Abstract
Poly-β-hydroxybutyrate (PHB) is a thermoplastic polyester accumulated intracellularly by many microorganisms under unfavorable growth conditions. The features of PHB are biodegradable and biocompatible, and the physical properties are similar to polypropylene, which has attracted industrial attention as an environmentally degradable plastic for a wide range of agricultural, marine, and medical applications and appropriate substitutes for hydrocarbon-based plastics. Starch is a renewable carbon source from plant sources available abundantly in large quantities throughout the globe and has recently been used as a carbon source for PHB production. The utilization of starch in PHB production needs enzymatic hydrolysis for starch degradation since many microorganisms do not produce these enzymes natively. This suggests there is a need for exploitation of bacterial culture for the co-production of the starch-hydrolyzing enzyme (amylolytic bacteria) as well as PHB. Some bacteria have been reported capable to convert starch into PHB directly, which are from the genus Bacillus. The process of PHB production from starch by amylolytic bacteria is simultaneous saccharification and fermentation (SSF). The mechanism of bacteria synthesizing PHB from starch is divided into two groups, namely, the growth-associated PHB synthesis and the non-growth-associated PHB synthesis. The utilization of starch for PHB production is an economic strategy to reduce production costs of PHB as well as its applications in various fields.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
9.1 Introduction
Polyhydroxyalkanoate (PHA) is known as bioplastics produced by microbes or also called microbial bioplastics which were investigated by Beijerinck in 1888 under a microscope as granules inside cells, whereas PHA composition was discovered by Lemoigne in 1927 (Byrom 1987). Polyhydroxyalkanoates (PHAs) are the most versatile bioplastics with properties similar to petroleum-based plastics (Anderson and Dawes 1990; Madison and Huisman 1999). As a family of biodegradable and biocompatible polyesters, PHA could be developed as environmentally friendly bulk plastics, provided the production cost is competitive (Anderson and Dawes 1990; Madison and Huisman 1999; Lenz and Marchessault 2005). Poly-β-hydroxybutyrate (PHB) is the most common PHA and was first described by Lemoigne, which is accumulated by Bacillus megaterium (Byrom 1987). PHB is a biopolymer synthesized by microbes and accumulated as intracellular granules and functions as carbon and energy reserves (Madison and Huisman 1999; Lenz and Marchessault 2005). This polymer was introduced as a prototype of degraded thermoplastics (Byrom 1987) that can solve the problem of plastic pollution. PHB is composed of 3-hydroxybutyrate monomers strung together by β-bonds (Fig. 9.1) (Madison and Huisman 1999; Lenz and Marchessault 2005; Zilliges and Damrow 2017). Carboxyl groups from one monomer bind to another hydroxyl monomer group with ester bonds (Anderson and Dawes 1990; Madison and Huisman 1999; Zilliges and Damrow 2017).
The advantages of PHB as a bioplastic lie in its properties such as heat-resistant, easily formed, not easily broken, and biodegradable (Anderson and Dawes 1990). PHB also has biocompatible properties of organ systems in the human body so that it is potentially used in the medical field (Madison and Huisman 1999; Lenz and Marchessault 2005). Some of the PHB bioplastic products that have been marketed under the Biopol trademark from England and Wella from Germany are combs, toothbrushes, bags, trash cans, and packaging bottles (Byrom 1987; Lee 1996). PHB production as bioplastics is constrained due to high production costs so that it cannot compete with synthetic plastics (Byrom 1987; Lee 1996). Therefore, various efforts are needed to reduce the cost of PHB production such as the discovery of strains of superior PHB-producing bacteria and the use of alternative carbon sources that are cheaper than glucose. Starch is an inexpensive substrate and can be generated from various agricultural wastes. Therefore, starch may be a good candidate to replace the more expensive carbon sources for the industrial production of PHB (Kim 2000; Halami 2008; Ramadas et al. 2009).
9.2 Overview of Starch as a Substrate for PHB Production
The substrates commonly used for polyhydroxyalkanoate (PHA) production are simple carbohydrates such as glucose and sucrose (Lee 1996). The substrate used for PHA production can determine the type, characteristic, quality, and quantity of polymer synthesized by microbes (Byrom 1987; Kim 2000). For example, when glucose is the sole substrate, then a polymer formed is poly-β-hydroxybutyrate (PHB), but if the substrate is a mixture of glucose and propionic acid, the product formed is a copolymer poly-β-hydroxybutyrate-co-poly-hydroxyvalerate (PHBV) (Byrom 1987). Currently, the use of inexpensive carbon sources in the biosynthesis of PHA is predicted to reduce its production cost. Some study reported that the use of complex substrates such as starch, as the carbon source for the production of PHB, can reduce its production cost since it is inexpensive and widely available to users (Kim and Chang 1998; Kim 2000; Gonzalez-Garcıa et al. 2011; Halami 2008; Yanti and Muhiddin 2016).
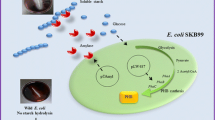
Starch is a polysaccharide of glucose made of two types of α-d-glucan chains, amylose and amylopectin. Starch molecules produced by each plant species have specific structures and compositions (such as the length of glucose chains or the amylose/amylopectin ratio), and the protein and fat content of the storage organs may vary significantly (Egharevba 2019). Starch is very potential as a substrate for PHB production since the monomer of starch is glucose; besides that, the carbon content is high, while the nitrogen and phosphorus levels are low (Alcazar-Alay and Meireles 2015; Yanti et al. 2009). PHB will be produced by bacteria when glucose is the sole carbon source, and the nutrients in the growth media are not balanced, namely, excessive carbon content, while other nutrient contents such as nitrogen, phosphorus, and kalium are limited (Byrom 1987; Anderson and Dawes 1990). Several types of starch can be used as substrates to produce PHB. The types of starch that have been explored as substrates to produce PHB are shown in Table 9.1. Nevertheless, before being used as a convenient carbon source for most of the known PHA-producing strains, nowadays the majority of the complex carbohydrates need treatment such as hydrolysis (Hassan et al. 1997; Kim and Chang 1998). Several PHB productions using starch as substrates perform stages of hydrolysis of starch using enzyme (Syamsu et al. 2006; Haas et al. 2008; Krueger et al. 2012) and chemical compounds (Yu 2001; Jiang et al. 2016). This pretreatment step implies an extra cost and can cause environmental problems whether chemicals are used in the hydrolysis process. These problems can be overcome by finding bacterial strains that are able to hydrolyze starch and synthesize PHB simultaneously.
9.3 Poly-β-hydroxybutyrate (PHB)-Producing Microbes
Poly-β-hydroxybutyrate (PHB) is a polymer produced by microbes, mainly from the prokaryotic group (Anderson and Dawes 1990). Prokaryotic microbial groups that can accumulate PHB include Gram-negative bacteria (Byrom 1987; Sudesh et al. 2000), Gram-positive bacteria (Najimudin et al. 1997; Sudesh et al. 2000; Aslim et al. 2002; Yilmaz et al. 2005; Yanti et al. 2019), and Cyanobacteria (Sudesh et al. 2000). PHB-producing bacteria from the Gram-negative group are strains belonging to the genera Alcaligenes, Azospirillum, Beijerinckia, Chromobacterium, Chromatium, Cupriavidus, Derxia, Ferrobacillus, Hyphomicrobium, Methylobacterium, Pseudomonas, Rhodospirillum, Spirillum, Vibrio and Zoogloea. Rhizobium, Rhodopseudomonas, Rhodospillus, Rhodospillillum, Rhodospillillum, Zephromogillus, Rhodospillum, and Zephromogillus (Sudesh et al. 2000), and Gram-positive bacteria groups are strains of the genus Actinomyces, Bacillus, Micrococcus, Nocardia, and Streptomyces (Byrom 1987; Lenz and Marchessault 2005; Yanti et al. 2019), while strains belonging to the genus Chlorogloea and Spirulina are a member of the Cyanobacteria group (Byrom 1987; Lenz and Marchessault 2005).
In general, PHB-producing bacteria use simple substrates such as glucose. However, not many of these bacteria can use complex substrates such as starch to produce PHB. PHB-producing bacteria that have been used on an industrial scale such as Cupriavidus necator and Alcaligenes latus are highly dependent on glucose as a substrate in producing PHB, causing high production costs (Anderson and Dawes 1990; Lenz and Marchessault 2005). Utilization of bacteria that can use starch as a cheaper substrate to produce PHB can reduce production costs (Kim and Chang 1998; Kim 2000; Halami 2008). However, these bacteria must have amylolytic activity to use starch to produce PHB bioplastics efficiently. Therefore, searching for amylolytic bacteria producing PHB is an effort that can be done to reduce the production cost of PHB.
Several studies have been conducted to explore indigenous amylolytic bacteria that can be utilized in producing PHB using starch as a substrate (Halami 2008; Margino et al. 2014; Yanti et al. 2019). Margino et al. (2000) succeeded in isolating amylolytic bacteria that accumulated PHB from tapioca industrial waste and were identified as genera Bacillus and Pseudomonas. Halami (2008) obtained indigenous bacterial isolates that were able to produce PHB from starch substrates and were identified as Bacillus cereus. Yanti et al. (2019) also obtained indigenous bacterial isolates from the sago starch processing area that were able to produce PHB from sago starch substrates and were identified as members of the genus Bacillus. In general, bacteria that can convert various types of starch into bioplastic PHB directly are the genus Bacillus, namely, from the species Bacillus megaterium, Bacillus subtilis, and Bacillus cereus (Halami 2008; Yanti et al. 2019). Besides the genus Bacillus, a bacterial strain of the genera Azotobacter (Kim 2000), Haloferax (Lillo and Rodriguez-Valera 1990), Saccharophagus (Gonzalez-Garcıa et al. 2011), and Micrococcus (Margino et al. 2014) was reportedly capable of producing PHB from starch (Table 9.1). Out of these, Bacillus spp. are found to be more efficient for PHB production due to their higher stability and reproducibility under environmental stress (Shivalkar and Prabha 2017). These bacteria are very potential to be used to produce PHB bioplastics using various types of starch industrially.
9.4 PHB Detection
Poly-β-hydroxybutyrate (PHB) is a polymeric ester which functions as an energy and carbon reserve in prokaryotic cells. PHB exists as discrete inclusions or granules in the cell (Ostle and Holt 1982). To start an extensive search of bacterial population of PHB producers, it was necessary to develop simple qualitative and quantitative methods of PHB content estimation in living bacterial cells. PHB detection in bacterial cells is done by the staining method. Sudan Black, Nile blue, and Nile red are stains that can be used to detect PHB granules (Ostle and Holt 1982; Amara 2008). The three stains have a high affinity for PHB granules (Ostle and Holt 1982), so they are good for screening for PHB-producing bacteria. Redzwan et al. (1997) reported the use of Sudan Black B staining technique as a first-line screening for PHB-producing bacteria from nature, needed to screen various bacterial collections in a short time by using special staining for the detection of PHB granules. Hartman (1940) was the first to suggest the use of Sudan Black B, as a bacterial fat stain. The lipophilic stain Sudan Black B has long been regarded as a dye with particularly high affinity for PHAs (Murray et al. 1994). The Sudan Black B staining was used as the first line of qualitative observation of PHB production for the bacterial species as also suggested by certain workers (Phanse et al. 2011). Different bacterial species such as Bacillus subtilis NRRL-B-941, Bacillus licheniformis B-NRRL 1001, Bacillus cereus NRRL-B-3711, Bacillus megaterium NRRL-B-3712, Bacillus thuringiensis 798 (Phanse et al. 2011; Asad et al. 2016), and Bacillus megaterium PSA10 (Yanti et al. 2009) were screened based on qualitative tests using a Sudan Black B staining. In several studies, the detection of PHB accumulation using Sudan Black staining has been carried out macroscopically, namely, the coloring of bacterial colonies on the agar plate (Asad et al. 2016; Yanti et al. 2019). Sudan Black staining method was microscopically performed by coloring the bacterial cells using Sudan Black solution 0.2% (w/v) in 70% ethanol for 10 min and then washing with xylene for 10 s and then painting with safranin. Observation using a phase-contrast microscope has shown that PHB granules in cells are blackish blue while vegetative cells are red (Fig. 9.2a). PHB detection using Sudan Black staining macroscopically is done by staining bacterial colonies grown on an agar plate medium containing starch 1% (w/v) as carbon source using Sudan Black solution 0.02% (w/v) dissolved in 96% ethanol for 10 min and then washing with 100% ethanol. The dark-blue-colored colonies were taken as positive for PHA production (Fig. 9.2b) (Yanti et al. 2019).
Detection of PHB granule accumulation in bacteria. (a) Micrographs showing PHB granule with Sudan Black staining under a phase-contrast microscope. (b) PHB-accumulating colonies with Sudan Black staining (+, positive PHB; −, negative PHB). (c) Micrographs of PHB granule with Nile blue under a fluorescence microscope. (d) The PHB-accumulating colonies, after Nile blue A staining, showed bright orange fluorescence on irradiation with UV light (+, positive PHB; −, negative PHB). (e, f) PHB granules under the transmission electron microscope. Black arrows indicate PHB granules inside the cells
Besides Sudan Black stain, Nile blue A is another stain for the detection of PHB granules in bacteria and is, in fact, superior to Sudan Black B since it is not as easily washed from the cell by decolorization procedures (Ostle and Holt 1982; Mascarenhas and Aruna 2017). Ostle and Holt (1982) advocated the use of Nile blue A, a water-soluble basic oxazine dye that has a greater affinity and higher specificity than Sudan Black for PHB detection, and gives a bright orange fluorescence on exposure to ultraviolet light. The oxazine form of the dye Nile blue is responsible for the fluorescent staining of PHB (Ostle and Holt 1982; Mascarenhas and Aruna 2017). Other inclusion bodies, such as glycogen and polyphosphate, do not stain with Nile blue A, thus emphasizing its usefulness. PHB detection using a Nile blue staining could be done microscopically and macroscopically. The method of observing PHB granules in bacterial cells (microscopically) uses Nile blue stain as follows: spread bacterial cell preparations on an object glass and then drip with Nile blue solution and then heat at a temperature of 55 °C using a hot plate for 10 min. After staining, the slide preparation is washed with running water to remove excess dyes and then washed with 8% (v/v) acetic acid for 1 min. The preparations are washed with running water and then dried. Dry preparations were observed using a fluorescence microscope, and PHB granules in bacterial cells will fluoresce when observed with a fluorescence microscope (Ostle and Holt 1982) (Fig. 9.2c). Kitamura and Doi (1994) first demonstrated the viable colony method on agar plates; they induced the isolates to accumulate PHA by culturing in E2 medium containing 2% (w/v) glucose before Nile blue A staining. The PHA-accumulating colonies, after Nile blue A staining, showed bright orange fluorescence on irradiation with UV-light at 312 wavelengths (Fig. 9.2d), and their fluorescence intensity increased with an increase in PHA content of the bacterial cells (Kitamura and Doi 1994). PHB granules formed intracellularly were observed using transmission electron microscopy (TEM). Observation of PHB granules using TEM will clarify PHB in cells. Sample preparation for the observation of PHB granules with TEM was carried out based on the method described by Rodríguez-Contreras et al. (2013). The sample was fixed with a mixture of 2% (v/v) glutaraldehyde solution, 3% (w/v) paraformaldehyde, 5% (w/v) sucrose, and 0.1 mol/L sodium cacodylate. Furthermore, the bacterial cell was dehydrated using an ethanol solution and then planted in a polymerized polymer at 60 °C overnight. Bacterial cells embedded in the resin are then sliced using ultra-microtomes with a thickness of about 70 nm. After that, the samples were examined using a transmission electron microscope. PHB granules in bacterial cells observed with a TEM microscope are shown in Fig. 9.2e, f. Exploration of amylolytic bacteria (the ability to hydrolyze starch), as well as PHB-producing, is needed to find bacteria that can convert starch into bioplastic PHB directly. Amylolytic bacterial screening and PHB-producing can be demonstrated as the viable colony method on agar plates containing starch 1% (w/v) as carbon sources. Detection of amylolytic ability, using Lugol’s iodine whereas PHB accumulation using Sudan black staining (Yanti et al. 2019). The clear zone formed around the bacterial colonies on starch agar media indicates amylolytic activity (starch hydrolysis), while the blackish-blue color of the colonies indicates the bacterial cells can accumulate PHB (Fig. 9.3).
9.5 Downstream Processing of PHB (Recovery and Purification)
PHB is synthesized in bacterial cells, so PHB must be extracted from cells to be purified before being used as bioplastics. Several solvent extraction processes have been developed to recover PHB from biomass. For example, PHB can be extracted from bacterial cells including various acids (HCl, H2SO4), alkalies (NaOH, KOH, and NH4OH), and surfactants namely dioctyl sulfosuccinate sodium salt (AOT), hexadecyltrimethylammonium bromide (CTAB), sodium dodecyl sulfate (SDS), polyoxyethylene-p-tert-octylphenol (Triton X-100), and polyoxyethylene (20) sorbitan monolaurate (Tween 20) (Choi and Lee 1999) and chloroform (Jacquel et al. 2008). SDS is an efficient chemical for PHB isolation from recombinant E. coli, but the price is expensive and it has a waste disposal problem (Choi and Lee 1999). NaOH and KOH were also efficient and economical for the recovery of P(3HB) (Choi and Lee 1999). A less complex procedure is the use of a differential digestion method employing sodium hypochlorite. Although simple and effective, this method has been avoided because sodium hypochlorite can cause a decrease in the molecular weight of PHB as much as 50% (Lee 1996). Ramsay et al. (1990) reported that by optimizing the conditions of sodium hypochlorite digestion and by balancing the ratio of hypochlorite to non-PHB biomass, PHB of 95% purity with an average molecular weight of 600,000 was recovered from Alcaligenes eutrophus. PHB is hydrophobic, while lyophilized cells are hydrophilic. When PHB is isolated from the cell by the action of hypochlorite, it will immediately migrate to the chloroform phase avoiding severe degradation. Chloroform can, at least partially, protect the PHB molecules from further destructive action of the hypochlorite (Ramsay et al. 1990). Sodium hydroxide (NaOH) can be used as a substitute for sodium hypochlorite in the PHB extraction process because it can obtain up to 97% PHB. Another advantage of sodium hydroxide compared to sodium hypochlorite is it is inexpensive and environmentally friendly (Choi and Lee 1999).
9.6 Metabolism of Poly-β-hydroxybutyrate (PHB)
9.6.1 Synthesis of PHB
The mechanism of PHB synthesis from starch by bacteria occurs in two stages, namely, the hydrolysis of starch to glucose and the synthesis of glucose into PHB. The one-step bioconversion of starch into PHB can be done by using microorganisms that are able to degrade or digest starch into glucose and then the fermentation glucose into PHB (Yanti et al. 2013; Yanti and Muhiddin 2016). Bacteria that can convert starch into bioplastic PHB directly in one stage are amylolytic bacteria, which can hydrolyze starch into simple sugars and at the same time are able to synthesize PHB also (Halami 2008; Yanti et al. 2013; Yanti and Muhiddin 2016). This process is called as simultaneous saccharification and fermentation (SSF). In the process of SSF, the glucose produced by the hydrolyzing enzymes is consumed immediately by the fermenting microorganism present in the culture (Taherzadeh and Karimi 2007). SSF process has many advantages to use as a fermentation strategy; they are the risk of contamination is low and the number of vessels required is reduced, resulting in lower capital cost of the process (Taherzadeh and Karimi 2007).
The pathway of PHB synthesis from starch by amylolytic bacteria gets completed in several steps. Starch is hydrolyzed into glucose by the amylase enzyme, and then the glucose is converted into pyruvate via the Embden-Meyerhof-Parnas pathway for subsequent entry into the synthesis of PHB. PHB synthesis is carried out by a series of enzymes, namely, (1) β-ketothiolase (acetyl-CoA acyltransferase) which catalyzes the dimerization of acetic acid CoA (acetyl-CoA) derivatives into acetoacetyl-CoA, (2) acetoacetyl-CoA reductase that catalyzes the hydrogenation of acetoacetyl CoA that becomes [R]-3-hydroxybutyryl-CoA which is a PHB monomer, and (3) PHB synthase that catalyzes the polymerization of PHB monomers to PHB (Braunegg et al. 1998; Lenz and Marchessault 2005). The mechanism of PHB synthesis from starch is presented in Fig. 9.4. Under conditions of unbalanced growth (excessive carbon but other nutrients are limited), NADH oxidase activity in the Krebs cycle decreases, thereby increasing the amount of NADH (Byrom 1987; Braunegg et al. 1998). The increase in NADH will inhibit the activity of citrate synthase so that acetyl-CoA and oxaloacetate (OAA) are not converted into citrate and free CoA and cause acetyl-CoA acetyltransferase (β-ketothiolase) to become active to condense acetyl-CoA to acetoacetyl-CoA which is the initial compound of the polymerization of PHB (Doi et al. 1992; Braunegg et al. 1998). As long as the condition of the medium has not changed, microbes remain to accumulate PHB in their cells, but if the carbon concentration in the medium declines, the microbes will degrade PHB polymers (depolymerization) to obtain the energy needed for growth (Byrom 1987). As a result, the amount of PHB in the cell decreased (Byrom 1987; Brandl et al. 1990).
9.6.2 Degradation of PHB
The enzymes that play a role in the degradation of PHB polymers are (1) depolymerase or hydrolase, which is an enzyme that hydrolyzes PHB granules to [R]-3-hydroxybutyric acid; (2) specific dehydrogenase, which is an enzyme that converts [R]-3-hydroxybutyric acid to acetoacetic acid; and (3) acetoacetyl-CoA synthetase, which is an enzyme that converts acetoacetic acid into acetoacetyl-CoA (Lenz and Marchessault 2005). The mechanisms of PHB degradation are presented in Fig. 9.4. The PHB synthesis and degradation process are influenced by nutrient content and incubation time. Margino et al. (2000) and Yanti et al. (2013) reported that Alcaligenes eutrophus and several types of Bacillus accumulate PHB in a medium that is limited in nitrogen in an incubation period of 48–72 h. Therefore, to obtain PHB with the best quality and maximum amount, it is necessary to have the right and specific nutrient content and incubation time for each type of microbes.
9.7 Fermentation Process
Bacteria that are used for the production of PHB can be classified into two groups, depending on the culture conditions to PHB accumulation. The first group of bacteria accumulating the polymer PHB during the stationary growth phase (non-growth-associated) requires limitation of essential nutrients such as nitrogen and oxygen and the presence of excess carbon source for the efficient synthesis of PHB. The representative bacteria belonging to this group include Alcaligenes eutrophus (Lee 1996), Bacillus subtilis 25, and Bacillus megaterium 12 (Yuksekdag et al. 2004) and Bacillus subtilis PPK5 (Yanti et al. 2013). On the other hand, the second group of bacteria accumulating the polymer during the growth (exponential) phase (growth-associated) does not require nutrient limitation for PHB synthesis. Some of the bacteria included in this group are Alcaligenes latus (Lee 1996), Bacillus mycoides (Thakur et al. 2001), Bacillus sphaericus NCIM 5149 (Ramadas et al. 2009), and Bacillus megaterium PSA10 (Yanti et al. 2013, 2020). The culture conditions required for PHB biosynthesis are important criteria to be taken into consideration for the development of cultivation techniques used in the large-scale production of PHB (Chee et al. 2010).
The fermentation process of PHB using starch by amylolytic bacteria consists of two mechanisms. In the first group, amylolytic bacteria hydrolyze the starch to glucose which is then used for growth that synthesizes PHB directly so that glucose does not accumulate in the fermentation media. In this mechanism, bacteria synthesize PHB together with their growth (growth-associated) (Fig. 9.5a). Bacteria that belong to this group are Bacillus megaterium PSA10 (Yanti et al. 2013, 2020). In the second group, amylolytic bacteria hydrolyze starch to glucose, and then glucose is accumulated in the fermentation media. In this process, PHB began synthesized at the late exponential phase or in early stationary phase of growth after the high glucose levels in the medium, and PHB synthesis is increased after growth decline (non-growth-associated) (Fig. 9.5b). Bacteria included in this group are Bacillus subtilis PPK5 (Yanti et al. 2013, 2019).
9.8 Characteristics of PHB
PHB is an aliphatic homopolymer that has thermoplastic properties with good mechanical properties and has a similar characteristic to polypropylene (PP). A comparison of physical characteristics between PHB and polypropylene is shown in Table 9.2. PHB is an intracellular product, if PHB still inside the cell are amorphous, but after going through an extraction process using an organic solvent, PHB will change into a highly crystalline. The high crystallinity causes PHB to be a rigid but brittle material. The PHB fragility caused is not resistant to pressure. PHB melting temperature (175 °C) which is approaching its thermal degradation temperature (200 °C) caused a limitation in the processing process (Madison and Huisman 1999). According to Kim and Rhee (2003), this weakness can be improved by copolymerizing poly-β-hydroxybutyrate (PHB) and polyhydroxyvalerate (PHV) into a copolymer of poly-hydroxybutyrate-co-polyhydroxyvalerate (PHB-co-HV) that is more flexible and has low process temperature. PHB characteristics such as crystallinity and tensile strength, depending on the molecular weight of the polymer. The amount of molecular weight is influenced by the strain of microorganism used, the cultivation conditions, and the purity of PHB (Pena et al. 2014). The average molecular weight of PHB varies from 2 to 4 × 103 kDa. The molecular weight depends on the ability of microbes to accumulate the produced polymer, conditions of growth, and extraction method (Rajan et al. 2018). Bourque et al. (1995) state that the molecular weight of PHB can be reduced during the polymer processing process. Lafferty et al. (1988) added that the reduction in PHB molecular weight can occur throughout the extraction process from biomass.
According to Hrabak (1992), PHB has polypropylene-like characteristics with three unique properties, namely, thermoplastic, 100% waterproof, and 100% biodegradable. PHB has several superior characteristics such as water vapor resistance and insoluble in water, and these characteristics distinguished the PHB with other biodegradable plastics (Lenz and Marchessault 2005). PHB also has good impermeability to oxygen. PHB can dissolve in various solvents such as chloroform, methylene chloride, ethylene chloride, pyridine, or dichloromethane/ethanol mixture (Choi and Lee 1999). The solubility of PHB in some solvents is shown in Table 9.3.
9.9 Applications of Bioplastic PHB
PHB can be applied as plastic because it is biodegradable, thermoplastic, and piezoelectric and has the ability to depolymerize PHB into a d-3-hydroxybutyric acid monomer (Lafferty et al. 1988). Generally, PHB application is divided into three areas, namely, medical and pharmaceutical, agricultural, and packaging (Mathuriya and Yakhmi 2017; Koller 2018). Some examples of practical applications of PHB are shown in Table 9.4. PHB polymers produced by Gram-positive bacteria have the potential to be used for medical equipment. According to Valappil et al. (2007), the use of Gram-positive bacteria as a producer of PHB for medical purposes has advantages compared to Gram-negative bacteria. Gram-negative bacteria have lipopolysaccharides (LPS) in their cell walls which can cause immunogenic reactions, while Gram-positive bacteria do not have LPS (Valappil et al. 2007).
References
Alcazar-Alay SC, Meireles MAA (2015) Physicochemical properties, modifications, and applications of starches from different botanical sources. Food Sci Technol (Campinas) 35(2):215–236
Amara AA (2008) Polyhydroxyalkanoates: from basic research and molecular biology to application. IIUM Eng J 9(1):37–73
Anderson AJ, Dawes EA (1990) Occurrence, metabolism, metabolic role, and industrial uses of bacterial polyhydroxyalkanoates. Microbiol Rev 54(4):450–472
Asad UR, Aslam A, Masood R, Muhammad N, Aftab MN, Ajmal R, Ikram U-H (2016) Production and characterization of thermostable bioplastic (poly-β-hydroxybutyrate) from Bacillus cereus NRRL-B-3711. Pak J Bot 48(1):349–356
Aslim B, Yuksekdag ZN, Beyatli Y (2002) Determination of PHB growth quantities of certain Bacillus species isolated from soil. Turkish Electronic J Biotechnol 19:24–30
Bourque D, Pomerleau Y, Groleau D (1995) High-cell-density production of poly-β-hydroxybutyrate (PHB) from methanol by Methylobacterium extorquens: production of high-molecular-mass PHB. Appl Microbiol Biotechnol 44:367–376
Brandl H, Gross RA, Lenz RW, Fuller RC (1990) Plastics from bacteria and for bacteria: poly(β-hydroxyalkanoates) as natural, biocompatible, and biodegradable polyesters. Adv Biochem Eng Biotechnol 41:77
Braunegg G, Lefebvre G, Genser KF (1998) Polyhydroxyalkanoates, biopolyesters from renewable resources: physiological and engineering aspects. J Biotechnol 65:127–161
Byrom D (1987) Polymer synthesis by microorganisms: technology and economics. Tibtech 5:246–250
Chee JY, Yoga SS, Lau NS, Ling SC, Abed RMM, Sudesh K (2010) Bacterially produced polyhydroxyalkanoate (PHA): converting renewable resources into bioplastics. In: Current research, technology and education topics in applied microbiology and microbial biotechnology. Formatex Research Center, Badajoz, pp 1395–1404
Choi J, Lee SY (1999) Efficient and economical recovery of poly (3-hydroxybutyrate) from recombinant Escherichia coli by simple digestion with chemicals. Biotechnol Bioeng 62:546–553
Doi Y, Kawaguchi Y, Koyama N, Nakamura S, Hiramitsu M, Yoshida Y, Kimura H (1992) Synthesis and degradation of polyhydroxyalkanoates in Alcaligenes eutrophus. FEMS Microbiol Rev 103:103–108
Egharevba HO (2019) Chemical properties of starch and its application in the food industry. https://doi.org/10.5772/intechopen.87777
Gonzalez-Garcıa Y, Rosales MA, Gonzalez-Reynoso O, Sanjuan-Duenas R, Cordova J (2011) Polyhydroxybutyrate production by Saccharophagus degradans using raw starch as carbon source. Eng Life Sci 11(1):59–64
Haas R, Jin B, Zepf FT (2008) Production of poly (3-hydroxybutyrate) from waste potato starch. Biosci Biotechnol Biochem 72(1):253–256
Halami PM (2008) Production of polyhydroxyalkanoate from starch by the native isolate Bacillus cereus CFR06. World J Microbiol Biotechnol 24:805–812
Hartman TL (1940) The use of Sudan Black B as a bacterial fat stain. Stain Technol 15:23–28
Hassan MA, Shirai Y, Karim MIA, Hashimoto K (1997) Simultaneous process for the treatment of agro-industrial wastewaters and the production of bacterial bioplastics. Ann Rep IC Biotechnol 20:708–709
Hrabak O (1992) Industrial production of poly-β-hydroxybutyrate. FEMS Microbiol Rev 103:251–256
Jacquel N, Lo CW, Wei YH, Wu HS, Wang S (2008) Isolation and purification of bacterial poly(3-hydroxyalkanoates). Biochem Eng J 39:15–27. https://doi.org/10.1016/j.bej.2007.11.029
Jiang G, Hill DJ, Kowalczuk M, Johnston B, Adamus G, Irorere V, Radecka I (2016) Carbon sources for polyhydroxyalkanoates and an integrated biorefinery. Int J Mol Sci 17:1–21. https://doi.org/10.3390/ijms17071157
Kim BS (2000) Production of poly(3-hydroxybutyrate) from inexpensive substrates. Enzym Microb Technol 27:774–777
Kim BS, Chang HN (1998) Production of poly (3-hydroxybutyrate) from starch by Azotobacter chroococcum. Biotechnol Lett 20(2):109–112
Kim DY, Rhee Y (2003) Biodegradation of microbial and synthetic polyesters by fungi. Appl Microbiol Biotechnol 61:300–308. https://doi.org/10.1007/s00253-002-1205-3
Kitamura S, Doi Y (1994) Staining method of poly(3-hydroxyalkanoic acids) producing bacteria by Nile blue. Biotechnol Tech 8(5):345–350
Koller M (2018) Biodegradable and biocompatible polyhydroxy-alkanoates (PHA): auspicious microbial macromolecules for pharmaceutical and therapeutic applications. Molecules 23(362):1–20
Krueger CL, Radetski CM, Bendia AG, Oliveira IM, Castro-Silva MA, Rambo CM, Antonio RV, Lima AOS (2012) Bioconversion of cassava starch by-product into Bacillus and related bacteria polyhydroxyalkanoates. Electron J Biotechnol 15(3):8. https://doi.org/10.2225/vol15-issue3-fulltext-6
Lafferty RM, Korstko B, Korsatko W (1988) Microbial production of poly (3-hydroxybutyric acid). In: Rehm HJ, Reed G (eds) Biotechnology, vol 6. Verlagsgesellschaft, Weinheim, pp 135–176
Lee SY (1996) Plastic bacteria? Progress and prospect for polyhydroxyalkanoate production in bacteria. Tibtech 14:431–438
Lenz RW, Marchessault RH (2005) Bacterial polyesters: biosynthesis, biodegradable plastics, and biotechnology. Biomacromolecules 6(1):1–8
Lillo JG, Rodriguez-Valera F (1990) Effects of culture conditions on poly(beta-hydroxybutyric acid) production by Haloferax mediterranei. Appl Environ Microbiol 56(8):2517–2521
Madison LL, Huisman GW (1999) Metabolic engineering of poly (3-hydroxyalkanoates): from DNA to plastics. Microbiol Mol Biol Rev 63(1):21–53
Margino S, Martani E, Yuswanto S, Sembiring L (2000) Isolasi dan Seleksi Bakteri Penghasil Plastik Terdegradasi, Poli-β-hidroksibutirat. Biologi 2(10):583–597. [Indonesian]
Margino S, Martani E, Prameswara A (2014) Poly-β-hydroxybutyrate (phb) production by amylolytic Micrococcus sp. PG1 isolated from soil polluted arrowroot starch waste. Int J Biotech 19(1):56–63
Mascarenhas J, Aruna K (2017) Screening of polyhydroxyalkanoates (PHA) accumulating bacteria from diverse habitats. J Global Biosci 6(3):4835–4848
Mathuriya AS, Yakhmi JV (2017) Polyhydroxyalkanoates: biodegradable plastics and their applications. In: Martínez L, Kharissova O, Kharisov B (eds) Handbook of ecomaterials. Springer, Cham. https://doi.org/10.1007/978-3-319-48281-1_84-1
Murray RGE, Doetsch RN, Robinow CF (1994) Determinative and cytological light microscopy. In: Gerhardt P, Murray RGE, Wood WA, Krieg NR (eds) Manual of methods for general microbiology. American Society for Microbiology, Washington, DC, pp 21–41
Najimudin N, Aguestin A, Few LL, Lim CH, Razip MS, Azizan MN, Isa AM (1997) Isolation of gram positive polyhydroxyalkanoate producers from malaysian soil. Annu Rep IC Biotechnol 20:710–714
Ostle AG, Holt JG (1982) Nile blue A as a fluorescent stain for poly-β-hydroxybutyrate. Appl Environ Microbiol 44(1):238–241
Pena C, Castillo T, García A, Millán M, Segura D (2014) Biotechnological strategies to improve production of microbial poly-(3-hydroxybutyrate): a review of recent research work. Microb Biotechnol 7(4):278–293. https://doi.org/10.1111/1751-7915.12129
Phanse N, Chincholikar A, Patel B et al (2011) Screening of PHA (polyhydroxyalkanoate) producing bacteria from diverse sources. Int J Biosci 1(6):27–32
Rajan KP, Thomas SP, Gopanna A, Chavali M (2018) Polyhydroxybutyrate (PHB): a standout biopolymer for environmental sustainability. In: Martínez L, Kharissova O, Kharisov B (eds) Handbook of ecomaterials. Springer, Cham. https://doi.org/10.1007/978-3-319-48281-1_92-1
Ramadas NV, Singh SK, Soccol CR, Pandey A (2009) Polyhydroxybutyrate production using agro-industrial residue as substrate by Bacillus sphaericus NCIM 5149. Braz Arch Biol Technol 52:17–23
Ramsay BA, Lomaliza K, Chavarie C, Dube B, Bataille P, Ramsay JA (1990) Production of poly-(β-hydroxybutyric-co-β-hydroxyvaleric) acids. Appl Environ Microbiol 56(7):2093–2098
Redzwan G, Gan SN, Tan IKP (1997) Isolation of polyhydroxyalkanoate producing bacteria from an integrated-farming pond and palm-oil mill effluent ponds. World J Microbiol Biotechnol 13:707–709
Rodríguez-Contreras A, Koller M, De Sousa Dias M, Calafell-Montfort M, Braunegg G, Marqués-Calvo M (2013) High production of poly(3-hydroxybutyrate) from a wild Bacillus megaterium Bolivian strain. J Appl Microbiol 114:1378–1387. https://doi.org/10.1111/jam.12151
Shivalkar YK, Prabha R (2017) Polyhydroxybutyrate as bio-degradable plastic – a review. IOSR J Environ Sci Toxicol Food Technol 11(5):10–12
Sudesh K, Abe H, Doi Y (2000) Synthesis, structure and properties of polyhydroxyalkanoates: biological polyester. Prog Polym Sci 25:1503–1555
Syamsu K, Fauzi AM, Hartoto L, Suryani A, Atifah N (2006) Utilization of hydrolyzed sago starch as main substrate for the production of bioplastic poly hydroxy butyrate (PHB) by Ralstonia eutropha on fed-batch cultivation system. In: Proceedings The Era of Bioscience Challenge and Hope. The Fifth Regional IMT-GT Uninet Conference & International Seminar 2006. Faculty of Agriculture Universitas Sumatera Utara, Medan, 22–23 June 2006
Taherzadeh MJ, Karimi K (2007) Enzyme-based hydrolysis processes for ethanol from lignocellulosic materials: a review. Bioresources 2(4):707–738
Thakur PS, Borah B, Baruah SD, Nigam JN (2001) Growth-associated production of poly-β-hydroxybutyrate by Bacillus mycoides. Folia Microbiol 46(6):488–494
Valappil SP, Boccaccini AR, Bucke C, Roy EI (2007) Polyhydroxyalkanoates in gram positive bacteria: insight from the genera Bacillus and Streptomyces. Anton Leeuw 91:1–17
Yanti NA (2013) Produksi Bioplastik Poli-Β-Hidroksibutirat Oleh Bakteri Amilolitik Yang Diisolasi Dari Tepung Sagu Basah Menggunakan Berbagai Macam Substrat Pati. In: Prosiding Seminar Nasional ke-22 Perhimpunan Biologi Indonesia. Purwokerto, Jawa Tengah, Indonesia: Fakultas Biologi Unsoed. [Indonesian]
Yanti NA, Muhiddin NH (2016) Bioconversion of sago starch to bioplastic poly-β-hydroxybutyrate (PHB) by local strain bacterial Bacillus megaterium PSA10. J Chem Pharm Res 8(7):918–923
Yanti NA, Sembiring L, Margino S (2009) Production of Poly-β-hydroxybutyrate (PHB) from sago starch by the native isolate Bacillus megaterium PSA10. Int J Biotechnol 14(1):1111–1116
Yanti NA, Sembiring L, Margino S, Muhiddin NH (2013) A study on production of poly-β-hydroxybutyrate bioplastic from sago starch by indigenous amylolytic bacteria. Int J Biotechnol 18(2):144–150
Yanti NA, Sembiring L, Margino S, Muhiddin HN, Ahmad SW, Ardiansyah (2019) Polyphasic identification of amylolytic bacteria producing bioplastic poly-β-hydroxybutyrate (PHB). Sains Malays 48(12):2663–2673
Yanti NA, Margino S, Sembiring L (2020) Proses Produksi Bioplastik Poli-β-hidroksibutirat (PHB) dari Substrat Pati Sagu menggunakan Bacillus megaterium PSA10. Indonesian Patent IDP000068082, 13 Mar 2020
Yilmaz M, Soran H, Beyatli Y (2005) Determination of poly-β-hydroxybutyrate (PHB) production by some Bacillus spp. World J Microbiol Biotechnol 21:565–566
Yu J (2001) Production of PHA from starchy wastewater via organic acids. J Biotechnol 86(2):105–112
Yuksekdag ZN, Aslim B, Beyatli Y, Mercan N (2004) Effect of carbon and nitrogen sources and incubation times on poly-β-hydroxybutyrate (PHB) synthesis by Bacillus subtilis 25 and Bacillus megaterium 12. Afr J Biotechnol 3(1):63–66
Zilliges Y, Damrow R (2017) Quantitative determination of poly-β-hydroxybutyrate in Synechocystis sp. PCC 6803. Bio-protocol 7(14):e2402. https://doi.org/10.21769/BioProtoc.2402
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2021 The Author(s), under exclusive licence to Springer Nature Singapore Pte Ltd.
About this chapter
Cite this chapter
Yanti, N.A., Sembiring, L., Margino, S., Ahmad, S.W. (2021). Bacterial Production of Poly-β-hydroxybutyrate (PHB): Converting Starch into Bioplastics. In: Kuddus, M., Roohi (eds) Bioplastics for Sustainable Development. Springer, Singapore. https://doi.org/10.1007/978-981-16-1823-9_9
Download citation
DOI: https://doi.org/10.1007/978-981-16-1823-9_9
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-16-1822-2
Online ISBN: 978-981-16-1823-9
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)