Abstract
Cold plasma has garnered much attention in the last decade as food processing technology with potential in food preservation and managing the safety of food with minimum influence on quality characteristics. The action of cold plasma on pathogens is multi-targets, mostly including the cellular envelopes, cell membrane, lipids, proteins, and DNA via interaction with the reactive species in cold plasma and liquid medium. The multi-target high germicidal action of cold plasma on important food pathogens will undoubtedly play a vital role in the smooth adoption of this technology in fruit and vegetable juice and milk processing industries. Also, pH decline increased lipid oxidation, and changes in color, antioxidant activity, phenolic, and flavonoid concentrations were observed after cold plasma treatments. Therefore, intensified efforts are needed to address some of the quality issues relating to pH decline and heightened lipid oxidation in oily liquid food products, as this has a considerable influence on safety and consumer perception.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Nonthermal plasma
- Plasma-treated liquid food
- Plasma-activated water chemistry
- Microbial inactivation
- Quality attributes
11.1 Introduction
Food spoilage and poisoning from pathogens have become a topic of concern to food processors and researchers. If these pathogens are not controlled, they can contaminate food products and pose a significant threat to human health as a result of food-borne illnesses. The estimation of foodborne illnesses can be utilized to create food safety policies and strategies that can drastically reduce the episodes of food-borne outbreaks. The World Health Organization data of 2010 showed food-borne diseases have led to about 600 million illnesses and 420,000 premature deaths in most of the low- and middle-income countries (Sub-Saharan Africa, South Asia, and Southeast Asia). These translated to an estimated cost of $95.2 billion per annum, with an annual food-borne illness treatment cost of around $15 billion (World-Bank 2018). Likewise, the European Union reported about 88 million tons of food wastage every year estimated at a loss of $177.12 (€143) billion in value (Scherhaufer et al. 2018; Stenmarck et al. 2016). The economic burden of food-borne illnesses in the United States is over $15.5 billion, with about 9.4 million illnesses annually. The United State Center for Disease Control (CDC) food-borne estimates in 2011 showed that about 40 million people have become sick due to food-borne outbreak, among which 128,000 cases were hospitalized, and 3000 death were recorded (CDC 2018; Hoffmann et al. 2015).
Numerous pathogens have been associated with food-borne diseases and food spoilage, and these include Salmonella typhi that causes typhoid, Vibrio cholera that results in cholera, and Escherichia coli O157:H7 which causes illnesses including hemolytic uremic syndrome, characterized by renal failure and hemolytic anemia which can lead to permanent kidney dysfunction. The spores of Bacillus species were also associated with many food spoilage and poisoning (Lin et al. 2005; Malik et al. 2014). These pathogens and many others must be contained to prevent cross-contamination along the food value chain as well as during handling operations in the food industry. Therefore, the need for an effective processing strategy capable of decontaminating or killing of food pathogens is one of the most critical steps for the hazard analysis and critical control point (HACCP) system in the food industry.
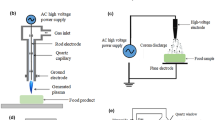
In this regard, the importance of cold plasma in ensuring food safety has been studies on many food products. The cold plasma produced a variety of reactive species that come in contact with food constituents and microorganisms, thus resulting in many reactions, which leads to microbial inactivation. Some chapters in this book have discussed some of the reactions involved in the antimicrobial efficacy of cold plasma. Cold plasma has been applied in various fruit and vegetable beverages (Fig. 11.1). This chapter will focus on the interactions of cold plasma reactive species with liquid foods and its antimicrobial effects from a food safety perspective.
11.2 Cold Plasma at Gas-Liquid Interphases
In order to fully understand the role of cold plasma in ensuring the safety of food products, one needs to understand its behavior in air and liquid phases. Cold plasma generated at gas-liquid interphase produces reactive species such as hydrogen peroxide (H2O2), hydroxyl radical (OH∙), nitric acid (HNO3), and ozone (O3) that have high antimicrobial efficacies (Liao et al. 2018b; Muhammad et al. 2018c). Liu et al. (2015) demonstrated how fast plasma species could be transferred from the point of generation to the air region. At the air gap region, reactive oxygen species (ROS) such as O3 and H2O2 can diffuse to the downstream liquid surface over a distance of 0.01 m within 1 s of discharge. However, the concentration of H2O2 declined due to larger Henry’s coefficient, whereas the concentration of the O3 remained similar to that at the exit of the plasma generation region. H2O2 is highly soluble in water compared to O3 and OH∙, and has a longer lifespan, thus making it an important ROS in microorganisms inactivation (Liao et al. 2018b; Liu et al. 2015). To further explain the diffusion of reactive species in air and water, Shimizu et al. (2011) studied the thermal flow pattern and chemical components transport using the platinum electrode under a water surface dielectric barrier discharge plasma system. The researchers found that the diffusion of reactive species towards the water surface was influenced by the airflow rate visibly demonstrated by the circular pattern. The plasma flume interaction with water resulted in pH reduction, thus affirming the dissolution of reactive species, including O3, H2O2, and nitrous acid (HNO2). These reactive species are distributed within the water molecules via convective transport, as explained by the discoloration of methyl red solution as the flow pattern progressed. This phenomenon highlighted how microorganisms in liquid could be inactivated when in contact with plasma-activated liquids. It is worth noting that only long-live plasma reactive species such as atomic oxygen (O), O3, H2O2, or nitric oxide (NO) can diffuse through the liquid medium and interact with microorganisms (Zuizina et al. 2012). Other reactive species of antimicrobial importance that are found in plasma-treated water include OH∙, peroxynitrite (ONOO−), nitrite (NO2−), and nitrate (NO3−) (Julák et al. 2018; Muhammad et al. 2019a). Their generation and importance with regard to food safety will be discussed in subsequent sections.
11.2.1 Understanding the Interaction of Cold Plasma Reactive Species with Water Molecules Using Atmospheric Air as Inducer Gas
Water subjected to cold plasma treatment at predefined condition is termed plasma-activated water (PAW) (Liao et al. 2020). Reactive oxygen and nitrogen species (RONS) are generated in PAW by several chemical reactions involving the plasma flume and the water molecules with atmospheric air as working gas. In cold plasma generation, atomic oxygen (O), nitrogen (N2), and OH∙ are excited at the gaseous plasma interphase and are transported into the water and converted to other RONS, including H2O2, NO2−, and NO3− at the liquid interphase (Lukes et al. 2014). The attack of high-energy electrons (e−) creates the reactive species in plasma discharge and combine with water molecules to produce many aqueous reactive species and radicals. The acidic RONS among them such as NO2− and NO3− increase the water acidity, and consequently enhanced the bactericidal efficacy of the water (Cao et al. 2018; Shen et al. 2016). Some of the reactions involved in the formation of reactive species are summarized below. When the e− energy is higher than that of the ionization energy of water molecules, the water molecules dissociate as a result of electron collision, thereby forming hydrogen radicals (H∙), OH∙, and other cations, as shown in reactions (11.1), (11.11), and (11.12) (Cao et al. 2018; Zhou et al. 2018).
According to Joshi et al. (1995), Laroussi and Leipold (2004), and Cao et al. (2018), the radicals can react with each other and produce other reactive species as illustrated in reactions (11.2)–(11.14).
OH∙ is among the crucial species in cold plasma discharges and is formed mainly as a result of electron striking water molecules with a short lifetime of few nanoseconds. Nevertheless, OH∙ can recombine to give a dimer H2O2 as in reaction (11.9) (Cao et al. 2018). Meanwhile, O3 is another essential ROS with strong oxidizing power similar to that of O and is produced from the reaction of O and O2 is demonstrated in reaction (11.6) (Muhammad et al. 2019b; Surowsky et al. 2014b). The solubility of H2O2 is higher in water compared to O3 and OH∙ and has a longer lifespan for microorganisms inactivation (Liao et al. 2018a; Liu et al. 2015). In addition to the above-mentioned RONS, ONOO− is another RONS of antimicrobial importance. Its instability has rendered this compound to have a less biological effect (Julák et al. 2018). In contrast to this, Machala et al. (2013) established that ONOO− is produced in plasma-treated liquids and correlated its antimicrobial efficacy with the amount that is produced. Although few researchers have reported its biological activity against some important food pathogens, its instability and production level is too low to be regarded as a key antibacterial agent alone and therefore is less explored during PAW studies. Notwithstanding, the contribution of ONOO− in PAW is the decomposition of the solution into highly reactive OH∙ and nitroxyl (NO2∙) (Slosky and Vanderah 2015). Shen et al. (2016) also reported ONOO− contribution during the cell death of Staphylococcus aureus by interacting with H+, NO2−, and H2O2 as indicated in reaction (11.15). On the other hand, studies have demonstrated that Escherichia coli cytochrome bd is resistant to damages caused by ONOO−. This rapidly triggers the degradation of ONOO− to NO2 thus, suggesting that ONOO− was more of a catalyst for the formation of more reactive NO2 (Borisov et al. 2015; Dezest et al. 2017).
11.3 Antimicrobial Mechanisms of Cold Plasma in Liquid Media
The mechanism of inactivation varies depending on the microorganisms (vegetative cells and spores). Bacterial spores are generally more resistant to cold plasma treatment than vegetative bacteria. Also, Gram-positive bacteria are more resistant to cold plasma treatment than Gram-negative bacteria. This is because they possess thicker peptidoglycan that allows them to resist external stress, thus block the influx of cold plasm reactive species through the cellular envelopes. Peptidoglycan is an essential biomolecule in the cell wall that increases bacterial rigidity and survival instinct (Liao et al. 2018b; López et al. 2019). The roles of reactive species in cold plasma for microbial inactivation are in different pathways, as showcased in Fig. 11.2. There are the physical means via the generation of electric fields, and ultraviolet (UV) radiation, and chemical means that are as a result of low pH and the action of RONS and their interaction with water.
Microorganisms exposed to cold plasma experienced continuous bombardment, shrinkage, and etching by charged particles due to electrostatic field at the cell membrane (Lunov et al. 2016; Schlüter and Fröhling 2014). Some microorganisms change shape from rod-shape to round shape to minimize the effect of electrostatic forces from the charged particles. When this effect becomes too high and unbearable, the integrity of the cell membrane is compromised, thereby allowing the inflow of RONS (Dezest et al. 2017; Fröhling and Schlüter 2015; Ha et al. 2016; Joshi et al. 2011). Electric fields generated in cold plasma have lethal effects on microorganisms such as electroporation (Lukes et al. 2012). This phenomenon rendered the cell membrane permeable and encouraged the influx of RONS into the cell. Cell shrinkage and damages of the cell membrane with loss of cytoplasmic membrane integrity are other pathways in which microorganisms are inactivated (López et al. 2019). The role of UV in microbial inactivation is to prevent bacterial replication via dimerization of thymine bases in DNA strands (Laroussi and Leipold 2004). The interaction of UV with genetic constituents, including DNA, increases with the continuous etching of the cell membrane (Moisan et al. 2002).
The low acidic pH has a synergistic antibacterial effect with RONS in PAW. Lower acidic environmental is more favorable for the reactive species to penetrate cell walls, and the presence of reactive species in such acidic conditions reduces the resistance of bacteria. Furthermore, the H+ in PAW flow into the intracellular of the bacterial cell, thereby disrupting the intracellular pH homeostasis, eventually lead to cell death (Xu et al. 2019, 2020; Zhang et al. 2016).
On the other hand, RONS as the most active components of cold plasma have caused the oxidation and degradation of cell biomolecules (Laroussi and Leipold 2004). RONS such as OH∙, NO∙, excited N2 and O2, O3, and H2O2 induced etching and erosion of cell membrane, lesion, and subsequent reaction with cell macromolecules (Park et al. 2015). Membrane lipid, especially polyunsaturated fatty acid, is one of the first to be attacked by RONS because it is located near the cell surface and has a high affinity to RONS. The peroxidation of lipid by O2 oxidation leads to lipid hydroperoxide that resulted in the production of Malondialdehyde (MDA) as a secondary product. MDA is used as the biomarker for oxidative stress. The formation of MDA in E. coli submitted to plasma increased with exposure time and can react with protein and DNA, thereby causing irreversible damage and eventual cell death (Alkawareek et al. 2014; Joshi et al. 2011; Liao et al. 2017).
Protein oxidation is another path in which microbial cells are targeted and can occur in different processes such as direct amino acid oxidation by H2O2 and recombination of lipid peroxidation by-products during the oxidation of cell membranes (Weber et al. 2015). Protein carbonylation is an irreversible change in the microbial cell, and this type of oxidation was reported in E. coli due to the interaction with H2O2 (Dezest et al. 2017).
11.4 Cold Plasma Processing of Fruit and Vegetable Juices
11.4.1 Effects on Microorganisms Inactivation
The safety of fruit and vegetable juices is critical in controlling food-borne outbreaks. The selection of appropriate processing techniques that will minimize microbial spoilages is essential. Generally, the presence of spoilage microorganisms in the range greater than 4 log CFU/mL in food products is responsible for their spoilage. Therefore, the choice of biological decontamination strategy that will drastically reduce the population of the microorganisms and minimize quality changes is needed. Thermal pasteurization of fruit and vegetable juices has been utilized for decades in the fruit juices preservation and extension of shelf life. However, this is accompanied by some quality changes that impaired the nutritional and organoleptic properties of the juices (Petruzzi et al. 2017). Fruit and vegetable juices are healthy, functional food products with several health benefits and disease prevention properties (Muhammad et al. 2018b). Cold plasma, due to its low temperature and high bactericidal efficacy, has been utilized for the treatment of thermolabile fruit juices to ensure their microbial safety, functionality, and nutritional attributes are preserved. Table 11.1 summarized the recent cold plasma inactivation of food pathogens in fruit and vegetable juices. Hosseini et al. (2020) reported 6 log CFU/mL inactivation of Escherichia coli in sour cherry juice after 9 min of cold plasma treatment. Apple juice exposed to cold plasma treatment for 30 min resulted in a 5.6 log CFU/mL reduction in Zygosaccharomyces rouxii. The scanning electron microscopy (SEM) images of the plasma-treated yeast cells showed some pores formation and contraction, with visible damages to the cell wall (Wang et al. 2020). The inactivation of a similar microorganism in apple juice resulted in about 5 log CFU/mL reduction after 90 W and 140 s of plasma treatment (Xiang et al. 2018). Another cold plasma treatment of apple juice by Liao et al. (2018a) showed 3.98–4.3 log CFU/mL inactivation of E. coli in less than 40 s at input powers ranging from 30 to 50 W. The researcher attributed the high microbial inactivation to the accumulation of H2O2, O3, and NO3− in the treated apple juice. Similarly, Surowsky et al. (2014a) obtained 5 log CFU/mL reduction of Citrobacter freundii in apple juice subjected to cold plasma treatment for 480 s after 24 h of storage. The treated cells showed severe deformation with rough surfaces, discrete ridges, and pores. Dasan and Boyaci (2018) compared the inactivation efficacy of cold plasma on E. coli in different fruit juices. The results revealed inactivation efficacies of 1.59, 1.43, 4.02, and 3.34 log CFU/mL reductions in orange, tomato, apple, and sour cherry juices, respectively. The study further linked the lower inactivation obtained in orange and tomato juices to different food matrices. Clear juices such as apple juice result in more log reduction than cloudy juices or juice with high suspended solids such as tomato juice. DBD plasma used in the inactivation of Staphylococcus aureus, E. coli, and C. albicans in orange juice showed more than 5 log CFU/mL after 25 s of treatment. The researchers explained that the reduction rate in the viable counts were higher in E. coli, then followed by S. aureus, and C. albicans being the least (Shi et al. 2011). In plasma-treated tangerine juice, E. coli at an initial concentration of 7 log CFU/mL was inactivated below the detection limit of 1 log CFU/mL after 3 min of plasma treatment (Yannam et al. 2018).
Starek et al. (2019) carried out cold plasma inactivation of numerous microorganisms in tomato juice. The inactivation results obtained were 3.45, 3.55, 3.32, 2.93, and 3.69 log CFU/mL reductions for mesophilic bacteria, yeast, molds, Candida albicans, and S. cerevisiae, respectively. Similarly, when a strawberry juice was exposed to cold plasma treatment for 10 min, a 1 log CFU/mL reduction in total aerobic bacteria was recorded (Mehta and Yadav 2020). A high-voltage atmospheric cold plasma (HVACP) employed in the inactivation of Salmonella enterica serovar Typhimurium in orange juice revealed more than 5 log CFU/mL reduction after 30 s of treatment using a mixture of 65% O2 + 30% N2 + 5% CO2 (MA65) as working gas. Meanwhile, treatment of 120 s using air and M65 gas with subsequent storage for 24 h inactivated the pathogen by 2.9 and 4.7 log CFU/mL, respectively. The morphological changes observed in the treated S. enterica included shrinkage and lysed cells, thus demonstrated obvious damages and loss of integrity (Xu et al. 2017). A similar HVACP cold plasma equipment was utilized by Pankaj et al. (2017) for the inactivation of S. cerevisiae in white grape juice. The microorganism was inactivated by 7.4 log CFU/mL within 4 min of plasma exposure. In cold plasma treatment of blueberry juice, Hou et al. (2019) obtained a 7.2 log CFU/mL reduction during the inactivation of Bacillus sp. after 6 min treatment at 1% O2 concentration. The antimicrobial potency and mode of action of plasma reactive species are based on cell wall etching and perforation, and cell permeabilization. ROS such as H2O2 and hydroperoxy radicals (∙HO2) have induced DNA and RNA damage and oxidation of protein and lipid at the cell level (Liao et al. 2018b; López et al. 2019; Surowsky et al. 2014a).
One of the main challenges facing cold plasm inactivation of pathogens in liquid food products is the availability of standardized equipment that can ensure uniformity in the generation and distribution of RONS within the liquid media that can achieve efficient inactivation. With the current trend in the activation efficacy, it is difficult to unify the pathogens inactivation due to many factors including microorganisms strain (bacteria, spores, fungi, or yeast), initial microbial load, Gram-positive or Gram-negative, treatment conditions (input voltage or power, gas type, duration, mode of exposure, and discharge type), and claim high pasteurization as stipulated by authorities for liquid foods. The United States Food and Drug Administration (FDA) has specified a minimum benchmark of 5 log CFU/mL for pathogens inactivation in fruit and vegetable juices or milk by novel emerging technologies be achieved before claiming pasteurization (FDA 2004).
11.4.2 Effects of Cold Plasma on Quality of Juices
The quality of processed food products determined consumers’ acceptance. Processing strategies should be applied to promote food safety, as well as preserved the quality of the treated food products. pH, acidity, and color are quality parameters that determine the freshness of beverages. Many studies have reported declines in the pH of fruit juices after cold plasma treatments (Dasan and Boyaci 2018; Liao et al. 2018a; Mehta and Yadav 2020; Pankaj et al. 2017; Shi et al. 2011; Starek et al. 2019; Wang et al. 2020; Xu et al. 2017). The acidification of plasma-treated foods usually depends on the treatment duration, input power, juices type, and inducer gas used. The increase in acidity has been associated with numerous plasma RONS reactions with food substrates and bioactive compounds. Among them is the degradation of amino acid by-products by ROS, with the formation of acidic compounds (Bußler et al. 2015). In addition, the interaction of water molecules and with charged ions also led to the formation of hydronium ions (Muhammad et al. 2018c).
Other notable changes in cold plasma-treated juices included changes in the color of apple juice after cold plasma treatment. The color of the apple juice became yellowish and lighter, with no dramatic changes in the volatile compounds as compared with the untreated juice (Liao et al. 2018a; Wang et al. 2020). Dasan and Boyaci (2018) also observed the quality of orange, tomato, apple, and sour cherry juices not visibly impaired in terms of color after cold plasma treatment. A similar assertion was made by Hou et al. (2019), where the original color of the treated blueberry juice remained unchanged.
11.4.3 Effects on the Functional Components, Vitamins, and Antioxidant Constituents
Phenolic compounds are bioactive components in fruit and vegetable juices. Their uptake into the body induces numerous health benefits such as anti-inflammatory, antimicrobial properties, and antioxidant. Cold plasma processing of fruit and vegetable juices caused some qualitative and quantitative changes in the composition and functionality of the bioactive components. Anthocyanins are water-soluble bioactive components found in the cell vacuole of plants. The exposure of these plant materials to cold plasma breaks up the cell membrane and leads to the release of intracellular substances, including bioactive compounds into the extracellular environment. This leads to enhanced mass transfer and reactions with RONS; consequently, the bioactive components are extracted faster (Muhammad et al. 2018b). Many studies have recorded accumulation or otherwise in total phenolic and anthocyanin contents in plasma-treated food products. The total phenolic content of the treated blueberry juice was significantly increased following plasma treatment from 2 to 4 min in the presence of 1% O2. On extending the treatment time, a significant reduction in anthocyanin and antioxidant activity was obtained (Hou et al. 2019). In contrast, Liao et al. (2018a) did not observe significant changes in total phenolic content and antioxidant capacity of apple juice treated at the lower input powers of 30 and 40 W. Accumulation of 10–15% in phenolic contents of orange, tomato, apple, and sour cherry juices was described by Dasan and Boyaci (2018) after cold plasma processing. In plasma-treated strawberry juice, an increment in total phenolic contents was discovered following 10 min of plasma treatment (Mehta and Yadav 2020). Likewise, the total phenolic and flavonoid contents and antioxidant capacity of white grape juice were found to reduce with an increase of plasma treatment time. The researchers opined that these decreases were comparable to those recorded with thermal pasteurization of the juice (Pankaj et al. 2017). The anthocyanin content of processed sour cherry juice was reduced by 4% after 9 min of cold plasma treatment (Hosseini et al. 2020).
Fruit and vegetable juices are natural sources of vitamins such as riboflavin (B2), pyridoxine (B6), biotin, vitamin A, C, and E, carotenoids, thiamin (B1), and lycopene. Some of these vitamins are stable during processing while others (carotenoids, lycopene, thiamin (B1), and vitamin A, C, and E) are degraded (Altemimi et al. 2017; Muhammad et al. 2018b; Pankaj et al. 2018). Cold plasma can exert its effect on vitamins present in juices during processing. Starek et al. (2019) also observed increases of 11% and 13% in the quantity of lycopene and carotenoid, respectively, during cold plasma processing of tomato juice. Sour cherry juice subjected to 9 min of cold plasma treatment experienced a 21% reduction in vitamin C content (Hosseini et al. 2020). The vitamin C content of the orange juice was also reduced by 22% after cold plasma treatment with air as inducer gas (Xu et al. 2017). A similar loss of 5% in vitamin C contents was observed in tomato juice after 5 min of treatment (Starek et al. 2019). Following the cold plasma treatment, a slight decrease in vitamin C content was noticed, and the shelf life of the orange juice was extended (Shi et al. 2011). Contrarily, a significant reduction in vitamin C in blueberry juice was obtained with an increase in cold plasma treatment time (Hou et al. 2019), meanwhile a decrease in vitamin C content of strawberry juice was equally recorded (Mehta and Yadav 2020). These changes in the concentrations of vitamins were mostly linked to the oxidation influence of ROS such as O2, O3, and OH∙.
11.5 Cold Plasma Processing of Milk
11.5.1 Effects of Cold Plasma on Microbial Inactivation
The presence of background microflora in milk determines its microbial safety limits and freshness. The total background microflora in such products ranging from 3.0 to 5.2 log CFU/mL for unpasteurized milk (Corrales et al. 2012). Even though consumption of milk can boost the health and well-being of individuals, they can also harbor pathogens that could pose health hazards to consumers due to poor handling. Several pathogens have been associated with milk in the past, and some, including L. monocytogenes and Bacillus cereus spores, can withstand and survive post-pasteurization environments (Olivier et al. 2005). The pasteurization technique that is employed for controlling milk pathogens should be in such a way that it does not harm the milk quality while providing the necessary antimicrobial effects required. In the last decades, studies have demonstrated that achieving a higher inactivation rate in milk is more complicated than in juices or model solutions due to the complex microstructure of milk. Corrales et al. (2012) associated the low microbial inactivation by ultraviolet radiation (UV-C) in tiger nut milk to turbidity due to suspended solids. Likewise, the inactivation of milk with pulsed electric field was also influenced by the presence of fat in the milk (Grahl and Märkl 1996). In cold plasma preservation of milk, encouraging results were recorded with regard to microbial safety. Table 11.2 has summarized cold plasma-related studies of milk inactivation. Gurol et al. (2012) recorded a 4.18 log CFU/mL reduction in the E. coli population in milk after 3 min of plasma treatment. Reductions of 2.43, 2.40, and 2.46 log CFU/mL for E. coli, L. monocytogenes, and S. typhimurium, respectively, were reported in cold plasma-treated milk by Kim et al. (2015) following 10 min exposure. Another study explored an important milk pathogen (Prototheca zopfii) that causes mastitis, which results in low milk production and quality deterioration in cows. The cold plasma treatment of this milk revealed more than 2 log CFU/mL reduction of P. zopfii (Tyczkowska-Sieron et al. 2018).
Unlike in the studies mentioned above, higher logarithmic inactivation from the milk of plant origin was reported. This could probably be due to their lower fat and protein contents as compared to dairy milk. When tiger nut milk was submitted to cold plasma treatment, the microflora of the milk was reduced to an undetectable limit after 12 min of treatment (Muhammad et al. 2018a). Similarly, a 5.28-log reduction of Bacillus cereus at input powers ranging from 39 to 46 W was obtained during the cold plasma processing of tiger nut milk (Muhammad et al. 2019b). It is worthwhile mentioning that irrespective of the milk origin, cold plasma can be relied upon to ensure the microbial safety of the treated milk.
11.5.2 Effects of Cold Plasma Treatment on Quality Attributes of Milk
The cold plasma-treated milk also experiences some quality changes, as indicated in Table 11.2, mainly due to reaction with some plasma immanent species. Similar to fruit juices, the pH of plasma-treated milk was found to decline with increases of treatment time and input power (Gurol et al. 2012; Kim et al. 2015; Muhammad et al. 2018a, 2019b). Besides, slight changes in color parameters were observed in both treated milk from animal and plant origin (Gurol et al. 2012; Kim et al. 2015; Muhammad et al. 2019b).
11.5.3 Influence of Cold Plasma on Bioactive Components of Milk
Milk is among the healthy food with abundant functional ingredients. The bioactive components are altered due to cold plasma treatment. The concentrations of the fatty acids in the plasma-treated milk were not altered except butyric acid and caprylic acid (Kim et al. 2015). Meanwhile, the total phenolic content of tiger nut milk was significantly increased in a time- and input power-dependent manner after cold plasma treatment. In contrast, the antioxidant capacity and total flavonoid content were markedly reduced in similar trends (Muhammad et al. 2019b).
Another critical quality issues associated with cold plasma treatment of milk is lipid oxidation. Many researchers have reported an escalation of lipid oxidation in cold plasma-treated foods (Kim et al. 2015; Muhammad et al. 2018a). This is expected as plasma contains many oxidizing ROS. The lipid oxidation induced by ROS could negatively influence the acceptability and shelf life of food products. Although it is a general belief that cold plasma does not produce waste by-products, the level of lipid oxidation occurs in high oxygen plasma food called for safety scrutiny. To date, no document has an in-depth study of the safety issues of the secondary metabolite generated in plasma-treated milk. One study, however, emphasized the need to investigate the cold plasma influence on lipids (Gavahian et al. 2018). This could reduce the adverse effects on the sensory characteristics of the food products and clear the related health concerns.
11.6 Conclusions
Cold plasma is a nonthermal strategy for managing the safety of food products and has achieved tremendous bactericidal efficacy in the last two decades concerning liquid food products. This chapter presented a recap of cold plasma inactivation of pathogens with high microbial risks in liquid food products by the lethal actions of cold plasma. Even though cold plasma has enormous contributions to food safety, it still presents some challenges related to the quality of the treated products. Already, some of the products such as milk and juices are complex multi-component food products containing proteins, starch, vitamins, lipids, phenolics, and antioxidant constituents, and their reaction with cold plasma RONS become more complicated to understand the chemical reaction mechanisms. This is one of the drawbacks that require further attention to realize its full-scale application in food industries. A tailored cold plasma treatment that targets high inactivation efficacy for a specific food matrix (liquid or solid foods) with negligible quality changes can offer a solution to this challenge. Therefore, the design of cold plasma equipment, standardization of protocols for pathogens inactivation using the various plasma equipment, choice of inducer gas, and treatment duration are critical process conditions that should be optimized to achieve effective pathogens inactivation with minimum or negligible quality changes.
References
Alkawareek MY, Gorman SP, Graham WG et al (2014) Potential cellular targets and antibacterial efficacy of atmospheric pressure nonthermal plasma. Int J Antimicrob Agents 43(2):154–160
Altemimi A, Lakhssassi N, Baharlouei A et al (2017) Phytochemicals: extraction, isolation, and identification of bioactive compounds from plant extracts. Plants 6(42):1–23
Borisov VB, Forte E, Siletsky SA et al (2015) Cytochrome bd from Escherichia coli catalyzes peroxynitrite decomposition. Biochim Biophys Acta Biomembr 1847(2):182–188
Bußler S, Herppich WB, Neugart S et al (2015) Impact of cold atmospheric pressure plasma on physiology and flavonol glycoside profile of peas (Pisum sativum ‘Salamanca’). Food Res Int 76:132–141
Cao Y, Qu G, Li T et al (2018) Review on reactive species in water treatment using electrical discharge plasma: formation, measurement, mechanisms and mass transfer. Plasma Sci Technol 20:103001
CDC (2018) Burden of foodborne illness: findings. https://www.cdc.gov/foodborneburden/2011-foodborne-estimates.html. Accessed 17 Apr 2020
Corrales M, De Souza PM, Stahl MR et al (2012) Effects of the decontamination of a fresh tiger nuts’ milk beverage (horchata) with short wave ultraviolet treatments (UV-C) on quality attributes. Innov Food Sci Emerg Technol 13:163–168
Dasan BG, Boyaci IH (2018) Effect of cold atmospheric plasma on inactivation of Escherichia coli and physicochemical properties of apple, orange, tomato juices, and sour cherry nectar. Food Bioprocess Technol 11(2):1–10
Dezest M, Bulteau AL, Quinton D et al (2017) Oxidative modification and electrochemical inactivation of Escherichia coli upon cold atmospheric pressure plasma exposure. PLoS One 12(3):1–18
FDA (2004) Guidance for industry: juice hazard analysis critical control point hazards and controls guidance. 1st Edition. FDA Guidance Documents website. https://www.fda.gov/regulatory-information/search-fda-guidance-documents/guidance-industry-juice-hazard-analysis-critical-control-point-hazards-and-controls-guidance-first. Accessed 27 Apr 2020
Fröhling A, Schlüter O (2015) Flow cytometric evaluation of physico-chemical impact on Gram-positive and Gram-negative bacteria. Front Microbiol 6:1–21
Gavahian M, Chu YH, Mousavi Khaneghah A et al (2018) A critical analysis of the cold plasma induced lipid oxidation in foods. Trends Food Sci Technol 77:32–41
Grahl T, Märkl H (1996) Killing of microorganisms by pulsed electric fields. Appl Microbiol Biotechnol 45:148–157
Gurol C, Ekinci FY, Aslan N et al (2012) Low temperature plasma for decontamination of E. coli in milk. Int J Food Microbiol 157(1):1–5
Ha L, Patil S, Boehm D et al (2016) Mechanisms of inactivation by high-voltage atmospheric cold plasma differ for Escherichia coli and Staphylococcus aureus. Appl Environ Microbiol 82(2):450–458
Hoffmann S, Maculloch B, Batz M (2015) Economic burden of major foodborne illnesses acquired in the United States. Economic Information Bulletin. https://www.ers.usda.gov/webdocs/publications/43984/52807_eib140.pdf
Hosseini SM, Rostami S, Hosseinzadeh Samani B et al (2020) The effect of atmospheric pressure cold plasma on the inactivation of Escherichia coli in sour cherry juice and its qualitative properties. Food Sci Nutr 8(2):870–883
Hou Y, Wang R, Gan Z et al (2019) Effect of cold plasma on blueberry juice quality. Food Chem 290:79–86
Joshi AA, Locke BR, Arce P et al (1995) Formation of hydroxyl radicals, hydrogen peroxide and aqueous electrons by pulsed streamer corona discharge in aqueous solution. J Hazard Mater 41(1):3–30
Joshi SG, Cooper M, Yost A et al (2011) Nonthermal dielectric-barrier discharge plasma-induced inactivation involves oxidative DNA damage and membrane lipid peroxidation in Escherichia coli. Antimicrob Agents Chemother 55(3):1053–1062
Julák J, Hujacová A, Scholtz V et al (2018) Contribution to the chemistry of plasma-activated water. Plasma Phys Rep 44(1):125–136
Kim H-J, Yong HI, Park S et al (2015) Microbial safety and quality attributes of milk following treatment with atmospheric pressure encapsulated dielectric barrier discharge plasma. Food Control 47:451–456
Laroussi M, Leipold F (2004) Evaluation of the roles of reactive species, heat, and UV radiation in the inactivation of bacterial cells by air plasmas at atmospheric pressure. Int J Mass Spectrom 233(1–3):81–86
Liao X, Liu D, Xiang Q et al (2017) Inactivation mechanisms of nonthermal plasma on microbes: a review. Food Control 75:83–91
Liao X, Li J, Muhammad AI et al (2018a) Application of a dielectric barrier discharge atmospheric cold Plasma (Dbd-Acp) for Escherichia coli inactivation in apple juice. J Food Sci 83(2):401–408
Liao X, Muhammad AI, Chen S et al (2018b) Bacterial spore inactivation induced by cold plasma. Crit Rev Food Sci Nutr 59(16):1–11
Liao X, Xiang Q, Cullen PJ et al (2020) Plasma-activated water (PAW) and slightly acidic electrolyzed water (SAEW) as beef thawing media for enhancing microbiological safety. LWT 117:108649
Lin CTJ, Jensen KL, Yen ST (2005) Awareness of foodborne pathogens among US consumers. Food Qual Prefer 16(5):401–412
Liu ZC, Liu DX, Chen C et al (2015) Physicochemical processes in the indirect interaction between surface air plasma and deionized water. J Phys D Appl Phys 48(49):495201
López M, Calvo T, Prieto M et al (2019) A review on nonthermal atmospheric plasma for food preservation: mode of action, determinants of effectiveness, and applications. Front Microbiol 10:622
Lukes P, Locke BR, Brisset J (2012) Aqueous-phase chemistry of electrical discharge plasma in water and in gas–liquid environments. In: Parvulescu VI, Magureanu M, Lukes P (eds) Plasma chemistry and catalysis in gases and liquids. Wiley, Hoboken, NJ, pp 243–308
Lukes P, Dolezalova E, Sisrova I et al (2014) Aqueous-phase chemistry and bactericidal effects from an air discharge plasma in contact with water: evidence for the formation of peroxynitrite through a pseudo-second-order post-discharge reaction of H2O2 and HNO2. Plasma Sources Sci Technol 23:015019
Lunov O, Zablotskii V, Churpita O et al (2016) The interplay between biological and physical scenarios of bacterial death induced by nonthermal plasma. Biomaterials 82:71–83
Machala Z, Tarabova B, Hensel K et al (2013) Formation of ROS and RNS in water electro-sprayed through transient spark discharge in air and their bactericidal effects. Plasma Processes Polym 10(7):649–659
Malik A, Erginkaya Z, Ahmad S et al (eds) (2014) Food process: strategies for quality assessment, Food engineering series. Springer, New York
Mehta D, Yadav SK (2020) Impact of atmospheric nonthermal plasma and hydrothermal treatment on bioactive compounds and microbial inactivation of strawberry juice: a hurdle technology approach. Int J Food Sci 26(1):3–10
Moisan M, Barbeau J, Crevier M-C et al (2002) Plasma sterilization. Methods and mechanisms. Pure Appl Chem 74(3):349–358
Muhammad AI, Li Y, Liao X et al (2018a) Effect of dielectric barrier discharge plasma on background microflora and physicochemical properties of tiger nut milk. Food Control 96:119–127
Muhammad AI, Liao X, Cullen PJ et al (2018b) Effects of nonthermal plasma technology on functional food components. Compr Rev Food Sci Food Saf 17(5):1379–1394
Muhammad AI, Xiang Q, Liao X et al (2018c) Understanding the impact of nonthermal plasma on food constituents and microstructure—a review. Food Bioprocess Technol 11(3):463–486
Muhammad AI, Chen W, Liao X et al (2019a) Effects of plasma-activated water and blanching on microbial and physicochemical properties of tiger nuts. Food Bioprocess Technol 12(10):1721–1732
Muhammad AI, Lv R, Liao X et al (2019b) Modeling the inactivation of Bacillus cereus in tiger nut milk treated with cold atmospheric pressure plasma. J Food Prot 82(11):1828–1836
Olivier SP, Jayarao BM, Almeida RA (2005) Foodborne pathogens in milk and the dairy farm environment: food safety and public health implications. Foodborne Pathog Dis 2(2):115–137
Pankaj SK, Wan Z, Colonna W et al (2017) Effect of high voltage atmospheric cold plasma on white grape juice quality. J Sci Food Agric 97(12):4016–4021
Pankaj SK, Wan Z, Keener KM (2018) Effects of cold plasma on food quality: a review. Foods 7(1):4
Park JH, Kumar N, Park DH et al (2015) A comparative study for the inactivation of multidrug resistance bacteria using dielectric barrier discharge and nanosecond pulsed plasma. Sci Rep 5:13849
Petruzzi L, Campaniello D, Speranza B et al (2017) Thermal treatments for fruit and vegetable juices and veverages: a literature overview. Compr Rev Food Sci Food Saf 16(4):668–691
Scherhaufer S, Moates G, Hartikainen H et al (2018) Environmental impacts of food waste in Europe. Waste Manag 77:98–113
Schlüter O, Fröhling A (2014) Cold plasma for bioefficient food processing. Encyclopedia Food Microbiol 2:948–953
Shen J, Tian Y, Li Y et al (2016) Bactericidal effects against S. aureus and physicochemical properties of plasma activated water stored at different temperatures. Sci Rep 6:28505
Shi X, Zhang G, Wu X et al (2011) Effect of low-temperature plasma on microorganism inactivation and quality of freshly squeezed orange juice. IEEE Trans Plasma Sci 39(7):1591–1597
Shimizu T, Iwafuchi Y, Morfill GE (2011) Formation of thermal flow fields and chemical transport in air and water by atmospheric plasma. New J Phys 13:053025
Slosky LM, Vanderah TW (2015) Therapeutic potential of peroxynitrite decomposition catalysts: a patent review. Expert Opin Ther Pat 25(4):443–466
Starek A, Pawłat J, Chudzik B et al (2019) Evaluation of selected microbial and physicochemical parameters of fresh tomato juice after cold atmospheric pressure plasma treatment during refrigerated storage. Sci Rep 9(1):1–11
Stenmarck Å, Jensen C, Quested T et al. (2016) Estimates of European food waste levels. In Fusions. https://www.eu-fusions.org/phocadownload/Publications/Estimates of European food waste levels.pdf%5Cnhttps://phys.org/news/2016-12-quarter-million-tonnes-food-logistics.html#nRlv
Surowsky B, Fröhling A, Gottschalk N et al (2014a) Impact of cold plasma on Citrobacter freundii in apple juice: inactivation kinetics and mechanisms. Int J Food Microbiol 174:63–71
Surowsky B, Schlüter O, Knorr D (2014b) Interactions of nonthermal atmospheric pressure plasma with solid and liquid food systems: a review. Food Eng Rev 7(2):82–108
Tyczkowska-Sieron E, Markiewicz J, Grzesiak B et al (2018) Short communication: cold atmospheric plasma inactivation of Prototheca zopfii isolated from bovine milk. Int J Dairy Sci 101(1):118–122
Wang Y, Wang Z, Zhu X et al (2020) Application of electrical discharge plasma on the inactivation of Zygosaccharomyces rouxii in apple juice. LWT-Food Sci Technol 121:108974
Weber D, Davies MJ, Grune T (2015) Determination of protein carbonyls in plasma, cell extracts, tissue homogenates, isolated proteins: focus on sample preparation and derivatization conditions. Redox Biol 5:367–380
World-Bank (2018) Foodborne illnesses cost US$ 110 billion per year in low- and middle-income countries. 2019/072/AGR website. https://www.worldbank.org/en/news/press-release/2018/10/23/food-borne-illnesses-cost-us-110-billion-per-year-in-low-and-middle-income-countries. Accessed 27 Mar 2020
Xiang Q, Liu X, Li J et al (2018) Effects of dielectric barrier discharge plasma on the inactivation of Zygosaccharomyces rouxii and quality of apple juice. Food Chem 254(136):201–207
Xu L, Garner AL, Tao B et al (2017) Microbial inactivation and quality changes in orange juice treated by high voltage atmospheric cold plasma. Food Bioprocess Technol 10:1778–1791
Xu H, Zhu Y, Cui D et al (2019) Evaluating the roles of OH radicals, H2O2, ORP and pH in the inactivation of yeast cells on a tissue model by surface micro-discharge plasma. J Phys D Appl Phys 52(39):395201
Xu H, Ma R, Zhu Y et al (2020) A systematic study of the antimicrobial mechanisms of cold atmospheric-pressure plasma for water disinfection. Sci Total Environ 703:134965
Yannam SK, Estifaee P, Rogers S et al (2018) Application of high voltage electrical discharge plasma for the inactivation of Escherichia coli ATCC 700891 in tangerine juice. LWT-Food Sci Technol 90:180–185
Zhang Q, Ma R, Tian Y et al (2016) Sterilization efficiency of a novel electrochemical disinfectant against Staphylococcus aureus. Environ Sci Technol 50(6):3184–3192
Zhou R, Zhou R, Prasad K et al (2018) Cold atmospheric plasma activated water as a prospective disinfectant the crucial role of peroxynitrite. Green Chem 20:5276–5284
Zuizina D, Patil S, Cullen PJ et al (2012) Atmospheric cold plasma inactivation of Escherichia coli in liquid media inside a sealed package. J Appl Microbiol 114:778–787
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2022 Zhejiang University Press
About this chapter
Cite this chapter
Muhammad, A.I. (2022). Application of Cold Plasma in Liquid Food Products. In: Ding, T., Cullen, P., Yan, W. (eds) Applications of Cold Plasma in Food Safety. Springer, Singapore. https://doi.org/10.1007/978-981-16-1827-7_11
Download citation
DOI: https://doi.org/10.1007/978-981-16-1827-7_11
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-16-1826-0
Online ISBN: 978-981-16-1827-7
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)