Abstract
Agricultural production continues to be forced by a salt stress that can reduce crop yield quantity and quality. Nitric oxide (NO), a signaling molecule, plays multiple roles in plant growth and development and in response to salt stress. Nutrient management strategy is critical for salt stress alleviation in plants. NO is recognized as a main participant in response to changes in nutrient availability. In particular, potassium (K) and nitrogen (N) are important nutrient for plants, as both nutrient carry out most of the biochemical and physiological processes or vital functions in metabolism, growth, and stress adaptation in plants. The following chapter focuses on the synthesis of NO with emerging role of K and N nutrients under salt stress. The synthesis of NO and its effects on plant growth, morphology, and plant metabolism are discussed. The physiological and molecular mechanisms of K and N function with NO in plant salt stress resistance are reviewed. This study summarizes a vital role of NO synthesis in modulating the homeostasis of K and N and its nutrition in plants and highlights the future needs for research about the role of K and N with NO synthesis under salt stress in agriculture.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
13.1 Introduction
The world population is increasing rapidly and will become from its existing number of approximately 7 billion to 9.4 billion by the year 2050 (FAO 2009; United States Census Bureau 2012). For an expanding world population, a substantial increase in crop production is required to meet the food demands of future generations while conserving ecological resources of our world. However, agricultural production continues to be repressed by abiotic factors such as drought, salinity, cold, frost, etc., that cause severe negative impact on the gross agronomy and reduce the quantity and quality of crop production (Wang et al. 2013). However, in abiotic stress, plant exposed to salt stress becomes a major concern in the world. Salt stress has impaired chlorophyll biosynthesis due to downregulation of gene expression and influenced growth, development, and yield of crops. The reduced photosynthesis affected by salt stress is attributed to decline in chlorophyll fluorescence, perturbation of thylakoid membrane fluidity, and consequent decline in CO2 assimilation and protein trafficking to chloroplasts (Fatma et al. 2021). Photosynthetic inhibition in plants under salt stress results from the inhibition of the activation of Rubisco and the Calvin cycle reactions together with the decrease in stomatal conductance and intercellular CO2 concentration (Fatma et al. 2016). It is, therefore, important to understand the mechanism of salt-induced physiological dysfunctions in crops of agricultural importance.
Plants have developed a wide range of mechanisms to resist a variety of stressed conditions. In particular, nitric oxide (NO), a crucial gaseous signaling molecule which has attracted much attention because of its diverse role in physiological responses in plants, ranges from germination and senescence to photosynthesis and cellular redox balance (Nabi et al. 2019; Jahan et al. 2020). Nitric oxide plays an important role in resistance to salt stress by its antioxidant properties and also by inducing the activity of ROS-scavenging enzymes to alleviate oxidative stress (Fatma et al. 2016). The study of Lopez-Carrion et al. (2008) showed the relationship between NO and the induction of proline in response to salt stress and suggested that NO could mitigate the damage associated with salt stress. Moreover, several studies also suggested that the coordination between NO and nutritional signaling plays an important role in salt tolerance (Jahan et al. 2020; Fatma et al. 2021).
Among the mineral nutrients, potassium (K) and nitrogen (N) are well-characterized nutrients in plant defense mechanisms and plays a critical role in plant growth and metabolism, and it contributes greatly to the survival of plants that are under various biotic and abiotic stresses. An essential nutrient, K is the most abundant cation in plants and plays essential roles in enzyme activation, protein synthesis, photosynthesis, osmoregulation, stomatal movement, energy transfer, phloem transport, cation-anion balance, and stress resistance (Marschner 2012). Similarly, N is also an essential macronutrient and is a constituent of many vital compounds of plants (Krapp 2015; Jahan et al. 2020). Recent reports have highlighted a role of NO in N assimilation and N uptake in plants and have suggested that NO is a vital signaling molecule of the nitrate-sensing pathway (Balotf et al. 2018), and it shows connection between N assimilation and NO synthesis.
There are several publications individually on the K and N under abiotic stress (Abbasi et al. 2015; Iqbal et al. 2015; Jahan et al. 2020) and NO, as important plant growth regulator under salt stress (Fatma et al. 2016; Sehar et al. 2019). However, the information on the role and underlying mechanism of K and N nutrients with NO in plants under salt exposure is scanty. Here, we summarize some of the old and current information regarding the role of NO synthesis in some of the responses to K and N nutrients under salt stress. The available chapter clearly established that NO biosynthesis and perception largely affect the homeostasis of K and N, as a mineral nutrient with a strong influence on plant growth and development. An abundant understanding of the interaction between NO and nutrients, K and N under salt stress will provide new strategies for improving crop potency and development under changing environment. In this chapter, the effect of NO pathway on K and N homeostasis under salt stress and plant responses is reviewed and discussed. The information supporting a role for NO synthesis in the homeostasis of K and N nutrient responses under salt stress is critically analyzed.
13.2 Basic Biochemistry of Nitric Oxide
Nitric oxide is a small-sized highly diffusible gas and a ubiquitous bioactive molecule. Nitric oxide acts a versatile signal molecule in plants since it possesses unique chemistry. Nitric oxide is a diatomic inorganic gaseous molecule and is comprised of an atom each of N and O compose NO, where seven electrons from N and eight electrons from O are involved to form an uncharged molecule (N≡O). Therefore, NO functions through interactions with cellular targets via either redox or additive chemistry. Moreover, depending on its concentration and the site of production, NO varyingly influences major physiological and molecular processes in plants, wherein its both positive and negative effects may be perceptible (Rather et al. 2020). Nitric oxide transmits its bioactivity, and eventual physiological/biochemical and molecular changes occur in plants mainly as a result of its interaction with proteins, abscisic acid, auxins, osmolytes (such as proline and glycine betaine), and nonenzymatic antioxidants (such as reduced GSH). Nitric oxide modulates the activity of proteins through nitrosylation and probably tyrosine nitration. In fact, the protein tyrosine nitration and the production of nitrite (ONOO−) occur as a result of the reaction of NO with ROS such as superoxide anions. Additionally, NO-accrued cysteine S-nitrosylation yields S-nitrosothiols (SNOs). NO-GSH reaction produces S-nitrosoglutathione (GSNO) that, in turn, can be transported to other cells/tissues (and help NO travel long distance) and be converted into oxidized glutathione (GSSG) and NH3 by GSNO reductase (GSNOR) (reviewed by Rather et al. 2020). Interaction of NO with plant lipids such as nitro-fatty acids (NO2-FA) (Sánchez-Calvo et al. 2013; Fazzari et al. 2014) and mitogen-activated protein kinase (MAPK) (Ye et al. 2013) results in cell signaling processes and the modulation of plant stress responses. On the other, NO interaction with abscisic acid and auxins results in inhibition of the programmed cell death along with the reduction of detrimental stress factor (Nabi et al. 2019; Sharma et al. 2020). Nitric oxide can also act as a Ca2+-mobilizing messenger (Besson-Bard et al. 2008). Nitric oxide-mediated increased cellular levels of the major osmolytes (such as proline and glycine betaine) have also been reported in plants (Ahmad et al. 2016; Khan et al. 2020).
13.3 Biosynthesis of Nitric Oxide
The major sites of NO biosynthesis in plants are protoplasts, chloroplasts, mitochondria, and peroxisome. Nitric oxide can be synthesized in plants both by enzymatic as well as nonenzymatic systems. Enzymatic systems contributing NO synthesis in plants include nitrate reductase (NR), nitrite: NO reductase (NiNOR), and NOS-like enzymes. The major nonenzymatic mechanisms for NO generation include chemical reduction of NO2− at acidic pH, carotenoids in the presence of light, and at acidic pH in the presence of a reductant such as ascorbic acid (reviewed by Ferreira and Cataneo 2010). Further, enzymatic and nonenzymatic pathways for NO synthesis in plants have been classified as either oxidative or reductive pathways depending on the substrate involved (Fig. 13.1). To date, several pathways for NO biosynthesis have been discovered in plants. There are two pathways included in plant tissues: enzymatic and nonenzymatic pathways (Sánchez-Calvo et al. 2013; Rather et al. 2020).
A brief discussion on the major enzymatic and nonenzymatic pathways involved in NO synthesis is presented hereunder.
13.3.1 Enzymatic Pathways
The reductive pathways for NO synthesis depend on NO2− as a primary substrate and include NR, plasma membrane-associated nitrite:NO reductase (NiNOR), and other molybdoenzymes (such as xanthine oxidoreductase, XOR), and also mitochondrial and chloroplastic electron transport chains (Galatro et al. 2020).
13.3.1.1 Nitrate Reductase (NR)
Nitrate reductase (NR) is molybdoenzyme and is involved in catalysis of the first and rate-limiting step in nitrate assimilation, where nitrate (NO3−) is reduced to nitrite (NO2−) in the presence of NADPH. Notably, NR pathway is among the best-identified pathway for NO biosynthesis in plants. NO2− thus formed is reduced to NO via NR itself or electron transport chain in mitochondria. Nitrate reductase can undergo a regulatory switch from its preferential high-affinity substrate NO3− (Km nitrate = <40 μM) to NO2− (low affinity; Km nitrite = 100 μM) and producing NO (Mur et al. 2013). The generation of NO via NR has been reported to require both low oxygen concentration and cellular pH. Additionally, NR-mediated NO generation may also occur during closure of stomata, flowering, and formation of lateral roots and, most importantly, during abiotic stress responses (Moreau et al. 2010; Prochazkova et al. 2014).
13.3.1.2 Xanthine Oxidoreductase (XOR)
Xanthine oxidoreductase (XOR), a peroxisomally located and Mo-containing ubiquitous enzyme exists in two interconvertible forms, namely, xanthine oxidase (XO) and xanthine dehydrogenase (XDH). Xanthine oxidoreductase reduces NO2− to NO at the expense of NADH under anaerobic conditions (Corpas et al. 2008). In particular, XO can reduce organic and inorganic NO3− and NO2− and eventually release NO (Godber et al. 2000). On the other, Wang et al. (2010) noticed the involvement of XDH in NO synthesis in roots of Lupinus albus grown under phosphate (PO4−) deficiency.
13.3.1.3 Nitrite:NO Reductase (NiNOR)
NO-forming nitrite reductase (NOFNiR) is a dual system that also includes NR as a necessary partner. Ni:NOR does not require the Mo-center of NR; however, it depends on the NR electron transport chain from NADPH to heme for the production of NO. An in vitro study has indicated cytochrome c as an electron donor (Wilson et al. 2008). NR:NiNOR system can sense nitrate availability in the soil (Meyer and Stöhr 2002), whereas Ni-NOR-mediated NO production regulates root infection by mycorrhizal fungi (Moche et al. 2010).
13.3.1.4 Nitric Oxide Synthase (NOS)-Like Enzymes
Plants possess strategies for using the sources of N other than NO3− and NO2− in the production of NO. To this end, NOS-like enzymes act as an important arsenal in NO3−-independent synthesis of NO. NOS-like enzymes deaminate L-arginine into L-citrulline and eventually NO in the presence of NADPH and O2 (Rümer et al. 2009). NOS activity was measured in a number of plants including Pisum sativum (Corpas et al. 2006), Glycine max chloroplasts (Simontacchi et al. 2004), Sorghum bicolor seed embryonic axes (Jasid et al. 2006), and Pisum sativum and Zea mays tissues (Barroso et al. 1999; Ribeiro et al. 1999). Search for the presence of transcripts encoding NOS proteins in land plants (>1000 species) resulted in no typical NOS sequences (Jeandroz et al. 2016). Only a few algae species including the green alga Ostreococcus tauri was among photosynthetic organisms that contained NOS orthologs (Foresi et al. 2010; Jeandroz et al. 2016; Santolini et al. 2017). In Glycine max primary roots, NOS-like enzyme (and NR also) has been proposed to regulate the gravitropic root response (Hu et al. 2005).
13.3.1.5 Other Notable Enzymes
The enzymatic production of NO can also be mediated by a number of other enzymes including copper amine oxidase 1, polyamine oxidases, horseradish peroxidase, cytochrome P450, catalase and hemoglobin (reviewed by Prochazkova et al. 2014). To this end, del Río et al. (2004) reported the release of NO via cytochrome P450-catalyzed oxidation of N-hydroxy-arginine (NOHA) in the presence of NADPH and O2.
13.3.2 Nonenzymatic Pathways
Various nonenzymatic pathways are associated with NO generation in plants (Prochazkova et al. 2014). The oxidative pathways do not require NO2− as a substrate and comprise NO production from arginine, polyamines (PAs), or hydroxylamine. In Arabidopsis thaliana seedlings, PAs induced rapid biosynthesis of NO (Tun et al. 2006). Employing fluorimetry and fluorescence microscopy (using the NO-binding fluorophores DAF-2 and dye diaminorhodamine 4 M (DAR-4 M), a fluorescent probe for NO), an exogenous application of PAs putrescine, spermidine, and spermine to Arabidopsis seedlings was reported to induce therein the production of NO. Ascorbate (AsA) can mediate the reduction of NO2− and produce NO (and also dehydroascorbic acid at acidic pH) (Bethke et al. 2004; del Río et al. 2004). Earlier, carotenoids-mediated reduction of NO2 into NO was reported in Spinacia oleracea (Cooney et al. 1994).
13.4 Nitric Oxide Synthesis on Potassium and Nitrogen
13.4.1 Nitric Oxide Synthesis Affects Potassium Homeostasis in Plants
Potassium is a major nutrient and main inorganic cation in plant cells which interacts with negative charges on nucleic acids and proteins and maintains cytosolic pH homeostasis. Inside the cell, K acts as a cofactor activating specific enzymes and also work in maintaining the membrane potential (Maathuis 2009; Dreyer and Uozumi 2011). The maintenance of potassium ion (K+) homeostasis enables plants to activate the metabolic pathway for setting the osmotic potential for plant growth and movement (Buet et al. 2019). The movement of K+ nutrition within the cells and between plant organs includes several transporters (Véry et al. 2014; Santa-María et al. 2018; Buet et al. 2019). Accordingly, there are several ways in which K+ nutrition interacts with NO like in root architecture, where NO generally exerts a specific effect on K+ transport and works in the stomata. The indirect effects of NO on root elongation associated with defective K+ nutrition are likely to occur, as observed in plants lacking K+ channels (AKT1 activity), in which root length proved to be hypersensitive to the SNP as a NO donor (Xia et al. 2014). Besides, SNP stimulates K+ efflux, while the cPTIO as NO scavenger reduces it, indicating that these K+ currents are associated with NO signaling. High levels of NO increase the content of pyridoxal 5′-phosphate, an active form of vitamin B6, which in turn inhibits the activity of AKT1 and ultimately decreased the K+ content in the plant cell (Xia et al. 2014). Similarly, Song et al. (2018) observed that Nicotiana tabacum cultivar with low K+ susceptibility have decreased total root length, root volume, and number of first-order lateral roots under K+ deficiency with increased levels of NO while tolerant cultivar did not show any effect. This study suggested that NO plays an important role in modulating the growth of first-order lateral roots as susceptible cultivar roots showed increased levels of NO after K+ limitation. In support of this piece of evidence, the addition of cPTIO, L-NAME, or tungstate resulted in an increase in the first-order root length. Therefore, NO mediates K+ homeostasis by the negative regulation of K+ uptake via K+ channels. The hormone NO regulate K+ channel indirectly, in which case NO integrates with protein phosphorylation and intracellular Ca2+ release signals to inactivate the inward-rectifying K+ channels and close stomata (Garcia-Mata et al. 2003; Sokolovski et al. 2005). The NO seems to play a role in water-stress signaling, and its situation within ABA-related signaling pathways and its relationship to ion transport that drives stomatal movement have remained unclear. However, Garcia-Mata et al. (2003) demonstrated that NO acts on inward-rectifying K+ channels and anion channels by activating ryanodine-sensitive Ca2+ channels of intercellular Ca2+ stores to elevate [Ca2+] in Vicia faba guard cells. At these very low levels, NO had no influence on IK,out, consistent with the Ca2+ insensitivity of these K+ channels. However, NO could be expected to possess additional effects on stomatal behavior at higher concentrations. Oxidative stress in plants is known to suppress stomatal closure (Willmer and Fricker 1996) and, in some circumstances, can suppress IK,out (Kohler et al. 2003) and promote stomatal opening (Black and Black 1979). Indeed, NO can bond covalently with the SH residues of Cys to make S-nitrosothiols, and this easy reaction is the basis of the many regulatory cascades (Stamler et al. 2001; Ahern et al. 2002), including vascular homeostasis and endotoxic shock in animals (Liu et al. 2004). In subsequent experiments, a reversible decrease in IK,out with moderate elevation to sub-micromolar NO has been observed and has explored the effects of NO on the IK,out in vivo. The key observations were that a NO-mediated block of IK,out is suppressed by reducing reagents, especially British anti-Lewisite (BAL; 2,3-dimercapto-1-propanol), and is mimicked by the oxidizing reagent phenylarsine oxide that targets vicinal SH residues. These findings indicate that the IK,out can become locked down under nitrosative stress and led to propose that NO action on IK out is mediated by direct S-nitrosylation of Cys residues closely associated with the ion channel. In other words, NO inactivate the activity of outward-rectifying K+ channel in Vicia faba guard cell through direct S-nitrosylation of cysteinyl residues, the not-yet identified channel, or its closely attached protein (Sokolovski and Blatt 2004). Additionally, Zhang et al. (2018) showed that NO-mediated alteration in K+ homeostasis was known as a result of work with Arabidopsis roots subjected with excess iron. An exposure of toxic concentrations of iron to the root tips increased the levels of NO and leads to growth arrest, which was in part related to NO-induced alteration in K+ homeostasis (Arnaud et al. 2006). These findings revealed a pivotal role of NO synthesis in modulating the homeostasis of K and its nutrition in plants.
13.4.2 Nitric Oxide Synthesis Affects Nitrogen Uptake and Homeostasis in Plants
Nitrogen is an essential macronutrient and component of many biological molecules like proteins and nucleotides. Chlorophyll molecules also have N which makes it critical for growth and development of plants (Wang et al. 2012; O’Brien et al. 2016). Several enzymes and intermediates are involved in N uptake and metabolism, including NR, NiR, glutamate synthase (GS), glutamate dehydrogenase (GDH), and glutamine synthetase (GOGAT) (Andrews et al. 2013; Zhang et al. 2020). The main sources of N for the plants are NO3− and NH4+ which are easily taken up by the plants. There are many reports that suggest a connection between the N assimilation and NO production in plants. It has been observed that not only the amount of N but its form of supply (NO3− or NH4+) also affects the NO production in plants (Caro and Puntarulo 1998; Jin et al. 2009; Sun et al. 2015; Zhu et al. 2016). For the production of NO, nitrite (NO2−) and arginine are the main substrates and are derived through the N assimilation pathway. Mainly, NO is produced by the reduction of nitrate to nitrite using NADH as major electron donor in the cytosol. However, plants may have optimized the use of NO2− as a main source for NO synthesis (Jeandroz et al. 2016; Santolini et al. 2017). Nitrate reductase is a key enzyme in N metabolism and synthesizes NO as a by-product of NO2− decomposition in a reaction catalyzed by NR (Klepper 1987). Besides NR, plants can generate NO under acidic conditions from HNO2 in the presence of ascorbate or GSH (Wendehenne et al. 2004). On the basis of root response and expression of NR and nsHbs which are involved in NO synthesis and scavenging, respectively, it was suggested that NO3− play role in NO production (Trevisan et al. 2011). The role of N (NO3− or NO3− / NH4+) has been confirmed in the root response through the involvement of NR and NO either using the in situ chemical detection of NO and application of tungstate (inhibit NO synthesis) or NO inhibitor [cPTIO; 2-(4-carboxyphenyl)-4,4,5,5-tetramethylimidazoline-1- oxyl-3-oxide] in maize and rice (Manoli et al. 2014; Sun et al. 2015). Moreover, Sun et al. (2015) observed increase in NO content in lateral root and root tip region of Nanguang rice cultivar (with high-nitrate response) which grows under limited NO3− conditions, and this NO accumulation was mainly from an NIA2-dependent NR source. Similarly, Zea mays plants showed increase in 4-amino-5-methylamino-2′,7′-difluorofluorescein diacetate (DAF-FM DA) fluorescence (corresponding to NO detection) in the first minutes after NO3− treatment in the meristematic apex and transition zone. After study of the transcriptome and proteome, Trevisan et al. (2015) found that the transition zone was critical in detecting NO3− and also stated that NO3−-mediated response was induced by NO. Recently, Nejamkin et al. (2020) observed that deficiency of N retards the growth (leaf area, protein content, and seed yield) of Nicotiana tabacum plants that showed lesser expression of NOS enzyme. In plants, NO is produced from NO2 through photoconversion by carotenoids and reaction with NRs (Rockel et al. 2002) and with glycine decarboxylase (Chandok et al. 2003). In NR-deficient Arabidopsis, stomata fail to close in ABA (Desikan et al. 2002). Furthermore, NO scavengers suppress ABA action in closing stomata, and NO donors promote closure in the absence of ABA (Neill et al. 2002; Garcia-Mata and Lamattina 2002). In N metabolism, NR is a key enzyme and a source of NO, and its activity may be exaggerated by NO levels (Chamizo-Ampudia et al., 2017). In Triticum aestivum leaf, NR activity is negatively modulated by NO released from NO donor (SNP or GSNO); concurrently, nitrate content significantly increased, indicating that the substrate for NR activity was present in amounts enough to be not a limiting factor for NR activity (Rosales et al. 2011). Nevertheless, some in vitro and in vivo experiments have confirmed the generation of NO via NR with low oxygen concentration and cellular pH as two of the most important requirements for the activity of NR (Prochazkova et al. 2014; Sharma et al. 2020). The NO generation through NR has been reported in many plant species, such as Helianthus annuus, Spinacia oleracea, Zea mays, Triticum aestivum, Malaxis monophyllos, and Aloe vera (Xu and Zhao 2003).
13.5 Influence of Nitric Oxide Synthesis on Potassium and Nitrogen Homeostasis Under Salt Stress
Salt stress closely associated with nutrient deficiency especially for K and N nutrients due to competitive inhibition of ion transporters and decline of nutrient uptake in the plant roots. As a result, diverse signaling molecules must be integrated in order to accomplish K and N balance under salt stress at a whole plant or cellular level. Hence, the study can assume that NO as a versatile hormone has an important function in this process. The study focuses the role of NO synthesis and its signaling on K and N homeostasis under salt stress.
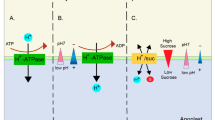
One of the major limiting elements of plant growth is K, and crops often suffer from low K+. The availability of K+ varies with environmental conditions, such as drought and soil density (Kuchenbuch et al. 1986; Liebersbach et al. 2004). Therefore, plants may frequently experience K+ deficiency. Nitric oxide production is altered when plants are subjected to abiotic or biotic stresses (Lamattina et al. 2003; Leitner et al. 2009). High salt limits agriculture yield, induces a fast endogenous NO accumulation in plants (Bai et al. 2011; Wang et al. 2009), and triggers enhanced Na+ influx and reduced K+ absorption in the root (Zhu 2003). Both endogenously produced NO and exogenously applied NO have been proposed to enhance plant salt tolerance (Zhang et al. 2006; Zhao et al. 2007; Molassiotis et al. 2010; Chen et al. 2010) by attenuating high salt-induced increases in the Na+ to K+ ratio. Genetic analysis showed that K+ nutrition, but not Na+, plays critical role in plant salt tolerance (Zhu et al. 1998). In a study, Xia et al. (2014) found that NO reduces the activity of the K+ channel (AKT1) in Xenopus oocytes and protoplasts under conditions of adequate K supply under salt stress. They suggested the possible roles of NO on K+ uptake in response to low-K+ condition rather than its roles on root growth by speculating that low-K+ -induced NO accumulation would have a feedback modulation of K+ uptake through downregulating several K+ transporters. Zhang et al. (2007) reported that NO enhanced salt tolerance in Populus euphratica callus under salinity by increasing the K+/Na+ ratio, where H2O2 was involved in the increase of (PM) H+-ATPase activity. Ruan et al. (2002) reported that NO stimulated proline accumulation under salt stress, owing to NO-induced increase in K+ in Triticum aestivum seedling roots under salt stress conditions. Sung and Hong (2010) concluded that NO mediates K+/Na+ homeostasis and antioxidant defense in NaCl-stressed callus cells of two contrasting Populus euphratica. Accordingly, these studies suggest that K+ homeostasis is important as a signal in growth and development of plants under salt stress conditions and function mainly as redirecting the energy from metabolic reactions to defense responses.
Nitrogen can be absorbed from the soil either in inorganic forms such as NO3− and NH4+ or in organic forms, mostly as free amino acids (Kiba et al. 2011), but NO3− is one of the commonest N forms available in plants in high pH soils and aerobic condition, which is often affected by salt stress (Masclaux-Daubresse et al. 2010; Li et al. 2019; Feng et al. 2020). Plants may have optimized the use of NO2− as a main source for NO (Jeandroz et al. 2016; Santolini et al. 2017). In addition, in NO regulation of NR activity, NO seems to modulate N uptake and distribution systems (Simon et al. 2013; Dong et al. 2015). Schinko et al. (2010) reported that nitrate assimilation involved in generating NO as a by-product. Nitrate reductase activity, as the first enzyme in the nitrate assimilation process (Iqbal et al. 2015; Khanna et al. 2021), has been shown to decrease in salt-stressed leaves of different plant species, including Morus rubra (Surabhi et al. 2008), Helianthus annuus and Carthamus tinctorius (Jabeen and Ahmad 2011), Cucumis sativus seedlings (Li et al. 2019), Triticum aestivum (Sehar et al. 2019; Khanna et al. 2021), as well as Vigna radiata (Hussain et al. 2020). However, the supplementation of N with NO nullified the toxicity of salt stress and increased the NR activity. In Zea mays roots, NO has been reported as a key signal in nitrate sensing (Trevisan et al. 2011; Manoli et al. 2014), and NO improve capacity of N uptake by regulating lateral root initiation and the rate of uptake of N (Sun et al. 2015), signifying that NO concerned in regulating N uptake in plant; nonetheless, the physiological and molecular mechanisms of NO on N assimilation under salt stress remain unclearly. Recently, Jahan et al. (2020) suggested that the application of NO plus split application of N and S more significantly promoted assimilation of N and S, through increased N- and S-use efficiency, photosynthesis, and growth in mustard plants under salt stress. Sehar et al. (2019) reported that decrease N content and NR activity under salt stress are restoration with the use of SNP. They also reported the positive role of NO on N assimilation. Moreover, the application of NO improved the assimilation of N- and S and antioxidant metabolism which confer tolerance against Cd stress in Vigna radiata (Hasan et al. 2020). In Oryza sativa, N uptake and accumulation is inhibited by salt stress, but those negative effects can be alleviated by NO. Furthermore, NO regulates the expression of genes related to N uptake and salt resistance in Oryza sativa plants (Huang et al. 2020).
The increase in NO levels after ABA perception in guard cells depends on the NR activity as it was confirmed by Chen et al. (2016) using the nia1nia2 Arabidopsis mutant, which lacks the two genes coding for NR. The double mutant showed reduced NO synthesis and lower leaf K content, and its stomata exhibited ABA insensitivity; however, they responded to exogenous NO addition. According to this, ABA-induced stomatal closure involves NO derived from NR activity which contributes to the inhibition of inward currents mediated by the KAT1 and AKT1 K channels through a Ca2+-dependent mechanism in Arabidopsis guard cells (Chen et al. 2016). The study suggests that NO synthesis would be involved in the control of long-distance transport of K+ and also understood in terms of the mutual interaction between K+ and N nutrition. To conclude, the available reports suggest a main role for NO synthesis in modulating K+ accumulation and N homeostasis in plants, which may be particularly relevant when plants are under salt stress conditions (Fig. 13.2). In view of the complexity of mineral nutrients homeostasis with signaling hormone in plants, a truly multidisciplinary program is the only mode to make progress in understanding the incorporation of ion transport in response to nutrient deficiency and salinity.
Schematic representation of the major effect of nitric oxide and their involvement in potassium and nitrogen homeostasis in plants for salt tolerance. The figure shows that NO reacts with GSH and leads to the formation of GSNO in the presence of GSNOR enzyme. This metabolite can be converted to GSSG and NH3 in ascorbate-glutathione cycle and decreases the content of oxidative stress (H2O2; TBARS) for salt tolerance. Supply of K and N increases the content of NO and maintains the homeostasis of K and N. Additionally, supply of K helps in maintaining the K+/Na+ ratio through the increase in the accumulation/synthesis of NO. Arrow (---) indicates signaling between NO and GSH. GSH reduced glutathione, GSNO S-nitrosoglutathione, GSNOR S-nitrosoglutathione reductase, GSSG oxidized glutathione, H2O2 hydrogen peroxide, K potassium, K+/Na+ ratio of potassium ion to sodium ion, N nitrogen, NADH nicotinamide adenine dinucleotide hydride, NADPH nicotinamide adenine dinucleotide phosphate hydrogen, NH3 ammonia, NO nitric oxide, NO3− nitrate, NO2− nitrite, NOS nitric oxide synthase, NR nitrate reductase, TBARS thiobarbituric acid reactive substances
13.6 Conclusion
Plants are constantly exposed to salt stress that adversely affects their yield and growth. In order to acclimatize under salt stress, plants initiate their defense mechanism. The synthesis of NO and its signaling is one such defense response that may be activated in response to stress associated with the changes in K and N nutrients. Nitric oxide plays an important role under salt stress in plants, whether there is increase or decrease in the level of this hormone. It develops an adaptive mechanism to tolerate salt stress in plants by modulating the homeostasis of K and N nutrition in plants, but whether they act dependently or independently on each other is debatable. The interaction between N availability and NO is well known, and relationship of N with K is also studied. However, the influence of K on NO and salt stress is still to be explored. The underlying mechanism between K and N with NO can be manipulated for adjusting plants to the changing environment for sustainable agricultural development. However, future studies are needed to deepen our knowledge about the role of NO in the modulation of the physiological and molecular mechanisms associated with other plant hormones and nutrients in order to improve crop growth under various stresses. Current understanding is carried out to build the databases of gene expression, proteomic changes, and metabolic pathways and is pointed in the right direction. Such a data pool is constantly reanalyzed and reinterpreted as we progress in our study.
References
Abbasi GH, Akhtar J, Ahmad R, Jamil M, Anwar-ul-Haq M, Ali S, Ijaz M (2015) Potassium application mitigates salt stress differentially at different growth stages in tolerant and sensitive maize hybrids. Plant Growth Regul 76(1):111–125
Ahern GP, Klyachko VA, Jackson MB (2002) cGMP and S-nitrosylation: two routes for modulation of neuronal excitability by NO. Trends Neurosci 25:510–517
Ahmad P, Abdel Latef AA, Hashem A, Abd_Allah EF, Gucel S, L-SP T (2016) Nitric oxide mitigates salt stress by regulating levels of osmolytes and antioxidant enzymes in chickpea. Front Plant Sci 7:347
Andrews M, Raven JA, Lea PJ (2013) Do plants need nitrate? The mechanisms by which nitrogen form affects plants. Ann Appl Biol 163(2):174–199
Arnaud N, Murgia I, Boucherez J, Briat JF, Cellier F, Gaymard F (2006) An iron-induced nitric oxide burst precedes ubiquitin-dependent protein degradation for Arabidopsis AtFer1 ferritin gene expression. J Biol Chem 281:23579–23588
Bai X, Yang L, Yang Y, Ahmad P, Yang Y, Hu X (2011) Deciphering the protective role of nitric oxide against salt stress at the physiological and proteomic levels in maize. J Proteome Res 10(10):4349–4364
Balotf S, Islam S, Kavoosi G, Kholdebarin B, Juhasz A, Ma W (2018) How exogenous nitric oxide regulates nitrogen assimilation in wheat seedlings under different nitrogen sources and levels. PLoS One 13:0190269
Barroso JB, Corpas FJ, Carreras A, Sandalio LM, Valderrama R, Palma JM, Lupiáñez JA, del Río LA (1999) Localization of nitric-oxide synthase in plant peroxisomes. J Biol Chem 274:36729–36733
Besson-Bard A, Pugin A, Wendehenne D (2008) New insights into nitric oxide signaling in plants. Annu Rev Plant Biol 59:21–39
Bethke PC, Badger MR, Jones RL (2004) Apoplastic synthesis of nitric oxide by plant tissues. Plant Cell 16:332–341
Black CR, Black VJ (1979) The effects of low concentrations of Sulphur dioxide on stomatal conductance and epidermal cell survival in field bean (Vicia faba L). J Exp Bot 30:291–298
Buet A, Galatro A, Ramos-Artuso F, Simontacchi M (2019) Nitric oxide and plant mineral nutrition: current knowledge. J Exp Bot 70(17):4461–4476
Caro A, Puntarulo S (1998) Nitric oxide decreases superoxide anion generation by microsomes from soybean embryonic axes. Physiol Plant 104:357–364
Chamizo-Ampudia A, Sanz-Luque E, Llamas A, Galvan A, Fernandez E (2017) Nitrate reductase regulates plant nitric oxide homeostasis. Trends Plant Sci 22:163–174
Chandok MR, Ytterberg AJ, van Wijk KJ, Klessig DF (2003) The pathogen-inducible nitric oxide synthase (iNOS) in plants is a variant of the P protein of the glycine decarboxylase complex. Cell 113(4):469–482
Chen ZH, Wang Y, Wang JW et al (2016) Nitrate reductase mutation alters potassium nutrition as well as nitric oxide-mediated control of guard cell ion channels in Arabidopsis. New Phytol 209:1456–1469
Chen J, Xiao Q, Wu F, Dong X, He J, Pei Z, Zheng H (2010) Nitric oxide enhances salt secretion and Na+ sequestration in a mangrove plant, Avicennia marina, through increasing the expression of H+-ATPase and Na+/H+ antiporter under high salinity. Tree Physiol 30(12):1570–1585
Cooney RV, Harwood PJ, Custer LJ, Franke AA (1994) Light-mediated conversion of nitrogen dioxide to nitric oxide by carotenoids. Environ Health Perspect 102:460–462
Corpas FJ, Barroso JB, Carreras A, Valderrama R, Palma JM, León AM, Sandalio LM, del Río LA (2006) Constitutive arginine-dependent nitric oxide synthase activity in different organs of pea seedlings during plant development. Planta 224:246–254
Corpas FJ, Carreras A, Esteban FJ, Chaki M, Valderrama R, del Rio LA, Barroso JB (2008) Localization of S-nitrosothiols and assay of nitric oxide synthase and S-nitrosoglutathione reductase activity in plants. Methods Enzymol 437:561–574
Desikan R, Griffiths R, Hancock J, Neill S (2002) A new role for an old enzyme: nitrate reductase-mediated nitric oxide generation is required for abscisic acid-induced stomatal closure in Arabidopsis thaliana. Proc Natl Acad Sci U S A 99(25):16314–16318
Dong F, Simon J, Rienks M, Lindermayr C, Rennenberg H (2015) Effects of rhizopheric nitric oxide (NO) on N uptake in Fagus sylvatica seedlings depend on soil CO2 concentration, soil N availability and N source. Tree Physiol 35(8):910–920
Dreyer I, Uozumi N (2011) Potassium channels in plant cells. FEBS J 278:4293–4303
Fatma M, Iqbal N, Gautam H, Sehar Z, Sofo A, D’Ippolito I, Khan NA (2021) Ethylene and Sulfur Coordinately modulate the antioxidant system and ABA accumulation in mustard plants under salt stress. Plants (Basel) 10(1):180
Fatma M, Masood A, Per TS, Khan NA (2016) Nitric oxide alleviates salt stress inhibited photosynthetic performance by interacting with sulfur assimilation in mustard. Front Plant Sci 7:521
Fatma M, Masood A, Per TS, Rasheed F, Khan NA (2016) Interplay between nitric oxide and sulfur assimilation in salt tolerance in plants. Crop J 4(3):153–161
Fazzari M, Trostchansky A, Schopfer FJ, Salvatore SR, Sánchez-Calvo B, Vitturi D et al (2014) Olives and olive oil are sources of electrophilic fatty acid nitroalkenes. PLoS One 9(1):e84884
Feng H, Fan X, Miller AJ, Xu G (2020) Plant nitrogen uptake and assimilation: regulation of cellular pH homeostasis. J Exp Bot 71(15):4380–4392
Ferreira LC, Cataneo AC (2010) Nitric oxide in plants: a brief discussion on this multifunctional molecule. Sci Agric 67(2):236–243
Food and Agricultural Organization (FAO) (2009) How to feed the world in 2050. Available via http://www.fao.org/fileadmin/templates/wsfs/docs/expert_paper/How_to_Feed_the_World_in_2050.pdf
Foresi N, Correa-Aragunde N, Parisi G, Caló G, Salerno G, Lamattina L (2010) Characterization of a nitric oxide synthase from the plant kingdom: NO generation from the green alga Ostreococcus tauri is light irradiance and growth phase dependent. Plant Cell 22(11):3816–3830
Galatro A, Ramos-Artuso F, Luquet M, Buet A, Simontacchi M (2020) An update on nitric oxide production and role under phosphorus scarcity in plants. Front Plant Sci 11:413
Garcia-Mata C, Lamattina L (2002) Nitric oxide and abscisic acid cross talk in guard cells. Plant Physiol 128(3):790–792
Garcia-Mata C, Gay R, Sokolovski S, Hills A, Lamattina L, Blatt MR (2003) Nitric oxide regulates K+ and Cl− channels in guard cells through a subset of abscisic acid-evoked signaling pathways. Proc Natl Acad Sci 100:11116–11121
Godber BL, Doel JJ, Sapkota GP, Blake DR, Stevens CR, Eisenthal R, Harrison R (2000) Reduction of nitrite to nitric oxide catalysed by xanthine oxidoreductase. J Biol Chem 275:7757–7763
Hasan S, Sehar Z, Khan NA (2020) Gibberellic acid and Sulfur-mediated reversal of cadmium-inhibited photosynthetic performance in Mungbean (Vigna radiata L.) involves nitric oxide. J Plant Growth Regul 39(4):1605–1615
Hu X, Neill SJ, Tang Z, Cai W (2005) Nitric oxide mediates gravitropic bending in soybean roots. Plant Physiol 137:663–670
Huang J, Zhu C, Hussain S, Huang J, Liang Q, Zhu L, Cao X, Kong Y, Li Y, Wang L, Li J (2020) Effects of nitric oxide on nitrogen metabolism and the salt resistance of rice (Oryza sativa L.) seedlings with different salt tolerances. Plant Physiol Biochem 155:374–383
Hussain SJ, Khan NA, Anjum NA, Masood A, Khan MIR (2020) Mechanistic elucidation of salicylic acid and Sulphur-induced defence systems, nitrogen metabolism, photosynthetic, and growth potential of mungbean (Vigna radiata) under salt stress. J Plant Growth Regul:1–17
Iqbal N, Umar S, Khan NA (2015) Nitrogen availability regulates proline and ethylene production and alleviates salinity stress in mustard (Brassica juncea). J Plant Physiol 178:84–91
Jabeen N, Ahmad R (2011) Foliar application of potassium nitrate affects the growth and nitrate reductase activity in sunflower and safflower leaves under salinity. Not Bot Horti Agrobot Cluj Napoca 39(2):172–178
Jahan B, Al Ajmi MF, Rehman MT, Khan NA (2020) Treatment of nitric oxide supplemented with nitrogen and sulfur regulates photosynthetic performance and stomatal behavior in mustard under salt stress. Physiol Plant 168:490–510
Jasid S, Simontacchi M, Bartoli CG, Puntarulo S (2006) Chloroplasts as a nitric oxide cellular source. Effect of reactive nitrogen species on chloroplastic lipids and proteins. Plant Physiol 142:1246–1255
Jeandroz S, Wipf D, Stuehr DJ, Lamattina L, Melkonian M, Tian Z, Zhu Y, Carpenter EJ, Wong GK, Wendehenne D (2016) Occurrence, structure, and evolution of nitric oxide synthase-like proteins in the plant kingdom. Sci Signal 9(417):re2
Jin CW, Du ST, Zhang YS, Lin XY, Tang CX (2009) Differential regulatory role of nitric oxide in mediating nitrate reductase activity in roots of tomato (Solanum lycocarpum). Ann Bot 104:9–17
Khan MN, AlSolami MA, Basahi RA, Siddiqui MH, Al-Huqail AA, Abbas ZK et al (2020) Nitric oxide is involved in nano-titanium dioxide-induced activation of antioxidant defense system and accumulation of osmolytes under water-deficit stress in Vicia faba L. Ecotoxicol Environ Saf 190:110152
Khanna RR, Jahan B, Iqbal N, Khan NA, AlAjmi MF, Rehman MT, Khan MIR (2021) GABA reverses salt-inhibited photosynthetic and growth responses through its influence on NO-mediated nitrogen-sulfur assimilation and antioxidant system in wheat. J Biotechnol 325:73–82
Kiba T, Kudo T, Kojima M, Sakakibara H (2011) Hormonal control of nitrogen acquisition: roles of auxin, abscisic acid, and cytokinin. J Exp Bot 62:1399–1409
Klepper LA (1987) Nitric oxide emissions from soybean leaves during in vivo nitrate reductase assays. Plant Physiol 85:96–99
Kohler B, Hills A, Blatt MR (2003) Control of guard cell ion channels by hydrogen peroxide and abscisic acid indicates their action through alternate signaling pathways. Plant Physiol 131:385–388
Krapp A (2015) Plant nitrogen assimilation and its regulation: a complex puzzle with missing pieces. Curr Opin Plant Biol 25:115–122
Kuchenbuch R, Claassen N, Jungk A (1986) Potassium availability in relation to soil-moisture. Plant Soil 95:221–231
Lamattina L, García-Mata C, Graziano M, Pagnussat G (2003) Nitric oxide: the versatility of an extensive signal molecule. Annu Rev Plant Biol 54:109–136
Leitner M, Vandelle E, Gaupels F, Bellin D, Delledonne M (2009) NO signals in the haze: nitric oxide signalling in plant defence. Curr Opin Plant Biol 12(4):451–458
Li S, Li Y, He X, Li Q, Liu B, Ai X, Zhang D (2019) Response of water balance and nitrogen assimilation in cucumber seedlings to CO2 enrichment and salt stress. Plant Physiol Biochem 139:256–263
Liebersbach H, Steingrobe B, Claassen N (2004) Roots regulate ion transport in the rhizosphere to counteract reduced mobility in dry soil. Plant Soil 260:79–88
Liu L, Yan Y, Zeng M, Zhang J, Hanes MA, Ahearn G, McMahon TJ, Dickfeld T, Marshall HE, Que LG et al (2004) Essential roles of S-nitrosothiols in vascular homeostasis and endotoxic shock. Cell 116:617–628
Lopez-Carrion AI, Castellano R, Rosales MA, Ruiz JM, Romero L (2008) Role of nitric oxide under saline stress: implications on proline metabolism. Biol Plant 52(3):587
Maathuis FJ (2009) Physiological functions of mineral macronutrients. Cur Opin Plant Biol 12:250–258
Manoli A, Begheldo M, Genre A, Lanfranco L, Trevisan S, Quaggiotti S (2014) NO homeostasis is a key regulator of early nitrate perception and root elongation in maize. J Exp Bot 65:185–200
Marschner P (2012) Marschner’s mineral nutrition of higher plants, 3rd edn. Academic Press, London, pp 178–189
Masclaux-Daubresse C, Daniel-Vedele F, Dechorgnat J, Chardon F, Gaufichon L, Suzuki A (2010) Nitrogen uptake, assimilation and remobilization in plants: challenges for sustainable and productive agriculture. Ann Bot 105(7):1141–1157
Meyer C, Stöhr C (2002) Soluble and plasma membrane bound enzymes involved in nitrate and nitrite metabolism. In: Foyer CH, Noctor G (eds) Photosynthetic nitrogen assimilation and associated carbon metabolism. Kluwer Academic Publishers, Dordrecht, pp 49–62
Moche M, Stremlau S, Hecht L, Göbel C, Feussner I, Stöhr C (2010) Effect of nitrate supply and mycorrhizal inoculation on characteristics of tobacco root plasma membrane vesicles. Planta 231:425–436
Molassiotis A, Tanou G, Diamantidis G (2010) NO says more than ‘YES’ to salt tolerance: salt priming and systemic nitric oxide signaling in plants. Plant Signal Behav 5(3):209–212
Moreau M, Lindermayr C, Durner J, Klessig DF (2010) NO synthesis and signalling in plants—where do we stand? Physiol Plant 138:372–383
Mur LA, Mandon J, Persijn S, Cristescu SM, Moshkov IE, Novikova GV et al (2013) Nitric oxide in plants: an assessment of the current state of knowledge. AoB Plants 5:pls052
Nabi RBS, Tayade R, Hussain A, Kulkarni KP, Imran QM, Mun B-G, Yun B-W (2019) Nitric oxide regulates plant responses to drought, salinity, and heavy metal stress. Environ Exp Bot 161:120–133
Neill SJ, Desikan R, Clarke A, Hancock JT (2002) Nitric oxide is a novel component of abscisic acid signaling in stomatal guard cells. Plant Physiol 128(1):13–16
Nejamkin A, Foresi N, Mayta ML, Lodeyro AF, Del Castello F, Correa-Aragunde N, Carrillo N, Lamattina L (2020) Nitrogen depletion blocks growth stimulation driven by the expression of nitric oxide synthase in tobacco. Front Plant Sci 11:312
O’Brien JA, Vega A, Bouguyon E, Krouk G, Gojon A, Coruzzi G, Gutiérrez RA (2016) Nitrate transport, sensing, and responses in plants. Mol Plant 9:837–856
Prochazkova D, Haisel D, Pavlikova D (2014) Nitric oxide biosynthesis in plants-the short overview. Plant Soil Environ 60(3):129–134
Rather BA, Masood A, Sehar Z, Majid A, Anjum NA, Khan NA (2020) Mechanisms and role of nitric oxide in phytotoxicity-mitigation of copper. Front Plant Sci 11:675
Ribeiro EA Jr, Cunha FQ, Tamashiro WM, Martins IS (1999) Growth phase-dependent subcellular localization of nitric oxide synthase in maize cells. FEBS Lett 445:283–286
del Río LA, Corpas FJ, Barroso JB (2004) Nitric oxide and nitric oxide synthase activity in plants. Phytochemistry 65:783–792
Rockel P, Strube F, Rockel A, Wildt J, Kaiser WM (2002) Regulation of nitric oxide (NO) production by plant nitrate reductase in vivo and in vitro. J Exp Bot 53(366):103–110
Rosales EP, Iannone MF, Groppa MD, Benavides MP (2011) Nitric oxide inhibits nitrate reductase activity in wheat leaves. Plant Physiol Biochem 49(2):124–130
Ruan H, Shen W, Ye M, Xu L (2002) Protective effects of nitric oxide on salt stress-induced oxidative damage to wheat (Triticum aestivum L.) leaves. Chin Sci Bull 47(8):677–681
Rümer S, Kapuganti JG, Kaiser WM (2009) Oxidation of hydroxylamines to NO by plant cells. Plant Signal Behav 4:853–855
Sánchez-Calvo B, Barroso JB, Corpas FJ (2013) Hypothesis: nitro-fatty acids play a role in plant metabolism. Plant Sci 199-200:1–6
Santa-María GE, Oliferuk S, Moriconi JI (2018) KT-HAK-KUP transporters in major terrestrial photosynthetic organisms: a twenty years’ tale. J Plant Physiol 226:77–90
Santolini J, André F, Jeandroz S, Wendehenne D (2017) Nitric oxide synthase in plants: where do we stand? Nitric Oxide 63:30–38
Schinko T, Berger H, Lee W, Gallmetzer A, Pirker K, Pachlinger R, Strauss J (2010) Transcriptome analysis of nitrate assimilation in Aspergillus nidulans reveals connections to nitric oxide metabolism. Mol Microbiol 78(3):720–738
Sehar Z, Masood A, Khan NA (2019) Nitric oxide reverses glucose-mediated photosynthetic repression in wheat (Triticum aestivum L.) under salt stress. Environ Exp Bot 161:277–289
Sharma A, Soares C, Sousa B, Martins M, Kumar V, Shahzad B et al (2020) Nitric oxide-mediated regulation of oxidative stress in plants under metal stress: a review on molecular and biochemical aspects. Physiol Plant 168(2):318–344
Simon J, Dong F, Buegger F, Rennenberg H (2013) Rhizospheric NO affects N uptake and metabolism in Scots pine (Pinus sylvestris L.) seedlings depending on soil N availability and N source. Plant Cell Environ 36(5):1019–1026
Simontacchi M, Jasid S, Puntarulo S (2004) Nitric oxide generation during early germination of sorghum seeds. Plant Sci 167:839–847
Sokolovski S, Blatt MR (2004) Nitric oxide block of outward-rectifying K+ channels indicate direct control by protein nitrosylation in guard cells. Plant Physiol 136(4):4275–4284
Sokolovski S, Hills A, Gay R, Garcia-Mata C, Lamattina L, Blatt MR (2005) Protein phosphorylation is a prerequisite for intracellular Ca2+ release and ion channel control by nitric oxide and abscisic acid in guard cells. Plant J 43(4):520–529
Song W, Xue R, Song Y et al (2018) Differential response of first-order lateral root elongation to low potassium involves nitric oxide in two tobacco cultivars. J Plant Growth Regul 37:114–127
Stamler JS, Lamas S, Fang FC (2001) Nitrosylation: the prototypic redox-based signaling mechanism. Cell 106:675–683
Sun H, Li J, Song W, Tao J, Huang S, Chen S, Hou M, Xu G, Zhang Y (2015) Nitric oxide generated by nitrate reductase increases nitrogen uptake capacity by inducing lateral root formation and inorganic nitrogen uptake under partial nitrate nutrition in rice. J Exp Bot 66:2449–2459
Sung CH, Hong JK (2010) Sodium nitroprusside mediates seedling development and attenuation of oxidative stresses in Chinese cabbage. Plant Biotechnol Rep 4(4):243–251
Surabhi GK, Reddy AM, Kumari GJ, Sudhakar C (2008) Modulations in key enzymes of nitrogen metabolism in two high yielding genotypes of mulberry (Morus alba L.) with differential sensitivity to salt stress. Environ Exp Bot 64(2):171–179
Trevisan S, Manoli A, Begheldo M, Nonis A, Enna M, Vaccaro S, Caporale G, Ruperti B, Quaggiotti S (2011) Transcriptome analysis reveals coordinated spatiotemporal regulation of hemoglobin and nitrate reductase in response to nitrate in maize roots. New Phytol 192(2):338–352
Trevisan S, Manoli A, Ravazzolo L, Botton A, Pivato M, Masi A, Quaggiotti S (2015) Nitrate sensing by the maize root apex transition zone: a merged transcriptomic and proteomic survey. J Exp Bot 66:3699–3715
Tun NN, Santa-Catarina C, Begum T, Silveira V, Handro W, Floh EI, Scherer GF (2006) Polyamines induce rapid biosynthesis of nitric oxide (NO) in Arabidopsis thaliana seedlings. Plant Cell Physiol 47(3):346–354
United States Census Bureau. (2012) International Data Base. Total midyear population for the world: 1950–2050
Véry AA, Nieves-Cordones M, Daly M, Khan I, Fizames C, Sentenac H (2014) Molecular biology of K+ transport across the plant cell membrane: what do we learn from comparison between plant species? J Plant Physiol 171(9):748–769
Wang YY, Hsu PK, Tsay YF (2012) Uptake, allocation and signaling of nitrate. Trends Plant Sci 17:458–467
Wang H, Liang X, Wan Q, Wang X, Bi Y (2009) Ethylene and nitric oxide are involved in maintaining ion homeostasis in Arabidopsis callus under salt stress. Planta 230(2):293–307
Wang BL, Tang XY, Cheng LY, Zhang AZ, Zhang WH, Zhang FS, Liu JQ, Cao Y, Allan DL, Vance CP, Shen JB (2010) Nitric oxide is involved in phosphorus deficiency-induced cluster-root development and citrate exudation in white lupin. New Phytol 187:1112–1123
Wang M, Zheng Q, Shen Q, Guo S (2013) The critical role of potassium in plant stress response. Int J Mol Sci 14(4):7370–7390
Wendehenne D, Durner J, Klessig DF (2004) Nitric oxide: a new player in plant signalling and defence responses. Curr Opin Plant Biol 7:449–455
Willmer C, Fricker MD (1996) Stomata. Chapman and Hall, London, pp 1–375
Wilson ID, Neill SJ, Hancock JT (2008) Nitric oxide synthesis and signalling in plants. Plant Cell Environ 31:622–631
Xia J, Kong D, Xue S, Tian W, Li N, Bao F, Hu Y, Du J, Wang Y, Pan X, Wang L, Zhang X, Niu G, Feng X, Li L, He Y (2014) Nitric oxide negatively regulates AKT1-mediated potassium uptake through modulating vitamin B6 homeostasis in Arabidopsis. Proc Natl Acad Sci U S A 111:16196–16201
Xu YC, Zhao BL (2003) The main origin of endogenous NO in higher non-leguminous plants. Plant Physiol Biochem 41(9):833–838
Y Y, Li ZH, Xing DA (2013) Nitric oxide promotes MPK6-mediated caspase-3-like activation in cadmium-induced Arabidopsis thaliana programmed cell death. Plant Cell Environ 36(1):1–5
Zhang L, Li G, Wang M, Di D, Sun L, Kronzucker HJ, Shi W (2018) Excess iron stress reduces root tip zone growth through nitric oxide-mediated repression of potassium homeostasis in Arabidopsis. New Phytol 219(1):259–274
Zhang F, Wang Y, Yang Y, Wu HAO, Wang DI, Liu J (2007) Involvement of hydrogen peroxide and nitric oxide in salt resistance in the calluses from Populus euphratica. Plant Cell Environ 30(7):775–785
Zhang NN, Zou H, Lin XY, Pan Q, Zhang WQ, Zhang JH, Chen J (2020) Hydrogen sulfide and rhizobia synergistically regulate nitrogen assimilation and remobilization during N deficiency-induced senescence in soybean. Plant Cell Environ 43(5):1130–1147
Zhang Y et al (2006) Nitric oxide enhances salt tolerance in maize seedlings through increasing activities of proton-pump and Na+/H+ antiport in the tonoplast. Planta 224(3):545–555
Zhao MG, Tian QY, Zhang WH (2007) Nitric oxide synthase-dependent nitric oxide production is associated with salt tolerance in Arabidopsis. Plant Physiol 144(1):206–217
Zhu JK (2003) Regulation of ion homeostasis under salt stress. Curr Opin Plant Biol 6(5):441–445
Zhu JK, Liu J, Xiong L (1998) Genetic analysis of salt tolerance in Arabidopsis: evidence for a critical role of potassium nutrition. Plant Cell 10(7):1181–1191
Zhu CQ, Zhu XF, Hu AY, Wang C, Wang B, Dong XY, Shen RF (2016) Differential effects of nitrogen forms on cell wall phosphorus remobilization are mediated by nitric oxide, pectin content, and phosphate transporter expression. Plant Physiol 171:1407–1417
Author information
Authors and Affiliations
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2022 The Author(s), under exclusive license to Springer Nature Singapore Pte Ltd.
About this chapter
Cite this chapter
Fatma, M., Bashri, G., Rasheed, F., Jahan, B., Per, T.S., Khan, N.A. (2022). Nitric Oxide Synthesis Affects Potassium and Nitrogen Homeostasis in Plants for Salt Tolerance. In: Iqbal, N., Umar, S. (eds) Role of Potassium in Abiotic Stress. Springer, Singapore. https://doi.org/10.1007/978-981-16-4461-0_13
Download citation
DOI: https://doi.org/10.1007/978-981-16-4461-0_13
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-16-4460-3
Online ISBN: 978-981-16-4461-0
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)