Abstract
Nanotechnology is an emergent field of research and innovation applied to nanoelectronics, nanomaterials, nanobiology, nanomedicine, among other areas. Particularly in medicine it has been used to improve the disease diagnosis through novel instrumentation as well as the treatment efficacy through the encapsulation and controlled release of bioactive compounds. Overall, nanotechnology can provide novel tools and techniques to biomedical research, namely in proteomics, genetics and regenerative medicine. Polymer nanofibers are one of the nanostructures that have been used in nanomedicine, especially those fabricated by electrospinning. The development of drug delivery systems, wound dressing materials, tissue regeneration and disease diagnosis are the most representative examples about the use of electrospun nanofibers. Their use in medical microbiology has mostly contributed to an improvement in the treatment of microbial infections through the increase of response capacity of active pharmaceutical ingredients but also into the reduction of side effects. In this chapter, a comprehensive overview about the manufacturing techniques and the most common polymers used for nanofibers production is discussed. The relation between the usefulness of these nanofibers with their composition, their size, and orientation is pointed out. Their medical applications on microbiology, namely the local administration of antibiotic drugs in infected areas, the development of wound dressing materials to improve the healing process and the possibility to raise or replace biological functions without organ transplantation by the use of artificial materials were analysed covering the developments accomplished over the last 10 years. It is noticed a lack of information about the formation of biofilms on the surface of nanofibrous scaffolds. Moreover, more studies must be done considering the nanoscale morphology and the type of polymer used, which seems to be crucial to prevent infections by microorganisms across medical applications.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Nanosciences
- Medical microbiology
- Nanofibers
- Biomedical engineering
- Drug delivery
- Wound dressing
- Tissue engineering
4.1 Introduction
Nanoscience and nanoengineering are emerging and promising interdisciplinary research areas from nanotechnology. The prefix ‘nano’ derives from the Greek νᾶνος (Latin nanus), meaning “dwarf”. The General Conference on Weights and Measures officially endorsed the usage of nano as a standard prefix in 1960 and depicts one thousand millionths of a meter (10−9 m) (Vert et al. 2012). The technology operated at nanoscale dimensions (1–100 nm) in material, physical, chemical, biological, and environmental sciences (Fig. 4.1) makes this content so important as the industrial revolution (Barhoum et al. 2019; Thangadurai et al. 2020a, b).
Examples of nanoparticles sizes. (Reproduced from Gnach et al. (2015) with permission from The Royal Society of Chemistry)
The scientific story of nanotechnology applied to nanoelectronics, nanomaterials, nanobiology, nanomedicine, etc. started in the middle of the last century. Richard Feynman, an American physicist, and Nobel Prize laureate, introduced the concept of nanotechnology in 1959, giving the first lecture about this subject “There’s Plenty of Room at the Bottom” (Feynman 1960), at California Institute of Technology at the American Physical Society meeting.
Particularly in medicine, nanotechnology has been used in three main areas (Jain 2019), which includes: (a) rapid diagnosis, auxiliary treatment and transport of active substances; (b) scientific research in molecular medicine like genetics and proteomics through the production of modified microorganisms; (c) regenerative medicine by the creation of biomaterials and nanostructures with potential clinical implications (Peran et al. 2013). In the last few years, promising therapies and diagnosis devices resorting to nanoparticles were used to face the increasing incidences of cancer, cardiovascular, respiratory, musculoskeletal, and neurodegenerative diseases (Patra et al. 2018). The first generation of nanoparticle-based therapy comprised lipid systems like liposomes and micelles that could include gold or magnetic nanoparticles to change membrane fluidity for drug delivery system (Park et al. 2006). Diferent types of nanomaterials are frequently considered in designing the target-specific drug delivery systems namely in drugs with poor water solubility and less absorption ability (Mira et al. 2020; Ye et al. 2020); and they stay in the blood circulatory system for an extended period of time with an easier penetration in the tissue system, facilitating the uptake of the drugs by cells. Therefore, the increase of drug delivery efficiency in the target location is possible. They have been also referred concerning their nanostructure on the biological effects. Several parameters such as size, zeta potential, surface chemistry, mechanical property, shape and other inherent biophysical/chemical characteristics contribute to enhance drug delivery effectiveness (Venkataraman et al. 2011; Prasad et al. 2017). However, toxicity and exposure data, combined with therapeutic properties, have been contributed to the risk assessment evaluation on the use of nanomaterials in consumer products, improving its regulation by governmental organizations (FDA 2012; Hardy et al. 2018; SCCS 2020; Tsuji et al. 2006).
The most current types of nanomaterials used in medical biology are carbon-based materials, metal-based materials, dendrimers, composites, polymers, etc. (Azonano 2017; Gubala et al. 2018). Carbon-based materials are composed mainly of carbon and can be prepared with different shapes: hollow spheres, ellipsoids or tubes; metal-based materials include quantum dots, nanogold, nanosilver and metal oxides, such as titanium dioxide; dendrimers are nanosized polymers built from branched units, whose surface can be tailored to perform specific chemical functions; composites combine different nanomaterials or different sizes of bulk-type materials. Polymers are another type of nanostructured materials that can be prepared in different morphologies such as nanofibers, nanoparticles, nanowires, nanospheres, and others (Lu et al. 2017). They have been used in medical care, such as drug delivery, tissue engineering, wound dressing, diagnosis systems, etc. Among them, nanofibers are highlighted once they have been proposed to support many developments guided to therapeutic solutions such as scaffolds structures, functionalization for tissue engineering and regenerative medicine; delivery systems for drugs/proteins/genes; bioactive wound dressings; membranes for different medical applications including filtration and dialysis; and biosensing for disease diagnostics and/or prognosis.
Fibers are a bioinspired nanostructure in the way that we recognize from either continuous filaments or elongated objects. The history of fiber production by humanity, such as wild silk threads is dated to c. 2450–2000 BCE. The spindle for the manufacture of cotton and wool fibers used in the production of clothing was invented around 1300 BCE. This practice slowly evolved into the textile industry in the 1880s (Xue et al. 2019). The history of synthetic nanofiber technology started in the late 1500s with William Gilbert work (Burgess 2012). This English physician observed that when a piece of charged amber was brought near a spherical drop of water on a dry surface, the amber “pulls the nearest parts out of their position and draws it up into a cone.” (Barhoum et al. 2019). This was the first reported case of electrospraying, which was later renamed into electrospinning.
The electrospinning process for nanofibers production was recognized and registered as a patent in 1902 (Cooley 1902). Later in 1914 John Zeleny studied the behaviour of fluid droplets at the end of metal capillaries that formed fine fibers, which contributed to the development of the mathematical model explaining the behaviour of fluids under electrostatic forces (Tucker et al. 2012). Until 1995 electrospinning process was employed for over three decades in industries to produce various products, namely spun and tubular products, nonwoven fabrics, grafts, etc. (Doshi and Reneker 1995). For further details, the reader shall check the work published by Tucker et al. (2012) and Xue et al. (2019). Since that time, the term electrospinning becomes more popular and the number of publications has been increasing exponentially every year (Fig. 4.2).
Nanofibers can be prepared from natural or synthetic polymers that result in different mechanical properties and applications. The most common materials used are organic polymers in the form of either solution or melt. Synthetic polymers include poly(vinyl alcohol) (PVA), poly(lactic acid) (PLA), polycaprolactone (PCL), polyurethane (PU), poly(lactic-co-glycolic acid) (PLGA), poly(vinylpyrrolidone) (PVP), polyethylene oxide (PEO), poly(ethylene-co-vinylacetate) (PEVA), etc. (Sharma et al. 2015). Their main advantages are the low-cost, tailored architecture, controllable degradation rate, and mass production (Nair and Laurencin 2007). Natural polymers include collagen, silk fibroin (SF), heparin, keratin, gelatin, proteins, and polysaccharides such as cellulose acetate (CA), pectin, chitosan, and alginate (Sharma et al. 2015). These natural polymers are highlighted by their biocompatibility, which induces low antigenic response. However, their price and poor water stability hinder their use in nanofibers production. Nonetheless, these natural polymers could be combined with synthetic polymers to fabricate hybrid nanofibers with better mechanical properties (Homaeigohar and Boccaccini 2020).
The rheological properties of the polymer, dictated by its molecular weight, and its electrical properties determine its chain entanglement. For instance, lowering the molecular weight tends to generate beads rather than fibers. All the molecules that can have self-assemble and generate enough chain entanglement can be used for nanofibers production. Overall, lower viscosity and lower density of surface charges favour the formation of beaded nanofibers, whereas reduction of surface tension makes the beads disappear gradually (Xue et al. 2019). A variety of compounds can also be combined to produce composite nanofibers structures to have improved and inimitable physical, chemical, and biological properties (Polini and Yang 2017).
Interestingly, the choice of the polymer and the technique used for nanofibers production is based on its final application. Particularly in nanomedicine, the physico-chemical properties of the used polymer should be carefully evaluated since they affect the biocompatibility, cytotoxicity, and physical features (diameter, shape, and form) of the final nanofibers. In the present chapter, we focus on the use of nanofibers in biomedical applications, highlighting their importance in medical microbiology. PCL, PLGA, and gelatine have been used in the fabrication of nanofibers for the delivery of antibiotics and wound dressing materials with antibacterial properties. Parallelly, SF, PLA, and CA have been used for tissue engineering and drug delivery systems, while PEVA, PVA, and chitosan have been proposed, as promising polymers, for drug delivery applications (Table 4.1).
4.2 Manufacturing Techniques
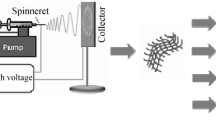
There are several techniques to make nanofibers, which can be divided into non-electrospinning and electrospinning techniques (Fig. 4.3). The first group includes drawing techniques, spinneret-based tunable engineered parameter method, phase separation, self-assembly, template synthesis, freeze-drying synthesis, and interfacial polymerization of nanofibers according (Alghoraibi and Alomari 2019). The second group includes electrospinning, which is the most commonly used technique to generate nanofibers for research applications and has also demonstrated the most promising results in terms of biomedical applications. Different structures such as hollow, flat, and ribbon-shaped, with controlled, inter- and intrafiber porosity can be fabricated depending on the application purposes. Many advantages for the use of the electrospinning method have been referred such as the straightforward setup, the ability to mass-produce continuous nanofibers from various polymers, and the capability to generate ultrathin fibers with controllable diameters, compositions, and orientations (Al-Enizi et al. 2018; X. Z. Gao et al. 2019; W. J. Lu et al. 2014).
Schematic representation of nanofibers production methods. (Reproduced from Yildiz et al. (2020). Copyright © 2020 with permission from Elsevier)
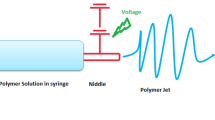
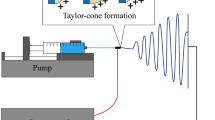
Electrospinning technique was firstly implemented with a simple apparatus for nanofibers production. A syringe containing polymer solution or melted polymer, with a charged metallic needle tip, a grounded or oppositely charged collector, and a high-voltage power supply, are the main constituents. The electrospinning process can be divided into four stages:
(a) charging of the liquid droplet and formation of Taylor cone or cone-shaped jet (Fig. 4.4); the equilibrium reached between the electrostatic charges (resulted by the application of an electric field) and the surface tension of the solution, is responsible for the Taylor cone formation. Up this voltage point, the jet is ejected from the tip of the Taylor cone; (b) extension of the charged jet along a straight line; (c) thinning of the jet in the presence of an electric field and growth of electrical bending instability (also known as whipping instability); (d) solidification and collection of the jet as solid fiber(s) on a grounded collector (Xue et al. 2019). The jet passes through a region of instability, where the solution’s solvents evaporate and are deposited onto the substrate as a fiber (Kitto et al. 2019).
Electrospinning technique for nanofibers production. (Reprinted with permission from Xue et al. (2019). Copyright © 2019 American Chemical Society)
Nowadays, there are available electrospinning systems with needleless technology for a high-throughput production for pilot scale and industrial-scale production (Ske 2020). In this new electrospinning platform, a rotating cylindrical spinneret with the application-specific rotating collectors makes possible the fabrication of many fibrous architectures with various fiber diameter and orientation. The fiber’s properties are determined by the applied voltage, the flow rate, and the distance between the spinneret and the collector (Xue et al. 2019).
As previously mentioned organic polymers can be applied to solution electrospinning or melt electrospinning. The most used one is the solution electrospinning where the solid nanofibers are formed after solvent evaporation and deposited on the collector (Xue et al. 2019). Biocompatible and biodegradable synthetic polymers, such as PCL and PLGA, have been directly electrospun into nanofibers and further explored as scaffolds for biomedical applications (Eren Boncu et al. 2020; Tahmasebi et al. 2020). On the other hand, natural biopolymers, such as SF, fibrinogens, dextran, casein, chitosan, alginate, collagen, and gelatin, have been electrospun into nanofibers from their solutions.
The structure and morphology of the nanofibers produced by solution electrospinning are determined by the polymer (ex: molecular weight), by the solvent (suitable for polymer dissolution), processing parameters, and atmospheric conditions. Some polymers such as polyethylene and polypropylene are not suitable for solution electrospinning due to their low solubility in solvents. In those situations, fibers are produced by melting electrospinning resorting to hot circulating fluids, electrical heating tape, or laser (Xue et al. 2019).
The nanofibers obtained by electrospinning can have meticulous orientations and dimensions, which makes this technique a powerful fiber manufacturing tool. The possibility to fabricate nanofibers with controlled/uncontrolled structure and ordered/non ordered orientation empathize their potential and acceptability for biomedical applications, which require well-arranged alignment and special structures (Greiner and Wendorff 2007).
Nanofibers use is increasing in several medical areas as already described. Particularly in medical microbiology, two important aspects of their use should be noticed. The first is related to their development and application in tissue engineering and the strategies to avoid biofilm formation that can affect their medical viability. The second is related with their use as a drug delivery system for antibiotics that help to optimize the therapy, reduce the concentrations, and reduce or eliminate the lateral effects of the drug. The use of wound dressing based on nanofibers has also been considered concerning microbiological aspects, namely the antibacterial and anti-inflammatory properties of some polymers or the incorporation of bioactive substances for that purpose.
4.3 Medical Applications on Microbiology
As already mentioned nanofibers have been widely used in biomedical applications. Their attractive properties such as the ratio of high surface area and volume as well as the high porosity with small pores enhance their use because of the similar characteristics with the extracellular matrix. This novel morphology emphasize cell behaviour and offers promising support for cells, which is quite attractive for tissue engineering purposes (Al-Enizi et al. 2018; X. Z. Gao et al. 2019; W. J. Lu et al. 2014; Yoshimoto et al. 2003). A plethora of other biomedical applications using nanofibers have been reported, especially drug delivery systems (Abid et al. 2019), biological wound dressings (Homaeigohar and Boccaccini 2020) and medical diagnosis (Rezaei and Mahmoudifard 2019). Particularly in medical microbiology, the controlled release of active pharmaceutical ingredients for bacterial infection therapy, wound healing materials incorporating antimicrobial substances and the fabrication of effective biosensing systems for disease diagnosis are the representative examples of these types of applications (Fig. 4.5). Nonetheless, nanofibers can also be used for early detection, cure, and diagnosis of cancer but this subject will not be described in this chapter. For further details, the reader shall check the work published by Abid et al. (2019).
Key of biomedical applications of nanofiber composites (Bacakova et al. 2020)
4.3.1 Drug Delivery Systems
Drug delivery systems refer to a technical system that comprehensively regulates the distribution of drugs in living organisms in space, time, and dosage (Ghosh and Murthy 2006; Prasad et al. 2017). It uses controlled-release materials that allow a slow and continuous release of the drug in the human body, maintaining its effective concentration for a long period. An ideal delivery system should release drug molecules in a controlled manner, preserving their bioactivity, optimizing their effectiveness, and reducing their side effects (Bongio et al. 2010; Mottaghitalab et al. 2015). Therefore, the development of novel and effective drug delivery systems for the controlled release of bioactive molecules is of critical importance in field medicine, particularly in medical microbiology. The recent advances in nanomaterials research opened new possibilities, allowing the production of numerous nanostructured carriers including liposomes, dendrimers, and nanoparticles (Mody et al. 2014) as well as electrospun nanofibers (Cleeton et al. 2019). The latter has been used for drug administration purposes due to their high surface-to-volume ratio. This feature increases the efficiency of the encapsulation and the stability of the drug release, as well as the ease of processing and the cost-benefit ratio (Ulubayram et al. 2015).
The selection of polymers is very important in the fabrication of electrospun nanofibers for drug delivery systems since their features like water solubility, polarity, and molecular weight would determine the properties of the final fiber (Ye et al. 2020). One of the polymers that attracted attention was chitosan due to its biodegradability, biocompatibility, and safety characteristics. However, electrospinning pure chitosan is very difficult due to the repulsive forces between its ionic groups (Desai et al. 2008). Meanwhile, PVP is a synthetic hydrophilic polymer with well spinnable and mechanical properties that can be used alone or combined with other polymers to fabricate electrospinning fibers (Ye et al. 2020).
Another important aspect is related to the solvent system, which is fundamental for the optimization of electrospinning by altering the rheological and electrostatic properties of the polymer solutions. This feature was recently demonstrated after the encapsulation of linezolid, a new generation antibiotic against methicillin-resistant Staphylococcus aureus (MRSA) infections, into electrospun PLGA nanofibers using different solvents (Fig. 4.6). The researchers found that mono solvent solutions were not spinnable but solvents combined at different ratios allowed the production of nanofibers (Eren Boncu et al. 2020). In this work, the best combination was attained with dichloromethane and dimethylformamide at 50:50 ratio (v/v), providing a controlled release of linezolid for 13 days.
Schematic illustration of drug incorporation strategies into nanofibers. (a) Blend electrospinning, where drugs and polymers are co-dissolved in solvents to be spun; (b) emulsion electrospinning, where drug solutions are emulsified into immiscible polymer solutions, followed by spinning; (c) co-axial electrospinning, where drug and polymer solutions are separately spun through two concentric nozzles; (d) post-immobilization, where drugs are conjugated onto fabricated nanofiber matrices through physical or chemical interaction. (Reproduced from (J. Wang and Windbergs 2017). Copyright © 2017 with permission from Elsevier)
The mechanism and the amount of drug-loaded inside the nanofibers is also a key factor for the development of highly effective nanofibers as drug delivery systems. There are several ways to incorporate the drugs inside the fibers including blended electrospinning, emulsion electrospinning, co-axial electrospinning, and surface modification (Fig. 4.6) (Wang and Windbergs 2017). Drug molecules can be directly embedded into the polymer fiber matrix or be attached to the fiber surface. Nonetheless, the mechanism release of the drug from the electrospun fibers is also an important aspect, which can be affected by different factors such as the drug content, drug distribution in nanofibers and fiber diameter distribution (Al-Enizi et al. 2018; Verreck et al. 2003).
The first study related to the use of electrospun fibers as drug delivery system came out in 2002, in which tetracycline hydrochloride, an antibiotic used for periodontal disease treatment, was loaded into PLA, PEVA, and PLA/PEVA blend (50:50, w/w) nanofibers (Kenawy et al. 2002). The results pointed out that the release profile was dependent on the nature of the used polymer and the amount of the loaded drug. The best results showed a smooth release of the drug over about 5 days using electrospun PEVA and PLA/PEVA (50:50) mats. In recent years, electrospinning has been used to the synthesis of polymer nanofibers capable of sustained release of several pharmaceutical drugs such as antibiotics (Y. J. Chen et al. 2020a; Shahi et al. 2017; Song et al. 2017; Y. N. Yang et al. 2020) and anti-cancer substances (Y. Liu et al. 2020; Sedghi et al. 2017; Yan et al. 2020). The most recent and representative examples are summarized in Table 4.1, which included ceftazidime, vancomycin, emodin, doxorubicin, and paclitaxel drugs.
Although the techniques for incorporating drugs into nanofibers have proven to be somewhat effective, it was identified a fast pharmaceutical drug dissolution (or bioactive substance) on electrospun nanofibers surface, which is known as burst release (Al-Enizi et al. 2018). To circumvent this drawback, core-shell composite nanofibers can be fabricated using co-axial electrospinning, in which two miscible or immiscible materials can be electrospun together (Huang et al. 2006). Using this approach, drugs can be coated by an outer shell of a polymeric layer to enhance the release of these materials. An interesting study reported the variation of the physical and chemical properties of the core and shell solutions to control the release of a hydrophilic drug, metoclopramide hydrochloride (Tiwari et al. 2010). The results clearly indicated a more controllable release pattern with the core-shell fibers using either PLC, PLA, and PLGA comparing to the respective monolithic fibers.
On the other hand, the use of core-shell nanofibers can simplify the use of pharmaceutical drugs with poor solubility in water and low oral bioavailability. The co-axial electrospinning nanofibers with emodin drug encapsulated in the hygroscopic cellulose acetate sheath is a representative example (Fig. 4.7a) (Ye et al. 2020). The results showed a biphasic drug release profile of emodin with an initial rapid release followed by a slower sustained release (Fig. 4.7b). In another work, co-axial electrospinning was used to prepare core-shell nanofibers containing amoxicillin trihydrate loaded in SF as core and PVA in the shell (Ojah et al. 2019). The fabricated nanofibers showed a biphasic drug profile with prolonged antibacterial activity against the Gram-negative Escherichia coli (E. coli) and the Gram-positive Staphylococcus aureus (S. aureus) bacteria. A similar approach was described in the fabrication of PVA/PCL core-shell nanofibers, in which PVA and PCL formed the core and shell layers, respectively (Yan et al. 2020).
Electrospun nanofibers used for emodin encapsulation and release. (a) Transmission electron microscopy image of core-shell structure nanofiber containing emodin. (b) Cumulative release curves of emodin from nanofibers and raw emodin. (a) Nanofibers containing emodin, (b) raw emodin. The insert profiles show the release behavior of nanofibers and raw emodin during the initial 12 h. (Ye et al. 2020)
The remarkable progress in this field of nanotechnology, particularly in nanomaterials science, conducted on the development of new drug delivery systems based on electrospun nanofibers. The recent research trends refer to the incorporation of nanoparticles into polymer nanofibers to develop new medical treatment strategies. An interesting example refers to the incorporation of vancomycin-loaded gelatine nanospheres into SF nanofibers through a colloidal electrospinning technique (Song et al. 2017). The results showed a more sustained release of vancomycin from nanofibrous membranes fortified with gelatine B nanospheres than nanosphere-free membranes. This strategy provides an extended antibacterial effect against S. aureus. Additionally, SF/graphene oxide nanofibers were fabricated by blended electrospinning, and the antibacterial activity was further evaluated (Wang et al. 2018). The results showed an increase of the inhibitory effect against S. aureus and E. coli which was enhanced by the addition of graphene oxide.
The combination of natural or synthetic drugs with electrospun mats for the development of drug delivery systems has been proved to be an attractive alternative to eliminate microorganisms. The major problems like low solubility and poor bioavailability of the drugs can be overcome by their incorporation into nanofibrous scaffolds.
These excellent outcomes of nanofibers as drug delivery, combined with their mechanical properties leverage their use as wound dressing materials.
4.3.2 Wound Dressing
Recently, electrospun nanofibers have been used as materials for wound healing purposes, which is a physiological process that occurs normally as a response to tissue damage to maintain its functionality and integrity (Monaco and Lawrence 2003). An ideal polymer for wound dressing should be noncytotoxic, biodegradable, hemocompatible, easy to remove, and capable of maintaining the moisture content over the wound surface (Wang et al. 2007). Another relevant characteristic should be their impermeability to bacteria, avoiding the formation of biofilms at the surface that could infect the wound. In general, the excellent properties of nanofibers accomplish these criteria, particularly the high oxygen porosity and the similar texture to the natural extracellular matrix in the skin, which largely contributed to the development of new medical materials (Fayemi et al. 2018). Polymer nanofibers can be used by themselves without any additives or in combination with bioactive plant extracts or synthetic materials used to improve their efficiency (Table 4.1). Mussel adhesive proteins (Kim et al. 2015) and honey-derived products (Sarhan and Azzazy 2017) could also be used to fabricate nanofibers with enhanced wound healing capability. Some of the representative examples of materials incorporated into electrospun nanofibers are displayed in Fig. 4.8.
Among the natural herbal extracts, there are several examples stated in the scientific literature. In one case, the well-known antioxidant properties of Aloe vera were enhanced by its incorporation into electrospun PLGA nanofibrous membrane, stimulating the proliferation and activity of fibroblasts (Garcia-Orue et al. 2017). The wound-healing effect was even boosted by loading the nanofibers with a recombinant human epidermal growth factor, which acted as a mediator in the healing process. Electrospun nanofibers synthetized from PVA and chitosan blends loaded with extract of Bidens pilosa were also referred (Kegere et al. 2019). The results showed an increase in the antibacterial activity of the composite nanofibers against S. aureus and E. coli. When compared with either herbal extract or chitosan alone, suggesting their potential use as wound healing material. The herbal extract of the traditional Centella asiatica was also incorporated into gelatin nanofibers using electrospinning technique, promoting fibroblast proliferation and collagen synthesis, which enhanced the wound healing process (Yao et al. 2017). Other examples in the literature refer to moringa leaf extracts with antimicrobial properties incorporated into polyacrylonitrile electrospun nanofibers (Fayemi et al. 2018). Chemical substances obtained from herbal extracts can also be used such as the salvianolic acid B (Salvia miltiorrhiza) and bromelain (Ananas comosu) that were co-loaded into electrospun core-shell nanofibers (Shoba et al. 2017). In this case, the bromolein was first released from PVA/gelatin shell to remove the formed eschar as a result of its proteolytic activity while the salvianolic acid was loaded into the core of PCL to be released later and promote the formation of new blood vessels.
The recent advances in nanomaterials have drawn the attention of researchers to the development of novel wound dressings. An interesting example describes the incorporation of different contents of silver nanoparticles into chitosan nanofibers by electrospinning process (Lee et al. 2014). The composite nanofibers showed a higher degree of effectiveness against P. aeruginosa and MRSA when compared to the pure chitosan nanofibers. Additionally, as the number of nanoparticles increased in the fibers, its antibacterial properties were enhanced against both microorganisms. In another work, gold nanoparticles modified with 6-aminopenicillanic acid as antibacterial active ingredients were loaded into PCL/gelatin nanofibers by co-electrospinning to fabricate biocompatible wound dressings against multidrug-resistant bacteria (X. Yang et al. 2017). Zinc oxide nanoparticles were also used to produce composite scaffolds based on oxidized cellulose and the synthetic polyethylene glycol polymer (Shefa et al. 2019). The nanofiber composite was obtained by the freeze-drying method and showed biodegradable and biocompatible characteristics. The incorporation of zinc oxide enhanced the blood/fibrin coot formation and antibacterial properties, proving the potential of the proposed scaffold to be used as a hemostatic agent while inhibiting the spreading of bacterial infections. Other applications for cutaneous wound healing reported the use of graphene oxide to enhance the antibacterial activities of nanofibers, such as the SF/graphene oxide-blended nanofibers (Wang et al. 2018) and the scaffolds based in PU and CA as polymers blended with a reduced graphene oxide/silver nanocomposite and the natural polyphenolic compound called curcumin (Esmaeili et al. 2020).
Antimicrobial peptides are other group of effective broad-spectrum antibiotics that can be used for the treatment of both Gram negative and Gram positive bacteria (K. V. R. Reddy et al. 2004; Inamuddin et al. 2021). Accordingly, these short peptides have been incorporated in electrospun nanofibers for the fabrication of wound dressings. An interesting example describes the loading of the Crabrolin, a synthetic peptide, into PCL electrospun nanofibers. An extended linear release profile of the antimicrobial peptide was observed (Eriksen et al. 2013). In a different work, lysozyme and niasin were incorporated into an electrospun nanofibrous scaffold synthesized with a blend of water soluble polymers, PVA and poly(acrylic acid) and showed a stronger antibacterial activity against S. aureus, proving the great potential to treat infected wounds (Amariei et al. 2018). Lysozyme was loaded into electrospun nanofibrous mat based in chitosan-ethylenediaminetetraacetic acid blended with PVA (Charernsriwilaiwat et al. 2012). In animal wound healing studies, lysozyme loaded nanofibers exhibited an accelerated healing rate compared to the control (gauze) (Fig. 4.9). In another study, a synthetic single chain peptide derived from the proline-rich antimicrobial peptide dimer A3-APO was loaded into PVA electrospun nanofibers (Sebe et al. 2016). The results indicate an improvement in the appearance of wounds treated with APO-loaded nanofibers, reducing significantly the wound size and its bacterial presence. Other examples reported the incorporation of the synthetic Cys-KR12 into SF electrospun nanofibers (Song et al. 2016) and the co-loading of pam3CSK4 peptide and vitamin D3 into core-shell nanofibrous (S. Chen et al. 2017a).
Presence of wound healing at 1, 4, 7, and 10 days after adding (a) 30% lysozyme loaded CS–EDTA/PVA nanofiber mats, (b) gauze (−ve control), and (c) commercial antibacterial gauze dressing (Sofra-tulle®) (+ve control). (Reproduced from (Charernsriwilaiwat et al. 2012). Copyright © 2012 with permission from Elsevier)
As previously described, electrospun nanofibers have been used successfully to develop new solutions for wound healing agents. Natural and synthetic polymers have been used so far, including chitosan, SF, PVA, PU, PLA, and PCL, among others. Parallelly, several antibacterial materials such as bioactive molecules and nanoparticles were already incorporated into nanofibrous mats to prevent wound infection. The versatility of polymers and the tremendous research in nanomaterials, namely in bioactive nanoparticles will certainly contribute to the appearance of novel and cost-effective wound dressings.
4.3.3 Tissue Engineering
Tissue engineering is an interesting research field for the development of functional replacements for damaged tissues namely for musculoskeletal (including bone, cartilage, ligament, and skeletal muscle), skin, vascular and neural tissues (O’Brien 2011; Vasita and Katti 2006). It involves the use of a biocompatible scaffold capable of mimic the biological function and structure of the natural extracellular matrix, which is essential for the formation of new viable tissue. The similarity of electrospun nanofibers with the extracellular matrix offers a promising approach to develop fibrous scaffolds for cells growing, migration, and seeding. In recent years, different artificial polymeric scaffolds have been developed for bone and vascular tissue engineering, among others (Al-Enizi et al. 2018; W. J. Lu et al. 2014). Some representative examples are summarized in Table 4.1, including the formation of nanofibrous scaffolds for cell attachment and proliferation as well as the fabrication of nanofibers able to incorporate and deliver different growth factors. Nonetheless, the formation of biofilms on the surface of such nanofibrous scaffolds may be a ubiquitous problem (Kurtz and Schiffman 2018), leading to bacterial infections at the implanted place and severe health problems. To reduce the need for the use of antibacterial agents, anti-fouling nanofibers to repel microbes and proteins for as long as possible, delaying their binding to solid material is one of the areas with great impact on tissue engineering. Electrospun nanofiber mats will keep having a vital role in controlling the local interactions with microorganisms across medical applications (Kurtz and Schiffman 2018).
To understand the interaction between bacteria and the polymer, Fabrizio De Cesare recently studied the effect of the nanofiber diameter on the attachment of bacteria (De Cesare et al. 2019), using electrospun PCL nanofibers and Burkholderia terrícola bacteria cells as models. The results showed that bacteria attached preferentially to nanofibers with ≈100 nm diameter. Thus, materials could be created to reduce or prevent the adhesion of bacteria by adjusting the size of the fibers, acting as well as antimicrobials for the biocontrol of diseases induced by pathogens in medicine (De Cesare et al. 2019).
Nonetheless, not only in the area of microbiology these nanofiber compounds have attracted interest. Various biodegradable and biocompatible polymers, including either natural and/or synthetic polymers have been tested in the nanofibers production. An interesting example refers to the natural polymer chitin and its derivative, chitosan, for the fabrication of nanofibrous scaffolds applied mainly in bone tissue engineering (Tao et al. 2020). Similarly, a composite scaffold made of chitosan, alginate, collagen, and hydroxyapatite was fabricated by electrospinning to decrease the collagen solubility at the implanted place (Yu et al. 2013). The nanofibers were applied in studies in vitro showing a reduction of the collagen decomposition by 35% in 10 days, which contributed to the cell spreading, attachment, mineralization, and proliferation. Synthetic polymers have been also used for nanofibers production, such as the attractive study describing the synthesis of a tubular nanofibrous structure with a 6 mm internal diameter by blended electrospinning of polyethylene terephthalate and PCL (Nejad et al. 2020). The suitable mechanical properties of the attained nanofibers, combined with the biocompatibility of both polymers, make this material a good candidate for artificial vessel replacement.
Natural polymers can also be combined with synthetic polymers to produce hybrid nanofibers with better mechanical properties. An example of this valuable strategy was demonstrated by combining SF, an animal-derived protein, whose use has been hindered by the poor mechanical properties and the high biodegradation rate, with the synthetic polymer Nylon 6, which has excellent mechanical strength and a low decomposition rate (Yuan et al. 2020). These composite nanofibrous mats were loaded with lysozyme and collagen. The results showed a high potential for clinical therapy of pelvic organ prolapse. Among the natural polymers, peptides and proteins have drawn the attention of researchers, particularly in the field of tissue engineering. The great advantage relies on the fact that they are basic components of cells and have many vital functions in the body, which provides a high degree of compatibility (Yildiz et al. 2020). Besides, they are biodegradable materials and thus can be easily removed from the body without surgical intervention. Different peptide/protein nanofibers have been described in the literature using either plant or animal-derived proteins. Plant-derived proteins used in nanofibers synthesis include zein (Zhang et al. 2015), soy protein (Ramji and Shah 2014), and gluten (N. Reddy and Yang 2008) while animal-derived proteins can be listed as casein (Selvaraj et al. 2018), SF (Y. Chen et al. 2017b; Y. Gao et al. 2018), bovine serum albumin (Valmikinathan et al. 2009; Won et al. 2012), elastin (Machado et al. 2013), fibrinogen (Z. Liu et al. 2015), keratin (Rajabi et al. 2020) and gelatin (Aldana and Abraham 2017), among others. The effect of nanofibers based on peptide/protein scaffolds has been demonstrated in various in vivo studies but this material is not available on the market, yet (Yildiz et al. 2020).
Another interesting approach for tissue engineering involves the use of growth factors alone or in combination with cells or other biochemical materials for cellular reparation (Laurencin et al. 1999). These growth factors usually behave as mediators of basic cell functions through their binding to specific receptors (Anitua et al. 2012). However, due to their instability, they have a short half-life time, losing their effectiveness in a short period. Therefore, there is an urgent demand to develop new methods capable of delivering these growth factors, maintaining their stability and activity. Regarding this aspect, electrospun nanofibers have great potential due to the ability to incorporate and deliver these growth factors (Shin et al. 2012). An interesting example refers to the use of electrospun gelatin nanofibers to incorporate the fibroblast growth factor 2 (FGF-2), providing the increased cell proliferation when compared with control nanofibers (Lee et al. 2016). In another study, an electrospun core-shell nanofiber scaffold loaded with osteogenic enhancer fibroblast growth factors, FGF-18 and FGF-2, was fabricated for repairing bone defects. The core was based on PEO loaded with the FGF-2 and the FGF-18, which was first incorporated into bioactive glass nanospheres for a long-term delivery, while the outer shell was synthesized from PCL (Kang et al. 2015). The in vitro cellular studies showed significant stimulation of cell proliferation and the induction of cellular mineralization. Histological images at 6 weeks were examined after H&E and Masson’s trichrome staining (Fig. 4.10), showing the formation of new bone with similar morphology to old bone, particularly observed in the scaffold loaded with FGF18/FGF2.
Histological images of the harvested samples at 6 weeks, optically examined after H&E (left column) and Masson’s trichrome staining (right column): (a) blank control, (b) scaffold only, and (c) FGF2/FGF18-scaffold. Enlarged images of the FGF2/FGF18-loaded scaffold group in the region of new bone formation revealed an immature woven bone structure with osteocytes, bone lining cells and vessel forming cells. Dashed lines indicate defect margins. OB, old bone; NB, new bone. Scale bars in (a–c): 500 μm; and in the enlarged image: 100 μm. (Reproduced from Kang et al. (2015). Copyright © 2015 with permission from Elsevier)
Vascular endothelial growth factor, involved in angiogenesis, was also incorporated into electrospun nanofibers (Rosa et al. 2017). The fabricated scaffolds based on PGLA provided a sustained release of the growth factor over 6 days, enhancing the cell adhesion without any remarkable toxicity. In another case, co-axial electrospinning was used to encapsulate a recombinant form of this vascular growth factor and PEO in the core of nanofibers, while a mixture of PCL and PEG were used to synthetize the shell (Zigdon-Giladi et al. 2017). The in vitro cellular studies demonstrated an endothelial cell migration 80-fold higher with the loaded nanofibers when compared to the scaffold without growth factor. Studies in mouses confirmed that nanofibers loaded with the vascular growth factor can stimulate cell migration into the scaffold within three days and significantly enhanced blood vessels formation within 14 days, whereas control scaffolds contained few vessels. The incorporation and release of nerve growth factor (Whitehead et al. 2018), platelet derived growth factor (Bertoncelj et al. 2014) and connective tissue growth factor using different nanofibers are other representative examples (Xu et al. 2019).
Nowadays, electrospun nanofibers have been used to develop new strategies in tissue engineering area. The results achieved so far are at embrionary stage but the replacement of bone and vascular tissue in animals was already accomplished. An important aspect of the use of nanofibers, although the biocompatibility and noncytotoxic features, is related to the formation of biofilms at the polymer’s surface, which can lead to severe infections. Further work should be performed to understand this behaviour and overcome this drawback.
4.4 Future Perspectives
The importance of nanoscale science and technology in different fields is remarkable. The development of various nanostructured materials has been improved medical care not only from the perspective of early disease diagnosis but also in novel therapeutic strategies. Particularly in microbiology, nanomedicine has been contributed to improving the treatment efficiency on microbial infections through the increase of response capacity of active pharmaceutical ingredients but also into the reduction of side effects.
A plethora of nanostructures has been used for medical biology applications, in which nanofibers represent an attractive and powerful approach. The excellent properties of nanofibers and the versatility of available polymers, either natural or synthetic, highlight their use in medicine.
In this chapter, the use of nanofibers for the local administration of antibiotic drugs in infected areas by microorganisms is described. The development of wound dressing materials based on nanofibers has also been described to improve the healing process, by incorporating bioactive substances capable of eliminate bacteria and reduce the inflammation.
Tissue engineering based on nanofibers have an excellent potential to create functional materials or technologies that mimics the structural organization of natural tissues. The possibility to raise or replace biological functions without organ transplantation by the use of artificial materials that enable the seeding and proliferation of cells into the material structure is a present goal. The potential of these nanostructured materials is highlighted by the functional and mechanical properties of electrospun nanofibers similar to the extracellular matrix. However, it is scarce the data about the formation of biofilms on the surface of such nanofibrous scaffolds, considering the nanoscale morphology and the type of polymer used. These studies are crucial to controlling the local interactions with microorganisms across medical applications.
Overall, nanofibers show to be a reliable and promising approach in nanomedicine, particularly in medical microbiology.
References
Abid S, Hussain T, Raza ZA, Nazir A (2019) Current applications of electrospun polymeric nanofibers in cancer therapy. Mater Sci Eng C Mater Biol Appl 97:966–977. https://doi.org/10.1016/j.msec.2018.12.105
Aldana AA, Abraham GA (2017) Current advances in electrospun gelatin-based scaffolds for tissue engineering applications. Int J Pharm 523(2):441–453. https://doi.org/10.1016/j.ijpharm.2016.09.044
Al-Enizi A, Zagho M, Elzatahry A (2018) Polymer-based electrospun nanofibers for biomedical applications. Nanomaterials 8(4):259. https://doi.org/10.3390/nano8040259
Alghoraibi I, Alomari S (2019) Handbook of nanofibers. Springer
Amariei G, Kokol V, Boltes K, Letón P, Rosal R (2018) Incorporation of antimicrobial peptides on electrospun nanofibres for biomedical applications. RSC Adv 8(49):28013–28023. https://doi.org/10.1039/C8RA03861A
Anitua E, Alonso R, Girbau C, Aguirre JJ, Muruzabal F, Orive G (2012) Antibacterial effect of plasma rich in growth factors (PRGF®-Endoret®) against Staphylococcus aureus and Staphylococcus epidermidis strains. Clin Exp Dermatol 37(6):652–657. https://doi.org/10.1111/j.1365-2230.2011.04303.x
Awasthi GP, Maharjan B, Shrestha S, Bhattarai DP, Yoon D, Park CH, Kim CS (2020) Synthesis, characterizations, and biocompatibility evaluation of polycaprolactone-MXene electrospun fibers. Colloids Surf A 586:124282. https://doi.org/10.1016/j.colsurfa.2019.124282
Azonano (2017) Classification of nanomaterials, the four main types of intentionally produced nanomaterials. https://www.azonano.com/article.aspx?ArticleID=1872
Bacakova L, Pajorova J, Tomkova M, Matejka R, Broz A, Stepanovska J et al (2020) Applications of nanocellulose/nanocarbon composites: focus on biotechnology and medicine. Nanomaterials 10(2):196. https://doi.org/10.3390/nano10020196
Barhoum A, Rasouli R, Yousefzadeh M, Rahier H, Bechelany M (2019) Handbook of nanofibers. Springer
Bertoncelj V, Pelipenko J, Kristl J, Jeras M, Cukjati M, Kocbek P (2014) Development and bioevaluation of nanofibers with blood-derived growth factors for dermal wound healing. Eur J Pharm Biopharm 88(1):64–74. https://doi.org/10.1016/j.ejpb.2014.06.001
Bongio M, van den Beucken JJJP, Leeuwenburgh SCG, Jansen JA (2010) Development of bone substitute materials: from ‘biocompatible’ to ‘instructive’. J Mater Chem 20(40):8747–8759. https://doi.org/10.1039/C0JM00795A
Burgess R (2012) Understanding nanomedicine an introductory textbook. Pan Stanford Publishing, Singapore
Charernsriwilaiwat N, Opanasopit P, Rojanarata T, Ngawhirunpat T (2012) Lysozyme-loaded, electrospun chitosan-based nanofiber mats for wound healing. Int J Pharm 427(2):379–384. https://doi.org/10.1016/j.ijpharm.2012.02.010
Chen S, Ge L, Gombart AF, Shuler FD, Carlson MA, Reilly DA, Xie J (2017a) Nanofiber-based sutures induce endogenous antimicrobial peptide. Nanomedicine 12(21):2597–2609. https://doi.org/10.2217/nnm-2017-0161
Chen Y, Yang W, Wang W, Zhang M, Li M (2017b) Bombyx mori silk fibroin scaffolds with Antheraea pernyi silk fibroin micro/nano fibers for promoting EA. hy926 cell proliferation. Materials (Basel) 10(10):1153. https://doi.org/10.3390/ma10101153
Chen Y, Qiu YY, Chen WBF, Wei QF (2020a) Electrospun thymol-loaded porous cellulose acetate fibers with potential biomedical applications. Mater Sci Eng C 109:110536. https://doi.org/10.1016/j.msec.2019.110536
Chen J, Zhang TH, Hua WK, Li PY, Wang XF (2020b) 3D porous poly(lactic acid)/regenerated cellulose composite scaffolds based on electrospun nanofibers for biomineralization. Colloids Surf A 585:124048. https://doi.org/10.1016/j.colsurfa.2019.124048
Cleeton C, Keirouz A, Chen XF, Radacsi N (2019) Electrospun nanofibers for drug delivery and biosensing. ACS Biomater Sci Eng 5(9):4183–4205. https://doi.org/10.1021/acsbiomaterials.9b00853
Cooley JF (1902) Apparatus for electrically dispersing fluids. Google Patents US692631A
De Cesare F, Di Mattia E, Zussman E, Macagnano A (2019) A study on the dependence of bacteria adhesion on the polymer nanofibre diameter. Environ Sci Nano 6(3):778–797. https://doi.org/10.1039/C8EN01237G
Desai K, Kit K, Li J, Zivanovic S (2008) Morphological and surface properties of electrospun chitosan nanofibers. Biomacromolecules 9(3):1000–1006. https://doi.org/10.1021/bm701017z
Doshi J, Reneker DH (1995) Electrospinning process and applications of electrospun fibers. J Electrost 35(2–3):151–160. https://doi.org/10.1016/0304-3886(95)00041-8
Eren Boncu T, Ozdemir N, Uskudar Guclu A (2020) Electrospinning of linezolid loaded PLGA nanofibers: effect of solvents on its spinnability, drug delivery, mechanical properties, and antibacterial activities. Drug Dev Ind Pharm 46(1):109–121. https://doi.org/10.1080/03639045.2019.1706550
Eriksen THB, Skovsen E, Fojan P (2013) Release of antimicrobial peptides from electrospun nanofibres as a drug delivery system. J Biomed Nanotechnol 9(3):492–498. https://doi.org/10.1166/jbn.2013.1553
Esmaeili E, Eslami-Arshaghi T, Hosseinzadeh S, Elahirad E, Jamalpoor Z, Hatamie S, Soleimani M (2020) The biomedical potential of cellulose acetate/polyurethane nanofibrous mats containing reduced graphene oxide/silver nanocomposites and curcumin: antimicrobial performance and cutaneous wound healing. Int J Biol Macromol 152:418–427. https://doi.org/10.1016/j.ijbiomac.2020.02.295
Fayemi OE, Ekennia AC, Katata-Seru L, Ebokaiwe AP, Ijomone OM, Onwudiwe DC, Ebenso EE (2018) Antimicrobial and wound healing properties of polyacrylonitrile-moringa extract nanofibers. Acs Omega 3(5):4791–4797. https://doi.org/10.1021/acsomega.7b01981
FDA (2012) FDA’s approach to regulation of nanotechnology products. https://www.fda.gov/science-research/nanotechnology-programs-fda/fdas-approach-regulation-nanotechnology-products
Feynman RP (1960) There’s plenty of room at the bottom. Eng Sci 23(5):22–36
Gao Y, Shao W, Qian W, He J, Zhou Y, Qi K et al (2018) Biomineralized poly (l-lactic-co-glycolic acid)-tussah silk fibroin nanofiber fabric with hierarchical architecture as a scaffold for bone tissue engineering. Mater Sci Eng C 84:195–207. https://doi.org/10.1016/j.msec.2017.11.047
Gao XZ, Han SY, Zhang RH, Liu GT, Wu J (2019) Progress in electrospun composite nanofibers: composition, performance and applications for tissue engineering. J Mater Chem B 7(45):7075–7089. https://doi.org/10.1039/c9tb01730e
Garcia-Orue I, Gainza G, Gutierrez FB, Aguirre JJ, Evora C, Pedraz JL et al (2017) Novel nanofibrous dressings containing rhEGF and Aloe vera for wound healing applications. Int J Pharm 523(2):556–566. https://doi.org/10.1016/j.ijpharm.2016.11.006
Ghosh PK, Murthy RS (2006) Microemulsions: a potential drug delivery system. Curr Drug Deliv 3(2):167–180. https://doi.org/10.2174/156720106776359168
Gnach A, Lipinski T, Bednarkiewicz A, Rybka J, Capobianco JA (2015) Upconverting nanoparticles: assessing the toxicity. Chem Soc Rev 44(6):1561–1584. https://doi.org/10.1039/c4cs00177j
Greiner A, Wendorff JH (2007) Electrospinning: a fascinating method for the preparation of ultrathin fibres. Angew Chem Int Ed 46(30):5670–5703. https://doi.org/10.1002/anie.200604646
Gubala V, Johnston LJ, Liu ZW, Krug H, Moore CJ, Ober CK et al (2018) Engineered nanomaterials and human health: part 1. Preparation, functionalization and characterization (IUPAC technical report). Pure Appl Chem 90(8):1283–1324. https://doi.org/10.1515/pac-2017-0101
Hardy A, Benford D, Halldorsson T, Jeger MJ, Knutsen HK, More S et al (2018) Guidance on risk assessment of the application of nanoscience and nanotechnologies in the food and feed chain: part 1, human and animal health. EFSA J 16(7):5327. https://doi.org/10.2903/j.efsa.2018.5327
Homaeigohar S, Boccaccini AR (2020) Antibacterial biohybrid nanofibers for wound dressings. Acta Biomater 107:25–49. https://doi.org/10.1016/j.actbio.2020.02.022
Huang ZM, He CL, Yang A, Zhang Y, Han XJ, Yin J, Wu Q (2006) Encapsulating drugs in biodegradable ultrafine fibers through co-axial electrospinning. J Biomed Mater Res A 77(1):169–179. https://doi.org/10.1002/jbm.a.30564
Inamuddin, Ahamed MI, Prasad R (2021) Advanced antimicrobial materials and applications. Springer, Singapore. ISBN 978-981-15-7098-8. https://www.springer.com/gp/book/9789811570971
Jain KK (2019) The handbook of nanomedicine. Humana Press and Springer, Totowa, NJ
Kang MS, Kim J-H, Singh RK, Jang J-H, Kim H-W (2015) Therapeutic-designed electrospun bone scaffolds: mesoporous bioactive nanocarriers in hollow fiber composites to sequentially deliver dual growth factors. Acta Biomater 16:103–116. https://doi.org/10.1016/j.actbio.2014.12.028
Kegere J, Ouf A, Siam R, Mamdouh W (2019) Fabrication of poly(vinyl alcohol)/chitosan/bidens pilosa composite electrospun nanofibers with enhanced antibacterial activities. Acs Omega 4(5):8778–8785. https://doi.org/10.1021/acsomega.9b00204
Kenawy E-R, Bowlin GL, Mansfield K, Layman J, Simpson DG, Sanders EH, Wnek GE (2002) Release of tetracycline hydrochloride from electrospun poly(ethylene-co-vinylacetate), poly(lactic acid), and a blend. J Control Release 81(1):57–64. https://doi.org/10.1016/S0168-3659(02)00041-X
Khoshbakht S, Asghari-Sana F, Fathi-Azarbayjani A, Sharifi Y (2020) Fabrication and characterization of tretinoin-loaded nanofiber for topical skin delivery. Biomater Res 24:8. https://doi.org/10.1186/s40824-020-00186-3
Kim BJ, Kim S, Oh DX, Masic A, Cha HJ, Hwang DS (2015) Mussel-inspired adhesive protein-based electrospun nanofibers reinforced by Fe(iii)–DOPA complexation. J Mater Chem B 3(1):112–118. https://doi.org/10.1039/C4TB01496K
Kitto T, Bodart-Le Guen C, Rossetti N, Cicoira F (2019) 25 - processing and patterning of conducting polymers for flexible, stretchable, and biomedical electronics. In: Ostroverkhova O (ed) Handbook of organic materials for electronic and photonic devices, 2nd edn. Woodhead Publishing, pp 817–842
Kurtz IS, Schiffman JD (2018) Current and emerging approaches to engineer antibacterial and antifouling electrospun nanofibers. Materials (Basel) 11(7):1059. https://doi.org/10.3390/ma11071059
Laurencin CT, Ambrosio AMA, Borden MD, Cooper JA, J. (1999) Tissue engineering: orthopedic applications. Annu Rev Biomed Eng 1(1):19–46. https://doi.org/10.1146/annurev.bioeng.1.1.19
Lee SJ, Heo DN, Moon JH, Ko WK, Lee JB, Bae MS et al (2014) Electrospun chitosan nanofibers with controlled levels of silver nanoparticles. Preparation, characterization and antibacterial activity. Carbohydr Polym 111:530–537. https://doi.org/10.1016/j.carbpol.2014.04.026
Lee H, Lim S, Birajdar MS, Lee S-H, Park H (2016) Fabrication of FGF-2 immobilized electrospun gelatin nanofibers for tissue engineering. Int J Biol Macromol 93:1559–1566. https://doi.org/10.1016/j.ijbiomac.2016.07.041
Liu Z, Li S, Su L, Sun K, Wu X, Wu F et al (2015) Novel superhydrophilic poly(l-lactic acid-co-ε-caprolactone)/fibrinogen electrospun patch for rat abdominal wall reconstruction. J Biomater Appl 30(2):230–238. https://doi.org/10.1177/0885328215577732
Liu Y, Wang Q, Lu Y, Deng HB, Zhou X (2020) Synergistic enhancement of cytotoxicity against cancer cells by incorporation of rectorite into the paclitaxel immobilized cellulose acetate nanofibers. Int J Biol Macromol 152:672–680. https://doi.org/10.1016/j.ijbiomac.2020.02.184
Lu WJ, Sun JS, Jiang XY (2014) Recent advances in electrospinning technology and biomedical applications of electrospun fibers. J Mater Chem B 2(17):2369–2380. https://doi.org/10.1039/c3tb21478h
Lu Y, Shah KW, Xu JW (2017) Synthesis, morphologies and building applications of nanostructured polymers. Polymers 9(10):506. https://doi.org/10.3390/polym9100506
Machado R, da Costa A, Sencadas V, Garcia-Arévalo C, Costa CM, Padrão J et al (2013) Electrospun silk-elastin-like fibre mats for tissue engineering applications. Biomed Mater 8(6):065009. https://doi.org/10.1088/1748-6041/8/6/065009
Mamidi N, Zuniga AE, Villela-Castrejon J (2020) Engineering and evaluation of forcespun functionalized carbon nano-onions reinforced poly (epsilon-caprolactone) composite nanofibers for pH-responsive drug release. Mater Sci Eng C 112:110928. https://doi.org/10.1016/j.msec.2020.110928
Mira A, Sainz-Urruela C, Codina H, Jenkins SI, Rodriguez-Diaz JC, Mallavia R, Falco A (2020) Physico-chemically distinct nanomaterials synthesized from derivates of a poly(anhydride) diversify the spectrum of loadable antibiotics. Nanomaterials (Basel) 10(3):486. https://doi.org/10.3390/nano10030486
Mody N, Tekade RK, Mehra NK, Chopdey P, Jain NK (2014) Dendrimer, liposomes, carbon nanotubes and PLGA nanoparticles: one platform assessment of drug delivery potential. AAPS PharmSciTech 15(2):388–399. https://doi.org/10.1208/s12249-014-0073-3
Monaco JL, Lawrence WT (2003) Acute wound healing an overview. Clin Plast Surg 30:1), 1–1),12. https://doi.org/10.1016/s0094-1298(02)00070-6
Mottaghitalab F, Farokhi M, Shokrgozar MA, Atyabi F, Hosseinkhani H (2015) Silk fibroin nanoparticle as a novel drug delivery system. J Control Release 206:161–176. https://doi.org/10.1016/j.jconrel.2015.03.020
Nair LS, Laurencin CT (2007) Biodegradable polymers as biomaterials. Prog Polym Sci 32(8):762–798. https://doi.org/10.1016/j.progpolymsci.2007.05.017
Nejad MR, Yousefzadeh M, Solouk A (2020) Electrospun PET/PCL small diameter nanofibrous conduit for biomedical application. Mater Sci Eng C 110:110692. https://doi.org/10.1016/j.msec.2020.110692
O’Brien FJ (2011) Biomaterials & scaffolds for tissue engineering. Mater Today 14(3):88–95. https://doi.org/10.1016/S1369-7021(11)70058-X
Ojah N, Saikia D, Gogoi D, Baishya P, Ahmed GA, Ramteke A, Choudhury AJ (2019) Surface modification of core-shell silk/PVA nanofibers by oxygen dielectric barrier discharge plasma: studies of physico-chemical properties and drug release behavior. Appl Surf Sci 475:219–229. https://doi.org/10.1016/j.apsusc.2018.12.270
Park SH, Oh SG, Mun JY, Han SS (2006) Loading of gold nanoparticles inside the DPPC bilayers of liposome and their effects on membrane fluidities. Colloids Surf B 48(2):112–118. https://doi.org/10.1016/j.colsurfb.2006.01.006
Patra JK, Das G, Fraceto LF, Campos EVR, Rodriguez-Torres MDP, Acosta-Torres LS et al (2018) Nano based drug delivery systems: recent developments and future prospects. J Nanobiotechnol 16:71. https://doi.org/10.1186/s12951-018-0392-8
Peran M, Garcia MA, Lopez-Ruiz E, Jimenez G, Marchal JA (2013) How can nanotechnology help to repair the body? Advances in cardiac, skin, bone, cartilage and nerve tissue regeneration. Materials 6(4):1333–1359. https://doi.org/10.3390/ma6041333
Polini A, Yang F (2017) Physicochemical characterization of nanofiber composites. In: Nanofiber composites for biomedical application. Elsevier, pp 97–115
Prasad R, Pandey R, Varma A, Barman I (2017) Polymer based nanoparticles for drug delivery systems and cancer therapeutics. In: Kharkwal H, Janaswamy S (eds) Natural polymers for drug delivery. CAB International, London, pp 53–70
Rajabi M, Ali A, McConnell M, Cabral J (2020) Keratinous materials: structures and functions in biomedical applications. Mater Sci Eng C 110:110612. https://doi.org/10.1016/j.msec.2019.110612
Ramji K, Shah RN (2014) Electrospun soy protein nanofiber scaffolds for tissue regeneration. J Biomater Appl 29(3):411–422. https://doi.org/10.1177/0885328214530765
Reddy N, Yang Y (2008) Self-crosslinked gliadin fibers with high strength and water stability for potential medical applications. J Mater Sci Mater Med 19(5):2055–2061. https://doi.org/10.1007/s10856-007-3294-0
Reddy KVR, Yedery RD, Aranha C (2004) Antimicrobial peptides: premises and promises. Int J Antimicrob Agents 24(6):536–547. https://doi.org/10.1016/j.ijantimicag.2004.09.005
Rezaei Z, Mahmoudifard M (2019) Pivotal role of electrospun nanofibers in microfluidic diagnostic systems—a review. J Mater Chem B 7(30):4602–4619. https://doi.org/10.1039/c9tb00682f
Rosa AR, Steffens D, Santi B, Quintiliano K, Steffen N, Pilger DA, Pranke P (2017) Development of VEGF-loaded PLGA matrices in association with mesenchymal stem cells for tissue engineering. Braz J Med Biol Res 50(9):e5648. https://doi.org/10.1590/1414-431x20175648
Safdari M, Shakiba E, Kiaie SH, Fattahi A (2016) Preparation and characterization of Ceftazidime loaded electrospun silk fibroin/gelatin mat for wound dressing. Fibers Polym 17(5):744–750. https://doi.org/10.1007/s12221-016-5822-3
Sarhan WA, Azzazy HM (2017) Apitherapeutics and phage-loaded nanofibers as wound dressings with enhanced wound healing and antibacterial activity. Nanomedicine 12(17):2055–2067. https://doi.org/10.2217/nnm-2017-0151
SCCS (2020) The SCCS guidance on the safety assessment of nanomaterials in cosmetics comment. Regul Toxicol Pharmacol 112:104611. https://doi.org/10.1016/j.yrtph.2020.104611
Sebe I, Ostorhazi E, Fekete A, Kovacs KN, Zelko R, Kovalszky I et al (2016) Polyvinyl alcohol nanofiber formulation of the designer antimicrobial peptide APO sterilizes acinetobacter baumannii-infected skin wounds in mice. Amino Acids 48(1):203–211. https://doi.org/10.1007/s00726-015-2080-4
Sedghi R, Shaabani A, Mohammadi Z, Samadi FY, Isaei E (2017) Biocompatible electrospinning chitosan nanofibers: a novel delivery system with superior local cancer therapy. Carbohydr Polym 159:1–10. https://doi.org/10.1016/j.carbpol.2016.12.011
Selvaraj S, Thangam R, Fathima NN (2018) Electrospinning of casein nanofibers with silver nanoparticles for potential biomedical applications. Int J Biol Macromol 120:1674–1681. https://doi.org/10.1016/j.ijbiomac.2018.09.177
Shahi RG, Albuquerque MTP, Munchow EA, Blanchard SB, Gregory RL, Bottino MC (2017) Novel bioactive tetracycline-containing electrospun polymer fibers as a potential antibacterial dental implant coating. Odontology 105(3):354–363. https://doi.org/10.1007/s10266-016-0268-z
Shahini Shams Abadi M, Mirzaei E, Bazargani A, Gholipour A, Heidari H, Hadi N (2020) Antibacterial activity and mechanism of action of chitosan nanofibers against toxigenic Clostridioides (clostridium) difficile isolates. Ann Ig 32(1):72–80. https://doi.org/10.7416/ai.2020.2332
Shalumon KT, Anulekha KH, Nair SV, Nair SV, Chennazhi KP, Jayakumar R (2011) Sodium alginate/poly(vinyl alcohol)/nano ZnO composite nanofibers for antibacterial wound dressings. Int J Biol Macromol 49(3):247–254. https://doi.org/10.1016/j.ijbiomac.2011.04.005
Sharma J, Lizu M, Stewart M, Zygula K, Lu Y, Chauhan R et al (2015) Multifunctional nanofibers towards active biomedical therapeutics. Polymers 7(2):186–219. https://doi.org/10.3390/polym7020186
Shefa AA, Taz M, Hossain M, Kim YS, Lee SY, Lee BT (2019) Investigation of efficiency of a novel, zinc oxide loaded TEMPO-oxidized cellulose nanofiber based hemostat for topical bleeding. Int J Biol Macromol 126:786–795. https://doi.org/10.1016/j.ijbiomac.2018.12.079
Shin SH, Purevdorj O, Castano O, Planell JA, Kim HW (2012) A short review: recent advances in electrospinning for bone tissue regeneration. J Tissue Eng 3(1):2041731412443530. https://doi.org/10.1177/2041731412443530
Shoba E, Lakra R, Syamala Kiran M, Korrapati PS (2017) Fabrication of core–shell nanofibers for controlled delivery of bromelain and salvianolic acid B for skin regeneration in wound therapeutics. Biomed Mater 12(3):035005. https://doi.org/10.1088/1748-605x/aa6684
Ske (2020) Electrospinning system—needleless version. http://www.ske.it/index.php/product/electrospinning-system-needleless-electrospinning/
Song DW, Kim SH, Kim HH, Lee KH, Ki CS, Park YH (2016) Multi-biofunction of antimicrobial peptide-immobilized silk fibroin nanofiber membrane: implications for wound healing. Acta Biomater 39:146–155. https://doi.org/10.1016/j.actbio.2016.05.008
Song J, Klymov A, Shao J, Zhang Y, Ji W, Kolwijck E et al (2017) Electrospun nanofibrous silk fibroin membranes containing gelatin nanospheres for controlled delivery of biomolecules. Adv Healthc Mater 6(14):1700014. https://doi.org/10.1002/adhm.201700014
Tahmasebi A, Enderami SE, Saburi E, Islami M, Yaslianifard S, Mahabadi JA et al (2020) Micro-RNA-incorporated electrospun nanofibers improve osteogenic differentiation of human-induced pluripotent stem cells. J Biomed Mater Res A 108(2):377–386. https://doi.org/10.1002/jbm.a.36824
Tao FH, Cheng YX, Shi XW, Zheng HF, Du YM, Xiang W, Deng HB (2020) Applications of chitin and chitosan nanofibers in bone regenerative engineering. Carbohydr Polym 230:115658. https://doi.org/10.1016/j.carbpol.2019.115658
Thangadurai D, Sangeetha J, Prasad R (2020a) Nanotechnology for food, agriculture, and environment. Springer International Publishing, Cham. ISBN 978-3-030-31937-3. https://www.springer.com/gp/book/9783030319373
Thangadurai D, Sangeetha J, Prasad R (2020b) Functional bionanomaterials. Springer International Publishing, Cham. ISBN 978-3-030-41464-1. https://www.springer.com/gp/book/9783030414634
Tiwari SK, Tzezana R, Zussman E, Venkatraman SS (2010) Optimizing partition-controlled drug release from electrospun core–shell fibers. Int J Pharm 392(1):209–217. https://doi.org/10.1016/j.ijpharm.2010.03.021
Tsuji JS, Maynard AD, Howard PC, James JT, Lam CW, Warheit DB, Santamaria AB (2006) Research strategies for safety evaluation of nanomaterials, part IV: risk assessment of nanoparticles. Toxicol Sci 89(1):42–50. https://doi.org/10.1093/toxsci/kfi339
Tucker N, Stanger JJ, Staiger MP, Razzaq H, Hofman K (2012) The history of the science and technology of electrospinning from 1600 to 1995. J Eng Fibers Fabr 7:63–73. https://doi.org/10.1177/155892501200702S10
Uehara TM, Paino IMM, Santos FA, Scagion VP, Correa DS, Zucolotto V (2020) Fabrication of random and aligned electrospun nanofibers containing graphene oxide for skeletal muscle cells scaffold. Polym Adv Technol 31(6):1437–1443. https://doi.org/10.1002/pat.4874
Ulubayram K, Calamak S, Shahbazi R, Eroglu I (2015) Nanofibers based antibacterial drug design, delivery and applications. Curr Pharm Des 21(15):1930–1943. https://doi.org/10.2174/1381612821666150302151804
Valmikinathan CM, Defroda S, Yu X (2009) Polycaprolactone and bovine serum albumin based nanofibers for controlled release of nerve growth factor. Biomacromolecules 10(5):1084–1089. https://doi.org/10.1021/bm8012499
Vasita R, Katti DS (2006) Nanofibers and their applications in tissue engineering. Int J Nanomed 1(1):15–30. https://doi.org/10.2147/nano.2006.1.1.15
Venkataraman S, Hedrick JL, Ong ZY, Yang C, Ee PLR, Hammond PT, Yang YY (2011) The effects of polymeric nanostructure shape on drug delivery. Adv Drug Deliv Rev 63(14–15):1228–1246. https://doi.org/10.1016/j.addr.2011.06.016
Verreck G, Chun I, Peeters J, Rosenblatt J, Brewster ME (2003) Preparation and characterization of Nanofibers containing amorphous drug dispersions generated by electrostatic spinning. Pharm Res 20(5):810–817. https://doi.org/10.1023/A:1023450006281
Vert M, Doi Y, Hellwich KH, Hess M, Hodge P, Kubisa P et al (2012) Terminology for biorelated polymers and applications (IUPAC recommendations 2012). Pure Appl Chem 84(2):377–408. https://doi.org/10.1351/pac-rec-10-12-04
Wang J, Windbergs M (2017) Functional electrospun fibers for the treatment of human skin wounds. Eur J Pharm Biopharm 119:283–299. https://doi.org/10.1016/j.ejpb.2017.07.001
Wang Z-G, Wan L-S, Xu Z-K (2007) Surface engineerings of polyacrylonitrile-based asymmetric membranes towards biomedical applications: an overview. J Membr Sci 304(1):8–23. https://doi.org/10.1016/j.memsci.2007.05.012
Wang S-D, Ma Q, Wang K, Chen H-W (2018) Improving antibacterial activity and biocompatibility of bioinspired electrospinning silk fibroin nanofibers modified by graphene oxide. Acs Omega 3(1):406–413. https://doi.org/10.1021/acsomega.7b01210
Whitehead TJ, Avila COC, Sundararaghavan HG (2018) Combining growth factor releasing microspheres within aligned nanofibers enhances neurite outgrowth. J Biomed Mater Res A 106(1):17–25. https://doi.org/10.1002/jbm.a.36204
Won JJ, Nirmala R, Navamathavan R, Kim HY (2012) Electrospun core–shell nanofibers from homogeneous solution of poly(vinyl alcohol)/bovine serum albumin. Int J Biol Macromol 50(5):1292–1298. https://doi.org/10.1016/j.ijbiomac.2012.04.007
Xu R, Zhang Z, Toftdal MS, Møller AC, Dagnaes-Hansen F, Dong M et al (2019) Synchronous delivery of hydroxyapatite and connective tissue growth factor derived osteoinductive peptide enhanced osteogenesis. J Control Release 301:129–139. https://doi.org/10.1016/j.jconrel.2019.02.037
Xue JJ, Wu T, Dai YQ, Xia YN (2019) Electrospinning and electrospun nanofibers: methods, materials, and applications. Chem Rev 119(8):5298–5415. https://doi.org/10.1021/acs.chemrev.8b00593
Yan EY, Jiang JY, Yang XY, Fan LQ, Wang YW, An QL et al (2020) pH-sensitive core-shell electrospun nanofibers based on polyvinyl alcohol/polycaprolactone as a potential drug delivery system for the chemotherapy against cervical cancer. J Drug Deliv Sci Technol 55:101455. https://doi.org/10.1016/j.jddst.2019.101455
Yang X, Yang J, Wang L, Ran B, Jia Y, Zhang L et al (2017) Pharmaceutical intermediate-modified gold nanoparticles: against multidrug-resistant Bacteria and wound-healing application via an electrospun scaffold. ACS Nano 11(6):5737–5745. https://doi.org/10.1021/acsnano.7b01240
Yang YN, Lu KY, Wang P, Ho YC, Tsai ML, Mi FL (2020) Development of bacterial cellulose/chitin multi-nanofibers based smart films containing natural active microspheres and nanoparticles formed in situ. Carbohydr Polym 228:115370. https://doi.org/10.1016/j.carbpol.2019.115370
Yao C-H, Yeh J-Y, Chen Y-S, Li M-H, Huang C-H (2017) Wound-healing effect of electrospun gelatin nanofibres containing centella asiatica extract in a rat model. J Tissue Eng Regen Med 11(3):905–915. https://doi.org/10.1002/term.1992
Ye P, Wei S, Luo C, Wang Q, Li A, Wei F (2020) Long-term effect against methicillin-resistant Staphylococcus aureus of emodin released from coaxial electrospinning nanofiber membranes with a biphasic profile. Biomol Ther 10(3):362. https://doi.org/10.3390/biom10030362
Yildiz A, Kara AA, Acarturk F (2020) Peptide-protein based nanofibers in pharmaceutical and biomedical applications. Int J Biol Macromol 148:1084–1097. https://doi.org/10.1016/j.ijbiomac.2019.12.275
Yoshimoto H, Shin YM, Terai H, Vacanti JP (2003) A biodegradable nanofiber scaffold by electrospinning and its potential for bone tissue engineering. Biomaterials 24(12):2077–2082. https://doi.org/10.1016/S0142-9612(02)00635-X
Yu C-C, Chang J-J, Lee Y-H, Lin Y-C, Wu M-H, Yang M-C, Chien C-T (2013) Electrospun scaffolds composing of alginate, chitosan, collagen and hydroxyapatite for applying in bone tissue engineering. Mater Lett 93:133–136. https://doi.org/10.1016/j.matlet.2012.11.040
Yuan MQ, Dai FF, Li D, Fan YQ, Xiang W, Tao FH et al (2020) Lysozyme/collagen multilayers layer-by-layer deposited nanofibers with enhanced biocompatibility and antibacterial activity. Mater Sci Eng C 112:110868. https://doi.org/10.1016/j.msec.2020.110868
Zhang Y, Cui L, Che X, Zhang H, Shi N, Li C et al (2015) Zein-based films and their usage for controlled delivery: origin, classes and current landscape. J Control Release 206:206–219. https://doi.org/10.1016/j.jconrel.2015.03.030
Zigdon-Giladi H, Khutaba A, Elimelech R, Machtei EE, Srouji S (2017) VEGF release from a polymeric nanofiber scaffold for improved angiogenesis. J Biomed Mater Res A 105(10):2712–2721. https://doi.org/10.1002/jbm.a.36127
Zupancic S, Rijavec T, Lapanje A, Petelin M, Kristl J, Kocbek P (2018) Nanofibers with incorporated autochthonous bacteria as potential probiotics for local treatment of periodontal disease. Biomacromolecules 19(11):4299–4306. https://doi.org/10.1021/acs.biomac.8b01181
Acknowledgments
This work was supported by the National Funds through FCT/MCTES—Portuguese Foundation for Science and Technology within the scope of the project UIDB/50006/2020. Renato Gil thanks FCT (Fundação para a Ciência e Tecnologia) and ESF (European Social Fund) through POCH (Programa Operacional Capital Humano) for his PhD grant SFRH/BD/131504/2017.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2021 The Author(s), under exclusive license to Springer Nature Singapore Pte Ltd.
About this chapter
Cite this chapter
Gil, R.L., Amorim, C.G., Rodríguez-Díaz, J.M., Araújo, A.N., Montenegro, M.C.B.S.M. (2021). Nanofibers in Medical Microbiology. In: Maddela, N.R., Chakraborty, S., Prasad, R. (eds) Nanotechnology for Advances in Medical Microbiology. Environmental and Microbial Biotechnology. Springer, Singapore. https://doi.org/10.1007/978-981-15-9916-3_4
Download citation
DOI: https://doi.org/10.1007/978-981-15-9916-3_4
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-15-9915-6
Online ISBN: 978-981-15-9916-3
eBook Packages: Earth and Environmental ScienceEarth and Environmental Science (R0)