Abstract
Metastasis is the leading cause of cancer-related mortality. The tumor microenvironment per se is a key player regulating the invasion and metastasis of cancer cells. Cancer cells residing in the tumor microenvironment as well as in transit during metastasis are exposed to various chemical and mechanical cues which contribute to their invasiveness. A plethora of studies since the last decade has shed light on the role of physical forces in tumor initiation and progression, iteratively underscoring the importance of cellular mechanobiology in the context of cancer. One of the emerging mechanobiological phenomena observed in cancer cells is autophagy. This chapter accounts for the various mechanical stimuli experienced by cancer cells in vivo and highlights the importance of mechanically-induced autophagy in the tumor milieu.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
8.1 Introduction
Solid tumors begin with the abnormal proliferation of cells in a confined area within the body. In due course of growth, cancerous cells from the periphery of the tumor start dislodging into the bloodstream and lymphatic ducts. What follows is the process called metastatic dissemination. Some of the dislodged cells survive through the bloodstream and migrate to the “foreign soil” of distant tissues to form a secondary tumor (Chaffer 2011). During this process, mechanical cues, alongside chemical signals, may activate myriad signaling pathways in metastatic cancer cells (Janmey and Miller 2011). While chemical signals include growth factors and soluble ligands, biophysical cues may arise in the form of matrix stiffness, confinement, topography, shear stress, compression, and mechanical stretching (Chaudhuri et al. 2018; Stylianopoulos et al. 2018). The subsequent section accounts for the genesis of mechanical cues in the tumor microenvironment.
8.2 Genesis of Mechanical Cues in the Tumor Microenvironment
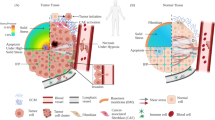
As cancer progresses, solid tumors keep growing in confined spaces within normal tissue of a host and become increasingly rigid. An increase in the number of cells within a tumor causes stiffening of the tissue. This occurs due to the addition of cancer cells, stromal cells, and extracellular matrix constituents, resulting in higher elastic modulus than normal tissue, as high as one order of magnitude (Jain et al. 2014; Samani et al. 2007). Tumor growth generates compressive and stretching forces within the tumor and also between the tumor and the host tissue. Mathematical models have predicted that compressive stress at the tumor interior may exceed 40 kPa (Stylianopoulos et al. 2013; Voutouri et al. 2014; Roose et al. 2003). Confined growth of solid tumor leads to distortion of associated tumor vessels, which in turn induce both solid and fluid stresses, that foster tumor progression. While invading the dense matrix of the tumor stroma, cancer cells squeeze and deform through narrow pores. Naturally, when the cell passes through confinements of subnuclear dimensions, the nucleus is exposed to high deformation which may affect cellular behavior through a process called mechanotransduction. Biophysical properties thus influence the efficacy of cancer cell invasion and subsequent metastases (Talmadge and Fidler 2010). Inside the vasculature of the body, cancer cells experience shear stress generated by the interstitial flow at the tumor site and hemodynamic flow during metastasis. Fluid shear forces could affect both survival and invasiveness of circulating tumor cells (CTCs) (Mitchell and King 2013; Ma et al. 2017). Constricted lymphatic vessels may lead to enhanced interstitial fluid velocities (Chary and Jain 1989). Hemodynamic shear stresses, ranging from 0.5–30.0 dyn/cm2, may arise due to the movement of blood along the surface of the cancer cell and is dependent on both fluid viscosity and flow rates (Mitchell and King 2013; Wirtz et al. 2011). Contact of tumor cells with endothelial cells, as tumor cells enter and leave the vasculature through the processes of intravasation and extravasation respectively, may also lead to the generation of shear stresses (Northcott et al. 2018). A landscape of various mechanical forces in the tumor microenvironment is depicted in Fig. 8.1.
Origin of various mechanical stresses in the tumor microenvironment. The growth of a primary tumor generates solid stress due to radially inward compression and circumferential tension or stretching of tumor cells by neighboring cells and the surrounding matrix. Also, the cancer cells are exposed to low shear stresses in the form of interstitial lymphatic flows within the stromal matrix. During hematogenous metastasis, intravasation of metastatic cells into the bloodstream exposes the cells to higher magnitudes of shear stress that the cells experience until they get arrested and extravasate at a secondary tumor site
8.3 Impact of Mechanical Cues on Cancer Cells
The influence of physical forces on tumor growth has been studied through numerous in vitro models (Huang et al. 2018). This is intuitive given the fact that the tumor microenvironment is exposed to several factors like chemical signals, multiple cell–cell interactions, and mechanical stimuli. To study the exclusive effects of mechanical cues on tumor progression, scientists have resorted to a reductionist approach. A host of accumulating evidence has highlighted the role of biophysical cues in cancer development (Das et al. 2019a). This section discusses the various approaches employed to apply various mechanical stimuli to cancer cells in vitro and the effects observed thereof. One of the first challenges in this regard was to measure stress levels of developing solid tumors. Helmlinger et al. were the first to reveal such measurements using tumor spheroids of colon and breast cancer cells embedded in increasing concentrations of agarose (Helmlinger et al. 1997). Since then, several improvisations have been made to mimic the compressive environment of growing tumors in vitro, most of them being cancer spheroid models or their modifications (shown in Fig. 8.2a–c). Compression to cancer cells grown in monolayers have been applied using piston-based systems or simply by applying appropriate weights to bead encapsulated cancer cells (Tse et al. 2012; Kim et al. 2017). Compression alters gene expression in cancer cells in turn affecting invasion and metastasis (Tse et al. 2012; Koike et al. 2002; Kalli et al. 2018). However, excessive solid stress may play an inhibitory role by reducing the rate of proliferation while inducing apoptosis (Helmlinger et al. 1997; Cheng et al. 2009; Kaufman et al. 2005; Delarue et al. 2014). The compression of blood and lymphatic vessels may reduce perfusion and create a hypoxic microenvironment that promotes tumor progression (Jain et al. 2014; Jain 2014). In order to metastasize, tumor cells must survive through the circulation while migrating to a distant location. The time a cancer cell spends in circulation and the magnitude of shear stress it experiences on its way determines its survival (Fan et al. 2016). The shear-dominant microenvironment of metastasizing cancer cells has been mimicked in vitro by generating fluid flows though setups like parallel-plate flow chambers, peristaltic pumps, microfluidic platforms, and hypodermic needles (Ma et al. 2017; Lien et al. 2013; Das et al. 2011, 2018; Barnes et al. 2012) (see Fig. 8.2d–f). Shear stress of the physiological range (0.5–3 Pa) has been shown to inhibit proliferation but stimulate migration and adhesion of tumor cells (Mitchell and King 2013; Ma et al. 2017; Avvisato et al. 2007; Xiong et al. 2017). However, a high magnitude of stresses caused tumor cell death (Regmi et al. 2017). Tumor cells must show resistance to the various mechanical stresses if they have to travel and colonize at a secondary metastatic site. It is one of the major reasons why cancer cells must undergo various cellular adaptations (Northcott et al. 2018). The subsequent section discusses myriad cellular adaptive responses that may be elicited in cancer cells experiencing microenvironmental stresses.
(a–f) Schematic of the various experimental setups implemented for performing mechanobiological studies of cancer cells. (a) Cancer spheroids embedded in increasing concentrations of culture-media equilibrated agarose and allowed to grow in the compressive environment, (b) weight applied to exert compression on a monolayer of cancer cells grown in a well of a cell culture plate, (c) weight applied to exert compression on agarose scaffolded alginate bead-encapsulated cancer cells, (d) parallel-plate flow chamber connected via silicone tubings to a peristaltic pump for applying fluid shear stress to cancer cells, (e) peristaltic pump connected to a microfluidic channel for applying fluid shear stress to cancer cells adhered on the microchannel floor, (f) programmable syringe pump to flow cancer cell suspension through a hypodermic needle for several passes in order to apply shear stress to the cells
8.4 Cellular Stress Response Mechanisms
A cell’s response to any kind of stress is a reaction to perturbations of ambient conditions. Such unfavorable conditions may or may not damage cellular macromolecules. Cells may either perform homeostasis to attain the former state or adopt a changed state, depending upon the severity and duration of incumbent stress. For instance, mild or moderate stresses may result in enhanced defense and repair processes. It is thus essential to study stress-adaptive mechanisms in order to comprehend the processes that cancer cells may undergo during their metamorphosis into the malignant state, leading to the identification of critical therapeutic targets (Das et al. 2019a; Milisav et al. 2012). Adaptive stress responses may occur through several mechanisms like damage repair, synthesis of protective molecules, and control of apoptosis induction. Cellular repair is brought about by alterations in gene expression patterns, miRNA-transcription, growth arrest, and so on. Protective molecules may be antioxidant enzymes like catalase, peroxidase, superoxide dismutase, etc. In many forms of cancers, tumor initiation, progression, and resistance to current anticancer therapies may be attributed to the overexpression of the anti-apoptotic proteins. Another important strategy that a cell may adopt to react to stress is the clearance of damaged organelles. For soluble proteins, this may occur through the ubiquitin-proteasome pathway (Shang and Taylor 2011), whereas for other cellular material, the autophagic pathway for degradation may be activated. Autophagy is an evolutionarily conserved catabolic process whereby cytosolic components are enclosed in sealed bilayered vesicles and then digested through the action of lysosomes. Autophagy helps in increasing nutrient availability to the cells through the clearance of toxic cellular materials and unfolded proteins influencing numerous physiological processes including homeostasis during cellular stresses (Das et al. 2019a). However, in the mechanobiological aspect of cancer, the role of autophagy had been less explored until the seminal work by King et al. showed that autophagy is induced as an immediate response to compressive stress (King et al. 2011). Autophagy may either contribute to cellular adaptation and survival or cellular death (Maiuri et al. 2007). At lower pressures, autophagy may also instigate mechanical signaling (King 2012). Reportedly, tumor cells upregulate autophagy in response to increased metabolic demands and cellular stresses (Yang et al. 2011). Although autophagy is known to be involved in several processes like modulation of cancer stem cell viability and differentiation, epithelial-to-mesenchymal transition, tumor cell dormancy, motility and invasion, resistance to anoikis, escape from immune surveillance, and so on, the direct implications of mechanically induced autophagy in the cancer scenario was least investigated until recent times (Mowers et al. 2017).
8.5 Implications of Mechanical Stress-Induced Autophagy in Cancer Metastasis
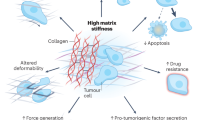
The differential roles of flow-induced shear forces in modulating cancer progression have started coming in the limelight (Swartz and Lund 2012). Lien et al. demonstrated that laminar shear stress in the range of 0.5–12 dyn/cm2, applied for more than 12 h, was able to induce autophagic and apoptotic death in cancer cells, but not in their normal counterparts (Lien et al. 2013). They found that laminar shear stress-induced autophagy acted not only as a parallel death-promoting mechanism but also as an independent death-inducing mechanism upstream of apoptosis. In contrast, Das et al. interestingly found that short pulses of laminar shear stress could elicit pro-survival autophagy in HeLa cells as an immediate response. Autophagy in this instance served as a protective mechanism that could delay apoptotic cell death (Das et al. 2018). In an independent study, Wang et al. found that inhibition of autophagy induced by fluid shear stress of 1.4 dyn/cm2, suppressed cellular migration, and invasion in hepatocellular carcinoma cells (Wang et al. 2018; Yan et al. 2019). Transit through the vasculature followed by arrest and extravasation at a distant location are some of the key steps of metastasis. Since these phenomena are short timescale processes, the immediate pro-survival autophagic response due to fluid shear may prove to be a crucial escape route of metastasizing cancer cells (Follain et al. 2018). Very recently, Das et al. (2019b) recreated a mechanically-compressed tumor microenvironment, in vitro, by applying appropriate compression to agarose-scaffolded HeLa cell-encapsulated alginate beads. They demonstrated that compression upregulates autophagy, which promotes turnover of paxillin, a crucial protein involved in cell migration, and secretion of active-matrix metalloproteinase 2 (MMP 2), leading to enhanced migration of HeLa cells (Das et al. 2019b). These evidences hint at the fact that compressive and shear forces in the tumor milieu may foster cancer progression at least partially, by upregulating autophagy. At the molecular level, King et al. demonstrated that mechanical induction of autophagy is independent of classical TOR/Akt pathway and AMPK signaling, which is the conventional route of autophagy induction in cells (King et al. 2011). However, the molecular pathways governing mechanically-induced autophagy remained completely obscure until 2013 when Lien et al. showed that shear forces could elicit autophagy in cancer cells through the BMPRIB/Smad 1/5/p38 MAPK axis (Lien et al. 2013). Later, Das et al. (2018), demonstrated that shear stress causes membrane perturbation which triggers lipid rafts, that is, cholesterol-rich nanodomains of cell membranes, to mediate the phosphorylation of p38 MAPKs which in turn leads to LC3 II/I conversion and autophagy induction in HeLa cells (Das et al. 2018). Fluid shear stress also induced the expression of Rho GTPases and cytoskeleton remodeling via the integrin/FAK pathway, leading to the upregulation of autophagy in HepG2 cells (Yan et al. 2019). The various manifestations of mechanical stress-induced autophagy in cancer cells are depicted in Fig. 8.3.
Manifestations of mechanical stress-induced autophagy in cancer cells. Shear stresses due to blood flows have been shown to trigger the phosphorylation of signaling molecules located on the cytoskeleton of cancer cells like integrins, focal adhesion kinases, and also of membrane lipid raft-associated cytoplasmic proteins like p38 MAPkinases, leading to activation of the autophagic cascade. Activation of focal adhesion kinases causes cytoskeletal rearrangement that also participates in the formation of autophagosomal components. Shear-induced autophagy imparts immediate resistance to shear-induced apoptosis while compression-induced autophagy upregulates secretion of MMP 2 and promote turnover of a crucial focal adhesion protein, Paxillin, thereby aiding in survival, invasion, and migration of cancer cells respectively
8.6 Conclusion and Perspective
Manipulation of mechanical forces in the tumor microenvironment to tame cancer is evolving as a new field termed “physical oncology”. This may be done by alterations of the physical characteristics of the stroma or by inhibition of cellular responses to the stiffening of the stroma (Northcott et al. 2018). The underlying goal remains the alleviation of the solid and fluid stresses within the tumor microenvironment. To this effect, extracellular matrix-degrading enzymes like Hyaluronidases have been implemented to release immobilized fluid for improving tissue compliance (Whatcott et al. 2011). TGF-β blockers and MMP inhibitors are some of the drugs that target ECM synthesis (Chaudhuri et al. 2018). Also, Losartan, an angiotensin inhibitor, has been used for vessel dilation to reduce IFP (Chauhan et al. 2013). Based on the knowledge of signaling networks operating downstream of focal adhesions, drugs targeted toward reducing actomyosin contractility have shown successful repression of tumor progression. Reports on potent therapeutic targets associated with mechanically-induced autophagy have started cropping up. Depletion of cholesterol by Methyl-beta cyclodextrin (MBCD) could lead to impairment of lipid raft mediated-p38 MAPK phosphorylation under fluid shear stress, thereby impeding the induction of pro-survival autophagy (Das et al. 2018). Cliengitide, an integrin inhibitor, could inhibit the activation of downstream FAK, thereby attenuating fluid shear stress-induced autophagy in HepG2 cells (Yan et al. 2019). It is imperative to further investigate the arsenal of mechanosensory elements of cancer cells that participate in mechanical stress-induced autophagy. For example, reports have suggested that intracellular Ca2+, a well-known regulator of autophagy, aids in the migration and proliferation of cancer cells (Filippi-chiela et al. 2016; Cui et al. 2017). Whether or not stretch-activated calcium channels participate in the mooted signal transduction pathway remains to be explored (Das et al. 2019a).
In summary, this chapter throws light on the origin of mechanical forces in the context of cancer and how these forces may govern cancer progression through the intervention of autophagy. Presently known implications of mechanical stress-induced autophagy in cancer include imparting immediate resistance to shear-induced apoptosis during metastasis and facilitating the migratory and invasive characteristics of cancer cells in the tumor microenvironment. It may thus be foreseen that the discovery of pathways related to mechanically-induced autophagy in cancer cells, may usher in an array of effective therapeutic molecules, in the near future.
References
Avvisato CL, Yang X, Shah S, Hoxter B, Li W, Gaynor R, Pestell R, Tozeren A, Byers SW (2007) Mechanical force modulates global gene expression and β-catenin signaling in colon cancer cells. J Cell Sci 120:2672–2682
Barnes JM, Nauseef JT, Henry MD (2012) Resistance to fluid shear stress is a conserved biophysical property of malignant cells. PLoS One 7:e50973
Chaffer CL (2011) A perspective on cancer cell metastasis. Science 331:1559–1564
Chary SR, Jain RK (1989) Direct measurement of interstitial convection and diffusion of albumin in normal and neoplastic tissues by fluorescence photobleaching. Proc Natl Acad Sci U S A 86:5385–5389
Chaudhuri PK, Low BC, Lim CT (2018) Mechanobiology of tumor growth. Chem Rev 118:6499–6515
Chauhan VP, Martin JD, Liu H, Lacorre DA, Jain SR, Kozin SV et al (2013) Angiotensin inhibition enhances drug delivery and potentiates chemotherapy by decompressing tumour blood vessels. Nat Commun 4:2516
Cheng G, Tse J, Jain RK, Munn LL (2009) Micro-environmental mechanical stress controls tumor spheroid size and morphology by suppressing proliferation and inducing apoptosis in cancer cells. PLoS One 4:e4632
Cui C, Merritt R, Fu L, Pan Z (2017) Targeting calcium signaling in cancer therapy. Acta Pharm Sin B 7:3–17
Das T, Maiti TK, Chakraborty S (2011) Augmented stress-responsive characteristics of cell lines in narrow confinements. Integr Biol (Camb) 3:684–695
Das J, Maji S, Agarwal T, Chakraborty S, Maiti TK (2018) Hemodynamic shear stress induces protective autophagy in HeLa cells through lipid raft-mediated mechanotransduction. Clin Exp Metastasis 35:135–148
Das J, Chakraborty S, Maiti TK (2019a) Seminars in cancer biology mechanical stress-induced autophagic response: a cancer-enabling characteristic? Semin Cancer Biol. https://doi.org/10.1016/j.semcancer.2019.05.017
Das J, Agarwal T, Chakraborty S, Maiti TK (2019b) Compressive stress-induced autophagy promotes invasion of HeLa cells by facilitating protein turnover in vitro. Exp Cell Res 381:201–207
Delarue M, Montel F, Vignjevic D, Prost J, Cappello G, Curie M, Curie I, De Recherche C (2014) Compressive stress inhibits proliferation in tumor spheroids through a volume limitation. Biophys J 107:1821–1828
Fan R, Emery T, Zhang Y, Xia Y, Sun J, Wan J (2016) Circulatory shear flow alters the viability and proliferation of circulating colon cancer cells. Sci Rep 6:27073
Filippi-chiela EC, Viegas MS, Thomé MP, Buffon A, Wink MR, Lenz G (2016) Modulation of autophagy by calcium signalosome in human disease. Mol Pharmacol 90:371–384
Follain G, Osmani N, Azevedo AS, Allio G, Mercier L, Karreman MA et al (2018) Hemodynamic forces tune the arrest, adhesion, and extravasation of circulating tumor cells. Dev Cell 45:33–52
Helmlinger G, Netti PA, Lichtenbeld HC, Melder RJ, Jain RK (1997) Solid stress inhibits the growth of multicellular tumor spheroids. Nat Biotechnol 15:778–783
Huang Q, Hu X, He W, Zhao Y, Hao S, Wu Q, Li S, Zhang S, Shi Fluid M (2018) Shear stress and tumor metastasis. Am J Cancer Res 8:763–777
Jain RK (2014) Perspective antiangiogenesis strategies revisited: from starving tumors to alleviating hypoxia. Cancer Cell 26:605–622
Jain RK, Martin JD, Stylianopoulos T (2014) The role of mechanical forces in tumor growth and therapy. Annu Rev Biomed Eng 16:321–346
Janmey PA, Miller RT (2011) Mechanisms of mechanical signaling in development and disease. J Cell Sci 124:9–18
Kalli M, Apageorgis PAP, Kretsi VAG, Tylianopoulos TRS (2018) Solid stress facilitates fibroblasts activation to promote pancreatic cancer cell migration. Ann Biomed Eng 46:657–669
Kaufman LJ, Brangwynne CP, Kasza KE, Filippidi E, Gordon VD, Deisboeck TS, Weitz DA (2005) Glioma expansion in collagen I matrices: analyzing collagen concentration-dependent growth and motility patterns. Biophys J 89:635–650
Kim BG, Gao M-Q, Kang S, Choi YP, Lee JH, Kim JE, Han HH, Mun SG, Cho NH (2017) Mechanical compression induces VEGFA overexpression in breast cancer via DNMT3A-dependent miR-9 downregulation. Cell Death Dis 8:e2646
King JS (2012) Mechanical stress meets autophagy: potential implications for physiology and pathology. Trends Mol Med 18:583–588
King JS, Veltman DM, Insall RH (2011) The induction of autophagy by mechanical stress. Autophagy 7:1490–1499
Koike C, Mckee TD, Pluen A, Ramanujan S, Burton K, Munn LL, Boucher Y, Jain RK (2002) Solid stress facilitates spheroid formation: potential involvement of hyaluronan. Br J Cancer 86:947–953
Lien S, Chang S, Lee P, Wei S, Chang MD, Chang J, Chiu J (2013) Mechanical regulation of cancer cell apoptosis and autophagy: roles of bone morphogenetic protein receptor, Smad1/5, and p38 MAPK. Biochim Biophys Acta Mol Cell Res 1833:3124–3133
Ma S, Fu A, Giap G, Chiew Y, Qian K (2017) Hemodynamic shear stress stimulates migration and extravasation of tumor cells by elevating cellular oxidative level. Cancer Lett 388:239–248
Maiuri MC, Zalckvar E, Kimchi A, Kroemer G (2007) Self-eating and self-killing: crosstalk between autophagy and apoptosis. Nat Rev Mol Cell Biol 8:741–752
Milisav I, Poljsak B, Šuput D (2012) Adaptive response, evidence of cross-resistance and its potential clinical use. Int J Mol Sci 13:10771–10806
Mitchell MJ, King MR (2013) Fluid shear stress sensitizes cancer cells to receptor-mediated apoptosis via trimeric death receptors. New J Phys 15:015008
Mowers EE, Sharifi MN, Macleod KF (2017) Autophagy in cancer metastasis. Oncogene 36:1619–1630
Northcott JM, Dean IS, Mouw JK, Weaver VM (2018) Feeling stress: the mechanics of cancer progression and aggression. Front Cell Dev Biol 6:1–12
Regmi S, Fu A, Luo KQ (2017) High shear stresses under exercise condition destroy circulating tumor cells in a microfluidic system. Sci Rep 7:1–12
Roose T, Netti PA, Munn LL, Boucher Y, Jain RK (2003) Solid stress generated by spheroid growth estimated using a linear poroelasticity model. Microvasc Res 66:204–212
Samani A, Plewes D, Samani A, Bishop J, Hagan JJO, Samani A (2007) Elastic moduli of normal and pathological human breast tissues: an inversion-technique-based investigation of 169 samples. Phys Med Biol 52:1565–1576
Shang F, Taylor A (2011) Ubiquitin-proteasome pathway and cellular responses to oxidative stress. Free Radic Biol Med 51:5–16
Stylianopoulos T, Martin JD, Snuderl M, Mpekris F, Jain SR, Jain RK (2013) Coevolution of solid stress and interstitial fluid pressure in tumors during progression: implications for vascular collapse. Cancer Res 73:3833–3841
Stylianopoulos T, Munn LL, Jain RK (2018) Reengineering the physical microenvironment of tumors to improve drug delivery and efficacy: from mathematical modeling to bench to bedside. Trends Cancer 4:292–319
Swartz MA, Lund AW (2012) Lymphatic and interstitial flow in the tumour microenvironment: linking mechanobiology with immunity. Nat Rev Cancer 12:210–219
Talmadge JE, Fidler IJ (2010) AACR centennial series: the biology of cancer metastasis: historical perspective. Cancer Res 70:5649–5669
Tse JM, Cheng G, Tyrrell JA, Wilcox-Adelman SA, Boucher Y, Jain RK, Munn LL (2012) Mechanical compression drives cancer cells toward invasive phenotype. Proc Natl Acad Sci U S A 109:911–916
Voutouri C, Mpekris F, Papageorgis P, Odysseos AD, Stylianopoulos T (2014) Role of constitutive behavior and tumor-host mechanical interactions in the state of stress and growth of solid tumors. PLoS One 9:e104717
Wang X, Zhang Y, Feng T, Su G, He J, Gao W, Shen Y, Liu X (2018) Fluid shear stress promotes autophagy in hepatocellular carcinoma cells. Int J Biol Sci 14:1277–1290
Whatcott CJ, Han H, Posner RG, Hostetter G, Von Hoff DD (2011) Targeting the tumor microenvironment in cancer: why hyaluronidase deserves a second look. Cancer Discov 1:291–296
Wirtz D, Konstantopoulos K, Searson PPC (2011) The physics of cancer: the role of physical interactions and mechanical forces in metastasis. Nat Rev Cancer 11:512–522
Xiong N, Li S, Tang K, Bai H, Peng Y, Yang H, Wu C, Liu Y (2017) Involvement of caveolin-1 in low shear stress-induced breast cancer cell motility and adhesion: roles of FAK/Src and ROCK/p-MLC pathways. Biochim Biophys Acta Mol Cell Res 1864:12–22
Yan Z, Su G, Gao W, He J, Shen Y, Zeng Y (2019) Fluid shear stress induces cell migration and invasion via activating autophagy in HepG2 cells. Cell Adhes Migr 13:1–12
Yang S, Wang X, Contino G, Liesa M, Sahin E, Mautner J, Tonon G, Haigis M, Shirihai OS, Doglioni C (2011) Pancreatic cancers require autophagy for tumor growth pancreatic cancers require autophagy for tumor growth. Genes Dev 25:717–729
Author information
Authors and Affiliations
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2020 Springer Nature Singapore Pte Ltd.
About this chapter
Cite this chapter
Das, J., Maiti, T.K. (2020). Mechanical Stress-Induced Autophagy: A Key Player in Cancer Metastasis. In: Bhutia, S.K. (eds) Autophagy in tumor and tumor microenvironment . Springer, Singapore. https://doi.org/10.1007/978-981-15-6930-2_8
Download citation
DOI: https://doi.org/10.1007/978-981-15-6930-2_8
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-15-6929-6
Online ISBN: 978-981-15-6930-2
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)