Abstract
Quinoa is a pseudocereal that has gained more attention in the last decades, due to its outstanding nutritional value. Quinoa has a very good protein quality and content, with a complete amino acid profile; it is also rich in minerals and bioactive compounds. However, quinoa, like other cereals and legumes, has phytate which inhibits the absorption of essential minerals. High content of phytate is usually associated with vegetarian diets and diets of rural areas of developing countries. Such diets may lead to mineral deficiencies. Fermentation of quinoa has been shown to be a very effective method for reducing the phytate content and therefore increasing the bioavailability of essential divalent minerals such as iron, calcium and zinc. Fermentation has also been investigated for its effect on improving the antioxidant capacity and content of phenolic compounds, which are considered health-promoting molecules. In addition, this chapter also presents information on the organoleptic changes that occur during quinoa fermentation, which in some cases were shown to be negative. Successful research has been done on the use of dry toasting, either before or after fermentation, to improve the sensory properties of the fermented quinoa. Fermented quinoa, besides having the attributes of being nutritionally adequate, safe and healthy, should also have good sensory properties, which are indispensable for its broad acceptability.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
15.1 Introduction
Quinoa (Chenopodium quinoa Willd.) is an Andean pseudocereal; it was called ‘the mother grain’ by the Incas; their argument was that quinoa is a very resistant crop with the ability to grow in arid and salty soils, like those found in the Andes of Bolivia and Chile. Moreover, it grows in conditions of water scarcity and has high resistance to extreme temperatures as low as −4 °C–38 °C (Jacobsen et al. 2003; Ruiz et al. 2014). Quinoa has a balanced nutrient profile characterized by good protein quantity and quality, which is in fact generally superior to those of cereal grains such as wheat and rice. It contains essential amino acids (i.e. lysine, methionine and threonine) that are particularly low in vegetal protein sources (Vilcacundo and Hernández-Ledesma 2017). Quinoa’s protein content has been reported to be between 13.1 and 16.7%, which is comparable to soy and milk protein (Vilcacundo and Hernández-Ledesma 2017). The reported protein quality for quinoa is 81–90%, close to that of casein which is 100%. Its protein digestibility is 83% also close to that of casein (91%) (D’Amico et al. 2017). Quinoa grains contain 7.8–14% of fibre (higher than rice 0.4%, wheat 2.7% and corn 1.7%), especially insoluble fibre; about 78% of its fibre content is insoluble and 22% soluble (Alvarez-Jubete et al. 2010). Quinoa is considered a good source of many micronutrients such as riboflavin, thiamine, folate and α- and β-tocopherol (Alvarez-Jubete et al. 2010; Repo-Carrasco-Valencia and Serna 2011). More importantly, seed has more Ca, Fe, Mn, Mg, Cu and K than other cereals (Konishi et al. 2004; Ruales and Nair 1993). Moreover, quinoa also contains a high amount of natural antioxidants with health-promoting properties, including saponins, phytosterols, phenolics, flavonoids, tocopherols and bioactive peptides (Alvarez-Jubete et al. 2010; Montemurro et al. 2019; Navruz-Varli and Sanlier 2016; Vega-Gálvez et al. 2010). Furthermore, there is a special interest on quinoa as gluten-free grain for people who are affected by gluten intolerances and coeliac disease (Jacobsen et al. 2003).
Quinoa has, however, some anti-nutritional compounds such saponins, which are responsible for the bitter taste and may be toxic in high concentrations; some saponins were found to form complexes with iron, thus reducing its bioavailability (Ruales and Nair 1993). Saponins are located on the outer layers of the grains, and can be removed by washing the grains thoroughly with water or by polishing the grains (Ruales and Nair 1993). Another compound found in seed is phytic acid, commonly found as salt phytates; it is negatively charged with 1–6 phosphate groups, which act as strong chelators of divalent cations. Thus, it inhibits the absorption of essential minerals such as zinc, iron and calcium by making them immersed in insoluble complexes (Weaver and Kannan 2002). Quinoa has also polyphenols, which are antioxidants, but they also have the ability to form insoluble complexes with divalent minerals, reducing their bioavailability (Petry et al. 2010; Sandberg 2002). On the other hand, polyphenols have antioxidant properties that have been widely studied. Polyphenols have shown to have health-promoting effects that are beyond modulation of oxidative stress (Scalbert et al. 2005).
Besides the nutritional quality, many health benefits are also reported, such as considerably positive effects on metabolic, cardiovascular and gastrointestinal health mostly studied in experimental in vivo models (mice, rats) (Noratto et al. 2019) but also few studies in humans (Bastidas et al. 2016; Navruz-Varli and Sanlier 2016; Noratto et al. 2019).
There is a growing trend on health-conscious consumers, who show preference towards more nutritious foods and value-added products from which they can get more than just taste but also good nutrients and possible health benefits. Innovations in food industry are increasing the opportunities for consumers to supplement or replace common cereal grains (rice, wheat, corn) with higher nutritional value options such as quinoa, and products derived. This highlights the importance of research on processing, with a special attention for populations that need to follow gluten-free diets, to maintain a good lifestyle without complications. There is agreement on the fact that development of new food products with improved nutritional quality and health-promoting effects is pursued for industries and demanded by consumers. Quinoa has become one of the prime alternative grains that motivates further research towards using conventional and innovative food processing methods to reduce its anti-nutritional compounds and improve its palatability.
15.2 Fermentation
Fermentation is an ancient food processing method which was first used in the East and Southeast Asian regions as an effective method for food preservation. Its use was mainly to change or improve sensory properties such as flavour, taste, colour and overall acceptability. Nowadays, fermentation of cereals and pseudocereals is getting more importance due to the interesting nutritional and sensorial changes that can be achieved in the food matrix. Furthermore, modification made by microflora during the fermentation process may release bioactive ingredients that are beneficial to human health.
Fermentation is a metabolic process, subjected to the effect of microbial enzymes (i.e. amylases, proteases and lipases), which liberates energy by biochemical transformation of carbohydrates, proteins and lipids into products with particular tastes, aromas and textures that are many times preferred by the consumers (Caplice and Fitzgerald 1999). In addition, the conditions achieved by fermentation (pH < 4.5) are essential to ensure the microbiological safety and shelf life of the product.
Currently, fermentation of cereals and pseudocereals is increasing in importance; during such fermentations there is a production of compounds that transform the organoleptic characteristics (i.e. aroma, texture, flavour) of the product. In addition, it has been reported that through fermentation, an improvement on nutritional properties can be achieved, which would in turn have a positive effect on human health (Bourdichon et al. 2012). Different authors have shown that cereals and pseudocereals, rich in nutrients, are particularly good medium for microbial fermentations. For example, quinoa is rich in polysaccharides, which are used during fermentation as a source of carbon and energy by the microorganisms. Seeds, besides carbohydrates, also contain other growth factors such as vitamins and minerals, which create a good environment for microorganism growth (Montemurro et al. 2019). Cereals and pseudocereals contain indigenous microbiota, lactic acid bacteria (LAB), mould and enterobacteria that compete for nutrients during fermentation; thus, it is very common to add starter culture in fermentation, to ensure that desirable bacteria are grown above the least desirable. During fermentation conditions such as pH, temperature, water, salt concentration and composition of the food matrix can be modified in order to favour the growth of desirable bacteria (Castro-Alba et al. 2019b, c; Rollan et al. 2019; Salovaara and Gänzle 2011).
Lactic acid fermentation, carried out by laboratory, is one of the preferred processes for fermentation of cereals and pseudocereals; it was shown that through this method, the nutritional and functional qualities of foods can be improved in various ways. It was reported that seeds decrease mineral inhibitors such as phytates and tannins (Castro-Alba et al. 2019b, c; Rollan et al. 2019), and increase the antioxidant capacity and total phenolic content (Rocchetti et al. 2019; Rollan et al. 2019). These changes can promote a positive effect on the nutritional status, immune system and overall health of the consumers.
The group of microorganisms that have been used to ferment and preserve foods since ancient times includes microorganisms from Lactobacillus, Pediococcus, Streptococcus and Leuconostoc genera; they are generally recognized as safe (GRAS). During fermentation, catabolism is undertaken via two main routes: homo- and heterofermentative (Axelsson et al. 1989). Microorganisms produce lactic acid from hexoses, and since they lack functional haem-linked electron transport chains and Krebs cycle, they obtain the needed energy through substrate phosphorylation (Caplice and Fitzgerald 1999).
The ones classified as homofermenters are Pediococcus, Lactococcus, Streptococcus and some lactobacilli. They produce lactic acid as the major end product of glucose fermentation. Heterofermenters such as Weissella, Leuconostoc and some lactobacilli produce, via the hexose monophosphate pathway, equimolar amounts of lactate, CO2 and ethanol from glucose (Caplice and Fitzgerald 1999).
Various strains of microorganisms have been used for seed fermentation; one of the most common fermentation products is sourdough bread, for which the following LAB strains have been used, L. plantarum, L. brevis, Lc lactis, Leuc. mesenteroides, Leuc. citreum and E. casseliflavus (Ruiz Rodríguez et al. 2016). Rizzello et al. (2016) have reported that strains such as L. plantarum, P. pentosaceus and L. rossiae are good inocula for fermentation, based on the best acidification, release of free amino acids and growth capability (Rizzello et al. 2016). The number of cereal products obtained by LAB fermentation is quite ample; however, the number of fermented products is more limited and slowly increasing. Table 15.1 shows a summary of fermented products obtained with different types of fermentative microorganisms.
15.2.1 Fermentation Effect on Phytate Content and Mineral Bioavailability
Even though quinoa has an outstanding nutritional profile, it also has compounds such as phytates that can inhibit the bioavailability of essential minerals. This is particularly important in rural areas in developing countries, where the common is monotonous and plant-based diets containing little or no animal products. This problem also concerns to vegetarian and vegan diets, which are trendier in developed countries. In such diets, bioavailability of minerals is low, due to the lack of meat products and the presence of phytate. Quinoa grains contain phytates in a range of 8.44–22.8 g/kg (Castro-Alba et al. 2019a; Lazarte et al. 2015; Tang et al. 2015). It is reported that phytate in cereals is located in the aleurone layers adherent to bran fraction; however, in quinoa, phytate is evenly distributed in the endosperm (Ruales and Nair 1993).
Phytate (myo-inositol hexakisphosphate, IP6) is the main phosphorus storage compound in pseudocereals and cereals; it is a strong chelator that binds divalent mineral cations to form insoluble compounds (Weaver and Kannan 2002). The resulting complexes are difficult for humans to hydrolyse during gastrointestinal digestion, thus making the minerals less available for absorption. Phytate forms insoluble complexes with nutritionally important essential minerals such as zinc, iron and calcium, therefore having an adverse health effect, particularly in populations that follow plant-based diets (Sandberg 2002; Troesch et al. 2013; Weaver and Kannan 2002). The stability of complexes formed by phytic acid and divalent minerals is influenced by pH, the number of phosphate groups in the phytate ring and the molar concentrations of phytate and mineral present in the food matrix. Thus, the more phytate in the food, the least bioavailable are the divalent essential minerals. Also, in the food matrix are found enzymes such as phytases, which are a subgroup of acid phosphatases. Phytase has the ability to hydrolyse phytates (IP5, IP6) and inorganic phosphate (Pi) (Sandberg and Andlid 2002), through hydrolysis of phytate. During hydrolysis, the phosphate groups are removed and thus divalent minerals are released, and their solubility increases making them more bioavailable for absorption during gastrointestinal digestion (Sandberg and Andlid 2002). This is one of the main drivers to investigate different quinoa processing techniques to achieve a significant reduction of phytate content.
Fermentation has been successfully used to reduce the phytate content of cereals and pseudocereals, including quinoa. The principle by which the phytate content is reduced during fermentation is enzymatic hydrolysis taking place during the fermentation (Fig. 15.1). The pH and temperature of fermentation can activate the endogenous phytase in the food matrix and initiate phytate hydrolysis, where ions of divalent minerals will be set free of the phosphate groups, thus being more available for absorption and utilization in the body (Sandberg 2002; Sandberg and Andlid 2002). Phytase can also be secreted by microorganisms to the food matrix during fermentation or in in vitro experiments. Some microorganisms showed to efficiently degrade phytate as a source of phosphorus and energy required for growth (Sandberg and Andlid 2002). Some bacteria showed to be able to degrade phytate during growth by producing extracellular phytases, for example, E. coli, Bacillus subtilis, Pseudomonas spp. and Klebsiella terrigena have been able to manifest phytase activity (Greiner and Alminger 1999; Greiner et al. 2001).
Enzymatic hydrolysis of phytate (adapted from Lazarte 2014)
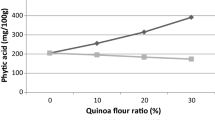
Quinoa fermentation has been investigated by various researchers from different countries in South America and Europe. Castro-Alba et al. (2019b) reported the degradation of phytate in quinoa grains and flour fermented spontaneously and with Lactobacillus plantarum 299v. Their findings (Fig. 15.2a) showed that phytate content was reduced by 47–51% in grains during fermentation, while a phytate reduction of up to 83–85% was achieved when flour was fermented. The authors argued that fermentation of flour is more effective than grains since phytate is not only located in the seed coat but in the entire food matrix. Quinoa flour also presents an increased surface area than the grains, which allow for an easier diffusion of water and nutrients, and probably more contact of phytase and phytate for the enzymatic hydrolysis (Castro-Alba et al. 2019b). The mechanism by which phytate is reduced during fermentation is the activation of endogenous phytase at the fermentation conditions (pH range 4–5) which leads to enzymatic hydrolysis of phytate. Castro-Alba et al. (2019b) reported a reduction of pH from 6.2 to 3.82 and an increase of lactic acid from 14.1 to 75.2 g/kg (Fig. 15.2b); such conditions are reported to be favourable for activation of endogenous phytase activity. It is also reported that quinoa fermented with selected starters of LAB had a phytase activity of 2.75 times higher than phytase activity in raw flour, which may support the theories of phytate reduction during fermentation (Rizzello et al. 2016). Another hypothesis is that during fermentation, certain microbial strains are capable of exhibiting phytase activity; this was shown in various studies of fermentation of cereals (Cizeikiene et al. 2015; De Angelis et al. 2003). However, there is scarce information on phytase activity of autochthonous quinoa LAB. Lactobacillus plantarum CRL2106 is one of the few strains isolated from quinoa that showed phytase activity (730 ± 25 U/mL) (Carrizo et al. 2016).
Fermentation of quinoa grains and flour, spontaneous and with L. plantarum 299v (Lp) degradation during fermentation of quinoa. (a) Phytate reduction and (b) changes of pH and lactic acid content. QFS quinoa flour spontaneous, QFL quinoa flour with Lp;,QGS quinoa grains spontaneous, QGL quinoa grains with Lp (Castro-Alba 2019)
To further elucidate the differences between spontaneous fermentation and fermentation with added bacteria Lactobacillus plantarum 299v, Castro-Alba et al. (2019b) analysed the kinetics of phytate degradation in these fermentations. The degradation rate of phytate was suggested to follow a first-order reaction, proportional to the available phytate for degradation. It was shown that the addition of inoculum L. plantarum induced a faster phytate degradation, demonstrated by the degradation rate constants (Kphy), −0.25 h−1 for fermentation with inoculum and −0.16 h−1 for spontaneous. The use of L. plantarum 299v resulted in a faster pH reduction and a higher production of lactic acid than in a spontaneous fermentation; these conditions may have facilitated the faster phytate degradation during fermentation. Table 15.2 shows a summary of studies of fermentation; it presents the microorganisms used, fermentation parameters and yield of phytate reduction.
15.2.2 Fermentation Effect on Phenolic Compounds and Total Antioxidant Capacity
Seeds are reported to contain phenolic compounds such as flavonoids (i.e. glycosidic forms of the flavonoids, kaempferol and quercetin) and phenolic acids (Alvarez-Jubete et al. 2010; Rocchetti et al. 2017). Bound phenolics are especially important for their ability to establish complexes with the food matrix components. Later on, phenolic compounds are released during gastrointestinal digestion, making them available for bacterial microflora and promoting antioxidant environment (Rocchetti et al. 2018). Phenolic compounds have gained greater importance in the last years as health-promoting compounds; evidence supports their positive effect on the prevention of osteoporosis and cardiovascular diseases; it was also suggested that they have a positive role in the prevention of diabetes and neurodegenerative diseases (Scalbert et al. 2005).
It has been reported that fermentation has a significant effect on increasing the total phenolic compounds (TCP) and antioxidant capacity. It was explained that the changes on bioactive compounds, such as polyphenols and other antioxidants, during fermentation of cereals and pseudocereals are due to the metabolic activity of the microorganisms (Đorđević et al. 2010; Katina et al. 2007a).
Quinoa fermentation performed with two different strains of S. cerevisiae showed an increase of TCP levels by about 55% with respect to raw quinoa grains (Carciochi et al. 2016). TCP was also reported to be twofold higher sourdough bread as compared to raw quinoa (Rizzello et al. 2016). During fermentation, various microbial enzymes may prompt cell structure breakdown and hydrolyse insoluble and esterified phenolic compounds, thus facilitating their release into the food matrix (Đorđević et al. 2010). Enzymes such as esterase were also reported to be activated during sourdough fermentation conditions; this enzyme has the ability to hydrolyse complex phenolic compounds and their glycosylated structures into corresponding phenolic acids (Nionelli et al. 2014; Rizzello et al. 2016). However, other authors have reported that there were not significant changes in TCP in cooked and fermented quinoa with isolated bacteria (P. pentosaceus, L. paracasei) (Rocchetti et al. 2019). It seems that prior treatments, such cooking may affect the changes in TCP; another important factor may be the type of bacteria strains used for fermentation.
Antioxidant capacity has also been reported to increase during fermentation. Antioxidant capacity can be measured by different methods such as DPPH (radical scavenging activity), ABTS (radical scavenging assay), FRAP (ferric-reducing ability power) and ORAC (radical scavenging). In the study presented by Carciochi et al. (2016), the results of DPPH•, ABTS and FRAP showed similar trends in the antioxidant activity of quinoa grains before and after fermentation (Fig. 15.3). In experiments where seed was fermented with two strains of S. cerevisiae, DPPH showed a significant increase of 33 and 43%, ABTS increased by 22 and 27% and FRAP increased by 50 and 51%. In the case of spontaneous fermentation, the changes in antioxidant capacity were not significant. A 72% increase in antioxidant activity (DPPH•) was also reported for quinoa sourdough bread, fermented with bacteria (Rizzello et al. 2016). Other authors have analysed the changes in antioxidant capacity as FRAP and ORAC in cooked fermented quinoa with P. pentosaceus and L. paracasei; interestingly their results after fermentation showed that FRAP decreased to not-detectable levels, while ORAC showed a significant increase after fermentation (Rocchetti et al. 2019). The authors discussed the results indicating that the increase in antioxidant capacity may be affected by various factors such as the pH, temperature, fermentation time, microorganism species, aerobic conditions as well as solvent and water ratios during extraction. Further studies on the effect of fermentation on TCP and antioxidant capacity using other type of microorganisms and varying fermentation conditions would add valuable information to this field.
Total phenolic content (TCP) and antioxidant capacity (DPPH•, ABTS•+ and FRAP) values of raw and processed quinoa seeds (Carciochi et al. 2016). (*Fermentation baker’s yeast = Saccharomyces cerevisiae NBRC 2375, brewer’s yeast = Saccharomyces cerevisiae NBRC 1951) and natural fermentation = spontaneous
Fermentation also showed an effect on the phenolic profile. In raw quinoa, four free phenolic acids were identified (p-hydroxybenzoic, p-coumaric, ferulic and vanillic acids), and two flavonoids (kaempferol and quercetin) (Carciochi et al. 2016; Tang et al. 2015). A significant increase on the content of phenolic acids (i.e. p-OH-benzoic acid, vanillic acid, and p-coumaric acid) was observed after fermentation of seeds with two strains of S. cerevisiae (Table 15.3). It was argued that the increase on the content of phenolic acids was due to hydrolytic enzymes, produced by bacteria, capable of releasing insoluble bound-phenolic acids and/or conjugated phenolic acids from the grains (Carciochi et al. 2016). This mechanism of increased phenolic acids during fermentation has also been reported for other food matrices, for example, rye (Katina et al. 2007b) and wheat bran fermentation (Moore et al. 2007). Another theory backing up these changes is that the reduction of pH (4.9–5.5) during fermentation may be optimum for activation of endogenous or microbial enzymes that degrade the cell wall and facilitate the release of phenolic acids (Carciochi et al. 2016; Katina et al. 2007b). On the other hand, the same authors reported that the content of flavonoid compounds (quercetin and kaempferol) was decreased to not-detectable levels after the fermentation (Carciochi et al. 2016). This decrease was attributed to the more acidity conditions reached during fermentation pH <4 (3.92) (Carciochi et al. 2016). Low pH conditions were reported for other food matrices to significantly reduce the activity of cell wall-degrading enzymes, thus interfering in the release of insoluble-bound phenolic compounds (Hur et al. 2014). The magnitude of changes in the content of phenolic compounds will depend upon the microorganisms used as starter culture, as well as the end pH of the fermentation (Svensson et al. 2010). Further studies appear to be worthwhile to elucidate the different mechanisms that lead to variations on phenolic compounds during fermentation.
15.3 Animal and Human Studies on the Nutritional and Health Benefits
The mineral bioavailability of fermented flour was investigated in an animal study; iron in the liver and zinc content in the femur of rats were used as indicators of iron and zinc bioavailability, respectively (Castro-Alba 2019). The author found that the concentration of iron in the liver of rats fed fermented seed increased by 55% compared to rats fed unfermented flour. Similarly the zinc in the femur increased by 54% for rats fed the fermented diet (Castro-Alba 2019). It was discussed that the mechanism by which the iron bioavailability is increased in fermented foods is due to the phytate reduction, which is related to the increased solubility of minerals in the small intestine, facilitating then their absorption (Sandberg 2002; Schlemmer et al. 2009). Moreover, fermented seed has shown higher content of organic acids, such as lactic acid (Castro-Alba 2019; Castro-Alba et al. 2019b, c; Valencia et al. 1999), which could in turn improve the absorption of iron in the small intestine (Bering et al. 2006). In another animal study, Carrizo et al. (2020) reported an increase in vitamin B2 (riboflavin) and B9 (folate) content in mice fed pasta made of fermented quinoa compared to animals feed control pasta made of unfermented flour. In addition it was shown that fermentation increases resistant starch and delays gastric emptying by organic acids produced during fermentation; this condition tends to reduce the glycaemic index (GI) of fermented cereals and pseudocereals (Östman et al. 2005; Scazzina et al. 2009). In this regard, it was found in an animal study that rats fed diets with fermented nutrition showed a decrease on blood glucose and lipid levels, indicating that fermentation potentiates the ability to reduce the GI (de Oliveira Lopes et al. 2019). Other researchers have associated the presence of phytochemicals and bioactive compounds with its glucose-lowering effect (Paśko et al. 2010). There is a huge potential for further investigation on this topic; in general low GI diets have shown to be healthier and effective in the prevention and control of obesity, diabetes and cardiovascular diseases (Brand-Miller et al. 2003). While some studies have already been conducted on the positive effects of quinoa on reducing glucose and cholesterol and preventing obesity, diabetes and cardiovascular diseases (Bastidas et al. 2016; Navruz-Varli and Sanlier 2016; Noratto et al. 2019; Paśko et al. 2010; Ruiz et al. 2017), it remains relevant to further investigate the health effects of fermented seeds.
15.4 Dry Roasting
It is quiet documented that fermentation of cereals may positively influence sensory properties of end products due to the production of flavour-enhancing compounds (Rollan et al. 2019). However, during fermentation of pseudocereals, it has been challenging to achieve a good palatability and acceptable sensory properties, attributed to the production of off-flavour compounds from sulphur amino acids (Di Renzo et al. 2018). Within all microorganisms, it was found that dough fermented with microbial strains had better palatability and sensory properties that can be further improved (Corsetti and Settanni 2007; Montemurro et al. 2019). Dry roasting has been investigated for some authors as a process to improve the sensory properties of fermented products.
Dry roasting is a heating process that has been used since ancient times to cook and enhance flavour of raw quinoa. Factors such as particle size, temperature and time of process should be taken into account to obtain an appetizing dry-roasted food product. Seeds can be dry roasted as whole grains or as a flour. Grain’s size ranges between 1.0 and 2.6 mm (Bertero et al. 2004), being this size small enough to dry roasting in a tray in an oven or in frying pan on the stove. Temperature is an important parameter to be controlled during dry roasting process. Temperatures between 120 °C and 200 °C have been used for dry roasting (Brady et al. 2007; Castro-Alba et al. 2019c; Nickel et al. 2016). The temperature of the process is directly related to the development of flavour compounds. A quinoa sample that is treated at different temperatures develops different flavour profiles. The dry roasting time is interlinked with the temperature. Usually high dry roasting temperature is applied together with short dry roasting time, or vice versa.
It has been reported that there are different effects of dry roasting on antinutrient and mineral inhibitors present in grains. Regarding saponin content, Brady et al. (2007) reported that the chemical profile of flour changed after dry roasting at 200 °C for 10 min. They suggested that dry roasting resulted in degradation of saponins due to the increasing in the content of a triterpenoid structure, an aglycon of the major saponin present. On the other side, Nickel et al. (2016) found that there was not a significant change in saponin content of washed quinoa grains heat-treated at 200 °C for 10 min.
Phytate content can be also diminished by heat treatments. Castro-Alba (2019) showed that dry roasting had a significant effect on phytate content of grains. The grains were subjected to a heating treatment at 120 °C for 5 min resulting in a 20% phytate degradation from the initial levels, which can have a positive influence on improving the bioavailability of divalent minerals.
Phenolic compounds can also be affected in their composition after dry roasting. Nickel et al. (2016) reported that the heat treatment at 200 °C for 15 min significantly decreased the content of total phenolic compounds in washed grains, and they suggested this reduction was mainly due to the high temperature used during this treatment. It was also shown that the antioxidant activity was decreased, mainly because this activity is directly correlated with the total phenolic content. Conversely, it was shown that the total phenolic and total flavonoid content increased due to increase in dry roasting temperature, while the processing time had a minor but significant effect (Carciochi et al. 2016). As a result of the increase in these compounds, the antioxidant activity of dry-roasted quinoa grains was increased between 78 and 135% compared to non-dry-roasted grains.
15.5 Sensory Properties of Roasted Seeds
To improve the flavour and colour of fermented quinoa, Castro-Alba (2019) have performed dry roasting at different stages of their process. In a process, grains before lactic acid fermentation were dry roasted at 120 °C for 5 min, and in another process flour, after fermentation, was dry roasted at 120 °C for 3 min. In these processes, the temperature and the pH range (4.28–6.70) were appropriate parameters to develop flavour and colour compounds through the Maillard reaction, which occurs between amino acids and sugars (Fayle and Gerrard 2002). Quinoa has a high content of amino acids, e.g. lysine (5.6–6.0 g/100 g protein) (Repo-Carrasco et al. 2003), which is the main amino acid involved in the Maillard reaction. Moreover, the sugar content of raw grains is increased after lactic acid fermentation due to starch hydrolysis during this process (Dallagnol et al. 2013).
The typical flavour compounds formed during dry roasting are aldehydes, pyrazines, pyrroles and furfurals. Alkylpyrazines, acylpyridines, furans, furanones and pyranones are products of the Maillard reaction. Acylpyridines, which are regarded as unpleasant, are also formed during this reaction (Van Boekel 2006). It was also reported that the degradation of saponin content during dry roasting may have a positive influence on the sensory properties of quinoa. Less saponin content in quinoa grains may result in a more palatable quinoa product (Brady et al. 2007).
Colour development during the Maillard reaction is related to the formation of 5-hydroxymethylfurfural (HMF), which is a precursor to the formation of brown polymers called melanoidins (Parisi and Luo 2018). In this regard, it was reported that brown polymers were formed when grains were dry roasted at 130 °C (Carciochi et al. 2016). Castro-Alba (2019) reported that colour development during dry roasting of fermented flour was faster than in quinoa grains, attributed to the higher content of free amino acids and sugars as well as the pH range (4.0–5.0) which favoured the formation of colour compounds.
The acceptability of dry-roasted fermented seeds was evaluated by including it in different food products. It has been reported that the acceptability of porridges prepared with fermented flour that was dry roasted was comparable to the acceptability of a porridge prepared with dry-roasted flour (Castro-Alba et al. 2019c). Furthermore, there was no significant difference in the sensory properties of the products if dry roasting was conducted before or after fermentation; the products obtained similar colour, odour/aroma, taste, aftertaste and texture scores. The authors mention that this similarity is due to the fact that the dry roasting of grains develops flavour compounds and reduces the formation of off-flavour compounds during fermentation, as well as the reduction of volatile off-flavour compounds and formation of more flavour compounds during dry roasting of fermented flour.
The influence of roasting time on the sensory properties was also investigated; grains were roasted for 15, 30 and 45 min at 177 °C, where the sensory scores for appearance, colour, flavour, texture and overall acceptability were decreasing as the roasting time increased (Rothschild et al. 2015). Thus, to obtain acceptable sensory properties of quinoa products, it is important to find the most appropriate combination of time and temperature.
15.6 Conclusion
Quinoa is an interesting alternative to cereals, e.g. as gluten-free crop, which provides high amount of important essential minerals such as zinc, iron and calcium. However, it also contains phytates that inhibit the bioavailability of these minerals. Fermentation is an effective processing alternative to improve nutritional properties, e.g. reduce the phytate content and increase the bioavailability of limiting minerals. Moreover, fermentation has also shown an increase in the content of important polyphenols which could have positive health implications. Regrettably, off-flavours appear when it is fermented, which reduce the sensory properties. The addition of a well-balanced dry roasting process proved to significantly improve the taste of the final fermented product. In food industry, despite the nutritious and healthiness of food products, good sensory properties remain an essential requisite for broad acceptability of products. Therefore, further research on nutritious food development should be hand by hand with research on additional processes to improve the sensory properties of fermented products.
References
Alvarez-Jubete L, Arendt E, Gallagher E (2010) Nutritive value of pseudocereals and their increasing use as functional gluten-free ingredients. Trends Food Sci Technol 21:106–113
Axelsson L, Chung T, Dobrogosz W, Lindgren S (1989) Production of a broad spectrum antimicrobial substance by Lactobacillus reuteri. Microb Ecol Health Dis 2:131–136
Bastidas E, Roura R, Rizzolo D, Massanés T, Gomis R (2016) Quinoa (Chenopodium quinoa Willd), from nutritional value to potential health benefits: an integrative review. J Nutr Food Sci 6(3)
Bering S, Suchdev S, Sjøltov L, Berggren A, Tetens I, Bukhave K (2006) A lactic acid-fermented oat gruel increases non-haem iron absorption from a phytate-rich meal in healthy women of childbearing age. Br J Nutr 96:80–85
Bertero H, De la Vega A, Correa G, Jacobsen S, Mujica A (2004) Genotype and genotype-by-environment interaction effects for grain yield and grain size of quinoa (Chenopodium quinoa Willd.) as revealed by pattern analysis of international multi-environment trials. Field Crop Res 89:299–318
Bolívar-Monsalve J, Ceballos-González C, Ramírez-Toro C, Bolívar GA (2018) Reduction in saponin content and production of gluten-free cream soup base using quinoa fermented with Lactobacillus plantarum. J Food Process Preserv 42:1–1. https://doi.org/10.1111/jfpp.13495
Bourdichon F, Casaregola S, Farrokh C, Frisvad JC, Gerds ML, Hammes WP, Harnett J, Huys G, Laulund S, Ouwehand A (2012) Food fermentations: microorganisms with technological beneficial use. Int J Food Microbiol 154:87–97
Brady K, Ho C-T, Rosen RT, Sang S, Karwe MV (2007) Effects of processing on the nutraceutical profile of quinoa. Food Chem 100:1209–1216. https://doi.org/10.1016/j.foodchem.2005.12.001
Brand-Miller J, Hayne S, Petocz P, Colagiuri S (2003) Low–glycemic index diets in the management of diabetes: a meta-analysis of randomized controlled trials. Diabetes Care 26:2261–2267
Caplice E, Fitzgerald GF (1999) Food fermentations: role of microorganisms in food production and preservation. Int J Food Microbiol 50:131–149
Carbó R, Gordún E, Fernández A, Ginovart M (2020) Elaboration of a spontaneous gluten-free sourdough with a mixture of amaranth, buckwheat, and quinoa flours analyzing microbial load, acidity, and pH. Food Sci Technol Int 26:344–352. https://doi.org/10.1177/1082013219895357
Carciochi R, Galván-D’Alessandro L, Vandendriessche P, Chollet S, Carciochi RA, Galván-D’Alessandro L (2016) Effect of germination and fermentation process on the antioxidant compounds of quinoa seeds. Plant Foods Hum Nutr 71:361–367. https://doi.org/10.1007/s11130-016-0567-0
Carrizo SL, de Oca CEM, Laiño JE, Suarez NE, Vignolo G, LeBlanc JG, Rollán G (2016) Ancestral Andean grain quinoa as source of lactic acid bacteria capable to degrade phytate and produce B-group vitamins. Food Res Int 89:488–494
Carrizo SL, de LeBlanc A d M, LeBlanc JG, Rollán GC (2020) Quinoa pasta fermented with lactic acid bacteria prevents nutritional deficiencies in mice. Food Res Int 127:108735
Castro-Alba V (2019) Fermentation of quinoa, canihua and amaranth to degrade phytate and improve mineral bioavailability. Lund University
Castro-Alba V, Lazarte CE, Bergenståhl B, Granfeldt Y (2019a) Phytate, iron, zinc, and calcium content of common Bolivian foods and their estimated mineral bioavailability. Food Sci Nutr 7:2854–2865
Castro-Alba V, Lazarte CE, Perez-Rea D, Carlsson NG, Almgren A, Bergenståhl B, Granfeldt Y (2019b) Fermentation of pseudocereals quinoa, canihua, and amaranth to improve mineral accessibility through degradation of phytate. J Sci Food Agric 99:5239–5248
Castro-Alba V, Lazarte CE, Perez-Rea D, Sandberg AS, Carlsson NG, Almgren A, Bergenståhl B, Granfeldt Y (2019c) Effect of fermentation and dry roasting on the nutritional quality and sensory attributes of quinoa. Food Sci Nutr 7:3902–3911
Ceballos-González C, Bolívar-Monsalve J, Ramírez-Toro C, Bolívar GA (2018) Effect of lactic acid fermentation on quinoa dough to prepare gluten-free breads with high nutritional and sensory quality. J Food Process Preserv 42:1–1. https://doi.org/10.1111/jfpp.13551
Cizeikiene D, Juodeikiene G, Bartkiene E, Damasius J, Paskevicius A (2015) Phytase activity of lactic acid bacteria and their impact on the solubility of minerals from wholemeal wheat bread. Int J Food Sci Nutr 66:736–742
Corsetti A, Settanni L (2007) Lactobacilli in sourdough fermentation. Food Res Int 40:539–558
D’Amico S, Schoenlechner R, Tömösköszi S, Langó B (2017) Proteins and amino acids of kernels. In: Pseudocereals: chemistry and technology. pp 94–118
Dallagnol A, Pescuma M, Valdez G, Rollán G (2013) Fermentation of quinoa and wheat slurries by Lactobacillus plantarum CRL 778: proteolytic activity. Appl Microbiol Biotechnol 97:3129–3140. https://doi.org/10.1007/s00253-012-4520-3
De Angelis M, Gallo G, Corbo MR, McSweeney PL, Faccia M, Giovine M, Gobbetti M (2003) Phytase activity in sourdough lactic acid bacteria: purification and characterization of a phytase from Lactobacillus sanfranciscensis CB1. Int J Food Microbiol 87:259–270
de Oliveira Lopes C, Barcelos M d FP, de Goes Vieira CN, de Abreu WC, Ferreira EB, Pereira RC, de Angelis-Pereira MC (2019) Effects of sprouted and fermented quinoa (Chenopodium quinoa) on glycemic index of diet and biochemical parameters of blood of Wistar rats fed high carbohydrate diet. J Food Sci Technol 56:40–48
Di Renzo T, Reale A, Boscaino F, Messia MC (2018) Flavoring production in Kamut®, quinoa and wheat doughs fermented by Lactobacillus paracasei, Lactobacillus plantarum, and Lactobacillus brevis: a SPME-GC/MS Study. Front Microbiol 9:429
Đorđević TM, Šiler-Marinković SS, Dimitrijević-Branković SI (2010) Effect of fermentation on antioxidant properties of some cereals and pseudo cereals. Food Chem 119:957–963
Fayle SE, Gerrard JA (2002) The maillard reaction, vol 5. Royal Society of Chemistry
Greiner R, Alminger ML (1999) Purification and characterization of a phytate-degrading enzyme from germinated oat (Avena sativa). J Sci Food Agric 79:1453–1460
Greiner R, Muzquiz M, Burbano C, Cuadrado C, Pedrosa MM, Goyoaga C (2001) Purification and characterization of a phytate-degrading enzyme from germinated faba beans (Vicia faba var. Alameda). J Agric Food Chem 49:2234–2240
Hur SJ, Lee SY, Kim Y-C, Choi I, Kim G-B (2014) Effect of fermentation on the antioxidant activity in plant-based foods. Food Chem 160:346–356
Jacobsen S-E, Mujica A, Jensen C (2003) The resistance of quinoa (Chenopodium quinoa Willd.) to adverse abiotic factors. Food Rev Intl 19:99–109
Katina K, Laitila A, Juvonen R, Liukkonen K-H, Kariluoto S, Piironen V, Landberg R, Åman P, Poutanen K (2007a) Bran fermentation as a means to enhance technological properties and bioactivity of rye. Food Microbiol 24:175–186
Katina K, Liukkonen K-H, Kaukovirta-Norja A, Adlercreutz H, Heinonen S-M, Lampi A-M, Pihlava J-M, Poutanen K (2007b) Fermentation-induced changes in the nutritional value of native or germinated rye. J Cereal Sci 46:348–355
Konishi Y, Hirano S, Tsuboi H, Wada M (2004) Distribution of minerals in quinoa (Chenopodium quinoa Willd.) seeds. Biosci Biotechnol Biochem 68:231–234
Lazarte C (2014) Nutritional assessment in a rural area of bolivia. A study of zinc and iron deficiencies and bioavailability. Lund University
Lazarte CE, Carlsson N-G, Almgren A, Sandberg A-S, Granfeldt Y (2015) Phytate, zinc, iron and calcium content of common Bolivian food, and implications for mineral bioavailability. J Food Compos Anal 39:111–119
Ludena Urquizo FE, García Torres SM, Tolonen T, Jaakkola M, Pena-Niebuhr MG, Wright A, Repo-Carrasco-Valencia R, Korhonen H, Plumed-Ferrer C (2017) Development of a fermented quinoa-based beverage. Food Sci Nutr 5:602–608. https://doi.org/10.1002/fsn3.436
Montemurro M, Pontonio E, Rizzello CG (2019) Quinoa flour as an ingredient to enhance the nutritional and functional features of cereal-based foods. In: Flour and breads and their fortification in health and disease prevention. Elsevier, pp 453–464
Moore J, Cheng Z, Hao J, Guo G, Liu J-G, Lin C, Yu L (2007) Effects of solid-state yeast treatment on the antioxidant properties and protein and fiber compositions of common hard wheat bran. J Agric Food Chem 55:10173–10182
Navruz-Varli S, Sanlier N (2016) Nutritional and health benefits of quinoa (Chenopodium quinoa Willd.). J Cereal Sci 69:371–376
Nickel J, Spanier LP, Botelho FT, Gularte MA, Helbig E (2016) Effect of different types of processing on the total phenolic compound content, antioxidant capacity, and saponin content of Chenopodium quinoa Willd grains. Food Chem 209:139–143
Nionelli L, Curri N, Curiel JA, Di Cagno R, Pontonio E, Cavoski I, Gobbetti M, Rizzello CG (2014) Exploitation of Albanian wheat cultivars: characterization of the flours and lactic acid bacteria microbiota, and selection of starters for sourdough fermentation. Food Microbiol 44:96–107
Noratto GD, Murphy K, Chew BP (2019) Quinoa intake reduces plasma and liver cholesterol, lessens obesity-associated inflammation, and helps to prevent hepatic steatosis in obese db/db mouse. Food Chem 287:107–114. https://doi.org/10.1016/j.foodchem.2019.02.061
Östman E, Granfeldt Y, Persson L, Björck I (2005) Vinegar supplementation lowers glucose and insulin responses and increases satiety after a bread meal in healthy subjects. Eur J Clin Nutr 59:983–988
Parisi S, Luo W (2018) The importance of Maillard reaction in processed foods. In: Chemistry of maillard reactions in processed foods. Springer, pp 1–37
Paśko P, Zagrodzki P, Bartoń H, Chłopicka J, Gorinstein S (2010) Effect of quinoa seeds (Chenopodium quinoa) in diet on some biochemical parameters and essential elements in blood of high fructose-fed rats. Plant Foods Hum Nutr 65:333–338
Petry N, Egli I, Zeder C, Walczyk T, Hurrell R (2010) Polyphenols and phytic acid contribute to the low iron bioavailability from common beans in young women. J Nutr 140:1977–1982
Repo-Carrasco R, Espinoza C, Jacobsen S-E (2003) Nutritional value and use of the Andean crops quinoa (Chenopodium quinoa) and kañiwa (Chenopodium pallidicaule). Food Rev Intl 19:179–189
Repo-Carrasco-Valencia RA-M, Serna LA (2011) Quinoa (Chenopodium quinoa, Willd.) as a source of dietary fiber and other functional components. Food Sci Technol 31:225–230
Rizzello CG, Lorusso A, Montemurro M, Gobbetti M (2016) Use of sourdough made with quinoa (Chenopodium quinoa) flour and autochthonous selected lactic acid bacteria for enhancing the nutritional, textural and sensory features of white bread. Food Microbiol 56:1–13. https://doi.org/10.1016/j.fm.2015.11.018
Rocchetti G, Chiodelli G, Giuberti G, Masoero F, Trevisan M, Lucini L (2017) Evaluation of phenolic profile and antioxidant capacity in gluten-free flours. Food Chem 228:367–373
Rocchetti G, Chiodelli G, Giuberti G, Lucini L (2018) Bioaccessibility of phenolic compounds following in vitro large intestine fermentation of nuts for human consumption. Food Chem 245:633–640
Rocchetti G, Miragoli F, Zacconi C, Lucini L, Rebecchi A (2019) Impact of cooking and fermentation by lactic acid bacteria on phenolic profile of quinoa and buckwheat seeds. Food Res Intl (Ottawa, ON) 119:886–894. https://doi.org/10.1016/j.foodres.2018.10.073
Rollan GC, Gerez CL, LeBlanc JG (2019) Lactic fermentation as a strategy to improve the nutritional and functional values of pseudocereals. Front Nutr 6:98
Rothschild J, Rosentrater KA, Onwulata C, Singh M, Menutti L, Jambazian P, Omary MB (2015) Influence of quinoa roasting on sensory and physicochemical properties of allergen-free, gluten-free cakes. Int J Food Sci Technol 50:1873–1881
Ruales J, Nair BM (1993) Saponins, phytic acid, tannins and protease inhibitors in quinoa (Chenopodium quinoa, Willd) seeds. Food Chem 48:137–143. https://doi.org/10.1016/0308-8146(93)90048-K
Ruiz Rodríguez L, Vera Pingitore E, Rollan G, Cocconcelli PS, Fontana C, Saavedra L, Vignolo G, Hebert EM (2016) Biodiversity and technological-functional potential of lactic acid bacteria isolated from spontaneously fermented quinoa sourdoughs. J Appl Microbiol 120:1289–1301
Ruiz KB, Biondi S, Oses R, Acuña-Rodríguez IS, Antognoni F, Martinez-Mosqueira EA, Coulibaly A, Canahua-Murillo A, Pinto M, Zurita-Silva A (2014) Quinoa biodiversity and sustainability for food security under climate change. A review. Agron Sustain Dev 34:349–359
Ruiz A, Espinosa B, Santamaría CG, Fernández CJC, García MA, Méndez FS, Guillén IG, Rubia AJL, Ràzuri FJQ, Garrido AM, Román FJL (2017) Effect of quinua (Chenopodium quinoa) consumption as a coadjuvant in nutritional intervention in prediabetic subjects. Nutr Hosp 34:1163–1169
Salovaara H, Gänzle M (2011) Lactic acid bacteria in cereal-based products. In: Lahtinen S, Ouwehand AC, Salminen S, Von Wright A (eds) Lactic acid bacteria: microbiological and functional aspects, 4th edn. CRC Press, New York, pp 227–245
Sandberg A-S (2002) Bioavailability of minerals in legumes. Br J Nutr 88:281–285
Sandberg AS, Andlid T (2002) Phytogenic and microbial phytases in human nutrition. Int J Food Sci Technol 37:823–833
Scalbert A, Johnson IT, Saltmarsh M (2005) Polyphenols: antioxidants and beyond. Am J Clin Nutr 81:215S–217S
Scazzina F, Del Rio D, Pellegrini N, Brighenti F (2009) Sourdough bread: starch digestibility and postprandial glycemic response. J Cereal Sci 49:419–421
Schlemmer U, Frølich W, Prieto RM, Grases F (2009) Phytate in foods and significance for humans: food sources, intake, processing, bioavailability, protective role and analysis. Mol Nutr Food Res 53:S330–S375
Starzyńska-Janiszewska A, Stodolak B, Byczyński Ł, Gómez-Caravaca AM, Martin-Garcia B, Mickowska B (2019) Mould starter selection for extended solid-state fermentation of quinoa. LWT 99:231–237. https://doi.org/10.1016/j.lwt.2018.09.055
Svensson L, Sekwati-Monang B, Lutz DL, Schieber A, Ganzle MG (2010) Phenolic acids and flavonoids in nonfermented and fermented red sorghum (Sorghum bicolor (L.) Moench). J Agric Food Chem 58:9214–9220
Tang Y, Li X, Zhang B, Chen PX, Liu R, Tsao R (2015) Characterisation of phenolics, betanins and antioxidant activities in seeds of three Chenopodium quinoa Willd. genotypes. Food Chem 166:380–388
Troesch B, Jing H, Laillou A, Fowler A (2013) Absorption studies show that phytase from Aspergillus niger significantly increases iron and zinc bioavailability from phytate-rich foods. Food Nutr Bull 34:S90–S101
Valencia US, Sandberg A-S, Ruales J, Silvia (1999) Processing of quinoa (Chenopodium quinoa, Willd): effects on in vitro iron availability and phytate hydrolysis. Int J Food Sci Nutr 50:203–211
Van Boekel M (2006) Formation of flavour compounds in the Maillard reaction. Biotechnol Adv 24:230–233
Vega-Gálvez A, Miranda M, Vergara J, Uribe E, Puente L, Martínez EA (2010) Nutrition facts and functional potential of quinoa (Chenopodium quinoa willd.), an ancient Andean grain: a review. J Sci Food Agric 90:2541–2547
Vilcacundo R, Hernández-Ledesma B (2017) Nutritional and biological value of quinoa (Chenopodium quinoa Willd.). Curr Opin Food Sci 14:1–6. https://doi.org/10.1016/j.cofs.2016.11.007
Weaver CM, Kannan S (2002) Phytate and mineral bioavailability. Food Phytates 2002:211–223
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2021 The Author(s), under exclusive license to Springer Nature Singapore Pte Ltd.
About this chapter
Cite this chapter
Lazarte, C.E., Castro-Alba, V., Granfeldt, Y. (2021). Quinoa Fermentation and Dry Roasting to Improve Nutritional Quality and Sensory Properties. In: Varma, A. (eds) Biology and Biotechnology of Quinoa. Springer, Singapore. https://doi.org/10.1007/978-981-16-3832-9_15
Download citation
DOI: https://doi.org/10.1007/978-981-16-3832-9_15
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-16-3831-2
Online ISBN: 978-981-16-3832-9
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)