Abstract
In this study, orange, tomato, apple juices, and sour cherry nectar were exposed to an atmospheric pressure plasma jet. Plasma treatments were carried out using air as a precursor under constant gas flow (3000 L/h) at 650 W for different treatment times (30, 60, 90, and 120 s). After plasma processing, reduction of Escherichia coli, Hunter’s color parameters (L*, a*, b*), total phenolic content, and pH values were evaluated. The inactivation effect of cold atmospheric plasma (CAP) was investigated on E. coli, and the highest significant reductions were achieved in apple juice (4.02 ± 0.03 log CFU/mL) followed by sour cherry (3.34 ± 0.09 log CFU/mL), while the values in orange (1.59 ± 0.17 log CFU/mL) and tomato juices (1.43 ± 0.22 log CFU/mL) were lower, which could be attributed to the food matrix. Color parameters, except for apple juice, did not show significant changes after processing. Compared to untreated juice, plasma treatment yielded higher phenolic content from 10 to 15%, while pH values did not change significantly and the temperature remained below 40 °C after all plasma treatments. This study showed that CAP treatment had positive influences on phenolic stability and color change in all samples regardless of food intrinsic factors, while it was more effective on bacterial inactivation in clear juices than turbid ones. Our results indicate that atmospheric plasma appears to be a promising technology for microbial inactivation without causing undesirable changes in food product.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Nowadays, consumer demand for fresh food products which are healthier and tastier with minimum amount of chemical preservatives and/or processing conditions has been on the increase. Fruit juice as a healthy drink is also popular among today’s consumers. There is an ongoing trend among people towards consumption of juice since a number of studies reported nutritional and health benefits of fruit juice consumption (Echeverría and López 2014; Falguera and Ibarz 2014). Fruit juices are reported to contain high amounts of vitamins, minerals, and other essential nutrients that contribute to the functional property of food products (Dhuique-Mayer et al. 2005). Currently, manufacturing process of fruit juice is attracting both consumers’ and researchers’ attention and has become a hot topic in the industry.
These days, health-conscious consumers are looking out for some quality parameters in food products such as improved food safety, nutritional value, freshness, and flavors. Clean label foods which claim to be natural and fresh as well as free from chemical additives have gradually gained attention among consumers. Therefore, the food industry feels the need to use processing technologies capable of reducing additives while maintaining natural flavors and food quality, and this pursuit has increased development of new non-thermal processing technologies. Emerging technologies have to be able to deliver microbiologically stable products with elongated shelf life and high sensory and nutritional characteristics.
Conventional thermal methods are based on heating process that facilitates mass transfer between different phases of the system, consume lots of energy, and can significantly change the concentration, bioavailability, and bioactivity of phytochemicals in food (Barba et al. 2015a; Barba et al. 2015b; Zinoviadou et al. 2015; Buchner et al. 2006). Current tendency of consumers to prefer fresh fruit juice has been increased due to its much better organoleptic properties than pasteurized ones (Butz and Tauscher 2002). In order to reduce the adverse effects (loss of vitamins, flavor, and non-enzymatic browning) of thermal pasteurization, other methods that are effective in microorganism inactivation can be applied. In this respect, non-thermal processing as an alternative for juice quality preservation has become a promising technique to meet consumer’s expectations since it has the ability to inactivate microorganisms at ambient temperatures, thus avoiding negative effects of heat on flavor, color, and nutritional value of foods (Ferrari et al. 2010). New non-thermal technologies such as pulsed electric fields (Charles-Rodriguez et al. 2007), ultraviolet light (Noci et al. 2008), high hydrostatic pressure (Valdramidis et al. 2009), supercritical CO2 or N2O (Gasperi et al. 2009), membrane filtration (Gialleli et al. 2016), and ultrasound (Simunek et al. 2014) have been assessed for their effectiveness on microbiological stability of the final product as well as their impact on sensory characteristics. The biggest challenge about the use of non-thermal technologies for food processing is the inactivation of pathogenic microorganisms and food spoilage agents; however, these drawbacks can be overcome by using various methods.
Plasma, referred as the fourth state of matter, is a partially or completely ionized gas and a reactive atmosphere where a variety of energetic and charged species (electrons, positive and negative ions), radicals, neutral species (excited atoms and molecules), and photons (visible and UV) are formed mainly from collisions of energetic electrons with heavy particles (atoms, molecules, ions). These reactive plasma species are able to break down covalent bonds and initiate numerous chemical reactions significant for various technological applications (Surowsky et al. 2015; Mutlu et al. 1998; Gunaydin et al. 2010; Arjunan et al. 2015; Schluter et al. 2013; Biederman et al. 2001). Reactive atoms, charged particles, reactive oxygen species (ROSs), reactive nitrogen species (RONs), and UV photons, all able to inactivate microorganisms at different levels, are formed when air is used as plasma process gas (Mai-Prochnow et al. 2014). Reactive oxygen and nitrogen species interact with macromolecules, like lipids, amino acids, and nucleic acids, and cause changes that lead to microbial death or injury. In addition, charged particles accumulate on the surface of cell membrane and induce its rupture. UV photons also modify the DNA of microorganisms (Smet et al. 2016). However, reactive plasma species play a more dominant role in inactivation effect of atmospheric pressure plasma compared to the effect of charged particles and UV (Deng et al. 2006; Graves 2012). Reactive oxygen species (atomic oxygen (O), ozone (O3), and hydroxyl radicals (OH·)) generated during plasma in the presence of oxygen are known to be the most important components that provide plasma inactivation, cell damage, and cell death (Fridman et al. 2007; Moisan et al. 2001; von Woedtke et al. 2013). These active species are responsible for biological reactions ranging from intercellular DNA fracture and protein degeneration to oxidation of the outer membrane (Y. Ma et al. 2008). Due to the non-thermal characteristic of non-equilibrium plasma discharges (energetic electrons), these plasma processes offer unique combination of high reactivity at moderate temperatures, which is beneficial for treatment of temperature-sensitive substrates.
Cold atmospheric plasma (CAP) is an emerging technology that offers many potential applications. The findings of our previous studies showed the influence of cold plasma on solid surface decontamination of both foodstuffs and food-contact materials (Dasan et al. 2016a, b; 2017a, b). Plasma technology ensures microbial safety of food without addition of preservatives and allows processed food to maintain natural flavors and nutritional value of the original food material. Therefore, it is recognized as a minimal processing technology that ensures both food safety and flavor. Due to low operating temperatures and simultaneous high antimicrobial effects, cold plasma may be regarded as a future alternative for thermal pasteurization (Herceg et al. 2016). There are very few studies that investigated the possibility of using plasma to enhance quality and microbiological safety of liquid foods (Gurol et al. 2012; Ikawa et al. 2010; Surowsky et al. 2014). Cold atmospheric plasma as a non-thermal technology was investigated for polyphenol and color stability in pomegranate juices (Kovacevic et al. 2016b; Herceg et al. 2016), sour cherry juice (Garofulic et al. 2015), and chokeberry juice (Kovacevic et al. 2016a); for volatile components in tomato juice (Ma and Lan 2015); and for microorganism inactivation (Shi et al. 2011) and quality characteristics (Almeida et al. 2015) of orange juice. Cold atmospheric plasma has emerged to be a potential novel non-thermal technology for quality improvement of fruit juices. However, the literature still lacks a comparative study concerning the effects of cold plasma on both microbial degradation, considering food intrinsic factors that could influence resistance of microorganisms towards CAP treatment, and quality characteristics of various fruit juices. The aim of the present study was to examine influence of atmospheric cold plasma in apple, orange, tomato juices, and sour cherry nectar on (i) inactivation of artificially inoculated Escherichia coli, (ii) color stability, (iii) total phenolic content, and (iv) temperature and pH changes.
Materials and Methods
Juice Samples and Culture
In this study, clear and turbid fruit juices were selected as model food samples. Apple juice and sour cherry nectar were selected as clear, while orange and tomato juices as turbid juices. Pasteurized juice samples were obtained commercially from the same company from a local market in Ankara, Turkey. Samples were stored at 4 °C. E. coli ATCC 25922 was provided from Refik Saydam Hıfzıssıhha Microbiology Lab (Ankara, Turkey) on slant agar media. The bacterial strain was maintained in Brain Hearth Infusion (BHI) broth (Merck KGaA, Darmstadt, Germany) containing glycerol (1/1 V/V) at − 20 and − 80 °C. Viable counts were performed according to standard double-layer plate-counting method on Violet Red Bile Agar (VRBA) (Merck KGaA, Darmstadt, Germany) and expressed in log CFU/mL.
Test Microorganism and Inoculum Preparation
Two steps of regeneration procedure were followed. For the first regeneration, 100 μL of stock culture was inoculated into a 10-mL BHI broth and incubated at 37 °C for 18 h after 18 h in their early stationary phase of growth. Then, this procedure was repeated for the second step. Following the incubation, 1 mL of cell suspension was pipetted into 10 mL of each juice sample placed in a sterile Petri dish. The final concentrations of the juices were determined to be 6.33 ± 0.08, 6.50 ± 0.02, 6.16 ± 0.06, and 6.22 ± 0.08 log CFU/mL for orange, tomato, apple juices, and sour cherry nectar, respectively, with standard double-layer plate-counting method onto VRBA.
Cold Atmospheric Pressure Plasma System
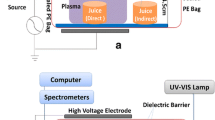
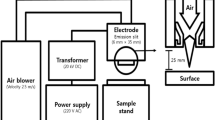
The atmospheric plasma system (Plasmatreat GmbH, Steinhagen, Germany) consisting of a 3 × 400 V–16 A power generator, a plasma jet, a high voltage transformer, and a pressure supply control unit was described in our previous studies (Dasan et al. 2017a, b) and it was used with some modifications (Fig. 1.). The plasma was generated through a high-voltage discharge with a single electrode, forming a plasma discharge that exits the jet nozzle at high velocity onto the sample surface. The atmospheric pressure plasma jet (APPJ) was mounted on an x-y motion system so that it could move in the determined x and y coordinates and scan the whole surface of material. Additionally, a double-nozzle system comprising two separate plasma nozzles which rotate in unison around the rotary axis to give a particularly intensive and uniform treatment was used. This rotary nozzle allows a homogeneous plasma treatment of wider surfaces with lower temperature increase in the treated area. The results from previous studies performed using the same plasma source showed that maximum inactivation effect on pathogenic microorganisms on solid surfaces (Dasan et al. 2016a) was achieved by applying 25 kHz frequency and 100% V that corresponds to 650 W (the highest power reachable for this APPJ) plasma power. Furthermore, the highest lethal effect of atmospheric plasma treatment was obtained using dry air as a plasma precursor. A gas flow of 3000 L/h was used to generate plasma from the tip of the needle electrode to the inner wall of the nozzle. Such plasma source produces a three-dimensional cone-shaped plasma jet that rotates around its own axis extending out of capillary tube to the length of about 30 mm with diameters of 15–30 mm at air gas flow of 3000 L/h.
Plasma Application on Fruit Juices—Inactivation of Escherichia coli
Each juice sample of 10 mL inoculated with 1 mL of E. coli suspension was spread evenly on glass Petri dishes (9.0 cm × 9.0 cm, thickness of 50 mm) for each experiment operated in atmospheric air with the room temperature of 20 °C. Following bacterial inoculation, the juice samples were immediately exposed to plasma treatment. Distance of plasma nozzle tip from the samples was fixed at 3.5 cm. CAP was applied using air as a precursor at a gas flow rate of 3000 L/h at 650 W plasma power, with a jet slide velocity of 5 m/min and scanning an area of 60–70 mm. In order to cool the system, an icepack was placed under Petri dishes. After CAP treatments of 30, 60, 90, and 120 s, the surviving bacterial counts were determined applying standard double-layer agar plate method onto VRBA. The plates were incubated at 37 °C for 24 h, and the results were expressed as log CFU/mL. The surviving bacterial counts after plasma processes were compared to the mean of three control samples, which are inoculated but untreated samples representing the initial bacterial concentration. In order to investigate the kinetics of bacterial cell inactivation, GInaFiT (Version 1.4), a freeware Add-inn for Microsoft® Excel, was used (Geeraerd et al. 2005). A two-fraction mathematical model for biphasic inactivation kinetics proposed by Cerf (1977) was selected which can be formulated as follows:
Herein, f is the fraction of the initial population in a subpopulation of first phase, (1- f) is the fraction of the initial population in a subpopulation of second phase, and k max1 and k max2 [1/time unit] are the specific inactivation rates of the two populations, respectively. This biphasic model can, if being used for time-varying conditions, be written under the form of two first-order differential equations, one for N 1 (first subpopulation) and one for N 2 (second subpopulation).
Effect of CAP on pH Value and Temperature
The pH values of the juice samples before and after plasma treatment were determined with a PHM210 pH meter (Radiometer, Denmark). Also, temperature changes occurring in juice samples after each plasma treatment time were measured with a digital thermometer (− 50/+ 300 °C) (Isolab, Laborgerate GmbH, Germany) which was inserted into the sample.
Colorimetric Evaluation
The measurements were performed using the L*a*b* color space (CIE LAB space) with Minolta Spectrophotometer CM-3600d (Japan). White and black ceramic plates were used for standardizing the instrument. According to CIE L*a*b* system, L* represents lightness index; a* and −a* redness and greenness; and b* and −b* yellowness and blueness. Colorimetric variables (L*, a*, b*) were measured and color change (ΔE* ab ), chroma (C*) and hue (H*) were calculated from:
where all ΔL* 2, Δa* 2, and Δb* 2 were calculated in reference to the untreated juices. ΔE* ab indicates the magnitude of color change after cold plasma treatment. All measurements were performed in triplicate.
Total Phenolic Compounds (Folin–Ciocalteau Assay)
Total phenolic content (TPC) was determined using the Folin–Ciocalteu assay (Singleton et al. 1999). In this method, 250 μL of juice samples (1:10 and 1:15 diluted with distilled water) was mixed with 1 mL of tenfold diluted Folin–Ciocalteu reagent (Sigma, Germany). After 5 min of equilibrium, 2 mL of 20% (w/v) sodium carbonate solution was added to the mixture and shaken thoroughly. After incubation at dark for 2 h at room temperature, absorbance of the solution against a reagent blank at 765 nm was measured with an Agilent 8453 UV–VIS spectrophotometer (Agilent Technologies, Inc., Santa Clara, CA). Quantification was based on standard curve ranged between 5 and 200 mg L−1 of gallic acid. The results were expressed as milligram gallic acid equivalent per liter of juice (mg GAE/L). The obtained data were expressed in terms of mean ± SD (standard deviation). All analyses were performed in triplicate.
Statistical Analysis
All experiments were carried out in triplicate. Data were subjected to factorial analysis of variance (ANOVA), and significance of differences among the treatments was determined with Tukey’s test (p < 0.05) using SPSS 13.0 statistical package (SPSS Inc., Chicago, IL).
Results and Discussion
Inactivation of Escherichia coli
CAP treatment at 650-W plasma power using air as plasma-forming gas with a flow rate of 3000 L/h for varying treatment times (30, 60, 90, and 120 s) was applied to orange, tomato, apple juices, and sour cherry nectar containing E. coli at initial levels between 6.18 and 6.50 log CFU/mL. The antimicrobial efficacy of CAP was compared among juice samples to determine the influence of food intrinsic factors. At the end of 120-s plasma treatment, E. coli were inactivated by 1.59 ± 0.17, 1.43 ± 0.22, 4.02 ± 0.03, and 3.34 ± 0.09 log CFU/mL in orange, tomato, apple juices, and sour cherry nectar, respectively (Fig. 2). Figure 2 showed that surviving colony numbers of the test strain decreased gradually with the increase of plasma exposure time.
The results showed that exposure to plasma has led to inactivation curves with two or three linear segments instead of a straight line. The inactivation data were fitted in the biphasic inactivation kinetics model and the obtained parameter values were given in Table 1. Similar multiphasic survival curves, each representing a different inactivation phase, were obtained in most studies investigating the lethal effect of plasma on microorganisms that are immobilized on the surface of solid foods to which plasmas are directly applied (Dasan et al. 2016a, b; Baier et al. 2014). Generally, these studies showed a reduction characteristic, which was fast at the beginning and slowed down after the fast initial phase because of microorganism distribution on the sample surface. This behavior could be attributed to roughness of the solid surfaces being treated with plasma and distribution of microorganisms on the surface (Hertwig et al. 2015). In our findings, as seen from k max values obtained at two inactivation phases (Table 1), however, reduction of E. coli cells in liquid juice samples was slow at the beginning and either accelerated after the initial phase or showed a similar tendency in both phases. This could be explained by the physical state of treated material where microorganisms are inoculated. When bacteria are inoculated into a liquid medium, neither ions nor electrons can interact directly with bacteria as they already react at plasma–liquid interface and do not penetrate very deep into liquid medium, and thus, the cells dispersed inside a liquid carrier are the most difficult to inactivate (Ikawa et al. 2010; Smet et al. 2017). As plasma exposure time of the samples was increased, generated plasma active species could accumulate on the surface in higher amounts than they were absorbed. Consequently, reduction of E. coli was faster in the latter phase. This inactivation kinetics was also observed in a study by Smet et al. (2016) in which the inactivation curves of Salmonella typhimurium cells treated in a liquid carrier presented a slower reduction in the initial phase followed by a log-linear inactivation phase. This slow inactivation kinetics reflects a resistant population and implies that the microorganisms need to reach a certain CAP treatment level before the cells are lethally damaged. These results clearly reveal that microorganism inactivation by plasmas is a complex process in which several factors can affect kinetics of the killing process. The CAP treatments of juice samples were not maintained after 2 min because many studies comparing the effect of plasma and thermal processing on juices have performed a total treatment of 2 min, which is also a valid process time in the industry (Kovacevic et al. 2016a; Garofulic et al. 2015).
It is clearly seen from the results that CAP was more effective on apple juice and sour cherry nectar than on orange and tomato juices. Both inactivation rates and the total reduction in E. coli concentrations were higher in clear juices. Different process parameters, including food intrinsic factors, are known to influence resistance of the cells against treatment (Smet et al. 2016). This result could be attributed to fluid properties and structure of juice samples. Turbid juices could have non-uniform cloudy particles (orange juice) or a homogeneous but more viscous structure (tomato juice). The solid particles in liquid sample or components that cause turbidity could enable bacteria cells to shield from plasma active species which are responsible for the killing process during treatment. However, CAP treatment was found to be more effective in clear juices. This higher inactivation effect could be attributed to clarity of the samples, since they do not contain any components that could attenuate reactivity of the plasma active species.
In their study where inhibition of E. coli in orange juice was investigated, Shi et al. (2011) achieved a 5-log reduction in 10 s. However, the plasma-applied sample volume was only 50 μL, which becomes significant in the case of plasma species forming during treatment because, as the volume of samples decrease, density of active plasma species responsible for cell destruction would increase, and consequently, total inactivation effect of plasma would clearly increase. Besides, 50 μL of sample would be spread as a thin layer which would act as a solid sheet surface that could be vulnerable to the access of plasma species. The penetration limitation of the active species formed during plasma process is the biggest restricting factor for the use of plasma technology in sterilization and decontamination treatments.
The pH values of aqueous solutions being treated by plasma were reported to be critical in inactivation of microorganisms. Ikawa et al. 2010 found that there is an approximate critical pH value of 4.7 for bactericidal effects, below which bacterial inactivation is extremely efficient and above which bacterial inactivation is relatively weak or hardly occurs. One of the most important findings in their study was that the combination of low pH (pH 3.5) and plasma jet exposure is essential for bacterial inactivation and the lack of either factor led to no bacterial inactivation. In our study, pHs of all juice samples were also below this critical value (Table 2); however, the main factor influencing the inactivation effect of plasma was clarity of the samples.
Effect of Plasma on T and pH of Juice Samples
The alterations occurring in temperature and pH of the samples during plasma treatment were measured after each treatment period. As seen in Fig. 3, the maximum temperature attained after 120 s of ACP treatment was below 40 °C. In this study, a rotating plasma nozzle which generates a three-dimensional cone-shaped plasma discharge moving in x-y direction was used. This continuous movement and avoidance of permanent contact points with the plasma jet provided homogenous sample treatment.
Non-equilibrium (non-thermal) plasmas under wet conditions, which are atmospheric pressure plasmas with low gas temperature, are typically less harmful to the treated surface but can cause some chemical reactions (especially sterilization) on the surface of a material. Such plasmas may be designed to emit very little UV but still act as a strong source of free radicals. Inactivation of bacteria and other microorganisms could be achieved using such low-temperature plasmas only with free radicals, instead of using UV or heat. Since penetration depth of free radicals on or near the surface is very limited, such plasmas may be used to sterilize heat-labile materials (Ikawa et al. 2010).
The pH values of orange, tomato, apple juices, and sour cherry nectar after plasma processing were in the ranges of 3.95–3.93, 4.31–4.33, 3.64–3.63, and 3.35–3.34, respectively. pH values of plasma-treated samples (Table 2) were not statistically different from non-treated sample (control). Although there are studies in which decreases of pH values in plasma-treated liquid samples (Shi et al. 2011; Ikawa et al. 2010) were reported, in our findings, plasma processing did not significantly affect pH of juice samples after application of different treatment times. Only slight changes were observed in orange and tomato juices and sour cherry nectar, whereas the pH difference change seen in apple juice after plasma exposure was not statistically different.
Color Analysis
Few studies have been conducted on physicochemical properties or bioactive components of food after plasma processing. Since this technology uses plasma active species including radicals, neutral species, UV photons, and charged particles, interaction with some food components is possible. Evaluation of physical appearance after processing of the food material would help to reveal the effect of plasma, since any change in color, texture, or aroma may indicate a possible chemical reaction with one or more food components.
In Table 3, color parameters for all experimental trials are shown. Among investigated samples, there was a visual difference in color for only treated apple juice compared to control, since color change ΔE* ab > 3.0 reflects barely noticeable color difference (Kovacevic et al. 2016b). In the case of orange juice, a* value represents the changes in greenness of the product. After 30 s of plasma treatment, the a* value decreased, indicating a possible decrease in greenness; however, only slight differences occurred after longer treatments. Similar to before, b* value, which represents yellowness, showed a statistically significant, but small downward trend with regard to treatment time. However, total change of color remained the same during studied treatment time, and statistically, no significant differences (p > 0.05) were observed. For tomato juice, no significant differences (p > 0.05) in color parameters were found due to varying plasma exposures.
Apple juice was the only sample in which a visual difference was observed after plasma processing. Firstly, as the samples were exposed to longer plasma treatments, the color of juice became darker, which was confirmed with decreasing L* values. The a* and b* values represent redness and yellowness of the product, respectively. Slight increases were observed in redness of the apple juice, while greater differences were obtained in yellowness where the color was slightly skidding into brownish. In addition, the greatest values for ΔE* ab were obtained in this sample. However, the change of color did not significantly differed (p > 0.05) with increasing plasma treatment time.
For sour cherry nectar, only slight changes were observed in a* values which represent redness of the samples regardless of plasma treatment time. However, total color change remained the same.
Literature reports various results with regard to color change and plasma treatment. In a study, for example, it was found that during a 3-min treatment, tomato and lettuce showed color changes that were higher than 4.47 and 9.63, respectively (Bermudez-Aguirre et al. 2013). In another study, it was reported that pomegranate juice became lighter after 7 min of plasma treatment with a total color change between 2.0 and 3.0 (Kovacevic et al. 2016b).
Total Phenolic Compounds
Results of TPC in juice samples after atmospheric plasma treatment are presented in Table 4. It can be observed that duration of plasma treatment highly influenced TPC. Compared to untreated juice, 120-s plasma-treated juices had higher amount of phenolic compounds. As it can be seen, TPC increased by 9.52, 14.81, 14.43, and 14.47% totally after 120 s of plasma treatment in orange, tomato, apple juices, and sour cherry nectar, respectively. Besides, TPC decreased at the beginning of plasma exposure, but significant increases were observed after 90 s of treatment. Although the mechanism is still unclear, these phenolic degradation results obtained at the beginning of plasma treatment are consistent with plasma treatment theory. Degradation of phenolic compounds after exposure to plasma treatment supports the theory of phenolic compounds’ reaction with plasma subsistent reactive oxygen species, like hydroxyl radicals, peroxyl radicals, atomic oxygen, and singlet oxygen (Brandenburg et al. 2007). This degradation of phenolics in the first part of treatment could be attributed to the known ability of phenolic compounds to scavenge free radicals (antioxidative capacity) which were generated with plasma. The significant increase of TPC in the latter part of plasma exposure could be explained by the ability of plasma reactive species (chemically reactive species, charged particles and UV photons) generated when treating the cloudy and clear juice samples with cold plasma, which possess sufficient energy to break covalent bonds and induce several chemical reactions that might increase cell membrane breakdown (Herceg et al. 2016). Since phenolic compounds in plant materials are mostly linked with plant cell wall polysaccharides and these chemical reactions caused by plasma active species may disintegrate the phenolic-cell wall matrix bonds (Khoddami et al. 2013), these effects could be attributed to enhanced phenolic extraction after longer plasma exposure. These results are consistent with possible mechanism of phenolic-plasma species interaction. In a study performed on the interactions of plasma reactive species with secondary plant metabolites in lamb’s lettuce after exposure to an atmospheric plasma jet, it was suggested that the interactions of reactive oxygen species like ·OH, O, O2, and Ar+ may lead to an erosion of epidermal tissue layers of lettuce by which flavonoids and other compounds accumulated in the central vacuoles of guard cells and epidermal cells are released (Grzegorzewski et al. 2011). Although the effects of plasma treatment on solid surfaces and liquid media are predicted to be different due to variety of reaction mechanisms undergo in both conditions, similar results were obtained in pomegranate juice treated with cold plasma in which TPC increased by 14.95–48.99% after varying plasma exposures (Herceg et al. 2016). There are other studies reporting that cold plasma treatment influences the content of phenolic compounds more positively compared to untreated juices (Kovacevic et al. 2016a; Garofulic et al. 2015). Considering our results and other valuable studies on fruit juices, it may be predicted that if the duration of plasma treatment is increased more than 120 s, the number and variety of free radicals would begin to increase and a second trend of phenolic degradation could start due to the scavenging ability of phenolics. In a study on plasma treatment of sour cherry juice, the highest concentration of phenolic acids was observed in the sample that was treated for the shortest time (3 min), while the sample with the lowest phenolic acid content underwent a treatment of 5 min (Garofulic et al. 2015). The optimization of plasma exposure time becomes a critical point when treating food materials which contain phenolic compounds at high concentrations. Nonetheless, due to the increase in TPC, phenolic stability of pomegranate juice treated with cold plasma may be a good alternative to traditional processing of juices such as pasteurization (Herceg et al. 2016).
Conclusion
This study has shown that CAP treatment can be used as an emerging technology for fruit juice processing as it does not affect quality of juice in terms of its phenolic content, color, and pH and provides a processing temperature below 40 °C while showing a great reduction in E. coli concentration. Although CAP treatment of materials at liquid state constitutes the problem of strong absorption of plasma active species generated during exposure by the liquid at the gas–liquid interface, the results achieved in this study showed that exposure of microorganism containing liquid food to atmospheric pressure plasma can also inactivate microorganisms suspended in the liquid. One of the most important results of this study was that the inactivation efficiency of CAP treatment on E. coli was higher in clear juice samples than in turbid ones. Due to the cloudy particles in turbid juices, bacteria cells could be sheltered from plasma reactive species that are generated during exposure and responsible for the killing mechanism of plasma and/or those active species could be adsorbed.
The optimal cold plasma treatment of this study could be potentially used as an alternative to conventional pasteurization due to the phenolic quality of fruit juices. Compared to untreated samples, plasma treatments showed higher total phenolic content (10–15%), which confirms that the plasma has a positive effect on stability of phenols.
Application of atmospheric non-thermal plasma to liquid food materials still needs further investigation in terms of the parameters affecting the inactivation mechanism allied to food matrix and also plasma–liquid interactions.
References
Almeida, F. D. L., Cavalcante, R. S., Cullen, P. J., Frias, J. M., Bourke, P., Fernandes, F. A. N., et al. (2015). Effects of atmospheric cold plasma and ozone on prebiotic orange juice. Innovative Food Science & Emerging Technologies, 32, 127–135.
Arjunan, K. P., Sharma, V. K., & Ptasinska, S. (2015). Effects of atmospheric pressure plasmas on isolated and cellular DNA—a review. International Journal of Molecular Sciences, 16(2), 2971–3016.
Baier, M., Gorgen, M., Ehlbeck, J., Knorr, D., Herppich, W. B., & Schluter, O. (2014). Non-thermal atmospheric pressure plasma: screening for gentle process conditions and antibacterial efficiency on perishable fresh produce. Innovative Food Science & Emerging Technologies, 22, 147–157.
Barba, F. J., Parniakov, O., Pereira, S. A., Wiktor, A., Grimi, N., Boussetta, N., et al. (2015a). Current applications and new opportunities for the use of pulsed electric fields in food science and industry. Food Research International, 77, 773–798.
Barba, F. J., Terefe, N. S., Buckow, R., Knorr, D., & Orlien, V. (2015b). New opportunities and perspectives of high pressure treatment to improve health and safety attributes of foods. A review. Food Research International, 77, 725–742.
Bermudez-Aguirre, D., Wemlinger, E., Pedrow, P., Barbosa-Canovas, G., & Garcia-Perez, M. (2013). Effect of atmospheric pressure cold plasma (APCP) on the inactivation of Escherichia coli in fresh produce. Food Control, 34(1), 149–157.
Biederman, H., Boyaci, I. H., Bilkova, P., Slavinska, D., Mutlu, S., Zemek, J., et al. (2001). Characterization of glow-discharge-treated cellulose acetate membrane surfaces for single-layer enzyme electrode studies. Journal of Applied Polymer Science, 81(6), 1341–1352.
Brandenburg, R., Ehlbeck, J., Stieber, M., von Woedtke, T., Zeymer, J., Schluter, O., et al. (2007). Antimicrobial treatment of heat sensitive materials by means of atmospheric pressure rf-driven plasma jet. Contributions to Plasma. Physics, 47(1–2), 72–79.
Buchner, N., Krumbein, A., Rohn, S., & Kroh, L. W. (2006). Effect of thermal processing on the flavonols rutin and quercetin. Rapid Communications in Mass Spectrometry, 20(21), 3229–3235.
Butz, P., & Tauscher, B. (2002). Emerging technologies: chemical aspects. Food Research International, 35(2–3), 279–284.
Cerf, O. (1977). A review. Tailing of survival curves of bacterial spores. Journal of Applied Microbiology, 42, 1–19.
Charles-Rodriguez, A. V., Nevarez-Moorillon, G. V., Zhang, Q. H., & Ortega-Rivas, E. (2007). Comparison of thermal processing and pulsed electric fields treatment in pasteurization of apple juice. Food and Bioproducts Processing, 85(C2), 93–97.
Dasan, B. G., Boyaci, I. H., & Mutlu, M. (2016a). Inactivation of aflatoxigenic fungi (Aspergillus spp.) on granular food model, maize, in an atmospheric pressure fluidized bed plasma system. Food Control, 70, 1–8.
Dasan, B. G., Mutlu, M., & Boyaci, I. H. (2016b). Decontamination of Aspergillus flavus and Aspergillus parasiticus spores on hazelnuts via atmospheric pressure fluidized bed plasma reactor. International Journal of Food Microbiology, 216, 50–59.
Dasan, B. G., Boyaci, I. H., & Mutlu, M. (2017a). Nonthermal plasma treatment of Aspergillus spp. spores on hazelnuts in an atmospheric pressure fluidized bed plasma system: impact of process parameters and surveillance of the residual viability of spores. Journal of Food Engineering, 196, 139–149.
Dasan, B. G., Onal-Ulusoy, B., Pawlat, J., Diatczyk, J., Sen, Y., & Mutlu, M. (2017b). A new and simple approach for decontamination of food contact surfaces with gliding arc discharge atmospheric non-thermal plasma. Food and Bioprocess Technology, 10, 650–661.
Deng, X. T., Shi, J. J., & Kong, M. G. (2006). Physical mechanisms of inactivation of Bacillus subtilis spores using cold atmospheric plasmas. IEEE Transactions on Plasma Science, 34(4), 1310–1316.
Dhuique-Mayer, C., Caris-Veyrat, C., Ollitrault, P., Curk, F., & Amiot, M. J. (2005). Varietal and interspecific influence on micronutrient contents in citrus from the Mediterranean area. Journal of Agricultural and Food Chemistry, 53(6), 2140–2145.
Echeverría, G., & López, M. L. (2014). Assessing juice quality: analysis of organoleptic properties of fruit juices. In V. Falguera & A. Ibarz (Eds.), Juice processing: quality, safety, and value-added opportunities (pp. 137–150). Boca Raton: CRC Press Taylor & Francis Group.
Falguera, V., & Ibarz, A. (2014). Squeezing fruits in the second decade of the twenty-first century: the current situation of the juice industry. In V. Falguera & A. Ibarz (Eds.), Juice processing: quality, safety, and value-added opportunities (pp. 1–12). Boca Raton: Taylor & Francis Group, LLC.
Ferrari, G., Maresca, P., & Ciccarone, R. (2010). The application of high hydrostatic pressure for the stabilization of functional foods: pomegranate juice. Journal of Food Engineering, 100(2), 245–253.
Fridman, G., Brooks, A. D., Balasubramanian, M., Fridman, A., Gutsol, A., Vasilets, V. N., et al. (2007). Comparison of direct and indirect effects of non-thermal atmospheric-pressure plasma on bacteria. Plasma Processes and Polymers, 4(4), 370–375.
Garofulic, I. E., Jambrak, A. R., Milosevic, S., Dragovic-Uzelac, V., Zoric, Z., & Herceg, Z. (2015). The effect of gas phase plasma treatment on the anthocyanin and phenolic acid content of sour cherry Marasca (Prunus cerasus var. Marasca) juice. Lwt-Food Science and Technology, 62(1), 894–900.
Gasperi, F., Aprea, E., Biasioli, F., Carlin, S., Endrizzi, I., Pirretti, G., et al. (2009). Effects of supercritical CO2 and N2O pasteurisation on the quality of fresh apple juice. Food Chemistry, 115(1), 129–136.
Geeraerd, A. H., Valdramidis, V. P., & Van Impe, J. F. (2005). GInaFiT, a freeware tool to assess non-log-linear microbial survivor curves. International Journal of Food Microbiology, 102, 95–105.
Gialleli, A. I., Bekatorou, A., Kanellaki, M., Nigam, P., & Koutinas, A. A. (2016). Apple juice preservation through microbial adsorption by nano/micro-tubular cellulose. Innovative Food Science & Emerging Technologies, 33, 416–421.
Graves, D. B. (2012). The emerging role of reactive oxygen and nitrogen species in redox biology and some implications for plasma applications to medicine and biology. Journal of Physics D-Applied Physics, IOP Publishing Ltd., 45(26).
Grzegorzewski, F., Ehlbeck, J., Schluter, O., Kroh, L. W., & Rohn, S. (2011). Treating lamb’s lettuce with a cold plasma—influence of atmospheric pressure Ar plasma immanent species on the phenolic profile of Valerianella locusta. Lwt-Food Science and Technology, 44(10), 2285–2289.
Gunaydin, B., Sir, N., Kavlak, S., Guner, A., & Mutlu, M. (2010). A new approach for the electrochemical detection of phenolic compounds. Part I: modification of graphite surface by plasma polymerization technique and characterization by Raman spectroscopy. Food and Bioprocess Technology, 3(3), 473–479.
Gurol, C., Ekinci, F. Y., Aslan, N., & Korachi, M. (2012). Low temperature plasma for decontamination of E. coli in milk. International Journal of Food Microbiology, 157(1), 1–5.
Herceg, Z., Kovacevic, D. B., Kljusuric, J. G., Jambrak, A. R., Zoric, Z., & Dragovic-Uzelac, V. (2016). Gas phase plasma impact on phenolic compounds in pomegranate juice. Food Chemistry, 190, 665–672.
Hertwig, C., Reineke, K., Ehlbeck, J., Knorr, D., & Schluter, O. (2015). Decontamination of whole black pepper using different cold atmospheric pressure plasma applications. Food Control, 55, 221–229.
Ikawa, S., Kitano, K., & Hamaguchi, S. (2010). Effects of pH on bacterial inactivation in aqueous solutions due to low-temperature atmospheric pressure plasma application. Plasma Processes and Polymers, 7(1), 33–42.
Khoddami, A., Wilkes, M. A., & Roberts, T. H. (2013). Techniques for analysis of plant phenolic compounds. Molecules, 18(2), 2328–2375.
Kovacevic, D. B., Kljusuric, J. G., Putnik, P., Vukusic, T., Herceg, Z., & Dragovic-Uzelac, V. (2016a). Stability of polyphenols in chokeberry juice treated with gas phase plasma. Food Chemistry, 212, 323–331.
Kovacevic, D. B., Putnik, P., Dragovic-Uzelac, V., Pedisic, S., Jambrak, A. R., & Herceg, Z. (2016b). Effects of cold atmospheric gas phase plasma on anthocyanins and color in pomegranate juice. Food Chemistry, 190, 317–323.
Ma, T. J., & Lan, W. S. (2015). Effects of non-thermal plasma sterilization on volatile components of tomato juice. International journal of Environmental Science and Technology, 12(12), 3767–3772.
Ma, Y., Zhang, G. J., Shi, X. M., Xu, G. M., & Yang, Y. (2008). Chemical mechanisms of bacterial inactivation using dielectric barrier discharge plasma in atmospheric air. IEEE Transactions on Plasma Science, 36(4), 1615–1620.
Mai-Prochnow, A., Murphy, A. B., McLean, K. M., Kong, M. G., & Ostrikov, K. (2014). Atmospheric pressure plasmas: infection control and bacterial responses. International Journal of Antimicrobial Agents, 43(6), 508–517.
Moisan, M., Barbeau, J., Moreau, S., Pelletier, J., Tabrizian, M., & Yahia, L. H. (2001). Low-temperature sterilization using gas plasmas: a review of the experiments and an analysis of the inactivation mechanisms. International Journal of Pharmaceutics, 226(1–2), 1–21.
Mutlu, M., Mutlu, S., Boyaci, I. H., Alp, B., & Piskin, E. (1998). High-linearity glucose enzyme electrodes for food industries: preparation by a plasma polymerization technique. Polymers in Sensors, 690, 57–65.
Noci, F., Riener, J., Walkling-Ribeiro, M., Cronin, D. A., Morgan, D. J., & Lyng, J. G. (2008). Ultraviolet irradiation and pulsed electric fields (PEF) in a hurdle strategy for the preservation of fresh apple juice. Journal of Food Engineering, 85(1), 141–146.
Schluter, O., Ehlbeck, J., Hertel, C., Habermeyer, M., Roth, A., Engel, K. H., et al. (2013). Opinion on the use of plasma processes for treatment of foods. Molecular Nutrition & Food Research, 57(5), 920–927.
Shi, X. M., Zhang, G. J., Wu, X. L., Li, Y. X., Ma, Y., & Shao, X. J. (2011). Effect of low-temperature plasma on microorganism inactivation and quality of freshly squeezed orange juice. IEEE Transactions on Plasma Science, 39(7), 1591–1597.
Simunek, M., Jambrak, A. R., Dobrovic, S., Herceg, Z., & Vukusic, T. (2014). Rheological properties of ultrasound treated apple, cranberry and blueberry juice and nectar. Journal of Food Science and Technology-Mysore, 51(12), 3577–3593.
Singleton, V. L., Orthofer, R., & Lamuela-Raventos, R. M. (1999). Analysis of total phenols and other oxidation substrates and antioxidants by means of Folin-Ciocalteu reagent. Oxidants and Antioxidants, Pt A, 299, 152–178.
Smet, C., Noriega, E., Rosier, F., Walsh, J. L., Valdramidis, V. P., & Van Impe, J. F. (2016). Influence of food intrinsic factors on the inactivation efficacy of cold atmospheric plasma: impact of osmotic stress, suboptimal pH and food structure. Innovative Food Science & Emerging Technologies, 38, 393–406.
Smet, C., Noriega, E., Rosier, F., Walsh, J. L., Valdramidis, V. P., & Van Impe, J. F. (2017). Impact of food model (micro)structure on the microbial inactivation efficacy of cold atmospheric plasma. International Journal of Food Microbiology, 240, 47–56.
Surowsky, B., Frohling, A., Gottschalk, N., Schluter, O., & Knorr, D. (2014). Impact of cold plasma on Citrobacter freundii in apple juice: inactivation kinetics and mechanisms. International Journal of Food Microbiology, 174, 63–71.
Surowsky, B., Schluter, O., & Knorr, D. (2015). Interactions of non-thermal atmospheric pressure plasma with solid and liquid food systems: a review. Food Engineering Reviews, 7(2), 82–108.
Valdramidis, V. P., Graham, W. D., Beattie, A., Linton, M., Mckay, A., Fearon, A. M., et al. (2009). Defining the stability interfaces of apple juice: implications on the optimisation and design of high hydrostatic pressure treatment. Innovative Food Science & Emerging Technologies, 10(4), 396–404.
von Woedtke, T., Reuter, S., Masur, K., & Weltmann, K. D. (2013). Plasmas for medicine. Physics Reports-Review Section of Physics Letters, 530(4), 291–320.
Zinoviadou, K. G., Galanakis, C. M., Brncic, M., Grimi, N., Boussetta, N., Mota, M. J., et al. (2015). Fruit juice sonication: implications on food safety and physicochemical and nutritional properties. Food Research International, 77, 743–752.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Dasan, B.G., Boyaci, I.H. Effect of Cold Atmospheric Plasma on Inactivation of Escherichia coli and Physicochemical Properties of Apple, Orange, Tomato Juices, and Sour Cherry Nectar. Food Bioprocess Technol 11, 334–343 (2018). https://doi.org/10.1007/s11947-017-2014-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11947-017-2014-0