Abstract
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) caused the outbreak of pneumonia which originated in Wuhan, China, at the end of 2019 has turned into a global pandemic—now termed coronavirus diseases 2019 (COVID-19). Like previously reported SARS-CoV strains, the newly discovered SARS-CoV-2 was also found to initiate the pathogenesis by binding with the angiotensin-converting enzyme 2 (ACE2), a receptor produced by various organs in the human body. Hence, COVID-19 is a viral multisystem disease which particularly infects the vascular system expressing ACE2 and reduced the ACE2 function; this further complexed by organ-specific pathogenesis related to the damage of cells expressing ACE2, such as alveolus, glomerulus, endothelium, and cardiac microvasculature. Under these conditions, it was advocated that the upregulation of ACE2 expression in predisposing individuals with aberrant renin–angiotensin system (RAS) level to advanced viral load on infection and relatively a greater number of cell death. Recently, a significant role of decreased ACE2 production and inequality between the RAS and ACE2/angiotensin-(1–7)/MAS (mitochondrial Ang system) after the onset of SARS-CoV-2 infection was established as a key factor for multiple organ injury in SARS-CoV-2-infected individuals. Furthermore, restoration of this imbalance has been suggested as a therapeutic approach to attenuate organ injuries in SARS-CoV-2 infection. Based on available data, this chapter presents the updated mechanism of the multi-organ diseases causes by COVID-19 via ACE2 which can be further helpful in the development of specific therapeutics.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
13.1 Introduction
A novel coronavirus, named severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), emerged at the end of 2019 in Wuhan, Hubei Province, China, and since then has spread to become a global pandemic known as coronavirus disease 2019 (COVID-19; Huang et al. 2020a, b; Zhu et al. 2020). SARS-CoV-2 is reported as comparatively highly contagious by comparison to two major human pandemic coronaviruses, namely, severe acute respiratory syndrome coronavirus (SARS-CoV) and the Middle East Respiratory Syndrome coronavirus (MERS-CoV). Since the outbreak of SARS-CoV-2, around 79 million cases of COVID-19 have been documented with nearly 1.7 million deaths worldwide by the end of December 2020.
Genetic analysis of SARS-CoV-2 using complete genome sequencing established that it shared 79.5% sequence similarity with SARS-CoV; pairwise protein sequence analysis for SARS-CoV-2 assisted in its classification under the category of SARS-related coronaviruses (Kirtipal et al. 2020; Yang et al. 2020). To note, both SARS-CoV and SARS-CoV-2 infect the host cells with the aid of a common cell receptor, namely, angiotensin-converting enzyme 2 (ACE2; Yang et al. 2020). Although the mean mortality rate for SARS-CoV-2 is lower than that of SARS and MERS; however, organ failures, such as acute cardiac injury, acute hepatic injury, acute kidney injury, and acute respiratory distress syndrome (ARDS), are widely recorded in serious SARS-CoV-2 infection. The receptor ACE2 is a homolog of angiotensin-converting enzyme (ACE) and spatially expressed in various organs and tissues as an essential factor for extensive biological activities; for instance, in many diseases, ACE2 is known to reduce the deleterious effect of the renin–angiotensin system (RAS; Donoghue et al. 2000; Patel et al. 2017; Santos et al. 2018). Surprisingly, the production of ACE2 protein is low in the lungs but high in type II pneumocytes—a cell type that also expresses TMPRSS2 (transmembrane protease serine 2) and also activates SARS-CoV-2 spike protein to dock with ACE2 receptor for transmission into the host cell (Davidson et al. 2020). Hence, this chapter discusses the role of ACE2 in SARS-CoV-2 infection and provides more comprehensive information on the targeted organs expressing ACE2 which can be beneficial in epidemic management.
13.2 RAS and ACE2 Relation
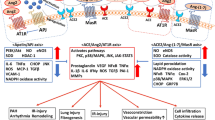
RAS, a complex cell signaling network, is known for essential functions in the regulation of electrolyte and fluid homeostasis, blood pressure, and control the functions of several organs, including the blood vessels, heart, and kidneys (Santos et al. 2018). Angiotensin II (Ang-II)—a most demonstrative bioactive peptide in the RAS network—extensively contributes to the advancement of cardiovascular diseases, such as myocardial infarction, cardiac failure, and hypertension (Keidar et al. 2007). In classic RAS, renin digests the angiotensinogen as substrate and forms decapeptide angiotensin I (Ang-I) followed by removal of two amino acids by ACE from Ang-I at the carboxyl terminus to yield Ang-II (Fig. 13.1). Till now, three Ang-II receptors are discovered and hold common attractions in the nanomolar range toward Ang-II (Keidar et al. 2007). Among these receptors, the binding of angiotensin type 1 receptor (AT1R) with Ang-II results in extracellular matrix remodeling, cell division, inflammatory responses, vasoconstriction, and blood coagulation, while angiotensin type 2 receptor (AT2R) responds to the said effects facilitated by AT1R (Horiuchi et al. 1999). However, the catalytic activity of ACE2 on Ang-I and Ang-II produces angiotensin-(1–7), which connects to the MAS receptor to vascular protection, anti-proliferation, anti-fibrosis, anti-inflammation, and vasodilation. Moreover, Ang-II can also attach to AT2R to reduce the aforementioned consequences intermediated by AT1R (Yang et al. 2020). In 2000, two independent research groups discovered a homolog of ACE named ACE2 that forms the heptapeptide angiotensin-(1–7) by removing the carboxy-terminal phenylalanine in Ang-II (Donoghue et al. 2000, Tipnis et al. 2000). Besides, the formation of angiotensin-(1–7) was also suggested without interruption of Ang-II under the varying influences of ACE2 and ACE (Fig. 13.1). Here, Ang-I is initially hydrolyzed by ACE2 to form angiotensin-(1–9), which is subsequently dissected by ACE to produce angiotensin-(1–7). Additionally, Ang-I was also reported to produce angiotensin-(1–7) by direct activities of endopeptidases and oligopeptidases (Santos et al. 2018). Remarkably, the classical pathway of Ang-II to angiotensin-(1–7) is relatively widespread due to the high affinity between ACE and Ang-I (Santos et al. 2018). Furthermore, Angiotensin-(1–7) also acted as a ligand and attaches with the G-protein-coupled receptor MAS and generates effect against that induced by Ang-II; and thus, applies various events in multiple organs or systems (Patel et al. 2017; Santos et al. 2018).
13.3 ACE2 as Potential Receptors for SARS-CoV-2
The virus instigates the infection cycle primarily through attachment with the aid of functional receptors on the host cell; the functional receptor regulates the transmission, proliferation, and clinical symptoms in the infected individuals (Groneberg et al. 2005; Kuba et al. 2013; Xu et al. 2020a). Thus, such functional receptors are projected as a key element in the avoidance and therapy of viral diseases. Under COVID-19, ACE2 has been recognized as the potential functional receptor required by SARS-CoV-2 for binding with host cells. However, some biological responses associated with viral receptors that could not be elucidated by ACE2 indicated that the functional receptor for SARS-CoV-2 binding is not quite clear. The coronaviruses contained spike protein (S-protein) for attachment with the host receptors; this S-protein shapes a trimer on the surface of virus structure, where each monomer contained a receptor-binding domain (RBD) that binds to the specific receptor on the membrane of the host cell (Li 2016). Interestingly, sequence analysis of S-protein RBD in SARS-CoV-2 suggested its high similarity to SARS-CoV’s RBD than MERS-CoV’s (Xu et al. 2020a). Furthermore, a close association of S-protein RBD in SARS-CoV-2 with human ACE2 molecules was confirmed by computer-guided homology modeling (Xu et al. 2020a). These results were also established by experimental studies using cell lines, for instance, HeLa cells with ACE2 expression were found sensitive to SARS-CoV-2 infection against cells deficient in ACE2 expression (Wan et al. 2020). Therefore, these results suggested the considerable role of ACE2 in SARS-CoV-2 infection and designated it as a potential functional receptor for SARS-CoV-2. Initially, SARS-CoV-2 S-protein structure in perfused conformation was determined using cryo-EM, where the prevalent state of S-protein trimer holds one of the three RBDs placed vertically in a receptor-accessible pose (Bian and Li 2021). Furthermore, structural and biophysical research revealed that the SARS-CoV-2 S-protein bound to ACE2 with a 10- to 20-fold higher affinity than the SARS-CoV S-protein, suggested its significant contribution to enhanced infectious behavior of SARS-CoV-2 against SARS-CoV (Wrapp et al. 2020), and hence, suggested as the significant factor that contributes to more contagious nature of SARS-CoV-2 against SARS-CoV. Subsequently, RBD in SARS-CoV-2 S-protein complexed with ACE2 crystal structure was also determined (Lan et al. 2020). This sequence of experimental work robustly reinforced the ACE2 protein in the host cells as the functional receptor of SARS-CoV-2 (Bian and Li 2021).
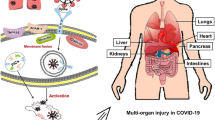
ACE2 receptor is nearly expressed with a varying degree in all the vital organs of the human body. The conventional immunohistochemical approach showed that the primary route of viral infection in the respiratory system is by type II alveolar epithelial cells that express ACE2. Besides, single-cell RNA-seq analysis revealed only weak expression of ACE2 on epithelial cells surface in the oral, nasal mucosa, and nasopharynx, directed to lungs as a main vulnerable organ for SARS-CoV-2 infection (Hamming et al. 2004; Zou et al. 2020a, b). ACE2 expression was also found on myocardial cells, bladder urothelial cells, and proximal tubule cells of the kidney as well as on enterocytes in the ileum of the small intestines (Hamming et al. 2004; Zhang et al. 2020; Zou et al. 2020a, b). Hence, it was suggested that cell-free and macrophage phagocytosis-associated virus can transmit to other organs of the host body with high expression of ACE2 from the lungs via blood circulation (Fig. 13.2). These assumptions are supported by the fact that ~67% of patients in the initial phase of SARS CoV infection suffered from diarrhea, while a considerable number of patients infected by SARS-CoV-2 also exhibited enteric symptoms (Leung et al. 2003; Liu et al. 2020b; Wang et al. 2020a). In this context, active viral replication has been documented in the enterocytes of the small intestine, and SARS-CoV-2 viral particles were also isolated from the fecal specimens of infected individuals (Cheung et al. 2020; Lamers et al. 2020).
Schematic presentation of the renin–angiotensin system (RAS) and ACE2/angiotensin-(1–7)/MAS axis (Yang et al. 2020)
Schematic representation for the SARS-CoV-2 infection on host cells expressing ACE2 receptor. Herein, initially, viral particles enter the lungs and bind with the cells via ACE2 cells with the aid of spike glycoproteins in the presence of other transmembrane proteinases, such as disintegrin metallopeptidase domain 17 (ADAM17) and transmembrane protease serine 2 (TMPRSS2), which assist the virus to enter into the cells allowing the virus to enter the cells. Following, the infected cells along with inflammatory cells agitated by the viral antigens results in the production of chemokines and pro-inflammatory cytokines (PICs) that further activate the inflammation and immunological reactions to prevent viral proliferation. Cell-free and macrophage-phagocytosed and cell-free viruses located in the blood are likely transferred to other organs of the host and typically afflict the cells abundant with ACE2 expression at local sites (Ni et al. 2020a, b)
13.4 Alliance of ACE2 with Multi-Organ Injury in SARS-CoV-2
The distribution of ACE2 in the different organs of the human body indicates the chances for SARS-CoV-like viral infection in the multiple organs. This is was supported by the autopsies of SARS-CoV-infected individuals, where damage in the organs such as the central nervous system, heart, liver, kidney, skeletal muscle, adrenal, and thyroid glands was observed apart from the mutilation in the lungs (Gu et al. 2005; Gu and Korteweg 2007). Likewise, the majority of seriously infected SARS-CoV-2 patients have had organ damage, including heart injury, acute lung injury, liver disease, acute kidney injury, and pneumothorax (Yang et al. 2020). Organ damaged, as seen in SARS and SARS-CoV-2, was commonly reported in MERS-CoV-infected patients, mostly in the gastrointestinal tract and kidneys, with the least occurrence of acute cardiac injury (Assiri et al. 2013; Alsaad et al. 2018; Hui et al. 2018; Hwang et al. 2019). To be evident, unlike SARS-CoV and SARS-CoV-2, MESR-CoV required Dipeptidyl-peptidase 4 (DPP4) as a functional receptor on host cells for entry, which expressed on activated leukocytes, multinucleated epithelial cells, pneumocytes, bronchial submucosal gland cells of the lungs, kidney epithelial cells, and small intestine epithelial cells (Boonacker and Noorden 2003; Lambeir et al. 2003; Raj et al. 2013). Moreover, DPP4 is sparsely expressed on the myocardial cells (Boonacker and Noorden 2003, Lambeir et al. 2003; Raj et al. 2013). Thus, these reports suggested the association of functional receptors with organ damage in the body (Fig. 13.3).
Conclusive results produced by a series of research on SARS-CoV suggested that pathogenesis of SARS-CoV-2 should be complex, including induction of inflammatory responses by the virus, excessive inflammatory cells recruitment, cytokines and chemokines expression, auto-antibodies formation, and insufficient interferon response (Gu and Korteweg 2007). To note, monocyte chemoattractant protein-1 (MCP-1), interferon-gamma-inducible protein 10 (IP-10), and chemokines plasma-like interleukin (IL)-1, IL-6, IL-12, IL-8, and pro-inflammatory cytokines (PICs) were observed with significant expression in the plasma of SARS-CoV-infected individuals (Wong et al. 2004, Zhang et al. 2004). These observations were also supported by the autopsy studies of SARS patients, where PICs and MCP-1 were noted in exceedingly expressed ACE2+ cells infested by SARS-CoV when compared to noninfected ACE2+ cells, indicating the role of virus-induced local immune-mediated damage (He et al. 2006). Considerably, similar elevated concentrations of PICs were discovered in the plasma of patients with a severe infection of SARS-CoV-2 (Huang et al. 2020a). Additionally, several studies documented that SARS-CoV caused downregulation of ACE2 expression in the infected cells and, hence, disrupts the physiological balance between ACE/ACE2 Ang-II/angiotensin-(1–7) consequently triggering severe damage to the organs in the body (Kuba et al. 2005, Haga et al. 2008, Oudit et al. 2009, Glowacka et al. 2010). Given that SARS-CoV-2 and SARS-CoV belong to the same family and used ACE2 as a functional receptor, ACE2 downregulation has been suggested as an essential factor to cause multiple organ damage in SARS-CoV-2-infected individuals (Fig. 13.3).
13.4.1 Acute Lung Injury
Although the mortality rate in SARS-CoV-2 infection is considerably lower in comparison to SARS-CoV and MERS-CoV, several SARS-CoV-2-infected individuals showed acute lung injury (ALI) (Leung et al. 2003, Yang et al. 2020). Remarkably, similar pathological features as observed in SARS and MERS, including severe diffuse alveolar damage, hyaline membrane formation, extensive edema, microthrombi formation, inflammatory infiltrates, organization, and fibrosis, were also noted in SARS-CoV-2 infection but relatively high cellular fibromyxoid exudates in the small airways and alveoli (Wichmann et al. 2020, Xu et al. 2020b). Interestingly, the clinical experiments have related the ACE insertion/deletion polymorphism along with the increased risk of ARDS (Marshall et al. 2002, Cruces et al. 2012), and elevated Ang-II levels in the lungs were associated with an increase in vascular permeability resulting in pulmonary edema (Marshall et al. 2004, Wsd et al. 2018). Moreover, various reports have suggested the defensive role of the ACE2/angiotensin-(1–7)/MAS axis in the lungs against inflammation, pulmonary arterial hypertension, fibrosis, cancer cell growth, tumor angiogenesis, and tumor metastasis (Imai et al. 2005, Feng et al. 2010, Jia 2016, Santos et al. 2018). From various studies on animal models of ALI, ACE2-knockout mice showed increased vascular permeability, enhanced lung edema, accumulation of neutrophils, and demonstrated obstruction in lung function in comparison to wild-type mice (Imai et al. 2005). Interestingly, AT1R blockers or recombinant human ACE2 protein injection in the ACE2-knockout mice exhibited considerable decrement in the degree of ALI (Imai et al. 2005). Of note, SARS-CoV infection was also noted with a significant reduction in ACE2 expression in the lungs of the infected mouse (Kuba et al. 2005, Cruces et al. 2012). Subsequent experimental data revealed that recombinant SARS-CoV spike-Fc can significantly bind to human and mouse ACE2, which produces a downregulation in cell-surface ACE2 expression (Kuba et al. 2005). Moreover, deteriorated effects were observed in acid-induced ALI in wild-type mice treated with spike-Fc protein, while no alternations were recorded in the ACE2-knockout mice for the severity of lung failure, indicated that the effect of spike protein on ALI is specific to ACE2 expression (Kuba et al. 2005). However, the individuals infected with SARS-CoV-2 showed elevated levels of Ang-II in the blood plasma, which was directly proportional to the viral load and lung injury (Liu et al. 2020c). Based on these reports, RAS and ACE2 downregulation have been suggested as an essential factor that contributes to pathogenesis of lung injury in SARS-CoV-2 infection.
13.4.2 Endothelial Disease
In severe COVID-19, evidence of acute myocardial injury, that is, increased level of cardiac troponins have been observed as a common event and associated with impaired prognosis. Under the observation of tissue tropism of SARS-CoV-2 against ACE2-expressing cells, another major target of the body more liable to infection is vascular endothelium, where both small and large arteries and veins abundantly expressed the ACE2 receptor (Hamming et al. 2004, Monteil et al. 2020). Thus, endothelial dysfunction has been noted as a common feature to key comorbidities that elevated the risk for severe SARS-CoV-2 infection, including diabetes mellitus, obesity, hypertension, coronary artery disease, or heart failure. Interestingly, the preliminary results also revealed that vascular endothelial cells can be infected by SARS-CoV-2 as evident from inflammation and endothelial injury in advanced cases of SARS-CoV-2 infection (Nägele et al. 2020). However, the essential role of endothelial cells is well established to regulate and maintain the blood coagulation and vascular homeostasis; exacerbation of endothelial dysfunction induced by SARS-CoV-2 infection is therefore assumed to produce impaired organ perfusion and procoagulant state that causes both macro- and microvascular thrombotic events. In this context, ACE inhibitors, angiotensin receptor blockers (ARBs), and statins were documented to improve endothelial dysfunction (Nägele et al. 2020). Moreover, endothelial injury and dysfunction have been suggested as results of direct infection by SARS-CoV-2, for instance, by stimulating intracellular oxidative stress and profound systemic inflammatory response (Kochetkov et al. 2018). Therefore, the potential relation of SARS-CoV-2 infection with endothelial injury projected a more severe SARS-CoV-2 infection in patients with preexisting endothelial dysfunction (Nägele et al. 2020).
13.4.3 Acute Cardiac Damage
Heart cells expressed a high concentration of ACE2, indicating its vulnerability to SARS-CoV-2 infection. For instance, autopsies of individuals diagnosed with SARS disclosed that 35% (7 of 20) of the SARS-CoV infected individuals had viral genome in cardiac tissue exhibited a comparatively high aggressive illness and earlier mortality rate (Oudit et al. 2009). Moreover, edema of the myocardial stroma, atrophy of cardiac muscle fibers, and inflammatory cell infiltration were also noted in patients with myocardial damage and SARS (Ding et al. 2003, Lang et al. 2003, Chong et al. 2004, Gu et al. 2005, Gu and Korteweg 2007). Of note, cardiac damage has been noted as a common feature in severely sick patients with SARS-CoV-2 while early acute myocardial damage was linked with an elevated risk of mortality (Ni et al. 2020a, b). Besides, ACE2/angiotensin-(1–7)/MAS axis positive role in the heart is already well established to induce vasorelaxation of coronary vessels, attenuate pathological cardiac remodeling, inhibit oxidative stress, and improve postischemic heart function (Jiang et al. 2014, Santos et al. 2018). It is important to mention that high expressions of ACE2 are normally observed at the initial stage of heart injury but gradually reduced with the progression of diseases (Keidar et al. 2007). For instance, knockout of ACE2 in mice causes myocardial hypertrophy and interstitial fibrosis, and hastens heart failure (Oudit et al. 2007, Zhong et al. 2010). Interestingly, both mice and humans infected with SARS-CoV exhibited a progressive downregulation in ACE2 expression on myocardial cells in the heart (Oudit et al. 2009). Recent studies also established hypertension as a comorbidity with severe disease in several patients (Ni et al. 2020a, b; Novel 2020*; Wang et al. 2020a). Thus, a considerable downregulation of ACE2 and upregulation of Ang-II in SARS-CoV-2 infected patients has been suggested to cause the overactivation of RAS and deactivation of angiotensin-(1–7) protective function that exacerbates and propagate cardiac injuries.
13.4.4 Damage to Digestive System
The gastrointestinal tract, particularly the intestine, has been documented as a potential target to SARS-CoV and SARS-CoV-2. For instance, unlike in the esophagus and stomach, SARS-CoV particles were only isolated from the epithelial cells of the intestinal mucosa (He et al. 2006, Gu and Korteweg 2007). Later, depletion of mucosal lymphoid tissue was discovered as a major pathological discovery in the intestines of individuals infected by SARS-CoV, while only mild focal inflammation was observed only in the gastrointestinal tract of infected patients by SARS-CoV (Shi et al. 2005). These findings provide the explanation to the non-sever and non-transient nature of SARS-CoV-2 in the gastrointestinal tract. Moreover, several individuals infected by SARS-CoV-2 exhibit a minor to mild increment in the serum levels of aspartate aminotransferase (AST) and/or alanine aminotransferase (ALT) during the viral infection (Chen et al. 2020b, Wang et al. 2020a). Also, postmortems results for SARS patients showed cellular infiltration, hepatocyte necrosis, and fatty degeneration in the liver (Gu and Korteweg 2007). Nevertheless, most infected individuals infected by SARS-CoV particles showed no viral load in the autopsied hepatic tissue samples (Gu and Korteweg 2007). This can be explained by the fact that Kupffer cells, hepatocytes, and the endothelial lining of the sinusoids lack ACE2 expression as evident from both immunohistochemistry and single-cell RNA-seq analyses; only cholangiocytes were found positive for the expression of ACE2 receptor (Hamming et al. 2004, Chai et al. 2020, Zou et al. 2020a, b). Interestingly, a high expression level of gamma-glutamyl transpeptidase (GGT)—a marker of cholangiocyte damage—was documented in some patients infected by SARS-CoV-2 (Fan et al. 2020). These results reveal that most acute hepatic damage is not induced by the viral infection but is highly likely caused by other factors, including hypoxia, systemic inflammation, and drug hepatotoxicity. Hence, SARS-CoV-2 induced injuries in the bile ducts which shows high expression of ACE2 on cholangiocytes required further investigation to established SARS-CoV-2 hepatic pathogenesis.
13.4.5 Acute Kidney Injury
The expression of ACE2 is considerably high in the kidney, chiefly in the apical membranes of proximal tubular epithelial cells, and suggested that the kidney is an additional susceptible organ to SARS-CoV-2 infection (Chen et al. 2020a, Zou et al. 2020a, b). A disturbance in the equilibrium between Ang-II and angiotensin-(1–7) induced by deficiency of ACE2 deficiency was also suggested to intensify the vulnerability for the kidney to acute kidney injury (AKI) caused by other factors (Ortiz-Melo and Gurley 2016). For instance, SARS-CoV was isolated in epithelial cells of the distal tubules while viral genomes were also detected in urinary samples of several infected individuals (Chan et al. 2004, Farcas et al. 2005). To note, urinary samples of some patients were also discovered with viral particles of SARS-CoV-2 (Wang et al. 2020b). Moreover, a retrospective data analysis of 536 patients infected by SARS-CoV-2 showed the development of acute renal damage in 6.7% of patients during COVID-19 (Chu et al. 2005). Another large cohort study conducted in New York on SARS-CoV-2-infected individuals revealed a 36.6% incidence of AKI (Hirsch et al. 2020).
13.4.6 Other Organ and Tissue Injuries
13.4.6.1 Olfactory Epithelium (OE) and Brain Infection Through Nasal Cavity
During the clinical course of SARS-CoV-2 infection, symptoms range from asymptomatic infection to severe acute respiratory distress, including involvement of multiple organs and even death. The SARS-CoV-2 infection was also documented to cause extrapulmonary complications such as neurologic disorders and has been constantly reported in the literature (Abboud et al. 2020). Recent studies established total anosmia or partial loss of smell as an early symptom of SARS-CoV-2 infection; this occurrence can be caused by different unidentified factors, for example, “cytokine storm” started in some infected individuals or by direct injury in the olfactory receptor neurons (ORNs) situated in the olfactory epithelium (Butowt and Bilinska 2020). The latter possibility is most likely to occur under the fact that cells in the OE significantly expressed the functional receptor used by SARS-CoV-2 to cause infection in humans. In this context, several gene expression databases have been documented that show a considerable level of ACE2 and TMPRSS2 in human and murine olfactory mucosa (Butowt and Bilinska 2020). OE is a constantly rejuvenating multilayer structure in mammals that contained both non-neuronal and neuronal cells. Recent major RNA-seq (transcriptome) analysis on both human and murine OE consistently revealed the expression of ACE2 in non-neuronal cells (Kanageswaran et al. 2015, Saraiva et al. 2015, Olender et al. 2016). Besides, TMPRSS2 expression seems to be elevated by comparison to that of ACE2 and observed in both neuronal and non-neuronal cells in the OE (Kanageswaran et al. 2015, Saraiva et al. 2015). Also, RNA-seq analysis showed even expression of the majority of the genes, except intriguingly mosaic TMPRSS2 expression was recorded in the subpopulation of mature ORNs (Saraiva et al. 2015). These results indicated that some of the olfactory neurons in the OE are considerably vulnerable to viral infection by comparison to other morphologically similar ORNs. Furthermore, SARS-CoV-2 was suggested to bypass the olfactory axonal route and directly pass from non-neuronal OCE cells to cerebrospinal fluid covered by olfactory nerves—situated near the cribriform—and then migrate to most of the regions in the brain such as medulla oblongata which holds the cardiorespiratory controlling nuclei (Harberts et al. 2011). Thereof, SARS-CoV-2 infection in the brain poses a considerable threat as several neurological impairments are reported, including stroke, epilepsy, and encephalitis. Although, expression of ACE2 is considerably low but well documented in the glia and neurons of the brain, however, specific sites for SARS-CoV-2 indection are not identified (Harberts et al. 2011).
13.4.6.2 Pancreas Disability
Pancreatic cells substantially expressed the ACE2 receptors, thereof, a potential target to SARS-CoV-2 infection (Pal and Banerjee 2020). Recent reports also documented elevated serum amylase and lipase levels in ~16% of patients at a severe stage of SARS-CoV-2 infection accompanied by significant pancreatic changes in 7% of the infected patients on CT scans (Liu et al. 2020a). Additionally, the clinical presentation of acute pancreatitis was also observed in the SARS-CoV-2-infected patients (Hadi et al. 2020). It is important to mention that ACE2/angiotensin-(1–7) display protective activity in diabetes by enhancing the pancreatic β cell survival, promoting insulin secretion, and decreasing insulin resistance (Santos et al. 2018). On this note, several SARS-CoV-infected patients with no diabetes and who had not been treated with steroids in comparison to non-SARS pneumonia patients developed insulin-dependent acute diabetes during hospitalization (Yang et al. 2006, Yang et al. 2010). Also, plasma glucose concentration and diabetes were observed as independent factors of mortality in SARS-CoV-infected patients (Yang et al. 2006). Moreover, postmortems of some SARS-CoV-infected individuals showed amyloid degeneration and atrophy in most of the pancreatic islets, indicating the islets damage induced by the virus (Lang et al. 2003). Thus, SARS-CoV-2 infection is stipulated to influence the pancreatic function as documented in SARS-CoV, and concentration of glucose levels should be diligently scrutinized in diabetic patients or under glucocorticoid treatment.
13.4.6.3 Skeletal Muscles Weakness
SARS-CoV-2 infection is also assumed to hold the ability to infect skeletal muscle, and a particular concern is the susceptibility of muscles connected with the respiratory pump, that is, the intercostal muscles and diaphragm (Ferrandi et al. 2020). Previous studies documented muscle weakness and high concentration of serum creatine kinase (CK) levels in ~30% of patients infected with SARS (Lee et al. 2003). Mild to moderate increases in CK levels were also recorded in the individuals infected by SARS-CoV-2 on hospitalization (Chen et al. 2020c). Although atrophy and myofiber necrosis were also noticed in the skeletal muscle tissues, electron microscopy analysis showed the absence of SARS-CoV particles (Leung et al. 2005, Gu and Korteweg 2007). Recently, a significant role of RAS was observed in the pathogenesis of several skeletal muscle disorders, and the ACE2/angiotensin-(1–7)/MAS axis was monitored to exert protective effects against muscle atrophy (Santos et al. 2018). Skeletal muscle myopathies are widely spread (D’Souza et al. 2013, Alway et al. 2014, Ryder et al. 2017, Maheshwari et al. 2020) and linked with certain populations defined at risk for SARS-CoV-2 infection (Guan et al. 2020, Wu and McGoogan 2020). However, infection of SARS-CoV-2 in the muscles and association of ACE2 downregulation with myopathy is not identified. The function of the diaphragm muscle is similarly obstructed in the course of aging caused by sarcopenia (Kelley and Ferreira 2017), while markers of systemic inflammation in chronic obstructive pulmonary disease (COPD) patients have been correlated with the severity of sarcopenic muscle loss (Byun et al. 2017). Therefore, the susceptibility of skeletal muscle to SARS-CoV-2 infection has been suggested to extend based on several myopathic circumstances correlated with comorbidities and aging (Ferrandi et al. 2020).
13.4.6.4 Damage to Central Nervous System Function
The neurons in the human brain widely express the ACE2 and contribute to the neural control of broad physiological functions, including, stress response and neurogenesis, cardiovascular, and metabolic activities (Santos et al. 2018, Alenina and Bader 2019, Katsi et al. 2019). For instance, experimental studies with mouse models infected with SARS-CoV showed infiltration of viral particles into the brain through the olfactory bulb followed by transneuronal transmission to other regions (Netland et al. 2008). To note, gustatory and olfactory dysfunctions were also observed in several patients infected by SARS-CoV-2, indicating the involvement of the olfactory bulb in viral infection (Lechien et al. 2020, Luers et al. 2020). Moreover, SARS-CoV viral particles were discovered in the human brain tissue specimens (Gu et al. 2005, Xu et al. 2005), and autopsies showed focal degeneration and edema in the brains of the infected individuals by SARS-CoV (Gu et al. 2005, Gu and Korteweg 2007). Likewise, several patients (78/214) showed neurologic manifestations in SARS-CoV-2, and the virus was discovered in the cerebrospinal fluid of a patient with encephalitis (Huang et al. 2020b, Mao et al. 2020). With the fact that SARS-CoV-2 possessed a higher affinity for the ACE2 as a functional receptor by comparison to SARS-CoV, the former has been implicit to infect and damage the central nervous system.
13.4.6.5 Blood Vessels Damage
Endothelial cells of small and large blood vessels expressed elevated levels of ACE2, while the vascular endothelium expresses angiotensin-(1–7) (Hamming et al. 2004, Santos et al. 2018). Moreover, ACE2/angiotensin-(1–7)/MAS axis stimulates antiproliferative, vasodilatory, and antithrombotic effects in the vasculature (Santos et al. 2018). In this context, SARS-CoV RNA was detected in the endothelia of the small veins (Zhang et al. 2003). Likewise, substantial increased plasma d-dimer levels were observed in individuals under severe SARS-CoV-2 infection (Chen et al. 2020b, Huang et al. 2020a, Yang et al. 2020), while the incidence of disseminated intravascular coagulation (DIC) was not rare at the initial stage of the disease. Inflammatory responses and viral infection cause severe injury to the integrity of the vascular endothelium, and this results in elevated permeability, microcirculation disturbance, and coagulation activation that has been assumed to contribute to organ injury in SARS-CoV-2.
13.5 Conclusions
RAS and ACE2/angiotensin-(1–7)/MAS axis are well established for contribution in various pathophysiological and physiological events. Both SARS-CoV and SARS-CoV-2 utilize the ACE2 as a functional receptor to infect the host cells. Due to the considerably higher expression of ACE2 in various organs and tissues of the human body, SARS-CoV-2 has the ability not only to infiltrate the lungs but can also damage the other organs with high ACE2 expression. The pathogenesis of COVID-19 is exceedingly complicated involving multiple factors; in addition to the direct viral effects, inflammatory and immune responses against the viral invasion, imbalance of the RAS and ACE2/angiotensin-(1–7)/MAS axis, and downregulation of ACE2 can also subsidize to the multiple organ damages in individuals infected by SARS-CoV-2. Thereof, the scientific community has recently engrossed its attention on ACE2 and ATIR, and their conceivable benefit/harms on the individuals infected by SARS-CoV-2 who experience pneumonia. However, some doubts still exist for the effect of respective inhibitors on SARS-CoV-2 infection. Thus, a potential approach to restoring the balance between RAS and ACE2/angiotensin-(1–7)/MAS has been looked upon as potential therapy to help diminish organ damages in patients infected by SARS-CoV-2.
References
Abboud H, Abboud FZ, Kharbouch H, Arkha Y, El Abbadi N, El Ouahabi A (2020) COVID-19 and SARS-Cov-2 infection: pathophysiology and clinical effects on the nervous system. World Neurosurg 140:49–53
Alenina N, Bader M (2019) ACE2 in brain physiology and pathophysiology: evidence from transgenic animal models. Neurochem Res 44:1323–1329
Alsaad KO, Hajeer AH, Balwi M, Moaiqel M, Oudah N, Ajlan A (2018) Histopathology of Middle East Respiratory Syndrome coronovirus (MERS-CoV) infection—clinicopathological and ultrastructural study. Histopathology 72:516–524
Alway SE, Myers MJ, Mohamed JS (2014) Regulation of satellite cell function in sarcopenia. Front Aging Neurosci 6:246
Assiri A, McGeer A, Perl TM, Price CS, Rabeeah AA, Cummings DA (2013) Hospital outbreak of Middle East Respiratory Syndrome coronavirus. N Engl J Med 369:407–416
Bian J, Li Z (2021) Angiotensin-converting enzyme 2 (ACE2): SARS-CoV-2 receptor and RAS modulator. Acta Pharm Sin B 11(1):1–12
Boonacker E, Noorden CJ (2003) The multifunctional or moonlighting protein CD26/DPPIV. Eur J Cell Biol 82(2):53–73
Butowt R, Bilinska K (2020) SARS-CoV-2: olfaction, brain infection, and the urgent need for clinical samples allowing earlier virus detection. ACS Chem Neurosci 11(9):1200–1203
Byun MK, Cho EN, Chang J, Ahn CM, Kim HJ (2017) Sarcopenia correlates with systemic inflammation in COPD. Int J Chron Obstruct Pulmon Dis 12:669
Chai X, Hu L, Zhang Y, Han W, Lu Z, Ke A, Zhou J, Shi G, Fang N, Fan J, Cai J, Fan J, Lan F (2020) Specific ACE2 expression in cholangiocytes may cause liver damage after 2019-nCoV infection. 2020.2002.2003.931766
Chan KH, Poon LL, Cheng VC, Guan Y, Hung IF, Kong J (2004) Detection of SARS coronavirus in patients with suspected SARS. Emerg Infect Dis 10(2):294–299
Chen L, Li X, Chen M, Feng Y, Xiong C (2020a) The ACE2 expression in human heart indicates new potential mechanism of heart injury among patients infected with SARS-CoV-2. Cardiovasc Res 116(6):1097–1100
Chen N, Zhou M, Dong X, Qu J, Gong F, Han Y (2020b) Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet 395:507–513
Chen T, Wu D, Chen H, Yan W, Yang D, Chen G, Ma K, Xu D, Yu H, Wang H, Wang T, Guo W, Chen J, Ding C, Zhang X, Huang J, Han M, Li S, Luo X, Zhao J, Ning Q (2020c) Clinical characteristics of 113 deceased patients with coronavirus disease 2019: retrospective study. BMJ 368:m1091
Cheung KS, Hung IFN, Chan PPY, Lung KC, Tso E, Liu R, Ng YY, Chu MY, Chung TWH, Tam AR, Yip CCY, Leung KH, Fung AY, Zhang RR, Lin Y, Cheng HM, Zhang AJX, To KKW, Chan KH, Yuen KY, Leung WK (2020) Gastrointestinal manifestations of SARS-CoV-2 infection and virus load in fecal samples from a Hong Kong cohort: systematic review and meta-analysis. Gastroenterology 159(1):81–95
Chong PY, Chui P, Ling AE, Franks TJ, Tai DY, Leo YS (2004) Analysis of deaths during the severe acute respiratory syndrome (SARS) epidemic in Singapore: challenges in determining a SARS diagnosis. Arch Pathol Lab Med 128:195–204
Chu KH, Tsang WK, Tang CS, Lam MF, Lai FM (2005) Acute renal impairment in coronavirus-associated severe acute respiratory syndrome. Kidney Int 67:698–705
Cruces P, Díaz F, Puga A, Erranz B, Donoso A, Carvajal C (2012) Angiotensin-converting enzyme insertion/deletion polymorphism is associated with severe hypoxemia in pediatric ARDS. Intensive Care Med 38:113–119
D’Souza DM, Al-Sajee D, Hawke TJ (2013) Diabetic myopathy: impact of diabetes mellitus on skeletal muscle progenitor cells. Front Physiol 4:379
Davidson AM, Wysocki J, Batlle D (2020) Interaction of SARS-CoV-2 and other coronavirus with ACE (angiotensin-converting enzyme)-2 as their main receptor. Hypertension 76(5):1339–1349
Ding Y, Wang H, Shen H, Li Z, Geng J, Han H (2003) The clinical pathology of severe acute respiratory syndrome (SARS): a report from China. J Pathol 200:282–289
Donoghue M, Hsieh F, Baronas E, Godbout K, Gosselin M, Stagliano N (2000) A novel angiotensin-converting enzyme-related carboxypeptidase (ACE2) converts angiotensin I to angiotensin 1–9. Circ Res 87:E1–E9
Fan Z, Chen L, Li J, Cheng X, Yang J, Tian C (2020) Clinical features of COVID-19-related liver functional abnormality. Clin Gastroenterol Hepatol 18:1561–1566
Farcas GA, Poutanen SM, Mazzulli T, Willey BM, Butany J, Asa SL (2005) Fatal severe acute respiratory syndrome is associated with multiorgan involvement by coronavirus. J Infect Dis 191:193–197
Feng Y, Wan H, Liu J, Zhang R, Ma Q, Han B (2010) The angiotensin-converting enzyme 2 in tumor growth and tumor-associated angiogenesis in non-small cell lung cancer. Oncol Rep 23:941–948
Ferrandi PJ, Alway SE, Mohamed JS (2020) The interaction between SARS-CoV-2 and ACE2 may have consequences for skeletal muscle viral susceptibility and myopathies. J Appl Physiol (1985) 129(4):864–867
Glowacka I, Bertram S, Herzog P, Pfefferle S, Steffen I, Muench MO (2010) Differential downregulation of ACE2 by the spike proteins of severe acute respiratory syndrome coronavirus and human coronavirus NL63. J Virol 84:1198–1205
Groneberg DA, Hilgenfeld R, Zabel P (2005) Molecular mechanisms of severe acute respiratory syndrome (SARS). Respir Res 6(1):8
Gu J, Gong E, Zhang B, Zheng J, Gao Z, Zhong Y (2005) Multiple organ infection and the pathogenesis of SARS. J Exp Med 202:415–424
Gu J, Korteweg C (2007) Pathology and pathogenesis of severe acute respiratory syndrome. Am J Pathol 170:1136–1147
Guan WJ, Liang WH, Zhao Y, Liang HR, Chen ZS, Li YM, Liu XQ, Chen RC, Tang CL, Wang T, Ou CQ, Li L, Chen PY, Sang L, Wang W, Li JF, Li CC, Ou LM, Cheng B, Xiong S, Ni ZY, Xiang J, Hu Y, Liu L, Shan H, Lei CL, Peng YX, Wei L, Liu Y, Hu YH, Peng P, Wang JM, Liu JY, Chen Z, Li G, Zheng ZJ, Qiu SQ, Luo J, Ye CJ, Zhu SY, Cheng LL, Ye F, Li SY, Zheng JP, Zhang NF, Zhong NS, He JX (2020) Comorbidity and its impact on 1590 patients with COVID-19 in China: a nationwide analysis. Eur Respir J 55(5):2000547
Hadi A, Werge MP, Kristiansen KT, Pedersen UG, Karstensen JG, Novovic S, Gluud LL (2020) Coronavirus disease-19 (COVID-19) associated with severe acute pancreatitis: case report on three family members. Pancreatology 20(4):665–667
Haga S, Yamamoto N, Nakai-Murakami C, Osawa Y, Tokunaga K, Sata T (2008) Modulation of TNF-alpha-converting enzyme by the spike protein of SARS-CoV and ACE2 induces TNF-alpha production and facilitates viral entry. Proc Natl Acad Sci U S A 105:7809–7814
Hamming I, Timens W, Bulthuis M, Lely A, Navis GJ, van Goor H (2004) Tissue distribution of ACE2 protein, the functional receptor for SARS coronavirus. A first step in understanding SARS pathogenesis. J Pathol 203(2):631–637
Harberts E, Yao K, Wohler JE, Maric D, Ohayon J, Henkin R, Jacobson S (2011) Human herpesvirus-6 entry into the central nervous system through the olfactory pathway. Proc Natl Acad Sci U S A 108(33):13734–13739
He L, Ding Y, Zhang Q, Che X, He Y, Shen H (2006) Expression of elevated levels of pro-inflammatory cytokines in SARS-CoV-infected ACE2+ cells in SARS patients: relation to the acute lung injury and pathogenesis of SARS. J Pathol 210:288–297
Hirsch JS, Ng JH, Ross DW, Sharma P, Shah HH, Barnett RL, Hazzan AD, Fishbane S, Jhaveri KD, Abate M, Andrade HP, Barnett RL, Bellucci A, Bhaskaran MC, Corona AG, Chang BF, Finger M, Fishbane S, Gitman M, Halinski C, Hasan S, Hazzan AD, Hirsch JS, Hong S, Jhaveri KD, Khanin Y, Kuan A, Madireddy V, Malieckal D, Muzib A, Nair G, Nair VV, Ng JH, Parikh R, Ross DW, Sakhiya V, Sachdeva M, Schwarz R, Shah HH, Sharma P, Singhal PC, Uppal NN, Wanchoo R, Chang BSF, Ng JH (2020) Acute kidney injury in patients hospitalized with COVID-19. Kidney Int 98(1):209–218
Horiuchi M, Akishita M, Dzau V (1999) Recent progress in angiotensin II type 2 receptor research in the cardiovascular system. Hypertension 33:613–621
Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y (2020a) Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 395:497–506
Huang YH, Jiang D, Huang JT (2020b) SARS-CoV-2 detected in cerebrospinal fluid by PCR in a case of COVID-19 encephalitis. Brain Behav Immun 87:149
Hui D, Azhar E, Kim Y, Memish Z, Oh M, Zumla A (2018) Middle East respiratory syndrome coronavirus: risk factors and determinants of primary, household, and nosocomial transmission. Lancet Infect Dis 18:e217–e227
Hwang SM, Na BJ, Jung Y, Lim HS, Seo JE, Park SA (2019) Clinical and laboratory findings of Middle East Respiratory Syndrome coronavirus infection. Jpn J Infect Dis 72:160–167
Imai Y, Kuba K, Rao S, Huan Y, Guo F, Guan B (2005) Angiotensin-converting enzyme 2 protects from severe acute lung failure. Nature 436:112–116
Jia H (2016) Pulmonary angiotensin-converting enzyme 2 (ACE2) and inflammatory lung disease. Shock 46:239–248
Jiang F, Yang J, Zhang Y, Dong M, Wang S, Zhang Q, Liu FF, Zhang K, Zhang C (2014) Angiotensin-converting enzyme 2 and angiotensin 1–7: novel therapeutic targets. Nat Rev Cardiol 11(7):413
Kanageswaran N, Demond M, Nagel M, Schreiner BS, Baumgart S, Scholz P, Altmüller J, Becker C, Doerner JF, Conrad H (2015) Deep sequencing of the murine olfactory receptor neuron transcriptome. PLoS One 10(1):e0113170
Katsi V, Maragkoudakis S, Marketou M, Tsioufis C, Parthenakis F, Tousoulis D (2019) The role of angiotensin-(1-7)/Mas axis and angiotensin type 2 receptors in the central nervous system in cardiovascular disease and therapeutics: a riddle to be solved. Curr Vasc Pharmacol 17:319–325
Keidar S, Kaplan M, Gamliel-Lazarovich A (2007) ACE2 of the heart: from angiotensin I to angiotensin (1-7). Cardiovasc Res 73:463–469
Kelley RC, Ferreira LF (2017) Diaphragm abnormalities in heart failure and aging: mechanisms and integration of cardiovascular and respiratory pathophysiology. Heart Fail Rev 22(2):191–207
Kirtipal N, Bharadwaj S, Kang SG (2020) From SARS to SARS-CoV-2, insights on structure, pathogenicity and immunity aspects of pandemic human coronaviruses. Infect Genet Evol 85:104502
Kochetkov SN, Ivanov AV, Khomich OA, Bartosch BJV (2018) Redox biology of respiratory viral infections. Viruses 10(8):392
Kuba K, Imai Y, Penninger JM (2013) Multiple functions of angiotensin-converting enzyme 2 and its relevance in cardiovascular diseases. Circ J 77(2):301–308
Kuba K, Imai Y, Rao S, Gao H, Guo F, Guan B (2005) A crucial role of angiotensin converting enzyme 2 (ACE2) in SARS coronavirus-induced lung injury. Nat Med 11:875–879
Lambeir AM, Durinx C, Scharpe S, De Meester I (2003) Dipeptidyl-peptidase IV from bench to bedside: an update on structural properties, functions, and clinical aspects of the enzyme DPP IV. Crit Rev Clin Lab Sci 40(3):209–294
Lamers MM, Beumer J, van der Vaart J, Knoops K, Puschhof J, Breugem TI, Ravelli RBG, van Schayck JP, Mykytyn AZ, Duimel HQ, van Donselaar E, Riesebosch S, Kuijpers HJH, Schipper D, van de Wetering WJ, de Graaf M, Koopmans M, Cuppen E, Peters PJ, Haagmans BL, Clevers H (2020) SARS-CoV-2 productively infects human gut enterocytes. Science 369(6499):50–54
Lan J, Ge J, Yu J, Shan S, Zhou H, Fan S, Zhang Q, Shi X, Wang Q, Zhang LJN (2020) Structure of the SARS-CoV-2 spike receptor-binding domain bound to the ACE2 receptor. Nature 581(7807):215–220
Lang ZW, Zhang LJ, Zhang SJ, Meng X, Li JQ, Song CZ (2003) A clinicopathological study of three cases of severe acute respiratory syndrome (SARS). Pathology 35:526–531
Lechien JR, Chiesa-Estomba CM, De Siati DR, Horoi M, Le Bon SD, Rodriguez A, Dequanter D, Blecic S, El Afia F, Distinguin L, Chekkoury-Idrissi Y, Hans S, Delgado IL, Calvo-Henriquez C, Lavigne P, Falanga C, Barillari MR, Cammaroto G, Khalife M, Leich P, Souchay C, Rossi C, Journe F, Hsieh J, Edjlali M, Carlier R, Ris L, Lovato A, De Filippis C, Coppee F, Fakhry N, Ayad T, Saussez S (2020) Olfactory and gustatory dysfunctions as a clinical presentation of mild-to-moderate forms of the coronavirus disease (COVID-19): a multicenter European study. Eur Arch Otorhinolaryngol 277(8):2251–2261
Lee N, Hui D, Wu A, Chan P, Cameron P, Joynt GM (2003) A major outbreak of severe acute respiratory syndrome in Hong Kong. N Engl J Med 348:1986–1994
Leung TW, Wong KS, Hui AC, Lai ST, Ng WF (2005) Myopathic changes associated with severe acute respiratory syndrome: a postmortem case series. Arch Neurol 62:1113–1117
Leung W, Chan P, Chan H, Wu A, Lee N (2003) Enteric involvement of severe acute respiratory syndrome-associated coronavirus infection. Gastroenterology 125:1011–1017
Li F (2016) Structure, function, and evolution of coronavirus spike proteins. Annu Rev Virol 3(1):237–261
Liu F, Long X, Zou W, Fang M, Wu W, Li W, Zhang B, Zhang W, Chen X, Zhang Z (2020a) Highly ACE2 expression in pancreas may cause pancreas damage after SARS-CoV-2 infection. 2020.2002.2028.20029181
Liu K, Fang YY, Deng Y, Liu W, Wang MF, Ma JP (2020b) Clinical characteristics of novel coronavirus cases in tertiary hospitals in Hubei Province. Chin Med J 133:1025–1031
Liu Y, Yang Y, Zhang C, Huang F, Wang F, Yuan J (2020c) Clinical and biochemical indexes from 2019-nCoV infected patients linked to viral loads and lung injury. Sci China Life Sci 63:364–374
Luers JC, Rokohl AC, Loreck N, Wawer Matos PA, Augustin M, Dewald F, Klein F, Lehmann C, Heindl LM (2020) Olfactory and gustatory dysfunction in coronavirus disease 2019 (COVID-19). Clin Infect Dis 71(16):2262–2264
Maheshwari J, Kolaitis N, Anderson M, Benvenuto L, Gao Y, Katz P, Wolters P, Golden J, Kukreja J, Hays SR (2020) Sarcopenia is associated with frailty in lung transplant candidates. J Heart Lung Transplant 39(4):S391
Mao L, Jin H, Wang M, Hu Y, Chen S, He Q, Chang J, Hong C, Zhou Y, Wang D, Miao X, Li Y, Hu B (2020) Neurologic manifestations of hospitalized patients with coronavirus disease 2019 in Wuhan, China. JAMA Neurol 77(6):683–690
Marshall RP, Gohlke P, Chambers RC, Howell DC, Bottoms SE, Unger T (2004) Angiotensin II and the fibroproliferative response to acute lung injury. Am J Physiol Lung Cell Mol Physiol 286:L156–L164
Marshall RP, Webb S, Bellingan GJ, Montgomery HE, Chaudhari B, McAnulty RJ (2002) Angiotensin converting enzyme insertion/deletion polymorphism is associated with susceptibility and outcome in acute respiratory distress syndrome. Am J Respir Crit Care Med 166:646–650
Monteil V, Kwon H, Prado P, Hagelkrüys A, Wimmer RA, Stahl M, Leopoldi A, Garreta E, Del Pozo CH, Prosper FJC (2020) Inhibition of SARS-CoV-2 infections in engineered human tissues using clinical-grade soluble human ACE2. Cell 181(4):905–913.e7
Nägele MP, Haubner B, Tanner FC, Ruschitzka F, Flammer AJ (2020) Endothelial dysfunction in COVID-19: current findings and therapeutic implications. Atherosclerosis 314:58–62
Netland J, Meyerholz DK, Moore S, Cassell M, Perlman S (2008) Severe acute respiratory syndrome coronavirus infection causes neuronal death in the absence of encephalitis in mice transgenic for human ACE2. J Virol 82:7264–7275
Ni W, Yang X, Liu J, Bao J, Li R, Xu Y, Guo W, Hu Y, Gao Z (2020a) Acute myocardial injury at hospital admission is associated with all-cause mortality in COVID-19. J Am Coll Cardiol 76(1):124–125
Ni W, Yang X, Yang D, Bao J, Li R, Xiao Y, Hou C, Wang H, Liu J, Yang D, Xu Y, Cao Z, Gao Z (2020b) Role of angiotensin-converting enzyme 2 (ACE2) in COVID-19. Crit Care 24(1):422
Olender T, Keydar I, Pinto JM, Tatarskyy P, Alkelai A, Chien MS, Fishilevich S, Restrepo D, Matsunami H, Gilad Y, Lancet D (2016) The human olfactory transcriptome. BMC Genomics 17(1):619
Ortiz-Melo DI, Gurley SB (2016) Angiotensin converting enzyme 2 and the kidney. Curr Opin Nephrol Hypertens 25:59–66
Oudit GY, Kassiri Z, Jiang C, Liu PP, Poutanen SM, Penninger JM (2009) SARS-coronavirus modulation of myocardial ACE2 expression and inflammation in patients with SARS. Eur J Clin Investig 39:618–625
Oudit GY, Kassiri Z, Patel MP, Chappell M, Butany J, Backx PH (2007) Angiotensin II-mediated oxidative stress and inflammation mediate the age-dependent cardiomyopathy in ACE2 null mice. Cardiovasc Res 75:29–39
Pal R, Banerjee M (2020) COVID-19 and the endocrine system: exploring the unexplored. J Endocrinol Invest 43(7):1027–1031
Patel S, Rauf A, Khan H, Abu-Izneid T (2017) Renin-angiotensin-aldosterone (RAAS): the ubiquitous system for homeostasis and pathologies. Biomed Pharmacother 94:317–325
Raj VS, Mou H, Smits SL, Dekkers DH, Müller MA, Dijkman R (2013) Dipeptidyl peptidase 4 is a functional receptor for the emerging human coronavirus-EMC. Nature 495:251–254
Ryder S, Leadley R, Armstrong N, Westwood M, De Kock S, Butt T, Jain M, Kleijnen J (2017) The burden, epidemiology, costs and treatment for Duchenne muscular dystrophy: an evidence review. Orphanet J Rare Dis 12(1):79
Santos R, Sampaio W, Alzamora A, Motta-Santos D, Alenina N, Bader M (2018) The ACE2/angiotensin-(1-7)/MAS axis of the renin-angiotensin system: focus on angiotensin-(1-7). Physiol Rev 98:505–553
Saraiva LR, Ibarra-Soria X, Khan M, Omura M, Scialdone A, Mombaerts P, Marioni JC, Logan DW (2015) Hierarchical deconstruction of mouse olfactory sensory neurons: from whole mucosa to single-cell RNA-seq. Sci Rep 5:18178
Shi X, Gong E, Gao D, Zhang B, Zheng J, Gao Z (2005) Severe acute respiratory syndrome associated coronavirus is detected in intestinal tissues of fatal cases. Am J Gastroenterol 100:169–176
Tipnis S, Hooper N, Hyde R, Karran E, Christie G, Turner A (2000) A human homolog of angiotensin-converting enzyme. Cloning and functional expression as a captopril-insensitive carboxypeptidase. J Biol Chem 275:33238–33243
Wan Y, Shang J, Graham R, Baric RS, Li F (2020) Receptor recognition by the novel coronavirus from Wuhan: an analysis based on decade-long structural studies of SARS coronavirus. J Virol 94(7):e00127–e00120
Wang D, Hu B, Hu C, Zhu F, Liu X, Zhang J, Wang B, Xiang H, Cheng Z, Xiong Y, Zhao Y, Li Y, Wang X, Peng Z (2020a) Clinical characteristics of 138 hospitalized patients with 2019 Novel coronavirus–infected pneumonia in Wuhan, China. JAMA 323(11):1061–1069
Wang W, Xu Y, Gao R, Lu R, Han K, Wu G (2020b) Detection of SARS-CoV-2 in different types of clinical specimens. JAMA 323:1843–1844
Wichmann D, SJ, Lütgehetmann M, Steurer S, Edler C, Heinemann A et al (2020) Autopsy findings and venous thromboembolism in patients with COVID-19. Ann Intern Med 173(4):268–277
Wong CK, Lam CW, Wu AK, Ip WK, Lee NL, Chan IH (2004) Plasma inflammatory cytokines and chemokines in severe acute respiratory syndrome. Clin Exp Immunol 136:95–103
Wrapp D, Wang N, Corbett KS, Goldsmith JA, Hsieh C-L, Abiona O, Graham BS, McLellan JS (2020) Cryo-EM structure of the 2019-nCoV spike in the prefusion conformation. Science 367(6483):1260–1263
Wsd T, Liao W, Zhou S, Mei D, Wong WF (2018) Targeting the renin-angiotensin system as novel therapeutic strategy for pulmonary diseases. Curr Opin Pharmacol 40:9–17
Wu Z, McGoogan JM (2020) Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: summary of a report of 72 314 cases from the Chinese Center for Disease Control and Prevention. JAMA 323(13):1239–1242
Xu J, Zhong S, Liu J, Li L, Li Y, Wu X (2005) Detection of severe acute respiratory syndrome coronavirus in the brain: potential role of the chemokine Mig in pathogenesis. Clin Infect Dis 41:1089–1096
Xu X, Chen P, Wang J, Feng J, Zhou H, Li X, Zhong W, Hao P (2020a) Evolution of the novel coronavirus from the ongoing Wuhan outbreak and modeling of its spike protein for risk of human transmission. Sci China Life Sci 63(3):457–460
Xu Z, Shi L, Wang Y, Zhang J, Huang L, Zhang C (2020b) Pathological findings of COVID-19 associated with acute respiratory distress syndrome. Lancet Respir Med 8:420–422
Yang JK, Feng Y, Yuan MY, Yuan SY, Fu HJ, Wu BY (2006) Plasma glucose levels and diabetes are independent predictors for mortality and morbidity in patients with SARS. Diabet Med 23:623–628
Yang JK, Lin SS, Ji XJ, Guo LM (2010) Binding of SARS coronavirus to its receptor damages islets and causes acute diabetes. Acta Diabetol 47:193–199
Yang X, Yu Y, Xu J, Shu H, Xia J, Liu H (2020) Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: a single-centered, retrospective, observational study. Lancet Respir Med 8:475–481
Zhang H, Li HB, Lyu JR, Lei XM, Li W, Wu G (2020) Specific ACE2 expression in small intestinal enterocytes may cause gastrointestinal symptoms and injury after 2019-nCoV infection. Int J Infect Dis 96:19–24
Zhang QL, Ding YQ, Hou JL, He L, Huang ZX, Wang HJ (2003) Detection of severe acute respiratory syndrome (SARS)-associated coronavirus RNA in autopsy tissues with in situ hybridization. Di Yi Jun Yi Da Xue Xue Bao 23:1125–1127
Zhang Y, Li J, Zhan Y, Wu L, Yu X, Zhang W (2004) Analysis of serum cytokines in patients with severe acute respiratory syndrome. Infect Immun 72:4410–4415
Zhong J, Basu R, Guo D, Chow FL, Byrns S, Schuster M (2010) Angiotensin-converting enzyme 2 suppresses pathological hypertrophy, myocardial fibrosis, and cardiac dysfunction. Circulation 122:717–728
Zhu N, Zhang D, Wang W, Li X, Yang B, Song J (2020) A novel coronavirus from patients with pneumonia in China, 2019. N Engl J Med 382:727–733
Zou X, Chen K, Zou J, Han P, Hao J, Han Z (2020a) Single-cell RNA-seq data analysis on the receptor ACE2 expression reveals the potential risk of different human organs vulnerable to 2019-nCoV infection. Front Med 14(2):185–192
Zou X, Chen K, Zou J, Han P, Hao J, Han Z (2020b) The single-cell RNA-seq data analysis on the receptor ACE2 expression reveals the potential risk of different human organs vulnerable to Wuhan 2019-nCoV infection. Front Med 14:185–192
Author information
Authors and Affiliations
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2021 The Author(s), under exclusive licence to Springer Nature Singapore Pte Ltd.
About this chapter
Cite this chapter
Bharadwaj, S., Dwivedi, V.D., Kang, S.G., Kirtipal, N., Sobti, R.C. (2021). Insights Into the Role of Angiotensin-Converting Enzyme 2 in the Onset of Severe Acute Respiratory Syndrome Coronavirus 2 Pathogenesis. In: Sobti, R.C., Dhalla, N.S., Watanabe, M., Sobti, A. (eds) Delineating Health and Health System: Mechanistic Insights into Covid 19 Complications. Springer, Singapore. https://doi.org/10.1007/978-981-16-5105-2_13
Download citation
DOI: https://doi.org/10.1007/978-981-16-5105-2_13
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-16-5104-5
Online ISBN: 978-981-16-5105-2
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)