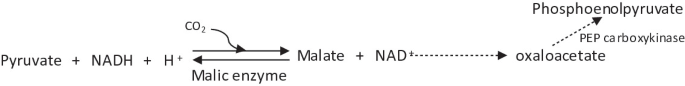Abstract
Tricarboxylic acid (TCA) cycle, also known as citric acid cycle and Krebs’ cycle, is the final step in complete oxidation of glucose under aerobic conditions. During aerobic respiration, pyruvate is oxidized to acetyl CoA by the action of the pyruvate dehydrogenase enzyme complex. The acetyl CoA thus generated enters the citric acid cycle. Tricarboxylic acid cycle starts with the condensation of acetyl CoA and oxaloacetate to form citrate and proceeds in a cyclic manner generating reductant, GTP and re-generating oxaloacetate. For complete oxidation of glucose, two rounds of TCA cycle are required. Various intermediates of the TCA cycle which act as precursors for various biosynthetic pathways such as synthesis of amino acids, heme, fats and lipids are discussed. Various reactions required for replenishing oxaloacetate for continuity of the TCA cycle are described. At the end, regulation of the TCA cycle is also discussed.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- TCA
- Citric acid
- Pyruvate dehydrogenase complex
- Malonate
- Hans krebs
- Malate–aspartate shuttle
- Glycerol phosphate shuttle
- Pyruvate carboxylase
- PEP carboxylase
- Energy charge
The central role of the tricarboxylic acid cycle (also known as TCA cycle/citric acid cycle/Krebs cycle) is the oxidation of acetyl CoA (derived from metabolism of amino acids, fatty acids and carbohydrates) to CO2 and H2O with simultaneous conservation of energy as reduced coenzymes and eventually ATP. Intermediates of citric acid cycle also serve as starting points in a number of synthetic reactions. Oxaloacetate and α-ketoglutarate are involved in amino acid synthesis, succinyl CoA is used in heme synthesis, and citrate is the source of acetyl CoA used in synthesis of fats, lipids and some amino acids. In eukaryotes, citric acid cycle operates in the mitochondrial matrix.
1 Historical Background of TCA Cycle
In the early 1900s, various experiments established that anaerobic suspensions of minced animal tissue transfer hydrogen atoms from organic acids (namely fumarate, succinate, citrate and malate) to other reducible compounds such as methylene blue dye. In 1935, Albert Szent-Gyorgyi discovered that small amounts of these organic acids caused oxygen uptake by a tissue suspension far in excess of the oxygen required to completely oxidize them to CO2 and H2O. He concluded that the organic acids acted catalytically on the oxidation of glucose or other carbohydrates. He also showed that malonate which is an inhibitor of succinate dehydrogenase, inhibited oxygen utilization (respiration). In 1937, Hans Krebs postulated the citric acid cycle based on previous findings by Szent-Gyorgyi and his own findings that citrate catalytically stimulates respiration and is converted to α-ketoglutarate and then to succinate. Also, oxaloacetate is converted to citrate by addition of two carbons. The source of these two carbons was later identified to be acetyl CoA by works of Frtiz Lipman (1947) and Severo Ochoa (1952) (Table 1).
2 Bridge Between Glycolysis and TCA Cycle—Pyruvate Dehydrogenase Complex
At the final step of the glycolytic cycle, phosphoenol pyruvate is converted to pyruvate. Under aerobic respiration, pyruvate is oxidized to acetyl CoA by the action of the pyruvate dehydrogenase enzyme complex. The acetyl CoA thus generated enters into the citric acid cycle. Thus, the pyruvate dehydrogenase enzyme complex acts as a bridge between the glycolytic pathway and the citric acid cycle.
Pyruvate dehydrogenase enzyme complex is present in both prokaryotes and eukaryotes where the reaction takes place in the mitochondrion. It is a multi-enzyme complex with an enormous size. The complex, a non-covalent assembly of three different enzymes, catalyzes five successive reactions involved in the conversion of pyruvate to acetyl CoA.
The three enzymes of the pyruvate dehydrogenase enzyme complex are pyruvate dehydrogenase (E1, also known as pyruvate decarboxylase), dihydrolipoate transacetylase (E2) and dihydrolipoate dehydrogenase (E3). In addition, the complex requires five coenzymes, namely thiamine pyrophosphate (TPP), FAD, lipoic acid, NAD + and coenzyme A. Each molecule of dihydrolipoate transacetylase contains two molecules of lipoic acid attached by an amide bond between the carboxyl group at the end of lipoic acid’s hydrocarbon chain and the terminal amino group of a lysine residue of the enzyme. TPP and FAD are catalyzing cofactors for pyruvate dehydrogenase and dihydrolipoate dehydrogenase, respectively, and are tightly bound to them.
The intermediates produced during the five successive reaction steps remain bound to the enzyme complex and are transferred from one active site to the other. The five-reaction sequence is thus an example of substrate channeling. The active sites of all the three enzymes are situated close to one another in such a way that the product of the first enzyme is the substrate for the second enzyme and is directly passed to the active site of the second enzyme.
Many molecules of each of these three enzymes are organized to form the enzyme complex. In gram-negative bacteria, pyruvate dehydrogenase complex has 60 subunits in three functional proteins (Table 2). In eukaryotes and gram-positive bacteria, it consists of 96 subunits in the three functional proteins. The pyruvate dehydrogenase complex is very large, and its size is several times bigger than a ribosome. This serves to channel the high pyruvate flux.
Pyruvate Dehydrogenase Complex in E. coli: In E. coli, the pyruvate dehydrogenase complex is 4.6 million Daltons in size and consists of 60 polypeptides (24 molecules of pyruvate dehydrogenase, 24 molecules of dihydrolipoate transacetylase, 12 molecules of dihydrolipoate dehydrogenase) (Fig. 1).
Reactions catalyzed by the pyruvate dehydrogenase complex:
-
Reaction 1—Pyruvate is decarboxylated to form the key reactive intermediate, hydroxyethyl thiamine pyrophosphate (HETPP) using pyruvate dehydrogenase. Hydroxytheyl derivative is bound to the reactive carbon of TPP, the coenzyme for pyruvate dehydrogenase.
-
Reaction 2—Oxidation of HETPP by transfer to the disulphide form of lipoic acid (bound to dihydrolipoate transacetylase) which itself is reduced to sulphydryl form. TPP is displaced, and the acetyl group is transferred to lipoic acid. This is also catalyzed by the action of pyruvate dehydrogenase.
-
Reaction 3—Transacetylation reaction wherein the acetyl group, bound as a thioester to the side chain of lipoic acid is transferred to CoA-SH forming acetyl CoA and reduced lipoic acid. This reaction is catalyzed by dihydrolipoate transacetylase.
-
Reaction 4—The reduced lipoic acid is reoxidized by FAD bound to dihydrolipoate dehydrogenase.
-
Reaction 5—The reduced FAD bound to dihydrolipoate dehydrogenase is reoxidized by NAD+. The reactions are summarized in Fig. 2.
Regulation of the pyruvate dehydrogenase complex: This reaction sequence is irreversible and regulated by several factors. In E. coli, pyruvate dehydrogenase complex is feedback inhibited by ATP, acetyl CoA and NADH. Further, PEP, AMP, NAD+ and CoA-SH accumulate when very small amounts of acetate flows into the citric acid cycle and allosterically activates the enzyme complex. Thus, the enzyme complex is turned off when sufficient fuel is available (as acetyl CoA) and when the cell has high levels of energy charge and reducing equivalents. Conversely, high energy demands and requirement of greater flux of acetyl CoA into the citric acid cycle turn on the enzyme complex (Fig. 3).
3 Tricarboxylic Acid Pathway/Krebs’s Cycle
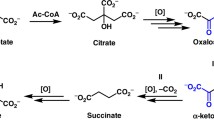
Tricarboxylic acid cycle starts with the condensation of acetyl CoA (generated by reactions of the pyruvate dehydrogenase complex) and oxaloacetate to form citrate by citrate synthase. After citrate formation, the pathway proceeds in a cyclic manner to generate oxaloacetate back. During this cycle, two moles of CO2, four moles of reductant (three NADH + H+ and one FADH2) and one GTP through substrate-level phosphorylation are generated (Fig. 4). Various steps are as follows:
Citric acid cycle.
-
1.
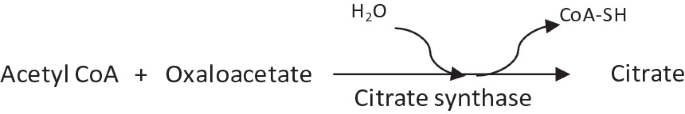
Condensation of acetyl CoA and oxaloacetate to form citrate by citrate synthase.
-
This reaction is irreversible and has equilibrium far in the direction of citrate synthesis.
-
This reaction uses an intermediate of the TCA cycle (oxaloacetate) and produces another intermediate (citrate).
-
The first substrate to bind to the enzyme, i.e., oxaloacetate induces a conformational change in the enzyme structure such that a binding site for the second substrate, acetyl CoA is created.
-
-
2.
Citrate is isomerized to isocitrate by aconitase, also known as aconitate hydratase.

-
This is a reversible reaction.
-
Aconitase occurs both in mitochondrial and cytoplasmic form.
-
-
3.
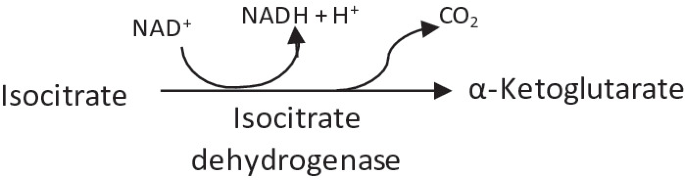
Decarboxylation and oxidation of isocitrate to α-ketoglutarate by isocitrate dehydrogenase.
-
This is a rate limiting step of the citric acid cycle.
-
It is an irreversible reaction.
-
Release of the first CO2 and reductant (NADH + H+) occurs.
-
There are two different forms of isocitrate dehydrogenase in all cells, one requiring NAD+ while the other requiring NADP+ as electron acceptor. The overall reactions catalyzed by these two forms are identical. In eukaryotic cells, the NAD-dependent enzyme is present in the mitochondrial matrix and functions in the TCA cycle. The NADP-dependent enzyme is present both in cytosol and mitochondrial matrix and leads to generation of NADPH, which is essential for reductive anabolic reactions. In case the NADH pool within mitochondria is very high, citrate can be diverted out to the cytoplasm where it will be acted upon by cytosolic aconitase and isocitrate dehydrogenase to produce NADPH.
-
-
4.
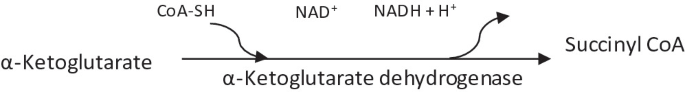
Oxidative decarboxylation of α-ketoglutarate by α-ketoglutarate dehydrogenase complex.
-
This reaction is irreversible and has equilibrium far in the direction of succinyl CoA.
-
Release of the second CO2 and reductant (NADH + H+) occurs
-
Both the structure and function of α-ketoglutarate dehydrogenase complex closely resemble that of pyruvate dehydrogenase complex.
-
It includes three enzymes, homologous to E1 (known as α-ketoglutarate dehydrogenase; E’1), E2 (known as dihydrolipolyl transsuccinylase or succinyl transferase; E’2) and E3 (dihydrolipoate dehydrogenase) of the pyruvate dehydrogenase complex. E3 of both the complexes is the same protein.
-
This complex also requires five coenzymes, namely enzyme-bound TPP, bound lipoic acid, FAD, NAD+ and coenzyme A.
-
-
5.
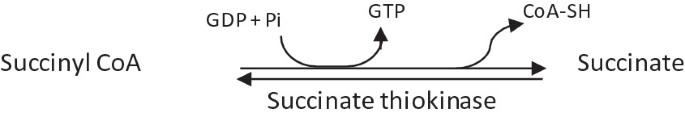
Cleavage of succinyl CoA to succinic acid by succinate thiokinase, also called as succinyl CoA synthetase.
-
It is a reversible reaction.
-
It is the only reaction yielding high-energy GTP by substrate-level phosphorylation.
-
-
6.
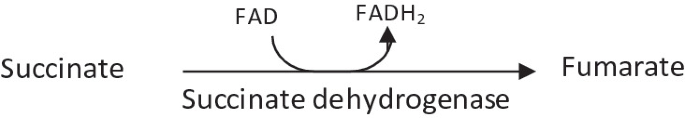
Succinate is oxidized to fumarate by succinate dehydrogenase.
-
It is an irreversible reaction.
-
Release of the third reductant (FADH2) occurs.
-
In this reaction, the electron acceptor is FAD instead of NAD+ as succinate does not have sufficient reducing power to reduce NAD+.
-
Malonate, an analog of succinate which is normally not present in cells, is a strong competitive inhibitor of succinate dehydrogenase. Its addition to mitochondria inhibits the activity of the citric acid cycle.
-
-
7.
Fumarate is hydrated to malate by fumarase, also known as fumarate hydratase.

-
It is a freely reversible reaction.
-
-
8.
Malate is oxidized to oxaloacetate by malate dehydrogenase.
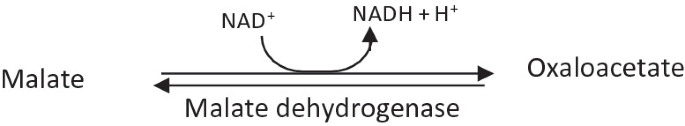
-
This is a reversible reaction.
-
Release of the fourth reductant (NADH + H+) occurs.
-
Malate dehydrogenase is present both in the mitochondrial matrix and the cytosol in case of eukaryotes.
-
Another enzyme malate: Quinone oxidoreductase (Mqo) catalyzes the oxidation of malate to oxaloacetate by donating electrons to quinone. Both Mqo and malate dehydrogenase can be active in the cell at the same time. However, Mqo does not have a very a significant physiological role in malate oxidation.
-
Stoichiometry of Complete Oxidation of Glucose
Starting from acetyl CoA, the stoichiometry of the citric acid cycle is presented in Table 3:
Acetyl CoA + 2H2O + 3NAD+ + FAD + GDP + Pi → 2CO2 + 3NADH + 3H+ + FADH2 + GTP + CoA-SH
Further, during the electron transport cycle, 2.5 ATPs are produced per NADH and 1.5 ATPs are produced per FADH2. Thus, one round of citric acid cycle yields 9 ATP and 1 GTP equivalent to 10 high-energy phosphates.
For complete oxidation of glucose, two rounds of TCA cycle are required. Thus, TCA cycle yields 20 ATP per glucose and total gain during complete oxidation via glycolysis till CO2 and H2O is 32 ATP (Table 4).
TCA cycle as an anabolic pathway
Intermediates of the TCA cycle act as precursors for various biosynthetic pathways such as synthesis of amino acids, heme, fats and lipids (Fig. 5). Oxaloacetate is used in synthesis of aspartate which in turn is involved in synthesis of five more amino acids. Succinyl CoA is utilized in the synthesis of lysine and methionine and synthesis of tetrapyrroles present in cytochromes and chlorophylls. α-ketoglutarate is used for glutamate synthesis which is the precursor for three more amino acids. Fumarate also acts as the precursor for aspartate in some bacteria. Since the TCA cycle can function in a catabolic manner and also anabolically as a source of precursors for biosynthetic pathways, it is referred to as an amphibolic pathway.
Anapleurotic reactions of TCA cycle
Intermediates of the cycle such as oxaloacetate, citrate, α-ketoglutarate and succinyl CoA act as precursors for various biosynthetic pathways are thus are constantly removed. The replenishment of these intermediates is important to maintain the cellular pool and continuity of the cycle. Cells have mechanisms of “refilling” the pools of citric acid cycle intermediates by pathways known as anapleurotic reactions. Under normal circumstances, there is a dynamic balance between the reactions involved in siphoning off the intermediates into other pathways and those responsible for their replenishment, such that the concentrations of these intermediates remain nearly constant.
Further, it is not necessary to replenish the intermediate that is being removed, as any intermediate can be replenished by feeding-in from any point in the cycle. Thus, cells need only one or two major anapleurotic reactions.
The continuity of the cycle is mostly maintained by maintaining the right levels of oxaloacetate. Oxaloacetate replenishment can occur by various pathways:
-
1.
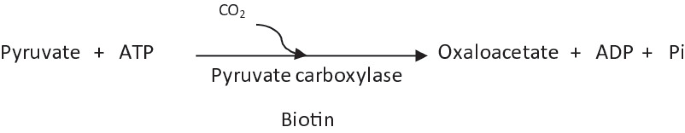
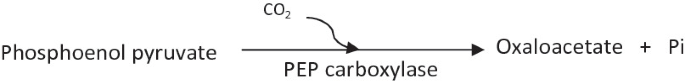
Sugars such as glucose are converted to pyruvate or phosphoenol pyruvate which can then be carboxylated to oxaloacetate via the action of enzymes pyruvate carboxylase and PEP carboxylase, respectively. Both these enzymes are widespread in bacteria. However, E. coli lacks pyruvate carboxylase. Further, PEP carboxylase is not found in fungi or animals.
-
(a)
Pyruvate carboxylase:
Pyruvate carboxylase contains biotin as a coenzyme. It is an allosteric enzyme that is positively regulated by acetyl CoA (higher levels of acetyl CoA signify higher requirement of oxaloacetate). In some eukaryotes such as Aspergillus niger, pyruvate carboxylase can be present both in mitochondria and cytosol.
-
(b)
PEP carboxylase:
In E. coli, PEP carboxylase is an allosteric enzyme. PEP carboxylase is negatively controlled by aspartate (which is formed from oxaloacetate and thus higher concentrations of aspartate signify higher concentrations of oxaloacetate) and positively controlled by acetyl CoA (higher concentration of acetyl CoA is a signal to produce higher amounts of oxaloacetate) (Fig. 6).
-
(a)
-
2.
Oxaloacetate is also replenished by the action of a third enzyme, commonly called malic enzyme. This enzyme catalyzes reductive carboxylation of pyruvate to malate which can then be oxidized to oxaloacetate.
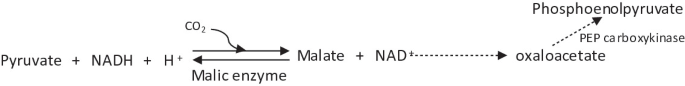
This is a reversible reaction. Malic enzyme, together with PEP carboxykinase, is important for growth on citric acid intermediates such as succinate or malate.
-
3.
In cases where there is excess availability of amino acids, they can be directly converted to oxaloacetate.
-
4.
In cases, where organic acids such as fumarate, succinate and malate are available in excess, they can be directly oxidized to oxaloacetate in the standard citric acid cycle reaction.
-
5.
The glyoxylate cycle can also contribute to replenishment of oxaloacetate by synthesis of malate.
Partitioning of Pyruvate between synthesis of acetyl CoA and oxaloacetate
Pyruvate has two major metabolic uses: oxidation of carbon via citric acid cycle for energy generation and providing precursors for biosynthetic pathways. Thus, pyruvate is partitioned between two main pathways—decarboxylation to acetyl CoA via activity of pyruvate dehydrogenase and carboxylation to oxaloacetate via activity of pyruvate carboxylase.
Acetyl CoA is a negative regulator for pyruvate dehydrogenase and a positive regulator for pyruvate carboxylase. Thus, in conditions where the intermediates of TCA cycle such as succinyl CoA or α-ketoglutarate are removed for biosynthesis, the levels of oxaloacetate would also decrease. Thus, the rate of citrate synthase reaction lowers and consequently acetyl CoA would accumulate. This leads to a decrease in activity of pyruvate dehydrogenase and increase in activity of pyruvate carboxylase thereby leading to increased production of oxaloacetate and continuation of TCA.
Regulation of TCA cycle
The regulation of the TCA cycle is largely governed by availability of substrate, energy charge and reducing equivalents in the cellular pool. Each of the three strongly exergonic steps in the cycle, those catalyzed by citrate synthase, isocitrate dehydrogenase, and α-ketoglutarate dehydrogenase, and the reactions catalyzed by pyruvate dehydrogenase complex can become the rate-limiting step under some circumstances (Fig. 7).
Under condition of high energy charge of the cell, i.e., high ATP/ADP + AMP ratio, citrate synthase, isocitrate dehydrogenase, and α-ketoglutarate dehydrogenase are inhibited. In addition, pyruvate dehydrogenase complex that synthesizes acetyl CoA (which is required for the first reaction of TCA cycle) is also negatively regulated by ATP. This regulation ensures that the TCA cycle will not oxidize excessive amount of pyruvate and acetyl CoA when ATP in the cell is plentiful. Conversely, when the energy charge in the cell is low, these enzymes are activated.
Availability of reducing equivalents, i.e., the ratio of NADH/NAD+ is the other major control of the cycle. This is due to substrate inhibition by NADH of the enzymes that use NAD+ as a substrate. This is desirable metabolically, since the operation of the cycle leads to an increase in this ratio. Electron transport and oxidative phosphorylation control the relative ratios of NAD+ and NADH. Under condition of low oxygen, the electron transport system is inhibited and NADH accumulates. This in turn decreases NAD+ availability for the citric acid cycle. Thus, the more “aerobic” the cellular environment is, the more the citric acid cycle runs. All the major checkpoints of the TCA cycle, i.e., activity of citrate synthase, isocitrate dehydrogenase, α-ketoglutarate dehydrogenase and also pyruvate dehydrogenase, are inhibited by NADH. Further, all these enzymes are activated at low levels of the reducing equivalents.
Substrate availability is another limitation of the cycle. Pyruvate dehydrogenase can be allosterically inhibited by acetyl CoA. Citrate synthase is regulated by the availability of its substrates, acetyl CoA and oxaloacetate. Citrate synthase is also inhibited by α-ketoglutarate in facultative gram-negative bacteria such as E. coli.
In addition to the above regulatory mechanisms, isocitrate dehydrogenase is also controlled by a reversible phosphorylation and dephosphorylation mechanism which is catalyzed by a bifunctional kinase/phosphatase. Phosphorylation inactivates the enzymes while dephosphorylation activates the enzymes. Thus, isocitrate dehydrogenase is normally present in the dephosphorylated form. Regulation by kinase/phosphatase comes into play only in the presence of acetate as the carbon source and more details will be provided while studying the glyoxylate cycle in Chap. 13: Alternate Tricarboxylic acid cycle.
Shuttling of intermediates and reductants between cytosol and mitochondria in eukaryotes
The glucose oxidation pathways, viz. glycolysis and pentose phosphate pathway, occur in cytosol while citric acid cycle operates in the mitochondrial matrix in case of eukaryotes. In prokaryotes, there is no such compartmentalization.
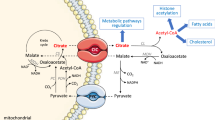
Shuttling of intermediates—The glycolytic by-product pyruvate is produced in cytosol and transported to mitochondria before it is further processed. There it is degraded by pyruvate dehydrogenase to acetyl CoA and reductant and enters into the TCA cycle. At the same time, as a part of anapleurotic reaction, mitochondrial pyruvate carboxylase converts a part of pyruvate to oxaloacetate to continue the cycle. Citrate, the first product of the TCA cycle, is also required for biosynthesis and is transported out of mitochondria. In the cytosol, citrate is acted upon by ATP citrate lyase to generate acetyl CoA which is required for synthesis of fatty acids, phospholipids and biomass generation. The citrate export from mitochondria is compensated by oxaloacetate import back into the mitochondria in the form of malate. This is feasible by conversion of oxaloacetate to malate by cytosolic malate dehydrogenase. Malate enters the mitochondria and gets converted back to oxaloacetate (Fig. 8).
Shuttling of reductant—The transport of reductants from the cytosol to the mitochondrial matrix occurs via two shuttle pathways, namely the malate–aspartate shuttle and the glycerol phosphate shuttle (Fig. 8).
-
1.
Malate–aspartate shuttle—In the first step, cytosolic malate dehydrogenase catalyzes the reaction of oxaloacetate and NADH + H+ to produce malate and NAD+. Thus, the reducing equivalents are attached to oxaloacetate to form malate. Malate thus produced is transported into the mitochondrial matrix. This import into the mitochondrial matrix is balanced by exporting out oxaloacetate formed by the action of mitochondrial malate dehydrogenase. During this reaction, NAD+ is reduced to form NADH + H+. Further, oxaloacetate cannot directly be transported into the cytosol and thus the export is in the form of aspartate. Mitochondrial aspartate aminotransferase converts oxaloacetate to aspartate using glutamate as an amino donor. In the reaction, glutamate is transformed into alpha-ketoglutarate. In the cytosol, aspartate is again converted back to oxaloacetate by cytosolic aspartate aminotransferase by transferring the amino group to α-ketoglutarate to form glutamate. The net effect of the malate–aspartate shuttle is purely in terms of redox. NADH + H+ in the cytosol is oxidized to NAD+ and NAD+ in the matrix is reduced to NADH + H+.
-
2.
Glycerol phosphate shuttle—The glycerol phosphate shuttle has a secondary role in transporting reducing equivalents. In the first step, cytosolic glycerol 3-phosphate dehydrogenase converts dihydroxyacetone phosphate (produced by isomerization of 3-phosphoglyceraldehyde) to glycerol 3-phosphate by oxidizing one molecule of NADH + H+ to NAD+. Glycerol 3-phosphate is transported into the mitochondrial matrix where it is converted back to dihydroxyacetone phosphate by mitochondrial glycerol-3-phosphate dehydrogenase, by reducing one molecule of FAD to FADH2. The dihydroxyacetone phosphate is exported back to the cytosol to complete the carbon balance.
4 Citric Acid Production by Aspergillus Niger: An Overview
Citric acid is a primary metabolite, and its production is well regulated in organisms. Citrate synthase, the enzyme that catalyzes production of citric acid, is regulated by several factors including energy charge and reductant. Further, citrate is one of the key inhibitors of the glycolytic pathway. Under such a tight regulation scheme, it is surprising that any organism can over-produce citric acid. Various studies have shown that citric acid producing mutants have devised several control mechanisms and many biochemical events jointly contribute to the over-production (Papagianni, 2007; Netik et al., 1997).
The accumulation of citric acid is strongly influenced by the composition of the medium and physiological conditions. In A. niger, citric acid over-production is observed during the idiophase (stationery growth phase) and under specific conditions, viz. (i) acidic pH ranging from 1.6–2.2; (ii) high carbon source (sugar concentration 12–25% w/v); (iii) Mn2+ deficiency; and (iv) limited nitrogen source (high NH4+ concentration) (Table 5).
Glucose uptake—Under high sugar concentrations, a low-affinity glucose transporter is induced that provides the high flux of glucose required for citrate production. Some reports also suggest that simple diffusion which is not dependent on any transport systems is the primary mechanism for glucose uptake under these conditions.
Conversion of glucose to pyruvate—During glucose fermentation by the glycolytic pathway, the key regulatory enzyme phosphofructokinase I (PFK I) is inhibited by both ATP and citrate. At the stationery growth phase, citrate concentration in the cell is high even during slow growth because of high biomass. This would normally lead to inhibition of PFK I. For citric acid over-production, this inhibition is overcome by accumulation of various positive effectors of PFK I. Manganese is a known inhibitor for PFK I, and thus, the medium is made to be deficient in Mn2+. Further, Mn2+ deficiency results in breakdown of cellular proteins which also contributes to an increase in intracellular concentration of ammonium. Ammonium ions further overcome citrate inhibition of PFK I. Furthermore, high sugar concentration leads to rapid production of fructose-2,6-bisphosphate, an activator of PFK I. These conditions lead to a flux through the glycolytic pathway and accumulation of pyruvate.
Citrate production—Some part of the accumulated pyruvate is transported to the mitochondrial matrix and enters TCA cycle as usual. High sugar concentration leads to accumulation of glycerol, an osmoprotectant which inhibits isocitrate dehydrogenase. On inhibition of isocitrate dehydrogenase, the unfavorable equilibrium of aconitase causes accumulation of large amounts of citrate rather than isocitrate. Moreover, the combination of high NH4+ concentration and high sugar concentration represses the synthesis of α-ketoglutarate dehydrogenase, thereby inhibiting citrate catabolism in the TCA cycle and favoring its overproduction. Thus, citric acid is an “overflow end product” due to high flux rates upstream and reduced flux rates downstream of the accumulation point.
Further, some part of the pyruvate accumulated due to the physiological conditions remain in the cytoplasm and is converted to oxaloacetate by cytosolic pyruvate carboxylase and then to malate by cytosolic malate dehydrogenase. Thus, the citrate accumulated in the mitochondrial matrix can be exported out to the cytoplasm in exchange for malate which is transported into the mitochondria.
Citrate export from mitochondria to cytosol—Citric acid is a tricarboxylic acid containing three carboxylic functional groups with three different values of pKa (3.1, 4.7 and 6.4). Thus, citric acid can occur in three ionization states depending upon the pH conditions. The normal ionization state of citrate at pH ~ 6 (prevalent in the cytosol) is citrate2− (citrate3−+H+). Citrate3−+H+ can be exchanged with malate (which is present as malate2− in the cytosol) via the tricarboxylate antiporter for delivery of citrate to the cytosol. The malate required for antiport exchange can be exported back to the cytosol via the dicarboxylate antiport, which accepts \({\text{HPO}}_{4}^{2 - }\). This has a dual role since oxidative phosphorylation creates a continuous demand for phosphate in mitochondria which is supplied via this exchange.
The citrate produced in mitochondria is also exchanged for α-ketoglutarate via another mitochondrial organic acid transporter known as citrate–oxoglutarate shuttle protein (Kirimura et al., 2019). The exchange of citric acid for cytoplasmic malate and oxoglutarate/α-ketoglutarate serves a dual purpose of exporting produced citric acid from mitochondria and importing TCA cycle intermediates. The oxaloacetate, which is used up during citric acid formation, is regenerated from these imported TCA cycle intermediates, malate and α-ketoglutarate. This continues citric acid production even when the TCA cycle is slowed down at isocitrate and α-ketoglutarate step.
Citrate export from cytosol to extracellular medium—The export of citric acid out of cytoplasm occurs by ΔpH-driven H+-symport-dependent system, active process and organic anion transporter.
There is a large pH gradient between the cytosol (which is at ~ pH 6) and the extracellular medium (which is at ~ pH 2). Thus, citric acid is present as citrate2− in the cytosol while it is almost undissociated at the pH of the extracellular medium. As per Mattey (1977), the cell membrane is permeable to citrate2− and citrate2− is excreted by passive diffusion along a gradient of dissociated citric acid. To prevent uptake of these citrate2− ions back in the cell, the extracellular pH is kept low. At low pH values, citrate2− ions get protonated, become undissociated and cannot enter the cell by the diffusion process. Thus, low extracellular pH keeps the external citrate2− concentrations at a negligible level and they cannot diffuse back into the cell. This is also the reason of different rates of citrate export observed at different external pH values.
Netik et al. (1997) demonstrated that citrate is excreted out of the cell by an active process requiring ATP. This export was also sensitive to metabolic inhibitors such as sodium azide and a proton conductor, carbonyl cyanide m-chlorophenyl hydrazone (CCCP). This system is dependent on Mn2+ and this dependency could only partially be fulfilled by other divalent metal ions. Further, it was observed that citrate was not exported out unless the fungal mycelium was deficient in Mn2+ ions, whereas the uptake of citrate from the medium was only detectable upon pre-cultivation of A. niger in a medium supplemented with Mn2+ ions. Thus, it has been postulated that citrate efflux as well as uptake is reciprocally regulated by manganese ions. Thus, presence of Mn2+ stimulates citrate uptake and inhibits efflux while deficiency of Mn2+ inhibits uptake and stimulates efflux. Manganese ions are responsible for differential transcriptional regulation of the uptake, and the excretion system and carrier inversion occur depending upon the Mn2+ levels.
Thus, Mn2+ deficiency has a dual role in citric acid over-production conditions. As described above, the primary role is preventing inhibition of PFK I and increasing the intracellular NH4+ concentration. The secondary role is stimulating the citrate efflux by active transport system. It directly follows that it is vital to carefully select the ingredients of the medium as well as bioreactor material to ensure that even traces of manganese are not present which may affect citric acid yields during over-production conditions (Fig. 9).
Furthermore, various experiments have established that the active transport system is not the main means of citrate export under citrate over-production conditions. A. niger possesses the active transport mechanism but does not use it when the external pH is low. Protein CexA, a citrate-H+ antiporter, is the main citric acid transporter in A. niger (Steiger et al., 2019). Its disruption completely abolishes citrate secretion into medium and reroutes the metabolism toward oxalic acid production. This cellular transport system is very important for A. niger cell as its over-expression leads to significant increase in citric acid secretion.
Summary
-
Tricarboxylic acid cycle, commonly known as TCA cycle/Krebs cycle, is the oxidation of acetyl CoA to CO2 and H2O with generation of reductants.
-
Hans Krebs in 1937 postulated the complete citric acid cycle based both on his own findings and some previous leads of Albert Szent-Gyorgyi and others.
-
TCA cycle initiates with acetyl CoA generation from decarboxylation of pyruvate produced during glycolytic pathway.
-
Decarboxylation of pyruvate may take place by pyruvate dehydrogenase complex in aerobic conditions in all organisms having aerobic metabolism.
-
Pyruvate dehydrogenase complex is a multi-enzyme complex that uses a number of coenzymes, viz. TPP, lipoic acid, FAD, NAD+ and CoA-SH.
-
TCA starts with condensation of acetyl CoA and oxaloacetate to form citrate by citrate synthase.
-
In a series of seven reactions, 6-carbon citric acid is broken down to 4-carbon oxaloacetate with generation of 2 mol of CO2, 3 mol of NADH + H+ and 1 mol FADH2 along with one high-energy phosphate bond GTP.
-
TCA cycle has a major anabolic role where precursors for amino acids, heme, fats and lipids pathways are generated.
-
For continuity of the TCA cycle, the major anapleurotic reaction for generation of oxaloacetate is carboxylation of pyruvate or PEP by pyruvate carboxylase and/or PEP carboxylase.
-
Partitioning of pyruvate between generation of acetyl CoA and oxaloacetate is largely regulated by acetyl CoA levels which is a positive regulator of pyruvate carboxylase.
-
TCA cycle is regulated by availability of substrate, energy and reducing equivalents in cellular pool.
Questions
-
1.
How pyruvate is oxidized to acetyl CoA in aerobic and anaerobic conditions?
-
2.
Name the different coenzymes required by pyruvate dehydrogenase enzyme.
-
3.
How many reductants are generated through one round of TCA cycle?
-
4.
Name any one enzyme which catalyzes an anapleurotic reaction in TCA cycle.
-
5.
How many ATPs are generated by one round of TCA?
-
6.
Name any one inhibitor of TCA cycle.
-
7.
Taking into account complete oxidation of one mole of glucose by EMP followed by TCA, what is the total ATP gain?
-
8.
Write the pyruvate dehydrogenase reaction for oxidation of pyruvate to acetyl CoA.
-
9.
How is oxaloacetate replenished during TCA cycle.
-
10.
In a eukaryotic cell, how does the NADH produced during glycolytic cycle enters the mitochondria for electron transport?
-
11.
Write different steps of TCA showing generation of reductant and high-energy phosphate.
References
Kirimura, K., Kobayashi, K., & Yoshioka, I. (2019). Decrease of citric acid produced by Aspergillus niger through disruption of the gene encoding a putative mitochondrial citrate-oxoglutarate shuttle protein. Bioscience, Biotechnology, and Biochemistry, 83(8), 1538–1546.
Mattey, M. (1977). Citrate regulation of citric acid production in Aspergillus niger. FEMS Microbiology Letters, 2(2), 71–74.
Netik, A., Torres, N. V., Riol, J. M. & Kubicek, C.P. (1997). Uptake and export of citric acid by Aspergillus niger is reciprocally regulated by manganese ions. Biochimica et Biophysica Acta (BBA) - Biomembranes, 1326(2), 287–294.
Papagianni, M. (2007). Advances in citric acid fermentation by Aspergillus niger: biochemical aspects, membrane transport and modeling. Biotechnology Advances, 25, 244–263.
Steiger, M. G., Rassinger, A., Mattanovich, D., & Sauer, M. (2019). Engineering of the citrate exporter protein enables high citric acid production in Aspergillus niger. Metabolic Engineering, 52, 224–231.
Author information
Authors and Affiliations
Rights and permissions
Copyright information
© 2021 Springer Nature Singapore Pte Ltd.
About this chapter
Cite this chapter
Gupta, R., Gupta, N. (2021). Tricarboxylic Acid Cycle. In: Fundamentals of Bacterial Physiology and Metabolism. Springer, Singapore. https://doi.org/10.1007/978-981-16-0723-3_12
Download citation
DOI: https://doi.org/10.1007/978-981-16-0723-3_12
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-16-0722-6
Online ISBN: 978-981-16-0723-3
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)