeta fibril formation and disaggregates preformed fibrils and protects against Abeta-induced cytotoxicty. Neurochem Int 52(4–5):741–750. https://doi.org/10.1016/j.neuint.2007.09.006
Eckburg PB, Bik EM, Bernstein CN, Purdom E, Dethlefsen L, Sargent M, Gill SR, Nelson KE, Relman DA (2005) Diversity of the human intestinal microbial flora. Science (New York, N.Y.) 308(5728):1635–1638. https://doi.org/10.1126/science.1110591
Fukumoto S, Tatewaki M, Yamada T, Fujimiya M, Mantyh C, Voss M, Eubanks S, Harris M, Pappas TN, Takahashi T (2003) Short-chain fatty cids stimulate colonic transit via intraluminal 5-HT release in rats. Am J Physiol Regul Integr Comp Physiol 284(5):R1269–R1276. https://doi.org/10.1152/ajpregu.00442.2002
Fushimi T, Suruga K, Oshima Y, Fukiharu M, Tsukamoto Y, Goda T (2006) Dietary acetic acid reduces serum cholesterol and triacylglycerols in rats fed a cholesterol-rich diet. Br J Nutr 95(5):916–924. https://doi.org/10.1079/bjn20061740
Gao Z, Yin J, Zhang J, Ward RE, Martin RJ, Lefevre M, Cefalu WT, Ye J (2009) Butyrate improves insulin sensitivity and increases energy expenditure in mice. Diabetes 58(7):1509–1517. https://doi.org/10.2337/db08-1637
GBD 2016 Neurology Collaborators (2019) Global, regional, and national burden of neurological disorders, 1990–2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol 18(5):459–480. https://doi.org/10.1016/S1474-4422(18)30499-X
Hansotia T, Drucker DJ (2005) GIP and GLP-1 as incretin hormones: lessons from single and double incretin receptor knockout mice. Regul Pept 128(2):125–134. https://doi.org/10.1016/j.regpep.2004.07.019
Kasubuchi M, Hasegawa S, Hiramatsu T, Ichimura A, Kimura I (2015) Dietary gut microbial metabolites, short-chain fatty acids, and host metabolic regulation. Nutrients 7(4):2839–2849. https://doi.org/10.3390/nu7042839
Lacassagne O, Kessler JP (2000) Cellular and subcellular distribution of the amino-3-hydroxy-5-methyl-4-isoxazole propionate receptor subunit GluR2 in the rat dorsal vagal complex. Neuroscience 99(3):557–563. https://doi.org/10.1016/s0306-4522(00)00204-9
Lal S, Kirkup AJ, Brunsden AM, Thompson DG, Grundy D (2001) Vagal afferent responses to fatty acids of different chain length in the rat. Am J Physiol Gastrointest Liver Physiol 281(4):G907–G915. https://doi.org/10.1152/ajpgi.2001.281.4.G907
Ley RE, Peterson DA, Gordon JI (2006) Ecological and evolutionary forces shaping microbial diversity in the human intestine. Cell 124(4):837–848. https://doi.org/10.1016/j.cell.2006.02.017
Lynch SV, Pedersen O (2016) The human intestinal microbiome in health and disease. N Engl J Med 375(24):2369–2379. https://doi.org/10.1056/NEJMra1600266
Mancuso C, Santangelo R (2014) Ferulic acid: pharmacological and toxicological aspects. Food Chem Toxicol 65:185–195. https://doi.org/10.1016/j.fct.2013.12.024
Merrill CA, Jonsson MAG, Minthon L, Ejnell H, C-son Silander H, Blennow K, Karlsson M, Nordlund A, Rolstad S, Warkentin S, Ben-Menachem E, Sjögren MJC (2006) Vagus nerve stimulation in patients with Alzheimer’s disease: additional follow-up results of a pilot study through 1 year. J Clin Psychiatry 67(8):1171–1178. https://doi.org/10.4088/jcp.v67n0801
Mori T, Koyama N, Guillot-Sestier M-V, Tan J, Town T (2013) Ferulic acid is a nutraceutical β-secretase modulator that improves behavioral impairment and alzheimer-like pathology in transgenic mice. PLoS One 8(2):e55774. https://doi.org/10.1371/journal.pone.0055774
O’Mahony SM, Clarke G, Borre YE, Dinan TG, Cryan JF (2015) Serotonin, tryptophan metabolism and the brain-gut-microbiome axis. Behav Brain Res 277:32–48. https://doi.org/10.1016/j.bbr.2014.07.027
Pérez-Reytor D, Puebla C, Karahanian E, García K (2021) Use of short-chain fatty acids for the recovery of the intestinal epithelial barrier affected by bacterial toxins. In: Frontiers in physiology, vol 12, p 721. https://doi.org/10.3389/fphys.2021.650313
Peterson CT (2020) Dysfunction of the microbiota-gut-brain Axis in neurodegenerative disease: the promise of therapeutic modulation with prebiotics, medicinal herbs, probiotics, and Synbiotics. J Evid-Based Integr Med 25:1–19. https://doi.org/10.1177/2515690X20957225
Reigstad CS, Salmonson CE, Rainey JF 3rd, Szurszewski JH, Linden DR, Sonnenburg JL, Farrugia G, Kashyap PC (2015) Gut microbes promote colonic serotonin production through an effect of short-chain fatty acids on enterochromaffin cells. FASEB J 29(4):1395–1403. https://doi.org/10.1096/fj.14-259598
Sjögren MJC, Hellström PTO, Jonsson MAG, Runnerstam M, Silander HC-S, Ben-Menachem E (2002) Cognition-enhancing effect of vagus nerve stimulation in patients with Alzheimer’s disease: a pilot study. J Clin Psychiatry 63(11):972–980. https://doi.org/10.4088/jcp.v63n1103
Srinivasan M, Sudheer AR, Menon VP (2007) Ferulic acid: therapeutic potential through its antioxidant property. J Clin Biochem Nutr 40(2):92–100. https://doi.org/10.3164/jcbn.40.92
Tagliabue A, Elli M (2013) The role of gut microbiota in human obesity: recent findings and future perspectives. Nutr Metab Cardiovasc Dis 23(3):160–168. https://doi.org/10.1016/j.numecd.2012.09.002
Thomas CM, Hong T, van Pijkeren JP, Hemarajata P, Trinh DV, Hu W, Britton RA, Kalkum M, Versalovic J (2012) Histamine derived from probiotic lactobacillus reuteri suppresses TNF via modulation of PKA and ERK signaling. PLoS One 7(2):e31951. https://doi.org/10.1371/journal.pone.0031951
Todesco T, Rao AV, Bosello O, Jenkins DJ (1991) Propionate lowers blood glucose and alters lipid metabolism in healthy subjects. Am J Clin Nutr 54(5):860–865. https://doi.org/10.1093/ajcn/54.5.860
Tomaro-Duchesneau C, Saha S, Malhotra M, Coussa-Charley M, Kahouli I, Jones ML, Labbé A, Prakash S (2012) Probiotic Ferulic acid esterase active lactobacillus fermentum NCIMB 5221 APA microcapsules for Oral delivery: preparation and in vitro characterization. Pharmaceuticals (Basel, Switzerland) 5(2):236–248. https://doi.org/10.3390/ph5020236
Verbeke KA, Boobis AR, Chiodini A, Edwards CA, Franck A, Kleerebezem M, Nauta A, Raes J, van Tol EAF, Tuohy KM (2015) Towards microbial fermentation metabolites as markers for health benefits of prebiotics. Nutr Res Rev 28(1):42–66. https://doi.org/10.1017/S0954422415000037
Westfall S, Lomis N, Kahouli I, Dia SY, Singh SP, Prakash S (2017) Microbiome, probiotics and neurodegenerative diseases: deciphering the gut brain axis. Cell Mol Life Sci 74(20):3769–3787. https://doi.org/10.1007/s00018-017-2550-9
Wu J, Zhang Y, Yang H, Rao Y, Miao J, Lu X (2016) Intestinal microbiota as an alternative therapeutic target for epilepsy. Can J Infect Dis Med Microbiol 2016:9032809. https://doi.org/10.1155/2016/9032809
Xu J, Zhang X, Qian Q, Wang Y, Dong H, Li N, Qian Y, Jin W (2018) Histamine upregulates the expression of histamine receptors and increases the neuroprotective effect of astrocytes. J Neuroinflam 15(1):41. https://doi.org/10.1186/s12974-018-1068-x
Yabe T, Hirahara H, Harada N, Ito N, Nagai T, Sanagi T, Yamada H (2010) Ferulic acid induces neural progenitor cell proliferation in vitro and in vivo. Neuroscience 165(2):515–524. https://doi.org/10.1016/j.neuroscience.2009.10.023
Yahfoufi N, Matar C, Ismail N (2020) Special issue : the gut microbiome and aging adolescence and aging : impact of adolescence inflammatory stress and microbiota alterations on brain development. Aging Neurodegen 75(7):1251–1257. https://doi.org/10.1093/gerona/glaa006
Yan J-J, Jung J-S, Kim T-K, Hasan A, Hong C-W, Nam J-S, Song D-K (2013) Protective effects of ferulic acid in amyloid precursor protein plus presenilin-1 transgenic mouse model of Alzheimer disease. Biol Pharm Bull 36(1):140–143. https://doi.org/10.1248/bpb.b12-00798
Yu L (2011) Neurogenesis-enhancing effect of sodium ferulate and its role in repair following stress-induced neuronal damage. World J Neurosci 01:9–18. https://doi.org/10.4236/wjns.2011.12002
Zhang M, Poplawski M, Yen K, Cheng H, Bloss E, Zhu X, Patel H, Mobbs CV (2009) Role of CBP and SATB-1 in aging, dietary restriction, and insulin-like signaling. PLoS Biol 7(11):e1000245. https://doi.org/10.1371/journal.pbio.1000245
Zhang L, Wang H, Wang T, Jiang N, Yu P, Chong Y, Fu F (2015) Ferulic acid ameliorates nerve injury induced by cerebral ischemia in rats. Exp Ther Med 9(3):972–976. https://doi.org/10.3892/etm.2014.2157
Author information
Authors and Affiliations
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2022 The Author(s), under exclusive license to Springer Nature Singapore Pte Ltd.
About this chapter
Cite this chapter
Prabhakar, P., Punnaveetil, S. (2022). Role of the Gut Microbiome and Its Modulation in Neurodegenerative Diseases. In: Elumalai, P., Lakshmi, S. (eds) Functional Foods and Therapeutic Strategies for Neurodegenerative Disorders. Springer, Singapore. https://doi.org/10.1007/978-981-16-6703-9_5
Download citation
DOI: https://doi.org/10.1007/978-981-16-6703-9_5
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-16-6702-2
Online ISBN: 978-981-16-6703-9
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)