Abstract
Spatial and non-spatial information, coming from medial perforant path (MPP) and lateral perforant path (LPP), respectively, is considered to be integrated on granule cell in dentate gyrus (DG) to play an important role in learning and memory. At both connected sites on dendrite, the phenomenon of learning and memory of spike timing-dependent plasticity (STDP) is known to be induced. Meanwhile, acetylcholine (ACh) is released from cholinergic terminals in DG when attentional processes are paid. And there are reports that ACh enhanced STDP in CA1 area. In order to investigate the ACh effects on STDP and its mechanism in DG, STDP-inducing protocol was applied to measure STDP on MPP or LPP in the presence of eserine, furthermore, the changes in baseline amplitude during the STDP protocol were investigated. As the results, STDPs at both sites were enhanced if ACh receptors were activated and then clarified that the baseline amplitude was one of the factors for the enhancement on MPP. These findings suggest that spatial and non-spatial information is strengthened in learning and memory if attentional processes are paid, but the underlain mechanisms are different.
Access provided by Autonomous University of Puebla. Download conference paper PDF
Similar content being viewed by others
1 Introduction
Dentate gyrus (DG) granule cells (GC) integrate spatial and non-spatial information. Spatial information is projecting from medial entorhinal cortex to medial dendrite of GC via medial perforant path (MPP) (Fyhn et al., 2004), while non-spatial information such as smell is projecting from lateral entorhinal cortex to distal dendrite via lateral perforant path (LPP) (Hargreaves et al., 2005). Meanwhile, it is known that acetylcholine (ACh) is released in the DG from the cholinergic terminals coming from the medial septum (Amaral et al., 2007) when attentional processes are paid, and ACh enhances spike timing-dependent plasticity (STDP) induced in the CA1 (Sugisaki et al., 2016). However, how ACh influences on STDP in DG, furthermore, what mechanisms are functioning on that cholinergically induced STDP are still not clear. In this study, we clarified the effect of ACh on STDP induced at dendrite sites on MPP or LPP, respectively, and then found baseline amplitude was an important factor for deciding an STDP magnitude on MPP. These results can be useful for clarifying information integration in the DG during attentional processes in the future.
2 Method
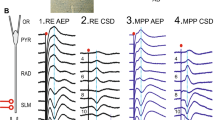
All procedures were approved by the Tamagawa University Animal Care and Used Committee. Hippocampal slices (400 \(\upmu \)m in thickness) were prepared from Wistar rats (20–25 days old), and whole cell patch clamp recordings were made from the soma of GC. Stimulating electrode was set on MPP or LPP to inject STDP-inducing protocol consisted of paired pulses (Fig. 1). The magnitude of STDP was defined as averaged EPSP slopes obtained from 20 to 30 min after STDP-inducing stimulus/averaged baseline EPSP slopes, while the lowest values of each membrane response induced by the paired pulses at the last 2 s of the STDP protocol were averaged as a baseline amplitude. Depending on the experiments, 2 \(\upmu \)M eserine, 1 \(\upmu \)M atropine and 10 \(\upmu \)M mecamylamine were added to ACSF 5 min. before the application of the stimuli until it was finished. 25 \(\upmu \)M Picrotoxin was added throughout the experiments. Analysis of variance (ANOVA) followed by Fisher’s PLSD test was used for statistical analysis (\(p<0.05\)).
3 Results
3.1 ACh Effects on STDP Enhancement
Before performing STDP experiments, MPP was clarified by observing a paired pulse depression (Petersen et al., 2013). First, STDP was measured and LTP was induced in the control condition (145.8 ± 10.5%, n=5, \(p<0.05\); Fig. 2), while larger LTP was observed if eserine was applied to influence ACh (176.8 ± 6.3%, n=5, \(p<0.01\), \(p<0.05\) vs. control), although no STDP was seen when blocking nicotinic ACh receptor (nAChR) and muscarinic ACh receptor (mAChR) activation by mecamylamine and atropine application, respectively, named non-ACh condition (118.9 ± 7.0%, n=5, N.S., N.S. vs. control, \(p<0.01\) vs. eserine).
These results show STDP with MPP stimulation was increased depending on ACh concentration. Then, similar experiments were performed replacing stimulating electrode on LPP. The position was confirmed by paired pulse facilitation (Petersen et al., 2013). As the results, LTD was induced in control condition (75.1 ± 8.6%, n=6, \(p<0.01\)), although STDPs were not observed despite of ACh existence (103.0 ± 1.6%, n=5, N.S., N.S. vs. control) or not existence (128.3 ± 1.9%, n=3, N.S., \(p<0.01\) vs. control, N.S. vs. eserine) conditions. Interestingly, small amount of ACh influenced STDP induction.
3.2 ACh Effects on Baseline Amplitude
As ACh effected on STDP enhancement at MPP stimulation, next baseline amplitude during the STDP stimulation was evaluated if some changes were occurred due to AChR activation. Then it was clarified that the baseline amplitude in the presence of eserine was higher (2.1 ± 0.4mV, n=5, \(p<0.05\); Fig. 3) than the one in non-ACh condition (0.9 ± 0.3 mV, n=5, \(p<0.05\), \(p<0.05\) vs. eserine).
These results suggest that the activation of AChRs effected on baseline amplitude. However, this tendency was not observed if replacing stimulating electrode on LPP that baseline amplitudes were similar in each condition (non-ACh: 1.2 ± 0.4 mV, n=3, N.S.; control: −0.06 ± 0.3 mV, n=5, N.S., N.S. vs. non-ACh; eserine: 0.4 ± 0.5 mV, n=5, N.S., N.S. vs. non-ACh, N.S. vs. control). These results suggest that the baseline amplitudes on LPP stimulation barely cooperated with STDP.
3.3 Contribution of mAChRs and nAChRs
As it was clarified that the ACh contributed to a larger STDP induction on MPP stimulation, next, the type of highly associated AChRs, mAChR or nAChR, was specified. Consequently, LTD was induced when only mAChRs were activated by mecamylamine application (56.5 ± 10.5%, n=4, \(p<0.01\), \(p<0.01\) vs. non-ACh; Fig. 4), though no STDP was observed if nAChRs were activated instead by atropine application (112.2 ± 5.9%, n=6, N.S., N.S. vs. non-ACh). Surprisingly, none of mAChRs nor nAChRs seemed to be cooperated with the larger STDP induction as it was observed in eserine condition (Fig. 2).
4 Conclusion
The direction and the magnitude of STDP in CA1 region are known to be decided by the postsynaptic Ca2\(^+\) level (Nishiyama et al. 2000; Aihara et al., 2007) according to BCM rule (Bienenstock et al., 1982). Therefore, it is likely that postsynaptic Ca2\(^+\) level in our results with ACh on MPP stimulation might have been higher than the one without ACh treatment as shown in Fig. 5. Also, similar fits were observed on LPP. Taken together, the activation of AChRs facilitated postsynaptic Ca2\(^+\) level according to BCM rule, especially, nAChRs were more responsible for STDP than mAChRs on MPP. However, the mechanism of STDP enhancement was not clear, so that we focused on baseline amplitude during the stimulation. Then it was clarified that the baseline amplitude on MPP was elevated by the activation of AChRs resulting in postsynaptic Ca2\(^+\) level elevation along with BCM rule. On the other hand, some other mechanisms besides the baseline amplitude may have drove on LPP.
In this study, as a first step for simplification, experiments were performed under interneuron-blocked network. However, if it is excited, nAChRs and mAChRs on interneurons may be contributed to suppress the magnitude of STDP (Sil’kis, 2003) to LTD direction due to the excitation of those modulated interneurons. Furthermore, it is known that different types of AChRs have different affinity that the concentration of ACh possibly influences on the magnitude of STDP through the relative strength of excitation and inhibition. Overall, this study is very important for clarifying the mechanisms of spatial and non-spatial information integration in accordance with attentional processes in the DG.
References
Aihara, T., Abiru, Y., Yamazaki, Y., Watanabe, H., Fukushima, Y., & Tsukada, M. (2007). The relation between spike-timing dependent plasticity and Ca2\(^+\) dynamics in the hippocampal CA1 network. Neuroscience, 145(1), 80–7.
Amaral, D. G., Scharfman, H. E., & Lavenex, P. (2007). The dentate gyrus: fundamental neuroanatomical organization (dentate gyrus for dummies). Progress in Brain Research, 163, 3–22.
Bienenstock, E. L., Cooper, L. N., & Munro, P. W. (1982). Theory for the development of neuron selectivity: orientation specificity and binocular interaction in visual cortex. Journal Neuroscience, 2(1), 32–48.
Fyhn, M., Molden, S., Witter, M. P., Moser, E. I., & Moser, M.-B. (2004). Spatial representation in the entorhinal cortex. Science, 305(5688), 1258–64.
Hargreaves, E. L., Rao, G., Lee, I., & Knierim, J. J. (2005). Major dissociation between medial and lateral entorhinal input to dorsal hippocampus. Science, 308(5729), 1792–4.
Nishiyama, M., Hong, K., Mikoshiba, K., Poo, M. M., & Kato, K. (2000). Calcium stores regulate the polarity and input specificity of synaptic modification. Nature, 408(6812), 584–8.
Petersen, R. P., Moradpour, F., Eadie, B. D., Shin, J. D., Kannangara, T. S., Delaney, K. R., et al. (2013). Electrophysiological identification of medial and lateral perforant path inputs to the dentate gyrus. Neuroscience, 252, 154–68.
Sil’kis, I. G. (2003). A possible mechanism for the effect of neuromodulators and modifiable inhibition on long-term potentiation and depression of the excitatory inputs to hippocampal principal cells. Neuroscience and Behavioral Physiology, 33(6), 529–41.
Sugisaki, E., Fukushima, Y., Fujii, S., Yamazaki, Y., & Aihara, T. (2016). The effect of coactivation of muscarinic and nicotinic acetylcholine receptors on ltd in the hippocampal ca1 network. Brain Research, 1649(Pt A), 44–52.
Acknowledgements
This work was supported by JSPS KAKENHI Grant Number JP16K00405.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2021 Springer Nature Singapore Pte Ltd.
About this paper
Cite this paper
Sugisaki, E., Fukushima, Y., Aihara, T. (2021). Acetylcholine Effects on STDP Induced on Spatial and Non-spatial Information in Dentate Gyrus. In: Lintas, A., Enrico, P., Pan, X., Wang, R., Villa, A. (eds) Advances in Cognitive Neurodynamics (VII). ICCN2019 2019. Advances in Cognitive Neurodynamics. Springer, Singapore. https://doi.org/10.1007/978-981-16-0317-4_9
Download citation
DOI: https://doi.org/10.1007/978-981-16-0317-4_9
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-16-0316-7
Online ISBN: 978-981-16-0317-4
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)