Abstract
Chemically, flavonoids are phenolic compounds. These are natural substances that have several pharmacological properties like anti-oxidative, anti-inflammatory, antimutagenic, and anti-carcinogenic and modulate key cellular enzyme function. Potent enzyme inhibitors for several enzymes like xanthine oxidase (XO), cyclooxygenase (COX), lipoxygenase, and phosphoinositide 3-kinase. Honey is a natural source of flavonoids. Non-alcoholic fatty liver disease (NAFLD) includes several diseases, ranging from steatosis to non-alcoholic steatohepatitis (NASH), fibrosis, cirrhosis, and hepatocellular carcinoma. Flavonoids have been used for the treatment of NAFLD. Groups of pathways involved in the pathogenesis of NAFLD support the treatment. Flavonoids have positive effects on insulin resistance, lipid metabolism, inflammation, and oxidative stress that are prominent pathophysiological pathways in NAFLD.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
17.1 Introduction
Honey is a natural substance which is used as a source of energy and has a lot of therapeutic effects. More than 200 substances are present in the honey that formed from the nectar of flowers by honeybees especially from the species Apis mellifera (Family: Apidae). Formation of honey is a stepwise process, in the whole process honey bees play a key role in transforming flower nectar into honey by the process of regurgitation, evaporation, and enzymatic alteration of saccharides present in nectar. The solute portion is mainly fructose (38–55%) and glucose (about 31%) monosaccharide’s solvated by the solvent is higher as compared to normal, so the solution is called supersaturated solution and sweetness is because of these solutes. Honey had been used by prehistoric populations, including the Greeks, Chinese, Egyptians, Romans, Mayans, and Babylonians, as a nutritional source and for its therapeutic effects. The main substance is fructose and glucose and also contains fructooligosaccharides (Chow et al. 2002), and apart from these it also contains many amino acids, vitamins, minerals, and enzymes (White 1979). This natural substance is very popular, insect-derived with several nutritional, cosmetic, therapeutic, and industrial values. As food, it is a balanced diet for both genders of all ages, not required to refrigerate, not at all ruin, stored at room temperature in a dry place and must be unopened (Samarghandian et al. 2017; Bansal et al. 2005). The standard physical properties which maintain the natural identity of honey are water activity of 0.56–0.62 and a pH value of 3.9 (Hassapidou et al. 2006; Babacan and Rand 2007). The high content of fructose makes it a natural sweetener, used from very ancient period of time (Babacan and Rand 2007), and is used as a sweating agent in beverage industries (Pataca et al. 2007). Globally, scientific advertisements given, in general magazines, journals, and natural products’ leaflets explain the therapeutic values as well its nutritional importance, convincing the human being to use it regularly (Inglett 1976). Composition variation of honey totally depends upon plants from where bees are feed. Apart from carbohydrates, the other natural substances like flavonoids, secondary metabolites of plants, frequently bound to sugars (glycosides) also available as aglycones. The structures of flavonoids have two aromatic rings and one heterocyclic ring. Based on the heterocyclic ring, flavonoids are divided into subclasses flavones, isoflavones, flavanols, flavanones, anthocyanidins, proanthocyanidin, and chalcones. Substances like phenolic acids, ascorbic acid, tocopherols, catalase (CAT), superoxide dismutase (SOD), reduced glutathione (GSH), Millard reaction products and peptides are present in honey that work against unwanted oxidation in the body, these have a synergistic antioxidant effect. All compounds of honey work against unwanted oxidation in the body, and have a synergistic antioxidant effect (Alvarez-Suarez et al. 2010; Johnston et al. 2005; Turkmen et al. 2006; Rakha et al. 2008; Al-Mamary et al. 2002). Flavonoids are a group of nutraceuticals, and about 5000 different flavonoid compounds are available in the seven classes.
All over the world, particularly in western countries, nonalcoholic fatty liver disease (NAFLD) is a common disease with ubiquity ranging from 6% to 35%, with a median of 20% (Vernon et al. 2011). Basically, this disease indicates a metabolic syndrome and includes a spectrum of diseases such as steatosis, nonalcoholic steatohepatitis, liver fibrosis, cirrhosis, and hepatocellular carcinoma. Patients of fatty liver have about 10–20% inflammation and fibrosis, possibly because a failure of antilipotoxic protection; nevertheless, inflammation may precede steatosis. The risk factor of NAFLD is obesity, hyperglycemia, insulin resistance, and hypertriglyceridemia. The most effective cause of morbidity and mortality is the development of the symptoms of diabetes and metabolic syndrome (Tilg et al. 2010; Farrell et al. 2006).
NAFLD is developed from multiple factors, the pathophysiological model does not follow a strict sequence (Fig. 17.1). In this model, metabolic disorders, oxidative stress, and inflammation (local and systemic) are major causes involved in the progression of NAFLD also, involved in insulin resistance and genetic susceptibility with histological characteristics of alcoholic liver (Ullah et al. 2019). This disease has been identified in four stages: (a) hepatic fat deposition (hepatic steatosis), (b) hepatic fat deposition with inflammation, (c) fibrosis, and (d) cirrhosis. The first and second stages are also known as nonalcoholic fatty liver (NAFL) and nonalcoholic steatohepatitis (NASH), respectively. In NASH condition over hepatic fat deposition and inflammation occur. Tenacious inflammation (hepatitis) liver cause’s scar tissue formation in the liver is called fibrosis. The acute form of NAFLD is cirrhosis, which is the fourth stage. Fibrosis leads to the cirrhosis where maximum liver cells’ structure and function are compromised. Finally the condition is failure of liver. In fibrosis condition, hepatocytes function properly, but in cirrhosis they fail to function. Generally, in adult population the NAFLD percentage is 5–20%, but this increase above 40% have patients of obesity and type 2 diabetic (Ullah et al. 2019; Clark 2006; Lazo et al. 2008). Because of the several factors accumulation of lipids in the liver. As the lipolysis increase, fatty acid uptake enhanced from the adipocytes or if the diets have high fat containing such phenomenon takes place. In liver deposition of triglyceride takes place if de novo lipogenesis increases, oxidation of fatty acid lessens and secretion of very-low-density lipoproteins occurs (VLDL). Accumulation of triglyceride droplets inside the hepatocytes may be protective, otherwise their conversion to nontriglyceride lipotoxic metabolites will be the cause of liver injury. The enzyme involved is in different steps of hepatic de novo lipogenesis is fatty acid synthase (FAS), and its functions are controlled by a complex network of nuclear receptors (Koo 2013; Neuschwander-Tetri 2010).
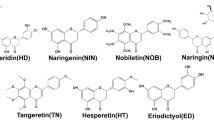
For treatment of the NAFLD examining the important pathways which materialize the necessary in the pathogenesis of the disease. It directed the patients to reduce the body mass index and improve insulin resistance by adopting physical exercise, dietary modification, bariatric surgery, and pharmacological treatments. Many times, NAFLD pathogenesis of conventional “two-hit” hypothesis is updated. In general, maximum patients have accumulation of lipid or steatosis which is because of obesity and insulin resistance. Development of steatohepatitis and fibrosis occurs because of several factors such as FFAs, inflammatory cytokines and adipokines, oxidative stress, and mitochondrial dysfunction in a complex interplay with a genetic predisposition. Plan for the treatment of NASH concentrates on enhancing the components of the metabolic syndrome like obesity and insulin resistance, and still no liver-specific agents are available. But the fine tune of any kind of mechanism is tangled in NASH pathogenesis which gives useful targets to prevent the development of fibrosis and its associated complications. The understandings of pathogenesis of NASH are helpful for the development of novel therapeutic strategies (Dowman et al. 2010). If the treatment of NASH not be done on time, it further grows to liver fibrosis, cirrhosis, and ultimately it is converted to hepatocellular carcinoma. At present, no any proper therapeutic approaches for the treatment of NAFLD and NASH has been found, but honey flavonoids are recommended as home remedy for the treatment to cure the disease. The other strategy in controlling and monitoring of NSFLD relies on diet, physical activity, and lifestyle modifications and metabolic disorder associated with NAFLD including hyperglycemia, insulin resistance, and hyperlipidemia. A regular use of honey flavonoids studied in patients of NAFLD found beneficial effects. Apart from the honey, foods that have flavonoids have several advantages for health. Basic role of flavonoids against the pathological condition are to their antioxidant activity. Sometimes these compounds directly interfere to block the signaling pathways which develop the disease process. All different subgroups of flavonoids have hepatoprotective effects (Akhlaghi 2016). Not only antioxidant but also other pharmacological effects such as anti-inflammatory and metabolic effects have been revealed, and this compound is considered as a bioactive compound. The different effects (Fig. 17.2) and subclasses (Table 17.1) are illustrated.
17.2 Pathophysiology of Nonalcoholic Fatty Liver Disease (NAFLD)
Nonalcoholic fatty liver disease (NAFLD) is a heterological disease, which as its name implies is deposition of fat in liver, which can be categorized into nonalcoholic fatty liver and nonalcoholic steatohepatitis (NASH), histologically. Presence of at least 5% hepatic steatosis without evidence of hepatocellular injury in the form of hepatocyte blooming is considered to be nonalcoholic fatty liver. While a least of 5% hepatic steatosis with inflammation and hepatocyte injury with or without fibrosis is considered to NASH (Ekstedt et al. 2015; Angulo et al. 2015). NASH clinical research network has given a spectrum of several histological patterns of NAFLD (Yeh and Brunt 2014) (Fig. 17.3) Out of all the patients diagnosed with NAFLD, only 5–10% patients will progress to NASH which makes it easier to transform into fibrosis, cirrhosis, and consequently hepatocellular cancer (Buzzetti et al. 2016).
Hepatic steatosis or fatty change is a major pathological marker for NAFLD. It is basically accumulation of fat droplets in the hepatocytes (Cairns and Peters 1983). Fat mainly includes free fatty acids and triglycerides which makes it a hallmark feature for NAFLD (Townsend and Newsome. 2016). There are various factors that can contribute to accumulation of fats in the hepatocytes which can broadly be categorized into genetics and unhealthy lifestyles. An unhealthy lifestyle mainly includes high calories intake mainly of glucose, fructose, and saturated fat, and this can further be accompanied by sedentary lifestyles and aging (Fig. 17.4) (Stefan et al. 2019). Earlier two-hit model of NAFLD has been proposed for the progression of the disease in which first hit included insulin resistance and hyperinsulinemia which altered hepatic pathways for uptake, synthesis, degradation of fat (FFA) in the liver and leads to fat accumulation in it (Buzzetti et al. 2016). The second hit is associated with fibro genesis which is a consequence of activation of several inflammatory events (Peverill et al. 2014). But the two-hit model has lost its importance for it being too simplistic to describe the intricacy of the NAFLD as NAFLD includes a multitude of factors in its development. Currently, a “multiple hit” theory describes the progression of NAFLD. These multiple factors mainly include unhealthy and sedentary lifestyles, diet, genetic, epigenetic, environmental factors, insulin resistance, and type-2 diabetes mellitus (Ayonrinde et al. 2015; Holterman et al. 2013). Complex involvement of hepatic resident cells and activated immune cells which includes Kupffer cells, T cells, and hepatic stellate cell (HSC) with inflammatory factors leads to progression and pathogenesis of NAFLD (Chen et al. 2016; De Vito et al. 2012) (Fig. 17.5) Markers for hepatic fibrosis and cirrhosis are chronic activation of hepatic stellate cells and apoptosis. While hepatic progenitor cells (HPCs) are associated with NASH and fibrosis (Nobili et al. 2012). Some evidences suggest that Kupffer cells mediate NAFLD pathogenies including immune tolerance and lipid homeostasis, differently (Dattaroy et al. 2016; Mouralidarane et al. 2013). A study reported that Kupffer cell triggers TGs accumulation and hepatic steatosis by interleukin-1 beta (IL-1β)-mediated suppression of peroxisome proliferator-activated reactor-α (PPAR-α) actions (Stienstra et al. 2010). Another study reported the altered functionality of peripheral T cells subpopulations in NASH (FerreyraSolari et al. 2012) Pathophysiology of NAFLD is still not certain and requires further investigation.
Pathogenesis of nonalcoholic fatty liver disease. Abbreviations FFAs free fatty acids, IKK β inhibitor of κB kinase-β, NF-κB nuclear factor kappa B, Mac macrophages, TNF-α tumor necrosis factor-α, IL-6 interleukin 6, IL-1 β interleukin 1 β, TGs triglycerides, NASH nonalcoholic steatohepatitis, HSC hepatic stellate cells, KC Kupffer cells
17.3 Therapeutic Effects of Flavonoids
Honey exerts a wide range of biological activity including anti-inflammatory, immune-strengthening, antitumor, and antioxidant effects (Cheng et al. 2007). Honey exerts neuroprotective effect via its antioxidant potential. Tualang honey has known to exert neuroprotective effect which was attributed to its high flavonoids content and some enzymes such as glucose oxidase, catalase and peroxidase, and non-enzymatic antioxidants such as ascorbic acid, α-tocopherol, and carotenoids (Azman et al. 2018).
Polyphenol play a central role in exerting the protective effect of honey in the nervous system. Polyphenol is able to counteract neurotoxicity induced by the ROS, which are neurotoxic, the deposition of misfolded proteins, such as beta amyloid hence imparting neuroprotection (Cianciosi et al. 2018).
Various microorganisms such as H. influenza, P. aeruginosa, S. aureus, K. pneumonia, and S. pyogenes are known to cause respiratory infections such as influenza, pneumonia, nosocomial infection, pneumonia in debilitated individual, and pharyngitis (Reham et al. 2016) Buckwheat honey which is higher in phenolic content is known to relief nocturnal cough and quality of sleep in children and parents, and the effect was found to be superior than dextromethorphan (Paul et al. 2007).
Honey is a rich source of natural antioxidants such as flavonoids, polyphenols (like quercetin, caffeic acid, acacetin, phenethyl ester (CAPE), galangin, and kaempferol), and monophenolics which are reported to exert cardioprotective effects (Khalil et al. 2010; Samarghandian et al. 2017). Some flavonoids such as rutin present in honey are known to enhance bioavailability of nitric oxide, a potent vasodilatation by increasing eNOS gene expression whose activity promotes the production of NO. Similarly, catechin and quercetin present in honey have negative effect on aortic atherosclerotic lesion development. Oxidative stress is the key cause of various diseases and disorders such as cancer, mutagenesis, aging, atherosclerosis, and many other degenerative diseases (Ahmed et al. 2008). Antioxidants seize the free radicals before any damage caused. Honey is known to be a rich source of antioxidant. Darker the honey, more is its antioxidant value. Antioxidant property of honey which is attributed to the presence of flavonoids, polyphenolics, Vitamin C, and monophenolics may be associated with the reduced cardiac failure. Flavonoids are known to exert antioxidant, antithrombotic, anti-ischemic, and vasorelaxant effects which exert protective effect against coronary heart disease. Flavonoids present in honey reduce the chances of coronary heart disorders via improving coronary vasodilatation, inhibiting oxidation of low-density lipoprotein, and reducing the ability of platelet to form blood clot. The flavonoids which are rich in honey are caffeic acid, quercetin, phenethyl ester, kaempferol, galangin, and acacetin (Samarghandian et al. 2017).
Antioxidant property of honey is attributed to its chemical constituents such as flavonoids and phenolic acids, carotenes, organic acids, sugars, amino acids, protein, Maillard reaction products. It is rich in several phenolics (viz., ferulic acids, p-coumarin, caffeic acids, ellagic acids), flavonoids (viz., quercetin, kaempferol, apigenin, pinocembrin, hesperetin, chrysin, and galangin), vitamins C and E, superoxide dismutase, and catalase. These antioxidants coordinate positively with each other to exert antioxidant potential (Vallianou et al. 2014). An antioxidant property of honey is related to its brightness. Darker honey has more antioxidant value. Phenolic compounds present in honey are responsible for its antioxidant property as phenolic level is correlated with its higher radical absorbance activity (Samarghandian et al. 2017).
Phenolic compounds such as pinocembrin and syringic acid present in honey exert anti-microbial effects (Cianciosi et al. 2010). The anti-fungal effect of honey was attributed to production of H2O2 and presence of volatile and phenolic compounds (Anand et al. 2019).
Honey with greater phenolic content exerts more antitumor effect (Erejuwa et al. 2014). The polyphenols and phenolic compounds present in honey are known to exert anti-leukemic potential against various leukemic cell lines (Ahmed et al. 2008). Honey is known to arrest cell cycle in the sub-G1 phase in bladder cancer cell lines, and this capacity of honey is attributed to presence of several flavonoids and phenolic compounds. Some of the flavonoids such as chrysin, quercetin, and kaempferol present in honey arrest the cell cycle in G0/G1, G1, and G2/M in various cancer cell lines (Anand et al. 2019). The phenolic component of honey also exerts anticancer effect by inducing apoptosis through mitochondrial membrane depolarization and modulating the expression of pro- and anti-apoptotic protein. It also increases the activation of caspase 3 and cleavage of poly (ADP-ribose) polymerase (PARP) in human colon cancer cell lines (Samarghandian et al. 2017). High levels of flavonoids present in honey exert anti-secretory mechanisms which contribute to its gastroprotective effect (Erejuwa et al. 2014). Honey also acts as a potent anti-inflammatory agent. Chrysin, galangin, and quercetin present in honey suppresses the activity of enzymes such as inducible nitric oxide synthase (iNOs) and cyclooxygenase-2 (COX-2) which are associated with the production of inflammatory cytokines. Manuka honey has been known to activate IL-10, IL-1, IL-6 (anti-inflammatory cytokine), TNF-α, and IL-1β (pro-inflammatory cytokines) through toll-like receptors (TLR) and growth factors PDGF and TGF-β.
17.4 Advantages of Honey Flavonoids
Honey is a natural product which is formed by honey bees from nectar of flower. Honey has been consumed by many civilization from ancient times and till now for its nutritional and medicinal benefits (Adebolu 2005; Ashrafi et al. 2005). There are evidences that indicate the therapeutic effects of honey as antioxidant (Ahmed et al. 2013), anti-inflammatory (Khalil et al. 2012), antibacterial (Attia et al. 2008), antidiabetic (Estevinho et al. 2008), respiratory, gastrointestinal (Abdulrhman et al. 2008) cardiovascular, and nervous (Ghosh and Playford 2003) system protective effects. Besides rich in carbohydrates, honey is also a source for flavonoids, polyphenols, reducing compounds, alkaloids, glycosides, cardiac glycosides, anthraquinone, and volatile compounds (White 1962). There are several flavonoids found in honey which include quercetin, kaempferol, myricetin, chrysin, galangin, and luteolin (Zand et al. 2000). Phenolic and flavonoid compounds have been shown to have anti-inflammatory and immunomodulatory effects in animal models, cell cultures (Fernandez-Cabezudo et al. 2013; Candiracci et al. 2012; Bilsel et al. 2002), and clinical trials (Leong et al. 2012). Honey flavonoids have shown to regulate/suppress the proteins like cyclooxygenase-2 (COX-2), inducible nitric oxide synthase (iNOS), ornithine decarboxylase, and tyrosine kinase. These are proteins in the process of inflammation (Viuda-Martos et al. 2008). Flavonoids and phenolic compounds also produce antioxidant effects and inhibit low-density lipoproteins from oxidizing (Kamaruzaman et al. 2014). Different types of honey have also shown inhibition of pro-inflammatory factors such as TNF-α, IL-6, and IL-1 β production (Hussein et al. 2012). Honey has shown immunomodulatory effects by short chain fatty acid (SCFA) fermentation agents, which is produced by slow absorption of honey (Samarghandian et al. 2017). Sufficient data exists which recommends the use of honey in a diseased condition, but further clinical examination can strengthen the therapeutic use of honey.
17.5 Limitations of Honey Flavonoids
Flavonoids interfere with a lot of molecular pathways that are involved in the progression of NAFLD, therefore it has shown beneficial effects. But these evidences are majorly based on pre-clinical studies where the disease model is artificially induced. This might differ from real NAFLD in humans. Another issue arises as flavonoid doses for animal studies were very high, extrapolation of these higher doses to human may cause ethical issues. Flavonoid–drug interaction is yet to be explored and studied (Srinivas et al. 2015; Akhlaghi 2016).
17.6 Conclusion
NAFLD pathophysiological model has multiple pathways, line up with steatosis and inflammation, and ultimately fibrosis, cirrhosis, liver failure, and hepatocellular carcinoma. Different flavonoids act on the pathways in different steps to control the disease. Flavonoids are bioactive compounds and are naturally present in plants. These bioactive compounds have been found to control lipid metabolism, insulin resistance, inflammation, and oxidative stress, the most important pathological processes in the etiology of NAFLD.
References
Abdulrhman M, El-Hefnawy M, Ali R, El-Goud AA (2008) Honey and type 1 diabetes mellitus. In: Liu CP (ed) Type-1 diabetes—complications, pathogenesis, and alternative treatments. Open InTech, Croatia
Adebolu TT (2005) Effect of natural honey on local isolates of diarrhea causing bacteria in Southwestern Nigeria. Afr J Biotechnol 4:1172–1174
Akhlaghi M (2016) Non-alcoholic fatty liver disease: beneficial effects of flavonoids. Phytother Res 30(10):1559–1571
Al-Mamary M, Al-Meeri A, Al-Habori M (2002) Antioxidant activities and total phenolics of different types of honey. Nutr Res 22:1041–1047
Alvarez-Suarez JM, Tulipani S, Romandini S, Bertoli E, Battino M (2010) Contribution of honey in nutrition and human health: a review. Mediterr J Nutr Metab 3:15–23
Anand S, Deighton M, Livanos G et al (2019) Agastache honey has superior antifungal activity in comparison with important commercial honeys. Sci Rep 9:181–197
Angulo P, Kleiner DE, Dam-Larsen S et al (2015) Liver fibrosis, but no other histologic features, is associated with long-term outcomes of patients with nonalcoholic fatty liver disease. Gastroenterology 149:389–397
Ashrafi S, Mastronikolas S, Wu CD (2005) Use of honey in treatment of aphthous ulcers IADR/AADR/CADR 83rd general session. Baltimore, MD, USA, pp 9–12
Attia WY, Gabry MS, El-Shaikh KA, Othman GA (2008) The anti-tumor effect of bee honey in Ehrlich ascitetumor model of mice is coincided with stimulation of the immune cells. J Egypt Public Health Assoc 15:169–183
Ayonrinde OT, Olynyk JK, Marsh JA, Beilin LJ, Mori TA, Oddy WH et al (2015) Childhood adiposity trajectories and risk of nonalcoholic fatty liver disease in adolescents. J Gastroenterol Hepatol 30:163–171
Azman KF, Zakaria R, Othman Z, Aziz CBA (2018) Neuroprotective effects of Tualang honey against oxidative stress and memory decline in young and aged rats exposed to noise stress. J Taibah Univ Sci 12(3):273–284
Babacan S, Rand AG (2007) Characterization of honey amylase. J Food Sci 72:C050–C055
Bansal V, Medhi B, Pandhi P (2005) Honey—a remedy rediscovered and its therapeutic utility. Kathmandu Univ Med J (KUMJ) 3:305–309
Bilsel Y, Bugra D, Yamaner S, Bulut T, Cevikbas U, Turkoglu U (2002) Could honey have a place in colitis therapy? Effects of honey, prednisolone, and disulfiram on inflammation, nitric oxide, and free radical formation. Dig Surg 19:306–311
Buzzetti E, Pinzani M, Tsochatzis EA (2016) The multiple-hit pathogenesis of non-alcoholic fatty liver disease (NAFLD). Metabolism 65(8):1038–1048
Cairns SR, Peters TJ (1983) Biochemical analysis of hepatic lipid in alcoholic and diabetic and control subjects. Clin Sci (Lond)() 65:645–652
Candiracci M, Piatti E, Dominguez-Barragán M, García-Antrás D, Morgado B, Ruano D et al (2012) Anti-inflammatory activity of a honey flavonoid extract on lipopolysaccharide-activated N13 microglial cells. J Agric Food Chem 60:12304–12311
Chen WEI, Wang X, Huang LI, Liu BO (2016) Hepcidin in non-alcoholic fatty liver disease regulated by the TLR4/NF-κBsignaling pathway. Exp Ther Med 11:73–76
Cianciosi D, Forbes-Hernández TY, Afrin S et al (2018) Phenolic compounds in honey and their associated health benefits: a review. Molecules 23(9):2322
Clark JM (2006) The epidemiology of nonalcoholic fatty liver disease in adults. J Clin Gastroenterol 40:S5–S10
Dattaroy D, Seth RK, Das S, Alhasson F, Chandrashekaran V, Michelotti G et al (2016) Sparstolonin B attenuates early liver inflammation in experimental NASH by modulating TLR4 trafficking in lipid rafts via NADPH oxidase activation. Am J Physiol Gastrointest Liver Physiol 310:G510–GG25
De Vito R, Alisi A, Masotti A, Ceccarelli S, Panera N, Citti A et al (2012) Markers of activated inflammatory cells correlate with severity of liver damage in children with nonalcoholic fatty liver disease. Int J Mol Med 30:49–56
Dowman JK, Tomlinson JW, Newsome PN (2010) Pathogenesis of non-alcoholic fatty liver disease. QJM 103(2):71–83
Ekstedt M, Hagström H, Nasr P et al (2015) Fibrosis stage is the strongest predictor for disease-specific mortality in NAFLD after up to 33 years of follow-up. Hepatology 61:1547–1554
Erejuwa OO, Sulaiman SA, Wahab MS (2014) Effects of honey and its mechanisms of action on the development and progression of cancer. Molecules 19(2):2497–2522
Estevinho L, Pereira AP, Moreira L, Dias LG, Pereira E (2008) Antioxidant and antimicrobial effects of phenolic compounds extracts of Northeast Portugal honey. Food Chem Toxicol 46:3774–3779
Fernandez-Cabezudo MJ, El-Kharrag R, Torab F, Bashir G, George JA, El-Taji H et al (2013) Intravenous administration of manuka honey inhibits tumor growth and improves host survival when used in combination with chemotherapy in a melanoma mouse model. PLoS One 8:e55993
Ghosh S, Playford RJ (2003) Bioactive natural compounds for the treatment of gastrointestinal disorders. Clin Sci (Lond) 104:547–556
Hassapidou M, Fotiadou E, Maglara E, Papadopoulou SK (2006) Energy intake, diet composition, energy expenditure, and body fatness of adolescents in Northern Greece. Obesity (Silver Spring) 14:855–862
Holterman A-XL, Guzman G, Fantuzzi G, Wang H, Aigner K, Browne A et al (2013) Nonalcoholic fatty liver disease in severely obese adolescent and adult patients. Obesity 21:591–597
Hussein SZ, Mohd Yusoff K, Makpol S, Mohd Yusof YA (2012) Gelam honey inhibits the production of proinflammatory, mediators NO, PGE (2), TNF-a, and IL-6 in carrageenan-induced acute paw edema in rats. Evid Based Complement Alternat Med 2012:109636
Inglett GE (1976) A history of sweeteners—natural and synthetic. J Toxicol Environ Health 2:207–214
Johnston JE, Sepe HA, Miano CL, Brannan RG, Alderton AL (2005) Honey inhibits lipid oxidation in ready-to-eat ground beef patties. Meat Sci 70:627–631
Kamaruzaman NA, Sulaiman SA, Kaur G, Yahaya B (2014) Inhalation of honey reduces airway inflammation and histopathological changes in a rabbit model of ovalbumin-induced chronic asthma. BMC Complement Altern Med 14:176
Khalil I, Moniruzzaman M, Boukraâ L, Benhanifia M, Islam A, Islam N et al (2012) Physicochemical and antioxidant properties of Algerian honey. Molecules 17:11199–11215
Koo SH (2013) Nonalcoholic fatty liver disease: molecular mechanisms for the hepatic steatosis. Clin Mol Hepatol 19:210–215
Leong AG, Herst PM, Harper JL (2012) Indigenous New Zealand honeys exhibit multiple anti-inflammatory activities. Innate Immun 18:459–466
Mouralidarane A, Soeda J, Visconti-Pugmire C, Samuelsson A-M, Pombo J, Maragkoudaki X et al (2013) Maternal obesity programs offspring nonalcoholic fatty liver disease by innate immune dysfunction in mice. Hepatology 58:128–138
Neuschwander-Tetri BA (2010) Nontriglyceride hepatic lipotoxicity: the new paradigm for the pathogenesis of NASH. Curr Gastroenterol Rep 12:49–56
Nobili V, Carpino G, Alisi A, Franchitto A, Alpini G, De Vito R et al (2012) Hepatic progenitor cells activation, fibrosis, and adipokines production in pediatricnonalcoholic fatty liver disease. Hepatology 56:2142–2153
Pataca LC, Borges Neto W, Marcucci MC, Poppi RJ (2007) Determination of apparent reducing sugars, moisture and acidity in honey by attenuated total reflectance Fourier transform infrared spectrometry. Talanta 71:1926–1931
Paul IM, Beiler J, McMonagle A, Shaffer ML, Duda L, Berlin CM Jr (2007) Effect of honey, dextromethorphan, and no treatment on nocturnal cough and sleep quality for coughing children and their parents. Arch Pediatr Adolesc Med 161(12):1140–1146
Peverill W, Powell LW, Skoien R (2014) Evolving concepts in the pathogenesis of NASH: beyond steatosis and inflammation. Int J Mol Sci 15:8591–8638
Rakha MK, Nabil ZI, Hussein AA (2008) Cardioactive and vasoactive effects of natural wild honey against cardiac malperformance induced by hyperadrenergic activity. J Med Food 11:91–98
Samarghandian S, Farkhondeh T, Samini F (2017) Honey and health: a review of recent clinical research. Pharm Res 9(2):121
Stefan N, Häring HU, Cusi K (2019) Non-alcoholic fatty liver disease: causes, diagnosis, cardiometabolic consequences, and treatment strategies. Lancet Diabetes Endocrinol 7(4):313–324
Stienstra R, Saudale F, Duval C, Keshtkar S, Groener JEM, van Rooijen N et al (2010) Kupffer cells promote hepatic steatosis via Interleukin-1 beta-dependent suppression of peroxisome proliferator-activated receptor alpha activity. Hepatology 51:511–522
Townsend SA, Newsome PN (2016) Non-alcoholic fatty liver disease in 2016. Br Med Bull 119:143–156
Turkmen N, Sari F, Poyrazoglu ES, Velioglu YS (2006) Effects of prolonged heating on antioxidant activity and colour of honey. Food Chem 95:653–657
Ullah R, Rauf N, Nabi G, Ullah H, Shen Y, Zhou YD, Fu J (2019) Role of nutrition in the pathogenesis and prevention of non-alcoholic fatty liver disease: recent updates. Int J Biol Sci 15(2):265
Vallianou NG, Gounari P, Skourtis A, Panagos J, Kazazis C (2014) Honey and its anti-inflammatory, anti-bacterial and anti-oxidant properties. Gen Med (Los Angel) 2:2
Vernon G, Baranova A, Younossi ZM (2011) Systematic review: the epidemiology and natural history of non-alcoholic fatty liver disease and non-alcoholic steatohepatitis in adults. Aliment Pharmacol Ther 34:274–285
Viuda-Martos M, Ruiz-Navajas Y, Fernández-López J, Pérez-Alvarez JA (2008) Functional properties of honey, propolis, and royal jelly. J Food Sci 73:R117–R124
White JW (1962) Composition of American honeys. Agricultural Research Service, USDA, Washington, DC
White JW (1979) Composition of honey. In: Crane E (ed) Honey: a comprehensive survey. Heinemann, London, pp 157–192
Yeh MM, Brunt EM (2014) Pathological features of fatty liver disease. Gastroenterology 147:754–764
Zand RS, Jenkins DJ, Diamandis EP (2000) Steroid hormone activity of flavonoids and related compounds. Breast Cancer Res Treat 62:35–49
Author information
Authors and Affiliations
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2020 Springer Nature Singapore Pte Ltd.
About this chapter
Cite this chapter
Taleuzzaman, M., Verma, R., Kala, C., Sharma, P., Gupta, D.K. (2020). Beneficial Effects of Honey Flavonoids in Nonalcoholic Fatty Liver Disease: An Update. In: Rehman, M.U., Majid, S. (eds) Therapeutic Applications of Honey and its Phytochemicals . Springer, Singapore. https://doi.org/10.1007/978-981-15-7305-7_17
Download citation
DOI: https://doi.org/10.1007/978-981-15-7305-7_17
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-15-7304-0
Online ISBN: 978-981-15-7305-7
eBook Packages: Chemistry and Materials ScienceChemistry and Material Science (R0)