Abstract
Mini PCNL has evolved as an important player in the scenario of a personalized approach to stone management. With regard to patient selection and postoperative management imaging plays an important role. In this chapter different imaging modalities will be discussed with focus on how preoperative diagnostic imaging contributes to selecting the right patient for the right treatment, and how postoperative evaluation of complications and stone free rate contribute to optimal patient management, taking into consideration burden of ionized radiation in a recurrent disease population through selective diagnostic imaging strategies.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
1 Introduction
Traditionally, percutaneous nephrolithotomy (PCNL) has been advocated for the treatment of patients with large and/or complex renal calculi. During recent years miniaturizing the access in PCNL (mini PCNL) has widened the indications for PCNL, and continuing evolutions in access and intracorporeal lithotripsy technology have increased the possibilities of a true Personalized Stone Approach (PSA), taking into consideration best available evidence, patients’ preferences, and expectations as well as surgeons’ clinical expertise (Fig. 3.1) [1]. In this perspective, imaging is an essential tool for patient selection, access planning, as well as for complication and outcome evaluation.
PSA in an EBM perspective. (PSA = Personalized Stone Approach; EBM = Evidence Based Medicine; [Adapted from Axelsson et al. World J Urol [1]])
2 Introducing the RALARA Principle and Imaging in Nephrolithiasis
Nephrolithiasis is often recurrent, and patients with kidney stones are at risk of high radiation exposure [2]. To reduce ionized radiation in stone patients it is of upmost importance to apply the ALARA principle. ALARA stands for “as low as reasonably achievable”, which should be an integral part of all activities that involve the use of ionized radiation to prevent unnecessary exposure as well as overexposure. Further details regarding the ALARA principle will be outlined in the next chapter.
In patients undergoing PCNL there are several additional factors contributing to high radiation exposure, including high body mass index (BMI), multiple tract access, complex renal anatomy, and increased stone burden [3]. Therefore, the concept of ALARA is of special importance in this group of patients. On the other hand, we need sufficient and reliable diagnostic imaging to define indication for treatment, for performing safe surgery, and for evaluation of outcome. In this respect, we introduce the concept of RALARA, “risk as low as reasonably achievable”, taking into consideration both 1) risk of performing the imaging procedure (radiation risk) and 2) risk of not performing the imaging procedure, potentially resulting in insufficient information for treatment decisions. Therefore, imaging strategy needs to be personalized (Fig. 3.2).
RALARA—Risk as Low as Reasonably Achievable. (Risk of performing imaging [ionized radiation hazards] should be weighed up against risk of not performing sufficient and reliable diagnostic imaging, in order to be able to select the right treatment for the right patient. In this clinical decision-making scenario, the concept of RALARA interacts dynamically with case and procedure complexity, patient’s consent and potential radiation risks for the individual patient)
2.1 Paediatric Nephrolithiasis
Since mini PCNL plays a particular role in management of paediatric urolithiasis, special attention should be on imaging modalities for paediatric upper urinary tract stone disease. Ideally radiation free imaging should be used; however, for planning PCNL procedures this may not be sufficient. Ultrasonography (US) is the preferred initial diagnostic examination in children with the advantages of being easily available and with no radiation exposure [4] (Fig. 3.3). However, US for diagnosis of urolithiasis and characterization of renal anatomy do have limitations. US accuracy is very operator dependent, and sensitivity and specificity for detection of renal stones have been reported to be 61–93% and 95–100%, respectively [4, 5]. Additionally, US often does not present renal and perirenal anatomy and details of stone burden accurately. Therefore, additional imaging is often necessary for treatment planning, especially if PCNL is considered. The other radiation free alternative, Magnetic Resonance Urography (MRU), is seldomly used [6]. Although Gadolinium-enhanced-excretory MRU has shown up to 90–100% sensitivity for urolithiasis diagnosis and is excellent for presenting anatomical details and severity of obstruction, the examination has considerable limitations, including longer procedure duration, need for general anaesthesia, motion artefacts, and high costs [4, 6]. Combining US and plain abdominal radiography (Kidney-Ureter-Bladder = KUB) with retrograde pyelography at surgery may be enough for PCNL surgical strategy; however, since ultra-low dose CT (ULD-CT) protocols with radiation doses close to KUB (0.5 mSv) and without limiting image quality in the paediatric population, ULD-CT has been suggested as standard prior to PCNL in children [7] (Fig. 3.4). Again, due to the extreme diversity of stone disease, especially in the population needing PCNL, a PSA imaging strategy in children should be applied, taking into consideration the concept of RALARA.
Ultralow dose computerized tomography [ULD-CT]. (ULD-CT in a 3-year-old boy with a very dense stone [mean HU 2501]. The examination could be designed with a radiation dose of 0.36 mSv, which is comparable or even less than a KUB. The boy was treated by mini-PCNL and Thulium fiber laser lithotripsy, since this very hard stone probably would have been Shock Wave Lithotripsy [SWL] resistant. In this way the imaging modality helped choosing the right treatment up front, thereby enabling a personalized stone approach [PSA])
Overall diagnostic imaging considerations concerning mini PCNL will be discussed in the following.
3 Preoperative Diagnostic Imaging
Preoperative imaging is considered the major tool for individualizing stone management; thereby enabling PSA [1]. Ideally imaging should characterize the stone, present renal and perirenal anatomy as well as estimate kidney function [8].
3.1 Stone Characteristics and Renal Anatomy
Previously, Intravenous Urography (IVU) (Fig. 3.5) was considered the gold standard for diagnosis and treatment planning of urolithiasis; however, nowadays CT has almost completely taken over the stone imaging scenario. For assessment of acute flank pain, Non-contrast CT (NCCT) with sensitivities and specificities for evaluating renal and ureteral calculi approaching 100% performs significantly better than IVU (evidence level 1a) [9,10,11,12]. Regarding treatment strategies in PCNL, CT examinations are of particular value for (1) assessment of stone characteristics (composition and volume) and (2) for defining renal anatomy, in order to choose optimal access size and site, which both are paramount in mini PCNL.
3.1.1 Stone Characteristics
CT-attenuation values, expressed as Hounsfield Units (HU), are widely used to estimate stone composition and hardness [13]. This may be of importance, when selecting endoscopic procedures (RIRS, mini PCNL) instead of SWL, since higher HU values (above 900–1200 HU) have been found to be independent predictors of SWL failure [13, 14]. It has been shown, however, that the correlation between HU and SWL failure is not linear despite identical stone composition, suggesting a multitude of factors involved (15). By using high-resolution detection of internal structure of renal calculi with helical CT, it was found that internal structure rather than HU of calcium oxalate monohydrate (COM) [15] and cystine stones [16] predicted lithotripsy fragility in vitro. COM and cystine stones of homogeneous structure required almost twice as many Shock Waves (SWs) to comminute than stones of similar mineral composition that exhibit internal structural features (void regions) that were visible by CT (Fig. 3.6). Hounsfield unit values of COM as well as cystine stones did not correlate with stone fragility. Thus, it seems that it is stone morphology, rather than X-ray attenuation, which correlates with fragility to SWs in COM and cystine stones, and these stone characteristics may be used for selection of patients to primary SWL or primary endoscopic treatment, such as mini PCNL, increasing efficacy of both (Fig. 3.7).
Non-contrast CT[NCCT] in bone window. (NCCT [left] demonstrating a branched stone in the upper pole of the left kidney that has a close relation to the spleen, which potentially would be a problem in an upper pole access. In the bone window it appears that the stone is heterogenous with void regions, which makes the stone easier fragmentable with SWL. Therefore, SWL was preferred, and after one SWL session the patient was almost stone free with only minor fragments left in the lower pole demonstrated on KUB [right]. Prior to SWL the patient had a JJ inserted to prevent adverse events of a Steinstrasse. In this way imaging helped personalizing treatment, focusing on both efficacy and safety)
Traditionally, stone diameters have been used to characterize stone burden. This is a routine that stems from the era of plain abdominal radiography (KUB) and IVU; however, with use of CT technology exact volume of stone burden is achievable, and volume seems to correlate better to treatment outcome and should be used in clinical as well as research settings [17, 18].
Whether a KUB should be added to the NCCT before stone treatment is a matter of debate [19]. KUB envisions radiopaque stones; including calcium stones, cystine and struvite stones, whereas uric acid stones are radiolucent (Fig. 3.8). This may be useful information during access as well as during endoscopy when evaluating residual fragments with fluoroscopy. This information may also be achieved using the CT planning image (CTI, Scout, Topogram, etc.), since it has been shown that kidney stones visible on CTI are also visible on KUB/fluoroscopy (positive predictive value 100%) [20]. Thus, adding a KUB seems to be unnecessary exposure of ionized radiation, if a CTI is available.
Low-dose 3-D CT. (In this 9-year-old boy initial ultrasonography gave the suspicion of a large stone in the lower part of the left kidney. For treatment planning a low-dose CT [1.7 mSv] was performed, and this examination gave the suspicion of a dual system [upper right], which was confirmed by a retrograde pyelography during surgery [lower right]. 3-D reformatting [left] helped deciding proper calyx for access in mini PCNL. Thus, the slightly higher radiation dose was justified by the additional information achieved, securing efficacious and safe surgery)
3.1.2 Renal Anatomy
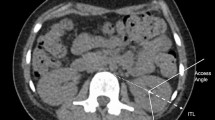
Regarding anatomical information, NCCT has been considered less suitable than IVU, and patients with complex stones or anatomy scheduled for PCNL may need additional imaging [13]. This can be done by a retrograde contrast study during surgery, which often is enough for a safe puncture. Additionally, this gives an impression of the dynamic anatomy of the collecting system, which may define need for a miniaturized access (narrow calyceal neck, diverticulum stones. Etc.). This also may be achieved by a contrast enhanced CT, which according to the Guidelines of European Association of Urology (EAU) should be done if renal stone removal is planned and the anatomy of the renal collecting system needs to be assessed [13, 21, 22]. Excretory contrast studies (ECT) may mask stones [8, 23]; however, viewing the images in the bone window most often will give valuable information regarding stone and calyceal system interrelations. In complex cases where access difficulties are anticipated, 3-D CT pyelography may be beneficial for detailed evaluation of stone burden and anatomy as well as for perirenal organ mapping, thereby helping to choose the right plane of access and at the same time avoiding injury to adjacent organs (Fig. 3.9). This may be of especial importance in patients with abnormal body habitus (Fig. 3.10). Three-dimensional CT pyelography demonstrates calculi in parallel calyces, calyceal orientation, and size of calyceal necks as well as presence of a calyceal diverticulum and other anatomical abnormalities, which may be highly valuable when deciding the best route of access, access size, and when performing combined endoscopic intrarenal surgery (ECIRS) (Fig. 3.11) [21, 24, 25]. In this way the advantages of a miniaturized access often become evident.
3.2 Perirenal Organ Mapping
Preoperative imaging should provide information regarding interpositioned organs (colon, spleen, liver, pleura and lung) within the planned percutaneous access route, thereby reducing risk of organ injury during PCNL [23]. In a study comparing CTs in supine and prone position, it was shown that colon is more often positioned behind the colon (retrorenal) in prone (10%) compared to the supine position (1.9%) [26], which may suggest a lower risk of colon injury in supine PCNL. Another study has demonstrated that colon is more often retrorenal on the left side, especially in women [27] (Fig. 3.12). This information may be used when planning patient positioning and access route. Theoretically, preoperative CT for PCNL planning should be performed with the patient in the same position in which surgery is planned [28]. In our practice we perform all CTs in supine position, and when the colon position is considered a problem, multiplanar reformatted images (3-D CT) are provided, since these often gives a more reliable estimate of risk of colon injury compared to evaluation of axial CT images [29].
If a supracostal puncture is planned, the relation of the access tract to the pleura and the lung must be considered. 3-DCT in both inspiratory and expiratory phases may be helpful in showing the relationships between the kidney and pleura/diaphragm/ribs [30]. It is generally recommended to do percutaneous puncture while the patient is in expiration [8].
3.3 Estimation of Renal Function
If the kidney function of the stone-bearing kidney is suspected to be severely decreased (reduced parenchymal thickness), a renogram/scintigraphy is considered mandatory for exact evaluation of the renal split function. The threshold deciding whether the patient should be offered PCNL or nephrectomy is depending on the total combined renal function, which must be evaluated by a clearance estimate (Fig. 3.13).
Plain chest X-ray. (PCNL was performed through an upper pole access for a right-sided partial staghorn stone [right]. Upper calyx was dilated and accessed just above costa 12. Surgery was uneventful, and patient was rendered stone free. Postoperatively, patient developed dyspnoea and pain at deep inspiration. Patient was hemodynamic stable with no haemoglobin drop. Plain chest X-ray showed pleural fluid accumulation on the right side. The pleural cavity was drained for clear fluid with an 8.3 Fr pigtail drain that could be removed two days postoperatively)
4 Postoperative Imaging
4.1 Evaluation of Complications
Although, miniaturized PCNL seems to have a lower complication rate, suspicion of procedure related complications postoperatively should prompt immediate imaging according to the specific clinical symptoms to limit serious sequelae.
Access above the ribs is associated with a higher risk of pleural injury [8]. In supra 12th and supra 11th accesses, hydro- or pneumothorax have been reported in up to 12% and 35%, respectively [8]. Chest fluoroscopy during surgery can be used to detect pleural complications, and this allows immediate drainage [8, 31]. If the patient develops symptoms indicative of pleural injury postoperatively, a chest X-ray or CT should be performed (Fig. 3.14).
Angiography with transarterial superselective embolization. (The patient presented with intermittent haemorrhage through the nephrostomy drain and haemoglobin drop 18 hours post-PCNL. Transarterial angiography was performed, showing an intrarenal pseudoaneurism that was treated by superselective embolization)
If the patient develops postoperative diarrhoea/haematochezia, signs of peritonitis, or passage of gas or faeces through the nephrostomy tract, a colonic perforation should be suspected, and such findings should prompt an abdominal CT, possibly with injection of contrast medium through the nephrostomy tube, if this has been placed [32]. Since colonic injuries are most often retroperitoneal, most of these can be managed conservatively.
Bleeding during and after PCNL is most often venous and usually self-limiting. Severe postprocedural haemorrhage is rarely seen in mini PCNL. However, if it happens, an arteriovenous fistula or a pseudoaneurysm must be suspected, and the patient should undergo immediate angiography with the possibility of performing superselective embolization, which is both a lifesaving and a nephron-sparing intervention [33]. Using B-mode with colour Doppler ultrasound for access guidance may avoid injury to the renal blood vessels during PCNL [34].
4.2 Evaluation of Residual Stones
Intraoperative imaging. Fluoroscopy during the PCNL procedure is used for nephroscopy guidance to detect residual stones. High magnification rotational fluoroscopy as an adjunct to aggressive nephroscopy has been shown to increase detection rate of residual calculi, and thereby may increase stone free rate (SFR) [35].
Postoperative imaging. Postoperative imaging for residuals helps deciding whether the patient needs additional treatment (repeat nephroscopy, SWL, ureteroscopy, etc.). Also, postoperative imaging is used for selecting patients that are candidates for metaphylaxis. Need of sensitive image studies is highlighted by the fact that patients with residual fragments are at higher risk for recurrence compared with patients rendered stone free [36]. In this perspective, KUB and nephrotomograms have been challenged, since these imaging modalities seem to overestimate SFR by 35% and 17% [8], respectively. In prospective series of patients undergoing PCNL for large and staghorn calculi, NCCT has been shown to be superior to KUB with regard to detecting residuals (NCCT sensitivity 100% compared to KUB sensitivity 46%) [8, 37,38,39]. The downside to conventional NCCT is radiation dose, and subsequently ultra-low dose CT protocols have been developed with radiation doses close to KUB [40], and in our experience such protocols may be equally good for postprocedural evaluation of SFR.
Ultrasonography (US) may be an appealing modality without radiation concerns for residual fragment evaluation; however, it has been documented that US has a poor sensitivity for residual fragment detection post-PCNL [41, 42]. Thus, it is evident that detection rate of residual stone burden is highly dependent on the applied imaging modality, which may influence clinical decision making. NCCT has the highest sensitivity for detecting residual fragments; however, less than half of patients with residual fragments on NCCT seem to experience a subsequent stone-related event [43], and thus early CT evaluation may lead to overtreatment. Taking into account the potential hazards of ionized radiation, this calls for a selective, personalized approach, in which the highly sensitive CT evaluation should be restricted to those patients, who have a high risk of residuals, and in whom residual calculi mandate aggressive treatment, for instance, infection and cystine stones. Timing of follow-up imaging has been a matter of debate. On the one hand, an early follow-up within the first days postoperatively may diagnose dust or residual fragments that will pass spontaneously without causing any adverse events, and as a consequence of this the EAU Guidelines propose imaging at four weeks to be most appropriate for evaluating stone free rate (SFR) [22, 44, 45]. On the other hand, early diagnosis of significant residual stone fragments will enable second-look nephroscopy in case a nephrostomy tube was placed. Thus, due to the diversity of stone disease follow-up timing of course also will have to be personalized, and according to above considerations a selective approach seems advisable [46].
5 Summary
Mini PCNL has evolved as an important treatment modality to enable a personalized approach to stone treatment (PSA). In this, imaging plays a crucial role for selection of the right patient to the right treatment. CT has emerged as the image modality of choice for defining stone burden and renal anatomy, as well as relationship of the kidney to adjacent organs. Also, with regard to complication management and detection of residual stone burden (SFR), CT plays an important role. However, both regarding diagnostic and follow-up imaging ionized radiation risk should be thoroughly considered, since stone formers are at increased risk of having cumulative doses of radiation, and in selective patients, such as children and severely recurrent stone formers, less radiation-heavy imaging modalities should be considered. In other words, the risks of ionized radiation should outweigh the risks of overlooking stone characteristics, anatomical details, and residual fragments (RALARA). Uroradiologists and urologists should work in close collaboration to design selective imaging protocols in such a way that the amount of ionized radiation is stratified and justified according to the clinical question.
References
Axelsson T, Cracco C, Desai M, Hasan MN, Knoll T, Montanari E, Pérez-Fentes D, Starub M, Thomas K, Williams JC Jr, Brehmer M, Osther PJS. Consultation on kidney stones, Copenhagen 2019: lithotripsy in percutaneous nephrolithotomy. World J Urol. 2020 Jul 29; https://doi.org/10.1017/s00345-020-03383-w.
Miller DT, Semins MJ. Minimizing radiation dose in management of stone disease: how to achieve ‘ALARA’. Curr Opin Urol, 2020 dec 31. Publish ahead of print. https://doi.org/10.1097/MOU.0000000000000845. Online ahead of print PMID: 33394609.
Lipkin ME, Preminger GM. Risk reduction strategy for radiation exposure during percutaneous nephrolithotomy. Curr Opin Urol. 2012;22(2):139–43. https://doi.org/10.1097/MOU.0b013e32834fc36a.
Grivas N, Thomas K, Drake T, Donaldson J, Neisius A, Petrik A, Ruhayel Y, Seitz C, Türk C, Skolarikos A. Imaging modalities and treatment of paediatric upper tract urolithiasis: a systematic review and update on behalf of the EAU uroliothiasis guidelines panel. J Ped Urology. 2010:612–24.
Robertson NP, Dillman JR, O’Hara SM, DeFoor WR Jr, Reddy PP, Giordano RM, et al. Comparison of ultrasound versus computed tomography for the detection of kidney stones in the pediatric population: a clinical effectiveness study. Pediatr Radiol. 2018;48:962–72.
Routh JC, Graham DA, Nelson CP. Trends in imaging and surgical management of pediatric urolithiasis at American pediatric hospitals. J Urol. 2010;184(4 suppl):1816–22.
Gedik A, Tutus A, Kayan D, Yilmaz Y, Bircan K. Percutaneous nephrolithotomy in pediatric patients: is computerized tomography a must? Urol Res. 2011;39:45–9.
Park S, Pearle MS. Imaging for percutaneous renal access and management of renal calculi. Urol Clin N Am. 2006:353–64.
Chen TT, Wang C, Ferrandino MN, Scales CD, Yoshizumi TT, Preminger GM, Lipkin ME. Radiation exposure during the evaluation and management of nephrolithiasis. J Urol. 2015;194:878–85.
Miller OF, Rineer SK, Reichard SR, Buckley RG, Donovan SM, Graham IR, Goff WB, Kane CJ. Prospective comparison of unenhanced spiral computed tomography and intravenous urogram in the evaluation of acute flank pain. Urology. 1998;52:982–7.
Pfister SA, Deckhart A, Laschke S, Dellas S, Otto U, Buitrago C, Roth J, Wiesner W, Bontgartz G, Gasser TC. Unenhanced helical computed tomography vs intervenous urography in patients with acute flank pain: accuracy and economic impact in a randomized prospective trial. Eur Radiol. 2003;13:2513–20.
Shine S. Urinary calculus IVU vs. CT renal stone? A critical appraised topic. Comput Biol Med. 2008;33:41–3.
Villa L, Giusti G, Knoll T, Traxer O. Imaging for urinary stones: update in 2015. Eur Urol Focus. 2016;2:122–9.
Ouzaid I, Al-qahtani S, Dominique S, et al. A 970 Hounsfield units (HU) threshold of kidney stone density on non-contrast computed tomography (NCCT) improves patients’ selection for extracorporeal shockwave lithotripsy (ESWL): evidence from a prospective study. BJU Int. 2012;110:E438–42.
Williams JC Jr, Saw KC, Paterson RF, Hatt EK, McAteer JA, Lingeman JE. Variability of renal stone fragility in shock wave lithotripsy. Urology. 2003;61:1092–6.
Zarse CA, Hameed TA, Jackson ME, Pischalnikov YA, Lingemen JE, JA MA, Williams JC Jr. CT visible internal stone structure, but not Hounsfield unit value, of calcium oxalate monohydrate (COM) calculi predicts lithotripsy fragility in vitro. Urol Res. 2007;35:201–6.
Kim SC, Burns EK, Lingeman JE, Paterson RF, McAteer JA, Williams JC Jr. Cystine calculi: correlation of CT-visible structure, CT number, and stone morphology with fragmentation by shock wave lithotripsy. Urol Res. 2007;35:319–24.
Panthier F, Doizi S, Illoul L, Berthe L, Traxer O. Developing free three-dimensional software for surgical planning for kidney stones: volume is better than diameter. Eur Urol Focus 2020; 23: S2405–4569(20)30161–9. https://doi.org/10.1016/j.euf.2020.06.003.
Brehmer M, Beckman MO, Magnusson A. Three-dimensional computed tomography planning improves percutaneous surgery. Scand J Urol. 2014;48(3):316–23.
Bariol SV, Tolley DA. What is the best imaging for stone management? BJU Int. 2005;95(1):4–5.
Graumann O, Osther SS, Spasojevic D, Osther PJ. Can the CT planning image determine whether a kidney stone is radiopaque on a plain KUB? Urol Res. 2012;40(4):333–7.
Patel U, Walkden RM, Ghani KR, Anson K. Three-dimensional CT pyelography for planning of percutaneous nephrolithotomy: accuracy of stone measurement, stone detection and pelvicalyceal reconstruction. Eur Radiol. 2009;19:1280–8.
European Association of Urology (EAU) guidelines on Urolithiasis. ISBN 978–94–92671-04-2. https://uroweb.org/guideline/urolithiasis/#note_42-44. Accessed 17 Jan 2021.
Nolte-Emsting C, Cowan N. Understanding multislice CT urography techniques: many roads lead to Rome. Eur Radiol. 2006;16:2670–86.
Thiruchelvam N, Mostafid H, Ubhayakar G. Planning percutaneous nephrolithotomy using multidetector computed tomography urography, multiplanar reconstruction and three-dimensional reformatting. BJU Int. 2005;95:1280–4.
Heyns CF, van Geldern WF. 3-dimensional imaging of the pelviocalyceal system by computerized tomographic reconstruction. J Urol. 1990;144:1335–8.
Hopper KD, Sherman JL, Lurthke JM, Ghaed N. The retrorenal colon in the supine and prone patient. Radiology. 1987;162:443–50.
Azhar RA, Szymanski KM, Lemercier E, Valenti D, Andonian S, Anidjar M. Visceral organ-to-percutaneous tract distance is shorter when patients are placed in the prone position on bolsters compared with the supine position. J Endourol. 2011;25:687–90.
Chalasani V, Bissoon D, Bhuvanagir AK, Mizzi A, Dunn IB. Should PCNL patients have a CT in the prone position preoperatively? Can J Urol. 2010;17:5082–6.
Traxer O. Management of injury to the bowel during percutaneous stone removal. J Endourol. 2009;23:1777–80.
Tuttle DN, Yeh BM, Meng MV, Breiman RS, Stoller ML, Coakley FV. Risk of injury to adjacent organs with lower-pole fluoroscopically guided percutaneous nephrostomy: evaluation with prone, supine, and multiplanar reformatted CT. J Vasc Interv Radiol. 2005;16:1489–92.
Ogan K, Corwin SC, Smith T, Watumull LM, Mullican MA, Cadeddu JA, Pearle MS. Sensitivity of chest fluoroscopy compared with chest CT and chest radiography for diagnosing hydropneumothorax in association with percutaneous nephrolithotomy. Urology. 2003;62:988–92.
Valdivia JG, Scarpa RM, Duvdevani M, Gross AJ, Nadler RB, Nutahare K, de la Rosette JJ. Supine versus prone position during percutaneous nephrolithotomy: a report from the clinical research Office of the Endourological Society Percutaneous Nephrolithotomy Global Study. J Endourol. 2011;25:1619–25.
Jain V, Ganpule A, Vyas J, Muthu V, Sabnis MRB, Rajapurkar MM, Desai MR. Management of non-neoplastic renal hemorrhage by transarterial embolization. Urology. 2009;74:522–7.
Lu M-H, Pu X-Y, Gao X, Zhou X-F, Qiu J-G, Si-Tu J. A comparative study of clinical value of single B-mode ultrasound guidance and B-mode combined with color doppler ultrasound guidance in mini-invasive percutaneous nephrolithotomy to decrease hemorrhagic complications. Urology. 2010;76:815–20.
Portis AJ, Laliberte MA, Drake S, Holtz C, Rosenberg MS, Bretzke CA. Intraoperative fragment detection during percutaneous nephrolithotomy: evaluation of high magnification rotational fluoroscopy combined with aggressive nephroscopy. J Urol. 2006;175:162–6.
Gettman MT, Pearle MS. Evaluation of residual stones following percutaneous nephrolithotomy. Braz J Urol. 2000;26:579–83.
Pearle MS, Watamull LM, Mullican MA. Sensitivity of noncontrast helical computerized tomography and plain film radiography compared to flexible nephroscopy for detecting residual fragments after percutaneous nephrolithotomy. J Urol. 1999;162:23–6.
Park J, Hong B, Park T, Park HK. Effectiveness of noncontrast computed tomography in evaluation of residual stones after percutaneous nephrolithotomy. J Endourol. 2007;21:684–7.
Osman Y, El-Tabey N, Rafai H, Elnahas A, Shoma A, Eraky I, Kenwy M, El-Kapany H. Detection of residual stones after percutaneous nephrolithotomy: role of nonenhanced spiral computerized tomography. J Urol. 2008;179:198–200.
Hyams ES, Shah O. Evaluation and follow-up of patients with urinary lithiasis: minimizing radiation exposure. Curr Urol Rep. 2010;11:80–6.
Fowler KAB, Locken JA, Duchesne JH, Williamson MR. US for detecting renal calculi with nonenhanced CT as a reference standard. Radiology. 2002;222:109–33.
Ulusan S, Koc Z, Tokmak N. Accuracy of sonography for detecting renal stone: comparison with CT. J Clin Ultrasound. 2007;35(5):256–61.
Gokce MI, Ozden E, Suer E, Gulpinar B, Gulpinar O, Tangal S. Comparison of imaging modalities for detection of residual fragments and prediction of stone related events following percutaneous nephrolithotomy. Int Braz J Urol. 2015;41(1):86–90.
Olvera-Posada D, Ali SN, Dion M, Alenezi H, Denstedt J, Razvi H. Natural history of residual fragments after percutaneous nephrolithotomy: evaluation of factors related to clinical events and intervention. Urology. 2016;97:46–50.
Tokas T, Habicher M, Junker D, Herrmann T, Jessen JP, Knoll T, Nagele U. Uncovering the real outcomes of active renal stone treatment by utilizing non-contrast computer tomography: a systematic review of the current literature. World J Urol. 2017;35(6):897–905.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2022 The Author(s), under exclusive license to Springer Nature Singapore Pte Ltd.
About this chapter
Cite this chapter
Osther, S.S., Osther, P.J.S. (2022). Diagnostic Imaging for Mini Percutaneous Nephrolithotomy. In: Agrawal, M.S., Mishra, D.K., Somani, B. (eds) Minimally Invasive Percutaneous Nephrolithotomy. Springer, Singapore. https://doi.org/10.1007/978-981-16-6001-6_3
Download citation
DOI: https://doi.org/10.1007/978-981-16-6001-6_3
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-16-6000-9
Online ISBN: 978-981-16-6001-6
eBook Packages: MedicineMedicine (R0)