Abstract
Increasing research evidence cites that the gut microbiota and its composition in the gastrointestinal tract greatly influence the host’s physiological and neuronal functions, immune and circulatory systems. A bidirectional communication or cross-talk comprising the microbiome and host exists between the brain and intestinal microbiota called microbiota-gut-brain (MGB) axis and neurological diseases have been thought to progress via the same. It serves various functions such as the production of neurons and neurotransmitters, maintenance of neuroendocrine system and also controls our response to stress and memorization. Any dysfunction in the axis impacts an individual’s behavior and disease pathogenesis eventually affecting the central nervous system. Pre-clinical and clinical data of microbiota-directed therapies using probiotics which are non-pathogenic live microorganisms, showed a significant association of gut microbiome dysbiosis and its increasing potential on the development of mental disorders in a host. Animal model studies have elucidated the significance of MGB axis though in humans, more substantial evidence is required. This chapter primarily outlines the associated benefits of the gut-microbiota axis and how a dysbiosis affecting the same is capable of triggering neurological disorders, often causing a defective development in brain function and molecular mechanisms of the gut-brain axis. A paradigmatic shift with the focus on microbiota-targeted therapy involving the modification of an individual’s gut microbiome with the aid of probiotics offers promising future prospects.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
10.1 Introduction
The gastrointestinal (GI) tract’s surface epithelium covers 32 m2 and stretches over 5 m in length (Helander and Fändriks 2014), anatomically GI organs are mesentery sectioned with each organ being composed of the mucosa, submucosa, muscular layer, and serosa. A pathogenic microorganism is an infectious, disease-causing agent which in the case of neurological disorders, negatively impacts the functioning of brain inducing both neurological and mental defects. Infectious diseases like HIV (caused by human immuno-deficiency virus) and Lyme disease (caused by Borrelia burgdorferi) can cause serious psychiatric and neurodegenerative effects on the host. Also in late stages of the parasitic infection, brain toxoplasmosis (triggered by Toxoplasma gondii) has indicated a possible link leading to a suicidal nature (Coccaro et al. 2016). These expressions of neurological infectious diseases are caused by the direct hindrance of neurotransmitter signaling by pathogens. Characterization of the gut microbiome has revolutionized our perception of gastrointestinal and metabolic processes. All bacteria are not pathogenic invaders, instead may have a potential function in maintaining immunity and homeostasis, this notion has impacted a major shift in neuroscience and neuropsychiatric research. The gastrointestinal (GI) microbiome is a distinctive assortment of commensal microorganisms inhabiting in diverse niches in the GI tract which substantially help both in the advancement and improvement of psychiatric and neurological disorders.
Probiotic microorganisms belong to diverse groups and play multiple roles for example short-chain fatty acid (SCFA) production, ferment undigested carbohydrates, synthesize vitamins and metabolites (Quigley 2013). Gestational age, delivery mode, diet, and antibiotic exposure regulate the influence the composition in gastrointestinal colonization by microorganisms (Fouhy et al. 2012). The important potential phyla present in human gut are Bacteroides and firmicutes (Bäckhed 2011). The other beneficial and opportunistic bacterial gut flora including Lactobacillus sp., Bifidobacterium sp., Propionobacteria, Enterococci, Peptostreptococci, etc. are a beneficial group but those such as Actinobacteria, Bacteroides sp., Clostridia, Peptococci, Enterobacteria, Streptococci, Staphylococci, and yeasts are of the opportunistic group (Joshi et al. 2018). The MGB axis is referred to as a bidirectional system of communication by the gut microbiome with the central nervous system (CNS) sending signals to the brain and vice versa (Fung et al. 2017). Behavioral factors like stress accelerate the corticotrophin-releasing hormone production in the hypothalamus, further activating the hypothalamic–pituitary–adrenal (HPA) axis (Belmaker and Agam 2008). The synthesis of neuroactive metabolic molecules by gut microbiota modulates the pathogenesis of several neurological diseases such as amyotrophic lateral sclerosis, Parkinson’s disease, multiple sclerosis, etc. (Girolamo et al. 2017). This chapter focuses on the cross-talk-mediated signaling of gut microbiota to the brain that impacts its functional development, summarizes the involvement of the gut-intestinal microbiota in the progression of neuropsychiatric disorders while making remarks on the potential therapeutic benefits of probiotics particularly in their actions against neurological disorders.
10.2 Microbiome-Gut-Brain Axis: A Bi-directional Communication System
10.2.1 Role and Developmental Role and Mechanism of Action of Gut-Brain Axis
The early life microbial colonization in an individual right after birth proceeds through a rapid development of unique site-specific microbial niches, and change in the microbiota composition from primitive in earlier life to mature form (Dominguez-Bello et al. 2016). “Barker Hypothesis” (1993) stated that the development of a fetus and its sensitivity to neurological and metabolic diseases is substantially affected by its intrauterine habitat in life. The GI tract instantaneously develops and facilitates for the colonization of microbiota. The pathogen Campylobacter jejuni stimulates behavioral abnormalities in early life, decreased motor function and increased anxiety (Forsythe et al. 2010). DNA methylation due to prenatal stress affects functional advancement of the HPA axis leading to hypersensitivity and glucocorticoids hypersecretion also decreased the binding capacity of the hippocampal glucocorticoid receptor (Murgatroyd et al. 2009). The cholinergic signaling synchronized with the HPA axis blocking nicotinic and muscarinic receptors causes the defect in barrier function which prevents an increase in macromolecular permeability (Gareau et al. 2007). Development in the womb is initially established and continued after the birth period but the emotions and storage of memories are controlled by the limbic system.
The process of new neuron formation known as neurogenesis takes place in specific regions of the brain throughout life (Fig. 10.1). Neurogenesis and memory endurance level have a complementary relationship between plasticity and the ability to encompass new information without deteriorating stored knowledge (Zhao et al. 2008). Microbiota communicates with CNS through neuronal pathways of the vagus nerve belonging to the parasympathetic division of ANS which regulates various functions such as gut motility, heart rate, and constriction of bronchi (Forsythe et al. 2010). Probiotic Lactobacillus rhamnosus sp. induces alteration in expression of GABA receptors in the brain resulting in reduced corticosterone and in an anxiolytic effect through signaling from the vagus nerve (Bravo et al. 2011). Probiotic mixture comprising B. animalis subsp. and Propionibacterium jensenii restored the imbalance in gut microbiota and attenuated activation of neonatal stress pathways in HPA axis. Presence of fatty acid composition also influences neurophysiologic conditions (Forsythe et al. 2010) which was observed in the immune conditions of stressed animals while experiencing neonatal maternal separation (Wall et al. 2012).
The development of brain and gut microbiome. During the prenatal stage brain and gut microbiota begins, in first 3 years is critical developmental stage. Disturbance in development can impact communication between these systems and it can also facilitate pathogenesis of neural development disorders for instance autism, IBS, attention-deficit hyperactivity disorders (ADHD), anxiety, and obesity (Ogbonnaya et al. 2015).
Antibiotics fundamentally show detrimental effects on neurodegenerative and neurodevelopment diseases disrupting gut microbiota involved in neuro-modulatory signaling. SCFAs derived from microbiota have an essential role in the functioning and promotion of microglial maturation (Barouei et al. 2012). When the levels of neurogenesis are low after birth, memories become more resistant to remodeling and a rise in the stability of memories dependent on the hippocampus (Erny et al. 2015). Decrease in neurogenesis due to cortex proliferative subventricular zone and microglia phagocytose neural precursor cells (NPC) arising a change in neural development (Akers et al. 2014) impacting memory formation and cognitive function.
These microglial cells are involved in CNS development at an early stage, and also in phagocytosis, antigen presentation, and regulation of inflammation during their lifetime. The amalgamation of bromodeoxyuridine (BrdU) in the hippocampus accentuated neurogenesis in adult germ-free mice (Cunningham et al. 2013).
The increased volume and modified morphology of dendrites indicated that, for amygdala and hippocampus, normal morphology and ultrastructure requirement of microbiota is significant (Borre et al. 2014). Probiotic Bifidobacterium longum and Lactobacillus helveticus combination can prevent a decrease in neurogenesis in the hippocampus region when influenced by stress (Luczynski et al. 2016). Astrocytes are various operational groups of glial cells, having functions such as neurotransmitter clearance, ion homeostasis, glycogen storage, maintenance of blood–brain barrier, and neuronal signaling additionally to their distinguished neuroinflammatory function (Ait-Belgnaoui et al. 2014). Toll-like receptor (TLR) signaling controls hippocampal neurogenesis in mice signifying the potential regulatory role of microbial components in neurogenesis. TLR4 KO mice demonstrated the formation of spatial reference memory and fear of learning and enhanced neurogenesis (Okun et al. 2012), whereas TLR2 inadequacy diminished both hippocampal volume and neurogenesis (Rolls et al. 2007).
Prenatal oral administration of probiotics to pregnant maternal indicated that lactation normalized high glucocorticoid (cortisol) secretion and restored corticotrophin-releasing hormone (CRH); for example, oral intake of genetically modified Enterococcus faecium to pregnant mice indicated offspring possessing the specific bacterial species in meconium and amniotic fluid (Jiménez et al. 2008) confirming that maternal microbial transmission in mammals is possible and maternal tryptophan along with serotonin (5-HT) neurotransmitter hormone is essential for neurodevelopment conversion led by the placenta that influences fetal brain development. Serotonin [5-hydroxytryptamine (5-HT)] is a biogenic amine neurotransmitter in the CNS and in gut where synthesis depends on the availability of its precursor, and tryptophan synthesis maintains cognitive activity and also regulates gastrointestinal secretion (Costedio et al. 2007). Tryptophan metabolism and availability depend on enteric microbiota and consequently impact the central serotonin concentrations as well as kynurenine, whereas the activity of tryptophan and indoleamine-2,3-dioxygenase enzymes induced altered enzyme activity in irritable bowel syndrome (IBS) (Schwarcz et al. 2012) and downstream neuroactive metabolites in the brain. The probiotic Bifidobacterium infantis sp. affects tryptophan metabolism along this pathway (Desbonnet et al. 2010). The gut communicates through hormonal signaling pathways to the brain resulting in the release of peptides in gut from entero-endocrine cells (such as orexin, ghrelin, and leptin), circadian pattern, sexual behavior, and anxiety (Cameron and Doucet 2007). Saresella M and colleagues (2017) (Saresella et al. 2017) reported that on MS patients where data supported the chance that diet would probably be utilized as a tool to regulate the immune system in anti-inflammatory way as a significance of changes in the gut microbiota.
Present results of this trial study show that a skewing of the constitution of the microbiota characterized by the plenty of Lachnospiraceae family, a diminish of IL-17-producing T CD4+ lymphocytes and PD-1 expressing T CD4+ lymphocytes, and an enhance of PD-L1 stating monocytes was considered in those individuals following a HV/LP diet. In these same patients, positive correlations between Lachnospiraceae and anti-inflammatory IL-10- and TGFβ-producing CD14+ monocytes, as well as between Lachnospiraceae and CD4+/CD25+/FoxP3+ T-reg lymphocytes were also observed. The concurrent development during initial postnatal life of the microbiota, gastrointestinal tract maturation, and neurogenesis of the hippocampus simultaneously forms the MGB axis. Identification of pathways and mechanisms of this complex interconnectedness has substantial therapeutic effects for several diseases.
10.2.2 Effects of Human Microbiome and Probiotics on ENS, ANS, and CNS
10.2.2.1 Effect of Human Microbiome and Probiotics on ENS
The enteric nervous system (ENS) or the “second brain” is a part of the peripheral and autonomic nervous system which tends to control GI tract functioning (Turner 2009). It is located within the GI tract wall, hence it remains protected from the intestinal luminal contents, and is composed of ganglia and millions of neurons. It serves several diverse functions such as the secretions from the gut and pancreas, reflexes, blood flow, gastrointestinal motility and physiology, GI-endocrine modulation, and protective reactions (Yan and Polk 2011). The gut microbiome helps in the overall development and maintenance of the intestinal barrier, prevents pathogenic production of emetic toxins, and also protects the intestinal sensory nerves from pathogenic invasion (Borthakur et al. 2008; Kamm et al. 2004). The excitatory irregularity of the ENS often caused as a result of gut dysbiosis has also been considerably reduced with the aid of probiotics (Bercik et al. 2011).
10.2.2.2 Effects of Human Microbiome and Probiotics on ANS
The autonomic nervous system (ANS) comprises the sympathetic and parasympathetic systems and is also composed of both motor and sensory neurons that transverse between the various internal organs and the central nervous system (CNS) (Azpiroz 2005). The sympathetic and parasympathetic systems together constitute the autonomic nervous system (ANS) which is also composed of both motor and sensory neurons that transverse between the various internal organs and the central nervous system (CNS) (Azpiroz 2005). The sympathetic nervous system prepares and aids the body in triggering the fight or flight response and subsequent reflexes, whereas the parasympathetic system on the other hand aids in returning the body functions adjusted by the former, from the excited/activated state to its normal stature (O’Mahony et al. 2011). Both prebiotics and probiotics regulate the generation of pro-inflammatory cytokines, thereby sustaining the intestinal barrier, promoting the ideal functioning of the brain-gut axis (although the supporting evidence is still under scrutiny), and exerting anti-inflammatory effects when administered in combination with certain fatty acid supplements (Desbonnet et al. 2010; Clarke et al. 2010; Wall et al. 2010).
10.2.2.3 Effects of Human Microbiome and Probiotics on Central Nervous System
The central nervous system (CNS) is majorly composed of the brain and spinal cord. It has a network of millions of neurons that transverse the comprehensive length of the body (Neufeld et al. 2011). One of the best examples for a CNS output includes human behavior and few studies have indicated the possible correlation between an individual’s neurochemical characteristics with that of his/her behavioral constitution (Heijtz et al. 2011). The crucial role of the gut microbiome on an individual’s CNS and corresponding mental status is already quite evident from several studies based on animal and human models (Galland 2014). The administration of probiotics has not only been found to positively impact the functioning of the human brain but has also shown to modify its neurochemistry both directly and indirectly. It has also markedly helped to decrease anxiety and depression with prolonged treatment (Bravo et al. 2011).
The mechanisms involved in the effect of probiotics on CNS include (Sharma and Kaur 2020):
-
(a)
Restoration of the functioning of the hypothalamic-pituitary-adrenal (HPA) axis that plays an essential role in our response to stress (Varghese and Brown 2001). Indirectly, it can act as a mediator in a number of neurological diseases such as anxiety, depression, IBS, etc. (Smith and Vale 2006). Increased activity of the HPA may be induced in conditions of prolonged stress and anxiety as reported in patients suffering from bipolar or depression associated disorders (Guilliams and Edwards 2010). Probiotic interventions that focus on the normalization of the HPA axis in patients experiencing psychotic disorders via regulating the levels of cortisol (CORT) or adrenocorticotropic hormone (ACTH) have given encouraging results (Savignac et al. 2015).
-
(b)
Regulation and production of brain-derived neurotrophic factor (BDNF) and SCFAs; neuronal factors such as BDNF are mostly proteins that aid in controlling the functioning of neurons including their differentiation, integrity, and survival (Edelmann et al. 2014). Neurodegenerative diseases are frequently accompanied by an impaired or abnormal expression of neurotrophic factors (Rao et al. 2007). A study by Ranuh et al. (2019)) demonstrated the direct linkage between the increased BDNF levels in the brain after probiotic administration and their corresponding stimulation of the gut-brain axis. When it comes to SCFAs, their increased production by the gut microbiome was shown to alleviate the oxidative stress induced during neurological disorders (Hamer et al. 2008).
-
(c)
Proto-oncogene activation and expression are likely to be enhanced in patients suffering from psychiatric disorders in particular Alzheimer’s disease and dementia (Lu et al. 1998). According to Smith et al. (2014)), probiotic administration can directly reinstate the expression of proto-oncogenes.
-
(d)
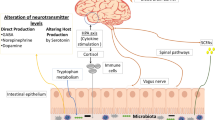
Stimulation of the vagus nerve, a major constituent of the parasympathetic nervous system by probiotic intervention, can help in the treatment of several neurological disorders like depression, anxiety, schizophrenia, IBS, etc. (Johnson and Wilson 2018). Enhanced activity of the vagus nerve helps to decrease the liberate pro-inflammatory cytokines such as TNF-α in stress-related disorders (Herman et al. 2016) (Fig. 10.2).
10.3 Neurological Diseases Influenced by Imbalance of Gut-Brain Axis
10.3.1 Amyotrophic Lateral Sclerosis
Amyotrophic lateral sclerosis (ALS) is a sequential neurodegenerative disorder. The symptoms include cramping, muscle spasm, weakness, muscle twitching, and stiffness, problems with coordination, speaking, breathing, and swallowing difficulties, weight loss, gastroparesis, and an increase in metabolic rate, relating to the death of motor neurons, spinal cord, and eventually the brain. Neuroinflammation is recognized as a disease driver for the development of ALS disorder (Skaper et al. 2018). Immune pathway de-regulation is a principal feature in the brain and spinal cord tissue affected with ALS (D’Erchia et al. 2017). ALS is of two types: (i) Sporadic ALS, signifying the most general form although causation is unknown, (ii) Familial ALS, occurring due to genetic changes (Steyn et al. 2018; Toepfer et al. 2000).
Modifications in monocytes, neutrophils, CD4+, CD8+, and natural killer T cells have been observed in patients with ALS causing progression in the disease rate (Perner et al. 2018; Zhou et al. 2017). Association of the gut microbiome and ALS in a transgenic mouse model with G93A genetic mutation of the superoxide dismutase gene (SOD1/SOD1 G93A) disclose disrupted BBB and increased permeability of the gut, reduced amount of butyrate-producing bacteria Peptostreptococcus and Butyrivibrio fibrisolvens resulting in an elevated level of serum and IL-17 (intestinal pro-inflammatory cytokine) as well as damaged intestinal wall due to decreased expression of E-cadherin and zonula occludens (ZO-1) (Wu et al. 2015). Furthermore, the mutant mice SOD1G93A treated with 2% butyrate in drinking water, improved intestinal barrier function by bacteria B. fibrisolvens as well as delayed symptoms like weight loss and even death, in comparison to control mice (Zhang et al. 2017). Butyrate-producing bacteria like Lachnospira, Anaerostipes, and Oscillibacter were in reduced levels in feces of ALS patients and indicated increased levels of Dorea spp. which synthesizes harmful end product ethanol in glucose metabolism (Fang et al. 2016). The therapeutic potential of probiotics for the improvement of the ALS condition is yet to be fully explored.
10.3.2 Epilepsy
It is a neurological globally prevalent disease. Symptoms like epileptic seizures are often experienced by the affected individuals and lack of proper medical care during severe seizures can even prove to be fatal (Gómez-Eguílaz et al. 2018). Although multiple therapeutic alternatives are in practice, their benefits remain limited or short-termed. The use of probiotics for the treating of pharmaco-resistant or refractory epilepsy (that often involve highly convulsive seizures) is already undergoing trials (Iannone et al. 2020). Such studies have revealed promising results by reducing the symptomatic seizures by over 50%, thus eventually contributing to a better quality of life among the patients (Krauss and Sperling 2011).
10.3.3 Autistic Spectrum Disorder
Autism or autistic spectrum disorder (ASD) comprises an extensive range of neuropsychiatric disorder exhibited mainly during infancy wherein affected children tend to experience numerous neurodevelopmental disabilities along with stereotypical or repetitive actions, communicational, and social interaction difficulties and intestinal problems (Lord et al. 2000; Horvath et al. 1999). Real-time PCR meta-analysis studies indicated autistic children have a lower copiousness of bacterial phyla comprising Proteobacteria, Bacteroidetes, Firmicutes, Bifidobacteria spp. and especially A. muciniphila mucolytic bacterium in their feces with comparison to controls (Cao et al. 2013; Wang et al. 2011). A probiotic consortium of Lactobacillus, Streptococcus, and Bifidobacterium showed considerable changes in fecal cytokine levels of ASD patients (Tomova et al. 2015).
Gut microbiota produces metabolites such as acetate, valerate, and propionate in lower levels in autistic patients. Also, ASD animal model MIA mouse resembles characteristics of ASD in mouse offspring which are caused due to gut-intestinal barrier defects and exhibited changes in significant microbial metabolite 4-ethyl-phenyl-sulfate (4EPS) associated with anxiety behaviors (Adams et al. 2011) and Bacteroides fragilis reverses gut-intestinal barrier non-regulation in the MIA mouse. Alternative ASD mouse model strain BTBR T+ Itpr3tf/J is directed by multiple genetic modifications inducing autism-like behavior leading to change in gut microbiota with a reduction in SCFAs, tryptophan, and bile acid metabolism (Meyza and Blanchard 2017). Simultaneously, these presymptomatic and symptomatic data substantiate the conception of a MGB axis dysfunction in ASD.
10.3.4 Dementia
It is a term collectively used to describe the various signs and symptoms of both cognitive and psychological impairment, often characterized by difficulties associated with communication, coordination, visual perception, memory loss, etc. Many conditions can contribute to dementia such as Alzheimer’s disease, Parkinson’s disease, brain injury, and traumas (Bachstetter et al. 2015). The administration of probiotics and prebiotics helps in stimulation of the functioning of the gut microbiome, in turn affecting the MGB axis (Cryan and Dinan 2012). With the advancement in age, the composition of gut microbiome changes denoted by the loss of a few beneficial microbes reduced heterogeneity and a possible increase of pathogenic microbes. Aging is probably one of the major risk factors that lead to the development of dementia and hence the microbiome-gut-brain axis is also likely to be a crucial factor in the development of dementia (as per the ongoing study references from Medical University of Graz, probiotics in dementia-undergoing clinical trials).
10.3.5 Multiple Sclerosis (MS)
Multiple sclerosis (MS) is a chronic autoimmune neurological disorder of the central nervous system. The neuropathological characteristics of MS include axonal damage, demyelination, progressive neurological disability, neurodegeneration, and abnormal T-cell-facilitated immune responses triggered against myelin antigens (Ota et al. 1990). The clinical disclosures include dizziness, vision loss, vertigo, motor dysfunction, pain, numbness, impaired coordination, fatigue, and depression. MS is mainly divided into four: (i) Progressive-relapsing MS (PRMS), (ii) Primary Progressive MS (PPMS), (iii) Secondary Progressive MS (SPMS), (iv) Relapsing-Remitting MS (RRMS) (Noseworthy et al. 2000).
Neuropathogenesis of MS is dependent upon important environmental factors like gut dysbiosis (Mowry and Glenn 2018). RRMS is distinguished by a reduction in bacteria responsible for generating T regulatory cells (Tregs) which are immune cells accountable to anti-inflammatory reactions, CD4+ T cells that generate tolerogenic dendritic cells, regulatory B cells, IL-10, and macrophage suppression. CD4+ T cells, dendritic cells, monocytes, or B cells produce pro-inflammatory reactions due to the increase in specific bacteria (Shahi et al. 2017). Germ-free mice having autoimmune encephalomyelitis (EAE) develop a substantially weakened pathology while the commensal microbiota in experimental mice stimulates CD4+ T cells which are myelin-specific and also obtained autoantibodies produced to myelin oligodendrocyte glycoprotein by B cells (Berer et al. 2011).
MS patient’s fecal gut microbiota has a different bacterial composition in comparison with controls (Shahi et al. 2017). Presence in increased concentration of Proteobacteria species like Acinetobacter calcoaceticus (Cekanaviciute et al. 2017), Pseudomonas, Mycoplasma, (Chen et al. 2016), Bilophila (Miyake et al. 2015) in white matter lesions; Akkermansia, Acinetobacter, Prevotella, Clostridium, Bacteroidetes, and Lactobacillus bacterial genera in MS patients gut microbiota induces production of SCFAs (Berer et al. 2011; Branton et al. 2016) and helps in the maintenance of immune cell for producing an anti-inflammatory reaction. The importance of bacteria Faecalibacterium in MS patients is its production of butyrate which increases the production of Tregs (Arpaia et al. 2013). Colonization of Clostridium perfringens type B in the human GI tract increases epsilon toxin level which deteriorates the blood–brain barrier (BBB), dominating oligodendrocyte and neuronal damage and also activating autoimmune demyelinating action (Rumah et al. 2013).
Oral administration of various combinations of probiotics like Lactobacillus species increased regulatory T cells and increase in IL-10 production and decreased IL-17 and (Takata et al. 2011), Streptococcus thermophiles, Bifidobacterium bifidum in both rat and mouse models improved clinical score of EAE and showed a decrease in Th1 and Th17 cells together with Tregs development (Kwon et al. 2013). Clinically, it was indicated that laboratory mice colonizing with MS patients gut microbiota increased the severity of experimental autoimmune encephalomyelitis (EAE) in MS animal models which can be reduced by the oral administration of Bifidobacterium animalis (Ezendam et al. 2008). An anti-inflammatory bacterium Prevotella histicola from human celiac disease patients has immunomodulatory potential which suppresses disease in EAE models through the initiation of tolerogenic dendritic cells, and FoxP3+ regulatory T cells and a decrease in pro-inflammatory Th1 and Th17 responses (Mangalam et al. 2017). Lactobacillus sp., Bifidobacterium bifidum, and Streptococcus thermophiles enhanced regulatory T cells, producing increased IL-10 and decreased TNF-α, IFN-c, IL-17, and reduced Th1 and Th17 cells along with the development of Tregs (Ezendam et al. 2008). These results produce evidence that the microbiome present in the human GI tract has a substantial impact on CNS-specific autoimmunity.
10.3.6 Alzheimer’s Disease
Alterations occurring to the gut microbiome have the ability to influence the advancement of neurological diseases such as Alzheimer’s Disease (AD), often recognized with a gradual decrease in cognitive abilities and memory and eventually in most cases, cause dementia (Mangalam et al. 2017). Although age is regarded as a notable risk factor for AD, the causative factors that majorly trigger it are still unclear (Kohler et al. 2016). There are several crucial factors that accompany the degenerative process of AD such as an impaired immune response, markers or signaling processes (Bhattacharjee and Lukiw 2013). Currently, no cure or treatment protocol is available for AD and the ways of disease progression by plaque and tangle formation or its further propagation in the brain still remain unknown (Aisen and Davis 1994). Probiotics possibly modulate and prevent the cognitive impairment in AD by the production of metabolites and neurotransmitters such as SCFAs and GABA. They help maintain a state of eubiosis, promote inflammatory responses and reduce cell damage caused by oxidative stress (Balin and Hudson 2014).
10.3.7 Anxiety and Depression
Anxiety is a psychological state of an individual characterized by apprehension or intense fear, associated with a lack of response of adaptation by the subject in a particular situation often commonly experience psychiatric disorders. Depression is a psychological state characterized by unhappiness or irritability and joint from various psycho-physiological alterations such as appetite distortions, sleep, constipation, and inability to experience work pleasure. Due to the intervention of the ANS, the two disorders generate alteration in the stability and constitution of the gut microbiota causing changes in colon mobility.
These patients exhibit increased inflammatory levels, dysfunction of the HPA axis, and neurotransmitter signaling. MDD patients showed significantly reduced Firmicutes and an increase in Bacteroidetes, Proteobacteria, and Actinobacteria but compared with controls confirmed the constitution of the altered gut microbiome. MDD patients also had increased Enterobacteriaceae, Prevotella, Klebsiella, Streptococcus, Clostridium, and Alistipes, decreased levels of Faecalibacterium, Bifidobacterium, Lactobacillus, Prevotellaceae and increase in Thermoanaerobacteraceae revealed that gut microbiota dysbiosis is significantly linked with metabolic changes of bile acids and tryptophan against the controls (Lin et al. 2017a). Desbonnet et al. 2008 (Desbonnet et al. 2010) observed peripheral HPA levels and concentrations of the serotonin precursor, tryptophan altered due to B. infantis for development of protective mechanisms preceding stress disclosure.
Germ-Free (GF) mice present an excessive liberation of adrenocorticotropic hormone (ACTH) and corticosterone resulting in stress. GF mice exhibit low anxiety suggesting that the intestinal microbiota affects the developmental behavior (Neufeld et al. 2011). Supplementing GF mice with Bifidobacterium infantis enhanced HPA stress response, comprising a reverse in an increase of plasma (ACTH) and corticosterone (Sudo et al. 2004). L. rhamnosus reduced stress-induced corticosterone and changed GABA receptor gene expression levels in the brain also signifying modulatory communication pathway as vagus nerve between the gut microbiome and the brain (Bravo et al. 2011). When fecal microbiota of MDD patients was transplanted to GF mice, induced metabolic disturbances in host and induced depression indicating dysfunctionality of gut microbiota playing a significant role in MDD.
Individuals with depression indicated higher levels of cytokines and immunoglobulins IgA and IgM, resulting in inflammatory processes and gastrointestinal disorders (Lima-Ojeda et al. 2017). Enteropathogens disturb mood through the immune system in humans. Reichenberg et al. (2001) indicated that following intravenous infusion with endotoxins from Salmonella abortus equi to healthy volunteers underwent an increase in anxiety, and depression levels. Alterations in the neuroendocrine pathways and several neurobiological mechanisms involving serotonin neurotransmitter reduction, reduction of dopamine in anxiety and noradrenaline in depression. The low doses of Campylobacter jejuni through oral administration can stimulate anxiogenic effects in mice (Lyte et al. 2006). The intake of probiotics has anti-inflammatory and antioxidant effects on depressed patients and their ability to regulate BDNF growth factor levels (Logan and Katzman 2005).
Lactobacillus rhamnosus JB-1 and combination of Lactobacillus helveticus R0052 and Bifidobacterium longum R0175 improved anxiolytic activity in specific-pathogen-free (SPF) rat and GF mice, reduced extreme HPA, changing GABA receptor level in particular brain regions, restored serotonin and norepinephrine levels in inflammatory stress response, and also promoted potential psychological properties in humans (Liang et al. 2015). The enteric microbiota shows a significant impact on potential therapeutic approach, modifying the host’s gut microbiota affecting neurochemical, behavioral, and immunological parameters appropriate to the brain-gut axis disorders provided health benefits to the host with psychobiotics as emerging treatment (Petschow et al. 2013).
10.3.8 Schizophrenia
Schizophrenia is a mental illness that disturbs the human mind’s functioning with severe occurrences of psychosis and passivity, followed by periods of normal mental activity (Grover et al. 2019). The prevalence of schizophrenia and autism has been correspondingly higher in patients suffering from C. difficile infection, possibly because the organism is known to synthesize a phenylalanine derivative in the gut that in turn regulates the levels of catecholamine in the brain (Argou-Cardozo and Zeidán-Chuliá 2018).
Further evidence from genetic studies focusing on twins and their adoption also suggests a linkage between the gut microbiome and schizophrenia. Patients suffering from schizophrenia often experience both mental and inflammatory stress, poor nutrition, and lactose sensitivity. These symptoms can be relieved with sufficient and proper probiotic administration (Ledochowski et al. 1998).
10.4 Psychobiotics
Probiotic microorganisms that may positively impact the psychological condition of a host upon adequate administration are referred to as psychobiotics (Dinan and Cryan 2013). The correlation of the gut microbiome with an individual’s psychological condition via the gut-brain axis is increasingly gaining importance. Hereby, gut microbiota has found to exert an anti-inflammatory effect along with a modulatory reaction on the functioning of neurotransmitters (Beck et al. 2019). It has often been implied that a healthy mind means a healthy body vice versa possibly indicating their close relationship and also the fact that the gut microbiome can have both a direct and indirect connection with the mental status of a host (Dinan et al. 2013).
The gut microbiome has the ability to shape and alter one’s thinking and mental abilities proving why it has been termed as the second brain. Dinan et al. (2013)) referred psychobiotics to some psychotropic bacteria colonizing the gut which could either positively or negatively influence the mental status of a host. Gut microbiota dysbiosis has also been associated with an unstable mental status of the host often leading to neurological disorders such as depression (Luczynski et al. 2016), autism disorders (Critchfield et al. 2011), schizophrenia (Grover et al. 2019), Parkinson’s disease (Holmqvist et al. 2014), Alzheimer’s disease, dementia (Kohler et al. 2016), etc. Psychobiotics are capable of releasing hormones and modulating the functioning of neurotransmitters which in turn act upon the gut-brain axis in Table 10.2 below (Ho et al. 2015). Studies reveal that the cytokines such as INFα and TNF-α have the ability to cause depression depending on their circulation and production levels, therefore a therapeutic dosage of psychobiotics is likely to alleviate such conditions and even improve mood swings (Petschow et al. 2013). Psychobiotics offer promising results in the treatment of various neurological diseases; targeting the gut microbiome and alteration of the same in a positive manner would greatly benefit the host (Rooks and Garrett 2016).
10.5 Therapeutic Manipulation, Implications, and Future Prospects
The significant functional and structural alteration of central nervous system can be concomitant with gut dysbiosis directing to the assumption that manipulation of gut microbiota is potentially a sensible method to confine clinical complications in neurological disorders. Certain treatments with antibiotics active against specific species are used to control intestinal flora of children with ASD and their GI tract showed a considerable negative impact on CNS with a reduction of pathogenic bacteria (Critchfield et al. 2011), but this method has only been partially successful and was found to follow a significant development in neurological disorder only during the administration of adequate drug dosage (Sandman et al. 2012). However, soon after treatment with certain phytochemicals reverses the neurological impairment in its initial phase (Tripathi et al. 2022).
A probiotic is defined as living microorganisms that aid in recovering the gut microbiota balance, with improvement in the integrity of the gut mucosal barrier and immunomodulation, ensure health benefits to the host, and IgA mucosal response to the benefit of the host when governed in acceptable amounts (D'Mello et al. 2015). Administration of probiotic bacteria modifies the bacterial composition of the gut, with an increase in protected positive strains and a reduction in negative strains. Furthermore, the concentration of specific bacterial products which causes immune system alterations, anti-anxiety effects (Curran et al. 2016), memory and learning improvements (Adler and Wong-Kee-You 2015), inflammation, modification of the CNS function and structure, and modulation of gene expression is reduced by probiotics when it exceeds the intestinal wall (Murgatroyd et al. 2009). This beneficial effect is different and strain-dependent.
Many presymptomatic and animal research investigations indicated the probiotic potential for treatment and prevention of numerous diseases, comprising of CNS and GI diseases, mainly using genera Lactobacillus and Bifidobacterium bacteria (Sánchez et al. 2017). Across the MGB axis, probiotics initiate brain function, for example anxiety and depression normalization (Wallace and Milev 2017); the brain neurological processing is influenced by the mechanism of probiotic supplements, for depression and anxiety in particular. For instance, chronic therapy with the probiotic Lactobacillus rhamnosus diminishes anxiety, depression, and stress responses merely in the existence of an intact vagus nerve, by reducing mRNA expression of GABAAα2 in the amygdala and prefrontal cortex, however, enhanced GABAAα2 mRNA expression in the hippocampus, therefore, vagus nerve identification plays a significant role in modulatory communication pathway between the gut, which is susceptible to probiotics or bacteria, and the brain (Bravo et al. 2011).
Bercik et al. (2011)) demonstrated a chronic administration of Bifidobacterium infantis in rats which was restricted by early age from maternal contact and possessed stress-related mood and gastrointestinal disorders resulting in immune normalization, reversal of behavioral deficiencies, and rehabilitation of basal noradrenaline concentrations in the brainstem. Moreover, Bifidobacterium longum NCC3001 and Bifidobacterium longum 1714 normalize anxiety-like behavior, with beneficial effect on cognition, Bifidobacteria infantis raised serotoninergic precursors and weakened inflammatory immune response, further indicated an antidepressant effect (Quigley et al. 2012) and hippocampal brain-derived neurotrophic factor (BDNF) in mice with infectious colitis (Bercik et al. 2011). In rats, the administration of Lactobacillus helveticus, Lactobacillus farciminis, and Lactobacillus rhamnosus can prevent chronic-stress-induced intestinal abnormalities and decreases psychological distress (Zareie et al. 2006). Lavasani et al. (2010)) observed that instead of administrating monostrain probiotic strains, three Lactobacillus strains such as L. paracasei DSM 13434, L. plantarum DSM 15312, and L. plantarum DSM 15313 in mice showed therapeutic efficiency on development of experimental autoimmune encephalomyelitis (EAE), by inhibiting disease progression (Boksa and El-Khodor 2003), gut microbiota modulation and improved destructive antisocial behavioral pattern, communication and anxiety problems in patients (Cai et al. 2015). Hsiao et al. (2013) described oral administration of Bacteroides fragilis which is a human commensal by a maternal immune activation (MIA) model of ASD in mouse offspring; modified the gut permeability, changed the composition of gut microbiota, and ameliorated the defects in communicative, stereotypic, with improved anxiety-like and sensorimotor communicative behaviors also Bacteroides thetaiotaomicron administration has also substantially improved abnormal behaviors and signifies that modulating therapies utilizing probiotics is a safer and efficient treatment for autism spectrum disorder (Cai et al. 2015).
Fecal microbiota transplantation (FMT) is used for the treatment of gastrointestinal diseases by introducing the enteric bacteria from healthy donor’s feces to affected/diseased individuals with the sole purpose of restoring gut microbiota balance or to effect in eubiosis. This is done through oral administration of capsules comprising of donor microbiota sample in freeze-dried form. So far the results have been promising without any adverse effects and hence maybe considered as a safe alternative strategy for the treatment in organ transplant recipients (Bajaj et al. 2017) and cancer patients (Hefazi et al. 2017). Although this mode of treatment would be most ideal for gut dysbiosis-related diseases for instance inflammatory bowel diseases, metabolic syndromes, etc. (Sayar and Cetin 2016).
Clustered and regularly interspaced short palindrome repeats (CRISPR) technology can be utilized to make genetic changes in probiotic microorganisms via gene editing. This technology in association with the bacterial neuroimmune and metabolic systems can be used to engineer genes through upregulation and downregulation of their expression via therapeutic signaling molecules and blocking exopolysaccharides produced by the bacteria (Petimar et al. 2019). CRISPR-CAS targets the bidirectional gut-brain axis changing the neurological and intestinal effects leading to neurodegeneration (Aliev et al. 2008). Also, CRISPR technology suppresses or silences the expression of bacterial inflammatory signaling molecules and endotoxins. CRISPR-CAS9 is also used to target antibiotic resistance through phage-specific 16 s sequences of Gram-negative bacteria; these CRISPR antimicrobials can be tailored to treat patient-specific dysbiosis without changing bacterial populations, yet help in achieving effective therapeutic potential (Martella et al. 2019; Konermann et al. 2015)
Daily consumption of a fermented milk product containing probiotics such as Bifidobacterium animalis subspp. sss, Lactobacillus bulgaricus, Streptococcus thermophilus, and Lactococcus lactis subspp. showed significant alteration of the activity of CNS and brain regions that control the treatment of sensation and emotion (Tillisch et al. 2013), and HTLV-1-associated myelopathy/tropical spastic paraparesis (TSP/HAM) patients were treated with oral administration of Lactobacillus casei strain Shirota and exhibited development of motor function due to improved activity of NK cell (Finegold et al. 2002). Furthermore, probiotic administration in early age could lower the neuropsychiatric disorder risk development subsequent in infancy and the structured neurological evaluation in children administrating probiotics disclosed a substantially lower prevalence of neurological abnormalities (Pärtty et al. 2015).
10.6 Conclusion
The potential probiotics existing in the gut microbiota of the human intestinal tract are composite and live microorganisms that perform well in microvilli advancement, maintaining homeostasis, immune-potentiation, prevention of pathogen colonization in the GI tract, and gut-brain axis regulation. It could be utilized as a neuroprotective nutraceutical for treating or preventing a wide range of neurological diseases. The probiotic development emphasizes on microbial psychobiotics to potentially treat and prevent psychiatric disorders. Postbiotics which are gut microbial signaling molecules should be exploited to identify targets for therapeutic benefits. Dysbiosis in gut microbiota can cause impairment of gut barrier function and inflammation in the gut-brain axis leading to mental disorders. The gut microbiota dysbiosis and neurological dysfunction have aroused its importance in several fields, including neuroscience, immunology, bioinformatics, and microbiology, which provide potential treatment approaches for a diverse set of neurological disorders. Clinical research on the potential impact of probiotic mechanism and manipulation can positively improve the understanding of neurodevelopmental disorders and also may help evaluate the microbial effect on the gut-brain axis, this should be comprehended more in the future. It remains unclear whether the genetic modification of gut microbiota is capable of resulting in neurological diseases as there still exists only a limited amount of information concerning such protective or beneficial bacteria. The therapeutic role of psychobiotics has been studied in animals but more efficient human clinical trials are required to confirm their roles in microbiome-targeted therapies for treating gut-brain disorders. Probiotics have also shown to play a prominent role in the neuropathogenesis of neural disorders by altered gut-brain axis function.
References
Adams JB, Johansen LJ, Powell LD, Quig D, Rubin RA (2011) Gastrointestinal flora and gastrointestinal status in children with autism–comparisons to typical children and correlation with autism severity. BMC Gastroenterol 11(1):22
Adler SA, Wong-Kee-You AM (2015) Differential attentional responding in caesarean versus vaginally delivered infants. Atten Percept Psychophys 77(8):2529–2539
Aisen PS, Davis KL (1994) Inflammatory mechanisms in Alzheimer’s disease: implications for therapy. Am J Psychiatry 151:1105–1113
Ait-Belgnaoui A, Colom A, Braniste V, Ramalho L, Marrot A, Cartier C, Tompkins T (2014) Probiotic gut effect prevents the chronic psychological stress-induced brain activity abnormality in mice. Neurogastroenterol Motil 26(4):510–520
Akers KG, Martinez-Canabal A, Restivo L, Yiu AP, De Cristofaro A, Hsiang HLL, Ohira K (2014) Hippocampal neurogenesis regulates forgetting during adulthood and infancy. Science 344(6184):598–602
Aliev G, Obrenovich ME, Reddy VP, Shenk JC, Moreira PI, Nunomura A, Perry G (2008) Antioxidant therapy in Alzheimer’s disease: theory and practice. Mini Rev Med Chem 8(13):1395
Argou-Cardozo I, Zeidán-Chuliá F (2018) Clostridium bacteria and autism spectrum conditions: a systematic review and hypothetical contribution of environmental glyphosate levels. Med Sci 6:29
Arpaia N, Campbell C, Fan X, Dikiy S, van der Veeken J, Deroos P, Rudensky AY (2013) Metabolites produced by commensal bacteria promote peripheral regulatory T-cell generation. Nature 504(7480):451–455
Azpiroz F (2005) Intestinal perception: mechanisms and assessment. Br J Nutr 93(S1):S7–S12
Bachstetter AD, Van Eldik LJ, Schmitt FA, Neltner JH, Ighodaro ET, Webster SJ, Patel E, Abner EL, Kryscio RJ, Nelson PT (2015) Disease-related microglia heterogeneity in the hippocampus of Alzheimer’s disease, dementia with Lewy bodies, and hippocampal sclerosis of aging. Acta Neuropathol Commun 3(1): 1–16
Bäckhed F (2011) Programming of host metabolism by the gut microbiota. Ann Nutr Metab 58(Suppl. 2):44–52
Bajaj JS, Kassam Z, Fagan A, Gavis EA, Liu E, Cox IJ, Williams R (2017) Fecal microbiota transplant from a rational stool donor improves hepatic encephalopathy: a randomized clinical trial. Hepatology 66(6):1727–1738
Balin BJ, Hudson AP (2014) Etiology and pathogenesis of late-onset Alzheimer’s disease. Curr Allergy Asthma Rep 14:013–0417
Barouei J, Moussavi M, Hodgson DM (2012) Effect of maternal probiotic intervention on HPA axis, immunity and gut microbiota in a rat model of irritable bowel syndrome. PLoS One 7(10) e46051
Barrett E, Ross RP, O’toole PW, Fitzgerald GF, Stanton C (2012) γ-Aminobutyric acid production by culturable bacteria from the human intestine. J Appl Microbiol 113(2):411–417
Beck BR, Park GS, Jeong DY, Lee YH, Im S, Song WH, Kang J (2019) Multidisciplinary and comparative investigations of potential psychobiotic effects of Lactobacillus strains isolated from newborns and their impact on gut microbiota and ileal transcriptome in a healthy murine model. Front Cell Infect Microbiol 9:269
Belmaker RH, Agam G (2008) Disorder MD. Major depressive disorder. N Engl J Med Overseas Ed 358:55–68
Bercik P, Park AJ, Sinclair D, Khoshdel A, Lu J, Huang X, Berger B (2011) The anxiolytic effect of Bifidobacterium longum NCC3001 involves vagal pathways for gut–brain communication. Neurogastroenterol Motil 23(12):1132–1139
Berer K, Mues M, Koutrolos M, Al Rasbi Z, Boziki M, Johner C, Krishnamoorthy G (2011) Commensal microbiota and myelin autoantigen cooperate to trigger autoimmune demyelination. Nature 479(7374):538–541
Bhattacharjee S, Lukiw WJ (2013) Alzheimer’s disease and the microbiome. Front Cell Neurosci 7:153
Boksa P, El-Khodor BF (2003) Birth insult interacts with stress at adulthood to alter dopaminergic function in animal models: possible implications for schizophrenia and other disorders. Neurosci Biobehav Rev 27(1–2):91–101
Borre YE, O’Keeffe GW, Clarke G, Stanton C, Dinan TG, Cryan JF (2014) Microbiota and neurodevelopmental windows: implications for brain disorders. Trends Mol Med 20(9):509–518
Borthakur A, Gill RK, Tyagi S, Koutsouris A, Alrefai WA, Hecht GA, Dudeja PK (2008) The probiotic Lactobacillus acidophilus stimulates chloride/hydroxyl exchange activity in human intestinal epithelial cells. J Nutr 138(7):1355–1359
Branton WG, Lu JQ, Surette MG, Holt RA, Lind J, Laman JD, Power C (2016) Brain microbiota disruption within inflammatory demyelinating lesions in multiple sclerosis. Sci Rep 6:37344
Bravo JA, Forsythe P, Chew MV, Escaravage E, Savignac HM, Dinan TG, Cryan JF (2011) Ingestion of Lactobacillus strain regulates emotional behavior and central GABA receptor expression in a mouse via the vagus nerve. Proc Natl Acad Sci 108(38):16050–16055
Breban M, Tap J, Leboime A, Said-Nahal R, Langella P, Chiocchia G, Sokol H (2017) Faecal microbiota study reveals specific dysbiosis in spondyloarthritis. Ann Rheum Dis 76(9):1614–1622
Cai S, Pang WW, Low YL, Sim LW, Sam SC, Bruntraeger MB, Richmond J (2015) Infant feeding effects on early neurocognitive development in Asian children. Am J Clin Nutr 101(2):326–336
Cameron J, Doucet E (2007) Getting to the bottom of feeding behaviour: who’s on top? Appl Physiol Nutr Metab 32(2):177–189
Cao X, Lin P, Jiang P, Li C (2013) Characteristics of the gastrointestinal microbiome in children with autism spectrum disorder: a systematic review. Shanghai Arch Psychiatry 25(6):342
Cekanaviciute E, Yoo BB, Runia TF, Debelius JW, Singh S, Nelson CA, Crabtree-Hartman E (2017) Gut bacteria from multiple sclerosis patients modulate human T cells and exacerbate symptoms in mouse models. Proc Natl Acad Sci 114(40):10713–10718
Chen J, Chia N, Kalari KR, Yao JZ, Novotna M, Soldan MMP, Weinshenker BG (2016) Multiple sclerosis patients have a distinct gut microbiota compared to healthy controls. Sci Rep 6(1):1–10
Clarke G, Fitzgerald P, Hennessy AA, Cassidy EM, Quigley EM, Ross P, Dinan TG (2010) Marked elevations in pro-inflammatory polyunsaturated fatty acid metabolites in females with irritable bowel syndrome. J Lipid Res 51(5):1186–1192
Coccaro EF, Lee R, Groer MW, Can A, Coussons-Read M, Postolache TT (2016) Toxoplasma gondii infection: relationship with aggression in psychiatric subjects. J Clin Psychiatry 77(3):334–341
Costedio MM, Hyman N, Mawe GM (2007) Serotonin and its role in colonic function and in gastrointestinal disorders. Dis Colon Rectum 50(3):376–388
Critchfield JW, Van Hemert S, Ash M, Mulder L, Ashwood P (2011) The potential role of probiotics in the management of childhood autism spectrum disorders. Gastroenterology research and practice
Cryan JF, Dinan TG (2012) Mind-altering microorganisms: the impact of the gut microbiota on brain and behaviour. Nat Rev Neurosci 13:701–712
Cunningham CL, Martínez-Cerdeño V, Noctor SC (2013) Microglia regulate the number of neural precursor cells in the developing cerebral cortex. J Neurosci 33(10):4216–4233
Curran EA, Khashan AS, Dalman C, Kenny LC, Cryan JF, Dinan TG, Kearney PM (2016) Obstetric mode of delivery and attention-deficit/hyperactivity disorder: a sibling-matched study. Int J Epidemiol 45(2):532–542
D’Erchia AM, Gallo A, Manzari C, Raho S, Horner DS, Chiara M, Locatelli F (2017) Massive transcriptome sequencing of human spinal cord tissues provides new insights into motor neuron degeneration in ALS. Sci Rep 7(1):1–20
Desbonnet L, Garrett L, Clarke G, Kiely B, Cryan JF, Dinan TG (2010) Effects of the probiotic Bifidobacterium infantis in the maternal separation model of depression. Neuroscience 170(4):1179–1188
Dinan, T. G., &Cryan, J. F. (2013). Melancholic microbes: a link between gut microbiota and depression?. Neurogastroenterology & Motility, 25(9), 713–719.
Dinan TG, Stanton C, Cryan JF (2013) Psychobiotics: a novel class of psychotropic. Biol Psychiatry 74(10):720–726
D'Mello C, Ronaghan N, Zaheer R, Dicay M, Le T, MacNaughton WK, Swain MG (2015) Probiotics improve inflammation-associated sickness behavior by altering communication between the peripheral immune system and the brain. J Neurosci 35(30):10821–10830
Dominguez-Bello MG, De Jesus-Laboy KM, Shen N, Cox LM, Amir A, Gonzalez A, Mendez K (2016) Partial restoration of the microbiota of cesarean-born infants via vaginal microbial transfer. Nat Med 22(3):250
Edelmann E, Lessmann V, Brigadski T (2014) Pre- and postsynaptic twists in BDNF secretion and action in synaptic plasticity. Neuropharmacology 76(Pt C):610–627
Erny D, de Angelis ALH, Jaitin D, Wieghofer P, Staszewski O, David E, Schwierzeck V (2015) Host microbiota constantly control maturation and function of microglia in the CNS. Nat Neurosci 18(7):965
Ezendam J, De Klerk A, Gremmer ER, Van Loveren H (2008) Effects of Bifidobacterium animalis administered during lactation on allergic and autoimmune responses in rodents. Clin Exp Immunol 154(3):424–431
Fang X, Wang X, Yang S, Meng F, Wang X, Wei H, Chen T (2016) Evaluation of the microbial diversity in amyotrophic lateral sclerosis using high-throughput sequencing. Front Microbiol 7:1479
Finegold SM, Molitoris D, Song Y, Liu C, Vaisanen ML, Bolte E, Collins MD (2002) Gastrointestinal microflora studies in late-onset autism. Clin Infect Dis 35(Supplement_1):S6–S16
Forsythe P, Sudo N, Dinan T, Taylor VH, Bienenstock J (2010) Mood and gut feelings. Brain Behav Immun 24(1):9–16
Fouhy F, Ross RP, Fitzgerald GF, Stanton C, Cotter PD (2012) Composition of the early intestinal microbiota: knowledge, knowledge gaps and the use of high-throughput sequencing to address these gaps. Gut microbes 3(3):203–220
Fung TC, Olson CA, Hsiao EY (2017) Interactions between the microbiota, immune and nervous systems in health and disease. Nat Neurosci 20(2):145
Galland L (2014) The gut microbiome and the brain. J Med Food 17(12):1261–1272
Gareau MG, Jury J, Perdue MH (2007) Neonatal maternal separation of rat pups results in abnormal cholinergic regulation of epithelial permeability. Am J Physiol Gastrointest Liver Physiol 293(1):G198–G203
Girolamo F, Coppola C, Ribatti D (2017) Immunoregulatory effect of mast cells influenced by microbes in neurodegenerative diseases. Brain Behav Immun 65:68–89
Gómez-Eguílaz M, Ramón-Trapero JL, Pérez-Martínez L, Blanco JR (2018) The beneficial effect of probiotics as a supplementary treatment in drug-resistant epilepsy: a pilot study. Benef Microbes 9(6):875–881
Grover S, Patil A, Kaur A, Garg G (2019) Probiotics: a potential immunotherapeutic approach for the treatment of schizophrenia. J Pharm Bioallied Sci 11(4):321–327
Guilliams TG, Edwards L (2010) Chronic stress and the HPA axis. The Standard 1(2):1–12
Hamer HM, Jonkers D, Venema K, Vanhoutvin S, Troost FJ, Brummer RJ (2008) Review article: the role of butyrate on colonic function. Aliment Pharmacol Ther 27(2):104–119
Hefazi, M., Patnaik, M. M., Hogan, W. J., Litzow, M. R., Pardi, D. S., & Khanna, S. (2017). Safety and efficacy of fecal microbiota transplant for recurrent Clostridium difficile infection in patients with cancer treated with cytotoxic chemotherapy: a single-institution retrospective case series. In: Mayo Clinic Proceedings (Vol. 92, No. 11, pp 1617–1624). Elsevier: Amsterdam.
Heijtz RD, Wang S, Anuar F, Qian Y, Björkholm B, Samuelsson A, Pettersson S (2011) Normal gut microbiota modulates brain development and behavior. Proc Natl Acad Sci 108(7):3047–3052
Helander HF, Fändriks L (2014) Surface area of the digestive tract–revisited. Scand J Gastroenterol 49(6):681–689
Herman JP, McKlveen JM, Ghosal S et al (2016) Regulation of the hypothalamic-pituitary-adrenocortical stress response. Compr Physiol 6(2):603–621
Ho JT, Chan GC, Li JC (2015) Systemic effects of gut microbiota and its relationship with disease and modulation. BMC Immunol 16:21
Holmqvist S, Chutna O, Bousset L, Aldrin-Kirk P, Li W, Björklund T, Li JY (2014) Direct evidence of Parkinson pathology spread from the gastrointestinal tract to the brain in rats. Acta Neuropathol 128(6):805–820
Horvath K, Papadimitriou JC, Rabsztyn A, Drachenberg C, Tildon JT (1999) Gastrointestinal abnormalities in children with autistic disorder. J Pediatr 135(5):559–563
Iannone L, Gómez-Eguílaz M, Citraro R, Russo E (2020) The potential role of interventions impacting on gut-microbiota in epilepsy. Expert Rev Clin Pharmacol 13. https://doi.org/10.1080/17512433.2020
Jiménez E, Delgado S, Maldonado A, Arroyo R, Albújar M, García N, Rodríguez JM (2008) Staphylococcus epidermidis: a differential trait of the fecal microbiota of breast-fed infants. BMC Microbiol 8(1):1–11
Johnson RL, Wilson CG (2018) A review of vagus nerve stimulation as a therapeutic intervention. J Inflamm Res 11:203–213
Joshi D, Roy S, Banerjee S (2018) Prebiotics: a functional food in health and disease. In: Mandal SC, Mandal V, Konishi T (eds) Natural Products & Drug Discovery. Elsevier, Amsterdam, pp 507–523
Jung IH, Jung MA, Kim EJ, Han MJ, Kim DH (2012) Lactobacillus pentosus var. plantarum C29 protects scopolamine-induced memory deficit in mice. J Appl Microbiol 113(6):1498–1506
Kamm K, Hoppe S, Breves G, Schröder B, Schemann M (2004) Effects of the probiotic yeast Saccharomyces boulardii on the neurochemistry of myenteric neurones in pig jejunum. Neurogastroenterol Motil 16(1):53–60
Kawashima K, Misawa H, Moriwaki Y, Fujii YX, Fujii T, Horiuchi Y, Kamekura M (2007) Ubiquitous expression of acetylcholine and its biological functions in life forms without nervous systems. Life Sci 80(24–25):2206–2209
Kleiman SC, Watson HJ, Bulik-Sullivan EC, Huh EY, Tarantino LM, Bulik CM, Carroll IM (2015) The intestinal microbiota in acute anorexia nervosa and during renourishment: relationship to depression, anxiety, and eating disorder psychopathology. Psychosom Med 77(9):969
Kohler CA, Maes M, Slyepchenko A, Berk M, Solmi M, Lanctôt KL, Carvalho AF (2016) The gut-brain axis, including the microbiome, leaky gut and bacterial translocation: mechanisms and pathophysiological role in Alzheimer’s disease. Curr Pharm Des 22(40):6152–6166
Konermann S, Brigham MD, Trevino AE, Joung J, Abudayyeh OO, Barcena C, Nureki O (2015) Genome-scale transcriptional activation by an engineered CRISPR-Cas9 complex. Nature 517(7536):583–588
Krauss GL, Sperling MR (2011) Treating patients with medically resistant epilepsy. Neurol Clin Pract 1(1):14–23
Kwon OI, Woo EJ, Du YP, Hwang D (2013) A tissue-relaxation-dependent neighboring method for robust mapping of the myelin water fraction. Neuroimage 74:12–21
Landete JM, de las Rivas B, Marcobal A, Munoz R (2008) Updated molecular knowledge about histamine biosynthesis by bacteria. Crit Rev Food Sci Nutr 48:697–714
Lavasani S, Dzhambazov B, Nouri M, Fåk F, Buske S, Molin G, Weström B (2010) A novel probiotic mixture exerts a therapeutic effect on experimental autoimmune encephalomyelitis mediated by IL-10 producing regulatory T cells. PLoS One 5(2) e9009
Ledochowski M, Sperner-Unterweger B, Fuchs D (1998) Lactose malabsorption is associated with early signs of mental depression in females: a preliminary report. Dig Dis Sci 43:2513–2517
Lee DH, Lee DH, Lee JS (2007) Characterization of a new antidementia β-secretase inhibitory peptide from Saccharomyces cerevisiae. Enzyme Microb Technol 42(1):83–88
Liang S, Wang T, Hu X, Luo J, Li W, Wu X, Jin F (2015) Administration of Lactobacillus helveticus NS8 improves behavioral, cognitive, and biochemical aberrations caused by chronic restraint stress. Neuroscience 310:561–577
Lima-Ojeda JM, Rupprecht R, Baghai TC (2017) “I am I and my bacterial circumstances”: linking gut microbiome, neurodevelopment, and depression. Front Psychiatry 8:153
Lin X, Chen Z, Jin L, Gao W, Qu B, Zuo Y, Yu M (2017a) Rasch analysis of the hospital anxiety and depression scale among Chinese cataract patients. PLoS One 12(9)
Lin P, Ding B, Feng C, Yin S, Zhang T, Qi X, Li Q (2017b) Prevotella and Klebsiella proportions in fecal microbial communities are potential characteristic parameters for patients with major depressive disorder. J Affect Disord 207:300–304
Logan AC, Katzman M (2005) Major depressive disorder: probiotics may be an adjuvant therapy. Med Hypotheses 64(3):533–538
Lord C, Cook EH, Leventhal BL, Amaral DG (2000) Autism spectrum disorders. Neuron 28(2):355–363
Lu W, Mi R, Tang H, Liu S, Fan M, Wang L (1998) Over-expression of c-fos mRNA in the hippocampal neurons in Alzheimer’s disease. Chin Med J (Engl) 111(1):35–37
Luczynski P, Whelan SO, O'Sullivan C, Clarke G, Shanahan F, Dinan TG, Cryan JF (2016) Adult microbiota-deficient mice have distinct dendritic morphological changes: Differential effects in the amygdala and hippocampus. Eur J Neurosci 44(9):2654–2666
Lynch SV, Pedersen O (2016) The human intestinal microbiome in health and disease. N Engl J Med 375(24):2369–2379
Lyte M, Li W, Opitz N, Gaykema RP, Goehler LE (2006) Induction of anxiety-like behavior in mice during the initial stages of infection with the agent of murine colonic hyperplasia Citrobacter rodentium. Physiol Behav 89(3):350–357
Mangalam A, Shahi SK, Luckey D, Karau M, Marietta E, Luo N, Rodriguez M (2017) Human gut-derived commensal bacteria suppress CNS inflammatory and demyelinating disease. Cell Rep 20(6):1269–1277
Martella A, Firth M, Taylor BJ, Göppert A, Cuomo EM, Roth RG, Fisher DI (2019) Systematic evaluation of CRISPRa and CRISPRi modalities enables development of a multiplexed, orthogonal gene activation and repression system. ACS Synthetic Biol 8(9):1998–2006
Mazzini L, Mogna L, De Marchi F, Amoruso A, Pane M, Aloisio I, Cantello R (2018) Potential role of gut microbiota in ALS pathogenesis and possible novel therapeutic strategies. J Clin Gastroenterol 52:S68–S70
Meyza KZ, Blanchard DC (2017) The BTBR mouse model of idiopathic autism–Current view on mechanisms. Neurosci Biobehav Rev 76:99–110
Miyake S, Kim S, Suda W, Oshima K, Nakamura M, Matsuoka T, Morita H (2015) Dysbiosis in the gut microbiota of patients with multiple sclerosis, with a striking depletion of species belonging to clostridia XIVa and IV clusters. PLoS One 10(9) e0137429
Mowry EM, Glenn JD (2018) The dynamics of the gut microbiome in multiple sclerosis in relation to disease. Neurol Clin 36(1):185–196
Murgatroyd C, Patchev AV, Wu Y, Micale V, Bockmühl Y, Fischer D, Spengler D (2009) Dynamic DNA methylation programs persistent adverse effects of early-life stress. Nat Neurosci 12(12):1559–1566
Nagy-Szakal D, Williams BL, Mishra N, Che X, Lee B, Bateman L, Peterson DL (2017) Fecal metagenomic profiles in subgroups of patients with myalgic encephalomyelitis/chronic fatigue syndrome. Microbiome 5(1):1–17
Neufeld KM, Kang N, Bienenstock J, Foster JA (2011) Reduced anxiety-like behavior and central neurochemical change in germ-free mice. Neurogastroenterol Motil 23(3):255. e119
Noseworthy JH, Lucchinetti C, Rodriguez M, Weinshenker BG (2000) Multiple sclerosis. N Engl J Med 343:938–952
O’Mahony SM, Hyland NP, Dinan TG, Cryan JF (2011) Maternal separation as a model of brain–gut axis dysfunction. Psychopharmacology (Berl) 214(1):71–88
Ogbonnaya ES, Clarke G, Shanahan F, Dinan TG, Cryan JF, O’Leary OF (2015) Adult hippocampal neurogenesis is regulated by the microbiome. Biol Psychiatry 78(4):e7–e9
Okun E, Barak B, Saada-Madar R, Rothman SM, Griffioen KJ, Roberts N, Arumugam TV (2012) Evidence for a developmental role for TLR4 in learning and memory. PLoS One 7(10)
Ota K, Matsui M, Milford EL, Mackin GA, Weiner HL, Hafler DA (1990) T-cell recognition of an immuno-dominant myelin basic protein epitope in multiple sclerosis. Nature 346(6280):183–187
Pärtty A, Kalliomäki M, Wacklin P, Salminen S, Isolauri E (2015) A possible link between early probiotic intervention and the risk of neuropsychiatric disorders later in childhood: a randomized trial. Pediatr Res 77(6):823–828
Perner C, Perner F, Stubendorff B, Förster M, Witte OW, Heidel FH, Grosskreutz J (2018) Dysregulation of chemokine receptor expression and function in leukocytes from ALS patients. J Neuroinflammation 15(1):99
Petimar J, O'Reilly É, Adami HO, van den Brandt PA, Buring J, English DR, Larsson SC (2019) Coffee, tea, and caffeine intake and amyotrophic lateral sclerosis mortality in a pooled analysis of eight prospective cohort studies. Eur J Neurol 26(3):468–475
Petrov VA, Saltykova IV, Zhukova IA, Alifirova VM, Zhukova NG, Dorofeeva YB, Mironova YS (2017) Analysis of gut microbiota in patients with Parkinson’s disease. Bull Exp Biol Med 162(6):734–737
Petschow B, Dore J, Hibberd P, Dinan T, Reid G, Blaser M et al (2013) Probiotics, prebiotics, and the host microbiome: the science of translation. Ann N Y Acad Sci 1306:1–17
Qian Y, Yang X, Xu S, Wu C, Song Y, Qin N, Xiao Q (2018) Alteration of the fecal microbiota in Chinese patients with Parkinson’s disease. Brain Behav Immun 70:194–202
Quigley EM (2013) Gut bacteria in health and disease. Gastroenterol Hepatol 9(9):560
Quigley MA, Hockley C, Carson C, Kelly Y, Renfrew MJ, Sacker A (2012) Breastfeeding is associated with improved child cognitive development: a population-based cohort study. J Pediatr 160(1):25–32
Ranuh R, Athiyyah AF, Darma A et al (2019) Effect of the probiotic Lactobacillus plantarum IS-10506 on BDNF and 5HT stimulation: role of intestinal microbiota on the gut-brain axis. Iran J Microbiol 11(2):145–150
Rao JS, Ertley RN, Lee H-J et al (2007) n-3 polyunsaturated fatty acid deprivation in rats decreases frontal cortex BDNF via a p38 MAPK-dependent mechanism. Mol Psychiatry 12(1):36–46
Reichenberg A, Yirmiya R, Schuld A, Kraus T, Haack M, Morag A, Pollmächer T (2001) Cytokine-associated emotional and cognitive disturbances in humans. Arch Gen Psychiatry 58(5):445–452
Rolls A, Shechter R, London A, Ziv Y, Ronen A, Levy R, Schwartz M (2007) Toll-like receptors modulate adult hippocampal neurogenesis. Nat Cell Biol 9(9):1081–1088
Rooks MG, Garrett WS (2016) Gut microbiota, metabolites and host immunity. Nat Rev Immunol 16:341–352
Rumah KR, Linden J, Fischetti VA, Vartanian T (2013) Isolation of Clostridium perfringens type B in an individual at first clinical presentation of multiple sclerosis provides clues for environmental triggers of the disease. PLoS One 8(10) e76359
Sánchez B, Delgado S, Blanco-Míguez A, Lourenço A, Gueimonde M, Margolles A (2017) Probiotics, gut microbiota, and their influence on host health and disease. Mol Nutr Food Res 61(1):1600240
Sandman CA, Davis EP, Buss C, Glynn LM (2012) Exposure to prenatal psychobiological stress exerts programming influences on the mother and her fetus. Neuroendocrinology 95(1):8–21
Saresella M, Mendozzi L, Rossi V, Mazzali F, Piancone F, LaRosa F, Clerici M (2017) Immunological and clinical effect of diet modulation of the gut microbiome in multiple sclerosis patients: a pilot study. Front Immunol 8:1391
Savignac HM, Tramullas M, Kiely B, Dinan TG, Cryan JF (2015) Bifidobacteria modulate cognitive processes in an anxious mouse strain. Behav Brain Res 287:59–72
Sayar GH, Cetin M (2016) Psychobiotics: the potential therapeutic promise of microbes in psychiatry. Klinik Psikofarmakol Bülteni 26(2):93–102
Schwarcz R, Bruno JP, Muchowski PJ, Wu HQ (2012) Kynurenines in the mammalian brain: when physiology meets pathology. Nat Rev Neurosci 13(7):465–477
Shahi SK, Freedman SN, Mangalam AK (2017) Gut microbiome in multiple sclerosis: the players involved and the roles they play. Gut microbes 8(6):607–615
Sharma V, Kaur S (2020) The Effect of Probiotic Intervention in Ameliorating the Altered Central Nervous System Functions in Neurological Disorders: A Review. Open Microbiol J 14(1)
Shishov VA, Kirovskaya TA, Kudrin VS, Oleskin AV (2009) Amine neuromediators, their precursors, and oxidation products in the culture of Escherichia coli K-12. Appl Biochem Microbiol 45(5):494–497
Skaper SD, Facci L, Zusso M, Giusti P (2018) An inflammation-centric view of neurological disease: beyond the neuron. Front Cell Neurosci 12:72
Smith SM, Vale WW (2006) The role of the hypothalamic-pituitary-adrenal axis in neuroendocrine responses to stress. Dialogues Clin Neurosci 8(4):383
Smith CJ, Emge JR, Berzins K et al (2014) Probiotics normalize the gutbrain- microbiota axis in immunodeficient mice. Am J Physiol Gastrointest Liver Physiol 307(8):G793–G802
Steyn FJ, Ioannides ZA, van Eijk RP, Heggie S, Thorpe KA, Ceslis A, Henderson RD (2018) Hypermetabolism in ALS is associated with greater functional decline and shorter survival. J Neurol Neurosurg Psychiatry 89(10):1016–1023
Sudo N, Chida Y, Aiba Y, Sonoda J, Oyama N, Yu XN, Koga Y (2004) Postnatal microbial colonization programs the hypothalamic–pituitary–adrenal system for stress response in mice. J Physiol 558(1):263–275
Takata K, Kinoshita M, Okuno T, Moriya M, Kohda T, Honorat JA, Sakoda S (2011) The lactic acid bacterium Pediococcusacidilactici suppresses autoimmune encephalomyelitis by inducing IL-10-producing regulatory T cells. PLoS One 6(11) e27644
Tan AH, Chong CW, Song SL, Teh CSJ, Yap IKS, Loke MF, Lim SY (2018) Altered gut microbiome and metabolome in patients with multiple system atrophy. Mov Disord Clin Pract Soc 33(1):174
Tap J, Derrien M, Törnblom H, Brazeilles R, Cools-Portier S, Doré J, Simrén M (2017) Identification of an intestinal microbiota signature associated with severity of irritable bowel syndrome. Gastroenterology 152(1):111–123
Thomas CA, Hong T, van Pijkeren JP, Hemarajata P, Trinh DV, Hu W, Britton RA, Kalkum M, Versalovic J (2012) Histamine derived from probiotic Lactobacillus reuteri suppress TNF via modulation of PKA and ERK signalling. PLoS One 7(2) e31951
Thursby E, Juge N (2017) Introduction to the human gut microbiota. Biochem J 474(11):1823–1836
Tillisch K, Labus J, Kilpatrick L, Jiang Z, Stains J, Ebrat B, Mayer EA (2013) Consumption of fermented milk product with probiotic modulates brain activity. Gastroenterology 144(7):1394–1401
Toepfer CF, Klauser A, Riepl RL, Muller-Felber W, Pongratz D, M. (2000) Gastrointestinal dysfunction in amyotrophic lateral sclerosis. Amyotroph Lateral Scler Other Motor Neuron Disord 1(1):15–19
Tomova A, Husarova V, Lakatosova S, Bakos J, Vlkova B, Babinska K, Ostatnikova D (2015) Gastrointestinal microbiota in children with autism in Slovakia. Physiol Behav 138:179–187
Tripathi AK, Ray AK, Mishra SK (2022) Molecular and pharmacological aspects of piperine as a potential molecule for disease prevention and management: evidence from clinical trials. Beni-Suef Univ J Basic Appl Sci 11(1):1–24
Tsavkelova EA, Botvinko IV, Kudrin VS, Oleskin AV (2000) Detection of neurotransmitter amines in microorganisms with the use of high-performance liquid chromatography. Dokl Biochem 372(1–6):115
Turner JR (2009) Intestinal mucosal barrier function in health and disease. Nat Rev Immunol 9(11):799–809
Varghese FP, Brown ES (2001) The hypothalamic-pituitary-adrenal axis in major depressive disorder: a brief primer for primary care physicians. Prim Care Companion J Clin Psychiatry 3(4):151
Wall R, Ross RP, Shanahan F, O’Mahony L, Kiely B, Quigley E, Stanton C (2010) Impact of administered bifidobacterium on murine host fatty acid composition. Lipids 45(5):429–436
Wall R, Marques TM, O’Sullivan O, Ross RP, Shanahan F, Quigley EM, Fouhy F (2012) Contrasting effects of Bifidobacterium breve NCIMB 702258 and Bifidobacterium breve DPC 6330 on the composition of murine brain fatty acids and gut microbiota. Am J Clin Nutr 95(5):1278–1287
Wallace CJ, Milev R (2017) The effects of probiotics on depressive symptoms in humans: a systematic review. Ann Gen Psychiatry 16(1):14
Wang L, Christophersen CT, Sorich MJ, Gerber JP, Angley MT, Conlon MA (2011) Low relative abundances of the mucolytic bacterium Akkermansiamuciniphila and Bifidobacterium spp. in feces of children with autism. Appl Environ Microbiol 77(18):6718–6721
Wu S, Yi J, Zhang YG, Zhou J, Sun J (2015) Leaky intestine and impaired microbiome in an amyotrophic lateral sclerosis mouse model. Physiol Rep 3(4)
Yan F, Polk DB (2011) Probiotics and immune health. Curr Opin Gastroenterol
Yeon SW, You YS, Kwon HS, Yang EH, Ryu JS, Kang BH, Kang JH (2010) Fermented milk of Lactobacillus helveticus IDCC3801 reduces beta-amyloid and attenuates memory deficit. J Funct Foods 2(2):143–152
Zareie M, Johnson-Henry K, Jury J, Yang PC, Ngan BY, McKay DM, Sherman PM (2006) Probiotics prevent bacterial translocation and improve intestinal barrier function in rats following chronic psychological stress. Gut 55(11):1553–1560
Zhang YG, Wu S, Yi J, Xia Y, Jin D, Zhou J, Sun J (2017) Target intestinal microbiota to alleviate disease progression in amyotrophic lateral sclerosis. Clin Ther 39(2):322–336
Zhang M, Ma W, Zhang J, He Y, Wang J (2018) Analysis of gut microbiota profiles and microbe-disease associations in children with autism spectrum disorders in China. Sci Rep 8(1):1–9
Zhao C, Deng W, Gage FH (2008) Mechanisms and Functional Implications of Adult Neurogenesis. Cell 132
Zhou T, Ahmad TK, Gozda K, Truong J, Kong J, Namaka M (2017) Implications of white matter damage in amyotrophic lateral sclerosis. Mol Med Rep 16(4):4379–4392
Zhuang ZQ, Shen LL, Li WW, Fu X, Zeng F, Gui L, Zheng P (2018) Gut microbiota is altered in patients with Alzheimer’s disease. J Alzheimers Dis 63(4):1337–1346
Author information
Authors and Affiliations
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2022 The Author(s), under exclusive license to Springer Nature Singapore Pte Ltd.
About this chapter
Cite this chapter
Jose, J.V., Aliya, S. (2022). Potential Role of Probiotics on Gut Microbiota in Neurological Disease. In: Tripathi, A.K., Kotak, M. (eds) Gut Microbiome in Neurological Health and Disorders. Nutritional Neurosciences. Springer, Singapore. https://doi.org/10.1007/978-981-19-4530-4_10
Download citation
DOI: https://doi.org/10.1007/978-981-19-4530-4_10
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-19-4529-8
Online ISBN: 978-981-19-4530-4
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)