Abstract
Intracellular potassium (K+) homeostasis is an essential requirement for the optimum processing of plant metabolism and overall functioning of plants. It is regulated by K+ ion uptake, efflux, and intracellular and long-distance translocation, which is arbitrated by a great amount of K+-selective and nonselective channels and transporters placed at both plasma and vacuolar membranes. Various abiotic stresses like drought, salinity, water-logging stress, etc. led to drastic deterioration of intracellular potassium homeostasis. These stresses aggravate a K+ channel and transporter expression along with the posttranslational control of their actions and optimization of K+ absorption and consequently cause programmed cell death. Though there are certain specialized approaches which regulate the action of K+ channels and transporters by membrane potential, cytosolic Ca2+, reactive oxygen species, polyamines, plant growth regulators, and gasotransmitters are related to the adaptive plant responses to the unfavorable environment. Therefore, this chapter mainly provides an insight into the molecular strategies associated with the potassium uptake and homeostasis during different abiotic stress conditions.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
3.1 Introduction
Oxidative burst caused by various abiotic stresses, for instance drought, salinity, temperature, heavy metal stress, etc., caused severe loss to plant productivity globally, and plant response to a combination of different environmental stresses is unlike to the response developed by the plant to individual stress (Fahad et al. 2017). These abiotic stresses in combination or alone caused imbalances in the redox cell homeostasis due to the generation of a plethora of reactive oxygen species (ROS) such as hydrogen peroxide (H2O2), superoxide (O2•−), and radicals (Kaur et al. 2018). For the normal functioning of the plant metabolism and their survival, plants require water (H2O) and essential mineral nutrients; however, to maintain the cellular homeostasis under stress conditions, plant genome stimulates expression of different sets of genes to ensure availability of nutrients and H2O (Mitra 2018). In this context, level of intracellular potassium (K+) homeostasis is altered by various abiotic stresses. K+ after N and P is the third most required essential macronutrient by plants for their healthy life span (Srinivasarao and Kundu 2017).
Plants contain 2–10% of K of their dry weight, and cytoplasmic K+ amount is approximately 100 mM; however, vacuole may have 20–200 mM of K+ (Gierth and Mäser 2007), and apoplastic amount of K+ may vary between 10 and 200 mM and may increase up to 500 mM (White and Karley 2010; Wang et al. 2013). Several cellular proteins especially membrane proteins which are often called transporters and channels are beneficial in K+ uptake from the outer environment and transport it to various plant tissues, and the upregulation of the K status decreases the ROS generation in plants. K decreases the activity of nicotinamide adenine dinucleotide phosphate (NADPH) oxidases and regulates the photosynthetic electron transport activity which helps to reduce ROS (Hasanuzzaman et al. 2018). There are two types of K+ transporters on the basis of their affinity for K+: one is high-affinity transporters which are active at low concentration of external K+, and the other one is low-affinity channels which are active at high concentration of external K+ (Wang and Wu 2013).
Intracellular K+ homeostasis is recognized as a beneficial essential macronutrient for the normal functioning of plant metabolism ultimately, overall plant productivity and its essentiality are related to its multiple roles in plants like enzyme activation, osmoregulation, maintenance of cell turgor pressure, regulation of membrane polarization, and cytoplasmic pH regulation (Barragán et al. 2012), some others are regulation of cell elongation, stomatal movements, tropisms, phloem solute transport, ion balancing, photosynthesis process functioning, protein synthesis, carbohydrate translocation, and metabolism, and as most of these processes are directly involved in plant adaptation to hostile environment, K+ uptake, transport, and homeostasis play a vital role in strengthening plant tolerance to abiotic stresses (Shabala and Pottosin 2014). Over 50 different cytoplasmic enzymes are regulated by K+; therefore, impairment of the K+ homeostasis caused by different stresses results in severe damage to plant metabolism both in root and leaf tissues (Almeida et al. 2017).
3.2 Potassium Uptake and Transport Under Abiotic Stress Conditions
Potassium (K+) is an essential macronutrient for the growth, development, and metabolic processes of plants. It is an obligatory element and rich cation in plant cells which plays a prime role in physiological processes, viz., photosynthesis, transportation, and stomatal and signal regulation (Clarkson and Hanson 1980). It also has a modulatory function in key biochemical channels related to carbohydrate, sugar, and energy metabolism and protein and enzyme activation (Marschner 2012). Potassium is a chief fertilizer complex in physiological, biochemical, and metabolical vibrant processes to augment plant growth, physiology, development, yield, quality, and environmental adversity tolerance. Furthermore, these cations play principal osmotic functions, i.e., cellular turgor, maintenance of cytosolic pH, and membrane potential along with the proton motive force in plant system (Maathuis 2009; Marschner 2012). Potassium is also considered as a foremost macronutrient in tolerance to abiotic stresses, i.e., osmotic, drought, salinity, temperature, etc. Thus to maintain a resistance against adverse stresses, plants advance energy for the uptake of K+ ions and their distribution throughout the plant (Amtmann et al. 2008; Sharma et al. 2013).
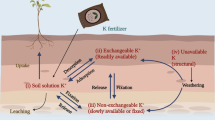
Potassium is predominantly absorbed through roots into the xylem sap and transported aboveground to plant parts by transpiration-driven mass flow process (Wegner 2015) (Fig. 3.1). Plants take up potassium either in the form of a cation (K+) or salt form, viz., KNO3, KCl, K2CO3, etc., from soils. Among these, KCl is the most routinely used for agronomic crops (Kafkafi et al. 2001). The uptake of K+ by root system depends on the properties of the soil like texture, pH, organic, alkalinity, and moisture content of soil. A few soluble forms of K compound, namely, K silicate minerals, also exist in certain soils at a very low concentration. Thus, microorganisms like fungi and bacteria accelerate solubility of K and play a key role in transformation to soluble forms via exchange, acidification, and chelation methods (Masood and Bano 2016). However, in sandy soil circumstances, potassium is given in the form of foliar spray. But, the efficiency of the foliar application is dependent on the absorption, penetration, and sufficient leaf area capacity into leaves (Ling and Silberbush 2002; Hasanuzzaman et al. 2018).
Potassium is taken up from the soil through xylem and translocated to different cellular compartments from the external surroundings which requires many proteins called transporters and channels which are present at the surface of the cell membrane (Fig. 3.1). Potassium is highly mobile in plants, and its translocation takes place through both xylem (root to shoot) and phloem (from source to sink). In the whole translocation process from the soil to the different plant organs, K+ transverse numerous tissues cell membranes via specific K+ membrane transport systems (Fig. 3.1). For the effective transportation and uptake of K+ via various plant tissues to cellular compartments, a coordinated molecular mechanism of sensing, signalling, and protein channels are primarily processes.
Potassium ion uptake in different plant parts shows dual-affinity mechanism on the basis of their affinity for K+. It can be classified as high affinity where K+ uptake is mediated by K+ transporters at a low concentration of external K+ and low-affinity K+ uptake mechanism facilitated mainly by K+ channels that are active at a higher concentration, usually at more than 0.3 mM external K+ (Wang and Wu 2013; Cherel et al. 2014) (Fig. 3.1). Over the past few years, progression in molecular approaches and their advanced implementations enhanced the understanding and involvement of high-affinity and low-affinity transporters in diverse crop species including Oryza sativa, Hordeum vulgare L., Capsicum annuum L., and Arabidopsis thaliana (Pyo et al. 2010; Wang and Wu 2013; Nieves-Cordones et al. 2014; Hasanuzzaman et al. 2018).
Therefore, K+ uptake from soil and translocation into cellular compartments are accomplished through numerous membrane transporter proteins and channel families, viz., Shaker family channels include the tandem-pore K (TPK), voltage-dependent, and two-pore channels (TPC) (Hedrich 2012), cation-proton antiporter transport families include CHX, NHX, and KEA antiporters (Sze and Chanroj 2018), and the carrier transport families are KT/HAK/KUP, HKT uniporters, and symporters (Hamamoto et al. 2015; Li et al. 2018a) (Fig. 3.1). All these transporters and channels have difference in their energetic coupling, affinity, and selectivity for uptake and transport of K+ in plant cells (Ward et al. 2009; Sharma et al. 2013). The pH and voltage potential gradients caused by H+-ATPase gated energy for K+ influx across the cell membrane of root cells in apoplasmic solution are appropriate at less than 1 mM K+ (Maathuis and Sanders 1994). H+/K+ symporters, such as NHXs and CHXs, mediate this process and play an important function in K+ homeostasis in vacuolated plant cells (Leidi et al. 2010; Barragán et al. 2012). The efficiency of K channels and transporters might be enriched by overexpressing their underlying genes or enhancing their protein activity. The increasing expression of genes encoding high-affinity systems plays an important role in K+ influx when K+ is in short supply (Shin and Schachtman 2004; Wang et al. 2015).
It is important to uphold normal growth of plants by taking an optimal amount of K+ via high-affinity K+ transporter uptake system in roots from the soil (Cherel et al. 2014; Ragel et al. 2019). So, K+ carrier transporters might be grouped into four major families like KT/HAK/KUP, CHX, Trk/HKT, and KEA (Liang et al. 2020). These channels and transporter proteins play a vital action to strengthen tolerance mechanism in plants to various abiotic stresses, viz., salt, temperature, drought, heavy metal, etc. (Bose et al. 2014; Song et al. 2015). The KT/HAK/KUP-gated transporters are the leading potassium ion transporter families. These plasma membrane transporter proteins mediated K+ uptake over a broad range of concentrations. For the homeostasis maintenance of ions in plants, numerous K+ transporters have been recognized in relation to their functions either influx or efflux from the cellular compartments and within the whole plant (Grabov 2007; Liang et al. 2020).
Over a few years, KT/HAK/KUP membrane transporters have been enumerated in diversified annual crop species like barley, rice, maize, weeds, tomato, pear, and Arabidopsis (Vallejo et al. 2005; Zhang et al. 2012; Song and Su 2013; Han et al. 2016; Li et al. 2018b). In Arabidopsis thaliana comprehensively 71 K+ membrane transporters and protein channels have been documented for the distribution of potassium ions (Wang and Wu 2013; Sharma et al. 2013).
The transportation of potassium ions from soil to cellular compartments is carried out via enormous genes. These genes are virtually grouped into different transporter and channel families, viz., Shaker-type K+-gated channels, antiporter K+/H+ genes, pore K+ channels, KUP/HAK/KT transporters, HKT transporters, cyclic nucleotide-gated channels, and glutamate receptors (Anschütz et al. 2014). Thus, the interplay of biomarker genes has a putative role in K+ influx and K+ efflux in plants.
Wang and Wu (2013) confirmed that channels and transporters encoded by different genes have diverse functions to redox homeostasis in plants. At root level, the K+ uptake is primarily mediated by two proteins, AKT1 and HAK5, since these two proteins are expressed in the roots which have high-affinity transporters to mediate adequate K+ uptake for plant growth (Pyo et al. 2010; Li et al. 2014; Yang et al. 2014) (Fig. 3.1).
These K+ transporters and channels are considered not only as the major components for uptake and translocation of K+ but also as potential K+ sensors for plant responses to K+ deficiency. Still, the regulatory mechanisms of many K+ transporters and channels remain unknown. Hence, forthcoming investigations should give consideration to functional characterization of these membrane channels and transporters and to the regulatory mechanisms of these components. Analyzing the molecular approaches among various channels and transporters to potassium uptake is also an important task, one that would benefit our understanding of the complex signalling network for plant responses under abiotic stress.
3.3 Potassium Homeostasis Under Abiotic Stress Conditions
Potassium (K+) is an essential plant nutrient, and despite of the fact that most of the soils are rich in potassium, yet the demand for K fertilizer is increasing owing to the lesser amounts of bioavailable forms of K+ to plants and the leaching and runoff from the upper soil layers, contributing to K+ deficiencies in agricultural soils. Potassium is one of the vital structural elements in plants as well as it regulates several physiological and biochemical processes in cells and organs like carbohydrate metabolism, photosynthesis, protein synthesis, enzyme activation, and stomatal movements.
3.3.1 Potassium and Abiotic Stress Tolerance in Plants
Recent research outcomes credit potassium to provide abiotic stress tolerance in plants. K helps to regulate the osmotic balance under salt stress conditions and also maintains the ion homeostasis. It helps the plants to adapt themselves to water deficiency by regulating the stomatal opening under drought stress conditions. K has also been reported to enhance antioxidant defense system of plants, thus providing them oxidative stress protection under various environmental adversities. Potassium retains the photosynthetic electron transport activity by reducing the activity of nicotinamide adenine dinucleotide phosphate (NADPH) oxidases leading to ROS alleviation. The molecular mechanisms of K-induced abiotic stress tolerance in plants might include an insight into the cellular signalling provided by this element alone or in association with other signalling molecules and phytohormones (Hasanuzzaman et al. 2018).
3.3.1.1 Salinity Stress
A high concentration of soluble salts in the soil refers to as high soil salinity where NaCl is the most soluble and widespread salt. Saline soils show an electrical conductivity (EC) of 4 dS/m (corresponding to approximately 40 mM NaCl) and significantly reduce the growth and yield of most crops. Salinity is often considered as an intrinsic problem in coastal areas and river deltas, but excessive use of irrigation with poor-quality water and insufficient drainage in inland areas lead to the buildup of secondary salinization of the soil surface (Thomson et al. 2010). Salinity poses two major threats to plants:
-
1.
Osmotic stress due to excess solutes outside the roots that reduces the soil water potential and hence water absorption affecting cell turgor and expansion, promoting biosynthesis of ABA, lowering stomatal conductance, hampering carbon assimilation and biomass production, and thus decreasing the yield (Munns and Tester 2008)
-
2.
The ionic stress due to excessive influx of sodium ions (Na+) and/or chloride (Cl−) ions into the plant leading to interruption of metabolic processes and inducing tissue necrosis and early leaf senescence (Roy et al. 2014)
Salinity stress inhibits plant root growth due to osmotic effect and ion toxicity which decreases nutrient uptake and translocation, especially that of potassium K+ (Wang et al. 2013), which is a vital macronutrient that plays essential functions related to cellular metabolism like osmotic regulation, turgor maintenance, enzyme activation, regulation of the membrane potential, protein synthesis, and cytoplasmic homeostasis (Almeida et al. 2017). Decrease in K+ content was observed in the roots and shoots of Vigna subterranea (L.) Verdc due to salinity stress, while increase in Na+ concentration has been reported by Taffouo et al. (2010).
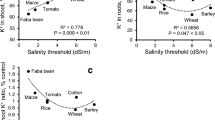
Sodium exhibits a strong inhibitory effect on K+ uptake by cells via inhibiting K+ transporters, i.e., AKT1 (hyperpolarization-activated inward-rectifying K+ channel) (Fuchs et al. 2005) and HAK5 (carrier-type HUP/HAK/KT transport) (Nieves-Cordones et al. 2010), that are present in the plasma membrane of root cells. Further, K+ leakage occurs due to salinity-induced membrane depolarization and decreased membrane integrity. Na+ competes with K+ for major binding sites in enzymatic reactions and cellular metabolism like protein synthesis and ribosome functions due to similarity in their physicochemical properties, i.e., ionic radius, ion hydration energy, etc. (Marschner 1995; PPI 1998). Activity of over 50 different cytoplasmic enzymes that require K+ for functioning is inhibited by Na+ ions. Hence, the disruption of the K+ homeostasis and higher Na+ concentrations are toxic for cell metabolism and lead to severe metabolism impairment, both in root and leaf tissues. However, exogenous potassium application has been shown to positively affect the plant root and shoot growth during salinity stress (Saida et al. 2014). Fayez and Bazaid (2014) found that the shoot fresh weight and height of the Hordeum vulgare plants under 150 mM NaCl stress improved when treated with potassium. Similar results have been reported by Amjad et al. (2016) in Solanum lycopersicum under 75 mM NaCl stress, Merwad (2016) in Beta vulgaris cultivars, and Chakraborty et al. (2016) in peanut (Arachis hypogea L.) under salt stress. Thus, maintaining the cytosolic K+ contents at a constant level is important for plant metabolic processes (Flowers et al. 2015; Shabala et al. 2016).
Maintaining the cellular K content above a certain threshold and maintaining a high cytosolic K+/Na+ ratio are crucial for plant growth and salt tolerance. Mansour (2014) has associated the increased P-ATPases activity with salt stress tolerance and has attributed this to the repolarization of the NaCl-induced depolarized plasma membrane. This significantly reduces Na+ influx via depolarization-activated NSCCs and through depolarization-activated outward-rectifying K+ channels (e.g., GORK) (Adams and Shin 2014) and also NSCCs (Sun et al. 2009), which help to restore higher K+/Na+ levels (Sun et al. 2009). The higher P-ATPase activity under salt stress also energizes the active transport that excludes Na+ from root cells, a process dependent of the SOS1 Na+/H+ antiporter (Gaxiola et al. 2007). High-affinity potassium transporters (HKT) have been shown to mediate Na+-specific transport either channel-like Na+ uniport (class I or HKT1 group) or Na+-K+ symport (class II or HKT2 group) which have vital roles in plant Na+ tolerance (Su et al. 2015). Generally, dicot species have only a few HKT genes in their genomes preferably encoding class I HKT proteins, whereas monocots have multiple HKT genes of both classes (Suzuki et al. 2016; El Mahi et al. 2019). Figure 3.2 represents the integrative model of action of various factors involved in potassium homeostasis (Assaha et al. 2017).
PM H+-ATPases are at the center of K+ uptake under salt stress and low-K+ conditions. The activity of SOS1 (salt overly sensitive 1), SA (salicylic acid), HO (heme oxygenase), and ROS (reactive oxygen species) contributes in stabilizing the membrane potential, by regulating the activity of H+-ATPase. This stable membrane potential is favorable for K+ uptake by HAK and AKT1 which are sensitive to membrane depolarization. Membrane depolarization by ROS will lead to K+ efflux through NSCCs (nonselective cation channels) and KORs (K+ outward-rectifying channels), but application of exogenous Ca2+ and the activity of SOS1 can alleviate this condition. In the vacuole, the tonoplast two-pore K+ channels (TPK1) are important in replenishing lost cytosolic K+ from vacuolar pools, while in the chloroplast, thylakoid localized TPK3 is essential in regulating membrane potentials and protein gradients that drive ATP synthesis and the dissipation of excess energy during light photosynthetic reactions, thus enhancing plant fitness. Red lines indicate inhibition, while black arrows indicate activation (modified after Assaha et al. 2017)
3.3.1.2 Drought Stress
Drought stress is one of the main factors limiting yield in many crops. Preserving K+ homeostasis and maintaining a high cytosolic K+/Na+ ratio are important strategies of the plants for coping with drought stress (Scoffoni et al. 2017). H+-ATPase in the plasma membrane of mesophyll cells maintains membrane potential to regulate K+ transmembrane transport (Falhof et al. 2016). Lower drought resistance has been observed in transgenic Arabidopsis (plasma membrane H+-ATPase mutants aha1–6 and aha1–7) due to membrane depolarization-induced higher K+ efflux (Yan et al. 2015). Increased root hair cell plasma membrane H+-ATPase was associated with drought stress tolerance in oats (Avena sativa L.) (Gong et al. 2010) and wheat seedlings (Liu et al. 2005). The exogenous supply of potassium however provides drought stress tolerance as has been observed in olive (Benlloch-González et al. 2008) and sunflower leaves (Benlloch-González et al. 2010). An external supply of K2CO3 has been reported to significantly increase shoot potassium content and improve drought resistance in the drought-tolerant variety SN16 relative to the intolerant variety JM22 (Wei et al. 2013). Presence of potassium in adequate amounts in plants facilitates in improving the ability of plants to tolerate drought stress by maintaining high turgor pressure and low osmotic potential through osmotic adjustment (Egilla et al. 2005) Plasma membrane-located AKT/KAT K+ uptake channels like Os-AKT1 (Li et al. 2014) and AtAKT1 are activated by plasma membrane hyperpolarization (Daszkowska-Golec and Szarejko 2013). Drought stress management requires the hydraulic uncoupling of guard cells from the surrounding mesophyll cells (Becker et al. 2003), and Shaker-like depolarization-activated outward-rectifying K+ (GORK in Arabidopsis) channels at the guard cell plasma membrane plays a crucial role in this process.
The organic osmolyte production especially proline is also increased by exogenous supply of K under drought conditions. Pro accumulation plays a highly protective role in the plants exposed to drought stress, and it is involved in osmotic adjustments (Teixeira and Pereira 2007). Several studies have revealed an increase in Pro through K application, e.g., in Oryza sativa (Pandey et al. 2004), Brassica napus (Din et al. 2011), Triticum aestivum (Jatav et al. 2012), Zea mays (Zhang et al. 2014), and Gossypium hirsutum (Zahoor et al. 2017) under drought stress conditions. The external application of potassium has also been found to enhance root growth that increases the root surface area under drought conditions, which ultimately enhances the water uptake by plant cells (Römheld and Kirkby 2010). Under drought conditions, excess ROS production in plants leads to excessive cellular lipid peroxidation, leading to an increase in the cellular membrane permeability followed by enhanced electrolyte leakage (EL) and malondialdehyde (MDA) content (Fazeli et al. 2007). Soleimanzadeh et al. (2010) reported significant decrease in MDA content under water shortage conditions in sunflower (Helianthus annuus L.) on adequate supply of K which clearly indicates the role of K in mitigating oxidative stress. A relationship between the aquaporin activities and the K channel/transporter has been demonstrated by Kanai et al. (2011). The results of the study showed K deficiency to significantly alter the K+-channel activity, resulting in a variation in the root hydraulic conductance and signal transduction with consequent changes in the aquaporin activity. Thus, the reduction in the root hydraulic conductance and water supply for transpiration was suppressed under K deficiency. Guo et al. (2007) observed a positive correlation between water uptake and K absorption in their work on common bean (Phaseolus vulgaris L.). Potassium was found to mediate the xylem hydraulic conductance resulting in maintenance of cell turgor, stomatal movements, and sufficient gas exchange as part of the drought adaptations as these events helped to maintain water balance in plants (Oddo et al. 2011). Drought-stressed plants commonly experience xylem cavitation, and increased K+ availability has been observed to reduce its occurrence (Trifilò et al. 2011). Figure 3.3 illustrates the involvement of K in plant tolerance under drought stress.
Role of potassium under drought stress (modified after Hasanuzzaman et al. 2018)
3.3.1.3 Extreme Temperature Stress
Plants suffer from extreme temperature stress at the temperature more than the optimum temperature for plant growth and development. Extreme temperatures disrupt various biochemical reactions and plant metabolisms (Hasanuzzaman et al. 2013). K plays a significant role in coping with temperature stress as it helps to activate the various physiological and metabolic processes such as photosynthesis, respiration, and nutrient homeostasis.
3.3.1.3.1 High Temperature Stress
Potassium plays a significant role in increasing the tissue water potentiality that assists in high temperature stress tolerance. It has been observed that plants accumulate various types of osmolytes to overcome the damage caused by the heat stress, and potassium is suggested to work as an osmolyte that helps to maintain stomatal conductance and prevent damage (Azedo-Silva et al. 2004). K-deficient plants produce ROS under stress conditions, and such plants have been found to be benefitted by supplementation with adequate amounts of K that helps plants by supporting protein synthesis, stimulating various enzymatic reactions, assisting in carbohydrate production, and increasing the WUE (Cakmak 2005). Foliar application of K has been shown to alleviate the heat stress in wheat plants by preventing leaf damage, increasing the photosynthetic ability, and enhanced translocation and accumulation of photosynthates (Dias and Lidon 2010).
3.3.1.3.2 Chilling/Freezing Stress
Chilling/freezing stress causes dehydration and loss of apoplastic water and downregulation of K-regulated mechanisms such as photosynthesis and carbon assimilation, metabolism, and phloem activity. Such freezing-induced dehydration can be ameliorated by adequate supply of K that adjusts the osmotic potential (Wang et al. 2013) and leads to better ROS defense ultimately resulting in greater stress tolerance (Farooq et al. 2008).
3.3.1.4 Stress Due to High Light Intensity
High light intensity causes photooxidative damages and rapid leaf chlorosis leading to impaired photosynthesis (Choi et al. 2002). In cases of severe stress, the photosynthetic ability, RuBisCO activity, quantum yield, and electron transport are disrupted (Lu et al. 2017). The damage to plant system in terms of enhanced leaf chlorosis, decreased photoassimilate utilization, and phloem translocation has been correlated with the insufficient amounts of K in plants facing high light intensity stress (Schumann et al. 2017). Hence, plants receiving high light intensity show requirement of K in a great quantity to utilize the absorbed high light for CO2 fixation, and source-sink relation.
3.3.1.5 Stress Due to Waterlogging
At least 10% of the global agricultural land has been affected by waterlogging which is an important barrier for crop production as the root zone of waterlogged plants faces a severe shortage of oxygen supply (hypoxia or anoxia). This disrupts the respiration process in roots leading to energy shortage in the cells. Plants growing in waterlogged soils face a “physiological drought” that causes reduction in stomatal conductivity by severalfold (Ou et al. 2011, Polacik and Maricle 2013), thus affecting nutrient delivery to the shoot. Increase in the xylem sap osmolality during prolonged flooding and root growth arrest due to hypoxia result in a significant reallocation of root K+ toward the shoot. Due to reduced root capacity to take up K+, the roots experience a severe K+ deficiency. Shoot potassium content also decreases severalfold in plants exposed to prolonged waterlogging (Smethurst et al. 2005, Board 2008).
Hence, the key mechanism to save the plants growing in waterlogged condition is avoiding K loss, at the time of hypoxia or anoxia (Teakle et al. 2013). Several researchers have reported the effect of exogenous K application for ameliorating the adverse effects of waterlogging. Higher application of K to soil or foliage has been found to increase plant height, photosynthetic capacity, chlorophyll content, and greater nutrient uptake in cotton plants (Ashraf et al. 2011) and improvement of nonstructural carbohydrates (NSC) contents, photosynthetic pigment content, and higher antioxidative activity as well as lower lipid peroxidation in rice plants (Dwivedi et al. 2017). It also results in a temporary upregulation of H+-ATPase activity reducing K+ loss via KOR channels.
Elemental soil toxicity due to the changes in the soil redox potential also contributes to the negative impact on root K+ homeostasis under flooded condition (Khabaz-Saberi and Rengel 2010; Shabala 2011). Zeng et al. (2013) observed a massive increase in the amount of available Mn and Fe in the soil solution often to above toxic levels within a few days of onset of waterlogging. Other reports also show similar patterns of soil elemental toxicity, e.g., a fivefold increase in Fe content in waterlogged Eucalyptus nitens plants reported by Close and Davidson (2003), and fivefold increase in root Fe level in waterlogged lucerne reported by Smethurst et al. (2005). Elemental toxicity leads to production of ROS species, causing lipid peroxidation, damage to DNA and proteins, pigment breakdown, and impairment of various enzymatic activities. Hydroxyl radicals, the most reactive and detrimental among ROS, activate both K+-selective outward-rectifying (Demidchik et al. 2010) and nonselective K+ permeable (Zepeda-Jazo et al. 2011) channels, resulting in a massive K+ leak from the cytosol. In addition to elemental toxicity of waterlogged soils due to inorganic phytotoxins like Fe2+, Mn2+, and H2S, the organic substances like ethanol, acetaldehyde, various short-chain fatty acids, and phenolics have also been found in significant abundance in waterlogged soils as a result of anaerobic metabolism in both plants and rhizosphere microorganisms (Armstrong and Armstrong 1999, Shabala 2011). These secondary metabolites have detrimental effects on K+ uptake and accumulation, both at the whole plant and cell-specific levels (Pang et al. 2007). TEA+, a known blocker of voltage-gated Shaker-type K+ channels, has been found to strongly inhibit net K+ efflux induced by physiological concentrations of hydroxybenzoic and acetic acids in barley roots (Pang et al. 2007). Although the K+ leakage in flooded roots is certainly detrimental for overall plant nutrition and is responsible for K+ deficiency in crops grown in waterlogged soils, it might also play a possible beneficial role in aerenchyma formation by eliminating cortical cells via PCD mechanism (programmed cell death) that seems to get triggered by the increased caspase-like activity in K+-depleted cells (Shabala and Pottosin 2014).
3.3.1.6 Heavy Metal Stress
Rapid industrialization has led to increase in the heavy metal contamination of agricultural soils worldwide (Hasanuzzaman and Fujita 2012). The plants growing in such soils experience deleterious effects on their growth and development. Heavy metal toxicity causes major disturbances and alterations in the physiological and metabolic processes like uptake of essential nutrients, stomatal mechanism, membrane functions, photosynthesis, activities of various enzymes, and reduction of the water potential and generation of excess ROS (Emamverdian et al. 2015). Hence, remediation of these contaminants in soils or increasing plant tolerance or resistance to heavy metal stress is a matter of utmost concern for plant scientists. Among various other strategies implied for enhancing plant stress tolerance, using K as a plant protector against metal toxicity is quite promising as K plays a crucial role in the activation of several enzymes, synthesis of various proteins, photosynthetic activity, osmoregulation, stomatal movement, transfer of energy, phloem transport, cation-anion balance, and stress resistance (Wang et al. 2013). Potassium has been reported to play a significant role in ameliorating Cd-induced oxidative damage in broad bean (Vicia faba L.; Siddiqui et al. 2012). Song et al. (2015) have also provided the evidence that K helped in mitigating the Zn toxicity in peach plants by improving the photosynthesis and antioxidant defense system and maintaining plant nutritional balance.
3.3.2 Potassium-Induced Abiotic Stress Signalling
The K content of the soil does not remain constant over the growing period of a crop due to the varying environmental conditions. Fluctuations in the K+ availability are sensed by plant roots, and when they sense K deficiency, a series of events occurs in the plant at the molecular level to cope with this condition. Under K-deficient conditions, two distinct Ca2+ signals get induced that are read by CBL1/9. The calcium sensors CBL1 and CBL2 regulate CIPK23 (a protein kinase) that activates the K+ transporter AKT1 by phosphorylation (Behera et al., 2017). Potassium channels such as NSCC and GORK are very sensitive to ROS, and this is the primary reason for the K pool reduction in the cytosol under stress conditions (Demidchik 2014). Phytohormones such as ethylene, auxin, cytokinin, and JA are also involved in low K-induced signalling processes involving the regulation of high-affinity K+ transporter HAK5 transcription and expression (Schachtman 2015). The involvement of microRNAs in plant nutrient homeostasis has also been reported in many studies, and the gene chip overexpression of OsmiR399 has been found to increase the plant nutrient contents including the contents of K+ (Hu et al. 2015). It is suggested by various authors that a complex pathway induced by K signalling might be involved in ensuring the optimum K level in the plants. Figure 3.4 depicts the signalling components including the Ca2+ signalling, ROS, microRNA, membrane potential, and phytohormones involved from signal perception to adaptive responses (Wang and Wu 2013; Wang and Wu 2017).
K-induced signalling in the plants. ROS reactive oxygen species; NSCC nonselective cation channel; HAK5 and AKT1 K+ transporter; CBL1/9 calcineurin B-like proteins; CIPK23 A protein kinase (modified after Hasanuzzaman et al. 2018)
Cytosolic potassium homeostasis and the ability of various tissues to retain potassium under stress conditions are considered important for stress tolerance in plants, but recent studies suggest that stress-induced K+ efflux might also mediate growth and development under various adverse environmental conditions and can be further considered as a switch between metabolic and defense responses. The physiological rationale behind it is that K+ is known to be an activator of a very large number (>70) of metabolic enzymes (Anschütz et al. 2014). Under control conditions, when cytosolic K+ concentrations are high, the major bulk of available energy is directed toward cell metabolism as the enzymes regulated by K are active. When plants are confronted by stress conditions, the available energy diminishes as ATP production declines, and a large pool of ATP is redirected toward defense reactions. With stress progression and increase in stress severity and stress duration, the amount of energy available for defense is quickly reduced to zero, and the cell dies. To avoid or delay the cell death, cell metabolism needs to be shut down to prevent the competition for energy between metabolic and defense responses. It is suggested that the cell uses K+ efflux as a metabolic switch and decreases the cytosolic [K+] to subthreshold levels to inactivate numerous metabolic reactions, allowing a redistribution of the ATP pool toward defense responses (Fig. 3.5).
K+ as a metabolic switch for plant defense under stress conditions (modified after Shabala 2017)
GORK channels—guard cell outward-rectifying K+ channels (Véry et al. 2014)—play an important role in stomatal closure, and disruption of GORK K+ efflux might be viewed as the “metabolic switch” inhibiting energy-consuming anabolic reactions and saving energy for adaptation and repair (Demidchik 2014). The whole process seems to be tightly controlled. K+ efflux from the root must be confined to a relatively small root region so that the overall root potassium nutritional status is not compromised. The root apex is the most appropriate zone for this role as the cells in this zone are metabolically active and show much higher sensitivity to stress factors. It has an overall rate of K+ loss 10 -30 times higher compared to mature zone cells, and have less negative membrane potential. Moreover, it reflects lower H+-ATPase activity, thus depending more on K+ efflux as a means of restoring membrane potential. However, underdeveloped xylem tissue in this region and changes in the radial K+ fluxes will have no implications for long-distance K+ transport to the shoot and the overall volume of cells in the apex being much smaller as compared with the bulk of the root, hence such signalling by K+ loss will not affect the overall K+ nutrition (Shabala et al. 2016). The correlation between potassium and PCD events implies that a prolonged decrease in the cytosolic K+ level may be detrimental to cell viability, and, hence, signalling via K+ efflux should only be transient along with addition of brief cytosolic K+ spikes and Ca2+ and ROS messengers that would signal and shape plant adaptive stress responses. Figure 3.6 represents a model for cytosolic [K+] signalling (Shabala 2017). The three phases depicted include:
-
1.
Homeostatic phase (before stress)—cytosolic [K+] is maintained at a constant level in both zones. Higher metabolic demand for K+ and 10–15 mV less negative membrane potential in the apex maintain slightly higher cytosolic potassium levels. The optimal cytosolic [K+] levels are maintained in the mature zone by low-affinity K+ uptake mediated by AKT channels along the electrical gradient provided by H+-ATPase. In the root apex due to less negative membrane potential, a small but constant K+ leak via GORK channels occurs that needs to be compensated by the high-affinity K+ uptake mediated by HAK transporters.
-
2.
Signalling phase (onset of the stress)—depolarization of membrane potential in the apical zone to very low levels triggers a massive K+ efflux via GORK channels that is further exacerbated by the increase in GORK transcripts. This efflux partially restores the membrane potential and switches the cell’s operation from metabolic to defensive mode. Mature root cells also lose some K+ but to a lesser extent resulting in more-negative membrane potential initially.
-
3.
Recovery phase (after stress)—plant cells in both zones recover K+ loss due to stress-induced activation of H+-ATPase. To enable the root apical cells to regain optimal [K+], some K+ obtained by mature zone cells is redirected via symplast pathway to the apex.
Cytosolic [K+] signalling (modified after Shabala 2017)
The complex pathway of the reactive oxygen species-calcium-hormone signalling network is held responsible for sensing K+ deficiency in plants, and the elucidation and comprehensive understanding of these signalling pathways help in developing the genetic approaches using K+ transporters to increase K+ use efficiency (KUE) in plants under stress (Srivastava et al. 2020).
3.4 Factors Controlling Intracellular K+ Homeostasis in Plants
3.4.1 ROS
ROS are important regulatory agents in plants (Møller et al. 2007). By the onset of various stresses, the accumulation of ROS occurs in the cell, which has a destructive effect on the action of ion transporters, especially K+ ion in the cell (Shabala and cuin 2008; Demidchik et al. 2010). The ratio of K+ declined under abiotic stress (salt stress) which involves the formation of ROS. The ROS is elevated which leads to the activation of K+ permeable for nonselective cation channel (NSCC) and guard cell outward-rectifying K+ (GORK) channel. Thus, the potassium ion efflux from cytosol and its content was reduced (Anschütz et al. 2014), which in turn interrupt the homeostasis of cytosolic Na+-K+ ratio (Hauser and Horie 2010). Cytosolic K+ homeostasis was maintained by exhausting the vacuole K+ pool that results in turgor loss, and osmotic adjustment is done by synthesizing the organic osmolytes which leads to scavenging of ROS to retain the K+ ion in cytosol (Cuin et al. 2003; Shabala et al. 2006). It also activates the endonuclease and protease which leads to programmed cell death (Anschütz et al. 2014) as shown in Fig. 3.7.
3.4.2 Polyamines
Polyamines are the most important component in cells (Pegg 2016) which involve the role of polyamines in protein synthesis, cell division, etc. (Baronas and Kurata 2014; Handa et al. 2018). Ion transporter is also affected by polyamines like Spd, Spm, and Put (Roy et al. 2005). Inward rectifying of K+ channels (KIRC) is blocked by Spm during salt stress (Liu et al. 2000). NSCC and K+ efflux are also blocked by Spm under salt stress (Shabala et al. 2007). In barley seedling, the reduction of K+ efflux was observed by blocking VI-NSCC under the influence of exogenous spermidine during salinity stress (Zhao et al. 2007). Zhao et al. (2007) reported that Na+ accumulation is declined in roots which maintain a high level of K+ ions in shoots by affecting the Na+ and K+ currents in root cells by exogenous application of polyamine. In guard cells the inward K+ current is inhibited by the application of extracellular and intracellular polyamine (Liu et al. 2000). Vacuole K+ channel is affected by polyamines by inhibiting vacuolar NSCCs that increase K+/Na+ selectivity in tonoplast (Hamamoto et al. 2008). Prevention of the leakage of Na+ ion helps in restocking of cytosol with K+ through vacuolar K+ channels under the influence of polyamine (Zepeda-Jazo et al. 2008). Exogenous application of polyamine in pea mesophyll inhibited the VI-NSCC and reduced the loss of the K+ ions by decreasing the salt inducing membrane depolarization (Shabala et al. 2007). The role of PAs involves inhibition of NSCC and GORK to reduce K+ efflux by depolarization of potentials (Zepeda-Jazo et al. 2008). Depending on the growth condition and root zones, PAs improved NaCl-induced K+ efflux (Pandolfi et al. 2010). Activation role of PAs includes the induction of the depolarization of plasma membrane (Ozawa et al. 2010) which affects the transporter and generates driven force for the K+ efflux (Pottosin and Dobrovinskaya 2014) as shown in Fig. 3.8.
3.4.3 Plant Growth Regulators
There is a positive influence of K on the synthesis of plant hormones (Ashley et al. 2006). Alterations in auxin localization, its concentration, and its sensibility result in inhibition of lateral root growth under prolonged K+ shortage (Hafsi et al. 2014) and many auxin-associated gene responses to K+ deficiency in rice (Ma et al. 2012). MYB77, which is an Arabidopsis transcription factor, maintains the low K-dependent decline of the lateral root density through auxin signal transduction (Shin et al. 2007). GORK (guard cell outward-rectifying K+) channel activity and its expression level are expressively enhanced by elevated amounts of abscisic acid (ABA) and jasmonic acid and the expression of SKOR (stelar K+ outward rectifier) which is a K+ channel that is prevented by ABA during drought stress (Ragel et al. 2019). AtPP2CA involved in ABA signalling interacts with GORK and reduces its functioning independently from the phosphorylation status of the GORK protein (Lefoulon et al. 2016), and this PP2C not only regulates K+ channel activity by ABA signalling but also regulates SKOR activity. Cytokinins’ dependent and independent mechanisms maintain the HAK5 channel expression under low-K conditions, and in Arabidopsis, cytokinins also regulate HAK5 gene expression negatively under K starvation (Nam et al. 2012). OPEN STOMATA 1 (OST1), a crucial kinase in ABA signalling, phosphorylates K+ uptake transporter 6 (KUP6) in guard cells, and kup2 kup6 kup8 and kup6 kup8 GORK triple mutants (KUP2 and KUP8 are homologs of KUP6) display hypersensitivity to water deficit conditions as a result of defects in ABA-induced stomatal closure (Osakabe et al. 2013), showing that KUP6 and its homologs are involved in ABA signalling.
Ethylene is associated with the low-K signalling pathway by triggering the generation of ROS in roots, then altering root hair and primary root growth, and stimulating HAK5 expression in Arabidopsis (Jung et al. 2009). Low level of K+ in roots improved the amount of ethylene and the H2O2 level by mediating the expression of ethylene biosynthetic and signalling-related genes, and also of ROS metabolism-associated genes, and then, both ethylene and ROS escalated the transcription of HAK5 in Arabidopsis and tomato (Ródenas et al. 2018). Ethylene and ROS also stimulate the transcription of HAK5 under K-deficient environments (Schachtman 2015). Jasmonic acid is also involved in triggering comprehensible expression of a HAK5 in response to K+ deficiency, and it regulated the expression of rice K+ transporter (Chen et al. 2016). Augmented amounts of JA, 12-oxo-phytodienoic acid (OPDA), and hydroxy-12-oxo-octadecadienoic acids (HODs) were found through K-deficient environments in addition with the escalation of the 13-lipoxygenase (LOX) cascade, acknowledging the transcript phase of numerous biosynthetic enzymes with K association (Troufflard et al. 2010). Gene expression of K+ and its availability is decreased in the CORONATINE INSENSITIVE1 (coi1–16) mutant which is an important protein of JA signalling. However, K+ deficiency triggered the JA synthesis which enhanced the tolerance against pathogens and insects to the wild-type plants (Armengaud et al. 2010). Polyamines regulate stomatal movement by activating the K+ channel of the guard cell membrane, therefore showing that polyamines have important association with K at the cellular level (Liu et al. 2000).
3.4.4 Gasotransmitters
Gasotransmitters are the gaseous signalling molecules, for example, carbon monoxide (CO), nitric oxide (NO), nitrous oxide (N2O), and hydrogen sulfide (H2S), which vary from the usual signalling molecules due to their distinctive modes of action (Mustafa et al. 2009). Gasotransmitter accumulation has been reported to be enhanced in plants under the influence of abiotic stress conditions as the gasotransmitters increase stress tolerance in plants by regulating the action of numerous antioxidant enzymes (Yao et al. 2019). Gasotransmitters have been observed to provide cytoprotection for the potassium channel of mitochondria (Walewska et al. 2018). CO has been reported to regulate the number of ion channels of cell through the calcium-activated K+ as well as voltage-activated K+ receptors not only during normal conditions but also during pathogen infestation (Jaggar et al. 2005; Dallas et al. 2011; Duckles et al. 2015; Wilkinson et al. 2009). However, the mechanisms of actions behind modulation of the ion channels through carbon monoxide are not yet fully discovered; some studies suggest that sensitivity to CO is imparted toward certain ion channels through protein cofactors (Wilkinson and Kemp 2011). Similarly, in the plasma membrane, H2S has a variety of effects on the action of diverse potassium channels (Zhong 2010). In the HEK293 cells, hydrogen sulfide-mediated S-sulfhydration targets the Cys43 in the Kir6.1 subunit of the KATP channel and subsequently activates the ion channel (Mustafa et al., 2011). Further, it was observed that hydrogen sulfide also affects the activities of other Kir channels, i.e., Kir2 and Kir3 (Ha et al. 2018; Tang et al. 2005).
3.5 Regulation of K+ Uptake and Cellular Homeostasis by Molecular Approaches
The potassium transport systems (channels and transporters) are more complex and encoded by large gene families. The transport systems of same family are expressed into various tissues. This complex K+ sensing and signalling mechanism plays a role in the regulation of potassium ion uptake from the soil and transfer to different plant organs.
3.5.1 Regulation by NHX Transporters
It is genetically demonstrated that NHXs are involved in regulation of K+ homeostasis in Arabidopsis (Rodríguez-Rosales et al. 2008; Leidi et al. 2010; Bassil et al. 2011). Under control growth conditions, a single knockout mutant AtNHX1 displayed an altered phenotype including smaller cells and smaller leaves associated with altered K+ homeostasis. This is due to lower K+/H+ and Na+/H+ antiport activity (Apse et al. 2003; Sottosanto et al. 2004). AtNHX1 and 2 mutants showed reduction in rapidly elongating organs such as flowers, filaments, and hypocotyls of etiolated seedlings. In root and leaf cells of the double mutant, the vacuolar K+ content was about one-third of that from wild-type cells. The double mutant was highly sensitive to external K+ (nhx1nhx2 mutant has higher K+ cytosolic content), and this may indicate that these vacuolar NHX antiporters are the main moderators of cytosolic K+ uptake into the vacuole. It is also proposed that fluctuation of K+ content is essentially promoted by the activity of vacuolar NHX proteins (Bassil and Blumwald 2014). Stomatal movements require bidirectional K+ fluxes across the guard cells’ plasma and tonoplast membranes. Disruption in K+ accumulation in guard cells may affect the guard cell osmoregulation and stomatal movement (Andrés et al. 2014). Hence, it is suggested that NHX1 and NHX2 are the main transporters mediating K+ uptake to the vacuole.
3.6 Regulation of HAK/KUP/KT Transporters
3.6.1 Transcriptional Regulation
Transcriptional regulation is a universal mechanism for different plant species in response to K+-deficient stress (Wang and Wu 2013). Work with the DmHAK5 transporter showed that co-expression of the corresponding cRNA with that of CBL9/CIPK23 (but not DmHAK5 alone) brings about inward K+ in Venus flytrap and Rb+ currents in Xenopus oocytes that were invigorated by low external pH (Scherzer et al. 2015). Salt bladders of the halophyte Chenopodium quinoa that store salts to very high concentrations express a HAK-like activity and selective K+ uptake that was reliant on acidic external pH and by the CIPK23/CBL1 kinase module of Arabidopsis (Böhm et al. 2018). HAK1-like transporters are subject to complex transcriptional and posttranslational regulations; still research have been carried out almost exclusively in Arabidopsis AtHAK5 (Jung et al. 2009; Rubio et al. 2014; Ragel et al. 2015). In rice, adding NaCl in the external solution upregulates expression of OsHAK1 at high-K+ condition but downregulates its expression at low-K+ concentrations (Chen, et al. 2015). Furthermore, it has been commonly examined that the expression of HAK1-like genes is also regulated by other ions, particularly NH4+, NO3−, Na+, and Pi and not always in the same way (Nieves-Cordones et al. 2019). For example, the transcriptional expression of HAK5 in both tomato (cv. Micro-Tom) and Arabidopsis was also regulated by nitrate (NO3−) or phosphate (Pi) supply in response to low K+, but these regulations had no effect on the root K+ uptake rate of roots (Rubio et al. 2014) (Meng et al. 2016). It is shown that the expression of HAK5, RAP2.11 (encoding a transcriptional regulator of HAK5 (Kim et al. 2012)), and ANN1 (Laohavisit, et al., 2012) in the roots of nrt1.5 (a nitrate transporter) mutant is downregulated by NO3− starvation. The results suggest that on one hand HAK5, probably as well as other high-affinity K+ transporters, may be regulated transcriptionally or posttranscriptionally by low-K+ signal, while on the other hand, HAK5 may be involved in unknown functions related to plant NO3− and Pi deficiencies (Rubio et al. 2014). The root cell activity can be changed by growth medium containing NH4+, NO3−, Na+, and Pi. Thus, the effect of the salt stress and mineral inadequacy on transcriptional expression of certain HAK/KUP/KT genes may be linked with variations in the root cell membrane potentials (Rubio et al. 2014).
3.6.2 Regulation by Transcription Factors
The target sequences of many transcription factors (TFs) have been identified in the AtHAK5 promoter, and out of them ARF2 (auxin response factor 2) is described as a negative regulator of AtHAK5 transcription (Zhao et al. 2016). When K+ ions are present in large quantity, then channel-mediated K+ transport would be more favorable than cotransport (symport) through AtHAK5, and therefore AtHAK5 should be close (Zhao et al. 2016). Under such situations, within the AtHAK5 promoter, ARF2 binds to AuxREs (auxin-responsive elements) and represses transcription.
Under K+ deficiency, ARF2 is phosphorylated by a kinase and removed from the promoter and alleviates the repression on ATHAK5 transcription. Other transcription factors such as RAP2.11 bind to the ethylene-responsive element (ERE) and the GCC box of the AtHAK5 promoter, and its expression is upregulated by ethylene signalling, reactive oxygen species production, and calcium signalling (Kim et al. 2012). When K+ is again provided, ARF2 becomes dephosphorylated and represses the expression of AtHAK5 (Zhao et al. 2016). Thus, it is evident that regulation of the activity of TFs acting on AtHAK5 promoter (positively or negatively) is important to learn cooperatively the aggregation of the transcripts (Santa-María et al. 2018).
Potassium channels: These channels are further subdivided into two channels:
voltage-independent potassium channels and voltage-dependent channels:
3.7 Voltage-Independent Potassium Channels
3.7.1 Tandem-Pore Potassium Channels
These channels consist of six members, five tandem-pore channels (TPK1–TPK5), and a single subunit (KCO3) in Arabidopsis thaliana that resulted from chromosome segment duplication of a tandem-pore channel gene and subsequent partial deletion. All TPK/KCO channels are located at the vacuolar membrane and interact with 14-3-3 proteins (GRFs), showing regulation at the protein level. Cytosolic C-terminal part of these channels contains 1–2 Ca2+ binding, and N-terminal contains binding sites for 14-3-3 proteins. TPK channels are much dependent on Ca2+ ions which might be important for channel regulation (Latz et al. 2007a).
It has been proposed that TPK channels are involved in the K+ ion homeostasis of plant cells by providing controlled intracellular potassium transport from and into organelles. Recently the patch-clamp technique has revealed a mechano-sensitive nature of TPK channels recommending a role in osmoregulation. TPK1 has characteristics of K+-selective channels from Vicia faba specified in vivo with selectivity for potassium over sodium ions (Bihler et al. 2005; Gobert et al. 2007; Latz et al. 2007b). It was shown that TPK activity depends on cytosolic pH with probability of 6.7, decreasing 20–30% at physiological pH 7.5–7.8. Also, it has the highest affinity for calcium ions including calmodulin protein. It has been reported that TPK1 participates in K+ release from vacuole during stomatal closure, seed germination, and radicle growth (Gobert et al. 2007). There is higher abundance of TPK5 in senescent leaves and petals at mRNA levels (Voelker et al. 2010). TPK activity was observed in root cortex, vascular tissue, mesophyll cells, guard cells, and pollen grains using promoter-reporter gene (GUS) fusion (Czempinski et al. 2002).
3.7.2 Plant Kir-Like Channels
First Kir channels were identified from genome sequence databases by searching TPK1-related sequences in Arabidopsis. At biochemical level, KCO3 could be determined as a stable dimer (Rocchetti et al. 2012). The plants having mutant KCO3 gene show reduced growth under osmotic stress; hence, KCO3 gene plays an important role in osmoregulation. But the changed plant phenotype can be accomplished by expressing a mutant KCO3 gene with an inactive pore region. These results showed that the KCO3 serves its function independently to its ability to transport potassium ions under osmotic stress (Rocchetti et al. 2012). Thus, the above considerations revealed that these channels should be restored into the TPK family, instead of being expressed as a separate channel family.
3.8 Voltage-Dependent K+ Channels
These are the first Shaker-like, voltage-gated (VG) channels (AKT1) which are involved in nutrient uptake (Hirsch et al. 1998). The plant voltage-gated channels are phylogenetically related to animal Shaker channels and also include functional domains (Jegla et al. 2018). Four α-subunits of VG channels surround a central aqueous pore for K+ permeation. Each subunit (contains six transmembrane segments, i.e., S1–S6) can be divided into two different modules: a voltage-sensor domain (consists of four α-helices) contains positively charged residues that moves within the membrane in response to voltage which ultimately results in opening and closing of the channel. The physiological function and effect of plant Shaker channels on the plant are described in the following sections.
3.8.1 Regulation of AKT1 Channel
AKT1 is an inward-rectifying potassium channel involved in low and high potassium uptake in plants and is regulated by the protein kinase complex which consists of the kinase CIPK23 and one of the two alternative calcium-dependent regulatory subunits CBL1 and CBL9 (Li et al., 2006; Xu et al. 2006). AIP1, a 2C-type protein phosphatase (CIP kinases), was shown to bind and inactivate AKT1 (Lee et al. 2007). In principle, it is evidenced that phosphorylation/dephosphorylation regulates the activity of AKT1 channel, but it should be noted that no phosphorylation of AKT1 by CIPK23 and dephosphorylation by AIP1 have been revealed conclusively (Hashimoto et al. 2012). Instead it is suggested that mutual interaction of four components, CIPKs, CBLs, PP2Cs, and AKT1, inhibits the phosphorylation activity of the kinase and to dephosphorylate AKT1 (Lan et al. 2011). It is also indicated that CBL10 directly binds AKT1 and reduces its activity in a concentration-dependent and CIPK-independent manner (Ren et al. 2013). The phosphorylation-dephosphorylation system of CBL gives a strong regulatory network for the plant to respond to a wide range of environmental variations (Kudla et al. 2010).
3.8.2 Regulation via Heteromerization
Another regulatory subunit of Kin Shaker-like channels that alters the functionality of AKT1 is AtKC1. AtKC1-D increased the inhibition of AKT1 channel activity and confined K+ leakage through AKT1 under low-K+ conditions, thereby increasing the tolerance to nutrient stress (Wang et al. 2016). It is demonstrated that AtKC1 shifts activation threshold of AKT1 toward more-negative values and prevents K+ efflux through AKT1 under unfavorable conditions (Duby et al. 2008). It is established that at higher K+ concentrations, the pore of AKT1-AtKC1 heteromers collapses than that of AKT1homomers. Hence, heteromers comprise a more suitable obstructive K+ passage in the unfavorable outward direction.
3.9 Conclusion and Future Prospective
Intracellular K homeostasis is essential for the proper functioning of cultivar metabolic apparatuses and genotypes including development. The indispensability of K is due to its multifaceted appearance in genotypes such as stabilizing cell turgor pressure, cell expansion, opening and closing of stomata, osmotic adjustments, solute translocation in phloem, maintenance of membrane polarization, optimal photosynthetic activity, and many more. As many of these aspects are straightforwardly associated with plant acclimatization to adverse ecological situations, therefore K absorption, translocation, and homeostasis appear important to counteract abiotic factors. Our perception to the significance of K uptake strategy and its cellular homeostasis under stress environments has elevated substantially in recent years. Exposure to abiotic factors caused noteworthy interruption to intracellular K homeostasis which ultimately leads to huge K+ outflow. An intricate gene regulatory apparatus was found in cultivars to confirm K uptake and to uphold K+ homeostasis in plants under abiotic stress circumstances. Novel crop cultivars, which can absorb more K under hostile ecological circumstances and produce yield which is sufficient to fulfill the demands of human and animal, need to be developed. It is apparent that more studies, at both genetic and entire genotypic levels, are required to understand the mechanism of K homeostasis, its signalling, and regulators that alter their functioning.
References
Adams E, Shin R (2014) Transport, signaling, and homeostasis of potassium and sodium in plants. J Integr Plant Biol 56(3):231–249
Almeida DM, Oliveira MM, Saibo NJ (2017) Regulation of Na+ and K+ homeostasis in plants: towards improved salt stress tolerance in crop plants. Genet Mol Biol 40(1):326–345
Amjad M, Akhtar J, Murtaza B, Abbas G, Jawad H (2016) Differential accumulation of potassium results in varied salt-tolerance response in tomato (Solanum lycopersicum L) cultivars. Hortic Environ Biotechnol 57(3):248–258
Amtmann A, Troufflard S, Armengaud P (2008) The effect of potassium nutrition on pest and disease resistance in plants. Plant Physiol 133:682–691
Andrés Z, Pérez-Hormaeche J, Leidi EO, Schlücking K, Steinhorst L, McLachlan DH, Pardo JM (2014) Control of vacuolar dynamics and regulation of stomatal aperture by tonoplast potassium uptake. PNAS 111(17):E1806–E1814
Anschütz U, Becker D, Shabala S (2014) Going beyond nutrition: regulation of potassium homoeostasis as a common denominator of plant adaptive responses to environment. J Plant Physiol 171(9):670–687
Apse MP, Sottosanto JB, Blumwald E (2003) Vacuolar cation/H+ exchange, ion homeostasis, and leaf development are altered in a T-DNA insertional mutant of AtNHX1, the Arabidopsis vacuolar Na+/H+ antiporter. Plant J 36(2):229–239
Armengaud P, Breitling R, Amtmann A (2010) Coronatine-insensitive 1 (COI1) mediates transcriptional responses of Arabidopsis thaliana to external potassium supply. Mol Plant 3(2):390–405
Armstrong J, Armstrong W (1999) Phragmites die-back: toxic effects of propionic, butyric and caproic acids in relation to pH. New Phytol 142(2):201–217
Ashley MK, Grant M, Grabov A (2006) Plant responses to potassium deficiencies: a role for potassium transport proteins. J Exp Bot 57(2):425–436
Ashraf MA, Ahmad MSA, Ashraf M, Al-Qurainy F, Ashraf MY (2011) Alleviation of waterlogging stress in upland cotton (Gossypium hirsutum L) by exogenous application of potassium in soil and as a foliar spray. Crop Pasture Sci 62(1):25–38
Assaha DV, Ueda A, Saneoka H, Al-Yahyai R, Yaish MW (2017) The role of Na+ and K+ transporters in salt stress adaptation in glycophytes. Front Physiol 8:509
Azedo-Silva J, Osório J, Fonseca F, Correia MJ (2004) Effects of soil drying and subsequent re-watering on the activity of nitrate reductase in roots and leaves of Helianthus annuus. Funct Plant Biol 31(6):611–621
Baronas VA, Kurata HT (2014) Inward rectifiers and their regulation by endogenous polyamines. Front Physiol 5:325
Barragán V, Leidi EO, Andrés Z, Rubio L, De Luca A, Fernández JA, Pardo JM (2012) Ion exchangers NHX1 and NHX2 mediate active potassium uptake into vacuoles to regulate cell turgor and stomatal function in Arabidopsis. Plant Cell 24(3):1127–1142
Bassil E, Blumwald E (2014) The ins and outs of intracellular ion homeostasis: NHX-type cation/H+ transporters. Curr Opin Plant Biol 22:1–6
Bassil E, Tajima H, Liang YC, Ohto MA, Ushijima K, Nakano R, Blumwald E (2011) The Arabidopsis Na+/H+ antiporters NHX1 and NHX2 control vacuolar pH and K+ homeostasis to regulate growth, flower development, and reproduction. Plant Cell 23(9):3482–3497
Becker D, Hoth S, Ache P, Wenkel S, Roelfsema MRG, Meyerhoff O, Hedrich R (2003) Regulation of the ABA-sensitive Arabidopsis potassium channel gene GORK in response to water stress. FEBS Lett 554(1–2):119–126
Behera S, Long Y, Schmitz-Thom I, Wang XP, Zhang C, Li H, Wu WH (2017) Two spatially and temporally distinct Ca2+ signals convey Arabidopsis thaliana responses to K+ deficiency. New Phytol 213(2):739–750
Benlloch-González M, Arquero O, Fournier JM, Barranco D, Benlloch M (2008) K+ starvation inhibits water-stress-induced stomatal closure. J Plant Physiol 165(6):623–630
Benlloch-González M, Romera J, Cristescu S, Harren F, Fournier JM, Benlloch M (2010) K+ starvation inhibits water-stress-induced stomatal closure via ethylene synthesis in sunflower plants. J Exp Bot 61(4):1139–1145
Bihler H, Eing C, Hebeisen S, Roller A, Czempinski K, Bertl A (2005) TPK1 is a vacuolar ion channel different from the slow-vacuolar cation channel. Plant Physiol 139(1):417–424
Board JE (2008) Waterlogging effects on plant nutrient concentrations in soybean. J Plant Nutr 31:828–838
Böhm J, Messerer M, Müller HM, Scholz-Starke J, Gradogna A, Scherzer S, Ache P (2018) Understanding the molecular basis of salt sequestration in epidermal bladder cells of Chenopodium quinoa. Curr Biol 28(19):3075–3085
Bose J, Rodrigo-Moreno A, Shabala S (2014) ROS homeostasis in halophytes in the context of salinity stress tolerance. J Exp Bot 65(5):1241–1257
Cakmak I (2005) The role of potassium in alleviating detrimental effects of abiotic stresses in plants. J Plant Nutr Soil Sci 168(4):521–530
Chakraborty K, Bhaduri D, Meena HN, Kalariya K (2016) External potassium (K+) application improves salinity tolerance by promoting Na+-exclusion, K+-accumulation and osmotic adjustment in contrasting peanut cultivars. Plant Physiol Biochem 103:143–153
Chen G, Hu Q, Luo LE, Yang T, Zhang S, Hu Y, Xu G (2015) Rice potassium transporter O s HAK 1 is essential for maintaining potassium-mediated growth and functions in salt tolerance over low and high potassium concentration ranges. Plant Cell Environ 38(12):2747–2765
Chen Y, Ma J, Miller AJ, Luo B, Wang M, Zhu Z, Ouwerkerk PB (2016) OsCHX14 is involved in the K+ homeostasis in rice (Oryza sativa) flowers. Plant Cell Physiol 57(7):1530–1543
Cherel I, Lefoulon C, Boeglin M, Sentenac H (2014) Molecular mechanisms involved in plant adaptation to low K(+) availability. J Exp Bot 65:833–848
Choi S, Jeong S, Jeong W, Kwon S, Chow W, Park YI (2002) Chloroplast Cu/Zn-superoxide dismutase is a highly sensitive site in cucumber leaves chilled in the light. Planta 216(2):315–324
Clarkson DT, Hanson JB (1980) The mineral nutrition of higher-plants. Annu Rev Plant Physiol 31:239–298
Close DC, Davidson NJ (2003) Long-term waterlogging: nutrient, gas exchange, photochemical and pigment characteristics of Eucalyptus nitens saplings. Russ J Plant Physiol 50(6):843–847
Cuin TA, Miller AJ, Laurie SA, Leigh RA (2003) Potassium activities in cell compartments of salt-grown barley leaves. J Exp Bot 54(383):657–661
Czempinski K, Frachisse JM, Maurel C, Barbier-Brygoo H, Mueller-Roeber B (2002) Vacuolar membrane localization of the Arabidopsis ‘two-pore’ K+ channel KCO1. Plant J 29(6):809–820
Dallas ML, Boyle JP, Milligan CJ, Sayer R, Kerrigan TL, McKinstry C, Pearson HA (2011) Carbon monoxide protects against oxidant-induced apoptosis via inhibition of Kv2.1. FASEB J 25(5):1519–1530
Daszkowska-Golec A, Szarejko I (2013) Open or close the gate–stomata action under the control of phytohormones in drought stress conditions. Front Plant Sci 4:138
Demidchik V (2014) Mechanisms and physiological roles of K+ efflux from root cells. J Plant Physiol 171(9):696–707
Demidchik V, Cuin TA, Svistunenko D, Smith SJ, Miller AJ, Shabala S, Yurin V (2010) Arabidopsis root K+-efflux conductance activated by hydroxyl radicals: single-channel properties, genetic basis and involvement in stress-induced cell death. J Cell Sci 123(9):1468–1479
Dias AS, Lidon FC (2010) Bread and durum wheat tolerance under heat stress: a synoptical overview. Emir J Food Agric:412–436
Din J, Khan SU, Ali I, Gurmani AR (2011) Physiological and agronomic response of canola varieties to drought stress. J Anim Plant Sci 21(1):78–82
Duby G, Hosy E, Fizames C, Alcon C, Costa A, Sentenac H, Thibaud JB (2008) AtKC1, a conditionally targeted shaker-type subunit, regulates the activity of plant K+ channels. Plant J 53(1):115–123
Duckles H, Al-Owais MM, Elies J, Johnson E, Boycott H E, Dallas M L, Peers C (2015) T-type Ca2+ channel regulation by CO: a mechanism for control of cell proliferation in arterial chemoreceptors in physiology and pathophysiology. Springer, Cham, pp 291–300
Dwivedi SK, Kumar S, Bhakta N, Singh SK, Rao KK, Mishra JS, Singh AK (2017) Improvement of submergence tolerance in rice through efficient application of potassium under submergence-prone rainfed ecology of Indo-Gangetic Plain. Funct Plant Biol 44(9):907–916
Egilla JN, Davies FT, Boutton TW (2005) Drought stress influences leaf water content, photosynthesis, and water-use efficiency of Hibiscus rosa-sinensis at three potassium concentrations. Photosynthetica 43(1):135–140
El Mahi H, Pérez-Hormaeche J, De Luca A, Villalta I, Espartero J, Gámez-Arjona F, Lalanne E (2019) A critical role of sodium flux via the plasma membrane Na+/H+ exchanger SOS1 in the salt tolerance of rice. Plant Physiol 180(2):1046–1065
Emamverdian A, Ding Y, Mokhberdoran F, Xie Y (2015) Heavy metal stress and some mechanisms of plant defense response. Sci Wworld J. https://doi.org/10.1155/2015/756120
Fahad S, Bajwa AA, Nazir U, Anjum SA, Farooq A, Zohaib A, Ihsan MZ (2017) Crop production under drought and heat stress: plant responses and management options. Front Plant Sci 8:1147
Falhof J, Pedersen JT, Fuglsang AT, Palmgren M (2016) Plasma membrane H+-ATPase regulation in the center of plant physiology. Mol Plant 9(3):323–337
Farooq M, Basra SMA, Rehman H, Saleem BA (2008) Seed priming enhances the performance of late sown wheat (Triticum aestivum L) by improving chilling tolerance. J Agron Crop Sci 194(1):55–60
Fayez KA, Bazaid SA (2014) Improving drought and salinity tolerance in barley by application of salicylic acid and potassium nitrate. J Saudi Soc Agric Sci 13(1):45–55
Fazeli F, Ghorbanli M, Niknam V (2007) Effect of drought on biomass, protein content, lipid peroxidation and antioxidant enzymes in two sesame cultivars. Biol Plant 51(1):98–103
Flowers TJ, Munns R, Colmer TD (2015) Sodium chloride toxicity and the cellular basis of salt tolerance in halophytes. Ann Bot 115(3):419–431
Fuchs I, Stölzle S, Ivashikina N, Hedrich R (2005) Rice K+ uptake channel OsAKT1 is sensitive to salt stress. Planta 221(2):212–221
Gaxiola RA, Palmgren MG, Schumacher K (2007) Plant proton pumps. FEBS Lett 581(12):2204–2214
Gierth M, Mäser P (2007) Potassium transporters in plants—involvement in K+ acquisition, redistribution and homeostasis. FEBS Lett 581(12):2348–2356
Gobert A, Isayenkov S, Voelker C, Czempinski K, Maathuis FJ (2007) The two-pore channel TPK1 gene encodes the vacuolar K+ conductance and plays a role in K+ homeostasis. PNAS 104(25):10726–10731
Gong DS, Xiong YC, Ma BL, Wang TM, Ge JP, Qin XL, Li FM (2010) Early activation of plasma membrane H+-ATPase and its relation to drought adaptation in two contrasting oat (Avena sativa L) genotypes. Environ Exp Bot 69(1):1–8
Grabov A (2007) Plant KT/KUP/HAK potassium transporters: single family – multiple functions. Ann Bot 99(6):1035–1041
Guo S, Shen Q, Brueck H (2007) Effects of local nitrogen supply on water uptake of bean plants in a split root system. J Integr Plant Biol 49(4):472–480
Ha J, Xu Y, Kawano T, Hendon T, Baki L, Garai S, Logothetis DE (2018) Hydrogen sulfide inhibits Kir2 and Kir3 channels by decreasing sensitivity to the phospholipid phosphatidylinositol 4, 5-bisphosphate (PIP2). J Biol Chem 293(10):3546–3561
Hafsi C, Debez A, Abdelly C (2014) Potassium deficiency in plants: effects and signaling cascades. Acta Physiol Plant 36(5):1055–1070
Hamamoto S, Marui J, Matsuoka K, Higashi K, Igarashi K, Nakagawa T, Maeshima M (2008) Characterization of a tobacco TPK-type K+ channel as a novel tonoplast K+ channel using yeast tonoplasts. J Biol Chem 283(4):1911–1920
Hamamoto S, Horie T, Hauser F, Deinlein U, Schroeder JI, Uozumi N (2015) HKT transporters mediate salt stress resistance in plants: from structure and function to the field. Curr Opin Biotechnol 32:113–120
Han M, Wu W, Wu WH, Wang Y (2016) Potassium transporter KUP7 is involved in K+ acquisition and translocation in Arabidopsis root under K+-limited conditions. Mol Plant 9:437–446
Handa AK, Fatima T, Mattoo AK (2018) Polyamines: bio-molecules with diverse functions in plant and human health and disease. Front Chem 6:10
Hasanuzzaman M, Fujita M (2012) Heavy metals in the environment: current status, toxic effects on plants and phytoremediation. In: Phytotechnologies: remediation of environmental contaminants. CRC Press, Boca Raton, FL, pp 30–97
Hasanuzzaman M, Nahar K, Alam M, Roychowdhury R, Fujita M (2013) Physiological, biochemical, and molecular mechanisms of heat stress tolerance in plants. Int J Mol Sci 14(5):9643–9684
Hasanuzzaman M, Bhuyan MHM, Nahar K, Hossain M, Mahmud JA, Hossen M, Fujita M (2018) Potassium: a vital regulator of plant responses and tolerance to abiotic stresses. Agronomy 8(3):31
Hashimoto K, Eckert C, Anschütz U, Scholz M, Held K, Waadt R, Kudla J (2012) Phosphorylation of calcineurin B-like (CBL) calcium sensor proteins by their CBL-interacting protein kinases (CIPKs) is required for full activity of CBL-CIPK complexes toward their target proteins. J Biol Chem 287(11):7956–7968
Hauser F, Horie T (2010) A conserved primary salt tolerance mechanism mediated by HKT transporters: a mechanism for sodium exclusion and maintenance of high K+/Na+ ratio in leaves during salinity stress. Plant Cell Environ 33(4):552–565
Hedrich R (2012) Ion channels in plants. Physiol Rev 92:1777–1811
Hirsch RE, Lewis BD, Spalding EP, Sussman MR (1998) A role for the AKT1 potassium channel in plant nutrition. Science 280(5365):918–921
Hu B, Wang W, Deng K, Li H, Zhang Z, Zhang L, Chu C (2015) MicroRNA399 is involved in multiple nutrient starvation responses in rice. Front Plant Sci 6:188
Jaggar JH, Li A, Parfenova H, Liu J, Umstot ES, Dopico AM, Leffler CW (2005) Heme is a carbon monoxide receptor for large-conductance Ca2+-activated K+ channels. Circ Res 97(8):805–812
Jatav KS, Agarwal RM, Singh RP, Shrivastava M (2012) Growth and yield responses of wheat [Triticum aestivum l] to suboptimal water supply and different potassium doses. J Funct Environ Bot 2(1):39–51
Jegla T, Busey G, Assmann SM (2018) Evolution and structural characteristics of plant voltage-gated K+ channels. Plant Cell 30(12):2898–2909
Jung JY, Shin R, Schachtman DP (2009) Ethylene mediates response and tolerance to potassium deprivation in Arabidopsis. Plant Cell 21(2):607–621
Kafkafi U, Xu G, Imas P, Magen H, Tarchitzky J (2001) Potassium and chloride in crops and soils: the role of potassium chloride fertilizer in crop nutrition; IPI Research topics No 22. International Potash Institute, Horgen, Switzerland, p 220
Kanai S, Moghaieb RE, El-Shemy HA, Panigrahi R, Mohapatra PK, Ito J, Fujita K (2011) Potassium deficiency affects water status and photosynthetic rate of the vegetative sink in green house tomato prior to its effects on source activity. Plant Sci 180(2):368–374
Kaur H, Sirhindi G, Bhardwaj R, Alyemeni MN, Siddique KH, Ahmad P (2018) 28-homobrassinolide regulates antioxidant enzyme activities and gene expression in response to salt-and temperature-induced oxidative stress in Brassica juncea. Sci Rep 8(1):1–13
Khabaz-Saberi H, Rengel Z (2010) Aluminum, manganese, and iron tolerance improves performance of wheat genotypes in waterlogged acidic soils. J Plant Nutr Soil Sci 173(3):461–468
Kim MJ, Ruzicka D, Shin R, Schachtman DP (2012) The Arabidopsis AP2/ERF transcription factor RAP2 11 modulates plant response to low-potassium conditions. Mol Plant 5(5):1042–1057
Kudla J, Batistič O, Hashimoto K (2010) Calcium signals: the lead currency of plant information processing. Plant Cell 22(3):541–563
Lan WZ, Lee SC, Che YF, Jiang YQ, Luan S (2011) Mechanistic analysis of AKT1 regulation by the CBL–CIPK–PP2CA interactions. Mol Plant 4(3):527–536
Laohavisit A, Shang Z, Rubio L, Cuin TA, Véry AA, Wang A, Brownlee C (2012) Arabidopsis annexin1 mediates the radical-activated plasma membrane Ca2+-and K+-permeable conductance in root cells. Plant Cell 24(4):1522–1533
Latz A, Becker D, Hekman M, Müller T, Beyhl D, Marten I, Rapp UR (2007a) TPK1 a Ca2+-regulated Arabidopsis vacuole two-pore K+ channel is activated by 14-3-3 proteins. Plant J 52(3):449–459
Latz A, Ivashikina N, Fischer S, Ache P, Sano T, Becker D, Deeken R, Hedrich R (2007b) In planta AKT2 subunits constitute a pH-and Ca2+-sensitive inward rectifying K+ channel. Planta 225(5):1179–1191
Lee SC, Lan WZ, Kim BG, Li L, Cheong YH, Pandey GK, Luan S (2007) A protein phosphorylation/dephosphorylation network regulates a plant potassium channel. PNAS 104(40):15959–15964
Lefoulon C, Boeglin M, Moreau B, Véry AA, Szponarski W, Dauzat M, Chérel I (2016) The Arabidopsis AtPP2CA protein phosphatase inhibits the GORK K+ efflux channel and exerts a dominant suppressive effect on phosphomimetic-activating mutations. J Biol Chem 291(12):6521–6533
Leidi EO, Barragán V, Rubio L, El-Hamdaoui A, Ruiz MT, Cubero B, Pardo JM (2010) The AtNHX1 exchanger mediates potassium compartmentation in vacuoles of transgenic tomato. Plant J 61(3):495–506
Li L, Kim BG, Cheong YH, Pandey GK, Luan S (2006) A Ca2+ signaling pathway regulates a K+ channel for low-K response in Arabidopsis. PNAS 103(33):12625–12630
Li J, Long Y, Qi GN, Xu ZJ, Wu WH, Wang Y (2014) The Os-AKT1 channel is critical for K+ uptake in rice roots and is modulated by the rice CBL1-CIPK23 complex. Plant Cell 26(8):3387–3402
Li W, Xu G, Alli A, Yu L (2018a) Plant HAK/KUP/KT K(+) transporters: function and regulation. Semin Cell Dev Biol 74:133–141
Li Y, Peng L, Xie C (2018b) Genome-wide identification, characterization, and expression analyses of the HAK/KUP/KT potassium transporter gene family reveals their involvement in K+ deficient and abiotic stress responses in pear rootstock seedlings. Plant Growth Regul 85(2):187–198
Liang M, Gao Y, Mao T, Zhang X, Zhang S, Zhang H (2020) Characterization and expression of KT/HAK/KUP transporter family genes in willow under potassium deficiency, drought, and salt stresses. Biomed Res Int 6:1–12
Ling F, Silberbush M (2002) Response of maize to foliar vs soil application of nitrogen–phosphorus–potassium fertilizers. J Plant Nutr 25:2333–2342
Liu K, Fu H, Bei Q, Luan S (2000) Inward potassium channel in guard cells as a target for polyamine regulation of stomatal movements. Plant Physiol 124(3):1315–1326
Liu HP, Yu BJ, Zhang WH, Liu YL (2005) Effect of osmotic stress on the activity of H+-ATPase and the levels of covalently and noncovalently conjugated polyamines in plasma membrane preparation from wheat seedling roots. Plant Sci 168(6):1599–1607
Lu T, Meng Z, Zhang G, Qi M, Sun Z, Liu Y, Li T (2017) Sub-high temperature and high light intensity induced irreversible inhibition on photosynthesis system of tomato plant (Solanum lycopersicum L). Front Plant Sci 8:365
Ma TL, Wu WH, Wang Y (2012) Transcriptome analysis of rice root responses to potassium deficiency. BMC Plant Biol 12(1):161
Maathuis FJM (2009) Physiological functions of mineral macronutrients. Curr Opin Plant Biol 12:250–258
Maathuis FJM, Sanders D (1994) Mechanism of high-affinity potassium uptake in roots of Arabidopsis thaliana. Proc Natl Acad Sci U S A 91:9272–9276
Mansour MMF (2014) The plasma membrane transport systems and adaptation to salinity. J Plant Physiol 171(18):1787–1800
Marschner H (1995) Mineral nutrition of higher plants. Ann Bot 78:527–528
Marschner H (2012) Marschner’s mineral nutrition of higher plants, 3rd edn. Academic Press, London, pp 178–189
Masood S, Bano A (2016) Mechanism of potassium solubilization in the agricultural soils by the help of soil microorganisms. In: Meena V, Maurya B, Verma J, Meena R (eds) Potassium solubilizing microorganisms for sustainable agriculture. Springer, New Delhi, pp 137–147
Meng S, Peng JS, He YN, Zhang GB, Yi HY, Fu YL, Gong JM (2016) Arabidopsis NRT1 5 mediates the suppression of nitrate starvation-induced leaf senescence by modulating foliar potassium level. Mol Plant 9(3):461–470
Merwad ARM (2016) Efficiency of potassium fertilization and salicylic acid on yield and nutrient accumulation of sugar beet grown on saline soil. Commun Soil Sci Plant Anal 47(9):1184–1192
Mitra G (2018) Molecular approaches to nutrient uptake and cellular homeostasis in plants under abiotic stress. In: Plant nutrients and abiotic stress tolerance. Springer, Singapore, pp 525–590
Møller IM, Jensen PE, Hansson A (2007) Oxidative modifications to cellular components in plants. Annu Rev Plant Biol 58:459–481
Munns R, Tester M (2008) Mechanisms of salinity tolerance. Annu Rev Plant Biol 59:651–681
Mustafa AK, Gadalla MM, Snyder SH (2009) Signaling by gasotransmitters. Sci Signal. https://doi.org/10.1126/scisignal268re2
Mustafa AK, Sikka G, Gazi SK, Steppan J, Jung SM, Bhunia AK, Amzel LM (2011) Hydrogen sulfide as endothelium-derived hyperpolarizing factor sulfhydrates potassium channels. Circ Res 109(11):1259–1268
Nam YJ, Tran LSP, Kojima M, Sakakibara H, Nishiyama R, Shin R (2012) Regulatory roles of cytokinins and cytokinin signaling in response to potassium deficiency in Arabidopsis. PLoS One 7(10)
Nieves-Cordones M, Alemán F, Martínez V, Rubio F (2010) The Arabidopsis thaliana HAK5 K+ transporter is required for plant growth and K+ acquisition from low K+ solutions under saline conditions. Mol Plant 3(2):326–333
Nieves-Cordones M, Aleman F, Martinez V, Rubio F (2014) K(+) uptake in plant roots the systems involved, their regulation and parallels in other organisms. J Plant Physiol 171:688–695
Nieves-Cordones M, Ródenas R, Lara A, Martínez V, Rubio F (2019) The combination of K+ deficiency with other environmental stresses: what is the outcome? Physiol Plant 165(2):264–276
Oddo E, Inzerillo S, La Bella F, Grisafi F, Salleo S, Nardini A (2011) Short-term effects of potassium fertilization on the hydraulic conductance of Laurus nobilis L. Tree Physiol 31(2):131–138
Osakabe Y, Arinaga N, Umezawa T, Katsura S, Nagamachi K, Tanaka H, Yoshimura E (2013) Osmotic stress responses and plant growth controlled by potassium transporters in Arabidopsis. Plant Cell 25(2):609–624
Ou LJ, Dai XZ, Zhang ZQ, Zou XX (2011) Responses of pepper to waterlogging stress. Photosynthetica 49(3):339–345
Ozawa R, Bertea CM, Foti M, Ravishankar Narayana R, Arimura GI, Muroi A, Takabayashi J (2010) Polyamines and jasmonic acid induce plasma membrane potential variations in Lima bean. Plant Signal Behav 5(3):308–310
Pandey R, Agarwal RM, Jeevaratnam K, Sharma GL (2004) Osmotic stress-induced alterations in rice (Oryza sativa L) and recovery on stress release. Plant Growth Regul 42(1):79–87
Pandolfi C, Pottosin I, Cuin T, Mancuso S, Shabala S (2010) Specificity of polyamine effects on NaCl-induced ion flux kinetics and salt stress amelioration in plants. Plant Cell Physiol 51(3):422–434
Pang J, Ross J, Zhou M, Mendham N, Shabala S (2007) Amelioration of detrimental effects of waterlogging by foliar nutrient sprays in barley. Funct Plant Biol 34(3):221–227
Pegg AE (2016) Functions of polyamines in mammals. J Biol Chem 291(29):14904–14912
Polacik KA, Maricle BR (2013) Effects of flooding on photosynthesis and root respiration in saltcedar (Tamarix ramosissima) an invasive riparian shrub. Environ Exp Bot 89:19–27
Potash and Phosphate Institute (PPI) (1998) Potassium for Agriculture Better Crops with Plant Food, vol vol 32, N 3. Potash and Phosphate Institute, Atlanta
Pottosin I, Dobrovinskaya O (2014) Non-selective cation channels in plasma and vacuolar membranes and their contribution to K+ transport. J Plant Physiol 171(9):732–742
Pyo YJ, Gierth M, Schroeder JI, Cho MH (2010) High-affinity K+ transport in Arabidopsis: AtHAK5 and AKT1 are vital for seedling establishment and post-germination growth under low-potassium conditions. Plant Physiol 153:863–875
Ragel P, Ródenas R, García-Martín E, Andrés Z, Villalta I, Nieves-Cordones M, Rubio F (2015) The CBL-interacting protein kinase CIPK23 regulates HAK5-mediated high-affinity K+ uptake in Arabidopsis roots. Plant Physiol 169(4):2863–2873
Ragel P, Raddatz N, Leidi EO, Quintero FJ, Pardo JM (2019) Regulation of K+ nutrition in plants. Front Plant Sci 110:281
Ren XL, Qi GN, Feng HQ, Zhao S, Zhao SS, Wang Y, Wu WH (2013) Calcineurin B-like protein CBL 10 directly interacts with AKT 1 and modulates K+ homeostasis in Arabidopsis. Plant J 74(2):258–266
Rocchetti A, Sharma T, Wulfetange C, Scholz-Starke J, Grippa A, Carpaneto A, Pedrazzini E (2012) The putative K+ channel subunit AtKCO3 forms stable dimers in Arabidopsis. Front Plant Sci 3:251
Ródenas R, Nieves-Cordones M, Rivero RM, Martinez V, Rubio F (2018) Pharmacological and gene regulation properties point to the SlHAK5 K+ transporter as a system for high-affinity Cs+ uptake in tomato plants. Physiol Plant 162(4):455–466
Rodríguez-Rosales MP, Jiang X, Gálvez FJ, Aranda MN, Cubero B, Venema K (2008) Overexpression of the tomato K+/H+ antiporter LeNHX2 confers salt tolerance by improving potassium compartmentalization. New Phytol 179(2):366–377
Römheld V, Kirkby EA (2010) Research on potassium in agriculture: needs and prospects. Plant Soil 335(1–2):155–180
Roy P, Niyogi K, Sengupta DN, Ghosh B (2005) Spermidine treatment to rice seedlings recovers salinity stress-induced damage of plasma membrane and PM-bound H+-ATPase in salt-tolerant and salt-sensitive rice cultivars. Plant Sci 168(3):583–591
Roy SJ, Negrão S, Tester M (2014) Salt resistant crop plants. Curr Opin Biotechnol 26:115–124
Rubio F, Fon M, Ródenas R, Nieves-Cordones M, Alemán F, Rivero RM, Martínez V (2014) A low K+ signal is required for functional high-affinity K+ uptake through HAK5 transporters. Physiol Plant 152(3):558–570
Saida C, Houria B, Mébarek B (2014) Interactive effects of salinity and potassium on physio-morphological traits of tomato (Lycopersicon esculentum mill; var: heintz). Agri Biol J North Am 5(3):135–143
Santa-María GE, Oliferuk S, Moriconi JI (2018) KT-HAK-KUP transporters in major terrestrial photosynthetic organisms: a twenty years tale. J Plant Physiol 226:77–90
Schachtman DP (2015) The role of ethylene in plant responses to K+ deficiency. Front Plant Sci 6:1153
Scherzer S, Böhm J, Krol E, Shabala L, Kreuzer I, Larisch C, Neher E (2015) Calcium sensor kinase activates potassium uptake systems in gland cells of Venus flytraps. PNAS 112(23):7309–7314
Schumann T, Paul S, Melzer M, Dörmann P, Jahns P (2017) Plant growth under natural light conditions provides highly flexible short-term acclimation properties toward high light stress. Front Plant Sci 8:681
Scoffoni C, Sack L, Ort D (2017) The causes and consequences of leaf hydraulic decline with dehydration. J Exp Bot 68(16):4479–4496
Shabala S (2011) Physiological and cellular aspects of phytotoxicity tolerance in plants: the role of membrane transporters and implications for crop breeding for waterlogging tolerance. New Phytol 190(2):289–298
Shabala S (2017) Signalling by potassium: another second messenger to add to the list? J Exp Bot 68(15):4003–4007
Shabala S, Cuin TA (2008) Potassium transport and plant salt tolerance. Physiol Plant 133(4):651–669
Shabala S, Pottosin I (2014) Regulation of potassium transport in plants under hostile conditions: implications for abiotic and biotic stress tolerance. Physiol Plant 151(3):257–279
Shabala S, Demidchik V, Shabala L, Cuin TA, Smith SJ, Miller AJ, Newman IA (2006) Extracellular Ca2+ ameliorates NaCl-induced K+ loss from Arabidopsis root and leaf cells by controlling plasma membrane K+-permeable channels. Plant Physiol 141(4):1653–1665
Shabala S, Cuin TA, Pottosin I (2007) Polyamines prevent NaCl-induced K+ efflux from pea mesophyll by blocking non-selective cation channels. FEBS Lett 581(10):1993–1999
Shabala L, Zhang J, Pottosin I, Bose J, Zhu M, Fuglsang AT, Bacic A (2016) Cell-type-specific H+-ATPase activity in root tissues enables K+ retention and mediates acclimation of barley (Hordeum vulgare) to salinity stress. Plant Physiol 172(4):2445–2458
Sharma T, Dreyer I, Riedelsberger J (2013) The role of K(+) channels in uptake and redistribution of potassium in the model plant Arabidopsis thaliana. Front Plant Sci 4:224
Shin R, Schachtman DP (2004) Hydrogen peroxide mediates plant root cell response to nutrient deprivation. PNAS USA 101:8827–8832
Shin R, Burch AY, Huppert KA, Tiwari SB, Murphy AS, Guilfoyle TJ, Schachtman DP (2007) The Arabidopsis transcription factor MYB77 modulates auxin signal transduction. Plant Cell 19(8):2440–2453
Siddiqui MH, Al-Whaibi MH, Sakran AM, Basalah MO, Ali HM (2012) Effect of calcium and potassium on antioxidant system of Vicia faba L under cadmium stress. Int J Mol Sci 13(6):6604–6619
Smethurst CF, Garnett T, Shabala S (2005) Nutritional and chlorophyll fluorescence responses of lucerne (Medicago sativa) to waterlogging and subsequent recovery. Plant Soil 270(1):31–45
Soleimanzadeh H, Habibi D, Ardakani MR, Paknejad F, Rejali F (2010) Effect of potassium levels on antioxidant enzymes and malondialdehyde content under drought stress in sunflower (Helianthus annuus L). Am J Agric Biol Sci 5(1):56–61
Song ZZ, Su YH (2013) Distinctive potassium-accumulation capability of alligator weed (Alternanthera philoxeroides) links to high-affinity potassium transport facilitated by K+-uptake systems. Weed Sci 61(1):77–84
Song ZZ, Duan CL, Guo SL, Yang Y, Feng YF, Ma RJ, Yu ML (2015) Potassium contributes to zinc stress tolerance in peach (Prunus persica) seedlings by enhancing photosynthesis and the antioxidant defense system. Genet Mol Res 14(3):8338–8351
Sottosanto JB, Gelli A, Blumwald E (2004) DNA array analyses of Arabidopsis thaliana lacking a vacuolar Na+/H+ antiporter: impact of AtNHX1 on gene expression. Plant J 40(5):752–771
Srinivasarao C, Kundu S (2017) Potassium Management for Mitigation of biotic and abiotic stresses in plants under changing climates. Indian J Fert 46
Srivastava AK, Shankar A, Nalini Chandran AK, Sharma M, Jung KH, Suprasanna P, Pandey GK (2020) Emerging concepts of potassium homeostasis in plants. J Exp Bot 71(2):608–619
Su Y, Luo W, Lin W, Ma L, Kabir MH (2015) Model of cation transportation mediated by high-affinity potassium transporters (HKTs) in higher plants. Biol Proced Online 17(1):1–13
Sun J, Dai S, Wang R, Chen S, Li N, Zhou X, Zhang Z (2009) Calcium mediates root K+/Na+ homeostasis in poplar species differing in salt tolerance. Tree Physiol 29(9):1175–1186
Suzuki K, Yamaji N, Costa A, Okuma E, Kobayashi NI, Kashiwagi T, Schroeder JI (2016) OsHKT1; 4-mediated Na+ transport in stems contributes to Na+ exclusion from leaf blades of rice at the reproductive growth stage upon salt stress. BMC Plant Biol 16(1):1–15
Sze H, Chanroj S (2018) Plant endomembrane dynamics: studies of K(+)/H(+) antiporters provide insights on the effects of pH and ion homeostasis. Plant Physiol 177:875–895
Taffouo VD, Wamba OF, Youmbi E, Nono GV, Akoa A (2010) Growth, yield, water status and ionic distribution response of three bambara groundnut (Vigna subterranea (L) Verdc) landraces grown under saline conditions. Int J Bot 6(1):53–58
Tang G, Wu L, Liang W, Wang R (2005) Direct stimulation of KATP channels by exogenous and endogenous hydrogen sulfide in vascular smooth muscle cells. Mol Pharmacol 68(6):1757–1764
Teakle NL, Bazihizina N, Shabala S, Colmer TD, Barrett-Lennard EG, Rodrigo-Moreno A, Läuchli AE (2013) Differential tolerance to combined salinity and O2 deficiency in the halophytic grasses Puccinellia ciliata and Thinopyrum ponticum: the importance of K+ retention in roots. Environ Exp Bot 87:69–78
Teixeira J, Pereira S (2007) High salinity and drought act on an organ-dependent manner on potato glutamine synthetase expression and accumulation. Environ Exp Bot 60(1):121–126
Thomson MJ, de Ocampo M, Egdane J, Rahman MA, Sajise AG, Adorada DL, Gregorio GB (2010) Characterizing the Saltol quantitative trait locus for salinity tolerance in rice. Rice 3(2–3):148–160
Trifilò P, Nardini A, Raimondo F, Gullo MAL, Salleo S (2011) Ion-mediated compensation for drought-induced loss of xylem hydraulic conductivity in field-growing plants of Laurus nobilis. Funct Plant Biol 38(7):606–613
Troufflard S, Mullen W, Larson TR, Graham IA, Crozier A, Amtmann A, Armengaud P (2010) Potassium deficiency induces the biosynthesis of oxylipins and glucosinolates in Arabidopsis thaliana. BMC Plant Biol 10(1):172
Vallejo AJ, Peralta ML, Santa-Maria GE (2005) Expression of potassium-transporter coding genes, and kinetics of rubidium uptake, along a longitudinal root axis. Plant Cell Environ 28(7):850–862
Véry AA, Nieves-Cordones M, Daly M, Khan I, Fizames C, Sentenac H (2014) Molecular biology of K+ transport across the plant cell membrane: what do we learn from comparison between plant species? J Plant Physiol 171(9):748–769
Voelker C, Gomez-Porras JL, Becker D, Hamamoto S, Uozumi N, Gambale F, Dreyer I (2010) Roles of tandem-pore K+ channels in plants—a puzzle still to be solved. Plant Biol 12:56–63
Walewska A, Szewczyk A, Koprowski P (2018) Gas signaling molecules and mitochondrial potassium channels. Int J Mol Sci 19(10):3227
Wang Y, Wu H (2013) Potassium transport and signaling in higher plants. Annu Rev Plant Biol 64:451–476
Wang Y, Wu H (2017) Regulation of potassium transport and signaling in plants. Curr Opin Plant Biol 39:123–128
Wang M, Zheng Q, Shen Q, Guo S (2013) The critical role of potassium in plant stress response. Int J Mol Sci 14(4):7370–7390
Wang L, Wu X, Liu Y, Qiu Q-S (2015) AtNHX5 and AtNHX6 control cellular K+ and pH homeostasis in Arabidopsis: three conserved acidic residues are essential for K+ transport. PLoS One 10(12):e0144716
Wang XP, Chen LM, Liu WX, Shen LK, Wang FL, ZhouY WY (2016) AtKC1 and CIPK23 synergistically modulate AKT1-mediated low-potassium stress responses in Arabidopsis. Plant Physiol 170(4):2264–2277
Ward JM, Maser P, Schroeder JI (2009) Plant ion channels: gene families, physiology, and functional genomics analyses. Annu Rev Physiol 71:59–82
Wegner LH (2015) A thermodynamic analysis of the feasibility of water secretion into xylem vessels against a water potential gradient. Funct Plant Biol 42:828–835
Wei J, Li C, Li Y, Jiang G, Cheng G, Zheng Y (2013) Effects of external potassium (K) supply on drought tolerances of two contrasting winter wheat cultivars. PLoS One 8(7):e69737
White PJ, Karley AJ (2010) Potassium in cell biology of metals and nutrients. Springer, Berlin/Heidelberg, pp 199–224
Wilkinson WJ, Kemp PJ (2011) Carbon monoxide: an emerging regulator of ion channels. J Physiol 589(13):3055–3062
Wilkinson WJ, Gadeberg HC, Harrison AWJ, Allen ND, Riccardi D, Kemp PJ (2009) Carbon monoxide is a rapid modulator of recombinant and native P2X2 ligand-gated ion channels. Br J Pharmacol 158(3):862–871
Xu J, Li HD, Chen LQ, Wang Y, Liu LL, He L, Wu WH (2006) A protein kinase, interacting with two calcineurin B-like proteins, regulates K+ transporter AKT1 in Arabidopsis. Cell 125(7):1347–1360
Yan S, McLamore ES, Dong S, Gao H, Taguchi M, Wang N, Shen Y (2015) The role of plasma membrane H+-ATP ase in jasmonate-induced ion fluxes and stomatal closure in Arabidopsis thaliana. Plant J 83(4):638–649
Yang T, Zhang S, Hu Y, Wu F, Hu Q, Chen G, Cai J, Wu T, Moran N, Yu L (2014) The role of a potassium transporter OsHAK5 in potassium acquisition and transport from roots to shoots in rice at low potassium supply levels. Plant Physiol 166:945–959
Yao Y, Yang Y, Li C, Huang D, Zhang J, Wang C, Liao W (2019) Research progress on the functions of gasotransmitters in plant responses to abiotic stresses. Plants 8(12):605
Zahoor R, Zhao W, Abid M, Dong H, Zhou Z (2017) Potassium application regulates nitrogen metabolism and osmotic adjustment in cotton (Gossypium hirsutum L) functional leaf under drought stress. J Plant Physiol 215:30–38
Zeng F, Shabala L, Zhou M, Zhang G, Shabala S (2013) Barley responses to combined waterlogging and salinity stress: separating effects of oxygen deprivation and elemental toxicity. Front Plant Sci 4:313
Zepeda-Jazo I, Shabala S, Chen Z, Pottosin II (2008) Na+-K+ transport in roots under salt stress. Plant Signal Behav 3(6):401–403
Zepeda-Jazo I, Velarde-Buendía AM, Enríquez-Figueroa R, Bose J, Shabala S, Muñiz-Murguía J, Pottosin II (2011) Polyamines interact with hydroxyl radicals in activating Ca2+ and K+ transport across the root epidermal plasma membranes. Plant Physiol 157(4):2167–2180
Zhang Z, Zhang J, Chen Y, Li R, Wang H, Wei J (2012) Genome-wide analysis and identification of HAK potassium transporter gene family in maize (Zea mays L). Mol Biol Rep 39(8):8465–8473
Zhang L, Gao M, Li S, Alva AK, Ashraf M (2014) Potassium fertilization mitigates the adverse effects of drought on selected Zea mays cultivars. Turk J Bot 38(4):713–723
Zhao F, Song CP, He J, Zhu H (2007) Polyamines improve K+/Na+ homeostasis in barley seedlings by regulating root ion channel activities. Plant Physiol 145(3):1061–1072
Zhao S, Zhang ML, Ma TL, Wang Y (2016) Phosphorylation of ARF2 relieves its repression of transcription of the K+ transporter gene HAK5 in response to low potassium stress. Plant Cell 28(12):3005–3019
Zhong GZ (2010) Hydrogen Sulfide—a potent multichannel anti-arrhythmic drug. J Cardiovasc Dis Res 1(1):37
Author information
Authors and Affiliations
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2022 The Author(s), under exclusive license to Springer Nature Singapore Pte Ltd.
About this chapter
Cite this chapter
Kapoor, D. et al. (2022). Molecular Approaches to Potassium Uptake and Cellular Homeostasis in Plants Under Abiotic Stress. In: Iqbal, N., Umar, S. (eds) Role of Potassium in Abiotic Stress. Springer, Singapore. https://doi.org/10.1007/978-981-16-4461-0_3
Download citation
DOI: https://doi.org/10.1007/978-981-16-4461-0_3
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-16-4460-3
Online ISBN: 978-981-16-4461-0
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)