Abstract
The characteristics of neurodegenerative diseases (NDDs) are the loss of myelin sheath and neurons, which worsens and becomes dysfunctional over time. Alzheimer’s disease, Parkinson’s disease, and Huntington’s disease are among the most harmful brain diseases. Effective treatments for NDDs are usually unavailable because of the difficulty in obtaining therapeutic drugs. What’s more, the blood-brain barrier that selectively blocks the passage of substances is a dynamic interface between the brain tissue and blood. The presence of this barrier is effective in preventing some (mostly harmful) substances from entering the brain tissue from the blood, but it also causes the traditional drug transport systems to be unable to provide connectivity patterns and sufficient cellular structural repair, which is critical to functional recovery in brain diseases. Nanotechnology uses engineering equipment or materials to interact with biological systems, that is, to control and reduce side effects while inducing physiological responses through stimulation or interaction with targets, thereby completely changing the treatment NDDs. The nanomaterials have advantages in structure and performance and are designed as carriers to cross the blood-brain barrier to target location. Magnetic nanomaterials, as imaging agents or nanoprobes, have played an active role in the diagnosis of NDDs. The nanomaterials in clinical applications have not achieved the expected results, but it has made a breakthrough innovation, which points out the future development direction and lays a foundation for the application of nanotechnology in NDDs.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
3.1 Introduction
Neurodegenerative diseases (NDDs), including Alzheimer’s disease (AD), Parkinson’s disease (PD), and Huntington’s disease (HD), are caused by the accumulation of misfolded proteins, and they share some similarities in synaptic abnormalities and neuron loss [1, 2]. And NDDs can worsen over time, resulting in dysfunction. NDDs are caused by a variety of factors, including oxidative stress, mitochondrial dysfunction, and immune inflammation. Blocking one or two pathways does not significantly reduce overall neuronal dysfunction and loss. With the deepening of the research on NDDs, the use of the advantages of multi-approach and multi-target treatment has a good effect on improving the symptoms and regulating brain function. On the other hand, the pathological changes associated with the onset of NDDs are irreversible. When patients have cognitive impairment, the course of the disease is often in the middle stages. At this time, treatment can only slow down the development of the disease and cannot fundamentally reverse the damage of the neural network. The cause of NDD is still unclear, and NDD cannot be cured, which seriously threatens human health and daily life and places a huge burden on families and society. Although many theoretically effective drugs have been developed, their effectiveness is greatly reduced for the existence of the BBB (a dynamic interface between blood and brain tissue that selectively blocks substances) [3]. When various solutes in the blood enter the brain tissue from the capillaries in the brain, they enter more or less quickly, and some cannot even get through. The BBB can make the brain tissue suffer little or no damage from harmful substances in the circulating blood, so as to maintain the basic stability of the environment in the brain tissue, which has important biological significance for maintaining the normal physiological state of the central nervous system (CNS). But at the same time, this selective permeability of the BBB is also a huge challenge for the treatment of NDDs [4]. After the advent of the scanning tunneling microscope, nanotechnology was born. Research on nanotechnology focuses on the characteristics and applications of nanomaterials less than 100 nm [5]. Nanomaterials have superior properties in terms of size, morphology, biology, chemistry, physics, and characteristics [6, 7]. The drug delivery system of nanomaterials can overcome the BBB. This advantage can better assist in the diagnosis of neurological diseases [8]. The next section introduces the characteristics of nanomaterials and the BBB, as well as the advantages and challenges of nanomaterials used in NDD. It focuses on the introduction of nanomaterials as an effective method for the diagnosis and treatment of nonspecific diseases such as Parkinson’s disease (PD), Alzheimer’s disease (AD), and Huntington’s disease (HD).
3.2 BBB
Lewandowski proposed the concept of BBB in 1900. Initially, the blood-brain barrier was thought to be a barrier composed of brain capillary walls and glial cells, which can prevent substances in the blood (mainly harmful substances) from entering the CNS uncontrolled [3, 9,10,11]. The intact basement membrane, the glial membrane surrounded by astrocyte feet, pericytes, continuous capillary endothelium, and the tight junctions between the cells constitute the BBB [12,13,14]. Pericytes are located in the microvessels around the capillaries and plays a role in regulating BBB and supporting structures (Fig. 3.1a). At the same time, the tight junctions between endothelial cells form a network that limits proliferation. Chemical stability is maintained by the interaction of peripheral neurons with astrocytes. In addition, the formation of tight junctions is closely related to astrocyte foot processes. In summary, the abovementioned cells interact with transport proteins and regulatory enzymes, which together constitute the densest barrier. As the maintainer and protector of CNS stability, the blood-brain barrier strictly separates the nervous system from the vascular system, allowing only certain small molecules to pass through. However, BBB also inhibits the entry of drugs through the same mechanism, hindering the effective treatment and diagnosis of neurological diseases [13, 15,16,17]. When degenerative diseases occur in the CNS, the presence of BBB blocks the entry of most theoretically effective drugs. This is due to acidity and alkalinity, fat solubility, and high molecular weight [15, 18,19,20]. Diseases of the CNS can also affect the function and structure of the BBB, such as vascular cerebral hematoma. When vascular cerebral edema occurs, the endothelial cells of the cerebral capillaries are tightly connected and open, and the barrier permeability is significantly increased so that large molecules such as plasma albumin (molecular weight 69,000) can pass through the barrier. Severe brain injury leads to serious damage to the BBB, so serum proteins can also enter the brain through the barrier. As the damage is repaired, the influx of macromolecules into the brain stops first. The accelerated exchange of small molecules also disappeared after complete recovery, and the blood-brain barrier function was normal. Ionizing radiation, laser, and ultrasound can increase the permeability of the BBB.
(a) The schematic shows the structure of the BBB, which is formed by endothelial cells and surrounded by lamina and astrocytic perivascular endfeet. Pericytes and microglial cells are also presented. (b) The properties of nanocarriers such as type, charge, and shape among many others that affect the penetration and targeting of the BBB. (c) The various methods of transport of nanomaterials across the BBB for brain delivery. Copyright 2017, Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim
The capillaries in the brain and their adjacent areas do have some distinct structural characteristics compared with that in other tissues and organs. Cerebral capillaries lack the pores common to capillaries, or they are few and small. Endothelial cells overlap with each other and are tightly connected, effectively preventing the passage of macromolecules from their junctions. Endothelial cells are also surrounded by a continuous basement membrane. Beyond the basilar membrane, the perivascular foot (end foot) of many astrocytes accounts for approximately 85% of the surface of the brain capillaries. This constitutes multilayer membrane structure, forming the protective barrier of brain tissue. In pathological conditions, such as vascular cerebral edema, the close adhesion between endothelial cells opens; as a result of the loss of endothelial cell swelling overlap, many macromolecular substances can be exudated with the plasma filtrate capillary, which will damage the stability of the environment in the brain tissue, causing serious consequences. A variety of solutes in the blood travel from the capillaries of the brain to brain tissue at varying speeds, and some cannot get through at all. This is determined by the degree of binding to plasma proteins, the lipid solubility, and hydrophilicity. The solute in the blood has to pass through the endothelial cells, which constitutes the cerebral capillaries, to reach the tissue of the brain. The endothelial cell membrane is bilayer structure with lipid as the base, which is lipophilic, and the fat-soluble substances are easy to pass through. Therefore, the lipid solubility of the solute in the blood determines the difficulty and speed of its passage through the barrier. The more fat-soluble the solute is, the faster it gets through the BBB and into the brain. Thus, some drugs of the CNS can be modified, according to this law, to make it easier to enter the brain to exert effect of drugs more quickly. For example, barbitone, a central anesthetic, with weak lipophilicity, is slow to enter the brain tissue. When transformed into phenobarbital and gaining lipophilicity, barbitone can enter the brain tissue through the BBB easily and soon play its hypnotic anesthetic effect. If morphine is transformed into diacetyl morphine, it is easier to pass through the lipophilic endothelial cell membrane, reach the brain tissue, and perform its analgesic effect faster. Carotenoids are fat-soluble pigments, but astaxanthin is the only member of the carotenoid family that can cross the BBB. Regardless of whether the solutes are positively or negatively charged,they form hydrogen bonds with the oxygen atoms of water molecules when they dissolve in water. The more charged the solute, the stronger its ability to form hydrogen bonds and the worse its ability to pass through the blood-brain barrier. However, solutes such as water itself and glucose can enter the brain through the junction between endothelial cells and astrocytes due to their small molecular weight. Epinephrine and norepinephrine are difficult to get through the barrier because they are water-soluble and have many hydroxyl groups. Amino acids can cross the BBB, but amines have a harder time. Many compounds in plasma are bound to plasma proteins. Small molecules, such as hormones, cannot easily pass through the BBB when combined with plasma proteins and thus cannot exert their physiological effects. For example, nearly 99% of thyroxine binds to plasma protein and less than 1% is free. It has been proved that free thyroxine can readily enter the interstitial fluid of the brain. Thus, any drug which is able to prevent thyroxine from binding to the plasma protein can easily increase the amount of free thyroxine in the plasma, resulting in increased dose that passes the barrier.
In general, cell transport function is closely related to the lipophilicity and molecular weight of the transported substance to a large extent. Due to the effective efflux pump, although some drugs are lipoproteins, the drugs eventually return to the blood spontaneously [21, 22]. In addition, due to the tight connection between the cellular pathways and endothelial cells, it is difficult for large molecules to reach the brain to function. Therefore, a material that can overcome the selective permeability of the blood-brain barrier needs to be developed to successfully deliver the drug to the lesion [4, 23, 24].
3.3 Nanomaterials
As one of the emerging technologies with the greatest market application potential, the potential importance of nanotechnology is beyond doubt. The broad scope of nanotechnology includes nanomaterial technology, nanometer processing technology, nanometer measurement technology, and nanometer application technology. Nanomaterials have certain uniqueness. When the scale of nanomaterials is small to a certain extent, quantum mechanics must be used instead of traditional mechanics to describe their behavior. The reason why nanoparticles differ from large chunks is that their relatively large surface areas, known as ultra-microparticles, are covered with stepped structures that represent unstable atoms with high surface energy. Such atoms bond readily with foreign atoms and provide a large surface of reactive atoms due to particle size reduction. According to the content and characteristics of the research, the development history of nanoparticles can be divided into three stages after the advent of nanoparticle materials in the 1970s. It was divided into the first stage before 1990. In this stage, the research is focused on studying different methods of preparing nanoparticle powders in the laboratory, exploring the advantages of nanomaterials, and studying the evaluation methods. The research object is usually called nanocrystals or nanomaterials and is limited to single-phase materials. In the second stage, how to make full use of the physical and chemical properties of nanomaterials is the focus of research in this period. In the third stage (from 1994 to the present), the focus of nanomaterial research has shifted to the synthesis of nano-assembly systems, artificial assembly, and nanostructured material systems. This material is known internationally as a nanoscale mode material or a nano-assembly material system. Such systems are based on nanomaterial units, including nanoarray systems and mesoporous assembly systems. Specifically, nanoparticles, nanowires, and nanotubes are arranged in a multidimensional space to form a system. Fine-grained nanomaterials have many special properties such as macroscopic quantum tunneling effect, quantum size effect, dielectric confinement effect, surface effect, and volume effect compared with traditional solid materials. Therefore, nanomaterials have high microwave absorption characteristics. As shown in Fig. 3.2, the size, shape, charge, and delivery method of nanomaterials are beneficial to improve the permeability and bioavailability of drugs. What’s more, after these performance optimizations and upgrades, the ability of the material to penetrate the BBB can be further improved, and its therapeutic effect is better than traditional therapies.
Characterization of nanocarriers in several shapes. (A) FESEM images of different shapes of Fe3O4: (a) sphere, (b) spindle, (c) biconcave, (d) nanotube. (B) SEM images of polystyrene spheres (a) and elongated particles stretched from the 200 nm spheres (b). Scale bar, 1 μm. (C) TEM images of nanocarrier at pH 7.4 (a) and 5.8 (b), scale bar = 100 nm. (D) TEM images of morphologies in AuNRs (a) and AuNRs (b)–(d) at different steps. Copyright 2017, WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim
3.3.1 Size
Size is considered to be a crucial design factor, which directly affects the absorption of nanomaterials by the brain. Different material sizes can lead to special biological phenomena, including circulatory half-life and vascular penetration. The smaller the size of nanomaterials, the easier it is to cross the BBB, which means that it is more suitable for targeting and drug delivery. In principle, nanomaterials are generally considered to be better than 100 nm [25]. Although NPs of smaller size are easier to transport through the BBB, they cause limitations in encapsulation efficiency, rapid drug release, and surface energy limitations during endocytosis. When the size of the nanomaterial is less than 6 nm, it is easily filtered by the kidney and excreted from the body [26]. Nanomaterials around 20 nm are considered the ideal size for NDDs. It satisfies two conditions at the same time, escaping renal excretion and penetrating the BBB [21].
3.3.2 Shape
As shown in Fig. 3.2b, the nanomaterials have different shapes. In terms of spatial structure, the existing nanomaterials can be divided into four categories, namely, three-dimensional, two-dimensional, one-dimensional, and zero-dimensional. This depends on the nanomaterials meeting the nanoscale requirements in several dimensions. For example, nanorods and nanotubes meet the nanoscale in two dimensions of space, and they belong to the category of one-dimensional nanomaterials. Similarly, nanoparticles meet nanoscale in three dimensions and are classified as zero-dimensional nanomaterials [21]. In addition, studies have found that the entry of nanomaterials into cells, the circulation time of the drug throughout the body, and the choice of blood-brain barrier permeability are also affected by the shape of the nanomaterials. Round nanomaterials have the advantage of being easy to prepare, but they also have some shortcomings. The curvature of the round material limits the tight bonding with endothelial cells to a certain extent. After comparison, it is found that because the rod-shaped structure has a larger contact area with the receptor on the cell membrane surface, it has a tighter and more reliable bond and is easier to be taken up by cells. In summary, the shape of nanomaterials determines the permeability of the material to a certain extent. It is expected that improving the efficacy of therapeutic drugs and extending the cycle time can start from upgrading the shape of nanomaterials.
3.3.3 Charge
Because the cell membrane is negatively charged, it is generally believed that it is relatively easier for cells to take up nanomaterials with neutral and positive charges rather than negative charges. However, since the negatively charged nanomaterials have less binding to the proteins in the blood, the blood circulation time of the negatively charged materials is prolonged. In addition, under the premise of not destroying the integrity of the blood-brain barrier, negatively charged nanomaterials have strong permeability. Therefore, combining the advantages and disadvantages of these three types of materials, more negatively charged or neutral nanomaterials are selected in the application of NDD.
3.3.4 Delivery Methods
In order to overcome the selective permeability, improve the delivery efficiency, and improve the clinical effect, many explorations have been conducted on the drug delivery route of nano-drugs. Among them, intravenous administration is the most common way of administration. Through this method of administration, the nanomedicine can enter any vascular tissue including the brain. However, this method also has some problems that need to be solved urgently. How to avoid the rapid elimination of drugs from the body to prolong the time of systemic circulation and how to avoid accidental accumulation of drugs in nontarget organs, and thus more accumulation in target tissues and organs, are two problems that cannot be avoided by intravenous administration. In fact, the development of some new transportation methods has become a new research direction for scientists. Their view is that ultrasound-assisted nano-drug delivery has the potential to overcome the problems caused by these traditional delivery methods.
In summary, nanomaterials have the advantages of adding imaging agents, penetrating the vascular barrier, preventing drug degradation, and prolonging the time of drug in the systemic circulation. These have created more possibilities for the application of nanomaterials in NDD.
3.4 NDD
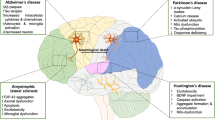
NDD is a complex degenerative disease of the CNS. It is characterized by a large number of irreversible loss of specific neurons, which eventually leads to chronic progressive disability or even death [27, 28]. Neurodegeneration is the gradual loss of neuronal structure and function, including neuronal death and glial cell balance, which can lead to cognitive impairment such as dementia [1, 29,30,31]. High glutamate concentration in the intercellular space can cause toxicity to neurons, resulting in aging and death of neurons. The excitotoxicity of glutamate is closely related to the occurrence and development of various NDDs. Nonspecific immunity is also called innate immunity. It protects the body through rapid response to various harmful substances. However, harmful substances also cause damage by activating the nonspecific immune, and such stimulation cannot be controlled [32, 34]. Mitochondria are important intracellular calcium stores. The exchange of calcium ions between the endoplasmic reticulum and mitochondria has a profound impact on cell fate. Failure to clear free radicals in time leads to an imbalance between the body’s oxidation and antioxidants. Under these stimuli, the endoplasmic reticulum releases its stored calcium ions, and then the mitochondria take up calcium ions, causing calcium overload and causing damage to the mitochondria. Mitochondrial damage will lead to the release of cytochrome c, triggering the formation of apoptosome, which activates Caspase-9, which in turn activates Caspase-3, the direct executor of apoptosis, resulting in neuronal cell apoptosis [32, 33]. Mitochondria are the power plants of most eukaryotes and also the only organelles containing DNA. Eighty to ninety percent of the energy required by cells comes from mitochondrial oxidative phosphorylation. The structure of mitochondria is divided into outer membrane, interstitial space, inner membrane, and matrix from outside to inside. The normal potential gradient between the structures is the basis for maintaining the normal function of mitochondria. Neurons can only obtain energy through aerobic metabolism of glucose consumption, and mitochondrial dysfunction will make neurons lack energy. Because of the complexity of brain function, the treatment of such diseases has been a difficult problem. With the development of nanomaterials and nanotechnology, it is believed that nanomaterials can penetrate the blood-brain barrier, assist in the diagnosis or treatment of NDD, and even participate in the improvement of patients’ motor symptoms and the regulation of nervous system functions. The following part will specifically illustrate the advantages and applications of nanomaterials from several typical NDDs.
3.4.1 AD
AD is considered to be one of the most common NDDs, which is an irreversible and progressive disease of the nervous system [35, 36]. At present, there is no effective way to improve symptoms and cure diseases. AD is characterized by the accumulation of proteins in the tangles and plaques of nerve fibers, the death of neurons and glial cells, and the impairment of cognitive function caused by aging or genetic mutations. In these disease states, tangles and plaque aggregates or other stimuli can lead to chronic inflammation. This neuroinflammation leads to the death and progression of disease of cells such as neurons, astrocytes, and oligodendrocytes. The main clinical symptoms of AD are learning and cognitive impairment and dementia [37,38,39]. In late stages of AD, patients will experience irritability, confusion, and behavioral changes [40, 41]. Amyloid plaques and neurofibrillary tangles (NFTs) are characteristic substances in the brain of AD patients [42,43,44]. The formation of amyloid plaques is due to the weakened metabolism of amyloid precursor proteins, and eventually the accumulation of these plaques leads to nerve damage [45]. Under physiological conditions, NFTs are pairs of helical filaments that support neuronal growth-related tau proteins. However, in the case of excessive phosphorylation, they are toxic to cells. The diffusible ligands and oligomers in toxic amyloid (Aβ) plaques are important factors that directly lead to neurotoxicity. The location and number of Aβ plaque deposition are directly related to the diagnosis of dementia and the number of neurons [27, 32,33,34, 46]. In the past, scientists believe that the breakthrough point in AD treatment lies in how to inhibit Aβ deposition and how to clear the already produced Aβ [47]. Based on the research on the mechanism of neurodegeneration, the concept of nerve cell protection has been put forward. There are three ways to protect nerve cells from degenerative changes: promoters that inhibit degenerative changes in nerve cells (such as microglia, nitric oxide), blocking the signal transduction process of degenerative changes of nerve cells, and activation of endogenous neuroprotective mechanisms (such as neurotrophic factors). With the deepening and complication of nanotechnology research in recent years, more possibilities for nanomaterials have been discovered and proposed, including the diagnosis, prevention, and treatment of AD [48, 49]. These nanomaterials, ranging in size from 6 to 100 nm, have significant advantages in preventing kidney excretion and crossing BBB. In addition, they also reduced the immune rejection of the host and greatly improved the biological safety of the material. Some nanomaterials realize their functions through specific binding with Aβ. In general, nanomaterials can be used as carriers to carry drugs across the BBB or as antioxidants and anti-apoptotic drugs to treat or prevent AD.
Tetrahedral DNA nanostructures (TDNs) are formed by four different DNAs (DNA, Table 3.1) based on the principle of basic complementary pairing (Fig. 3.3) [50,51,52,53,54,55,56,57]. In the past, it was found that drugs with neuroprotective or therapeutic effects on AD are harmful to nerve cells to a certain extent. From this aspect, TDNs have advantages in biosecurity and biocompatibility. What’s more, small size, special structure effect, and resistance to nuclease are also advantages of TDN [2, 56, 58,59,60,61,62,63,64,65,66,67,68]. Via upregulating ERK1/2 phosphorylation and activating ERK1/2 signaling pathway, TDNs have the potential to protect PC12 cells from Aβ25-35-induced apoptosis [57]. CCK-8 assay, flow cytometry, Western blot, real-time fluorescence quantitative PCR, immunofluorescence, and other techniques were used to verify that the nanomaterial can promote cell proliferation, inhibit apoptosis, restore nuclear morphology, and reduce intracellular reactive oxygen levels. Zhang and colleagues used polylactic acid-glycolic acid copolymer to deliver basic fibroblast growth factor to target Aβ oligomers in the brain. After specifically binding with Aβ, this nanomaterial assisted in the removal of Aβ [69]. Other nanomaterials including polyethylene glycol-AU and reconstituted high-density lipoprotein (rHDL) have similar effects as PEG-PLGA [47]. These nanomaterials with neuroprotective effects on AD have good biological safety and can cross the BBB.
The nanocomposite, called NC-KLVFF, is wrapped with protein molecules that contain a cross-linked Aβ-binding peptide (KLVFF) polymer layer prepared by in situ polymerization [2]. NC-KLVFF is able to significantly improve the morphology of Aβ polymer by forming nano-clusters. The nontoxic Aβ/NC-KLVFF complex, formed by NC-KLVFF and Aβ, can impact the aggregation of neurotoxic Aβ, leading to reduction of neurotoxicity of Aβ. Due to the decrease of Aβ oligomers, the inflammation and neuronal damage were also alleviated, and this had been proved in vivo. There are some other kinds of nanomaterials, including gold nanorod, apolipoprotein E3-reconstituted high-density lipoprotein ApoE3-rHDL, graphene oxide nanosheets, and poly(n-butylcyanoacrylate), which are also recognized as candidate for AD therapy and make an effect in three ways: (1) changing the morphology of Aβ oligomers and reducing the toxicity, (2) carrying drugs through the BBB and reaching the lesion, and (3) forming the sandwich structure with Aβ oligomers which is easier to be removed.
Regarding the application of nanomaterials in the diagnosis of NDD, it has been proposed that nanoscale diagnostic methods are very useful in detecting early Aβ oligomers. Nanoprobe is composed of magnetic nanostructure of MRI and Aβ oligomer antibody. After penetrating the BBB, it can effectively and selectively bind to the target. Finally, the detailed information of Aβ oligomers, including the location, size, and structure, can be detected by imaging techniques and further help to diagnose AD. Mirkin et al. applied nanomaterials as a DNA carrier to investigate the concentration of Aβ oligomers in the early cerebrospinal fluid (CSF) of AD patients. In brief, CSF was exposed to both gold nanoparticles to DNA-functionalized Aβ oligomer antibodies and magnetic nanoparticles bounding to Aβ oligomer antibodies. Subsequently, two kinds of antibodies specifically bind to Aβ oligomers, and a special sandwich complex used for Aβ oligomer detection was developed.
3.4.2 PD
The pathogenesis of PD is due to abnormal basal ganglia function, resulting in abnormal accumulation of Lewy bodies in the substantia nigra and the reduction of dopaminergic neurons [70,71,72]. PD, a motor disorder that eventually progresses to cognitive dissonance, also has an age and genetic basis, and protein aggregation is more complex than that of AD. Although most PD is idiopathic PD, some patients have known genetic mutations that complicate the search for new therapies. The exact cause of PD is unclear. It is currently believed that the degeneration and death of PD dopaminergic neurons can be related to genetic factors, environmental factors, oxidative stress, and aging [73,74,75,76]. Patients with PD will first experience tremor or awkward movements on one limb, which will further affect the contralateral limb. As the disease progresses, patients will experience clinical manifestations including static tremor, bradykinesia, stiffness, and postural gait disorders. Currently, drugs (levodopa preparation) are mainly used clinically as the main treatment for PD, but they can only slightly improve the symptoms and cannot prevent the progression of the disease [77]. Inhibition of neuronal apoptosis and abnormalities of α-synuclein are recognized as key to the treatment [72]. More and more nanomaterials have been designed for the diagnosis, prevention, and treatment of PD in recent years. These new nanomaterials can be used as drugs themselves or as carriers to carry drugs effectively into the brain through the blood-brain barrier, inhibit neuronal apoptosis, and reduce the accumulation of Lewy bodies, thereby preventing motor dysfunction and preventing the deterioration of the disease when compared with traditional drugs. In addition, magnetic nanomaterials can be used as an auxiliary means of MRI to diagnose PD early.
Tang et al. proposed a novel drug delivery system with few side effects, which improved the therapeutic effect [78]. They designed a nanoparticle to coat dopamine which was modified with borneol and lactoferrin (Lf-BNPs) and prepared by double emulsion solvent evaporation. This nanomaterial can promote the absorption of dopamine by SH-SY5Y, and it has low toxicity to cells after double modification. In addition, intranasal administration has been shown to effectively reduce striatum damage caused by 6-hydroxydopamine. Gan and his team designed a nanoparticle that has been shown to reduce pro-inflammatory cytokines and activate the B-cell pathway. This nanomaterial is prepared by coupling rabies virus glycoprotein (RVG29) [79]. Based on the effect of Zn on amyloid formation, Girigoswami used the human erythromycin amyloid model to compare the anti-amyloid capacity of ZnO nanoparticles (ZnO-NP) and ZnO nanoflowers (ZnO-NF) [80]. They designed a series of experiments and proved that ZnO-NF is more suitable for PC12 cell amyloid degradation than ZnO-NP due to the effect of surface ratio. In previous studies, deep brain stimulation (DBS) was considered an effective way to deal with PD. On this basis, many scientists are committed to applying nanomaterials and metal particles in this therapy. Xiao and colleagues modified the sensitive microelectrode with a nanocomposite of reduced graphene oxide and platinum nanoparticles (Pt/rGO) [81]. Microelectrode arrays (MEA) were used to monitor changes in dopamine concentration in real time after applying this modified nanomaterial to brain damage in PD animal models. Human-induced pluripotent stem cell (iPSC) transplantation has neuroprotective and repairing effects on PD. When combined with cyclosporin A (CsA), it can reduce the rejection of the host and improve the survival of iPSC. Yu et al. designed a nanoparticle of polylactic acid and glycolic acid containing cyclosporin and transplanted this nanocomposite together with iPSCs into the striatum of 6-hydroxydopamine-injured rats [82]. The measurement results of human marker Stem121 and the immunoreactivity of tyrosine hydroxylase (TH) indicated that the immune rejection of iPSC was greatly reduced after a month. Ahlawat et al. prepared a chitosan nano-molecule using particle gelation. This nanomaterial was subsequently demonstrated to have antioxidant and anti-apoptotic properties in the cell model of rotenone-induced PD [83].
The aforementioned new nanomaterials provide new possibilities for the application of nanomaterials in NDD, such as nanotechnology detection and partial discharge therapy. Nanoparticles have the advantages of size, shape, and charge, which can make them pass through BBB effectively. Combining these nanomaterials with certain traditional materials not only increases the ability of traditional drugs to penetrate the blood-brain barrier but also reduces host immune rejection caused by the new materials, achieving a win-win situation. In the next step, we will continue to explore the destination of metabolites of these new nanomaterials. Because whether they degrade or accumulate in the brain will adversely affect neurons.
3.4.3 HD
George Huntington first clearly described HD as an inherited NDDs [84]. It is a fatal neurodegenerative disorder characterized by mental, cognitive, and motor impairments. From the initial chromosomal localization to the detection of the Huntington protein gene, the genetic analysis of HD has been the leading study of hereditary neurological diseases. Studies have shown that an increase of more than 34 in CAG repeats leads to seizures in affected individuals. The mutant Huntington protein can accumulate, negatively affect mitochondrial function and metabolism, and inhibit the expression of brain-derived neurotrophic factor (BDNF) and other nutritional factors. Behavior disorder (delayed acquisition of motor skills), unconscious movement (dance disorder), and cognitive impairment (dementia) are the three major characteristics of HD [85]. Patients with HD were healthy and showed no signs before entering the symptoms. However, as the disease progresses and worsens, the patient may die due to serious complications such as inanition, fall, aspiration, restlessness, and difficulty swallowing [85]. The prevalence of HD was about 2.71 per 100,000 once reported in a systematic review [86]. The pathophysiological feature of HD is the abnormal CAG repeat amplification at the 5' end of the Huntington (Htt) gene (including extended polyglutamine extension). The gene is located at p 16.3 on chromosome 4, which causes the accumulation of abnormal unstable proteins [87]. The mutated Huntington protein can denature cells by altering the metabolism of neurons, causing damage to the striatum of the brain [88]. The severity of HD is closely related to the degree of repetition of polyglutamine sequence. Although the current research has not fully explained the mechanism of selective degeneration, it is generally believed to be closely related to inflammation, metabolic disorders, transcriptional disorders, and proteolytic changes [89].
Despite decades of progress made by clinicians, the treatment of HD still stops at improving symptoms. Tetrabenazine (TBZ), the only drug approved by the FDA to treat HD chorea, can inhibit vesicular monoamine transporter 2 (VMAT-2) [90]. However, when TBZ improves the symptoms of HD, it also brings serious side effects. Studies have shown that some participants who received TBZ treatment exhibited depression and suicidal behavior. Once use of TBZ is stopped, it may worsen chorea [91, 92]. In addition, there is currently no effective treatment that can improve cognitive symptoms. Depression is the most common psychotic symptom in HD. How to control refractory depression well is still a challenge.
The presence of mutant Htt protein is an important factor in the pathogenesis of HD, and silencing the expression of mutant Htt is considered to be one of the potential treatment methods for HD. Godinho synthesized a nanoparticle modified with β-cyclodextrin to send siRNA to the CNS. The study found that the expression level of mutant Htt protein in the experimental group using the nanomaterial was significantly reduced [93]. Debnath designed a new type of nanomaterial to successfully prevent the aggregation of mutant glutamine-containing Htt protein in neuronal cells. It consists of an ionic polymer shell of trehalose wrapped with an iron oxide core, which can reduce protein fibrillation in the extracellular space [94]. Peptide inhibitors (QBP1-QBP6, NT17, PGQ9P2, PGQ9P2,3, PGQ9P1,2,3, and P42) can inhibit abnormally aggregated polyglutamine and are therefore considered as one of the potential treatments for HD. In addition, Joshi and his colleagues verified the function of poly-D,L-lactide-co-glycolide nanoparticles loaded with PGQ9P2, QBP1, and NT17 in two classic cell models of HD and a Drosophila model [95]. Some researches designed some new kind of nanomaterials to eliminate the abnormal accumulation of Htt, which contains metal particles. Zhang found that MnFe2O4 nanoparticles can degrade mutant Htt protein through ubiquitin-proteasome system and reverse cell death in vitro. This material is coated with dextran as a surfactant to synthesize MnFe2O4 nanoparticles [96]. Ceccon designed nanomaterials capable of removing Htt protein targeting Met7 oxidation in the httNT domain, which is called TiO2 nanoparticle, and its surface has a catalytic oxidation effect [97].
Such nanomaterials containing metal particles do have the potential to inhibit or eliminate the accumulation of misfolded proteins. However, the solution of some problems needs more in-depth exploration. Specifically, the functions of some nanomaterials have only been verified in cells, and their ability to penetrate BBB and their effects on brain function need to be further verified by in vivo experiments. How to avoid the accumulation of metal particles and metabolites of the nanomaterials in the brain after the nanomaterials played their role, which affects safety, will be a difficult problem to be solved in the next step.
Solid lipid nanoparticles (SLNs) are colloidal carriers that can deliver hydrophobic drugs to the CNS. The advantages of using SLNs include no unpleasant odor or taste, the ability to cross BBB, and reduced dosage for efficient delivery [98]. Mitochondrial dysfunction is one of the important factors leading to HD, so a lot of research has focused on restoring mitochondrial function. Curcumin with anti-inflammatory and antioxidant functions was encapsulated in solid lipid nanoparticles (CSLN), and a new delivery system was established. The system was proved to be effective in treatment of HD animal model [98]. Thymoquinone (TQ), the main biologically active phytochemical component, is derived from the seeds of Nigella sativa. TQ has been shown to have the potential to be applied for many diseases. Ramachandran et al. wrapped TQ with SLNs and applied it to 3-NP-induced HD mouse model. It can be observed that the muscle strength and memory of HD mice after treatment are improved through the hook activity, space navigation task, forced swimming test, and string test [99]. They found that TQ-SLN can reduce NMDA receptor sensitization and resist neuroinflammation to relieve movement disorders. At the same time, it can also inhibit the activation of microglia [100]. Bhatt and his team synthesized SLN loaded with rosmarinic acid. The behavioral assessment of HD rats was significantly improved, when delivering the nanocomposite to the brain of Wistar rats by nasal administration [101].
Previous studies have found that cholesterol synthesis levels in HD mouse models are significantly reduced [102]. Thus, it is considered that cholesterol is one of the potential therapeutic targets of HD treatment due to the important role of cholesterol in physiologic function of cells. However, due to the effect of fat solubility, cholesterol cannot cross the BBB. Scientists thought that nanoparticles, wrapping cholesterol, can be new to overcome this difficulty. Valenza et al. designed a nanoparticle rich in cholesterol. After being injected into HD mice, the nanoparticles can significantly improve the cognitive function of mice [103]. Furthermore, Belletti et al. designed a nanoparticle called MIX-NPs, which can efficiently load cholesterol and can be absorbed by neuronal cells and release cholesterol in neuronal cells [104].
Current research supports the view that different treatments for HD are required at different stages. Thus, it is obvious that early diagnosis is particularly important throughout the whole treatment process. The combination of magnetic nanoparticles and imaging technology was introduced into the early diagnosis applications of HD. Liu and his team synthesized a nontoxic and PEGylated superparamagnetic oxidized nanoparticle (SPION), which contains amyloid oligomeric scFv antibody (W20). After intravenous injection of this composite material into the HD mouse model, it successfully provided signals for different focus areas and healthy areas [105]. Although the mechanism is unclear, numerous studies have illustrated a point that bone marrow mesenchymal stem cells (MSCs) have the potential to be used in the treatment of NDD [89]. In order to explore the specific mechanism, Moraes and his team proposed using SPION to label MSC. After applying SPIONs-MSCs to the HD rat model, it was observed that the behavior of mice was significantly improved and neurogenic brain damage was alleviated. The nanoparticles have great prospect in both treatment and diagnosis of HD. SPIONs-MSCs were detected in the HD lesion, shown by the results of MRI [106].
3.4.4 Other
Generally speaking, cerebral infarction (CI) is a cerebral tissue infarction caused by cerebral artery occlusion [107]. Huang et al. believed that embolization can be targeted by covalently binding magnetic nanoparticles in polyacrylic acid and tissue plasmin activator. In their research, they found that nanocomposites can reduce the area of CI mouse embolism caused by iron oxide and accelerate thrombolysis [108]. Mei and colleagues have designed a nanocomposite material that has been shown to inhibit the expansion of brain damage and reduce the area of cerebral infarction after brain injury. This material encapsulates tissue plasmid activators in self-assembled antioxidant nanoparticles [109]. So and colleagues first used cerebral artery occlusion (MCAO) to induce the construction of CI animal model and then encapsulated the acid salt in liposomes (LITA) and injected it into experimental animals [110]. MRI-assisted examination found that LITA reduced anterolateral ventricular (ALVv) [110]. The current research results show that that nanomaterials can play a role in assisting diagnosis and treatment, for example, imaging agents or carriers of small molecule drugs. However, it cannot be ignored that the metabolite of these nanomaterials is a problem of fate. This is also a problem to be solved in the research on nanomaterials in the future.
3.5 Conclusion
In conclusion, this review summarizes the latest applications and application prospects of nanomaterials in NDD. In the current research, some new nanomaterials have been developed as anti-apoptosis, antioxidant, and anti-inflammatory agents for NDD treatment. This type of material protects the vitality and function of nerve cells through anti-inflammatory and antioxidant properties and thus participates in the treatment of NDD. The accepted view is that the misfolding and abnormal aggregation of certain proteins lead to the occurrence of NDD. The original purpose of designing such nanomaterials was to treat NDD by inhibiting protein misfolding, reducing aggregation, or promoting clearance. In addition, some magnetic nanomaterials that can pass through the blood-brain barrier are good imaging agents and have the potential to detect and diagnose NDD with the help of MRI. In addition to the application and improvement of the ability to penetrate the blood-brain barrier, the application of nanomaterials in degenerative diseases is mainly focused on avoiding the accumulation of drugs in nontarget organs and host immune rejection. Although nanomaterials currently exhibit amazing advantages and potential in NDD applications, there are still some problems that need to be further explored and resolved. For example, it is not yet known whether the metabolites of nanomaterials will form polymers and accumulate in the brain, causing uncontrollable adverse consequences. At the same time, it is unknown whether these polymers are more likely to be intercepted by the BBB due to changes in the space organization. These problems require scientists to invest more time and energy to solve them, especially in the constantly evolving field of nanomaterials.
Abbreviations
- AD:
-
Alzheimer’s disease
- ALS:
-
Amyotrophic lateral sclerosis
- ALVv:
-
Anterolateral ventricular volume
- BBB:
-
Blood-brain barrier
- CI:
-
Cerebral infarction
- CsA:
-
Cyclosporin A
- CSF:
-
Cerebrospinal fluid
- HD:
-
Huntington’s disease
- Htt:
-
Huntingtin
- IPSCs:
-
Human-induced pluripotent stem cells
- LITA:
-
Liposome nanoparticles
- MCAO:
-
Middle cerebral artery occlusion
- MEA:
-
Microelectrode array
- MSC:
-
Mesenchymal stem cells
- NDD:
-
Neurodegenerative diseases
- NFTs:
-
Neurofibrillary tangles
- NanoCsA:
-
Nanoparticle cyclosporin A
- PD:
-
Parkinson’s disease
- PEG-AU:
-
Polyethylene glycol-AU
- PEG-PLGA:
-
Poly(lactic-co-glycolic acid)
- PLGA:
-
Poly-D,L-lactide-co-glycolide
- rHDL:
-
Reconstituted high-density lipoprotein
- SLNs:
-
Solid lipid nanoparticles
- SPIONs:
-
PEGylated superparamagnetic iron oxide nanoparticles
- TBZ:
-
Tetrabenazine
- TDNs:
-
Tetrahedral DNA nanostructures
- VMAT-2:
-
Vesicular monoamine transporter 2
- TH:
-
Tyrosine hydroxylase
- TQ:
-
Thymoquinone
- ZnO-NF:
-
ZnO nanoflowers
- ZnO-NP:
-
ZnO nanoparticles
References
Kritsilis M, Koutsoudaki PN, Evangelou K, Gorgoulis VG, Papadopoulos D. Ageing, cellular senescence and neurodegenerative disease. Int J Mol Sci. 2018;19:10.
Zhao Y, Cai J, Liu Z, Li Y, Zheng C. Nanocomposites inhibit the formation, mitigate the neurotoxicity, and facilitate the removal of beta-amyloid aggregates in Alzheimer’s disease mice. Nano Lett. 2019;19(2):674–83.
Daneman R, Prat A. The blood-brain barrier. Cold Spring Harb Perspect Biol. 2015;7(1):a020412.
Furtado D, Bjornmalm M, Ayton S, Bush AI, Kempe K, Caruso F. Overcoming the blood-brain barrier: the role of nanomaterials in treating neurological diseases. Adv Mater. 2018;30(46):e1801362.
Huo S, Li H, Boersma AJ, Herrmann A. DNA nanotechnology enters cell membranes. Adv Sci. 2019;6(10):1900043.
Atluri R, Jensen KA. Engineered nanomaterials: their physicochemical characteristics and how to measure them. Adv Exp Med Biol. 2017;947:3–23.
Rasmussen K, Rauscher H, Mech A, Riego Sintes J, Gilliland D, Gonzalez M, et al. Physico-chemical properties of manufactured nanomaterials - characterisation and relevant methods. An outlook based on the OECD testing programme. Regul Toxicol Pharmacol. 2018;92:8–28.
Singh MR. Application of metallic nanomaterials in nanomedicine. Adv Exp Med Biol. 2018;1052:83–102.
Stover PJ, Durga J, Field MS. Folate nutrition and blood-brain barrier dysfunction. Curr Opin Biotechnol. 2017;44:146–52.
Obermeier B, Verma A, Ransohoff RM. The blood-brain barrier. Handb Clin Neurol. 2016;133:39–59.
Serlin Y, Shelef I, Knyazer B, Friedman A. Anatomy and physiology of the blood-brain barrier. Semin Cell Dev Biol. 2015;38:2–6.
Rahman NA, Rasil A, Meyding-Lamade U, Craemer EM, Diah S, Tuah AA, et al. Immortalized endothelial cell lines for in vitro blood-brain barrier models: a systematic review. Brain Res. 1642;2016:532–45.
Abbott NJ, Ronnback L, Hansson E. Astrocyte-endothelial interactions at the blood-brain barrier. Nat Rev Neurosci. 2006;7(1):41–53.
Abbott NJ, Patabendige AA, Dolman DE, Yusof SR, Begley DJ. Structure and function of the blood-brain barrier. Neurobiol Dis. 2010;37(1):13–25.
Chakraborty A, de Wit NM, van der Flier WM, de Vries HE. The blood brain barrier in Alzheimer’s disease. Vasc Pharmacol. 2017;89:12–8.
Patching SG. Glucose transporters at the blood-brain barrier: function, regulation and gateways for drug delivery. Mol Neurobiol. 2017;54(2):1046–77.
Pozhilenkova EA, Lopatina OL, Komleva YK, Salmin VV, Salmina AB. Blood-brain barrier-supported neurogenesis in healthy and diseased brain. Rev Neurosci. 2017;28(4):397–415.
Jakki SL, Senthil V, Yasam VR, Chandrasekar MJN, Vijayaraghavan C. The blood brain barrier and its role in Alzheimer’s therapy: an overview. Curr Drug Targets. 2018;19(2):155–69.
Cai Z, Qiao PF, Wan CQ, Cai M, Zhou NK, Li Q. Role of blood-brain barrier in Alzheimer’s disease. JADA. 2018;63(4):1223–34.
Haley MJ, Lawrence CB. The blood-brain barrier after stroke: structural studies and the role of transcytotic vesicles. J Cereb Blood Flow Metab. 2017;37(2):456–70.
Tsou YH, Zhang XQ, Zhu H, Syed S, Xu X. Drug delivery to the brain across the blood-brain barrier using nanomaterials. Small. 2017;13:43.
Pardridge WM. CSF, blood-brain barrier, and brain drug delivery. Expert Opin Drug Deliv. 2016;13(7):963–75.
Grabrucker AM, Ruozi B, Belletti D, Pederzoli F, Forni F, Vandelli MA, et al. Nanoparticle transport across the blood brain barrier. Tissue Barriers. 2016;4(1):e1153568.
Nair KGS, Ramaiyan V, Sukumaran SK. Enhancement of drug permeability across blood brain barrier using nanoparticles in meningitis. Inflammopharmacology. 2018;26(3):675–84.
Wilson B, Samanta MK, Santhi K, Kumar KP, Paramakrishnan N, Suresh B. Poly(n-butylcyanoacrylate) nanoparticles coated with polysorbate 80 for the targeted delivery of rivastigmine into the brain to treat Alzheimer's disease. Brain Res. 2008;1200:159–68.
Ramanathan S, Archunan G, Sivakumar M, Tamil Selvan S, Fred AL, Kumar S, et al. Theranostic applications of nanoparticles in neurodegenerative disorders. Int J Nanomedicine. 2018;13:5561–76.
Vucic S, Kiernan MC. Transcranial magnetic stimulation for the assessment of neurodegenerative disease. Neurotherapeutics. 2017;14(1):91–106.
Gitler AD, Dhillon P, Shorter J. Neurodegenerative disease: models, mechanisms, and a new hope. Dis Model Mech. 2017;10(5):499–502.
Seeley WW. Mapping neurodegenerative disease onset and progression. Cold Spring Harb Perspect Biol. 2017;9:8.
Veldsman M, Egorova N. Advances in neuroimaging for neurodegenerative disease. Adv Neurobiol. 2017;15:451–78.
Poovaiah N, Davoudi Z, Peng H, Schlichtmann B, Mallapragada S, Narasimhan B, et al. Treatment of neurodegenerative disorders through the blood-brain barrier using nanocarriers. Nanoscale. 2018;10(36):16962–83.
Kim SH, Noh MY, Kim HJ, Oh KW, Park J, Lee S, et al. A therapeutic strategy for Alzheimer’s disease focused on immune-inflammatory modulation. Dement Neurocog Disorders. 2019;18(2):33–46.
Kamat PK, Kalani A, Rai S, Swarnkar S, Tota S. Mechanism of oxidative stress and synapse dysfunction in the pathogenesis of Alzheimer’s disease: understanding the therapeutics strategies. Mol Neurobiol. 2016;53(1):648–61.
Gutierrez IL, Gonzalez-Prieto M, Caso JR, Garcia-Bueno B, Leza JC, Madrigal JLM. Reboxetine treatment reduces neuroinflammation and neurodegeneration in the 5xFAD mouse model of Alzheimer’s disease: role of CCL2. Mol Neurobiol. 2019;19:01695.
Lane CA, Hardy J, Schott JM. Alzheimer’s disease. Eur J Neurol. 2018;25(1):59–70.
Vilasi S, Carrotta R, Ricci C, Rappa GC, Librizzi F. Inhibition of Abeta1-42 fibrillation by chaperonins: human Hsp60 is a stronger inhibitor than its bacterial homologue GroEL. ACS Chem Neurosci. 2019;10(8):3565–74.
Heupel-Reuter M, Kloppel S, Bauer JM, Voigt-Radloff S. Pharmacological interventions for apathy in Alzheimer’s disease. Z Gerontol Geriatr. 2019;52(5):457–9.
Ibrahim WW, Abdelkader NF, Ismail HM, Khattab MM. Escitalopram ameliorates cognitive impairment in D-galactose-injected ovariectomized rats: modulation of JNK, GSK-3beta, and ERK signalling pathways. Sci Rep. 2019;9(1):10056.
Jia J, Hu J, Huo X, Miao R, Zhang Y, Ma F. Effects of vitamin D supplementation on cognitive function and blood Abeta-related biomarkers in older adults with Alzheimer’s disease: a randomised, double-blind, placebo-controlled trial. J Neurol Neurosurg Psychiatry. 2019;90(12):1347–52.
Kang MJ, Kim SM, Han SE, Bae JH, Yu WJ. Effect of paper-based cognitive training in early stage of Alzheimer’s dementia. Dement Neurocog Disorders. 2019;18(2):62–8.
Bernard K, Gouttefangeas S, Bretin S, Galtier S, Robert P. A 24-week double-blind placebo-controlled study of the efficacy and safety of the AMPA modulator S47445 in patients with mild to moderate Alzheimer’s disease and depressive symptoms. Alzheimers Dement. 2019;5:231–40.
Tse KH, Herrup K. Re-imagining Alzheimer’s disease - the diminishing importance of amyloid and a glimpse of what lies ahead. J Neurochem. 2017;143(4):432–44.
Qiang W, Yau WM, Lu JX, Collinge J, Tycko R. Structural variation in amyloid-beta fibrils from Alzheimer’s disease clinical subtypes. Nature. 2017;541(7636):217–21.
Rajasekhar K, Chakrabarti M, Govindaraju T. Function and toxicity of amyloid beta and recent therapeutic interventions targeting amyloid beta in Alzheimer’s disease. Chem Commun. 2015;51(70):13434–50.
Wang Y, Guan X, Chen X, Cai Y, Ma Y. Choline supplementation ameliorates behavioural deficits and Alzheimer’s disease-like pathology in transgenic APP/PS1 mice. Mol Nutr Food Res. 2019;63:e1801407.
Tonnies E, Trushina E. Oxidative stress, synaptic dysfunction, and Alzheimer’s disease. JADA. 2017;57(4):1105–21.
Hajipour MJ, Santoso MR, Rezaee F, Aghaverdi H, Mahmoudi M, Perry G. Advances in Alzheimer’s diagnosis and therapy: the implications of nanotechnology. Trends Biotechnol. 2017;35(10):937–53.
Li Y, Li Y, Ji W, Lu Z, Liu L. Positively charged polyprodrug amphiphiles with enhanced drug loading and reactive oxygen species-responsive release ability for traceable synergistic therapy. J Am Chem Soc. 2018;140(11):4164–71.
Luo Q, Lin YX, Yang PP, Wang Y, Qi GB, Qiao ZY, et al. A self-destructive nanosweeper that captures and clears amyloid beta-peptides. Nat Commun. 2018;9(1):1802.
Li Q, Zhao D, Shao X, Lin S, Xie X. Aptamer-modified tetrahedral DNA nanostructure for tumor-targeted drug delivery. ACS Appl Mater Interfaces. 2017;9(42):36695–701.
Shi S, Lin S, Shao X, Li Q, Tao Z, Lin Y. Modulation of chondrocyte motility by tetrahedral DNA nanostructures. Cell Prolif. 2017;50:5.
Tian T, Zhang T, Zhou T, Lin S, Shi S, Lin Y. Synthesis of an ethyleneimine/tetrahedral DNA nanostructure complex and its potential application as a multi-functional delivery vehicle. Nanoscale. 2017;9(46):18402–12.
Lin S, Zhang Q, Zhang T, Shao X, Li Y. Tetrahedral DNA nanomaterial regulates the biological behaviors of adipose-derived stem cells via DNA methylation on Dlg3. ACS Appl Mater Interfaces. 2018;10(38):32017–25.
Ma W, Shao X, Zhao D, Li Q, Liu M. Self-assembled tetrahedral DNA nanostructures promote neural stem cell proliferation and neuronal differentiation. ACS Appl Mater Interfaces. 2018;10(9):7892–900.
Ma W, Xie X, Shao X, Zhang Y, Mao C, Zhan Y, et al. Tetrahedral DNA nanostructures facilitate neural stem cell migration via activating RHOA/ROCK2 signalling pathway. Cell Prolif. 2018;51(6):e12503.
Liu M, Ma W, Li Q, Zhao D, Shao X, Huang Q, et al. Aptamer-targeted DNA nanostructures with doxorubicin to treat protein tyrosine kinase 7-positive tumours. Cell Prolif. 2019;52(1):e12511.
Shao X, Ma W, Xie X, Li Q, Lin S. Neuroprotective effect of tetrahedral DNA nanostructures in a cell model of Alzheimer's disease. ACS Appl Mater Interfaces. 2018;10(28):23682–92.
Shi S, Lin S, Li Y, Zhang T, Shao X. Effects of tetrahedral DNA nanostructures on autophagy in chondrocytes. Chem Commun. 2018;54(11):1327–30.
Zhang Q, Lin S, Shi S, Zhang T, Ma Q. Anti-inflammatory and antioxidative effects of tetrahedral DNA nanostructures via the modulation of macrophage responses. ACS Appl Mater Interfaces. 2018;10(4):3421–30.
Zhang Y, Ma W, Zhu Y, Shi S, Li Q. Inhibiting methicillin-resistant Staphylococcus aureus by tetrahedral DNA nanostructure-enabled antisense peptide nucleic acid delivery. Nano Lett. 2018;18(9):5652–9.
Zhao D, Liu M, Li Q, Zhang X, Xue C. Tetrahedral DNA nanostructure promotes endothelial cell proliferation, migration, and angiogenesis via notch signaling pathway. ACS Appl Mater Interfaces. 2018;10(44):37911–8.
Zhou M, Liu NX, Shi SR, Li Y, Zhang Q. Effect of tetrahedral DNA nanostructures on proliferation and osteo/odontogenic differentiation of dental pulp stem cells via activation of the notch signaling pathway. Nanomedicine. 2018;14(4):1227–36.
Mao C, Pan W, Shao X, Ma W, Zhang Y. The clearance effect of tetrahedral DNA nanostructures on senescent human dermal fibroblasts. ACS Appl Mater Interfaces. 2019;11(2):1942–50.
Xie X, Shao X, Ma W, Zhao D, Shi S. Overcoming drug-resistant lung cancer by paclitaxel loaded tetrahedral DNA nanostructures. Nanoscale. 2018;10(12):5457–65.
Ge Y, Tian T, Shao XR, Lin SY, Zhang T. PEGylated protamine-based adsorbing improves the biological properties and stability of tetrahedral framework nucleic acids. ACS Appl Mater Interfaces. 2019;11(31):27588–97.
Liu N, Zhang X, Li N, Zhou M, Zhang T, Li S, et al. Tetrahedral framework nucleic acids promote corneal epithelial wound healing in vitro and in vivo. Small. 2019;15:e1901907.
Meng L, Ma W, Lin S, Shi S, Li Y, Lin Y. Tetrahedral DNA nanostructure-delivered DNAzyme for gene silencing to suppress cell growth. ACS Appl Mater Interfaces. 2019;11(7):6850–7.
Zhou M, Liu N, Zhang Q, Tian T, Ma Q, Zhang T, et al. Effect of tetrahedral DNA nanostructures on proliferation and osteogenic differentiation of human periodontal ligament stem cells. Cell Prolif. 2019;52(3):e12566.
Zhang C, Wan X, Zheng X, Shao X, Liu Q. Dual-functional nanoparticles targeting amyloid plaques in the brains of Alzheimer’s disease mice. Biomaterials. 2014;35(1):456–65.
Xuan M, Guan X, Huang P, Shen Z, Gu Q. Different patterns of gray matter density in early- and middle-late-onset Parkinson's disease: a voxel-based morphometry study. Brain Imaging Behav. 2019;13(1):172–9.
Filippini A, Gennarelli M, Russo I. Alpha-synuclein and Glia in Parkinson’s disease: a beneficial or a detrimental duet for the endo-lysosomal system? Cell Mol Neurobiol. 2019;39(2):161–8.
Chondrogiorgi M, Astrakas LG, Zikou AK, Weis L, Xydis VG. Multifocal alterations of white matter accompany the transition from normal cognition to dementia in Parkinson’s disease patients. Brain Imaging Behav. 2019;13(1):232–40.
Zhu YL, Sun MF, Jia XB, Cheng K, Xu YD. Neuroprotective effects of Astilbin on MPTP-induced Parkinson’s disease mice: glial reaction, alpha-synuclein expression and oxidative stress. Int Immunopharmacol. 2019;66:19–27.
Haghshomar M, Rahmani F, Hadi Aarabi M, Shahjouei S, Sobhani S, Rahmani M. White matter changes correlates of peripheral neuroinflammation in patients with Parkinson’s disease. Neuroscience. 2019;403:70–8.
Chahine LM, Dos Santos C, Fullard M, Scordia C, Weintraub D, Erus G, et al. Modifiable vascular risk factors, white matter disease and cognition in early Parkinson’s disease. Eur J Neurol. 2019;26(2):246.
Calderon-Garciduenas L, Reynoso-Robles R, Gonzalez-Maciel A. Combustion and friction-derived nanoparticles and industrial-sourced nanoparticles: the culprit of Alzheimer and Parkinson’s diseases. Environ Res. 2019;176:108574.
Dogan B, Akyol A, Memis CO, Sair A, Akyildiz U, Sevincok L. The relationship between temperament and depression in Parkinson’s disease patients under dopaminergic treatment. Psychogeriatrics. 2019;19(1):73–9.
Tang S, Wang A, Yan X, Chu L, Yang X. Brain-targeted intranasal delivery of dopamine with borneol and lactoferrin co-modified nanoparticles for treating Parkinson’s disease. Drug Deliv. 2019;26(1):700–7.
Gan L, Li Z, Lv Q, Huang W. Rabies virus glycoprotein (RVG29)-linked microRNA-124-loaded polymeric nanoparticles inhibit neuroinflammation in a Parkinson’s disease model. Int J Pharm. 2019;567:118449.
Girigoswami A, Ramalakshmi M, Akhtar N, Metkar SK, Girigoswami K. ZnO Nanoflower petals mediated amyloid degradation - an in vitro electrokinetic potential approach. Mater Sci Eng C. 2019;101:169–78.
Xiao G, Song Y, Zhang Y, Xing Y, Zhao HY, Xie J, et al. Microelectrode arrays modified with nanocomposites for monitoring dopamine and spike firings under deep brain stimulation in rat models of Parkinson’s disease. ACS Sens. 2019;4:1992–2000.
Yu SJ, Wang YC, Chang CY, Hsieh W, Chen S, Yang CS, et al. NanoCsA improves the survival of human iPSC transplant in hemiparkinsonian rats. Brain Res. 1719;2019:124–32.
Ahlawat J, Deemer EM, Narayan M. Chitosan nanoparticles rescue rotenone-mediated cell death. Mater Ther. 2019;12:7.
Huntington G. On chorea. George Huntington, MD. J Neuropsychiatry Clin Neurosci. 2003;15(1):109–12.
Walker FO. Huntington’s disease. Lancet. 2007;369(9557):218–28.
Pringsheim T, Wiltshire K, Day L, Dykeman J, Steeves T, Jette N. The incidence and prevalence of Huntington’s disease: a systematic review and meta-analysis. Mov Disord. 2012;27(9):1083–91.
The Huntington’s Disease Collaborative Research Group. A novel gene containing a trinucleotide repeat that is expanded and unstable on Huntington’s disease chromosomes. Cell. 1993;72(6):971–83.
Tellone E, Galtieri A, Ficarra S. Reviewing the biochemical implications of normal and mutated huntingtin in Huntington’s disease. Curr Med Chem. 2019;26:31.
Lee JK, Jin HK, Endo S, Schuchman EH, Carter JE, Bae JS. Intracerebral transplantation of bone marrow-derived mesenchymal stem cells reduces amyloid-beta deposition and rescues memory deficits in Alzheimer’s disease mice by modulation of immune responses. Stem Cells. 2010;28(2):329–43.
Tetrabenazine as antichorea therapy in Huntington disease: a randomized controlled trial. Neurology. 2006;66(3):366–72.
Frank S. Tetrabenazine as anti-chorea therapy in Huntington disease: an open-label continuation study. BMC Neurol. 2009;9:62.
Shen V, Clarence-Smith K, Hunter C, Jankovic J. Safety and efficacy of tetrabenazine and use of concomitant medications during long-term, open-label treatment of chorea associated with Huntington’s and other diseases. Tremor. 2013;3:4337.
Godinho BM, Ogier JR, Darcy R, O'Driscoll CM, Cryan JF. Self-assembling modified beta-cyclodextrin nanoparticles as neuronal siRNA delivery vectors: focus on Huntington’s disease. Mol Pharm. 2013;10(2):640–9.
Debnath K, Pradhan N, Singh BK, Jana NR, Jana NR. Poly(trehalose) nanoparticles prevent amyloid aggregation and suppress polyglutamine aggregation in a Huntington’s disease model mouse. ACS Appl Mater Interfaces. 2017;9(28):24126–39.
Joshi AS, Singh V, Gahane A, Thakur AK. Biodegradable nanoparticles containing mechanism based peptide inhibitors reduce polyglutamine aggregation in cell models and alleviate motor symptoms in a drosophila model of Huntington’s disease. ACS Chem Neurosci. 2019;10(3):1603–14.
Zhang L, Wei PF, Song YH, Dong L, Wu YD, Hao ZY, et al. MnFe2O4 nanoparticles accelerate the clearance of mutant huntingtin selectively through ubiquitin-proteasome system. Biomaterials. 2019;216:119248.
Ceccon A, Tugarinov V, Clore GM. TiO2 nanoparticles catalyze oxidation of Huntingtin exon 1-derived peptides impeding aggregation: a quantitative NMR study of binding and kinetics. J Am Chem Soc. 2019;141(1):94–7.
Sandhir R, Yadav A, Mehrotra A, Sunkaria A, Singh A, Sharma S. Curcumin nanoparticles attenuate neurochemical and neurobehavioral deficits in experimental model of Huntington’s disease. NeuroMolecular Med. 2014;16(1):106–18.
Ramachandran S, Thangarajan S. A novel therapeutic application of solid lipid nanoparticles encapsulated thymoquinone (TQ-SLNs) on 3-nitroproponic acid induced Huntington’s disease-like symptoms in wistar rats. Chem Biol Interact. 2016;256:25–36.
Ramachandran S, Thangarajan S. Thymoquinone loaded solid lipid nanoparticles counteracts 3-Nitropropionic acid induced motor impairments and neuroinflammation in rat model of Huntington’s disease. Metab Brain Dis. 2018;33(5):1459–70.
Bhatt R, Singh D, Prakash A, Mishra N. Development, characterization and nasal delivery of rosmarinic acid-loaded solid lipid nanoparticles for the effective management of Huntington’s disease. Drug Deliv. 2015;22(7):931–9.
Valenza M, Chen JY, Di Paolo E, Ruozi B, Belletti D, Ferrari Bardile C, et al. Cholesterol-loaded nanoparticles ameliorate synaptic and cognitive function in Huntington’s disease mice. EMBO Mol Med. 2015;7(12):1547–64.
Valenza M, Marullo M, Di Paolo E, Cesana E, Zuccato C, Biella G, et al. Disruption of astrocyte-neuron cholesterol cross talk affects neuronal function in Huntington's disease. Cell Death Differ. 2015;22(4):690–702.
Belletti D, Grabrucker AM, Pederzoli F, Menrath I, Vandelli MA, Tosi G, et al. Hybrid nanoparticles as a new technological approach to enhance the delivery of cholesterol into the brain. Int J Pharm. 2018;543(1-2):300–10.
Liu XG, Lu S, Liu DQ, Zhang L, Zhang LX, Yu XL, et al. ScFv-conjugated superparamagnetic iron oxide nanoparticles for MRI-based diagnosis in transgenic mouse models of Parkinson's and Huntington's diseases. Brain Res. 1707;2019:141–53.
Moraes L, Vasconcelos-dos-Santos A, Santana FC, Godoy MA, Rosado-de-Castro PH, et al. Neuroprotective effects and magnetic resonance imaging of mesenchymal stem cells labeled with SPION in a rat model of Huntington's disease. Stem Cell Res. 2012;9(2):143–55.
Kaviarasi S, Yuba E, Harada A, Krishnan UM. Emerging paradigms in nanotechnology for imaging and treatment of cerebral ischemia. J Control Release. 2019;300:22–45.
Huang L, Wang J, Huang S, Siaw-Debrah F, Nyanzu M, Zhuge Q. Polyacrylic acid-coated nanoparticles loaded with recombinant tissue plasminogen activator for the treatment of mice with ischemic stroke. Biochem Biophys Res Commun. 2019;516(2):565–70.
Mei T, Kim A, Vong LB, Marushima A, Puentes S. Encapsulation of tissue plasminogen activator in pH-sensitive self-assembled antioxidant nanoparticles for ischemic stroke treatment - synergistic effect of thrombolysis and antioxidant. Biomaterials. 2019;215:119209.
So PW, Ekonomou A, Galley K, Brody L, Sahuri-Arisoylu M. Intraperitoneal delivery of acetate-encapsulated liposomal nanoparticles for neuroprotection of the penumbra in a rat model of ischemic stroke. Int J Nanomedicine. 2019;14:1979–91.
Acknowledgments
This work was supported by the Sichuan Science and Technology Program (2019YFS0400) and the National Natural Science Foundation of China (81671031, 81470721).
Consent for Publication List of Abbreviations
Not applicable.
Conflict of Interest
The authors declare no conflict of interest, financial or otherwise.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2021 The Author(s), under exclusive license to Springer Nature Singapore Pte Ltd.
About this chapter
Cite this chapter
Cui, W., Fu, W., Zhang, T., Zhou, R., Zhang, T., Lin, Y. (2021). Application of Nanomaterials in Neurodegenerative Diseases. In: Lin, Y., Zhou, R. (eds) Advances in Nanomaterials-based Cell Biology Research. Springer, Singapore. https://doi.org/10.1007/978-981-16-2666-1_3
Download citation
DOI: https://doi.org/10.1007/978-981-16-2666-1_3
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-16-2665-4
Online ISBN: 978-981-16-2666-1
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)