Abstract
We describe a novel technique, Navigation and Endoscopic Assisted Tumor (NEAT) surgery in patients with benign bone tumors. The technique combines the advantages of both bone endoscopy and navigation guidance. It enables surgeons to perform intra-lesional tumor curettage in selected benign bone tumors in minimal access with less surgical trauma. The curettage procedure and the tumor cavity not only can be visualized with an endoscope but also can be assessed for tumor clearance with real-time feedback of navigation information on the preoperative CT images. It is particularly useful in benign bone tumors at difficultly accessed locations or tumors with irregular bone cavity and internal septae. The technique may avoid excessive bone removal to minimize the risk of fracture while preserving normal bone for better limb function. An initial learning curve, the facilities required, and a lack of long-term clinical results are some obstacles to its widespread use.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
1 Introduction
-
Benign bone tumors frequently occur in the knee region, namely distal femur and proximal tibia. They are more common in pediatric and young adults. Diagnosis includes simple bone cyst, aneurysmal bone cyst, fibrous dysplasia, enchondroma, chondroblastoma, and giant cell tumor of bone. Progressive bone destruction in chondroblastoma and giant cell tumor of bone at the juxta-articular locations often lead to bone pain, may even result in fracture or deformity due to weakened bone or damage of articular cartilage with secondary osteoarthritis [1, 2].
-
Given that the tumors are benign and the patients are in a young, active population, intra-lesional curettage is the mainstay of the surgical treatment. Tumor removal prevents further bone destruction, and yet preserves surrounding normal bone for joint function.
-
The conventional tumor curettage in chondroblastoma and giant cell tumor of bones requires large surgical exposure. A cortical window is created over the tumor, usually reaching the peripheral edge of the tumor so that it is wide enough to visualize the tumor cavity for tumor clearance. Inadequate tumor removal accounts for the high rate of recurrence that was reported to be between 10–35% in Chondroblastoma [3] and 18–53% in giant cell tumor of bones [4, 5]. Also, the bigger the cortical window is made, the weaker is the weight-bearing and juxta-articular bone of the knee joint. It may compromise early post-operative recovery or has a risk of fracture.
-
To address the potential undesirable effects of the conventional technique, endoscopic guided tumor curettage was reported in the surgical management of benign bone tumors [6,7,8,9]. The reports suggested that endoscopically guided curettage not only had the benefit of minimal access with less surgical trauma but also allowed visualization of the curettage procedure. The intramedullary cavity could be better assessed under direct and magnified endoscopic visualization in contrast to the open technique under naked eyes. Better visualization might facilitate complete tumor removal with less local tumor recurrence. Also, the endoscopic technique might avoid excessive curettage and minimize cartilage damage or risk of fracture [8, 10].
-
The endoscopic approach often requires an intraoperative 2D fluoroscopic image to locate the bone tumor, guide the site of the cortical window, and position the operating instruments. 2D images may not provide adequate bone information for the guidance if the tumors have complex geometry, multi-loculated components with internal calcified septae, or in a difficult accessed location such as distal radius, proximal femur, or pelvis. A modified method, Navigation Endoscopic Assisted tumor (NEAT) surgery, was reported [11]. A computer navigation system that has been applied in orthopedic tumor resection [12] was used in addition to endoscopic tumor curettage. The navigation system allows surgeons to get an instant and real-time visual feedback of the patients’ anatomy by referencing to the preoperative images about the spatial orientation of the extent of the tumor. Therefore, the NEAT surgery combines the advantages of navigation and endoscopic techniques to orientate and visualize the tumor cavity during the curettage procedure in a minimal access manner. It may facilitate adequate tumor clearance with the potential of minimizing tumor recurrence.
-
This chapter is to describe the details of the tumor curettage using the NEAT technique in a patient with chondromyxoid fibroma of the distal femur.
2 Indications
-
Biopsy-proven benign bone tumors (such as giant cell tumor of bone, enchondroma, chondromyxoid fibroma, aneurysmal bone cyst).
-
Benign bone tumors at the difficult access locations (such as distal radius, proximal femur, pelvis).
-
Benign bone tumors having multi-loculated components with internal septae, irregular peripheral bony borders, or close to articular cartilage at the subchondral bone, in which endoscopic curettage alone is difficult to ensure tumor clearance or avoid damage of the cartilage.
-
Denosumab-treated giant cell tumor of bone in which newly formed internal calcification makes the curettage difficult to ensure tumor clearance.
3 Contra-indications
-
Malignant bone tumors
4 Author Preferred Technique
4.1 Preoperative Planning
-
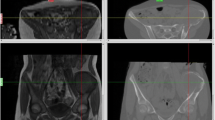
Preoperative plain radiograph, CT, and MR examinations of the knee were performed to delineate the extent of the tumor (Fig. 13.1a, b). The CT images were acquired at the same setting when the patient underwent a CT-guided tissue biopsy. Axial CT images (slices with 0.625 mm thickness) of the knee were obtained using a 16-detector scanner (General Electric LightSpeed, Milwaukee, WI).
-
The image datasets in Digital Imaging and Communications in Medicine (DICOM) format were imported into the CT-based navigation system (VectorVision, BrainLAB, iPlan Spine 2.0.1, Feldkirchen, Germany). The images were reformatted into different views. The extent of the bone tumor was mapped, and a 3D tumor model was generated (Fig. 13.2a). The sites of endoscopic portals were planned in the navigation system so that the entire tumor cavity could be reached and curetted by instruments (Fig. 13.2b–d).
-
Required instruments: a shoulder arthroscope (30° lens, 4 mmS.W.A.; Karl Storz, Tuttlingen, Germany); curettes, high-speed bone burr, and a CT-based navigation system with the navigation trackers and probe.
(a) The plain radiograph (anteroposterior view) of the right knee shows a mildly expansile osteolytic lesion with a well-defined sclerotic border at the medial condyle of the right femur in a 30-year-old patient. (b) The axial view of CT images of the same patient shows the osteolytic lesion at the medial femoral condyle with thinning of the cortex, close to the subchondral bone of the knee joint, and some irregularities of the inner tumor cavity. The CT-guided biopsy confirmed a benign bone tumor with the histological diagnosis of a chondromyxoid fibroma
Preoperative CT and MR images were imported and fused in the navigation system. The tumor was mapped (red). The extent of the tumor (arrows) could be examined in the 3D model (a), axial (b), reformatted sagittal (c), and coronal (d) images. The volume of the tumor could be calculated to estimate the amount of cement or bone graft needed for filling up the cavity after tumor curettage
4.2 Patient Positioning
-
The patient was positioned supine on an operating table with a radiolucent board (Fig. 13.3a). An ipsilateral thigh pneumatic tourniquet was applied to provide a bloodless surgical field during the bone curettage. The opposite leg was hanged on leg support to facilitate the intraoperative acquisition of the plain radiograph of the knee (Fig. 13.3b).
-
The navigation machine, with its tracking camera, was positioned at the rear end of the operating table (Fig. 13.4). An extra video monitor was located on the right side of the operating table so that the operating surgeon could watch the endoscopic images alone when performing bone curettage without navigation guidance (Fig. 13.5).
-
The endoscopic curettage procedure did not require fluid inflow, and the curettage procedure was performed with dry endoscopy.
(a) The patient was positioned supine with left leg hang on leg support (arrow). (b) The patient’s position facilitated the C-arm X-ray machine (asterisk) to acquire the anteroposterior and lateral views of the plain radiograph of the right knee for the navigation procedure. A patient tracker (yellow arrow) and a phantom tracker (white arrows) of the C-arm X-ray machine were mounted for the image registration of the plain radiographs
4.3 Portal Design
-
Two portals were used. Two cortical windows of about 1 cm in diameter were planned on the medial and middle aspect of the right distal femoral condyle so that the tumor cavity could be reached during curettage procedure (Fig. 13.6a–c). As the arthroscope, bone curettes, and high-speed burr are straight instruments, the two portals may be connected as one larger portal to facilitate the tumor clearance if necessary.
-
The tumor was removed through one working portal under direct magnified endoscopic visualization at the second viewing portal (Fig. 13.7). The curettage instruments and arthroscope took turns to change the portals for the tumor curettage.
The navigation display shows the locations of the working and viewing portals that were marked as pedicle screws (gray and green) in reformatted coronal (a), axial (b), 3D bone tumor model (c), and intraoperatively acquired plain radiograph (d) in the CT navigation spine system. The locations of the portals were planned so that the tumor cavity (orange) could be fully reached. After the image-to-patient registration, the planned location of the distal portal was identified by using the navigation probe (arrows)
4.4 A Detailed Description of the Technique
-
A patient’s tracker was attached to the lateral femoral condyle by inserting two parallel 4.5 mm pins so that the tracker would not hinder the working and viewing portals at the medial femoral condyle (Fig. 13.8).
-
To real-time tracking the operating bone, the navigation surgery requires a registration process in which the operative anatomy matches precisely to the preoperative CT images in the navigation software. A CT-fluoro matching technique was used. An ortho planar (anteroposterior and lateral) fluoroscopic images were intraoperatively acquired that was then matched to the virtual 3D bone model generated from the preoperative CT images in the navigation system (Fig. 13.9).
-
The registration accuracy was further verified by touching the navigation probe on the anatomic landmarks or the bone surface (Fig. 13.10). The navigation system was considered accurate only if there was exact matching between the image on the navigation console and the patient’s bone anatomy.
-
The skin incision over the planned portals was marked under navigation guidance (Fig. 13.11). A pneumatic tourniquet of the right thigh was put on.
-
The sites of the cortical bone window were identified and made as planned under navigation guidance (Fig. 13.6a–d).
-
After the central bulk of the tumor was curetted via the two portals (Fig. 13.12), tumor curettage was further performed at the tumor cavity with endoscopic assistance (Fig. 13.13a, b).
-
High-speed bone burr mounted with navigation trackers were calibrated to the navigation system so that the tip of the burr was correctly referenced and spatially visualized regarding the patient’s anatomy. The entire intra-osseous surface of the tumor cavity was further removed under the navigation guidance and direct endoscopic visualization (Fig. 13.14a–d). The excessive burring of the normal bone could be avoided to minimize fracture or at the subchondral area to reduce the chance of osteoarthritis.
-
The endoscopic visualization allowed us to visually identify the residual tumor on the walls of the tumor cavity while the navigation guidance confirmed the clearance was reaching the peripheral edge of the tumor with reference to preoperative CT images (Fig. 13.15a–d).
-
The tumor cavity was irrigated with hydrogen Peroxide and copious normal saline. Cement was injected into the bone cavity using a cement gun via one of the portals (Fig. 13.16a). A suction drain was inserted to prevent wound hematoma due to possible bleeding at the bone-cement junction, and the wound was closed by layers (Fig. 13.16b).
CT-fluoro matching was used for the image-to-patient registration in the navigation procedure. It involved the matching of the 3D model of the distal femur (arrow) generated from the preoperative CT images with intraoperatively acquired anteroposterior and lateral views of fluoroscopic images. The registration was considered accurate when the 3D bone model (blue) was manually moved until its outline overlapped precisely with that of fluoroscopic images
The spatial location of the tip of the navigated bone burr (arrow) could be real-time tracked under the navigation system with reference to the various reformatted CT images, (a) axial, (b) coronal views, and (c) 3D bone tumor model. (d) The bone burr (asterisk) could also be visualized with the endoscopic images
The adequacy of the tumor clearance could be further verified by using the navigation probe touching at the different sites of the curetted tumor cavity. The reformatted coronal (a), sagittal views (b), and 3D bone model (c) show that the tip of the navigation probe (arrows) was just beyond the peripheral edge of the tumor cavity near the subchondral region of the medial femoral condyle. (d) The navigation probe (asterisk) could also be visualized with the endoscopic images. The NEAT surgery could facilitate complete tumor clearance while avoiding excessive normal bone removal
4.5 Complications
-
Local tumor recurrence depends on the type of benign bone tumors undergoing tumor curettage and the adequacy of local tumor clearance. The technique may facilitate tumor surgeons to achieve better tumor control that may translate into a better clinical outcome.
-
Fracture due to the already weakened bone by tumor invasion or excessive bone burring during curettage procedure.
-
Late osteoarthritis as a result of the loss of subchondral bone by tumor involvement.
4.6 Post-operative Care
-
With the minimal access approach in terms of skin incision and cortical bone windows, the patient was allowed to resume full-weight bearing walking and immediate joint mobilization after the suction drain was removed 1 or 2 days after surgery.
-
A plain radiograph was taken after the drain was removed and then post-operative 1 month, every 3 months for the first 2 years, every 6 months for 3 years and then annually. Most of the tumor recurrence occurs at 2–3 years after surgery, and an annual plain radiograph was taken to detect late osteoarthritis (Fig. 13.17a, b).
5 Summary
-
The NEAT surgery combines the advantages of both bone endoscopy and navigation guidance. It enables surgeons to perform intra-lesional tumor curettage in selected benign bone tumors in minimal access with less surgical trauma. The curettage procedure and the tumor cavity not only can be visualized with an endoscope but also can be assessed for tumor clearance with real-time feedback of navigation information on the preoperative CT images.
-
The technique may avoid excessive bone removal to minimize the risk of fracture while preserving limb function. A learning curve is anticipated as it requires both expertise of endoscopic and navigation surgery. There is no long-term result to support the potential benefits that can be translated into a better outcome.
-
Currently, there is no commercial system and instruments dedicated to bone endoscopy. It would be helpful to design special curved instruments or protective portal sheath for endoscopic tumor surgery.
References
Farfalli GL, Slullitel PA, Muscolo DL, Ayerza MA, Aponte-Tinao LA. What happens to the articular surface after curettage for epiphyseal chondroblastoma? A report on functional results, arthritis, and arthroplasty. Clin Orthop Relat Res. 2017;475(3):760–6.
van der Heijden L, van de Sande MA, Heineken AC, Fiocco M, Nelissen RG, Dijkstra PD. Mid-term outcome after curettage with polymethyl methacrylate for giant cell tumor around the knee: higher risk of radiographic osteoarthritis? J Bone Joint Surg Am. 2013;95(21):e159.
Ramappa AJ, Lee FY, Tang P, et al. Chondroblastoma of bone. J Bone Joint Surg [Am]. 2000;82-A:1140–5.
Turcotte RE, et al. Giant cell tumor of long bone: a Canadian Sarcoma Group study. Clin Onhop Relat Res. 2002;397:248–58.
Hu P, Zhao L, Zhang H, et al. Recurrence rates and risk factors for primary giant cell tumors around the knee: a multicentre retrospective study in China. Sci Rep. 2016;6:36332.
Stricker SJ. Extraarticular endoscopic excision of femoral head chondroblastoma. J Pediatr Orthop. 1995;15(5):578–81.
Otsuka T, Kobayashi M, Yonezawa M, Kamiyama F, Matsushita Y, Matsui N. Treatment of chondroblastoma of the calcaneus with a secondary aneurysmal bone cyst using endoscopic curettage without bone grafting. Arthroscopy. 2002;18(4):430–5.
Choi Y, Kwak JM, Chung SH, Jung GH, Kim JD. Tumor treated by endoscopy. Clin Orthoped Surg. 2014;6:72–9.
Aiba M, Kobayashi Y, Waguri-Nagaya H, Goto J, Mizutani S, Yamada H, Okamoto M, Nozaki H, Mitsui S, Miwa M, Kobayashi K, Endo S, Saito T, Goto T. Otsuka, treatment of aneurysmal bone cysts using endoscopic curettage. BMC Musculoskelet. Disord. 2018;19(1):268.
Dietz JF, Kachar SM, Nagle DJ. Endoscopically assisted excision of digital enchondroma. Arthroscopy. 2007;23:678.e1–4.
Wong KC, Kumta SM, Tse LF, Ng WK, Lee KS. Navigation Endoscopic Assisted Tumor (NEAT) surgery for benign bone tumors of the extremities. Comput Aided Surg. 2010;15(1–3):32–9.
Wong KC, Kumta SM. Use of computer navigation in orthopedic oncology. Curr Surg Rep. 2014;2(4):47.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2021 The Author(s), under exclusive license to Springer Nature Singapore Pte Ltd.
About this chapter
Cite this chapter
Wong, K.C., Lau, H.W., Chiu, W.K., Kumta, S.M. (2021). Bone Endoscopy Around the Knee: Navigation Endoscopic Assisted Tumor (NEAT) Surgery for Benign Bone Tumors Around the Knee. In: Lui, T.H. (eds) Endoscopy of the Hip and Knee. Springer, Singapore. https://doi.org/10.1007/978-981-16-3488-8_13
Download citation
DOI: https://doi.org/10.1007/978-981-16-3488-8_13
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-16-3487-1
Online ISBN: 978-981-16-3488-8
eBook Packages: MedicineMedicine (R0)