Abstract
Herpes simplex virus (HSV) may infect various organs in the human body, of which the eye is one possible target. Ocular manifestations of HSV include blepharitis, conjunctivitis, keratitis, iritis, trabeculitis, or retinitis. HSV keratitis can manifest with epithelial, stromal, and/or endothelial disease. Both systemic and topical antiviral agents are available, and treatment indications depend on clinical presentation and response to therapy. Although HSV keratitis is typically diagnosed based on clinical findings, viral cultures and antigen detection methods are also available in atypical cases. After primary infection, the virus may establish latency in the trigeminal ganglia and cause future recurrences. In these cases, prophylactic antivirals may be indicated. Sequelae of HSV keratitis include neurotrophic keratopathy and corneal opacification, and both medical and surgical options exist to manage these outcomes. Future research may yield the development of an HSV vaccine, with positive early results in animal models.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
13.1 Background
Herpes simplex virus (HSV) is a ubiquitous double-stranded DNA virus that is a member of the human herpesvirus family. Humans are the only known natural reservoir for the virus. HSV is the etiology for several systemic infections including orolabial herpes, herpes gladiatorum, and herpes encephalitis [1]. When involving the ocular and periocular tissue, HSV can manifest as blepharitis, conjunctivitis, keratitis, iritis, trabeculitis, or retinitis. HSV keratitis is the most common etiology for infectious corneal blindness in developed countries [2]. The annual cost of treatment for this infectious condition reaches the tens of millions of dollars in the United States alone [3].
Disease may either occur as a primary infection or as a recurrence of latent disease. Primary infection with HSV typically occurs secondary to HSV spread through direct contact of infected lesions or secretions, such as tears or saliva, with mucous membranes of the host [4]. Asymptomatic individuals may shed HSV into their saliva, and one study found that 92% of asymptomatic HSV-infected subjects shed HSV-1 DNA from tears at least once during a 30-day period [5]. Interestingly, only 74% of subjects from this study were IgG positive for HSV-1, indicating that individuals who are seronegative can still harbor and shed HSV [5]. Many patients experience symptoms during primary infection; however, approximately two-thirds of primary HSV infections are unrecognized or asymptomatic.
Ocular HSV infection typically occurs secondary to the HSV-1 virus, although HSV-2 can also be responsible for HSV keratitis [1]. While HSV-2 is classically responsible for most genital herpes infections, HSV-1 and HSV-2 have been identified in similar numbers in the trigeminal and sacral ganglia at autopsy [6]. Therefore, host cell factors are the likely reason for the preference of HSV-1 for the facial area and HSV-2 for the genital area. In the United States, approximately 50% of the population harbors HSV-1 by 30 years of age, while at least 90% is infected by age 60 years [7, 8]. Studies from across different countries estimate the incidence of new cases of ocular HSV to range from 5 to 15 new cases per 100,000 individuals per year [4, 9,10,11,12]. This rate appears to be slowly trending upwards [4, 13].
13.2 Manifestations of HSV Keratitis
13.2.1 Epithelial Keratitis
Epithelial keratitis is the most common subtype of HSV keratitis [14]. Typical symptoms include redness, discharge, pain, and photophobia. With recurrent disease, chronic neurotrophic changes may result in fewer ocular pain symptoms. The differential diagnosis of HSV epithelial keratitis includes Acanthamoeba keratitis, varicella zoster virus (VZV) keratitis, Epstein-Barr virus keratitis, and epithelial regeneration lines.
The classic features of HSV epithelial keratitis are coarse, granular epithelial lesions that coalesce to form corneal dendrites [15]. A corneal dendrite is linear, branching, and exhibits terminal bulbs (Fig. 13.1). These dendrites are ulcerated and feature fluorescein staining at their base (Fig. 13.2) with rose bengal or lissamine green staining of the surrounding devitalized epithelial cells. Upon healing, a “ghost dendrite” can appear, characterized by subepithelial haze in a dendritic pattern. Dendrites present less frequently during recurrent disease. Other features of HSV epithelial keratitis include larger geographic ulcers (Fig. 13.3) or peripheral ulcers. The presence of a peripheral ulcer may result in misdiagnosis as an autoimmune-related keratitis.
Epithelial keratitis typically resolves without treatment in 2 weeks, but early initiation of antiviral therapy can reduce disease severity and hasten recovery [1]. Although epithelial debridement is a long-standing practice, there is strong evidence that debridement alone is insufficient therapy for HSV epithelial keratitis [16]. Several studies suggest that treatment with a combination of antiviral therapy and debridement results in faster healing than treatment with antiviral therapy alone [17, 18]. Debridement may be followed by amniotic membrane placement to potentially hasten recovery [19].
Medication options include systemic agents and topical therapy, with overall equal effectiveness between oral and ophthalmic options. In general, it is advisable to avoid or minimize topical corticosteroids in cases of HSV epithelial keratitis. Many cornea specialists prefer systemic over topical antiviral therapy due to its higher bioavailability, ease of administration, and the surface toxicity associated with many topical agents. Acyclovir and its derivatives contain nucleoside analogs that are selectively phosphorylated by viral and not host enzymes, therefore exhibiting few side effects. The analog is incorporated into viral DNA and results in chain termination, preventing further viral replication [20, 21]. Oral acyclovir reaches therapeutic levels in both tears and aqueous humor [22].
Oral treatment regimens include a 7–10 day course of acyclovir 400 mg three to five times daily, valacyclovir 500 mg two to three times daily, or famciclovir 250 mg two to three times daily. In cases of geographic ulcers, practitioners may extend treatment for 14–21 days and increase dosing to acyclovir 800 mg five times daily, valacyclovir 1 g three times daily, or famciclovir 500 mg two to three times daily. Compared with oral acyclovir, valacyclovir is better absorbed from the gastrointestinal tract with a threefold to fourfold higher circulating drug level, requiring a reduced frequency of administration and possibly improved patient adherence [23, 24]. Side effects of these medications include nausea, vomiting, diarrhea, or other gastrointestinal disturbances [24]. Oral antiviral agents should be employed with caution in elderly patients or in those with renal disease, as they have the potential to induce nephrotoxicity. In refractory cases, systemic valganciclovir has also shown successful results [25].
Although many clinicians prefer systemic to topical antiviral therapy, some cases of HSV keratitis may not respond to oral therapy alone, and can benefit from the addition of topical treatment [26]. While such selected cases exist, no large-scale study has demonstrated a benefit to dual topical and oral therapy for HSV keratitis compared to treatment with a single antiviral medication [27]. For decades, topical regimens for HSV epithelial keratitis included idoxuridine, vidarabine, and trifluridine 1%. These agents are limited primarily by toxicity, resulting in keratoconjunctivitis, allergic conjunctivitis, and punctal stenosis [28]. Overall, topical antiviral medications result in an allergic or toxic blepharoconjunctivitis or corneal epitheliopathy in 5–10% of patients [29].
Current topical regimens available in the United States for HSV epithelial keratitis include trifluridine 1% ophthalmic solution nine times daily for 7 days or ganciclovir 0.15% ophthalmic gel five times daily until the epithelium closes, followed by three times daily for 7 days. Many practitioners avoid using trifluridine 1% for more than 14–21 days due to toxicity. Acyclovir 3% ophthalmic ointment, which was approved by the United States Food and Drug Administration (FDA) in 2019, is better tolerated than trifluridine 1%, even with long-term use, but can cause blurred vision [30]. Ganciclovir 0.15% ophthalmic gel causes less visual disturbances and toxicity as compared to acyclovir 3% ointment with equivalent efficacy [31]. Despite having fewer side effects, ganciclovir 0.15% gel can also result in blurred vision and punctate keratitis [32].
Importantly, most topical antiviral medications fail to reach therapeutic levels in the aqueous humor, with the exception of acyclovir 3% ointment, and should not be used as monotherapy in cases of HSV keratouveitis [33]. Adequate aqueous concentrations of trifluridine 1% can be attained after epithelial debridement [34, 35]. Furthermore, topical therapy does not prevent reactivation of the virus from the sensory ganglia [30].
Long-term treatment with any of these medications can uncommonly result in the emergence of resistance due to viral mutations [36, 37]. Resistance to acyclovir has been shown to present more frequently in immunocompromised individuals those with recurrent ocular HSV infections [38]. Mutations in thymidine kinase are responsible for most cases of acyclovir resistance, and these strains are also resistant to valacyclovir, ganciclovir, and famciclovir [39, 40]. In these cases, other antiviral agents such as foscarnet, cidofovir, and trifluridine should be considered [40,41,42]. There is some evidence for corneal collagen cross-linking in the management of refractory cases of HSV keratitis, although recurrences may occur [43].
13.2.2 Stromal Keratitis
HSV stromal keratitis develops secondary to retained HSV antigens that initiate an antigen-antibody complement cascade, triggering an immune reaction [1]. The condition may exist with or without epithelial ulceration. The differential diagnosis of HSV stromal keratitis includes any other cause of interstitial keratitis including VZV keratitis, syphilis, Cogan’s syndrome, measles keratitis, and mumps keratitis.
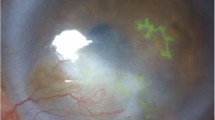
The pathogenesis of HSV stromal keratitis has been shown to involve T-cell activation and cytokine release [44, 45]. The condition may occur primarily or may present secondary to epithelial or endothelial disease. Findings on examination for stromal keratitis without epithelial ulceration include unifocal, multifocal (Fig. 13.4), or diffuse stromal infiltrates. Stromal edema or thinning may variably be present, and slit lamp examination may demonstrate an immune ring or neovascularization. Although these vessels may recede with time to form “ghost vessels,” persistent active vessels may result in lipid keratopathy and visually significant scarring (Fig. 13.5).
HSV stromal keratitis without epithelial ulceration is treated with prophylactic oral antiviral therapy and the addition of a topical corticosteroid, usually prednisolone acetate 1% ophthalmic suspension six to eight times daily. Corticosteroid alternatives include fluorometholone 0.1–0.25% suspension and difluprednate 0.05% emulsion. Prophylactic oral antiviral options include acyclovir 400 mg twice daily, valacyclovir 500 mg once or twice daily, or famciclovir 250 mg once or twice daily. Oral antiviral medications should be continued for at least as long as corticosteroids are in use.
The Herpetic Eye Disease Study (HEDS) established the importance of topical corticosteroids in the treatment of HSV stromal keratitis and demonstrated a decrease in persistent or progressive stromal keratitis as well as a shortened duration of keratitis with initiation of topical corticosteroids [46]. The corticosteroid should be tapered very slowly, often over at least 10 weeks, to the lowest dose required to control inflammation. Long-term corticosteroid maintenance therapy may be required in addition to oral antiviral prophylaxis. Topical cyclosporine 2% has shown comparable results to topical prednisolone 1% in best-corrected visual acuity and corneal densitometry after stromal keratitis [47]. There is some evidence that topical cyclosporine ranging in concentration from 0.05 to 2% may replace or reduce the need for topical corticosteroids in HSV stromal keratitis cases with steroid-induced glaucoma [48,49,50].
A more severe form of stromal keratitis, often referred to as necrotizing stromal keratitis, involves concomitant epithelial ulceration and stromal loss (Fig. 13.6). This less frequent manifestation is thought to be secondary to viral invasion of the corneal stroma. The differential diagnosis includes microbial keratitis, keratolysis from chemical injuries and autoimmune conditions, and neurotrophic keratopathy. This aggressive condition is typically more resistant to therapy and involves significant keratolysis, ulceration, thinning (Fig. 13.7), and possibly corneal perforation. Since this condition shares features of bacterial keratitis, cultures should be performed to rule out other sources of infection.
Significant stromal thinning demonstrated using slit beam in the eye with HSV stromal keratitis with epithelial ulceration seen in Fig. 13.6
Many physicians will treat stromal keratitis with epithelial ulceration with acyclovir 400–800 mg three to five times daily, valacyclovir 500 mg to 1 g three times daily, or famciclovir 250–500 mg two to three times daily. Topical trifluridine 1% and ganciclovir 0.15% demonstrate insufficient penetration of the corneal stroma, and therefore oral agents are preferred [35]. Judicious topical corticosteroids, often prednisolone 1% one to four times daily, may also be employed. Combining amniotic membrane grafting with medical therapy may help, but definitive data for the success of this approach has not been demonstrated [51]. Once the infection is controlled, oral antiviral therapy is reduced to a prophylactic dose and continued for as long as topical corticosteroids are used.
13.2.3 Endothelial Keratitis
HSV endothelial keratitis, also known as HSV endotheliitis, results from virus in the anterior chamber. Clinical findings include keratic precipitates, iritis, and stromal edema. The differential diagnosis of HSV endotheliitis includes any etiology of keratouveitis, Posner-Schlossman syndrome, and cytomegalovirus endothelial keratitis. In many cases, there is no prior known history of HSV epithelial or stromal keratitis. Endotheliitis may be subdivided based on the pattern of involvement as disciform, diffuse, or linear. Disciform endotheliitis is the most common form (Fig. 13.8), featuring a focal round area of stromal edema associated with underlying keratic precipitates and anterior uveitis [52]. Diffuse endotheliitis occurs less frequently, demonstrating keratic precipitates distributed throughout the endothelium. Diffuse endotheliitis may sometimes involve a retrocorneal plaque or a hypopyon, therefore potentially resembling a fungal infection. Finally, linear endotheliitis is characterized by a well-defined linear or serpiginous arrangement of keratic precipitates that demarcates edematous cornea from uninvolved and clear cornea.
The treatment regimen of HSV endotheliitis varies across practitioners, but active HSV in the anterior chamber necessitates systemic antiviral therapy instead of topical agents. Antiviral therapy is acyclovir 400 mg five times daily, valacyclovir 500 mg two to three times daily, or famciclovir 250 mg two to three times daily. Topical corticosteroids are typically initiated as well, usually prednisolone 1% six to eight times daily. Oral antiviral medication is continued until resolution of keratitis, and reduced to a prophylactic dose for as long as topical corticosteroids are being used. Linear endotheliitis may be more difficult to treat than the other patterns, and higher dosing of oral antivirals and corticosteroids may be indicated [1]. This would include acyclovir 400–800 mg five times daily, valacyclovir 500 mg to 1 g three times daily, or famciclovir 250–500 mg three times daily.
13.2.4 Keratouveitis
HSV keratouveitis may occur in association with epithelial, stromal, or endothelial disease. Anterior chamber inflammation occurs in up to 10% of patients with HSV keratitis [53]. Although the recognition of herpetic anterior uveitis is typically straightforward in the context of obvious herpetic lesions such as dendrites, it can be more challenging to identify the diagnosis in the absence of these findings. Clues pointing to the diagnosis of HSV keratouveitis include the presence of corneal scars or edema, decreased corneal sensation, geographically or diffusely distributed keratic precipitates, acutely elevated intraocular pressure, unilateral findings, and iris atrophy which can produce pupillary distortion and transillumination defects (Fig. 13.9) [30].
It is critical to distinguish HSV keratouveitis from VZV keratouveitis [54]. Typical concurrent or prior cutaneous lesions may provide strong support for VZV-related iritis, although these findings are not always present. Dendritic keratitis may help differentiate between the two, although it may be challenging to distinguish between HSV dendrites and VZV pseudodendrites, which are slightly elevated, polymorphous, exhibit less regular branching, demonstrate few terminal dilations, and have central rose bengal staining [30].
The treatment of HSV keratouveitis typically includes acyclovir 400–800 mg five times daily, valacyclovir 500 mg to 1 g three times daily, or famciclovir 250–500 mg three times daily. HEDS examined the effect of oral acyclovir in cases of herpetic keratitis on the duration and recurrence of anterior uveitis, and failed to identify a statistically significant treatment effect [55]. However, this arm of HEDS was relatively small and potentially underpowered [30]. Furthermore, patients were initially treated with both oral acyclovir and topical trifluridine, and therefore it may have been more difficult to identify a treatment effect of the oral acyclovir [30]. Hence, many practitioners will treat herpetic keratouveitis with high dose oral antiviral medication until resolution of infection, and continued maintenance therapy with oral antiviral medication is typically employed to decrease recurrences [56]. Patients are started on topical corticosteroids and a cycloplegic agent to prevent posterior synechiae development and for improved comfort.
13.3 Diagnosis
Early diagnosis of HSV keratitis enables immediate treatment initiation to reduce viral replication and hasten healing. Furthermore, inappropriate treatment can aggravate corneal inflammation and lead to visually debilitating results.
The diagnosis of HSV keratitis is primarily clinical, with classic features as described above. However, the diagnosis can be confounded by factors such as duration of disease, systemic illnesses, prior therapy, and previous keratoplasty, all of which can distort the appearance of HSV lesions [57]. In one study, 8% of HSV cases diagnosed based on clinical appearance were identified using polymerase chain reaction (PCR) as secondary to VZV [58]. When using PCR to confirm a clinical diagnosis of HSV epithelial keratitis, only a moderate correlation was detected, which was even poorer with atypical HSV lesions [57, 59]. Corneal lesions caused by cytomegalovirus, adenovirus, and fungal infections also share features with HSV keratitis and may cause misdiagnosis [7].
Viral culture and antigen detection methods [e.g., enzyme-linked immunosorbent assay (ELISA)] are available to detect HSV; however, the sensitivity of these techniques is inferior to nucleic acid amplification tests [60]. PCR can be used to identify HSV in cases of active viral replication, as is the case in epithelial keratitis [61]. Viral PCR has the advantages of rapid results and a higher sensitivity as compared to other methods [61]. Previous studies have demonstrated that PCR detects anywhere from 29.2 to 76% more cases of HSV as compared to viral culture [62]. Using tear collections of clinically suspected HSV keratitis cases, one study found a sensitivity of ELISA to be 49.2% and specificity to be 82.6%, whereas sensitivity of PCR was 55.8% and specificity 100% [63]. There is significant variation in studies comparing the rates of HSV detection by PCR and clinical diagnosis [59].
One advantage of PCR is that specimens may be obtained from the corneal surface or the anterior chamber, allowing for detection of epithelial or endothelial infection. Unfortunately, PCR has shown less utility for the identification of immune-mediated stromal keratitis, given that viral infection does not directly cause this condition [59]. In cases of endotheliitis in post-keratoplasty eyes, the findings may clinically mimic graft rejection; PCR analysis of aqueous humor can be particularly useful in this setting [64, 65].
One major limitation of PCR is that it may produce false negatives in patients with atypical herpetic lesions or in patients actively using antiviral therapy [66]. One report identified an 80% decrease in detectable virus among individuals who had been treated with acyclovir 400 mg twice daily [67]. In addition, several compounds utilized during clinical evaluation can also affect PCR results. For example, oxybuprocaine, a topical anesthetic used in combination with fluorescein to enhance visualization of HSV lesions, can decrease PCR yield by more than 2 logs (DNA copies/sample) [68]. Rose bengal and lissamine green may also interfere with the detection of HSV-DNA using PCR [69].
An alternative means of diagnosis is in vivo confocal microscopy (IVCM), in which viral keratitis is suggested by a lack of atypical organisms, presence of dendritic cells in the basal epithelial and subepithelial nerve plexus regions, decrease in the subepithelial nerve plexus, and hyperreflective keratocytes in the anterior stroma [70]. Although highly operator-dependent, one study demonstrated a sensitivity and specificity of IVCM in identifying HSV keratitis as 100% and 93.2%, respectively [71].
Newer techniques include tear film analyses, for which a combined assay for HSV immunoglobulins and HSV-DNA demonstrated a 98% positive predictive value for stromal keratitis [72]. Tear samples have also been employed to detect HSV epithelial keratitis, with PCR testing as well as evaluation for HSV-1 antigens by indirect immunofluorescence assays [73]. Tear collection analysis has been shown to yield lower detection rates for HSV endotheliitis compared to HSV epithelial keratitis [63]. Furthermore, viral loads in tear collections diminish after 11 days of illness, which could contribute to false negatives [63]. Other technologies include gene sequencing of intraocular samples [74].
13.4 Special Circumstances
13.4.1 Pediatric Disease
In infants, primary HSV keratitis typically occurs secondary to HSV-2, which is transmitted through antenatal, intrapartum, or postnatal exposures. Due to a weaker immune system, HSV infection in this population may have a severe course. Immediate and aggressive treatment is crucial to prevent amblyopia and poor visual results [75, 76].
Older children with HSV keratitis also tend to experience more severe infection than adults, increased frequency of recurrences, and worse corneal scarring and astigmatism, resulting in poorer visual outcomes and amblyopia [77,78,79]. Similarly, episodes of HSV stromal keratitis in children tend to be more severe [79]. Pediatric HSV keratitis is compounded by the difficulty in examining this population, and as a result the condition may be commonly misdiagnosed resulting in delayed treatment [79]. In addition, pediatric cases of HSV keratitis are more likely to present bilaterally, with rates of 3.4–26%, as compared to 1.3–12% in adults [7, 77, 78, 80,81,82,83]. Furthermore, children are more likely to experience HSV keratitis recurrences within the first year of an initial episode as compared to adults (45–50% vs. 18%, respectively) [77, 80, 84].
The treatment of pediatric HSV keratitis differs from adults. Topical trifluridine 1% is only FDA approved for children age six and above, whereas ganciclovir 0.15% is FDA approved in children over the age of 2 years. Acyclovir is safe in neonates, however valacyclovir is indicated in children age two years or above. Famciclovir, on the other hand, is only indicated in patients older than 18 years of age. Acyclovir dosing may vary based on experience of the physician, and one study advised a treatment dose of 12–80 mg/kg/day in divided doses, and a prophylactic dose of 12–20 mg/kg/day [85].
13.4.2 Bilateral Disease
Although most cases of HSV keratitis are unilateral, in approximately 1.3–12% of adult cases, bilateral infections occur. This presentation may occur in immunocompromised individuals [59, 81]. Additionally, bilateral HSV keratitis is more common in cases of primary as compared to recurrent HSV keratitis [12]. As described above, bilateral HSV keratitis is more prevalent in the pediatric age group.
In addition, patients with atopy are also more likely to suffer from bilateral HSV keratitis [86]. Atopic describes patients with a personal or family history of hay fever, asthma, or eczema. In general, patients with atopy exhibit an exaggerated reactivity to common environmental antigens that typically cause no response in other individuals; these antigens stimulate the production and activation of eosinophils. These individuals experience altered cell-mediated immunity and are therefore susceptible to HSV keratitis [87,88,89]. One study documented a 2.0–4.8-fold increased odds of developing ocular HSV in individuals with atopic disease compared to those without atopy [86]. Patients with atopy and HSV keratitis often have severe cases of infection with a poorer therapeutic response to topical antiviral agents as compared to oral therapy [87]. These patients may necessitate relatively higher doses of oral antivirals.
13.4.3 Recurrent Disease
Oftentimes, primary infection of the cornea is detected on clinical exam. However, initial disease may be subclinical and still establish latent infection. After primary infection, the virus can be subsequently transported in a retrograde fashion via sensory neurons, establishing latency in the trigeminal ganglia [90]. The virus remains asymptomatic in the sensory ganglia until reactivation, resulting in secondary or recurrent HSV infection. Each episode of recurrent keratitis increases the risk of subsequent episodes [12]. Recurrence rates of HSV keratitis are approximately 25% in the first year after initial disease and 33% by the second year [91]. Evidence indicates that short intervals between previous episodes of HSV keratitis are associated with similarly short intervals between future episodes [92]. Seroprevalence is affected by the level of exposure to the source of infection, and therefore is influenced by factors such as crowding, poor hygiene, and age [7].
The severity and probability of recurrent HSV keratitis is dependent on the virulence of the viral strain and the susceptibility of the host to this particular strain. The underlying mechanism of reactivation or recurrence remains largely unknown; however, several hypotheses have been proposed. One possible explanation is that a high quantity of latent viral copies and the number of latently infected neurons may overwhelm the cellular defense mechanisms against viral infection [93].
Local stressors are well-known triggers for reactivation of HSV keratitis, and infection has been documented to occur more frequently following intravitreal injections [94], after cataract surgery [95], and after corneal transplantation [96]. HSV keratitis has also been reported after laser-assisted in situ keratomileusis (LASIK) [97, 98], laser iridotomy [99], argon laser trabeculoplasty [100], phototherapeutic keratectomy (PTK) [101], and photorefractive keratectomy (PRK) [102].
Certain topical medications also result in a predisposition of the cornea to HSV recurrence. For example, topical prostaglandin analogs, used for the treatment of elevated intraocular pressure, have been shown to be associated with HSV epithelial keratitis [103, 104]. Prostaglandins are therefore best avoided in patients with a known history of HSV keratitis. Additionally, several forms of corticosteroids, including topical, intravitreal, or systemic formulations, can predispose to HSV epithelial keratitis [105,106,107].
HEDS found no association of ocular HSV recurrence with psychological stress, systemic infection, ultraviolet light exposure, menstruation, contact lens wear, or eye injury [46]. However, other individual studies have identified possible associations of recurrent HSV infection with systemic fever [108], menstruation [109], psychological stress [110], and upper respiratory tract infections [111].
13.4.4 Prophylaxis
HEDS investigated the benefit of acyclovir 400 mg twice daily for the prevention of recurrent ocular HSV over a 12 month period and found that this regimen reduced the recurrence of ocular disease by approximately 50% [46]. This finding established the recommendation for long-term prophylaxis. Additionally, 12 months of oral antiviral prophylaxis reduces episodes of both HSV epithelial and stromal keratitis, the latter of which was only significant in individuals with a known history of HSV stromal keratitis [112]. Given the degree of morbidity associated with HSV stromal keratitis, long-term oral antiviral prophylaxis is beneficial for individuals with a history of this condition.
Importantly, antiviral agents may not prevent the development of new stromal keratitis, which is immunomediated; however, by reducing viral load, the magnitude of the inflammatory response may be suppressed [7]. HEDS included patients treated for HSV epithelial keratitis with topical trifluridine and evaluated whether oral acyclovir 400 mg five times daily for 3 weeks reduced future episodes of HSV stromal keratitis. The study found no benefit of short-term systemic antivirals in preventing stromal keratitis [29].
Prophylaxis options include acyclovir 400 mg twice daily, valacyclovir 500 mg once or twice daily, or famciclovir 250 mg once or twice daily. A trial of patients with a history of recurrent ocular HSV found no difference between the efficacy of oral acyclovir 400 mg twice daily and valacyclovir 500 mg once daily in the prevention of recurrences [113]. A study comparing systemic and topical acyclovir 3% ointment for the prevention of HSV keratitis recurrence following corneal transplant found significantly better results in those treated with oral acyclovir [114]. Prophylaxis is typically continued for at least 1 year, but often for many years, and permanently after corneal transplantation.
Indications for prophylaxis include, but are not limited to, cases of multiple recurrences of any form of HSV keratitis, postoperatively in patients with a history of HSV ocular disease, and a history of ocular HSV in patients undergoing immunosuppressive therapy. Additional populations that may benefit from prophylaxis include immunosuppressed, monocular, and atopic individuals.
The host immune system plays a central role in dictating general susceptibility to ocular HSV infections and prevention of HSV recurrences. For example, patients with prior organ transplants are often immunocompromised due to systemic immunosuppressive medications. These patients face an increased risk of HSV infection and reactivation [115,116,117]. Other predisposing conditions for HSV infection and recurrence include long-standing diabetes mellitus, especially with poor glycemic control [118,119,120]. HIV positive patients have been shown to have a higher recurrence rate of HSV keratitis [121].
Finally, a study of patients with a history of recurrent HSV keratitis identified that recurrences were more likely in patients with ocular surgery within the prior 6 weeks. Among those with recurrences following ocular surgery, individuals on higher doses of oral acyclovir (average 1321 mg/day) faced fewer recurrences compared to those on lower doses (average 1000 mg/day) [122]. Hence, a higher dosing of prophylactic acyclovir may be appropriate in the perioperative period for patients with a history of HSV keratitis. This may include acyclovir 400 mg four or five times daily for a week preceding surgery until 1–2 weeks after surgery, although the regimen may vary based on the practitioner.
13.5 Management of Sequelae
13.5.1 Neurotrophic Keratopathy
The cornea is the most densely innervated tissue in the human body [123]. Corneal nerves are responsible for several reflexive, sensory, and trophic processes involving the ocular surface, and play a central role in the blink reflex, tear production, tear secretion, epithelial integrity, epithelial proliferation, and wound healing [124,125,126]. Prior episodes of herpetic keratitis may result in damage to corneal nerves, leading to neurotrophic keratopathy and corneal hypoesthesia. As the number of previous episodes of HSV keratitis increases, corneal sensitivity decreases [127]. A decrease in subbasal nerve corneal nerve fiber length and nerve branch density correlate with this decreased sensitivity [123].
Neurotrophic keratopathy may range in severity from mild epitheliopathy to oval-shaped ulceration. Patients often experience decreased corneal sensation, recurrent epithelial defects, or in more advanced cases, corneal ulceration, melting, and possibly perforation [128, 129]. Progressive corneal thinning and perforation may be managed with amniotic membrane grafting, corneal glue, tarsorrhaphy, conjunctival flap, patch grafting, or tectonic keratoplasty [130].
Corneal nerves have been demonstrated to regenerate in other instances of acute neuronal damage, such as after corneal refractive surgery [131, 132]. However, confocal microscopy studies of corneas previously infected by HSV found that nerves regenerate to a limited extent, which is often not clinically significant [123, 133, 134]. In one study, over an average follow-up of over 3 years, there was no significant change in corneal sensation compared to the initial visit as measured by Cochet-Bonnet esthesiometry (Luneau Ophthalmologie, Chartres, France), despite a small but statistically significant increase in central total corneal nerve density [133]. Another study showed that corneal sensation increased significantly at 6 months of follow-up, but did not reach normal levels [134].
Interestingly, subbasal nerve parameters of seemingly uninvolved contralateral eyes in patients with HSV keratitis have also been shown to be lower than controls [135,136,137]. The precise pathophysiology of contralateral eye changes remains unknown, although several hypotheses have been postulated. One possible explanation is that the central nervous system may regulate contralateral neuronal effects. Alternatively, there may exist subclinical viral expansion to the contralateral side [123].
Management of neurotrophic keratopathy typically begins with lubrication, bandage soft contact lens, serum tears, tarsorrhaphy, and amniotic membrane placement. Systemic antiviral prophylaxis should be continued indefinitely. Topical steroids may be employed judiciously if there is suspicion that inflammation is inhibiting reepithelialization. Recombinant human nerve growth factor (rhNGF) agents are being used increasingly in the management of neurotrophic keratopathy. The REPARO trial evaluated the efficacy of rhNGF agents in promoting corneal epithelialization in moderate-to-severe neurotrophic keratopathy [138]. Eyes treated with rhNGF agents achieved higher rates of healing at 4 and 8 weeks [138]. This effect appears to last for at least 1 year. The commercially available rhNGF agent, Oxervate (cenegermin-bkbj, Dompé, Milan, Italy), entered the United States market in January 2019 and is administered six times a day for 8 weeks. A scleral lens may also be a valuable therapeutic tool in treating neurotrophic keratopathy as it provides continuous hydration, protects the corneal epithelium, and optimizes vision [139]. Finally, surgical management is an option, with corneal neurotization demonstrating improved corneal sensation [140]. Confocal microscopy after neurotization has identified reinnervation at the level of the subbasal nerve plexus [141].
13.5.2 Corneal Opacification
Repeated epithelial, stromal, and endothelial disease increases the risk of fibrosis and neovascularization, with resulting visual loss [90]. Common findings include corneal edema, scarring, opacification, neovascularization, lipid keratopathy, band keratopathy, and irregular astigmatism (Fig. 13.10). In cases of stable, visually significant corneal scarring, surgical options may include deep anterior lamellar keratoplasty or penetrating keratoplasty. Ocular HSV is responsible for about 10% of all corneal transplants in the United Kingdom [142].
Penetrating keratoplasty in patients with a history of HSV keratitis involves several special considerations. Graft failure following penetrating keratoplasty for these patients may occur due to severe ocular surface disease, viral reactivation in the graft, or allograft rejection [143]. Many surgeons will perform a temporary tarsorrhaphy at the time of keratoplasty in eyes with a history of HSV ocular disease. HSV recurrences increase the risk of subsequent graft failure, and patients with a history of ocular HSV have a higher incidence of graft rejection compared to those with no history of HSV [144]. There are rare reports of transmission of HSV from the donor cornea [145].
Recurrences of HSV keratitis following penetrating keratoplasty may be reduced with oral antiviral prophylaxis; various reports have demonstrated that keratoplasty patients with a history of HSV keratitis treated with prophylactic acyclovir experience fewer recurrences of infection [146,147,148]. The recurrence rate of HSV keratitis after penetrating keratoplasty may be related to treatment length with oral acyclovir, and patients treated with oral acyclovir for 1 year after keratoplasty experienced a recurrence rate of 5% at 2 years [149]. On the other hand, individuals not treated with antiviral prophylaxis after penetrating keratoplasty experienced a 27–50% recurrence rate for HSV after 2 years [146, 150]. Many practitioners will continue antiviral systemic prophylaxis for as long as a patient is on topical corticosteroids, if not forever.
13.6 Future Directions
With continued research into HSV pathophysiology, potential new avenues for therapeutic agents have arisen. One area of active investigation is the development of a HSV vaccine, which has shown promising results for HSV keratitis in a murine model [151, 152]. While there are no FDA approved vaccines for the prevention of HSV keratitis infection or recurrence, many research trials are underway in this arena.
References
Valerio GS, Lin CC. Ocular manifestations of herpes simplex virus. Curr Opin Ophthalmol. 2019;30(6):525–31.
Farooq AV, Shah A, Shukla D. The role of herpesviruses in ocular infections. Virus Adapt Treat. 2010;2:115–23.
Lairson DR, Begley CE, Reynolds TF, Wilhelmus KR. Prevention of herpes simplex virus eye disease: a cost-effectiveness analysis. Arch Ophthalmol. 2003;121(1):108–12.
Liesegang TJ, Melton LJ 3rd, Daly PJ, Ilstrup DM. Epidemiology of ocular herpes simplex. Incidence in Rochester, Minn, 1950 through 1982. Arch Ophthalmol. 1989;107(8):1155–9.
Kaufman HE, Azcuy AM, Varnell ED, Sloop GD, Thompson HW, Hill JM. HSV-1 DNA in tears and saliva of normal adults. Invest Ophthalmol Vis Sci. 2005;46(1):241–7.
Obara Y, Furuta Y, Takasu T, Suzuki S, Suzuki H, Matsukawa S, Fujioka Y, Takahashi H, Kurata T, Nagashima K. Distribution of herpes simplex virus types 1 and 2 genomes in human spinal ganglia studied by PCR and in situ hybridization. J Med Virol. 1997;52(2):136–42.
Liesegang TJ. Herpes simplex virus epidemiology and ocular importance. Cornea. 2001;20(1):1–13.
Cohrs RJ, Randall J, Smith J, Gilden DH, Dabrowski C, van Der Keyl H, Tal-Singer R. Analysis of individual human trigeminal ganglia for latent herpes simplex virus type 1 and varicella-zoster virus nucleic acids using real-time PCR. J Virol. 2000;74(24):11464–71.
Labetoulle M, Auquier P, Conrad H, Crochard A, Daniloski M, Bouée S, El Hasnaoui A, Colin J. Incidence of herpes simplex virus keratitis in France. Ophthalmology. 2005;112(5):888–95.
Mortensen KK, Sjølie AK. Keratitis dendritica. An epidemiological investigation. Acta Ophthalmol. 1979;57(5):750–4.
Ribarić V. The incidence of herpetic keratitis among population. Ophthalmologica. 1976;173(1):19–22.
Darougar S, Wishart MS, Viswalingam ND. Epidemiological and clinical features of primary herpes simplex virus ocular infection. Br J Ophthalmol. 1985;69(1):2–6.
Young RC, Hodge DO, Liesegang TJ, Baratz KH. Incidence, recurrence, and outcomes of herpes simplex virus eye disease in Olmsted County, Minnesota, 1976-2007: the effect of oral antiviral prophylaxis. Arch Ophthalmol. 2010;128(9):1178–83.
Wilhelmus KR. Antiviral treatment and other therapeutic interventions for herpes simplex virus epithelial keratitis. Cochrane Database Syst Rev. 2015;1:CD002898.
Chang EJ, Dreyer EB. Herpesvirus infections of the anterior segment. Int Ophthalmol Clin. 1996;36(3):17–28.
Parlato CJ, Cohen EJ, Sakauye CM, Dreizen NG, Galentine PG, Laibson PR. Role of débridement and trifluridine (trifluorothymidine) in herpes simplex dendritic keratitis. Arch Ophthalmol. 1985;103(5):673–5.
Herbort CP, Buechi ER, Matter M. Blunt spatula debridement and trifluorothymidine in epithelial herpetic keratitis. Curr Eye Res. 1987;6(1):225–9.
Wilhelmus KR, Coster DJ, Jones BR. Acyclovir and debridement in the treatment of ulcerative herpetic keratitis. Am J Ophthalmol. 1981;91(3):323–7.
Cheng AMS, Tseng SCG. Self-retained amniotic membrane combined with antiviral therapy for herpetic epithelial keratitis. Cornea. 2017;36(11):1383–6.
Lisco A, Vanpouille C, Tchesnokov EP, Grivel JC, Biancotto A, Brichacek B, Elliott J, Fromentin E, Shattock R, Anton P, Gorelick R, Balzarini J, McGuigan C, Derudas M, Götte M, Schinazi RF, Margolis L. Acyclovir is activated into a HIV-1 reverse transcriptase inhibitor in herpesvirus-infected human tissues. Cell Host Microbe. 2008;4(3):260–70.
Tyring SK, Baker D, Snowden W. Valacyclovir for herpes simplex virus infection: long-term safety and sustained efficacy after 20 years’ experience with acyclovir. J Infect Dis. 2002;186(Suppl-1):S40–6.
Hung SO, Patterson A, Rees PJ. Pharmacokinetics of oral acyclovir (Zovirax) in the eye. Br J Ophthalmol. 1984;68(3):192–5.
Dias C, Nashed Y, Atluri H, Mitra A. Ocular penetration of acyclovir and its peptide prodrugs valacyclovir and val-valacyclovir following systemic administration in rabbits: an evaluation using ocular microdialysis and LC-MS. Curr Eye Res. 2002;25(4):243–52.
Perry CM, Faulds D. Valaciclovir. A review of its antiviral activity, pharmacokinetic properties and therapeutic efficacy in herpesvirus infections. Drugs. 1996;52(5):754–72.
Koseoglu ND, Strauss BR, Hamrah P. Successful management of herpes simplex keratitis with oral valganciclovir in patients unresponsive or allergic to conventional antiviral therapy. Cornea. 2019;38(6):663–7.
Carter SB, Cohen EJ. Development of herpes simplex virus infectious epithelial keratitis during oral acyclovir therapy and response to topical antivirals. Cornea. 2016;35(5):692–5.
Colin J, Chastel C, Kaufman HE, Kissling GE. Combination therapy for dendritic keratitis with acyclovir and vidarabine. J Ocul Pharmacol. 1987;3(1):39–42.
Naito T, Shiota H, Mimura Y. Side effects in the treatment of herpetic keratitis. Curr Eye Res. 1987;6(1):237–9.
Herpetic Eye Disease Study Group. A controlled trial of oral acyclovir for the prevention of stromal keratitis or iritis in patients with herpes simplex virus epithelial keratitis. The Epithelial Keratitis Trial. Arch Ophthalmol. 1997;115(6):703–12.
Cunningham ET Jr. Diagnosing and treating herpetic anterior uveitis. Ophthalmology. 2000;107(12):2129–30.
Wilhelmus KR. Therapeutic interventions for herpes simplex virus epithelial keratitis. Cochrane Database Syst Rev. 2008;1:CD002898.
Kaufman HE, Haw WH. Ganciclovir ophthalmic gel 0.15%: safety and efficacy of a new treatment for herpes simplex keratitis. Curr Eye Res. 2012;37(7):654–60.
Wilhelmus KR, Falcon MG, Jones BR. Herpetic iridocyclitis. Int Ophthalmol. 1982;4(3):143–50.
Pavan-Langston D, Nelson DJ. Intraocular penetration of trifluridine. Am J Ophthalmol. 1979;87(6):814–8.
Poirier RH, Kingham JD, de Miranda P, Annel M. Intraocular antiviral penetration. Arch Ophthalmol. 1982;100(12):1964–7.
Sauerbrei A, Deinhardt S, Zell R, Wutzler P. Testing of herpes simplex virus for resistance to antiviral drugs. Virulence. 2010;1(6):555–7.
Kudo E, Shiota H, Naito T, Satake K, Itakura M. Polymorphisms of thymidine kinase gene in herpes simplex virus type 1: analysis of clinical isolates from herpetic keratitis patients and laboratory strains. J Med Virol. 1998;56(2):151–8.
Frobert E, Cortay JC, Ooka T, Najioullah F, Thouvenot D, Lina B, Morfin F. Genotypic detection of acyclovir-resistant HSV-1: characterization of 67 ACV-sensitive and 14 ACV-resistant viruses. Antivir Res. 2008;79(1):28–36.
Duan R, de Vries RD, Osterhaus AD, Remeijer L, Verjans GM. Acyclovir-resistant corneal HSV-1 isolates from patients with herpetic keratitis. J Infect Dis. 2008;198(5):659–63.
Morfin F, Thouvenot D. Herpes simplex virus resistance to antiviral drugs. J Clin Virol. 2003;26(1):29–37.
Castelo-Soccio L, Bernardin R, Stern J, Goldstein SA, Kovarik C. Successful treatment of acyclovir-resistant herpes simplex virus with intralesional cidofovir. Arch Dermatol. 2010;146(2):124–6.
Kessler HA, Hurwitz S, Farthing C, Benson CA, Feinberg J, Kuritzkes DR, Bailey TC, Safrin S, Steigbigel RT, Cheeseman SH, McKinley GF, Wettlaufer B, Owens S, Nevin T, Korvick JA. Pilot study of topical trifluridine for the treatment of acyclovir-resistant mucocutaneous herpes simplex disease in patients with AIDS (ACTG 172). AIDS Clinical Trials Group. J Acquir Immune Defic Syndr Hum Retrovirol. 1996;12(2):147–52.
Khalili MR, Jahadi HR, Karimi M, Yasemi M. Corneal collagen cross-linking for treatment of bacterial and herpetic keratitis. J Clin Diagn Res. 2017;11(7):NC12–6.
Tang Q, Chen W, Hendricks RL. Proinflammatory functions of IL-2 in herpes simplex virus corneal infection. J Immunol. 1997;158(3):1275–83.
Rao P, Suvas S. Development of inflammatory hypoxia and prevalence of glycolytic metabolism in progressing herpes stromal keratitis lesions. J Immunol. 2019;202(2):514–26.
Kalezic T, Mazen M, Kuklinski E, Asbell P. Herpetic eye disease study: lessons learned. Curr Opin Ophthalmol. 2018;29(4):340–6.
Peyman A, Nayebzadeh M, Peyman M, Afshari NA, Pourazizi M. Topical cyclosporine-A versus prednisolone for herpetic stromal keratitis: a randomized controlled trial. Acta Ophthalmol. 2019;97(2):e194–8.
Gündüz K, Ozdemir O. Topical cyclosporin as an adjunct to topical acyclovir treatment in herpetic stromal keratitis. Ophthalmic Res. 1997;29(6):405–8.
Heiligenhaus A, Steuhl KP. Treatment of HSV-1 stromal keratitis with topical cyclosporin A: a pilot study. Graefes Arch Clin Exp Ophthalmol. 1999;237(5):435–8.
Rao SN. Treatment of herpes simplex virus stromal keratitis unresponsive to topical prednisolone 1% with topical cyclosporine 0.05%. Am J Ophthalmol. 2006;141(4):771–2.
Shi W, Chen M, Xie L. Amniotic membrane transplantation combined with antiviral and steroid therapy for herpes necrotizing stromal keratitis. Ophthalmology. 2007;114(8):1476–81.
Knickelbein JE, Hendricks RL, Charukamnoetkanok P. Management of herpes simplex virus stromal keratitis: an evidence-based review. Surv Ophthalmol. 2009;54(2):226–34.
Gaynor BD, Margolis TP, Cunningham ET Jr. Advances in diagnosis and management of herpetic uveitis. Int Ophthalmol Clin. 2000;40(2):85–109.
Liesegang TJ. Classification of herpes simplex virus keratitis and anterior uveitis. Cornea. 1999;18(2):127–43.
Herpetic Eye Disease Study Group. A controlled trial of oral acyclovir for iridocyclitis caused by herpes simplex virus. Arch Ophthalmol. 1996;114(9):1065–72.
Sudesh S, Laibson PR. The impact of the herpetic eye disease studies on the management of herpes simplex virus ocular infections. Curr Opin Ophthalmol. 1999;10(4):230–3.
Koizumi N, Nishida K, Adachi W, Tei M, Honma Y, Dota A, Sotozono C, Yokoi N, Yamamoto S, Kinoshita S. Detection of herpes simplex virus DNA in atypical epithelial keratitis using polymerase chain reaction. Br J Ophthalmol. 1999;83(8):957–60.
Rübben A, Baron JM, Grussendorf-Conen EI. Routine detection of herpes simplex virus and varicella zoster virus by polymerase chain reaction reveals that initial herpes zoster is frequently misdiagnosed as herpes simplex. Br J Dermatol. 1997;137(2):259–61.
Azher TN, Yin XT, Tajfirouz D, Huang AJ, Stuart PM. Herpes simplex keratitis: challenges in diagnosis and clinical management. Clin Ophthalmol. 2017;11:185–91.
Burrows J, Nitsche A, Bayly B, Walker E, Higgins G, Kok T. Detection and subtyping of Herpes simplex virus in clinical samples by LightCycler PCR, enzyme immunoassay and cell culture. BMC Microbiol. 2002;2:12.
Espy MJ, Ross TK, Teo R, Svien KA, Wold AD, Uhl JR, Smith TF. Evaluation of LightCycler PCR for implementation of laboratory diagnosis of herpes simplex virus infections. J Clin Microbiol. 2000;38(8):3116–8.
El-Aal AM, El Sayed M, Mohammed E, Ahmed M, Fathy M. Evaluation of herpes simplex detection in corneal scrapings by three molecular methods. Curr Microbiol. 2006;52(5):379–82.
Shoji J, Sakimoto T, Inada N, Kamei Y, Matsubara M, Takamura E, Sawa M. A diagnostic method for herpes simplex keratitis by simultaneous measurement of viral DNA and virus-specific secretory IgA in tears: an evaluation. Jpn J Ophthalmol. 2016;60(4):294–301.
Shin J, Ra H, Rho CR. Herpes simplex virus linear endotheliitis in a post-keratoplasty patient: a case report. Medicine (Baltimore). 2019;98(3):e14191.
Basak SK, Basak S. Recurrence of herpes simplex virus endotheliitis in a Descemet membrane endothelial keratoplasty graft: mimicking fungal interface infection. BMJ Case Rep. 2019;12(5):e229441.
Kowalski RP, Gordon YJ, Romanowski EG, Araullo-Cruz T, Kinchington PR. A comparison of enzyme immunoassay and polymerase chain reaction with the clinical examination for diagnosing ocular herpetic disease. Ophthalmology. 1993;100(4):530–3.
Wald A, Corey L, Cone R, Hobson A, Davis G, Zeh J. Frequent genital herpes simplex virus 2 shedding in immunocompetent women. Effect of acyclovir treatment. J Clin Invest. 1997;99(5):1092–7.
Goldschmidt P, Rostane H, Saint-Jean C, Batellier L, Alouch C, Zito E, Bourcier T, Laroche L, Chaumeil C. Effects of topical anaesthetics and fluorescein on the real-time PCR used for the diagnosis of Herpesviruses and Acanthamoeba keratitis. Br J Ophthalmol. 2006;90(11):1354–6.
Seitzman GD, Cevallos V, Margolis TP. Rose bengal and lissamine green inhibit detection of herpes simplex virus by PCR. Am J Ophthalmol. 2006;141(4):756–8.
Rosenberg ME, Tervo TM, Müller LJ, Moilanen JA, Vesaluoma MH. In vivo confocal microscopy after herpes keratitis. Cornea. 2002;21(3):265–9.
Wang YE, Tepelus TC, Vickers LA, Baghdasaryan E, Gui W, Huang P, Irvine JA, Sadda S, Hsu HY, Lee OL. Role of in vivo confocal microscopy in the diagnosis of infectious keratitis. Int Ophthalmol. 2019;39(12):2865–74.
Qiu J, Huang F, Wang Z, Xu J, Zhang C. The evaluation of diagnostic efficiency for stromal herpes simplex keratitis by the combination of tear HSV-sIgA and HSV-DNA. Graefes Arch Clin Exp Ophthalmol. 2017;255(7):1409–15.
Satpathy G, Mishra AK, Tandon R, Sharma MK, Sharma A, Nayak N, Titiyal JS, Sharma N. Evaluation of tear samples for Herpes Simplex Virus 1 (HSV) detection in suspected cases of viral keratitis using PCR assay and conventional laboratory diagnostic tools. Br J Ophthalmol. 2011;95(3):415–8.
Doan T, Pinsky BA. Current and future molecular diagnostics for ocular infectious diseases. Curr Opin Ophthalmol. 2016;27(6):561–7.
Vadoothker S, Andrews L, Jeng BH, Levin MR. Management of herpes simplex virus keratitis in the pediatric population. Pediatr Infect Dis J. 2018;37(9):949–51.
Matos RJC, Pires JMS, Cortesão D. Management of neonatal herpes simplex infection: a rare case of blepharoconjunctivitis and concurrent epithelial and stromal keratitis. Ocul Immunol Inflamm. 2018;26(4):625–7.
Chong EM, Wilhelmus KR, Matoba AY, Jones DB, Coats DK, Paysse EA. Herpes simplex virus keratitis in children. Am J Ophthalmol. 2004;138(3):474–5.
Beigi B, Algawi K, Foley-Nolan A, O’Keefe M. Herpes simplex keratitis in children. Br J Ophthalmol. 1994;78(6):458–60.
Liu S, Pavan-Langston D, Colby KA. Pediatric herpes simplex of the anterior segment: characteristics, treatment, and outcomes. Ophthalmology. 2012;119(10):2003–8.
Hsiao CH, Yeung L, Yeh LK, Kao LY, Tan HY, Wang NK, Lin KK, Ma DH. Pediatric herpes simplex virus keratitis. Cornea. 2009;28(3):249–53.
Souza PM, Holland EJ, Huang AJ. Bilateral herpetic keratoconjunctivitis. Ophthalmology. 2003;110(3):493–6.
Wilhelmus KR, Falcon MG, Jones BR. Bilateral herpetic keratitis. Br J Ophthalmol. 1981;65(6):385–7.
Uchio E, Hatano H, Mitsui K, Sugita M, Okada K, Goto K, Kagiya M, Enomoto Y, Ohno S. A retrospective study of herpes simplex keratitis over the last 30 years. Jpn J Ophthalmol. 1994;38(2):196–201.
Poirier RH. Herpetic ocular infections of childhood. Arch Ophthalmol. 1980;98(4):704–6.
Schwartz GS, Holland EJ. Oral acyclovir for the management of herpes simplex virus keratitis in children. Ophthalmology. 2000;107(2):278–82.
Prabriputaloong T, Margolis TP, Lietman TM, Wong IG, Mather R, Gritz DC. Atopic disease and herpes simplex eye disease: a population-based case-control study. Am J Ophthalmol. 2006;142(5):745–9.
Easty D, Entwistle C, Funk A, Witcher J. Herpes simplex keratitis and keratoconus in the atopic patient. A clinical and immunological study. Trans Ophthalmol Soc UK. 1975;95(2):267–76.
Garrity JA, Liesegang TJ. Ocular complications of atopic dermatitis. Can J Ophthalmol. 1984;19(1):21–4.
Rezende RA, Hammersmith K, Bisol T, Lima AL, Webster GF, Freitas JF, Rapuano CJ, Laibson PR, Cohen EJ. Comparative study of ocular herpes simplex virus in patients with and without self-reported atopy. Am J Ophthalmol. 2006;141(6):1120–5.
Tsatsos M, MacGregor C, Athanasiadis I, Moschos MM, Hossain P, Anderson D. Herpes simplex virus keratitis: an update of the pathogenesis and current treatment with oral and topical antiviral agents. Clin Exp Ophthalmol. 2016;44(9):824–37.
Herpetic Eye Disease Study Group. Acyclovir for the prevention of recurrent herpes simplex virus eye disease. N Engl J Med. 1998;339(5):300–6.
Shuster JJ, Kaufman HE, Nesburn AB. Statistical analysis of the rate of recurrence of herpesvirus ocular epithelial disease. Am J Ophthalmol. 1981;91(3):328–31.
Sawtell NM. Comprehensive quantification of herpes simplex virus latency at the single-cell level. J Virol. 1997;71(7):5423–31.
Derham AM, Chen E, Bunya VY, OʼMalley RE. Bilateral herpetic keratitis after bilateral intravitreal bevacizumab for exudative macular degeneration. Cornea. 2017;36(7):878–9.
Cho YK, Kwon JW, Konda S, Ambati BK. Epithelial keratitis after cataract surgery. Cornea. 2018;37(6):755–9.
Qi X, Wang M, Li X, Jia Y, Li S, Shi W, Gao H. Characteristics of new onset herpes simplex keratitis after keratoplasty. J Ophthalmol. 2018;2018:4351460.
Kamburoglu G, Ertan A. Peripheral herpes simplex keratitis following LASIK. J Refract Surg. 2007;23(8):742–3.
Jain V, Pineda R. Reactivated herpetic keratitis following laser in situ keratomileusis. J Cataract Refract Surg. 2009;35(5):946–8.
Hou YC, Chen CC, Wang IJ, Hu FR. Recurrent herpetic keratouveitis following YAG laser peripheral iridotomy. Cornea. 2004;23(6):641–2.
Reed SY, Shin DH, Birt CM, Rhee RK. Herpes simplex keratitis following argon laser trabeculoplasty. Ophthalmic Surg. 1994;25(9):640.
Vrabec MP, Durrie DS, Chase DS. Recurrence of herpes simplex after excimer laser keratectomy. Am J Ophthalmol. 1992;114(1):96–7.
Wulff K, Fechner PU. Herpes simplex keratitis after photorefractive keratectomy. J Refract Surg. 1997;13(7):613.
Wand M, Gilbert CM, Liesegang TJ. Latanoprost and herpes simplex keratitis. Am J Ophthalmol. 1999;127(5):602–4.
Ekatomatis P. Herpes simplex dendritic keratitis after treatment with latanoprost for primary open angle glaucoma. Br J Ophthalmol. 2001;85(8):1008–9.
Takeshita T. Bilateral herpes simplex virus keratitis in a patient with pemphigus vulgaris. Clin Exp Dermatol. 1996;21(4):291–2.
Gulkilik G, Demirci G, Ozdamar AM, Muftuoglu GI. A case of herpetic keratitis after intravitreal triamcinolone injection. Cornea. 2007;26(8):1000–1.
el-Antably SA, Atia HE. Ocular complications of corticosteroids. Bull Ophthalmol Soc Egypt. 1976;69(73):635–41.
Warren SL, Carpenter CM, Boak RA. Symptomatic Herpes, a sequela of artificially induced fever: incidence and C aspects; recovery of A virus from Herpetic vesicles, and comparison with a K strain of Herpes virus. J Exp Med. 1940;71(2):155–68.
Guinan ME, MacCalman J, Kern ER, Overall JC Jr, Spruance SL. The course of untreated recurrent genital herpes simplex infection in 27 women. N Engl J Med. 1981;304(13):759–63.
Dalkvist J, Wahlin TB, Bartsch E, Forsbeck M. Herpes simplex and mood: a prospective study. Psychosom Med. 1995;57(2):127–37.
Friedman E, Katcher AH, Brightman VJ. Incidence of recurrent herpes labialis and upper respiratory infection: a prospective study of the influence of biologic, social and psychologic predictors. Oral Surg Oral Med Oral Pathol. 1977;43(6):873–8.
Herpetic Eye Disease Study Group. Oral acyclovir for herpes simplex virus eye disease: effect on prevention of epithelial keratitis and stromal keratitis. Arch Ophthalmol. 2000;118(8):1030–6.
Miserocchi E, Modorati G, Galli L, Rama P. Efficacy of valacyclovir vs acyclovir for the prevention of recurrent herpes simplex virus eye disease: a pilot study. Am J Ophthalmol. 2007;144(4):547–51.
Ghosh S, Jhanji V, Lamoureux E, Taylor HR, Vajpayee RB. Acyclovir therapy in prevention of recurrent herpetic keratitis following penetrating keratoplasty. Am J Ophthalmol. 2008;145(2):198–202.
Papanicolaou GA, Meyers BR, Fuchs WS, Guillory SL, Mendelson MH, Sheiner P, Emre S, Miller C. Infectious ocular complications in orthotopic liver transplant patients. Clin Infect Dis. 1997;24(6):1172–7.
Korsager B, Spencer ES, Mordhorst CH, Andersen HK. Herpesvirus hominis infections in renal transplant recipients. Scand J Infect Dis. 1975;7(1):11–9.
Howcroft MJ, Breslin CW. Herpes simplex keratitis in renal transplant recipients. Can Med Assoc J. 1981;124(3):292–4.
Geerlings SE, Hoepelman AI. Immune dysfunction in patients with diabetes mellitus (DM). FEMS Immunol Med Microbiol. 1999;26(3-4):259–65.
Eliashiv A, Olumide F, Norton L, Eiseman B. Depression of cell-mediated immunity in diabetes. Arch Surg. 1978;113(10):1180–3.
Kaiserman I, Kaiserman N, Nakar S, Vinker S. Herpetic eye disease in diabetic patients. Ophthalmology. 2005;112(12):2184–8.
Hodge WG, Margolis TP. Herpes simplex virus keratitis among patients who are positive or negative for human immunodeficiency virus: an epidemiologic study. Ophthalmology. 1997;104(1):120–4.
Simon AL, Pavan-Langston D. Long-term oral acyclovir therapy. Effect on recurrent infectious herpes simplex keratitis in patients with and without grafts. Ophthalmology. 1996;103(9):1399–405.
Danileviciene V, Zemaitiene R, Gintauskiene VM, Nedzelskiene I, Zaliuniene D. Corneal sub-basal nerve changes in patients with herpetic keratitis during acute phase and after 6 months. Medicina (Kaunas). 2019;55(5):E214.
Oliveira-Soto L, Efron N. Morphology of corneal nerves using confocal microscopy. Cornea. 2001;20(4):374–84.
Guthoff RF, Wienss H, Hahnel C, Wree A. Epithelial innervation of human cornea: a three-dimensional study using confocal laser scanning fluorescence microscopy. Cornea. 2005;24(5):608–13.
Tavakoli M, Ferdousi M, Petropoulos IN, Morris J, Pritchard N, Zhivov A, Ziegler D, Pacaud D, Romanchuk K, Perkins BA, Lovblom LE, Bril V, Singleton JR, Smith G, Boulton AJ, Efron N, Malik RA. Normative values for corneal nerve morphology assessed using corneal confocal microscopy: a multinational normative data set. Diabetes Care. 2015;38(5):838–43.
Gallar J, Tervo TM, Neira W, Holopainen JM, Lamberg ME, Miñana F, Acosta MC, Belmonte C. Selective changes in human corneal sensation associated with herpes simplex virus keratitis. Invest Ophthalmol Vis Sci. 2010;51(9):4516–22.
Bonini S, Rama P, Olzi D, Lambiase A. Neurotrophic keratitis. Eye. 2003;17(8):989–95.
Semeraro F, Forbice E, Romano V, Angi M, Romano MR, Filippelli ME, Di Iorio R, Costagliola C. Neurotrophic keratitis. Ophthalmologica. 2014;231(4):191–7.
Tuli S, Gray M, Shah A. Surgical management of herpetic keratitis. Curr Opin Ophthalmol. 2018;29(4):347–54.
Latvala T, Linna T, Tervo T. Corneal nerve recovery after photorefractive keratectomy and laser in situ keratomileusis. Int Ophthalmol Clin. 1996;36(4):21–7.
Linna TU, Pérez-Santonja JJ, Tervo KM, Sakla HF, Alió y Sanz JL, Tervo TM. Recovery of corneal nerve morphology following laser in situ keratomileusis. Exp Eye Res. 1998;66(6):755–63.
Moein HR, Kheirkhah A, Muller RT, Cruzat AC, Pavan-Langston D, Hamrah P. Corneal nerve regeneration after herpes simplex keratitis: a longitudinal in vivo confocal microscopy study. Ocul Surf. 2018;16(2):218–25.
Zemaitiene R, Rakauskiene M, Danileviciene V, Use V, Kriauciuniene L, Zaliuniene D. Corneal esthesiometry and sub-basal nerves morphological changes in herpes simplex virus keratitis/uveitis patients. Int J Ophthalmol. 2019;12(3):407–11.
Hamrah P, Cruzat A, Dastjerdi MH, Zheng L, Shahatit BM, Bayhan HA, Dana R, Pavan-Langston D. Corneal sensation and subbasal nerve alterations in patients with herpes simplex keratitis: an in vivo confocal microscopy study. Ophthalmology. 2010;117(10):1930–6.
He J, Bazan NG, Bazan HE. Mapping the entire human corneal nerve architecture. Exp Eye Res. 2010;91(4):513–23.
Nagasato D, Araki-Sasaki K, Kojima T, Ideta R, Dogru M. Morphological changes of corneal subepithelial nerve plexus in different types of herpetic keratitis. Jpn J Ophthalmol. 2011;55(5):444–50.
Bonini S, Lambiase A2, Rama P3, Sinigaglia F4, Allegretti M4, Chao W4, Mantelli F4, REPARO Study Group. Phase II randomized, double-masked, vehicle-controlled trial of recombinant human nerve growth factor for neurotrophic keratitis. Ophthalmology. 2018;125(9):1332–43.
Shorter E, Harthan J, Nau CB, Nau A, Barr JT, Hodge DO, Schornack MM. Scleral lenses in the management of corneal irregularity and ocular surface disease. Eye Contact Lens. 2018;44(6):372–8.
Catapano J, Fung SSM, Halliday W, Jobst C, Cheyne D, Ho ES, Zuker RM, Borschel GH, Ali A. Treatment of neurotrophic keratopathy with minimally invasive corneal neurotisation: long-term clinical outcomes and evidence of corneal reinnervation. Br J Ophthalmol. 2019;103(12):1724–31.
Giannaccare G, Bolognesi F, Biglioli F, Marchetti C, Mariani S, Weiss JS, Allevi F, Cazzola FE, Ponzin D, Lozza A, Bovone C, Scorcia V, Busin M, Campos EC. In vivo and ex vivo comprehensive evaluation of corneal reinnervation in eyes neurotized with contralateral supratrochlear and supraorbital nerves. Cornea. 2020;39(2):210–4.
Ficker LA, Kirkness CM, Rice NS, Steele AD. The changing management and improved prognosis for corneal grafting in herpes simplex keratitis. Ophthalmology. 1989;96(11):1587–96.
Cockerham GC, Krafft AE, McLean IW. Herpes simplex virus in primary graft failure. Arch Ophthalmol. 1997;115(5):586–9.
Epstein RJ, Seedor JA, Dreizen NG, Stulting RD, Waring GO 3rd, Wilson LA, Cavanagh HD. Penetrating keratoplasty for herpes simplex keratitis and keratoconus. Allograft rejection and survival. Ophthalmology. 1987;94(8):935–44.
Cleator GM, Klapper PE, Dennett C, Sullivan AL, Bonshek RE, Marcyniuk B, Tullo AB. Corneal donor infection by herpes simplex virus: herpes simplex virus DNA in donor corneas. Cornea. 1994;13(4):294–304.
Akova YA, Onat M, Duman S. Efficacy of low-dose and long-term oral acyclovir therapy after penetrating keratoplasty for herpes simplex heratitis. Ocul Immunol Inflamm. 1999;7(1):51–60.
Barney NP, Foster CS. A prospective randomized trial of oral acyclovir after penetrating keratoplasty for herpes simplex keratitis. Cornea. 1994;13(3):232–6.
van Rooij J, Rijneveld WJ, Remeijer LJ, Beekhuis WH. A retrospective study on the effectiveness of oral acyclovir to prevent herpes simplex recurrence in corneal grafts. Eur J Ophthalmol. 1995;5(4):214–8.
Mayer K, Reinhard T, Reis A, Voiculescu A, Sundmacher R. Synergistic antiherpetic effect of acyclovir and mycophenolate mofetil following keratoplasty in patients with herpetic eye disease: first results of a randomised pilot study. Graefes Arch Clin Exp Ophthalmol. 2003;241(12):1051–4.
van Rooij J, Rijneveld WJ, Remeijer L, Völker-Dieben HJ, Eggink CA, Geerards AJ, Mulder PG, Doornenbal P, Beekhuis WH. Effect of oral acyclovir after penetrating keratoplasty for herpetic keratitis: a placebo-controlled multicenter trial. Ophthalmology. 2003;110(10):1916–9.
Dong LL, Tang R, Zhai YJ, Malla T, Hu K. DNA vaccine expressing herpes simplex virus 1 glycoprotein C and D protects mice against herpes simplex keratitis. Int J Ophthalmol. 2017;10(11):1633–9.
Tang R, Zhai Y, Dong L, Malla T, Hu K. Immunization with dendritic cell-based DNA vaccine pRSC-NLDC145.gD-IL21 protects mice against herpes simplex virus keratitis. Immunotherapy. 2018;10(3):189–200.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2021 Springer Nature Singapore Pte Ltd.
About this chapter
Cite this chapter
Syed, Z.A., Meghpara, B.B., Rapuano, C.J. (2021). Herpes Simplex Virus (HSV) Keratitis. In: Das, S., Jhanji, V. (eds) Infections of the Cornea and Conjunctiva. Springer, Singapore. https://doi.org/10.1007/978-981-15-8811-2_13
Download citation
DOI: https://doi.org/10.1007/978-981-15-8811-2_13
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-15-8810-5
Online ISBN: 978-981-15-8811-2
eBook Packages: MedicineMedicine (R0)