Abstract
Biofilms are defined as aggregates of microorganisms encased in a matrix of extracellular polymeric substances that are attached to a surface. Biofilms can form quickly in food industry environments, which results in serious hygienic problems and significant economic losses. Over the past decade, cold plasma, as a novel nonthermal technology, has shown great potential for safe and sustainable food production, including food decontamination, shelf-life extension, removal of toxins, and degradation of pesticides. This chapter presents an overview of the application of cold plasma for inactivation of microbial biofilms in vitro or on food products in detail. The factors affected the antibiofilm efficacy of cold plasma are well reviewed, including the characteristics of plasma generation, processing parameters of cold plasma, properties of microbial biofilms, and characteristics of the tested samples. The synergistic effect of reactive species, charged particles, UV emission, and electromagnetic fields are responsible for the antibiofilm efficacy of cold plasma. In addition, the synergistic antibiofilm effects of cold plasma combined with other hurdle strategies (such as plant essential oils, disinfecting agents, and chelating agents) are also well reviewed. The perspectives, research needs, and challenges in applying cold plasma for biofilms control in food industry are also discussed in this chapter.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
4.1 Biofilms in the Food Industry
Microbes such as bacteria, molds, yeasts, and viruses represent major threats for public health and food safety. In most natural environments, microorganisms predominantly exist as communities of sessile cells that develop as biofilms, which is generally recognized as a major threat causing infectious diseases and economic losses (Alvarez-Ordonez et al. 2019; Srey et al. 2013; Yuan et al. 2020).
4.1.1 Overview of Biofilms
As defined by the International Union of Pure and Applied Chemistry (IUPAC), biofilms are referred to as “aggregates of microorganisms in which cells are frequently embedded in a self-produced matrix of extracellular polymeric substances (EPS) that are adherent to each other and/or a surface” (Vert et al. 2012). Microorganisms generally can exist in biofilms on a wide variety of biotic and abiotic surfaces, such as living tissues, medical devices, industrial settings, water system piping, or natural aquatic systems (Donlan 2002). Biofilms may be composed of a single species or a mixture of many microbial species. Single-species biofilms in vitro have been extensively studied. However, most biofilms in nature are generally formed by a mixture of microbial species, including bacteria, fungi, yeasts, viruses (phages), algae, and protozoa Davey and O’toole 2000; Miquel et al. 2016). As compared with planktonic counterparts, microbial cells embedded in biofilms show enhanced resistance to stressful environmental conditions, e.g., antimicrobial agents, oxidative stress, desiccation, nutrient starvation, UV radiation, extreme temperature and pH, high salinity, and high pressure (Bridier et al. 2015; Flemming et al. 2016; Yin et al. 2019).
4.1.2 Biofilms in the Food Industry
Microbial biofilms are considered as potential sources of contamination and represent one of the main problems in a wide range of food industries, e.g., fresh produce, brewing, seafood processing, dairy processing, and meat processing (Srey et al. 2013; Yuan et al. 2020). Generally, foods provide a favorable environment for the growth and replication of most microorganisms. Various pathogenic and spoilage microorganisms can attach to food products and form biofilms on the surfaces (Srey et al. 2013). Enterobacter spp., Listeria spp., Salmonella spp., Bacillus spp., Vibrio spp., Aeromonas spp., Campylobacter spp., and psychrotrophic Pseudomonas spp. are the most common foodborne biofilm producers in the food industry (Galié et al. 2018; Srey et al. 2013).
Food contact surfaces are recognized as a major source of microbial contamination of food products during production, processing, transportation, packaging, and storage (Srey et al. 2013). Food contact surfaces refer to all surfaces that may contact with food products during the processing and packaging, such as cutting tools (e.g., knives and cutting boards), utensils, stockpots, prep tables, conveyor belts, and packaging material (Galié et al. 2018; Saad et al. 2019). These surfaces are typically made of a range of materials, such as stainless steel, plastic, wood, rubber, glass, and ceramics (Aviat et al. 2016). During food production and processing, microorganisms may adhere to the food contact surfaces and form biofilms, subsequently leading to cross-contamination in the food industry.
Biofilm-associated microorganisms may adversely affect the safety and quality of food products and result in the outbreaks of foodborne diseases (Srey et al. 2013; Yuan et al. 2020). In addition, biofilms are responsible for many problems in food production, such as equipment corrosion, reduced heat transfer efficiency, increased energy consumption, and blocked membrane pores (Alvarez-Ordonez et al. 2019; Poulsen 1999; Yuan et al. 2020). Thus, appropriate methods should be applied for the prevention of biofilm formation or biofilm removal in the food industry. However, biofilms are more resistant to antimicrobials compared to planktonic cells; it is a great challenge to make their elimination from food processing facilities.
4.1.3 Control Strategies for Biofilms
Some conventional strategies have been widely used for the control of biofilms in the food industry, e.g., chemical cleaning, enzymatic disruption, and steel coatings (Galié et al. 2018). For example, chemical disinfectants such as sodium hypochlorite, sodium hydroxide solutions, hydrogen peroxide, and peracetic acid are widely used for eradicating different kinds of microorganisms in food processing environments including wash water and food contact surfaces. However, the potential toxic effects of these chemical sanitizers have received great attention from the public in the last decades. Some toxic disinfection by-products (DBPs) such as trihalomethanes (THMs) and haloacetic acids (HAAs) are formed from reactions of natural organic matter with chlorine-based compounds and other disinfectants, which pose potential health risks to humans (de Castro et al. 2019; Hung et al. 2017). In the past few years, some novel technologies have been developed for the control of microbial biofilms in food processing, such as cold plasma, high hydrostatic pressure, ultrasound, high-pressure carbon dioxide, and bacteriophage (Erriu et al. 2014; Furukawa 2015; Galié et al. 2018).
4.2 Antibiofilm Activity of Cold Plasma
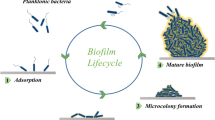
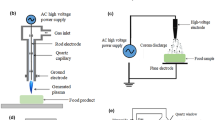
Cold plasma is a novel nonthermal food processing technology, which has shown great application in the food industry, e.g., inactivation of microorganisms, enzyme inactivation, removal of toxins, degradation of pesticides, shelf-life extension, and packaging modifications (Pan et al. 2019; Pankaj et al. 2018). The engineering principles of cold plasma is well reviewed in Chap. 1. In the past few years, the potential application of cold plasma in the control of microbial biofilms has attracted increasing attention (Fig. 4.1) (Gilmore et al. 2018; Gupta and Ayan 2019; Rao et al. 2020; Zhu et al. 2020).
Schematics of (a) DBD plasma and (b) atmospheric pressure plasma jet for biofilm eradication and control. (Adapted from Gilmore BF, Flynn PB, O’Brien S, et al. (2018) Cold plasmas for biofilm control: Opportunities and challenges. Trends Biotechnol 36(6): 627–638. Copyright (2020) with permission from Elsevier)
4.2.1 In Vitro Antibiofilm Activity of Cold Plasma
Several different types of cold plasma have been developed for food and biomedical application. Today, electrical discharge techniques have been commonly utilized to produce nonthermal plasma at atmospheric pressure, including dielectric barrier discharge (DBD), corona discharge (CD), atmospheric pressure plasma jet (APPJ), gliding arc discharge (GAD), resistive-barrier discharge (RBD), surface micro-discharge (SMD), floating electrode-dielectric barrier discharge (FE-DBD), radio-frequency discharge (RFD), plasma needle, and plasma pencil (Hoffmann et al. 2013; Sakudo et al. 2019). The application of cold plasma for in vitro biofilm inactivation is summarized in Table 4.1.
4.2.2 Cold Plasma for Biofilm Eradication on Food Products
The applications of cold plasma in the inactivation of bacterial biofilms are reviewed in Table 4.2. It should be noted that the antibiofilm effects of cold plasma on food products are generally lower than that of in vitro tests (Cui et al. 2016b). Thus, an extended time or power was required to reduce the mixed culture biofilm of bacteria on food product as compared with in vitro bacterial biofilms. The inactivation efficacy of cold plasma against biofilms formed on food products is affected by many factors, such as the types of microorganisms, biofilm formation conditions (such as temperature and time), and the nature of the produce’s surface (Cui et al. 2016b; Jahid et al. 2015; Patange et al. 2019; Srey et al. 2014).
The influences of cold plasma on the nutritional and sensory qualities of treated foods were well assessed in previous studies. As demonstrated by Srey et al. (2014), the cold plasma treatment at 750 mJ/cm2 did not cause any statistically significant changes in the color (L*, a*, and b*) and texture of cabbage and lettuce. In the study of Jahid et al. (2015), the 5-min cold plasma treatment did not cause significant changes in the color (L*, a*, and b*), texture, and sensory characteristics of lettuces. However, the obvious damages of lettuce surface were observed after being treated by cold plasma at 400 W for 5 min (Cui et al. 2016b). Therefore, the processing parameters of cold plasma should be well optimized to avoid resulting adverse effects on the nutritional and sensory qualities of treated food products.
4.2.3 Cold Plasma for Biofilm Eradication on Food Contact Surfaces
Several studies have investigated the effect of plasma on the biofilm inactivation of food contact surfaces, such as glass slides (Niemira et al. 2014; Vandervoort and Brelles-Marino 2014), stainless steel (Cui et al. 2016a, b), and plastic materials (Govaert et al. 2020a; Matthes et al. 2014; Pu et al. 2019; Theinkom et al. 2019). Niemira et al. (2014) studied the cold plasma-induced inactivation of Salmonella biofilms by using glass slides as a model food contact surface. The Salmonella biofilm was significantly inactivated by up to 2.1-log reduction within 15 s of cold plasma treatment. Cold plasma has also been used to control microbial biofilm contamination of 3D-printed food tools. In the research of Muro-Fraguas et al. (2020), 3D-printed poly-lactic acid (PLA) Petri dishes were coated by APPJ using acrylic acid (AcAc) and tetraethyl orthosilicate (TEOS). The results of crystal violet staining revealed that the total biofilm biomass of L. monocytogenes, P. aeruginosa, and E. coli formed on AcAc-coated Petri dishes by APPJ was decreased by 52.3%, 49.6%, and 35.9%, respectively, as compared with the untreated samples without plasma treatment. These findings suggested that plasma coatings can be used to reduce the bacterial attachment and biofilm formation on food contact surfaces.
4.3 Combination of Cold Plasma with Other Technologies
Although the individual treatments of cold plasma demonstrate the ability to inactivate mature biofilms, the limited diffusion of the reactive species inside the biofilms and the short lifetimes of chemical species cause adverse effects on the inactivation efficiency of cold plasma against biofilms. Consequently, the combination of cold plasma with other antimicrobial technologies is still required to improve the safety and quality characteristics of food products. Some new hurdle strategies have been developed, such as the combination treatments of cold plasma with plant essential oils (Cui et al. 2016a, b), disinfecting agents (Govaert et al. 2019b; Koban et al. 2013), vitamin C (Helgadóttir et al. 2017; Pandit et al. 2017a, b), chelating agents (Koban et al. 2013; Tschang and Thoma 2019), and bacteriophages (Cui et al. 2018). The mentioned descriptions are as follows.
4.3.1 Cold Plasma and Plant Essential Oils
Essential oils, a complex mixture of volatile organic compounds extracted from parts of plant materials (such as roots, stems, leaves, flowers, buds, fruits, and seeds), are well known for their excellent antibacterial and antibiofilm properties (Oh et al. 2017; Tariq et al. 2019). The combined treatment of cold plasma and plant essential oils has been well investigated for inactivation of biofilms (Cui et al. 2016a, b). According to Cui et al. (2016a), both Helichrysum italicum essential oil (0.5, 1, 2, and 4 mg/mL) and cold nitrogen plasma (400, 500, and 600 W) displayed significant eradication effect on E. coli O157:H7 biofilms in vitro. Following the combined treatment of Helichrysum italicum essential oil (0.5 mg/mL, 40 min) and cold plasma (400 W, 1 min), the population of S. aureus biofilm on 96-well plate and stainless steel were reduced by 6.26 and 5.50 log, respectively, which were significantly higher than that of the independent treatment (Cui et al. 2016a). The significant population reduction in S. aureus biofilms on different food contact surfaces (such as stainless steel, glass sheet, and plastic) was also observed after the synergetic treatment of Helichrysum italicum essential oil and cold plasma. The in vitro synergistic antibiofilm efficacy of cold nitrogen plasma and clove oil was also proved using the E. coli O157:H7 biofilm models grown on sterile stainless steel (Cui et al. 2016b).
Cui et al. (2016b) also evaluated the synergistic antibiofilm efficacy of cold nitrogen plasma and clove oil on fresh lettuce. The numbers of E. coli O157:H7 in biofilms on lettuce decreased by 0.81 and 2.26 log10 CFU/cm2 after the individual treatment of cold plasma (400 W, 3 min) or clove oil (4 mg/mL, 30 min), respectively. It was demonstrated that the E. coli O157:H7 biofilms on lettuce were decreased by 5.48 log10 CFU/cm2 following the synergetic treatment of cold nitrogen plasma (400 W, 3 min) and clove oil (1 mg/mL, 30 min), which was remarkably higher than that by using plasma or clove oil alone (Cui et al. 2016b). Moreover, the combined treatment of cold nitrogen plasma and clove oil has mild negative effect on the appearance, color, taste, and overall acceptability of lettuce.
4.3.2 Cold Plasma and Disinfectants
Disinfectants are extensively used to eliminate or inactivate microorganisms in the food industry. However, biofilms are more resistant to disinfectants than their planktonic counterparts (Bridier et al. 2011; Sanchez-Vizuete et al. 2015). Thus, the combining strategies should be applied to optimize biofilm control when using disinfectants. The synergistic effect of cold plasma and different disinfecting agents has been well evaluated, namely hydrogen peroxide, sodium hypochlorite, chlorhexidine, octenidine, ethylenediaminetetraacetic acid (EDTA), and polyhexanide (Govaert et al. 2019b; Koban et al. 2013). Govaert et al. (2019b) investigated the synergetic effect of DBD plasma and H2O2 on the inactivation of L. monocytogenes and S. Typhimurium biofilms in vitro. After DBD plasma treatment for 10 min, the 1- and 7-day-old L. monocytogenes biofilms decreased by 2.32 and 0.77 log10 CFU/cm2, respectively. Similar findings were also observed in S. Typhimurium. After the cold plasma or H2O2 treatment, the 1-day-old S. Typhimurium biofilms reduced between 1.96 and 3.70 log10 CFU/cm2. For the 1-day-old S. Typhimurium biofilms, the simultaneous H2O2 (0.05%, v/v) and DBD plasma resulted in significant differences between the combined log reduction value and the sum of corresponding individual treatments (p < 0.05). So the combined treatment of cold plasma and H2O2 may be used as a promising method for the inactivation of biofilms grown on food contact surfaces.
Koban et al. (2013) evaluated the combined antimicrobial effect of plasma with different agents (H2O2, sodium hypochlorite, chlorhexidine, octenidine, polyhexanide, and EDTA) against the monospecies (S. mutans) and multispecies dental biofilm models grown on Titanium discs in vitro. The combination of cold Ar plasma and all the tested compounds displayed additive antimicrobial effect against the used multispecies biofilms models.
4.3.3 Cold Plasma and Chelating Agents
Chelating agents, also known as chelators, can react with metal ions to form a stable complex, including ethylenediaminetetraacetic acid (EDTA), trisodium citrate (TSC), and ethylene glycol tetraacetic acid (EGTA). Previous studies have reported that chelating agents, such as EDTA, TSC, EGTA, and alizarin, can effectively destroy bacterial biofilms (Abraham et al. 2012; Banin et al. 2006; Bosma et al. 2010; Lee et al. 2016). Some studies have been conducted to investigate the bacterial biofilm reduction by the combined treatment of cold plasma and chelating agents (Tschang and Thoma 2019; Koban et al. 2013). Koban et al. (2013) found that the antibiofilm effect of Ar plasma against S. mutans biofilms was significantly increased by adding EDTA. This may be related to the EDTA-induced disruption of biofilm structure, which subsequently enhances the penetration of chemical species generated in the plasma into the biofilm matrix (Soares et al. 2010).
Consistent findings were also observed by Tschang and Thoma (2019), who studied the inactivation of E. coli, E. faecalis, and S. capitis biofilms induced by the combination of cold plasma and chelating agents (TSC, EDTA, EGTA, and alizarin). The results of colony count assay showed that the combined treatment of EDTA and TSC (10%, w/v) with cold plasma displayed synergistic effect on the inactivation of all the three bacterial biofilms, while the sequential treatment of alizarin and cold plasma had no significant effects on all three bacteria compared with cold plasma treatment alone (Tschang and Thoma 2019).
4.3.4 Cold Plasma and Vitamin C
Vitamin C, also known as ascorbic acid or L-ascorbic acid, is a water-soluble vitamin that is naturally present in fruits and vegetables. Vitamin C shows excellent antimicrobial and antibiofilm activities when used alone or in combination with other treatments (Majtan et al. 2020; Tajkarimi and Ibrahim 2011). Recent studies have proved that vitamin C can be used to enhance the inactivation capacity of cold plasma against biofilms (Helgadóttir et al. 2017; Pandit et al. 2017a, b). Helgadóttir et al. (2017) investigated the influences of vitamin C pretreatment on cold plasma-induced the inactivation of biofilms. The 48-h-old biofilms of B. subtilis, S. epidermidis, E. coli, and P. aeruginosa on glass coverslips were reduced by 2.0, 0.1, 1.0, and 0.4 log10 CFU/coverslips, respectively, after being exposed to cold plasma for 5 min. After the individual treatment of vitamin C for 15 min, the populations of B. subtilis, S. epidermidis, E. coli, and P. aeruginosa biofilms decreased by 1.0-, 0.3-, 0.8-, and 0.4-log reductions, respectively. The pretreatment with vitamin C could effectively strengthen the inactivation ability of cold plasma against the 48-h-old biofilms of S. epidermidis, E. coli, and P. aeruginosa. After the pretreatment of vitamin C for 15 min and cold plasma for 5 min, the biofilms of S. epidermidis, E. coli, and P. aeruginosa reduced by 0.8-, 1.7-, and 1.5-log reductions, which were higher than that of the individual treatment of vitamin C and cold plasma (Helgadóttir et al. 2017).
The pretreatment of vitamin C can inhibit the production of the matrix EPS and reduce the structural complexity of biofilms, which enhances the susceptibility of bacterial cells inside the biofilms to various chemical species generated in the plasma (Helgadóttir et al. 2017; Pandit et al. 2017a, b). Besides, vitamin C can exert predominantly pro-oxidant activity in the presence of iron, which leads to the generation of toxic free radicals (Timoshnikov et al. 2020; Vilchèze et al. 2013). Thus, the pro-oxidant activity of vitamin C may be one of the underlying mechanisms for the synergistic antibiofilm efficacy of cold nitrogen plasma and vitamin C.
4.3.5 Cold Plasma and Bacteriophages
Bacteriophages, also known as phages, are the viruses of bacteria. In recent years, the use of phages as an interesting alternative antibacterial approach has attracted great attention (Ferriol-González and Domingo-Calap 2020; Polaska and Sokolowska 2019). Cui et al. (2018) investigated the synthetic antibiofilm effects of the sequential treatment of cold plasma and phage techniques. The results showed that E. coli O157:H7 phages could effectively enhance the antibiofilm effects of cold plasma against E. coli O157:H7. Following the sequential treatment of cold plasma (400 W for 2 or 3 min) and phage techniques (5% or 10% for 30 min), the E. coli O157:H7 adhered on steel coupons were eliminated completely. Similar results were obtained in lettuces, cucumbers, and carrots. The initial concentration of E. coli O157:H7 was over 106 CFU/g for lettuces and cucumbers, while over 105 CFU/g for carrots. After the storage at 4 °C for 14 d, the population of E. coli O157:H7 in the control lettuce reduced to 3.79 log CFU/g. During the storage at 4 °C, the population of E. coli O157:H7 in lettuce treated by cold plasma and phages reduced to 1.21 log CFU/g on the third day, and no viable bacteria were detected on the ninth day. Similar results were also obtained in cucumbers and carrots. In addition, the sequential treatment of cold plasma and phages also effectively sustains the sensory quality of the types of vegetables.
4.3.6 Cold Plasma and Antibiotic
The combination of nonthermal plasma and antibiotic treatment was also used for the control of biofilms (Guo et al. 2018; Julák et al. 2020). Guo et al. (2018) studied the effect of cold plasma on the antibiotic sensitivity of methicillin-resistant Staphylococcus aureus (MRSA). The results indicated that the antibiotic sensitivity of MRSA increased after the sublethal treatment of both plasma and plasma-activated saline. The metabolic activity of S. epidermidis biofilms was decreased by 75% following the combination of cold plasma (15 min) and erythromycin (10 mg/L for 24 h), which was significantly higher than that of the NTP or erythromycin treatment alone (decreased by 46% and 47%, respectively). The improved decrease in the metabolic activity of E. coli biofilms was also observed after the subsequent combination of cold plasma and polymyxin B (Julák et al. 2020).
Taking together, the antimicrobial and antibiofilm properties of cold plasma can be effectively improved by combining with other mild physical or chemical intervention methods (Liao et al. 2020). However, the application of these synergetic methods in the food industry is not well investigated. Thus, further investigations are still required to assess the influences of these cold plasma-based hurdle interventions on microbiological safety and quality characteristics of food products. In subsequent work, the mechanisms underlying the synergistic antibiofilm efficacy of the cold plasma and other technologies also should be elucidated in more detail.
4.4 Factors Affecting the Antibiofilm Activity of Cold Plasma
In order to design effective methods for cold plasma-based biofilm removal, it is important to understand the factors affecting the antibiofilm activity of cold plasma. In general, the antibiofilm activity of cold plasma is affected by the characteristics of plasma generation, processing parameters of cold plasma, and properties of microbial biofilms (Alkawareek et al. 2012; Govaert et al. 2018; Gupta and Ayan 2019; Los et al. 2017; Puligundla and Mok 2017; Šimoncicová et al. 2019). These influencing factors should be taken into account for the practical application of cold plasma technologies in the food industry. The factors that affect the antibiofilm activity of cold plasma are reviewed in the present section (Fig. 4.2).
4.4.1 Characteristics of Plasma Generation
Generally, cold plasmas are generated by certain types of gas discharges at atmospheric pressure. So the discharge parameters affect the antibacterial and antibiofilm efficacy of cold plasma, including types of electrical discharge, device geometries, electrode configurations, voltage, frequency, input power, gas type, and flow rates (Alkawareek et al. 2012; Deng et al. 2007; Guo et al. 2019; Govaert et al. 2019a; Gupta and Ayan 2019; Han et al. 2016a, b; Hähnel et al. 2010; Matthes et al. 2014; Šimoncicová et al. 2019).
4.4.1.1 Types of Electrical Discharge
The most widely used plasma devices for biological samples are dielectric barrier discharge (DBD), atmospheric pressure plasma jets (APPJs), and corona discharge, which may affect the effectiveness of cold plasma. Govaert et al. (2019a) compared the antibiofilm efficacy of DBD plasma and surface barrier discharge (SBD) plasma. The obtained data suggested that the DBD configuration had the highest biofilm inactivation efficacy. Each discharge mode has its electrical characteristics, which affects the production of reactive chemical species and the antibacterial property. Therefore, the types of discharges should be appropriately chosen for the practical application of cold plasma technologies.
4.4.1.2 Voltage
The energy of electrical discharge is decided by applied voltage and frequency, thus generating different amounts of reactive species in cold plasma. Numerous studies have proved the effect of voltage levels on antimicrobial efficacy of cold plasma against bacteria (Deng et al. 2007; Govaert et al. 2019a; Han et al. 2016a, b; Patange et al. 2019). As reported by Patange et al. (2019), the inactivation abilities of cold plasma toward S. enterica, L. monocytogenes, and P. fluorescens in PBS were significantly enhanced when the applied voltage increased (60 to 80 kV). Similar inactivation patterns were also found in bacterial biofilms. Han et al. (2016a, b) investigated the effect of voltage levels (60, 70, and 80 kVRMS) on DBD plasma-mediated inactivation of E. coli, L. monocytogenes, and S. aureus biofilms in a meat model medium. The results demonstrated that the increase in the voltage levels led to further reductions in the number of bacteria inside the biofilms. This may be due to the more generation of extracellular reactive species in bacterial cells after being treated using DBD plasma with increased voltage levels (Han et al. 2016a, b).
4.4.1.3 Frequency
The plasma operating frequency also significantly affects the antibacterial activity of cold plasma (Alkawareek et al. 2012; Deng et al. 2007). As demonstrated by Deng et al. (2007), the inactivation of E. coli increased with the frequency. The populations of E. coli cells were reduced by 1.85-, 2.02-, 4.15-, and 5.60-log reduction after being treated by cold plasma for 30 s at 1, 1.5, 2.0, and 2.5 kHz, respectively. According to the studies of Alkawareek et al. (2012), the inactivation rate of P. aeruginosa biofilms after being exposed to 40 kHz plasma was higher than that of 20 kHz plasma. The D-values (the time taken to reduce the bacterial population by 90%) for P. aeruginosa biofilms with 40 kHz plasma jet were 15.97 s and 69.79 s for the first and the second inactivation phases, respectively, which were both higher than the 23.57 s and 128.20 s for 20 kHz plasma jet (Alkawareek et al. 2012). However, the gas temperature of the plasma jet discharged at 20 kHz was 39 °C, and the temperature increased to about 57 °C for 40 kHz plasma. The higher temperature of the plasma jet may limit its application for heat-sensitive fresh foods and food contact materials.
4.4.1.4 Input Power
The inactivation efficacy of cold plasma against microorganisms in planktonic and biofilm states is increased with an increase in input power (Cui et al. 2016a, b; Pina-Perez et al. 2020). Cui et al. (2016a) investigated the effect of different power on cold nitrogen plasma-mediated inactivation of biofilms. The results indicated that cold nitrogen plasma could inactivate S. aureus biofilm in an input power-dependent manner. The numbers of S. aureus biofilm on 96-well plates and stainless steel were reduced by 1.69- and 2.02-log reduction when treated with cold nitrogen plasma at 400 W for 1 min, respectively, which were significantly higher than that of samples treated with cold nitrogen plasma at 300 W for 1 min (Cui et al. 2016a). These results were consistent with those observed in cold nitrogen plasma-treated E. coli O157:H7 biofilms formed on stainless steel surface (Cui et al. 2016b).
4.4.1.5 Working Gas
The composition, humidity, and flow of working gases are the significant factors affecting the biofilm inactivation induced by cold plasma (Guo et al. 2019; Govaert et al. 2019a; Matthes et al. 2014). Matthes et al. (2014) investigated the influences of carrier gases on the antibiofilm properties of surface barrier-discharged (SBD) plasma. Three working gases were used in their work, which were Ar, Ar + 1% O2 (v/v), and Ar with 80% relative gas humidity (Ar + H2O), respectively. After being treated by SBD plasma for 300 s, P. aeruginosa biofilms were decreased by 4.88 log CFU/cm2 (Ar plasma), 2.97 log CFU/cm2 (Ar + O2 plasma), and 2.65 log CFU/cm2 (Ar + H2O plasma), respectively. Similar results were also observed by Guo et al. (2019), who investigated the influences of four working gases on the inactivation of methicillin-resistant S. aureus biofilms induced by surface discharge plasmas. Four working gases were used in this work, namely He+1% air, Ar + 1% air, synthetic air (a gas mixture of ~79% N2 and ~ 21% O2), and natural air (~78% N2, ~21% O2, and ~ 1% of rare gas). The experimental results demonstrated that the surface plasmas generated with Ar + 1% air and natural air had higher inactivation effect against S. aureus biofilms than that of plasmas generated with He+1% air and synthetic air. The S. aureus in biofilms were inactivated by 5.0-, 4.8-, 3.7-, and 2.3-log reduction by plasmas generated with Ar + 1% air, natural air, synthetic air, and He+1% air for 5 min, respectively (Guo et al. 2019). The composition of working gases may affect the kinds and concentrations of reactive chemicals produced by plasma discharge. As demonstrated by Guo et al. (2019), the levels of reactive species in the gas phase (such as 1O2, O2·−, ·NO2, and ONOO−) and liquid phase (such as H2O2, NO2−, and NO3−) produced by surface plasma with Ar + 1% air were remarkably higher than those generated with He+1% air. In addition, the working gases also affected the depths penetration of the reactive species in biofilms. The ROS penetration depths of plasma with Ar + 1% air, natural air, synthetic air, and He+1% air were 78 μm, 72 μm, 48 μm, and 16 μm, respectively, after 3-min plasma treatment; the changing trend was well in accordance with that of the antibiofilm properties of plasma with the four kinds of gases (Guo et al. 2019).
The antibiofilm activity of cold plasma is also influenced by the humidity of working gases. Hähnel et al. (2010) observed that the antimicrobial efficacy of dielectric barrier surface discharge plasma toward Bacillus spores was enhanced by increasing the relative air humidity up to 70%. However, as reported by Matthes et al. (2014), the antimicrobial efficacy of cold plasma generated with Ar against P. aeruginosa biofilms was decreased when increasing the relative humidity. After being exposed to Ar plasma for 300 s, P. aeruginosa biofilms were decreased by 4.88 log10 CFU/cm2, which was significantly higher than 2.65 log10 CFU/cm2 of Ar + H2O plasma. This may be due to the too high relative humidity of Ar used in the research of Matthes et al. (2014).
4.4.2 Processing Factors
The antibacterial and antibiofilm efficacy of cold plasma is also affected by the processing factors of cold plasma treatment, including the mode of plasma exposure, treatment time as well as the distance between the plasma source and the samples (Fridman et al. 2007; Gupta and Ayan 2019; Taghizadeh et al. 2015; Theinkom et al. 2019; Ziuzina et al. 2014).
4.4.2.1 Mode of Plasma Exposure
The applications of cold plasma for biofilm destruction can be classified into three different treatment modes: the direct plasma treatment, indirect plasma treatment, and hybrid plasma treatment (Czapka et al. 2018; Gupta and Ayan 2019; Keidar et al. 2013). Direct cold plasma treatment is defined as exposure of the samples placed directly under the plasma plume discharge (Morris et al. 2009; Ziuzina et al. 2014) or where the sample itself serves as an electrode that participates in the plasma discharge (Fridman et al. 2007). By contrast, the indirect or “remote” cold plasma treatment refers to the samples placed away from the plasma discharge area (Fridman et al. 2007; Morris et al. 2009; Ziuzina et al. 2014, 2015a). The hybrid plasma treatment represents a combination of direct and indirect plasma treatment (Keidar et al. 2013).
As described by Fridman et al. (2007), the bacteria inactivation induced by the direct treatment of DBD plasma was much faster than that of the indirect treatment. This may be because the cells are subjected to all plasma agents including reactive species, charged particles, photons, and electric field during the direct exposure (Laroussi 2020). However, contradictory findings have been reported by Ziuzina et al. (2015a), who evaluated the effect of direct or indirect DBD plasma treatment on the elimination of E. coli, L. monocytogenes, and S. aureus biofilms. According to the results of XTT assay, there was no significant difference between the antibiofilm efficacy of direct and indirect plasma exposure. So the plasma treatment mode should be fully considered in the practical application of cold plasma technologies.
4.4.2.2 Treatment Time
The antibiofilm performance of cold plasma is significantly affected by the treatment time. Generally, the inactivation efficacy of cold plasma against biofilm is increased with an increase in treatment time. As reported by Theinkom et al. (2019), the 48-h-old biofilms of E. faecalis were reduced by 1.8-, 1.8-, 2.6-, and 5.7-log reduction by surface micro-discharge plasma for 1, 3, 5, or 10 min, respectively. For 72-h-old biofilms of E. faecalis, the bacterial populations decreased by 1.7-, 2.0-, 2.4-, and 4.9-log reduction after being exposed to surface micro-discharge plasma for 1, 3, 5, or 10 min, respectively. Similar findings have also been observed in other studies (Cui et al. 2016a, 2016b; Han et al. 2016a, b; Niemira et al. 2018; Taghizadeh et al. 2015; Ziuzina et al. 2015a). According to Cui et al. (2016b), the E. coli O157:H7 biofilms on stainless steel decreased by 1.51 and 2.23 log CFU/cm2 after cold plasma treatment at 400 W for 1 or 3 min, respectively. Similar trends were found in E. coli O157:H7 biofilms formed on fresh lettuce. However, some obvious damage of lettuce surface was observed after exposure to cold nitrogen plasma at 400 W for 5 min (Cui et al. 2016b). Thus, the treatment time of cold plasma should be optimized when used for the food products.
4.4.2.3 Distance Between the Plasma Source and the SAMPLES
The distance between the plasma source and the sample also significantly affects the inactivation efficacy of cold plasma against biofilms. Taghizadeh et al. (2015) investigated the influences of the distance between the plasma nozzle and the sample on the inactivation of S. aureus biofilm. The survival rate of S. aureus biofilm on 96-well plate increased when upon increasing the distance between the plasma source and the sample. The S. aureus biofilm was effectively killed at 8 mm distance in comparison to the other distances (9, 10, and 11 mm). Almost no reduction in S. aureus biofilm was observed at a distance of 11 mm (Taghizadeh et al. 2015). Similar findings are also observed by Niemira et al. (2014). The distance between the plasma source and the sample may affect the amounts of reactive species that can reach the sample (Anzai et al. 2019). As reported by Anzai et al. (2019), the levels of reactive species (such as •OH, H2O2, and nitroxide radicals) are gradually decreased when the distance between the solution surface and the plasma jet nozzle increased. Thus, the inactivation efficiency of cold plasma against biofilm inactivation can be directed by varying the distance between the plasma source and the treated samples.
4.4.3 Properties of Microbial Biofilms
In general, the efficacy of cold plasma treatment is also determined by the biological properties of microbial biofilms, such as the microbial type, biofilm age, growth temperatures, the thickness of biofilm, the spatial structures and matrix components of biofilm, and initial inoculum (Govaert et al. 2018; Los et al. 2017; Puligundla and Mok 2017; Ziuzina et al. 2015a).
4.4.3.1 Type of Microorganism
The efficiency of cold plasma treatment against biofilms is strongly affected by phenotype and strain of bacteria studied. The tolerance of microbial biofilms to cold plasma is variable between species, strains of the same species. It has been reported that the biofilms of Gram-positive bacteria are more resistant to cold plasma treatments than that of Gram-negative bacteria (Los et al. 2017; Mai-Prochnow et al. 2016; Ziuzina et al. 2015a). As reported by Ziuzina et al. (2015a), the populations of Gram-negative E. coli in biofilms reduced to undetectable levels from an initial 5.4 log CFU/mL after the DBD plasma treatment for 60 s, whereas an extended treatment for 300 s was needed to inactivate the Gram-positive L. monocytogenes and S. aureus biofilms. After being treated by cold plasma for 10 min, the biofilms of Gram-positive B. subtilis, S. epidermidis, and K. carniphila decreased by 0.6-, 1-, and 2-log reduction, respectively, while the biofilms of Gram-negative species (P. aeruginosa, P. libanensis, and E. cloacae) showed high reduction ranging from 3.3 to 3.6 log reduction (Mai-Prochnow et al. 2016). These differences may due to the differences in the bacterial cell wall structure and the EPS (such as thickness, composition, and quantity) (Vu et al. 2009; Ziuzina et al. 2015a). For example, Gram-positive bacteria possess a thick (20–80 nm) cell wall than Gram-negative bacteria (<10 nm) (Mai-Prochnow et al. 2016). As analyzed by Mai-Prochnow et al. (2016), there is a good correlation of CFU log reduction of biofilms with cell wall thickness after 1, 3, and 10 min of cold plasma treatment. Czapka et al. (2018) also found that the biofilm of E. coli ATCC 10231 shown higher resistance to DBD plasma than biofilm of S. aureus ATCC 25922.
Generally, the single-species biofilms have been used extensively to assess the in vitro activity of antibiofilm approaches. However, most biofilms in nature are actually formed by multiple microbial species. Mixed-species biofilms are ubiquitous in the food industry, which are generally associated with higher resistance to disinfectants and antimicrobials (Burmolle et al. 2006; Jahid et al. 2015; Jahid and Ha 2014; Yuan et al. 2020). As founded by Jahid et al. (2015), the mixed cultures of S. Typhimurium and cultivable indigenous microorganisms (CIM) showed significantly greater resistance to cold plasma as compared to monoculture biofilms on lettuce. So more attention should be paid to the effect of cold plasma treatments on mixed-species biofilms on the surface of either real foods or packaging materials.
4.4.3.2 Biofilm Age
Biofilm age is also a decisive factor in determining the susceptibility of the resident microorganisms to disinfectants and antimicrobials treatment (Wong et al. 2010). The susceptibility of biofilms of different ages to cold plasma was well evaluated (Govaert et al. 2018, 2019b; Vleugels et al. 2005; Ziuzina et al. 2015b). Govaert et al. (2018) investigated the inactivation efficiency of DBD plasma against L. monocytogenes and S. Typhimurium biofilms with five different ages (1, 2, 3, 7, and 10 days). It was found out that the 7 days old L. monocytogenes and S. Typhimurium biofilms were more resistant toward DBD plasma treatment (Govaert et al. 2018). Similar findings were also reported by Ziuzina et al. (2015a) that the 48-h-old Salmonella, L. monocytogenes, and E. coli biofilms developed on lettuce exhibited higher resistance to cold plasma than 24-h-biofilms. The increased tolerance of the 7 days old biofilms to cold plasma may be due to the higher amount EPS matrix, which probably reduces the diffusion of the reactive species generated during plasma discharge into the biofilms, and, consequently, protects the biofilm-associated cells from DBD plasma-mediated cell death (Govaert et al. 2018; Jiang et al. 2017). Vleugels et al. (2005) also reported similar findings that 24-h-old P. agglomerans biofilms had stronger resistance to glow discharge plasma than that of 12-h-old samples. However, inconsistent findings were reported by Niemira et al. (2018) indicating that the biofilm age (24, 48, or 72 h) was not a significant factor influencing the inactivation efficiency of gliding arc plasma against E. coli O157:H7 biofilms.
4.4.3.3 Growth Temperatures of Biofilm
The temperature for biofilm formation also significantly affects the inactivation efficiency of cold plasma against bacterial biofilms. As reported by Patange et al. (2019), the mixed biofilms of L. monocytogenes and P. fluorescens formed on lettuce at 4 °C were more resistant to DBD plasma than that of biofilms formed at 15 °C. The results demonstrated that the DBD plasma treatment for 5 min significantly reduced L. monocytogenes and P. fluorescens in 48 h biofilm formed at 15 °C reduced to undetectable levels, while the cells in 48 h biofilm formed at 4 °C reduced by 4.0- and 2.1-log, respectively (Patange et al. 2019). In contrast, Jahid et al. (2014) reported that the biofilm populations of A. hydrophila formed on lettuce at higher temperatures (≥15 °C) displayed enhanced resistance to cold oxygen plasma. After 5 min of cold oxygen plasma treatment, a 5-log reduction was observed in biofilm populations of A. hydrophila formed on lettuce at 4 °C, 10 °C, and 15 °C. However, the populations of A. hydrophila in biofilms formed at 20 °C, 25 °C, and 30 °C were reduced maximally by 3.9-, 3.2-, and 3.0-log, respectively. These results may be related to the modulating effect of temperature on quorum sensing and biofilm formation of A. hydrophila on lettuce surfaces and stomata (Jahid et al. 2014).
4.4.3.4 Biofilm Matrices
The bacterial biofilm matrix is comprised of self-produced extracellular polymeric substances (EPS) like exopolysaccharide, proteins, extracellular DNA (eDNA), and extracellular RNA (eRNA) (Flemming and Wingender 2010; Karygianni et al. 2020). The EPS matrix offers effective protection microbial cells from a variety of unfavorable environmental stresses (Guillonneau et al. 2018; Yin et al. 2019). The polymeric matrix in biofilms may prevent the penetration of cold plasma into its deep layer, weakening its bactericidal capacity against bacterial cells at the bottom of the biofilms. The penetrating abilities of plasma reactive species are affected by plasma generation parameters (e.g., voltage, gas type), microbial biofilm properties, etc. As reported by Alkawareek et al. (2012), the cold plasma generated with a gas mixture (0.5% O2 + 99.5% He) can penetrate deeply and eradicate the P. aeruginosa biofilms of 40 to 80 μm thickness. In the work of Xiong et al. (2011), the cold oxygen plasma can penetrate the 15-μm-thick biofilms of P. gingivalis and effectively deactivate the biofilm cells. Thus, the matrices and thickness of bacterial biofilms are the significant factors affecting the inactivation efficiency of cold plasma (Gupta and Ayan 2019; Zhu et al. 2020).
4.4.4 Characteristics of the Treated Samples
In addition to the above-mentioned factors, the inactivation efficacy of cold plasma against biofilms formed on food products is also affected by the nature and characteristics of the tested samples (Cui et al. 2016b; Patange et al. 2019; Srey et al. 2014). The interactions between plasma and food products can be influenced by various factors, such as surface roughness, adsorption of diffusing plasma, components, and moisture, which may affect the resistance of microorganisms to cold plasma (Bhide et al. 2017; Yong et al. 2015). Bhide et al. (2017) showed that the microbial inactivation efficacy of cold plasma against E. aerogenes on sandpapers and the fruit peel surfaces significantly decreased as the surface roughness increased. For instance, the roughness values of the apple, orange, and cantaloupe peel surfaces were 6.12 μm, 13.07 μm, and 16.98 μm, respectively. After treatment with cold plasma for 492 s, the populations of E. aerogenes cells on the apple, orange, and cantaloupe peel surfaces decreased by 1.86-, 0.77-, and 0.61-log reduction, respectively. So the nature and characteristics of the food samples should be taken into consideration when applying cold plasma technologies in the preservation of foods.
4.5 Inactivation Agents of Cold Plasma
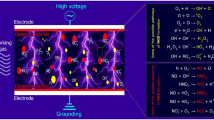
Plasma is an ionized gas composed of heat, UV emission, charged particles (electrons and ions), and reactive species (ROS, RNS, and free radicals), and electromagnetic fields (Ji et al. 2019; Weltmann and von Woedtke 2017). Many of these physical or chemical factors are well known to have antimicrobial efficacy (Niedźwiedź et al. 2019). It is widely recognized that the synergistic effect of these active agents is responsible for the antimicrobial and efficacy of cold plasma (Fig. 4.3) (Gilmore et al. 2018; Gupta and Ayan 2019; Laroussi 2005; Weltmann and von Woedtke 2017; Zhu et al. 2020).
4.5.1 Reactive Species
During plasma discharges, considerable amounts of reactive chemical species are generated through various collisional pathways such as hydroxyl radical (•OH), ozone (O3), hydrogen peroxide (H2O2), singlet oxygen (1O2), superoxide anion (O2•-), nitric oxide (NO), and nitrogen dioxide (NO2), which are well known to have antimicrobial efficacy (Laroussi 2005; Vatansever et al. 2013). Thus, it has always been recognized that the reactive species in cold plasma play an important role in its antimicrobial properties. These reactive species can directly or indirectly damage the structure (such as cell wall and cell membrane) and cellular components (e.g., DNA, proteins, and lipids) of microbial cells, which subsequently lead to the death of microorganisms (Han et al. 2016a, b; Liao et al. 2017a).
In the work of Schneider et al. (2011), they developed an experimental setup to effectively separate UV photons and reactive particles (such as accelerated ions and radicals) in a plasma jet with He gas flow. The complete-jet treatment showed the strongest antibacterial efficacy against E. coli, followed by the reactive particles-jet, while the UV photons-jet alone had little effect on the inactivation of E. coli cells at the same condition. These data indicated that the reactive species are mainly responsible for the microbial inactivation induced by cold plasma. Similar findings were also observed by Govaert et al. (2020b), who investigated the role of reactive species in the cold plasma-induced inactivation of biofilms by covering the biofilms with a quartz disc. The quartz disc can prevent all reactive species plasma, apart from UV photons, to interact with the biofilm samples (Herrmann et al. 1999). The results showed that the log reductions of L. monocytogenes and S. Typhimurium model biofilms obtained without quartz disc were quite higher than the corresponding values with quartz disc. In summary, the reactive species play important roles in the cold plasma-induced inactivation of microbial cells in both planktonic and biofilm states.
4.5.2 UV Radiation
The antibacterial and antibiofilm efficacy of UV radiation has been well identified. The ultraviolet photons are produced by radiative de-excitation of atoms or molecules during plasma discharge. Generally, the UV light produced by atmospheric pressure air cold plasma is less than that by low-pressure plasma (Lackmann et al. 2013; Laroussi 2009; Laroussi and Leipold 2004). The UV photons produced in the plasma may also contribute to its bactericidal and antibiofilm effect. In the work of Govaert et al. (2020b), the generation of UV light was detected by means of UV strips (wavelength ranging between 250 and 420 nm, intensities ranging between 5 and 60 mJ/cm2). After the DBD plasma treatment using He/O2 gas mixture at 21.88 V for 10 min, obvious color change in UV strips was observed, suggesting the generation of UV light during DBD discharge. The authors further investigated the role of UV light in the cold plasma-induced inactivation of biofilms. The remarkable inactivation of L. monocytogenes and S. Typhimurium biofilms was observed when the biofilms were covered with a quartz disc, which allowed UV photons and heat to pass through but prevented all chemical species in plasma (Govaert et al. 2020b). Therefore, ultraviolet photons may be involved in the biofilms inactivation induced by cold plasma, but the impact may be relatively weak (Lackmann et al. 2013; Schneider et al. 2011).
4.5.3 Charged Particles
Generally, the roles of reactive species and UV radiation in cold plasma-induced microbial inactivation have been well discussed, while much less attention is given to charged species (e.g., electrons and ions). Though the exact role of charged particles is not yet fully understood, their importance is indicated by several studies (Fridman et al. 2007; Laroussi 2002; Stoffels et al. 2008). It is suggested that charged particles may play a significant role in the electrostatic disruption of the outer membrane of E. coli cells (Laroussi 2002). They suggested the generated electrostatic force by charged particles can overcome the tensile strength of the membrane when sufficient charged particles are accumulated on the outer surface of bacterial cells, eventually leading to the disruption of the cell membrane structures (Laroussi 2002). According to Fridman et al. (2007), the direct treatment of cold plasma yielded better sterilization efficiency than the indirect treatment. This may be related to the charged particles, because the charged particles can only contact the microbial cells directly during the direct treatment of cold plasma (Laroussi 2009). Due to the complex structure of microbial biofilms, the charged particles may only contact the cells located on the surface layer of the biofilms. So much more work is needed to reveal the exact role of charged particles in cold plasma-induced eradication of biofilms.
4.5.4 Heat
Though the final temperature of cold plasma-treated samples increases in a time-depended way, heat is not an important factor in the inactivation process of bacterial cells (Laroussi 2009). As reported by Lacombe et al. (2017), the average surface temperature of berries increased to 46.6 °C from initial 23.2 °C after plasma jet treatment for 120 s at a working distance of 7.5 cm. The effect of plasma temperature on biofilm inactivation was also investigated (Fridman et al. 2007; Niemira et al. 2018; Vandervoort and Brelles-Marino 2014). Niemira et al. (2018) monitored the temperature of glass slides during the treatment of plasma jet, which was obtained with an infrared camera. Following cold plasma treatment at a 5 cm and 7 cm spacing, the maximum temperatures of the slides were 35.0–45.7 °C and 38.1–53.4 °C, respectively. Similar findings were also reported by Vandervoort and Brelles-Marino (2014) that the temperature of the coupon surface during cold plasma treatment (once a minute for 10 min) reached to 35 °C within a few minutes and remained constant over time. These obtained temperatures are too low to inactivate bacterial cells within the biofilms (Chmielewski and Frank 2004; Stringer et al. 2000). These data indicate that the thermal effects do not play an important role in the biofilm inactivation induced by cold plasma. However, to maintain the sensory and nutritional characteristics of food products, the untoward thermal effects of cold plasma on the samples should be avoided with appropriate measures such as forced air cooling (Lacombe et al. 2017).
4.5.5 Gas Flow
The effect of gas flow on the inactivation of the biofilm has also been assessed in previous studies (Joaquin et al. 2009; Vandervoort and Brelles-Marino 2014). Vandervoort and Brelles-Marino (2014) investigated the roles of gas flow (He flow of 20.4 L/min + N2 of 0.135 L/min) in cold plasma-induced inactivation of biofilms. The reduction level of P. aeruginosa biofilms was 5 to 10% after being exposed to gas in the absence of plasma (plasma source turned off), which was much lower than the inactivation level by cold plasma. The findings of this study were consistent with previous studies by Joaquin et al. (2009). The above data indicate that the inactivation of biofilms is due to plasma treatment and not due to excessive cell drying from the gas flow.
4.6 Antibiofilm Mechanisms of Cold Plasma
How cold plasma induces the inactivation of microbial biofilm is an interesting and complicated question. Several proposals have been suggested for the cold plasma-induced biofilm eradication (Gilmore et al. 2018; Gupta and Ayan 2019; Zhu et al. 2020).
4.6.1 The Roles of Reactive Species
The reactive species present in the plasma possess strong oxidizing potentials and are believed to be mainly responsible for the antimicrobial and antibiofilm efficacy of cold plasma. These reactive species can cause severe oxidative damages to cellular structures and important cellular components (such as DNA, proteins, and lipids), which subsequently led to the death of microorganisms (Han et al. 2016a, b; Liao et al. 2017a; Montie et al. 2000). For instance, oxidative DNA damage has been revealed in bacterial cells after exposure to cold plasma. Joshi et al. (2011) showed that the formation of 8-OHdG in planktonic E. coli cells, a ubiquitous marker of oxidative DNA damage, was significantly increased after being exposed to plasma treatment. The PCR results also revealed multisite genomic DNA strand breakage in E. coli and L. monocytogenes cells after DBD plasma treatment (Lu et al. 2014). DNA damage was also observed in L. monocytogenes and S. Typhimurium biofilms for most of the cold plasma treatment conditions (Govaert et al. 2020b). Joshi et al. (2011) assessed the malondialdehyde (MDA) levels in E. coli cells during plasma treatment. The results demonstrated that DBD plasma induced lipid peroxidation in intact E. coli cells in a time-dependent manner.
Besides the direct damage effect of reactive species, cold plasma also induces the generation of intracellular ROS or RNS in bacteria cells (Brun et al. 2018). In the work of Delben et al. (2016), the intracellular ROS levels in plasma-treated biofilms were measured with fluorescent probe dihydrorhodamine 123 (DHR 123). The results revealed that more cells in the biofilms of C. albicans and S. aureus exhibited green fluorescence compared with the untreated control, suggesting the presence of ROS in all plasma-treated biofilms. The consistent findings were also revealed by Govaert et al. (2020b).
4.6.2 Dispersion of Extracellular Matrix
In most biofilms, the matrix can contribute to more than 90% of the total dry mass, while the microorganisms account for less than 10% (Flemming and Wingender 2010). The biofilm matrix is mainly composed of water and EPS. EPS are the natural organic polymers of high molecular weight secreted by microbes, which are primarily comprised of polysaccharides, extracellular proteins, extracellular DNA (eDNA), and lipids (Flemming and Wingender 2010; Di Martino 2018). EPS can also act as a scaffold to protect the biofilm cells from a wide range of external environmental stresses, e.g., UV radiation, oxidizing agents, desiccation, antibiotics, and biocides (Vandervoort and Brelles-Marino 2014; Yin et al. 2019). In addition to their protective role, EPS also play important roles in the biofilms formation and development, such as mediating biofilm adhesion to surfaces, providing mechanical and structural stability, mediating, controlling gene regulation, facilitating quorum sensing, and acting as nutrient sources (Flemming and Wingender 2010; Hobley et al. 2015).
Though many studies have been conducted on the biofilm eradication induced by cold plasma, the damages inflicted to the biofilm matrix are not still well investigated. Vandervoort and Brelles-Marino (2014) studied the effect of DBD plasma on the P. aeruginosa biofilm matrix. By using the fluorescent dyes Calcofluor White and SYTO9, DBD plasma caused a reduction to almost complete removal of the biofilm matrix in a time-dependent manner. Besides, the noticeable lipid oxidation products and damaged DNA were also observed in P. aeruginosa biofilm matrix after plasma exposure. The above data suggest that the reactive agents in DBD plasma cause damages to polysaccharides, proteins, eDNA, lipids, and other components of the biofilm matrix. In addition, the disrupted heterogeneous biofilm structures were also observed in the work of Ziuzina et al. (2014) and Ziuzina et al. (2015a). These chemical and structural changes on the biofilm matrix may contribute to the plasma-mediated biofilm inactivation (Vandervoort and Brelles-Marino 2014; Ziuzina et al. 2014, 2015a).
4.6.3 Inhibition of Quorum Sensing
Quorum sensing (QS) is a type of population density-dependent cell to cell signaling system, which is based on the effect of low-molecular-weight extracellular signal molecules called autoinducers (AIs) (Rutherford and Bassler 2012). Autoinducers can be classified into three main types based on their structure and specific function: N-acyl-homoserine lactones (AHLs), autoinducing peptides (AIPs), and autoinducer-2 (AI-2). AHLs primarily exist in Gram-negative proteobacteria as well as some cyanobacteria and bacteroidetes, AIPs are mainly synthesized in Gram-positive bacteria, and the AI-2 QS system is found in both Gram-negative and -positive bacteria (Hense and Schuster 2015; Rutherford and Bassler 2012). QS signaling plays important roles in biofilm formation (such as attachment, maturation, aggregation, and dissolution), antimicrobial resistance development, and virulence factors secretion (Parsek and Greenberg 2005; Rutherford and Bassler 2012). Thus, the disruption of QS seems to be an attractive strategy for biofilm control (Muhammad et al. 2020; Sadekuzzaman et al. 2015).
Flynn et al. (2016) confirmed that the cold plasma caused chemical modification and degradation of AHL molecules in a time-dependent manner, which subsequently inhibited the production of QS-regulated virulence factors (such as pyoverdine and pyocyanin). Significant reductions of QS-regulated virulence factors, such as pyocyanin and elastase were also observed in P. aeruginosa following the exposure to DBD plasma (Ziuzina et al. 2015a). For the biofilms of non-hospital strains of P. aeruginosa (DBM 3777, ATCC 10145, and ATCC 15442), the production of AHLs was decreased after the cold plasma exposure, and the eradication of the biofilm was also achieved. These data suggested that cold plasma may reduce the formation of biofilm by inhibiting the AHL-dependent QS systems in non-hospital strains of P. aeruginosa (Paldrychová et al. 2019). The production of AHLs in hospital strains of P. aeruginosa was also significantly decreased after exposure to cold plasma (ATCC 27853 and DBM 3181), but this decrease did not result in significant changes in the total biofilm biomass and metabolic activity of the two stains. The biofilm formation of the two clinical isolates is probably controlled by another QS system, which is not affected by cold plasma (Paldrychová et al. 2019). Therefore, it is summarized that QS may be involved in the biofilm inactivation induced by cold plasma, while the underlying exact mechanisms are still not widely recognized.
4.6.4 Damage of Cytoplasmic Membranes
As reported in the literature, membrane damage is one of the primary mechanisms of microbial inactivation induced by cold plasma, including changes in membrane permeabilization, membrane potential, membrane depolarization, and membrane lipid peroxidation (Brun et al. 2018; Joshi et al. 2011; Kvam et al. 2012; Lu et al. 2014). Joshi et al. (2011) observed that the membrane potential in E. coli cells decreased in a dose-dependent way after exposure to DBD plasma. Govaert et al. (2020b) investigated the effect of cold plasma on the membrane integrity of the biofilm-associated bacterial cells by measuring the leakage of intracellular DNA, an indicator of membrane damage. The authors found that cold plasma caused leakage of intracellular DNA in L. monocytogenes and S. Typhimurium cells encased within biofilms, suggesting the severe destruction of membrane integrity (Govaert et al. 2020b).
4.7 Conclusion
This chapter reviews the unique opportunities of cold plasma for the control and eradication of biofilms on food products. Numerous studies have shown that cold plasma is a promising and multi-targeting approach to combat biofilms formed in food products and food processing environments. The antibiofilm efficacy of cold plasma is affected by plasma equipment, processing parameters, properties of microbial biofilms, etc. The synergistic effects of reactive species, charged particles, UV emission, and electromagnetic fields mainly contribute to the antibiofilm activity of cold plasma. In order to achieve a higher rate of biofilm inactivation, the cold plasma should be combined with other hurdle strategies, such as plant essential oils, disinfecting agents, and chelating agents. The present studies are mostly focused on the in vitro effect of cold plasma on microbial inactivation. In future studies, special attention should be paid to the application of cold plasma for biofilm eradication on food products and food processing environments. Besides, the influences of cold plasma on the nutritional and sensory qualities of food products should also be investigated in detail. In addition, microbial cells may enter into the sublethal injury or viable-but-nonculturable (VBNC) state after cold plasma exposure, which pose a potential threat to food safety (Liao et al. 2017b; Rowan et al. 2007; Ziuzina et al. 2015a). Therefore, further studies are still needed to clarify the stress responses elicited by food-associated microorganisms to cold plasma.
References
Abraham NM, Lamlertthon S, Fowler VG et al (2012) Chelating agents exert distinct effects on biofilm formation in Staphylococcus aureus depending on strain background: role for clumping factor B. J Med Microbiol 61(Pt 8):1062–1070
Alkawareek MY, Algwari QT, Laverty G et al (2012) Eradication of Pseudomonas aeruginosa biofilms by atmospheric pressure non-thermal plasma. PLoS One 7(8):e44289
Alvarez-Ordonez A, Coughlan LM, Briandet R et al (2019) Biofilms in food processing environments: challenges and opportunities. Annu Rev Food Sci Technol 10(1):173–195
Anzai K, Aoki T, Koshimizu S et al (2019) Formation of reactive oxygen species by irradiation of cold atmospheric pressure plasma jet to water depends on the irradiation distance. J Clin Biochem Nutr 64:187–193
Aviat F, Gerhards C, Rodriguez-Jerez JJ et al (2016) Microbial safety of wood in contact with food: a review. Comp Rev Food Sci Food Safety 15(3):491–505
Banin E, Brady KM, Greenberg EP (2006) Chelator-induced dispersal and killing of Pseudomonas aeruginosa cells in a biofilm. Appl Environ Microbiol 72(3):2064–2069
Bhide S, Salvi D, Schaffner DW et al (2017) Effect of surface roughness in model and fresh fruit systems on microbial inactivation efficacy of cold atmospheric pressure plasma. J Food Prot 80(8):1337–1346
Bosma JW, Siegert CEH, Peerbooms PGH et al (2010) Reduction of biofilm formation with trisodium citrate in haemodialysis catheters: a randomized controlled trial. Nephrol Dial Transplant 25(4):1213–1217
Bridier A, Briandet R, Thomas V et al (2011) Resistance of bacterial biofilms to disinfectants: a review. Biofouling 27(9):1017–1032
Bridier A, Sanchez-Vizuete P, Guilbaud M et al (2015) Biofilm-associated persistence of food-borne pathogens. Food Microbiol 45(Part B):167–178
Brun P, Bernabe G, Marchiori C et al (2018) Antibacterial efficacy and mechanisms of action of low power atmospheric pressure cold plasma: membrane permeability, biofilm penetration and antimicrobial sensitization. J Appl Microbiol 125(2):398–408
Burmolle M, Webb JS, Rao D et al (2006) Enhanced biofilm formation and increased resistance to antimicrobial agents and bacterial invasion are caused by synergistic interactions in multispecies biofilms. Appl Environ Microbiol 72(6):3916–3923
Chmielewski RA, Frank JF (2004) A predictive model for heat inactivation of Listeria monocytogenes biofilm on stainless steel. J Food Prot 67(12):2712–2718
Cui HY, Li W, Li CZ et al (2016a) Synergistic effect between Helichrysum italicum essential oil and cold nitrogen plasma against Staphylococcus aureus biofilms on different food-contact surfaces. Int J Food Sci Technol 51(11):2493–2501
Cui HY, Ma CX, Lin L (2016b) Synergetic antibacterial efficacy of cold nitrogen plasma and clove oil against Escherichia coli O157:H7 biofilms on lettuce. Food Control 66:8–16
Cui HY, Bai M, Yuan L et al (2018) Sequential effect of phages and cold nitrogen plasma against Escherichia coli O157:H7 biofilms on different vegetables. Int J Food Microbiol 268:1–9
Czapka T, Maliszewska I, Olesiak-Bańska J (2018) Influence of atmospheric pressure non-thermal plasma on inactivation of biofilm cells. Plasma Chem Plasma Process 38:1181–1197
Davey ME, O’toole GA (2000) Microbial biofilms: from ecology to molecular genetics. Microbiol Mol Biol Rev 64(4):847–867
de Castro ML, de Alencar FLS, Navoni JA et al (2019) Toxicological aspects of trihalomethanes: a systematic review. Environ Sci Pollut Res 26:5316–5332
Delben JA, Zago CE, Tyhovych N et al (2016) Effect of atmospheric-pressure cold plasma on pathogenic oral biofilms and in vitro reconstituted oral epithelium. PLoS One 11(5):e0155427
Deng SB, Ruan R, Mok CK et al (2007) Inactivation of Escherichia coli on almonds using nonthermal plasma. J Food Sci 72(2):62–66
Di Martino P (2018) Extracellular polymeric substances, a key element in understanding biofilm phenotype. AIMS Microbiol 4(2):274–288
Donlan RM (2002) Biofilms: microbial life on surfaces. Emerg Infect Dis 8(9):881–890
Erriu M, Blus C, Szmukler-Moncler S et al (2014) Microbial biofilm modulation by ultrasound: current concepts and controversies. Ultrason Sonochem 21(1):15–22
Ferriol-González C, Domingo-Calap P (2020) Phages for biofilm removal. Antibiotics 9:268
Flemming HC, Wingender J (2010) The biofilm matrix. Nat Rev Microbiol 8(9):623–633
Flemming HC, Wingender J, Szewzyk U et al (2016) Biofilms: an emergent form of bacterial life. Nat Rev Microbiol 14(9):563–575
Flynn P, Busetti A, Wielogorska E et al (2016) Non-thermal plasma exposure rapidly attenuates bacterial AHL-dependent quorum sensing and virulence. Sci Rep 6:26320
Fridman G, Brooks AD, Balasubramanian M et al (2007) Comparison of direct and indirect effects of non-thermal atmospheric-pressure plasma on bacteria. Plasma Process Polym 4(4):370–375
Furukawa S (2015) Studies on formation, control and application of biofilm formed by food related microorganisms. Biosci Biotechnol Biochem 79(7):1050–1056
Galié S, García-Gutiérrez C, Miguélez EM et al (2018) Biofilms in the food industry: health aspects and control methods. Front Microbiol 9:898
Gilmore BF, Flynn PB, O’Brien S et al (2018) Cold plasmas for biofilm control: opportunities and challenges. Trends Biotechnol 36(6):627–638
Govaert M, Smet C, Baka M et al (2018) Resistance of L. monocytogenes and S. Typhimurium towards cold atmospheric plasma as function of biofilm age. Appl. Sci 8(12):2702
Govaert M, Smet C, Vergauwen L et al (2019a) Influence of plasma characteristics on the efficacy of cold atmospheric plasma (CAP) for inactivation of Listeria monocytogenes and Salmonella Typhimurium biofilms. Innov Food Sci Emerg Technol 52:376–386
Govaert M, Smet C, Verheyen D et al (2019b) Combined effect of cold atmospheric plasma and hydrogen peroxide treatment on mature Listeria monocytogenes and Salmonella Typhimurium biofilms. Front Microbiol 10:2674
Govaert M, Smet C, Graeffe A et al (2020a) Inactivation of L. monocytogenes and S. Typhimurium biofilms by means of an air-based cold atmospheric plasma (CAP) system. Foods 9(2):157
Govaert M, Smet C, Walsh JL et al (2020b) Influence of plasma characteristics on the inactivation mechanism of cold atmospheric plasma (CAP) for Listeria monocytogenes and Salmonella Typhimurium biofilms. Appl Sci 10(9):3198
Guillonneau R, Baraquet C, Bazire A et al (2018) Multispecies biofilm development of marine bacteria implies complex relationships through competition and synergy and modification of matrix components. Front Microbiol 9:1960
Guo L, Xu RB, Zhao YM et al (2018) Gas plasma pre-treatment increases antibiotic sensitivity and persister eradication in methicillin-resistant Staphylococcus aureus. Front Microbiol 9:537
Guo L, Xu RB, Liu DX et al (2019) Eradication of methicillin-resistant Staphylococcus aureus biofilms by surface discharge plasmas with various working gases. J Phys D Appl Phys 52(42):425202
Gupta TT, Ayan H (2019) Application of non-thermal plasma on biofilm: a review. Appl Sci 9(17):3548
Hähnel M, von Woedtke T, Weltmann KD (2010) Influence of the air humidity on the reduction of Bacillus spores in a defined environment at atmospheric pressure using a dielectric barrier surface discharge. Plasma Process Polym 7(3–4):244–249
Han L, Patil S, Boehm D et al (2016a) Mechanisms of inactivation by high-voltage atmospheric cold plasma differ for Escherichia coli and Staphylococcus aureus. Appl Environ Microbiol 82(2):450–458
Han L, Ziuzina D, Heslin C et al (2016b) Controlling microbial safety challenges of meat using high voltage atmospheric cold plasma. Front Microbiol 7:977
He MW, Duan JW, Xu JL et al (2020) Candida albicans biofilm inactivated by cold plasma treatment in vitro and in vivo. Plasma Process Polym 17(4):e1900068
Helgadóttir S, Pandit S, Mokkapati VRSS et al (2017) Vitamin C pretreatment enhances the antibacterial effect of cold atmospheric plasma. Front Cell Infect Microbiol 7:43
Hense BA, Schuster M (2015) Core principles of bacterial autoinducer systems. Microbiol Mol Biol Rev 79(1):153–169
Herrmann HW, Henins I, Park J et al (1999) Decontamination of chemical and biological warfare (CBW) agents using an atmospheric pressure plasma jet (APPJ). Phys Plasmas 6(5):2284–2289
Hobley L, Harkins C, MacPhee CE et al (2015) Giving structure to the biofilm matrix: an overview of individual strategies and emerging common themes. FEMS Microbiol Rev 39(5):649–669
Hoffmann C, Berganza C, Zhang J (2013) Cold atmospheric plasma: methods of production and application in dentistry and oncology. Med Gas Res 3:21
Hung YC, Waters BW, Yemmireddy VK et al (2017) pH effect on the formation of THM and HAA disinfection byproducts and potential control strategies for food processing. J Integr Agric 16(12):2914–2923
Jahid IK, Ha SD (2014) The paradox of mixed-species biofilms in the context of food safety. Comp Rev Food Sci Food Safety 13(5):990–1011
Jahid IK, Han N, Ha SD (2014) Inactivation kinetics of cold oxygen plasma depend on incubation conditions of Aeromonas hydrophila biofilm on lettuce. Food Res Int 55:181–189
Jahid IK, Han N, Zhang CY et al (2015) Mixed culture biofilms of Salmonella Typhimurium and cultivable indigenous microorganisms on lettuce show enhanced resistance of their sessile cells to cold oxygen plasma. Food Microbiol 46:383–394
Ji W, Lee M, Kim G et al (2019) Quantitation of the ROS production in plasma and radiation treatments of biotargets. Sci Rep 9:19837
Jiang S, Chen S, Zhang CF et al (2017) Effect of the biofilm age and starvation on acid tolerance of biofilm formed by Streptococcus mutans isolated from caries-active and caries-free adults. Int J Mol Sci 18(4):713
Joaquin JC, Kwan C, Abramzon N et al (2009) Is gas-discharge plasma a new solution to the old problem of biofilm inactivation? Microbiology 155(Pt 3):724–732
Joshi SG, Paff M, Friedman G et al (2010) Control of methicillin-resistant Staphylococcus aureus in planktonic form and biofilms: a biocidal efficacy study of nonthermal dielectric-barrier discharge plasma. Am J Infect Control 38(4):293–301
Joshi SG, Cooper M, Yost A et al (2011) Nonthermal dielectric-barrier discharge plasma-induced inactivation involves oxidative DNA damage and membrane lipid peroxidation in Escherichia coli. Antimicrob Agents Chemother 55(3):1053–1062
Julák J, Vaňková E, Válková M et al (2020) Combination of non-thermal plasma and subsequent antibiotic treatment for biofilm re-development prevention. Folia Microbiol 65(5):863–869. https://doi.org/10.1007/s12223-020-00796-3
Karygianni L, Ren Z, Koo H et al (2020) Biofilm matrixome: Extracellular components in structured microbial communities. Trends Microbiol 28(8):668–681
Keidar M, Shashurin A, Volotskova O et al (2013) Cold atmospheric plasma in cancer therapy. Phys Plasmas 20(5):057101
Koban I, Geisel MH, Holtfreter B et al (2013) Synergistic effects of nonthermal plasma and disinfecting agents against dental biofilms in vitro. ISRN Dent Article 2013:573262
Kvam E, Davis B, Mondello F et al (2012) Nonthermal atmospheric plasma rapidly disinfects multidrug-resistant microbes by inducing cell surface damage. Antimicrob Agents Chemother 56(4):2028–2036
Lackmann JW, Schneider S, Edengeiser E et al (2013) Photons and particles emitted from cold atmospheric-pressure plasma inactivate bacteria and biomolecules independently and synergistically. J R Soc Interface 10(89):20130591
Lacombe A, Niemira BA, Gurtler JB et al (2017) Nonthermal inactivation of norovirus surrogates on blueberries using atmospheric cold plasma. Food Micro 63:1–5
Laroussi M (2002) Nonthermal decontamination of biological media by atmospheric-pressure plasmas: review, analysis, and prospects. EEE Trans Plasma Sci 30(4):1409–1415
Laroussi M (2005) Low temperature plasma-based sterilization: overview and state-of-the-art. Plasma Process Polym 2(5):391–400
Laroussi M (2009) Low-temperature plasma for medicine? IEEE Trans Plasma Sci 37(6):714–725
Laroussi M (2020) Cold plasma in medicine and healthcare: the new frontier in low temperature plasma applications. Front Phys. https://doi.org/10.3389/fphy.2020.00074
Laroussi M, Leipold F (2004) Evaluation of the roles of reactive species heat and UV radiation in the inactivation of bacterial cells by air plasmas at atmospheric pressure. Int J Mass Spectrom 233(1–3):81–86
Lee JH, Lee J, Ryu SY et al (2016) Calcium-chelating alizarin and other anthraquinones inhibit biofilm formation and the hemolytic activity of Staphylococcus aureus. Sci Rep 6:19267
Liao XY, Liu DH, Xiang QS et al (2017a) Inactivation mechanisms of non-thermal plasma on microbes: a review. Food Control 75:83–91
Liao XY, Xiang QS, Liu DH et al (2017b) Lethal and sublethal effect of a dielectric barrier discharge atmospheric cold plasma on Staphylococcus aureus. J Food Prot 80(6):928–932
Liao XY, Cullen PJ, Muhammad AI et al (2020) Cold plasma-based hurdle interventions: new strategies for improving food safety. Food Eng Rev 12:321–332. https://doi.org/10.1007/s12393-020-09222-3
Los A, Ziuzina D, Boehm D et al (2017) The potential of atmospheric air cold plasma for control of bacterial contaminants relevant to cereal grain production. Innov Food Sci Emerg Technol 44:36–45
Lu H, Patil S, Keener KM et al (2014) Bacterial inactivation by high-voltage atmospheric cold plasma: influence of process parameters and effects on cell leakage and DNA. J Appl Microbiol 116(4):784–794
Mai-Prochnow A, Clauson M, Hong J et al (2016) Gram positive and gram negative bacteria differ in their sensitivity to cold plasma. Sci Rep 6:38610
Majtan J, Sojka M, Palenikova H et al (2020) Vitamin C enhances the antibacterial activity of honey against planktonic and biofilm-embedded bacteria. Molecules 25(4):992
Marchal F, Robert H, Merbahi N et al (2012) Inactivation of gram-positive biofilms by low-temperature plasma jet at atmospheric pressure. J Phys D Appl Phys 45:345202
Matthes R, Hübner NO, Bender C et al (2014) Efficacy of different carrier gases for barrier discharge plasma generation compared to chlorhexidine on the survival of Pseudomonas aeruginosa embedded in biofilm in vitro. Skin Pharmacol Physiol 27:148–157
Miquel S, Lagrafeuille R, Souweine B et al (2016) Anti-biofilm activity as a health issue. Front Microbiol 7:592
Montie TC, Kelly-Wintenberg K, Roth JR (2000) An overview of research using the one atmosphere uniform glow discharge plasma (OAUGDP) for sterilization of surfaces and materials. IEEE Trans Plasma Sci 28(1):41–50
Morris AD, McCombs GB, Akan T et al (2009) Bactericidal effects on Geobacillus stearothermophilus and Bacillus cereus microorganisms. J Dent Hyg 83(2):55–61
Muhammad MH, Idris AL, Fan X et al (2020) Beyond risk: bacterial biofilms and their regulating approaches. Front Microbiol 11:928
Muro-Fraguas I, Sainz-García A, Gómez PF et al (2020) Atmospheric pressure cold plasma anti-biofilm coatings for 3D printed food tools. Innov Food Sci Emerg Technol 64:102404. https://doi.org/10.1016/j.ifset.2020.102404
Niedźwiedź I, Waśko A, Pawłat J et al (2019) The state of research on antimicrobial activity of cold plasma. Pol J Microbiol 68(2):153–164
Niemira BA, Boyd G, Sites J (2014) Cold plasma rapid decontamination of food contact surfaces contaminated with Salmonella biofilms. J Food Sci 79(5):917–922
Niemira BA, Boyd G, Sites J (2018) Cold plasma inactivation of Escherichia coli O157:H7 biofilms. Front Sustain Food Syst 2:UNSP 47
Oh SY, Yun W, Lee JH et al (2017) Effects of essential oil (blended and single essential oils) on anti-biofilm formation of Salmonella and Escherichia coli. J Anim Sci Technol 59(2):1–5
Paldrychová M, Vankova E, Scholtz V et al (2019) Effect of non-thermal plasma on AHL-dependent QS systems and biofilm formation in Pseudomonas aeruginosa: difference between non-hospital and clinical isolates. AIP Adv 9(5):055117
Pan YY, Cheng JH, Sun DW (2019) Cold plasma-mediated treatments for shelf life extension of fresh produce: a review of recent research developments. Compr Rev Food Sci Food Saf 18(5):1312–1326
Pandit S, Mokkapati VR, Helgadóttir SH et al (2017a) Combination of cold atmospheric plasma and vitamin C effectively disrupts bacterial biofilms. Clin Microbiol 6:3
Pandit S, Ravikumar V, Abdel-Haleem AM et al (2017b) Low concentrations of vitamin C reduce the synthesis of extracellular polymers and destabilize bacterial biofilms. Front Microbiol 8:2599
Pankaj SK, Wan ZF, Keener KM (2018) Effects of cold plasma on food quality: a review. Foods 7(1):4
Parsek MR, Greenberg EP (2005) Sociomicrobiology: the connections between quorum sensing and biofilms. Trends Microbiol 13(1):27–33
Patange A, Boehm D, Ziuzina D et al (2019) High voltage atmospheric cold air plasma control of bacterial biofilms on fresh produce. Int J Food Microbiol 293:137–145
Pina-Perez MC, Martinet D, Palacios-Gorba C et al (2020) Low-energy short-term cold atmospheric plasma: controlling the inactivation efficacy of bacterial spores in powders. Food Res Int 130:108921. https://doi.org/10.1016/j.foodres.2019.108921
Polaska M, Sokolowska B (2019) Bacteriophages–a new hope or a huge problem in the food industry. AIMS Microbiol 5(4):324–346
Poulsen LV (1999) Microbial biofilm in food processing. LWT-Food Sci Technol 32(6):321–326
Pu QK, Liu SJ, Huang H et al (2019) Sterilization effect of an atmospheric low temperature plasma jet on Candida albicans biofilm. J Sichuan Univ (Med Sci Ed) 50(3):339–343
Puligundla P, Mok C (2017) Potential applications of nonthermal plasmas against biofilm-associated micro-organisms in vitro. J Appl Microbiol 122(5):1134–1148
Rao YF, Shang WL, Yang Y et al (2020) Fighting mixed-species microbial biofilms with cold atmospheric plasma. Front Microbiol 11:1000
Rowan N, Espie S, Harrower J et al (2007) Evidence of lethal and sublethal injury in food-borne bacterial pathogens exposed to high-intensity pulsed-plasma gas discharges. Lett App Microbiol 46(1):80–86
Rutherford ST, Bassler BL (2012) Bacterial quorum sensing: its role in virulence and possibilities for its control. Cold Spring Harb Perspect Med 2(11):a012427
Saad M, Ong MHA, Osman NS et al (2019) Food contact surfaces’ hidden secrets and food handlers’ state of readiness. Asian J Qual Life 4(16):1–15
Sadekuzzaman M, Yang S, Mizan MFR et al (2015) Current and recent advanced strategies for combating biofilms. Compr Rev Food Sci Food Safety 14(4):491–509
Sakudo A, Yagyu Y, Onodera T (2019) Disinfection and sterilization using plasma technology: fundamentals and future perspectives for biological applications. Int J Mol Sci 20(20):5216
Sanchez-Vizuete P, Orgaz B, Aymerich S et al (2015) Pathogens protection against the action of disinfectants in multispecies biofilms. Front Microbiol 6:705
Schneider S, Lackmann JW, Narberhaus F et al (2011) Separation of VUV/UV photons and reactive particles in the effluent of a He/O2 atmospheric pressure plasma jet. Plasma Phys 44(29):295201
Šimoncicová J, Kryštofová S, Medvecká V et al (2019) Technical applications of plasma treatments: current state and perspectives. Appl Microbiol Biotechnol 103:5117–5129
Soares JA, Roque de Carvalho MA et al (2010) Effectiveness of chemomechanical preparation with alternating use of sodium hypochlorite and EDTA in eliminating intracanal Enterococcus faecalis biofilm. J Endod 36(5):894–898
Srey S, Jahid IK, Ha SD (2013) Biofilm formation in food industries: a food safety concern. Food Control 31(2):572–585
Srey S, Park SY, Jahid IK et al (2014) Reduction effect of the selected chemical and physical treatments to reduce L. monocytogenes biofilms formed on lettuce and cabbage. Food Res Int 62:484–491
Stoffels E, Sakiyama Y, Graves DB (2008) Cold atmospheric plasma: charged species and their interactions with cells and tissues. IEEE Trans Plasma Sci 36(4):1441–1457
Stringer SC, George SM, Peck MW (2000) Thermal inactivation of Escherichia coli O157:H7. J Appl Microbiol 88(S1):79–89
Taghizadeh L, Brackman G, Nikiforov A (2015) Inactivation of biofilms using a low power atmospheric pressure argon plasma jet; the role of entrained nitrogen. Plasma Process Polym 12:75–81
Tajkarimi M, Ibrahim SA (2011) Antimicrobial activity of ascorbic acid alone or in combination with lactic acid on Escherichia coli O157:H7 in laboratory medium and carrot juice. Food Control 22(6):801–804
Tariq S, Wani S, Rasool W et al (2019) A comprehensive review of the antibacterial, antifungal and antiviral potential of essential oils and their chemical constituents against drug-resistant microbial pathogens. Microb Pathog 134:103580. https://doi.org/10.1016/j.micpath.2019.103580
Theinkom F, Singer L, Cieplik F et al (2019) Antibacterial efficacy of cold atmospheric plasma against Enterococcus faecalis planktonic cultures and biofilms in vitro. PLoS One 14(11):e0223925
Timoshnikov VA, Kobzeva TV, Polyakov NE et al (2020) Redox interactions of vitamin C and iron: inhibition of the pro-oxidant activity by deferiprone. Int J Mol Sci 21:3967
Tschang CYT, Thoma M (2019) Biofilm inactivation by synergistic treatment of atmospheric pressure plasma and chelating agents. Clin Plasma Med 15:UNSP 100091
Vandervoort KG, Brelles-Marino G (2014) Plasma-mediated inactivation of Pseudomonas aeruginosa biofilms grown on borosilicate surfaces under continuous culture system. PLoS One 9(10):e108512
Vatansever F, de Melo WCA, Avci P et al (2013) Antimicrobial strategies centered around reactive oxygen species-bactericidal antibiotics, photodynamic therapy and beyond. FEMS Microbiol Rev 37(6):955–989
Vert M, Doi Y, Hellwich KH et al (2012) Terminology for biorelated polymers and applications (IUPAC recommendations 2012). Pure Appl Chem 84(2):377–410
Vilchèze C, Hartman T, Weinrick B et al (2013) Mycobacterium tuberculosis is extraordinarily sensitive to killing by a vitamin C-induced Fenton reaction. Nat Commun 4:1881
Vleugels M, Shama G, Deng XT et al (2005) Atmospheric plasma inactivation of biofilm-forming bacteria for food safety control. IEEE Trans Plasma 33(2):824–828
Vu B, Chen M, Crawford RJ et al (2009) Bacterial extracellular polysaccharides involved in biofilm formation. Molecules 14(7):2535–2554
Weltmann KD, von Woedtke T (2017) Plasma medicine-current state of research and medical application. Plasma Phys Control Fusion 59(1):014031
Wong HS, Townsend KM, Fenwick SG et al (2010) Comparative susceptibility of Salmonella Typhimurium biofilms of different ages to disinfectants. Biofouling 26(7):859–864
Xiong Z, Du T, Lu X et al (2011) How deep can plasma penetrate into a biofilm? Appl Phys Lett 98(22):221503
Yin W, Wang YT, Liu L et al (2019) Biofilms: the microbial “protective clothing” in extreme environments. Int J Mol Sci 20(14):3423
Yong HI, Kim HJ, Park S et al (2015) Evaluation of pathogen inactivation on sliced cheese induced by encapsulated atmospheric pressure dielectric barrier discharge plasma. Food Microbiol 46:46–50
Yuan L, Hansen MF, Roder HL et al (2020) Mixed-species biofilms in the food industry: current knowledge and novel control strategies. Crit Rev Food Sci Nutr 60(13):2277–2293
Zhu YL, Li CZ, Cui HY et al (2020) Feasibility of cold plasma for the control of biofilms in food industry. Trends Food Sci Technol 99:142–151
Ziuzina D, Patil S, Cullen PJ et al (2014) Dielectric barrier discharge atmospheric cold plasma for inactivation of Pseudomonas aeruginosa biofilms. Plasma Med 4(1–4):137–152
Ziuzina D, Boehm D, Patil S et al (2015a) Cold plasma inactivation of bacterial biofilms and reduction of quorum sensing regulated virulence factors. PLoS One 10(9):e0138209
Ziuzina D, Han L, Cullen PJ et al (2015b) Cold plasma inactivation of internalised bacteria and biofilms for Salmonella enterica serovar typhimurium, Listeria monocytogenes and Escherichia coli. Int J Food Microbiol 210:53–61
Author information
Authors and Affiliations
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2022 Zhejiang University Press
About this chapter
Cite this chapter
Xiang, Q., Niu, L., Bai, Y. (2022). Antibiofilm Application of Cold Plasma in Food Safety. In: Ding, T., Cullen, P., Yan, W. (eds) Applications of Cold Plasma in Food Safety. Springer, Singapore. https://doi.org/10.1007/978-981-16-1827-7_4
Download citation
DOI: https://doi.org/10.1007/978-981-16-1827-7_4
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-16-1826-0
Online ISBN: 978-981-16-1827-7
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)