Abstract
Autophagy is an intracellular catabolic process that helps in maintaining cellular homeostasis. Generally, it is involved in the recycling of unwanted proteins and damaged organelles but upon cellular stress, it helps in the survival of the cells. It is a tightly regulated process and any discrepancy in its regulation leads to the generation of many pathological abnormalities. During the early phase of cancer, it functions as a tumor suppressor whereas, at later stages, it facilitates tumor growth and helps in generating resistance to cancerous cells. Due to this functional switch of the pathway, many studies have been undertaken to find the mechanism behind its regulation in different cancer types and microRNAs (miRNAs) have been recently explored to be one of the regulatory factors. miRNAs are short non-coding RNAs that regulate the gene expression of most protein-coding genes post-transcriptionally. They control many important biological pathways including autophagic response in cancer. Their expression also gets dysregulated during different stages of cancer and thus gives a promising window of their utility as an attractive target during tumor therapy. Therefore, considering the potential of autophagy regulating miRNAs as future drug targets, this review is focused on recent advances in linking miRNAs to the regulation of autophagy pathway and their role in cancer and their implications in cancer treatment.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
4.1 Introduction
MicroRNAs (miRNAs) are small noncoding RNA molecules of 18–25 nucleotides that have a crucial role in gene regulation at the post-transcriptional level by controlling the stability and translation of mRNAs. They are produced as primary miRNAs (pri-miRNAs) and are subsequently processed to generate mature miRNAs through precursor miRNAs (pre-miRNAs). The mature miRNAs mainly interact with the 3′ untranslated region (UTR) of the target gene to regulate their expression (Ha and Kim 2014). But their interaction is not limited to 3′ UTR only, as many reports have suggested that they can bind to either 5′ UTR or different locations like promotor or gene coding regions as well (Broughton et al. 2016; O’Brien et al. 2018). miRNAs regulate many key biological processes like cell differentiation, growth, autophagy, migration, apoptosis, and so on, and are tightly regulated because their abnormal expression has been shown to be responsible in the development of many diseases (Fu et al. 2013; Paul et al. 2018; Tüfekci et al. 2014). They are secreted out of the cells to aid in signaling between cells and act as biomarkers for various diseases including cancer (Hayes et al. 2014; Huang 2017; Wang et al. 2016b). Upregulation or downregulation of specific miRNAs is reported in all cancer cell types like colon cancer, leukemia, breast cancer, lung cancer, and so on. (O’Brien et al. 2018). Dysregulation of miRNA biogenesis or expression is reported in various stages of cancer progression and regulates resistance to anti-cancer drugs (O’Brien et al. 2018).
Autophagy is a highly conserved cellular process involved in the recycling and digestion of damaged organelles, misfolded proteins, and intracellular pathogens by lysosomal degradation to maintain cell survival (He and Klionsky 2009; Mizushima et al. 2008). It is a continuous process undergoing in the cell at the basal level under normal conditions to maintain cellular homeostasis, but under stress conditions like starvation, infection, hypoxia, and so on, it gets upregulated (Mizushima et al. 2008). During stress conditions, autophagy plays a protective role by degrading damaged and unwanted cellular contents and recycling proteins to generate energy and free amino acids but hyperactivation of autophagy leads to death of the cell under stress, known as autophagic cell death (Mizushima et al. 2008). Being a key process, it is tightly regulated but abnormalities in the pathway arise and it leads to the development of many health issues including cancer (Frankel and Lund 2012; Jing et al. 2015). Many reports have been published showing the role of miRNA in autophagy regulation and cancer development (Gozuacik et al. 2017). In this review, we will briefly summarize the emerging connection between different miRNAs and autophagy pathways and how this regulation decides the fate of cancer cells.
4.2 miRNA Biogenesis
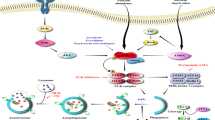
miRNAs play an important role in various physiological processes including cellular proliferation, differentiation, maturation, host–pathogen interaction, and many more (Demirci et al. 2016; Saçar et al. 2014). miRNAs are pervasive in the genome and originate from both coding genes as well as noncoding regions as primary transcripts by the cellular machinery (Grund and Diederichs 2010; Kim et al. 2009). The biogenesis of miRNA initiates in the nucleus followed by its transport into the cytoplasm where miRNA processing takes place to generate mature miRNA. The majority of miRNA genes are transcribed by RNA polymerase II or III to form long primary transcripts called pri-miRNA that contain hairpin (stem-loop) structure with some bulges formed due to base–pair mismatch (Krishnan and Damaraju 2018). The pri-miRNA possesses a 5′ 7-methylguanosine cap and a poly-A tail at the 3′ end and are in turn cleaved by a cellular RNAase Class II endonuclease III enzymes called Drosha (Gregory and Shiekhattar 2005). Drosha along with its cofactor DGC28/Pasha forms a microprocessor complex that specifically recognizes and cleaves the stem of pri-miRNA to liberate nearly 70–120 nucleotides shorter hairpin structure called pre-miRNA (Seitz and Zamore 2006; Shomron and Levy 2009). Following the formation of pre-miRNAs, they are transported to cytoplasm using exportin-5 (XPO5) in the presence of guanosine 5′ triphosphate bound Ras-related nuclear protein (RanGTP). XPO5 is a member from the karyopherin family and is mostly engaged in nuclear transport of structured RNAs including tRNAs, human Y1 RNA, and adenovirus VA1 RNA that possess 3′ overhang structure. Attachment of XPO5 to its cargo needs a minimum of 16 bp and a short 3′ overhang. Therefore, pre-miRNAs are properly processed by Drosha in order to be recognized and exported from the nucleus (Okada et al. 2009). Apart from its role in nucleocytoplasmic transport, Exportin-5 also stabilizes the pre-miRNA as well as prevents its degradation (Yi et al. 2003; Zeng and Cullen 2004). Once inside the cytoplasm, further processing of pre-miRNA takes place with the help of a double-stranded RNAase III enzyme Dicer. Dicer along with trans activation response RNA binding protein (TRBP) binds to 5′ phosphate and 3′ overhang at the base of stem loop of pre-miRNA and cuts both strands of the duplex at about two helical turns away from the base of stem loop. This cleavage by Dicer releases a double-stranded miRNA of ~21–24 nucleotides length called mature miRNA (Bartel 2004). One strand called the passenger strand in the newly generated double-stranded RNA undergoes degradation whereas the other strand known as guide RNA or mature RNA is loaded onto an Argonaute containing RNA induced silencing complex (RISC) by the help of RISC loading complex (RLC) that directs gene silencing. Selection of guide strand mostly depends on the thermodynamic stability and strand with less stability is selected by RISC (Khvorova et al. 2003). RISC is composed of mature miRNA, Dicer, TRBP, Argonaute protein 2 (AGO2), and protein kinase R activator (PACT) that possesses a regulatory role in both nucleus as well as cytoplasm. In the cytoplasm, the RISC complex targets the 3′UTR region of mRNA thereby results in translational repression (MacRae et al. 2008; Park and Shin 2014). Further, some of the miRNAs having nuclear localization signal are imported back to the nucleus. Mature miRNA along with Ago2 returns to the nucleus using a member of the karyopherin beta family protein called Importin-8 (Hwang et al. 2007; Wei et al. 2014). miRNA inside nucleus exhibits regulatory functions by targeting gene promoter region with the help of Argonaute proteins as well as through recruitment of epigenetic modifier proteins such as chromobox protein homolog 3 (CBX3), transcriptional intermediary factor 1beta (TIF1β), suppressor of variegation 3–9 homolog 1 (SUV39H1), euchromatic histone lysine methyltransferase 2 (EHMT2) that results in transcriptional gene silencing or gene activation (Kim et al. 2008; Liang et al. 2013; Salmanidis et al. 2014; Winter et al. 2009, Fig. 4.1).
4.3 Autophagy
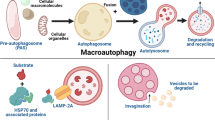
It is a lysosome mediated catabolic process that mainly occurs in response to nutrient starvation and stress conditions to ensure cell survival (Schneider and Cuervo 2014). This process is highly complex and fundamental in eukaryotes involving 20 dedicated autophagy-related (ATG) genes that coordinate the entire pathway starting from the formation of isolation membrane to degradation of cellular cargos (Mizushima 2019; Suzuki et al. 2017). The process is initiated by uncoordinated-51 like kinase (ULK, mammalian homolog of ATG1) complex and Vps34/PIK3C3 phosphatidylinositol 3-kinase (PtdIns3K) complex leading to the formation of cup-shaped isolation membrane. The isolation membrane then elongates, sequesters cytoplasmic targets, cellular cargos, damaged organelles, protein aggregates, long-lived proteins, and finally encloses to form a double membrane-bound organelle called an autophagosome. Phagophore elongation, expansion, and completion of autophagosome involve two ubiquitin-like conjugation complexes namely ATG12-ATG5-ATG16 and ATG8 conjugation systems. In the ATG12-ATG5-ATG16 conjugation system, ATG12 is catalyzed by E1-like enzyme, ATG7, and E2-like enzyme ATG10 to conjugate with ATG5. ATG12-ATG5 conjugate then interacts with ATG16L1 and forms a conglomerate that associates with autophagosome. In the ATG8 conjugation system, the pro-form of ATG8 is first cleaved into processed form by ATG4 leading to its activation by ATG7 and ATG12 (act as E3 like enzyme with ATG5). Active ATG8 is then transferred to E2-like enzyme ATG3 before conjugation with phosphatidylethanolamine (PE) and named as ATG8-PE which is present on the autophagosome membrane (Mizushima 2019). Mammalian homologs of ATG8 are known as microtubule-associated protein 1 light chain 3 (LC3) and gamma-aminobutyric acid receptor-associated protein (GABARAP). Its unlipidated (ATG8-I) or lipidated forms (ATG8-PE, ATG8-II) are generally referred to as LC3-I and LC3-II respectively. Upon recruitment, LC3-II stays on the autophagosome membrane until the culmination of the autophagic process. Completion of autophagosome biogenesis leads to its fusion with the lysosomes resulting in degradation of the engulfed material (Reggiori and Ungermann 2017). Cytoplasmic cargos are recognized and targeted to nascent autophagosome membrane by an interaction between molecular tags (such as polyubiquitin) and LC3 through adaptor proteins such as sequestosome 1 (p62/SQSTM1) and neighbor of BRCA1 gene 1 (NBR1) formation (Songane et al. 2012). Under normal physiological conditions, autophagy plays different crucial roles such as restoration of the amino acid pool and cellular ATP levels during nutrient deprivation condition, tumor growth inhibition, anti-aging, pre-implantation development, clearance of intracellular microbes and modulation of the innate and adaptive immune response, and so on. (Cecconi and Levine 2008; Deretic and Levine 2009; Mizushima and Komatsu 2011). Moreover, defects in the autophagic machinery have also been reported to be associated with numerous disease conditions including neurodegeneration, cancer, cardiovascular disorders, and infectious or inflammatory conditions (Choi et al. 2004). Because of the earlier multi-dimensional role, autophagy has been exploited in the past few years as a front-runner of host-directed therapy to get rid of different pathophysiological conditions including cancer.
4.4 Interplay Between miRNA and Autophagy (Fig. 4.2)
4.4.1 Autophagy Induction
Autophagy induction commences with activation of the ULK complex, consisting of the focal adhesion kinase family interacting protein of 200 kDa (FIP200), ULK1/ULK2, ATG101, and ATG13. ULK1 protein kinase is crucial to initiate autophagy whereas mTOR complex presents upstream acts as a suppressor of autophagy. Upon nutrient abundance, mTOR associates and dephosphorylates ATG13 and ULK1 leading to inhibition of ULK1 kinase activity and autophagy. But under starvation, mTOR dissociates from ULK1 that leads to its phosphorylation and subsequent activation of autophagy. A number of miRNAs directly or indirectly target the mTOR protein complex or many other proteins in the pathway. In hepatocellular carcinoma cells, miR-7 precisely targets mTOR and P70S6K (Fang et al. 2012). miR-199a and miR-101 are reported to target mTOR in different cancer cell types (Chen et al. 2012a; Fornari et al. 2010; Wang et al. 2013; Wu et al. 2013). ULK2 is a direct target of miR-885-3p to inhibit autophagy. It is reported that in squamous cell carcinoma, miR-885-3p gets upregulated upon cisplatin treatment. Aberrant expression of this miRNA leads to cell death and its suppression reverses the cisplatin-mediated reduction in cell viability (Huang et al. 2011). In prostate cancer cells, miR-26b also targets ULK2 to inhibit autophagy (Clotaire et al. 2016).
In melanoma cells, the miR-290-295 cluster targets ULK1 and ATG7, leading to suppression of glucose starvation mediated autophagic death. In C2C12 myoblast cells, miR-106b and miR-20a have shown to target and suppress ULK1 expression that upregulates the transcription factor c-Myc to inhibit leucine deprivation mediated autophagy (Wu et al. 2012). Transfecting cells with miR-106b and miR-20a inhibitors were found to restore the leucine deprivation mediated autophagy (Wu et al. 2012). Another study has found that miR-595 and miR-4487 target ULK1 to curb autophagy in neuroblastoma cells. In MCF7 cells, ULK1 is the direct target of miR-25 to inhibit autophagy (Wang et al. 2014). In multi drug-resistant MCF7 cells, isoliquiritigenin induces cell death, and autophagy by suppressing the expression of miR-25 and activating ULK1 (Wang et al. 2014).
4.4.2 Vesicle Nucleation
Vesicle nucleation involves recruiting proteins and lipids to form the autophagosome membrane. It starts with the activation of class III phosphatidylinositol 3-kinase-Beclin1 (class III PI3K-BECN1) complex. Human vacuolar protein sorting 34 (hVPS34), bax-interacting factor 1 (BIF-1), UV radiation resistance-associated gene (UVRAG), ATG14L, and RUN domain and cysteine-rich domain-containing, Beclin1-interacting protein (Rubicon) are considered as the main binding partners of the complex. Zhu et al. report for the first time, the role of miRNAs in regulating autophagy at this step (Zhu et al. 2009). They had shown that miR-30a directly targets BECN1 to inhibit autophagy. In this study, chronic myeloid leukemia cells were treated with imatinib or taxol and drug sensitivity amongst the cells was observed by miR-30a through BECN1 regulation (Zhu et al. 2009). In MCF-7 cells, the upregulation of miR-30a led to a reduction in Rapamycin mediated autophagy (Zhu et al. 2009; Zou et al. 2012). It is also reported to attenuate cisplatin-induced autophagy in Hela cells and sensitized them to chemotherapy (Zou et al. 2012). Recently it has been shown that chemoresistant osteosarcoma cells show decreased levels of miR-30a indicating their potential to inhibit autophagy (Xu et al. 2016). The role of miR-30a as an autophagy inducer or inhibitor is debatable and our group checked the effect of miR-30a-3p and -5p on autophagy and found that miR-30a-5p promotes autophagy whereas miR-30a-3p inhibits autophagy in human monocytic leukemic cell-line (THP-1 cells) upon infection with Mycobacterium tuberculosis (Behura et al. 2019).
In colon cancer cells, miR-409-3p is shown to block oxaliplatin mediated autophagy by targeting BECN1 and increases the sensitization of the cancerous cells to chemotherapy (Tan et al. 2016). miR-376a and miR-376b are also reported to target 3’UTR of BECN1 and ATG4C to inhibit autophagy activated by Rapamycin in lung and breast cancer cells (Korkmaz et al. 2012, 2013). This led to the proposal of “gas and brake model” stating that the autophagy activating stress signals can subsequently upregulate expression of various miRNAs that inhibit autophagy and these inhibitory miRNAs limit the hyperactivation of autophagy and ensure survival during prolonged stress conditions (Korkmaz et al. 2013; Tekirdag et al. 2016). Huang et al. have shown that miR-519a gets downregulated upon cisplatin treatment and overexpression of miR-519a blocks Cisplatin mediated autophagy by targeting BECN1 at its 3’UTR in squamous cell carcinoma cells (Huang et al. 2011). In breast cancer cells, irradiation mediated autophagy is reported to be blocked by miR-199-5p by targeting BECN1 and DRAM1 (Yi et al. 2013). In pancreatic cancer cells, miR-216a is shown to block irradiation mediated autophagy by targeting BECN1 (Zhang et al. 2015b). miR-384-5p is also reported to inhibit BECN1 expression to reduce autophagy in macrophages during atherosclerosis (Wang et al. 2016a). Huang et al. found that miR-374a and miR-630 can regulate levels of UVRAG, a BECN1 binding factor to inhibit autophagy (Huang et al. 2012). Another binding factor of class III PI3K-Beclin1 complex is identified as the direct target of miR-195 (Shi et al. 2013). miR-101 is reported to inhibit basal level autophagy and rapamycin-induced autophagy by directly targeting ras-related in brain 5A (RAB5A) protein (Frankel et al. 2011). RAB5A is a small GTPase molecule that induces the formation of autophagosome upon interaction with hVPS34 and BECN1 (Ravikumar et al. 2008). In human dermal fibroblasts, miR-23a inhibits activating molecule in BECN1-regulated autophagy protein 1 (AMBRA1) expression, a regulator of BECN1 and VPS34 complex to inhibit autophagy upon UV-B irradiation whereas transfection with miR-23a inhibitors restored the autophagy levels (Zhang et al. 2016).
4.4.3 Regulation of Elongation
Vesicle expansion involves two unique ubiquitin-like conjugation systems. In the initial reaction, ATG12 conjugates with ATG5 aided by ATG10 and ATG7 followed by the formation of a large multimeric conglomerate upon the interaction of ATG16L with ATG12-ATG5 complex. In the second reaction, LC3 lipidation begins with the conjugation of PE. This involves ATG4 mediated cleavage of LC3 at its C-terminal end to get cytosolic LC3-I. LC3-I further gets conjugated to PE to form LC3-II aided by ATG7 and E2 like enzyme ATG3 and E3 like enzyme ATG5-ATG12 and ATG16L complex.
In breast and liver cancer cells, miR-101, miR-376a, and miR-376b are shown to suppress autophagy by targeting the homologs ATG4D and ATG4C (Frankel et al. 2011; Korkmaz et al. 2012). ATG4 family of proteases are not only involved in the cleavage of LC3 but are also involved in the autophagosome closure, the fusion of lysosome with autophagosome, and LC3 recycling, thus they act as a crucial regulatory component of autophagy (Fujita et al. 2008; Kaminskyy and Zhivotovsky 2012). RAB5A is also involved in ATG5-ATG12 conjugation which has been shown to be inhibited by miR-101 (Frankel et al. 2011; Ravikumar et al. 2008). ATG5-ATG12 conjugation is also regulated by miR-630, miR-30a, miR-374a, and miR-181a (Huang et al. 2012; Yu et al. 2012).
Another study has shown that miR-204 can also inhibit autophagy in cardiomyocytes and renal clear cell carcinoma by regulating the conversion of LC3B by targeting its 3’UTR. miR-204 acts as a tumor suppressor gene and is generally downregulated in renal clear cell carcinoma (Mikhaylova et al. 2012; Xiao et al. 2011). In cervical cancer cells, miR-211 was found to downregulate LC3-I to LC3-II conversion (Liu et al. 2020). ATG5 is also reported to be a direct target of miR-224-3p, miR-374a, miR-181a, and miR-30a (Guo et al. 2015; Huang et al. 2012; Yu et al. 2012). Furthermore, ATG12 is shown to be suppressed by miR-30d, miR-630, and miR-200b (Huang et al. 2012; Yang et al. 2013). Huang et al. have also shown that miR-885-3p could affect the levels of ATG16L1 (Huang et al. 2011). miR 519a is suggested to inhibit the expression of both ATG16 and ATG10 (Huang et al. 2012). Many studies in the literature have shown ATG7 as the most targeted gene to inhibit autophagy by miRNAs. In hepatocellular carcinoma cells, miR-375 is shown to inhibit LC3-I to LC3-II conversion and regulate the expression of ATG7 inhibiting hypoxia-mediated autophagy thus protecting the cells against hypoxic stress and exerting tumor-suppressive activity (Chang et al. 2012). In a separate study, miR-199a-5p is reported to modulate autophagy by regulating ATG7 in hepatocellular carcinoma cells during hypoxia thus reducing their viability (Xu et al. 2012). Regulatory property of miR-20a on autophagy has been explained through ATG7 suppression leading to a further reduction in the levels of ATG16L1 (Sun et al. 2015a). In glioblastoma cells, the negative effect of miR-17 and miR-137 on starvation-induced autophagy was observed to be due to a decrease in ATG7 expression (Comincini et al. 2013; Zeng et al. 2015). The role of miR-96 in regulating autophagy during hypoxia stress in prostate cancer cells was found because of aberrant ATG7 levels (Ma et al. 2014).
4.4.4 Regulation of Retrieval
The process of retrieval is governed by ATG9 complex, a multi-spanning transmembrane protein. During retrieval, various membrane proteins and lipids are recruited to the growing autophagosome membrane (Frankel and Lund 2012). ATG9 is involved in the successful trafficking of endosomes containing lipids and proteins from the trans-Golgi network to autophagosome. ATG2A and ATG2B are involved in the closure of autophagosome vesicle (Velikkakath et al. 2012). miR-130a is reported to directly target ATG2B, inhibiting autophagy, and cell viability in human chronic lymphocytic leukemia (CLL) cells. miR-130a interferes with the retrieval of proteins and lipids to the growing phagophore membrane and inhibits the formation of ATG9-ATG2-ATG18 complex, leading to inefficient closure (Kovaleva et al. 2012). A tumor suppressor gene, miR-34a acts as an autophagic flux inhibitor by regulating ATG9A levels in mammalian cells (Kovaleva et al. 2012).
4.4.5 Regulation of Fusion
The fusion of the outer membrane of autophagosome and lysosome, leading to the formation of autophagolysosome is the final step of autophagy. This process is governed by a variety of RAB proteins. RAB7 along with LAMP1 and LAMP2 are the main players of the fusion process. In the prostate cancer cell line, RAB27A and LAMP3 are reported to be the targets of miR-205 (Pennati et al. 2014). miR-207 and miR-487-5p are shown to directly target LAMP2 (Bao et al. 2016; Tao et al. 2015). Bioinformatics analysis has shortlisted a series of miRNAs having potential involvement in the fusion step. This involves miR-142, miR-204, miR-98, miR-124, and miR-130 (Jegga et al. 2011). The predicted targets of these miRNAs are v-SNARE protein, LAMP1, and LAMP2. miR-351, miR-125, miR-630, and miR-374 are reported to regulate the levels of UVRAG (Fader et al. 2009). UVRAG is involved in the regulation of membrane curvature and endosomal trafficking leading to the maturation of autophagosomes through its interaction with BECN1 (Fader et al. 2009).
4.4.6 Regulation of miRNA Biogenesis Pathway by Autophagy
The levels of miRNAs in cancer cells are generally low due to the downregulation of major miRNA processing enzymes like Drosha and Dicer. These enzymes along with AGO2 are reported to be directly targeted for autophagosomal degradation. Inhibition of Dicer through the siRNA approach decreases LC3I and LC3II levels regardless of the presence or absence of Bafilomycin A1 (Kovaleva et al. 2012). Gibbings et al. have found that Dicer and AGO2 associate with the autophagy receptor NDP52 leading to their degradation (Gibbings et al. 2012). The autophagy-deficient cells show an increase in inactive Dicer-AGO2 complex and decreased ability of AGO2 to bind to miRNAs leading to a decrease in miRNA levels (Gibbings et al. 2012; Wampfler et al. 2015). They hypothesized that reduction in autophagy leads to an accumulation of inactive Dicer-AGO2 complexes and these inactive complexes can suppress the activity of active Dicer–AGO2 complexes. So, the degradation of the inactive Dicer-AGO2 complex is necessary for the proper functioning of Dicer–AGO2 complexes (Gibbings et al. 2012).
4.5 Role of Autophagy Regulating miRNAs and Cancer
Many autophagy regulating miRNAs are shown to regulate tumor growth, metabolism, migration, hypoxia, and response to drugs and radiotherapy. Some of the miRNAs modulating autophagy are being used as cancer biomarkers or anti-cancer agents due to their efficacy in regulating gene expression. Many groups have suggested the central role of miRNA in deciphering the outcome of cancer.
4.5.1 Cancer Cell Survival and Their Growth
Autophagy plays a major role in the growth of tumor cells and thus an important function of miRNAs is to regulate autophagy in cancer cells and control their growth and proliferation. In H1299, non-small lung cancer cells, overexpression of miR-143 by using mimics reduced their proliferation significantly by directly targeting ATG2b leading to autophagy inhibition (Wei et al. 2015). In medullary thyroid cancer cell lines, upregulation of miR-9-3p arrested cells at the G2 phase of cell cycle leading to inhibition of autophagy by decreasing the expression of major autophagy-related proteins like mTOR, ATG5, LAMP-1, and PIK3C3 which subsequently causes cell death (Gundara et al. 2015). Zhai et al. have shown that miR-502 inhibits autophagy by targeting RAB1B and p53 and overexpression of miR-502 reduced cell cycle progression and cell growth in vitro in colon cancer cells (Zhai et al. 2013). In another study, miR-204 is reported to suppress the growth of renal clear cell carcinoma cells in vitro and in mice by regulating LC3 expression. miR-204 is also known to target a tumor suppressor gene, von Hippel-Lindeu (VHL, Hall et al. 2014; Mikhaylova et al. 2012). VHL in turn suppresses the expression of transient receptor potential melastatin 3 (TRPM3) involved in renal clear cell carcinoma progression (Hall et al. 2014).
Feng et al. induced nutrient starvation in breast cancer cells and found that miR-372 expression is suppressed by Yin Yang 1 (YY1) leading to an increase in autophagy. Upregulating miR-372 leads to inhibition of autophagy and subsequent growth in cancer cells (Feng et al. 2014). Overexpression of miR-100 has been shown to reduce the cell viability of hepatocellular carcinoma cells. miR-100 directly targets the 3’UTR of mTOR to regulate its expression and activate ATG7, leading to autophagy induction that kills the cancer cells (Ge et al. 2014).
4.5.2 Cancer Cell Metabolism
The autophagy regulating miRNAs are also reported to regulate the metabolism and metabolic stress responses toward the tumor cells.
In colorectal adenoma, miR-124 was found to be downregulated (Taniguchi et al. 2015). Further studies on target validation showed that miR-124 targets polypyrimidine tsat binding protein 1 (PTB1). It regulates the splicing of pyruvate kinase muscle to isoform 1 (PKM1) or isoform 2 (PKM2, Taniguchi et al. 2015). PKM1 stimulates oxidative phosphorylation and is only found in normal tissues and cells, whereas PKM2 is exclusively present on constantly proliferating cells like cancer cells. In cancer cells, PKM2 boosts glycolysis even in the presence of abundant oxygen, thus helping the cancer cell metabolism and growth. So, overexpressing miR-124 suppresses PTB1 leading to a switch between the PKM isoforms. miR-124 preferentially favors PKM1 over PKM2 thus increasing ROS accumulation and oxidative phosphorylation in tumor cells (Taniguchi et al. 2015).
In lung cancer cells, A549 and H460, expression of miR-144 is suppressed. Upregulating its expression by using mimics blocked the proliferation of cancer cells and induced autophagy and apoptosis (Chen et al. 2015). Chen et al. showed that miR-144 targets TIGAR, a glycolysis and apoptosis regulator which is responsible for a reduction in oxidative burden and regulates cell energy for metabolism (Chen et al. 2015).
In melanoma cells, another set of miRNAs, that is, miR-290-295 cluster of miRNAs target the 3’UTR of ATG7 and ULK1 (Chen et al. 2012b). These sets of miRNAs confer resistance to metabolic stress-induced cell death in B16F1 melanoma cells by inhibiting autophagy. Glucose starvation-induced cell death in these cells by upregulating autophagy but overexpressing miR-290-295 cluster of miRNAs reversed this effect and helped in the survival of tumor cells (Chen et al. 2012b).
In malignant mesothelioma tissues, expression of miR-126 is found to be downregulated and it is shown to suppress cancer cell growth (Tomasetti et al. 2016). miR-126 suppressed IRS1 and decreased glucose uptake by the cells causing energy deprivation amongst the cancer cells. AMPK gets activated upon energy deprivation and further activates ULK1. miR-126 is also reported to alter the expression of other key proteins involved in the metabolism like acetyl co-A citrate and pyruvate dehydrogenase kinase (Tomasetti et al. 2016).
4.5.3 Hypoxia Responses
Due to irregular blood supply and abnormal vascularization, tumor cells develop a hypoxic environment. The hypoxic tumor cells rely on autophagy for their survival. Thus, a number of miRNAs regulate autophagy to control hypoxia-induced responses in cancer cells.
Upon hypoxia in prostate cancer cell lines DU145 and PC3, miR-124, and miR-144 were found to be downregulated (Gu et al. 2016). Overexpression of these miRNAs in prostate cancer cells led to a reduction in hypoxia-mediated autophagy and increased radiation-induced cell death (Gu et al. 2016). Another study has shown that miR-96 under moderate levels enhances hypoxia-mediated autophagy by suppressing mTOR (Ma et al. 2014). Whereas, overexpression of the same miRNA inhibited hypoxia-induced autophagy by targeting ATG7 (Ma et al. 2014).
miR-375 is known to be downregulated following hypoxia in hepatocellular carcinoma cell lines compared to normal liver tissues. It was found to target the 3’UTR of ATG7 to suppress hypoxia-mediated autophagy leading to cell death (Chang et al. 2012). In glioblastoma cells, hypoxia leads to the downregulation of miR-224-3p but on the contrary, overexpression of miR-224-3p reduced hypoxia-mediated autophagy leading to cell death. miR-224-3p is shown to directly target ATG5 and FIP200 to inhibit autophagy, and inhibition of miR-224-3p increased hypoxia-mediated autophagy (Guo et al. 2015). Hypoxia also leads to the upregulation of IL-6 production in glioblastoma cells. Previous study has shown that overexpression of IL-6 leads to the activation of autophagy (Xue et al. 2016). Increased IL-6 secretion leads to an increase in the level of miR-155-3p through STAT3 dependent signaling pathway. miR-155-3p is reported to directly target the CREB3 regulatory factor (CREBRF) leading to an increase in the expression of ATG5 and autophagy that enhanced the survival of the cells. Blocking IL-6 reduced autophagy, increased cancer cell death, and decreased the tumor burden. On the contrary, complementary strand miR-155-5p is reported to block autophagy by downregulating mTOR and causing cell cycle arrest. Therefore, miR-155-3p and miR-155-5p acts as a switch to determine the final autophagy-related outcome under hypoxic environment (Wan et al. 2014).
4.5.4 Angiogenesis
Autophagic activity is extremely important for angiogenesis and some miRNAs are shown to regulate autophagy-induced angiogenesis in tumor vascularization. In endothelial progenitor cells, miR-195 is reported to inhibit autophagy by targeting GABARAP like 1 (GABARAPL1) protein, and knocking down miR-195 led to stimulation of autophagy and promoted angiogenesis and cell growth under hypoxia (Mo et al. 2016). Inhibition of autophagy by the addition of 3-MA blocked all the above responses indicating the role of autophagy in tumor growth and angiogenesis (Mo et al. 2016). In prostate cancer cells, miR-212 was found to be downregulated and it is shown that miR-212 is able to inhibit autophagy by directly targeting the autophagy activator SIRT1 (Ramalinga et al. 2015). Overexpressing miR-212 led to the suppression of angiogenesis and the death of cancer cells (Ramalinga et al. 2015). Another study has shown that inhibition of miR-130a led to autophagy induction and initiated cell death in endothelial progenitor cells (Xu et al. 2014). The level of miR-1273g-3p is also elucidated to be increased upon glucose level fluctuations that induced autophagy and inhibited angiogenesis in cancer cells (Guo et al. 2016).
4.5.5 Autophagy Regulating miRNAs as Biomarkers in Cancer
Some of the miRNAs involved in the regulation of autophagy have been reported as potential biomarkers of cancer. A recent study by Fan et al. 2020 unraveled the link between miR-1246 expression and sensitivity of irradiation of non-small cell lung cancer (NSCLC) cells. They had shown that the expression of miR-1246 promoted the resistance of NSCLC cells toward irradiation through inhibition of mTOR and activation of autophagy. Furthermore, the study also established the prospective role of miR-1246 to act as a biomarker for predicting the efficacy of radiotherapy in NSCLC patients and a probable target for radiotherapy sensitization.
Another recent study (Guo et al. 2019) demonstrates the effect of miR-384 on tumor progression and autophagy in NSCLC cells. Based on a previous study (Fan et al. 2017), the authors of this paper found that the expression of miR-384 was significantly downregulated in NSCLC cells and reasoned that miR-384 can be a potential therapeutic target. They also found elevated expression of Collagen α-1 (X) chain (COL10A1) and co-related inverse relationship between miR-384 and COL10A1. Overexpression of miR-384 or inhibition in COL10A1 expression led to inhibition in NSCLC cell proliferation and tumor growth through autophagy induction. Overall, this study concluded that miR-384 can promote apoptosis and autophagy in NSCLC cells by downregulating COL10A1 and this potential can be used as a biomarker for the prediction of NSCLC (Guo et al. 2019).
A recent study demonstrated the effect of miR-1251-5p on autophagy and its consequence on tumor progression in ovarian cancer cells (Shao et al. 2019). Autophagy, being a complex process, is capable of either promoting or inhibiting tumorigenesis depending on the tissue and context (Chen and Debnath 2010; Kroemer et al. 2010; Yun and Lee 2018). In the above-mentioned study, miR-1251-5p mimics increased cell cycle progression and cell proliferation whereas overexpression of tumor-binding cofactor C (TBCC) increased the expression of p62 and α/β-tubulin along with inhibition of the expression of CDK4 and LC3BII which resulted in suppression of cell growth and autophagy. In ovarian cancer cells, effects on TBCC were rescued by miR-1251-5p and promoted tumor growth. Since miR-1251-5p can directly target TBCC and enhances autophagy leading to the promotion of carcinogenesis in ovarian cancer, it can be used as a biomarker to know the severity of the disease.
In breast cancer tissues, expression of miR-205 and miR-342 levels were found to be low (Savad et al. 2012) whereas the high expression of miR-155 and miR-493 is correlated as a better recovery of patients from cancer (Gasparini et al. 2014) and suppression of miR-30e and miR-27a is associated with worsening of the disease (Gasparini et al. 2014). In ovarian cancer, a decrease in miR-152 level is correlated with cisplatin resistance (He et al. 2015) and miR-29b expression is associated with recovery (Dai et al. 2014). A decrease in the expression of miR-212 in sera and tumor tissue of patients is used as a diagnosis for prostate cancer (Ramalinga et al. 2015).
4.5.6 Role of Autophagy Regulating miRNA in Tumor Therapy
Upon identification of miRNAs in 1993, its diagnostic application in different diseases has been demonstrated. miRNA plays a central role in controlling tumor suppression in cancer. It was found by Calin et al. that downregulation or deletion of miR-16-1 and miR-15a in 13q14 leads to the progression of CLL (Calin et al. 2002). Expression of miR-16-1 and miR-15a in CLL decreases the expression of B-cell lymphoma 2 (BCL2) that has been shown to be induced by heat shock and cell stress and prevent apoptosis of tumor cells, thereby giving credence to the fact that miRNAs can be used in cancer therapy (Tsujimoto 1989; Cimmino et al. 2005). Takamizawa et al. have found that in A549 lung adenocarcinoma cells, overexpression of miRNA let-7 inhibits the growth of cancer cells in vivo (Takamizawa et al. 2004). In epithelial ovarian cancer (EOC) cells, miR-199a regulates IKKβ expression, and inhibits the proliferation of these cells (Chen et al. 2008). Downregulation of miR-221 by isoflavone, bio response formulated 3,3′-diindolylmethane (BR-DIM) and difluorinated curcumin (CDF), inhibits proliferation of pancreatic cancer cells (Sarkar et al. 2013). In colorectal cancer (CRC), upregulation of miR-324-5p suppresses the proliferation of colorectal tumor cells and its invasion by targeting embryonic lethal abnormal vision-like protein 1 (ELAVL1, Gu et al. 2019). miR-331-3p is reported to reduce the expression of erythroblastic oncogene B-2 (ERBB-2) in prostate cancer (PCa, Epis et al. 2009). ERBB-2 associated with androgen receptor signaling that promotes cell proliferation proteins known to prevent apoptosis of tumor cells which was induced by heat shock and chemotherapeutics (Vernimmen et al. 2003). miR-199b-5b also suppresses tumor progression in breast cancer by inhibiting angiogenesis because miR-199b-5p treated mice showed a reduction in tumor size and the number of blood vessels in tumors and nearby tissues (Lin et al. 2019). miR-524 also affects tumor growth and angiogenesis by inhibiting the expression of angiopoietin-2 (He et al. 2014). Role of different miRNAs like miR-320, and miR-29b have been elucidated to suppress angiogenesis by inhibiting expression of neuropilin1 and Akt3 respectively (Wu et al. 2014; Li et al. 2017). In breast cancer cells, overexpression of miR-340 is elucidated to downregulate the level of ROCK1 and inhibits invasion, migration, and proliferation of tumor cells (Maskey et al. 2017). In hepatocellular carcinoma overexpression on miR-145 downregulates the ROCK1 expression and inhibits cell proliferation (Ding et al. 2016).
4.5.6.1 miRNA Regulates Autophagy and Inhibits Tumor Progression
Autophagy plays a dual role in cancer progression and suppression. Several studies say that autophagy promotes tumor survival by supplying nutrients to the stressed cancer cells (White and DiPaola 2009). In colorectal cancer stem cells, aberrant expression of miR-140-5p inhibits growth and interferes with autophagy through son of a mother against decapentaplegic (Smad 2) and ATG12 inhibition (Zhai et al. 2015). miR-502 has been reported to inhibit autophagy by suppressing Rab1 in colon cancer cells. It is also known to inhibit cancer cell growth and arrest cell cycle that impedes tumor progression (Zhai et al. 2013). In small cell lung cancer cells, overexpression of miR-143 decreases autophagy by targeting ATG2B leading to inhibition of cancer cell proliferation (Wei et al. 2015). Overexpression of miR-193b is reported to increase autophagy in oesophageal cancer cells and non-apoptotic cell death (Nyhan et al. 2016). On the contrary, miR-9-3p is known to inhibit autophagy and also decreases the expression of BCL-2 leading to apoptosis in medullary thyroid cancer cells (Gundara et al. 2015). Downregulation of autophagy was also manifested by miR-17-5p through a reduction in Beclin1 expression in paclitaxel resistance cancer cells (Chatterjee et al. 2014). Heterogenous unclear ribonucleoproteinA1 (hnRNP A1) that prevents apoptosis of cancerous cells by increasing connective tissue growth factor (CTGF) and CyclinD1 has been elucidated to be degraded through the autophagic pathway by miR-18a over-expression in colon cancer cells (Fujiya et al. 2014).
4.5.6.2 miRNA Inhibits Chemoresistance Tumor Cell Growth by Modulating Autophagy
In osteosarcoma cells, chemotherapy-induced autophagy imparts chemoresistance whereas miR-101 block the activation of autophagy in chemoresistance osteosarcoma cells and enhance their sensitivity to chemotherapy (Chang et al. 2014). In gastric cancer cells, autophagy is shown to help the tumor cells in survival against different drugs by converting them to chemoresistance cells. Overexpression of miR-23b-3p in multidrug resistance tumor cells, is reported to inhibit autophagy by targeting ATG12 and high-mobility group protein B2 (HMGB2) that increases sensitivity to different drugs (An et al. 2015). In hepatocarcinoma cells, autophagy prevents the apoptosis of cancerous cells induced by drug-like cisplatin and helps in promoting cell growth, whereas miR-101 overexpression has been reported to inhibit autophagy and induce apoptosis in cancerous cells (Xu et al. 2013). Similarly, in 5-fluorouracil treated colorectal cancer cells, miR-22 inhibits autophagy and promotes apoptosis for efficiently killing the tumor cells (Zhang et al. 2015a). HMGB1 has been shown to be targeted by miR-22 to inhibit autophagy that prevents cell proliferation, migration, and invasion of osteocarcinoma cells (Guo et al. 2014).
4.5.6.3 miRNA Inhibits Radioresistance Tumor Cells Growth by Modulating Autophagy
Autophagy helps the cancerous cells to survive from radiation treatment and makes them resistant to radiation. In breast cancer cells, autophagy induced radiation resistance is inhibited by miR-200 and makes the cells sensitized to radiation treatment (Sun et al. 2015b). Hypoxia also induced autophagy to produce radioresistance prostate cancer cells but overexpression of miR-124 and miR-144 decreases hypoxia-mediated autophagy and converts radioresistance tumor cells to radiosensitive cells (Gu et al. 2016).
4.6 Conclusion
miRNAs play a key role in regulating different biological processes. It has been predicted that around 60% of all the protein-coding genes are regulated by miRNA. As discussed in the review earlier, miRNAs directly or indirectly modulate autophagy in different cancer types under different physiological conditions and in response to different types of stress signals. Either a single miRNA can regulate the expression of different autophagy-related proteins/pathways or many different miRNAs can also control a single important autophagy-related protein and pathways. The same stress signal or stimuli can modulate the expression of different miRNAs in different cancer cell types. These miRNAs are generally termed as oncomirs and tumor suppressors. Most of these miRNAs are reported to regulate various autophagy-related genes. Autophagy plays an important role during cancer progression and spread. Dysregulation or aberrant expression of autophagy regulating miRNAs is involved in the development of different cancers. Due to the ability of autophagy regulating miRNAs to control cancer progression, they are being used in cancer treatment as they sensitize cells to chemotherapy and radiation therapy. They can also be used as cancer biomarkers to accurately predict the diagnosis of disease and to check the patient’s response to the treatment. Thus, miRNA manipulations by using antagomirs, mimics, gene therapy, gene delivery, or other strategies can be used for cancer treatment. Comprehensive knowledge of miRNAs and related networks might contribute to the efforts involving autophagy modulation as an innovative treatment approach.
Abbreviations
- ATG:
-
Autophagy-related
- CLL:
-
Chronic lymphocyte leukemia
- CoL10A1:
-
Collagen α-1(X) chain
- ELAVL1:
-
Embryonic lethal abnormal vision-like protein-1
- EOC:
-
Epithelial ovarian cancer
- FIP 2000:
-
Focal adhesion kinase
- hnRNP A1:
-
Heterogenous unclear ribonucleoprotein A1
- LAMP1:
-
Lysosomal-associated membrane protein 1
- LAMP2:
-
Lysosomal-associated membrane protein 2
- miRNA:
-
microRNA
- mTOR:
-
Mammalian target for rapamycin
- NSCLC:
-
Non-small cell lung cancer
- PE:
-
Phosphatidyl ethanolamine
- PIK3C3:
-
Phosphatidyl inositol 3 kinase catalytic subunit type 3
- PKM1:
-
Pyruvate kinase muscle isoform 1
- PKM2:
-
Pyruvate kinase muscle isoform 2
- PTB1:
-
Polypyrimidine tsat binding protein 1
- RISC:
-
RNA-induced silencing complex
- RLC:
-
RISC-loading complex
- ROS:
-
Reactive oxygen species
- TBCC:
-
Tumor-binding cofactor C
- TIGAR:
-
TP53 inducible glycolysis and apoptosis regulator
- TRPM3:
-
Transient receptor potential melastatin 3
- ULK:
-
Unc 51 like kinase
- UTR:
-
Untranslated region
- UVRAG:
-
UV radiation resistance-associated gene protein
- VHL:
-
Van hippel lindeu
References
An Y, Zhang Z, Shang Y, Jiang X, Dong J, Yu P, Nie Y, Zhao Q (2015) miR-23b-3p regulates the chemoresistance of gastric cancer cells by targeting ATG12 and HMGB2. Cell Death Dis 6:e1766
Bao L, Lv L, Feng J, Chen Y, Wang X, Han S, Zhao H (2016) miR-487b-5p regulates temozolomide resistance of lung cancer cells through LAMP2-medicated autophagy. DNA Cell Biol 35:385–392
Bartel DP (2004) MicroRNAs: genomics, biogenesis, mechanism, and function. Cell 116:281–297
Behura A, Mishra A, Chugh S, Mawatwal S, Kumar A, Manna D, Mishra A, Singh R, Dhiman R (2019) ESAT-6 modulates Calcimycin-induced autophagy through microRNA-30a in mycobacteria infected macrophages. J Infect 79:139–152
Broughton JP, Lovci MT, Huang JL, Yeo GW, Pasquinelli AE (2016) Pairing beyond the seed supports microRNA targeting specificity. Mol Cell 64:320–333
Calin GA, Dumitru CD, Shimizu M, Bichi R, Zupo S, Noch E, Aldler H, Rattan S, Keating M, Rai K (2002) Frequent deletions and down-regulation of micro-RNA genes miR15 and miR16 at 13q14 in chronic lymphocytic leukemia. Proc Natl Acad Sci 99:15524–15529
Cecconi F, Levine B (2008) The role of autophagy in mammalian development: cell makeover rather than cell death. Dev Cell 15:344–357
Chang Y, Yan W, He X, Zhang L, Li C, Huang H, Nace G, Geller DA, Lin J, Tsung A (2012) miR-375 inhibits autophagy and reduces viability of hepatocellular carcinoma cells under hypoxic conditions. Gastroenterology 143:177–187.e8
Chang Z, Huo L, Li K, Wu Y, Hu Z (2014) Blocked autophagy by miR-101 enhances osteosarcoma cell chemosensitivity in vitro. Sci World J 2014:794756
Chatterjee A, Chattopadhyay D, Chakrabarti G (2014) miR-17-5p downregulation contributes to paclitaxel resistance of lung cancer cells through altering beclin1 expression. PLoS One 9:e95716
Chen N, Debnath J (2010) Autophagy and tumorigenesis. FEBS Lett 584:1427–1435
Chen R, Alvero A, Silasi D, Kelly M, Fest S, Visintin I, Leiser A, Schwartz P, Rutherford T, Mor G (2008) Regulation of IKKβ by miR-199a affects NF-κB activity in ovarian cancer cells. Oncogene 27:4712–4723
Chen K, Fan W, Wang X, Ke X, Wu G, Hu C (2012a) MicroRNA-101 mediates the suppressive effect of laminar shear stress on mTOR expression in vascular endothelial cells. Biochem Biophys Res Commun 427:138–142
Chen Y, Liersch R, Detmar M (2012b) The miR-290-295 cluster suppresses autophagic cell death of melanoma cells. Sci Rep 2:808
Chen S, Li P, Li J, Wang Y, Du Y, Chen X, Zang W, Wang H, Chu H, Zhao G (2015) MiR-144 inhibits proliferation and induces apoptosis and autophagy in lung cancer cells by targeting TIGAR. Cell Physiol Biochem 35:997–1007
Choi SH, Lyu SY, Park WB (2004) Mistletoe lectin induces apoptosis and telomerase inhibition in human A253 cancer cells through dephosphorylation of Akt. Arch Pharm Res 27:68
Cimmino A, Calin GA, Fabbri M, Iorio MV, Ferracin M, Shimizu M, Wojcik SE, Aqeilan RI, Zupo S, Dono M (2005) miR-15 and miR-16 induce apoptosis by targeting BCL2. Proc Natl Acad Sci 102:13944–13949
Clotaire DZJ, Zhang B, Wei N, Gao R, Zhao F, Wang Y, Lei M, Huang W (2016) MiR-26b inhibits autophagy by targeting ULK2 in prostate cancer cells. Biochem Biophys Res Commun 472:194–200
Comincini S, Allavena G, Palumbo S, Morini M, Durando F, Angeletti F, Pirtoli L, Miracco C (2013) microRNA-17 regulates the expression of ATG7 and modulates the autophagy process, improving the sensitivity to temozolomide and low-dose ionizing radiation treatments in human glioblastoma cells. Cancer Biol Ther 14:574–586
Dai F, Zhang Y, Chen Y (2014) Involvement of miR-29b signaling in the sensitivity to chemotherapy in patients with ovarian carcinoma. Hum Pathol 45:1285–1293
Demirci MDS, Bağcı C, Allmer J (2016) Differential expression of toxoplasma gondii microRNAs in murine and human hosts. In: Non-coding RNAs and inter-kingdom communication. Springer, Cham, pp 143–159
Deretic V, Levine B (2009) Autophagy, immunity, and microbial adaptations. Cell Host Microbe 5:527–549
Ding W, Tan H, Zhao C, Li X, Li Z, Jiang C, Zhang Y, Wang L (2016) MiR-145 suppresses cell proliferation and motility by inhibiting ROCK1 in hepatocellular carcinoma. Tumor Biol 37:6255–6260
Epis MR, Giles KM, Barker A, Kendrick TS, Leedman PJ (2009) miR-331-3p regulates ERBB-2 expression and androgen receptor signaling in prostate cancer. J Biol Chem 284:24696–24704
Fader CM, Sánchez DG, Mestre MB, Colombo MI (2009) TI-VAMP/VAMP7 and VAMP3/cellubrevin: two v-SNARE proteins involved in specific steps of the autophagy/multivesicular body pathways. Biochim Biophys Acta Mol Cell Res 1793:1901–1916
Fan N, Zhang J, Cheng C, Zhang X, Feng J, Kong R (2017) MicroRNA-384 represses the growth and invasion of non-small-cell lung cancer by targeting astrocyte elevated gene-1/Wnt signaling. Biomed Pharmacother 95:1331–1337
Fan L, Wang J, Cao Q, Ding X, Li B (2020) Aberrant miR-1246 expression promotes radioresistance in non-small cell lung cancer: a potential prognostic biomarker and radiotherapy sensitization target. Am J Cancer Res 10:314
Fang Y, Xue JL, Shen Q, Chen J, Tian L (2012) MicroRNA-7 inhibits tumor growth and metastasis by targeting the phosphoinositide 3-kinase/Akt pathway in hepatocellular carcinoma. Hepatology 55:1852–1862
Feng L, Ma Y, Sun J, Shen Q, Liu L, Lu H, Wang F, Yue Y, Li J, Zhang S (2014) YY1-MIR372-SQSTM1 regulatory axis in autophagy. Autophagy 10:1442–1453
Fornari F, Milazzo M, Chieco P, Negrini M, Calin GA, Grazi GL, Pollutri D, Croce CM, Bolondi L, Gramantieri L (2010) MiR-199a-3p regulates mTOR and c-Met to influence the doxorubicin sensitivity of human hepatocarcinoma cells. Cancer Res 70:5184–5193
Frankel LB, Lund AH (2012) MicroRNA regulation of autophagy. Carcinogenesis 33:2018–2025
Frankel LB, Wen J, Lees M, Høyer-Hansen M, Farkas T, Krogh A, Jäättelä M, Lund AH (2011) microRNA-101 is a potent inhibitor of autophagy. EMBO J 30:4628–4641
Fu G, Brkić J, Hayder H, Peng C (2013) MicroRNAs in human placental development and pregnancy complications. Int J Mol Sci 14:5519–5544
Fujita N, Hayashi-Nishino M, Fukumoto H, Omori H, Yamamoto A, Noda T, Yoshimori T (2008) An Atg4B mutant hampers the lipidation of LC3 paralogues and causes defects in autophagosome closure. Mol Biol Cell 19:4651–4659
Fujiya M, Konishi H, Kamel MM, Ueno N, Inaba Y, Moriichi K, Tanabe H, Ikuta K, Ohtake T, Kohgo Y (2014) microRNA-18a induces apoptosis in colon cancer cells via the autophagolysosomal degradation of oncogenic heterogeneous nuclear ribonucleoprotein A1. Oncogene 33:4847–4856
Gasparini P, Cascione L, Fassan M, Lovat F, Guler G, Balci S, Irkkan C, Morrison C, Croce CM, Shapiro CL (2014) microRNA expression profiling identifies a four microRNA signature as a novel diagnostic and prognostic biomarker in triple negative breast cancers. Oncotarget 5:1174
Ge Y-Y, Shi Q, Zheng Z-Y, Gong J, Zeng C, Yang J, Zhuang S-M (2014) MicroRNA-100 promotes the autophagy of hepatocellular carcinoma cells by inhibiting the expression of mTOR and IGF-1R. Oncotarget 5:6218
Gibbings D, Mostowy S, Jay F, Schwab Y, Cossart P, Voinnet O (2012) Selective autophagy degrades DICER and AGO2 and regulates miRNA activity. Nat Cell Biol 14:1314–1321
Gozuacik D, Akkoc Y, Ozturk DG, Kocak M (2017) Autophagy-regulating microRNAs and cancer. Front Oncol 7:65
Gregory RI, Shiekhattar R (2005) MicroRNA biogenesis and cancer. Cancer Res 65:3509–3512
Grund S, Diederichs S (2010) microRNA biogenesis and its impact on RNA interference. In: RNA technologies and their applications. Springer, Berlin, pp 325–354
Gu H, Liu M, Ding C, Wang X, Wang R, Wu X, Fan R (2016) Hypoxia-responsive miR-124 and miR-144 reduce hypoxia-induced autophagy and enhance radiosensitivity of prostate cancer cells via suppressing PIM 1. Cancer Med 5:1174–1182
Gu C, Zhang M, Sun W, Dong C (2019) Upregulation of miR-324-5p inhibits proliferation and invasion of colorectal cancer cells by targeting ELAVL1. Oncol Res 27:515–524
Gundara JS, Zhao J, Gill AJ, Lee JC, Delbridge L, Robinson BG, McLean C, Serpell J, Sidhu SB (2015) Noncoding RNA blockade of autophagy is therapeutic in medullary thyroid cancer. Cancer Med 4:174–182
Guo S, Bai R, Liu W, Zhao A, Zhao Z, Wang Y, Wang Y, Zhao W, Wang W (2014) miR-22 inhibits osteosarcoma cell proliferation and migration by targeting HMGB1 and inhibiting HMGB1-mediated autophagy. Tumor Biol 35:7025–7034
Guo X, Xue H, Guo X, Gao X, Xu S, Yan S, Han X, Li T, Shen J, Li G (2015) MiR224-3p inhibits hypoxia-induced autophagy by targeting autophagy-related genes in human glioblastoma cells. Oncotarget 6:41620
Guo J, Sang Y, Yin T, Wang B, Yang W, Li X, Li H, Kang Y (2016) miR-1273g-3p participates in acute glucose fluctuation-induced autophagy, dysfunction, and proliferation attenuation in human umbilical vein endothelial cells. Am J Physiol Endocrinol Metab 310:E734–E743
Guo Q, Zheng M, Xu Y, Wang N, Zhao W (2019) MiR-384 induces apoptosis and autophagy of non-small cell lung cancer cells through the negative regulation of collagen α-1 (X) chain gene. Biosci Rep 39:BSR20181523
Ha M, Kim VN (2014) Regulation of microRNA biogenesis. Nat Rev Mol Cell Biol 15:509
Hall DP, Cost NG, Hegde S, Kellner E, Mikhaylova O, Stratton Y, Ehmer B, Abplanalp WA, Pandey R, Biesiada J (2014) TRPM3 and miR-204 establish a regulatory circuit that controls oncogenic autophagy in clear cell renal cell carcinoma. Cancer Cell 26:738–753
Hayes J, Peruzzi PP, Lawler S (2014) MicroRNAs in cancer: biomarkers, functions and therapy. Trends Mol Med 20:460–469
He C, Klionsky DJ (2009) Regulation mechanisms and signaling pathways of autophagy. Annu Rev Genet 43:67–93
He T, Qi F, Jia L, Wang S, Song N, Guo L, Fu Y, Luo Y (2014) MicroRNA-542-3p inhibits tumour angiogenesis by targeting angiopoietin-2. J Pathol 232:499–508
He J, Yu J-J, Xu Q, Wang L, Zheng JZ, Liu L-Z, Jiang B-H (2015) Downregulation of ATG14 by EGR1-MIR152 sensitizes ovarian cancer cells to cisplatin-induced apoptosis by inhibiting cyto-protective autophagy. Autophagy 11:373–384
Huang W (2017) MicroRNAs: biomarkers, diagnostics, and therapeutics. In: Bioinformatics in MicroRNA research. Springer, New York, pp 57–67
Huang Y, Chuang AY, Ratovitski EA (2011) Phospho-ΔNp63α/miR-885-3p axis in tumor cell life and cell death upon cisplatin exposure. Cell Cycle 10:3938–3947
Huang Y, Guerrero-Preston R, Ratovitski EA (2012) Phospho-ΔNp63α-dependent regulation of autophagic signaling through transcription and micro-RNA modulation. Cell Cycle 11:1247–1259
Hwang H-W, Wentzel EA, Mendell JT (2007) A hexanucleotide element directs microRNA nuclear import. Science 315:97–100
Jegga AG, Schneider L, Ouyang X, Zhang J (2011) Systems biology of the autophagy-lysosomal pathway. Autophagy 7:477–489
Jing Z, Han W, Sui X, Xie J, Pan H (2015) Interaction of autophagy with microRNAs and their potential therapeutic implications in human cancers. Cancer Lett 356:332–338
Kaminskyy V, Zhivotovsky B (2012) Proteases in autophagy. Biochim Biophys Acta Prot Proteomics 1824:44–50
Khvorova A, Reynolds A, Jayasena SD (2003) Functional siRNAs and miRNAs exhibit strand bias. Cell 115:209–216
Kim DH, Sætrom P, Snøve O, Rossi JJ (2008) MicroRNA-directed transcriptional gene silencing in mammalian cells. Proc Natl Acad Sci 105:16230–16235
Kim VN, Han J, Siomi MC (2009) Biogenesis of small RNAs in animals. Nat Rev Mol Cell Biol 10:126–139
Korkmaz G, Le Sage C, Tekirdag KA, Agami R, Gozuacik D (2012) miR-376b controls starvation and mTOR inhibition-related autophagy by targeting ATG4C and BECN1. Autophagy 8:165–176
Korkmaz G, Tekirdag KA, Ozturk DG, Kosar A, Sezerman OU, Gozuacik D (2013) MIR376A is a regulator of starvation-induced autophagy. PLoS One 8:e82556
Kovaleva V, Mora R, Park YJ, Plass C, Chiramel AI, Bartenschlager R, Döhner H, Stilgenbauer S, Pscherer A, Lichter P (2012) miRNA-130a targets ATG2B and DICER1 to inhibit autophagy and trigger killing of chronic lymphocytic leukemia cells. Cancer Res 72:1763–1772
Krishnan P, Damaraju S (2018) The challenges and opportunities in the clinical application of noncoding RNAs: the road map for miRNAs and piRNAs in cancer diagnostics and prognostics. Int J Genomics 2018:5848046
Kroemer G, Mariño G, Levine B (2010) Autophagy and the integrated stress response. Mol Cell 40:280–293
Li Y, Cai B, Shen L, Dong Y, Lu Q, Sun S, Liu S, Ma S, Ma PX, Chen J (2017) MiRNA-29b suppresses tumor growth through simultaneously inhibiting angiogenesis and tumorigenesis by targeting Akt3. Cancer Lett 397:111–119
Liang H, Zhang J, Zen K, Zhang C-Y, Chen X (2013) Nuclear microRNAs and their unconventional role in regulating non-coding RNAs. Protein Cell 4:325–330
Lin X, Qiu W, Xiao Y, Ma J, Xu F, Zhang K, Gao Y, Chen Q, Li Y, Li H (2019) MiR-199b-5p suppresses tumor angiogenesis mediated by vascular endothelial cells in breast cancer by targeting ALK1. Front Genet 10:1397
Liu S, Wang H, Mu J, Wang H, Peng Y, Li Q, Mao D, Guo L (2020) MiRNA-211 triggers an autophagy-dependent apoptosis in cervical cancer cells: regulation of Bcl-2. Naunyn Schmiedeberg’s Arch Pharmacol 393:359–370
Ma Y, Yang H-Z, Dong B-J, Zou H-B, Zhou Y, Kong X-M, Huang Y-R (2014) Biphasic regulation of autophagy by miR-96 in prostate cancer cells under hypoxia. Oncotarget 5:9169
MacRae IJ, Ma E, Zhou M, Robinson CV, Doudna JA (2008) In vitro reconstitution of the human RISC-loading complex. Proc Natl Acad Sci 105:512–517
Maskey N, Li D, Xu H, Song H, Wu C, Hua K, Song J, Fang L (2017) MicroRNA-340 inhibits invasion and metastasis by downregulating ROCK1 in breast cancer cells. Oncol Lett 14:2261–2267
Mikhaylova O, Stratton Y, Hall D, Kellner E, Ehmer B, Drew AF, Gallo CA, Plas DR, Biesiada J, Meller J (2012) VHL-regulated MiR-204 suppresses tumor growth through inhibition of LC3B-mediated autophagy in renal clear cell carcinoma. Cancer Cell 21:532–546
Mizushima N (2019) The ATG conjugation systems in autophagy. Curr Opin Cell Biol 63:1–10
Mizushima N, Komatsu M (2011) Autophagy: renovation of cells and tissues. Cell 147:728–741
Mizushima N, Levine B, Cuervo AM, Klionsky DJ (2008) Autophagy fights disease through cellular self-digestion. Nature 451:1069–1075
Mo J, Zhang D, Yang R (2016) MicroRNA-195 regulates proliferation, migration, angiogenesis and autophagy of endothelial progenitor cells by targeting GABARAPL1. Biosci Rep 36:e00396
Nyhan MJ, O’Donovan TR, Boersma AW, Wiemer EA, McKenna SL (2016) MiR-193b promotes autophagy and non-apoptotic cell death in oesophageal cancer cells. BMC Cancer 16:101
O’Brien J, Hayder H, Zayed Y, Peng C (2018) Overview of microRNA biogenesis, mechanisms of actions, and circulation. Front Endocrinol 9:402
Okada C, Yamashita E, Lee SJ, Shibata S, Katahira J, Nakagawa A, Yoneda Y, Tsukihara T (2009) A high-resolution structure of the pre-microRNA nuclear export machinery. Science 326:1275–1279
Park JH, Shin C (2014) MicroRNA-directed cleavage of targets: mechanism and experimental approaches. BMB Rep 47:417
Paul P, Chakraborty A, Sarkar D, Langthasa M, Rahman M, Bari M, Singha RS, Malakar AK, Chakraborty S (2018) Interplay between miRNAs and human diseases. J Cell Physiol 233:2007–2018
Pennati M, Lopergolo A, Profumo V, De Cesare M, Sbarra S, Valdagni R, Zaffaroni N, Gandellini P, Folini M (2014) miR-205 impairs the autophagic flux and enhances cisplatin cytotoxicity in castration-resistant prostate cancer cells. Biochem Pharmacol 87:579–597
Ramalinga M, Roy A, Srivastava A, Bhattarai A, Harish V, Suy S, Collins S, Kumar D (2015) MicroRNA-212 negatively regulates starvation induced autophagy in prostate cancer cells by inhibiting SIRT1 and is a modulator of angiogenesis and cellular senescence. Oncotarget 6:34446
Ravikumar B, Imarisio S, Sarkar S, O’Kane CJ, Rubinsztein DC (2008) Rab5 modulates aggregation and toxicity of mutant huntingtin through macroautophagy in cell and fly models of Huntington disease. J Cell Sci 121:1649–1660
Reggiori F, Ungermann C (2017) Autophagosome maturation and fusion. J Mol Biol 429:486–496
Saçar MD, Bağcı C, Allmer J (2014) Computational prediction of microRNAs from toxoplasma gondii potentially regulating the hosts’ gene expression. Genomics Proteomics Bioinformatics 12:228–238
Salmanidis M, Pillman K, Goodall G, Bracken C (2014) Direct transcriptional regulation by nuclear microRNAs. Int J Biochem Cell Biol 54:304–311
Sarkar S, Dubaybo H, Ali S, Goncalves P, Kollepara SL, Sethi S, Philip PA, Li Y (2013) Down-regulation of miR-221 inhibits proliferation of pancreatic cancer cells through up-regulation of PTEN, p27kip1, p57kip2, and PUMA. Am J Cancer Res 3:465
Savad S, Mehdipour P, Miryounesi M, Shirkoohi R, Fereidooni F, Mansouri F, Modarressi MH (2012) Expression analysis of MiR-21, MiR-205, and MiR-342 in breast cancer in Iran. Asian Pac J Cancer Prev 13:873–877
Schneider JL, Cuervo AM (2014) Autophagy and human disease: emerging themes. Curr Opin Genet Dev 26:16–23
Seitz H, Zamore PD (2006) Rethinking the microprocessor. Cell 125:827–829
Shao Y, Liu X, Meng J, Zhang X, Ma Z, Yang G (2019) MicroRNA-1251-5p promotes carcinogenesis and autophagy via targeting the tumor suppressor TBCC in ovarian cancer cells. Mol Ther 27:1653–1664
Shi G, Shi J, Liu K, Liu N, Wang Y, Fu Z, Ding J, Jia L, Yuan W (2013) Increased miR-195 aggravates neuropathic pain by inhibiting autophagy following peripheral nerve injury. Glia 61:504–512
Shomron N, Levy C (2009) MicroRNA-biogenesis and pre-mRNA splicing crosstalk. BioMed Res Int 2009:594678
Songane M, Kleinnijenhuis J, Netea MG, van Crevel R (2012) The role of autophagy in host defence against Mycobacterium tuberculosis infection. Tuberculosis 92:388–396
Sun K-T, Chen MY, Tu M-G, Wang I-K, Chang S-S, Li C-Y (2015a) MicroRNA-20a regulates autophagy related protein-ATG16L1 in hypoxia-induced osteoclast differentiation. Bone 73:145–153
Sun Q, Liu T, Yuan Y, Guo Z, Xie G, Du S, Lin X, Xu Z, Liu M, Wang W (2015b) MiR-200c inhibits autophagy and enhances radiosensitivity in breast cancer cells by targeting UBQLN1. Int J Cancer 136:1003–1012
Suzuki H, Osawa T, Fujioka Y, Noda NN (2017) Structural biology of the core autophagy machinery. Curr Opin Struct Biol 43:10–17
Takamizawa J, Konishi H, Yanagisawa K, Tomida S, Osada H, Endoh H, Harano T, Yatabe Y, Nagino M, Nimura Y (2004) Reduced expression of the let-7 microRNAs in human lung cancers in association with shortened postoperative survival. Cancer Res 64:3753–3756
Tan S, Shi H, Ba M, Lin S, Tang H, Zeng X, Zhang X (2016) miR-409-3p sensitizes colon cancer cells to oxaliplatin by inhibiting Beclin-1-mediated autophagy. Int J Mol Med 37:1030–1038
Taniguchi K, Sugito N, Kumazaki M, Shinohara H, Yamada N, Nakagawa Y, Ito Y, Otsuki Y, Uno B, Uchiyama K (2015) MicroRNA-124 inhibits cancer cell growth through PTB1/PKM1/PKM2 feedback cascade in colorectal cancer. Cancer Lett 363:17–27
Tao J, Liu W, Shang G, Zheng Y, Huang J, Lin R, Chen L (2015) MiR-207/352 regulate lysosomal-associated membrane proteins and enzymes following ischemic stroke. Neuroscience 305:1–14
Tekirdag KA, Akkoc Y, Kosar A, Gozuacik D (2016) MIR376 family and cancer. Histol Histopathol 31:841–855
Tomasetti M, Monaco F, Manzella N, Rohlena J, Rohlenova K, Staffolani S, Gaetani S, Ciarapica V, Amati M, Bracci M (2016) MicroRNA-126 induces autophagy by altering cell metabolism in malignant mesothelioma. Oncotarget 7:36338
Tsujimoto Y (1989) Stress-resistance conferred by high level of bcl-2 alpha protein in human B lymphoblastoid cell. Oncogene 4:1331–1336
Tüfekci KU, Öner MG, Meuwissen RLJ, Genç Ş (2014) The role of microRNAs in human diseases. In: miRNomics: MicroRNA biology and computational analysis. Eds. Malik Yousef and Jens Allmer. Springer, New York, pp 33–50
Velikkakath AKG, Nishimura T, Oita E, Ishihara N, Mizushima N (2012) Mammalian Atg2 proteins are essential for autophagosome formation and important for regulation of size and distribution of lipid droplets. Mol Biol Cell 23:896–909
Vernimmen D, Guéders M, Pisvin S, Delvenne P, Winkler R (2003) Different mechanisms are implicated in ERBB2 gene overexpression in breast and in other cancers. Br J Cancer 89:899–906
Wampfler J, Federzoni EA, Torbett BE, Fey MF, Tschan MP (2015) Low DICER1 expression is associated with attenuated neutrophil differentiation and autophagy of NB4 APL cells. J Leukoc Biol 98:357–363
Wan G, Xie W, Liu Z, Xu W, Lao Y, Huang N, Cui K, Liao M, He J, Jiang Y (2014) Hypoxia-induced MIR155 is a potent autophagy inducer by targeting multiple players in the MTOR pathway. Autophagy 10:70–79
Wang Y, Liu J, Liu C, Naji A, Stoffers DA (2013) MicroRNA-7 regulates the mTOR pathway and proliferation in adult pancreatic β-cells. Diabetes 62:887–895
Wang Z, Wang N, Liu P, Chen Q, Situ H, Xie T, Zhang J, Peng C, Lin Y, Chen J (2014) MicroRNA-25 regulates chemoresistance-associated autophagy in breast cancer cells, a process modulated by the natural autophagy inducer isoliquiritigenin. Oncotarget 5:7013
Wang B, Zhong Y, Huang D, Li J (2016a) Macrophage autophagy regulated by miR-384-5p-mediated control of Beclin-1 plays a role in the development of atherosclerosis. Am J Transl Res 8:606
Wang J, Chen J, Sen S (2016b) MicroRNA as biomarkers and diagnostics. J Cell Physiol 231:25–30
Wei Y, Li L, Wang D, Zhang C-Y, Zen K (2014) Importin 8 regulates the transport of mature microRNAs into the cell nucleus. J Biol Chem 289:10270–10275
Wei J, Ma Z, Li Y, Zhao B, Wang D, Jin Y, Jin Y (2015) miR-143 inhibits cell proliferation by targeting autophagy-related 2B in non-small cell lung cancer H1299 cells. Mol Med Rep 11:571–576
White E, DiPaola RS (2009) The double-edged sword of autophagy modulation in cancer. Clin Cancer Res 15:5308–5316
Winter J, Jung S, Keller S, Gregory RI, Diederichs S (2009) Many roads to maturity: microRNA biogenesis pathways and their regulation. Nat Cell Biol 11(3):228–234
Wu H, Wang F, Hu S, Yin C, Li X, Zhao S, Wang J, Yan X (2012) MiR-20a and miR-106b negatively regulate autophagy induced by leucine deprivation via suppression of ULK1 expression in C2C12 myoblasts. Cell Signal 24:2179–2186
Wu D, Huang H-J, He C-N, Wang K-Y (2013) MicroRNA-199a-3p regulates endometrial cancer cell proliferation by targeting mammalian target of rapamycin (mTOR). Int J Gynecol Cancer 23:1191–1197
Wu Y-Y, Chen Y-L, Jao Y-C, Hsieh I-S, Chang K-C, Hong T-M (2014) miR-320 regulates tumor angiogenesis driven by vascular endothelial cells in oral cancer by silencing neuropilin 1. Angiogenesis 17:247–260
Xiao J, Zhu X, He B, Zhang Y, Kang B, Wang Z, Ni X (2011) MiR-204 regulates cardiomyocyte autophagy induced by ischemia-reperfusion through LC3-II. J Biomed Sci 18:35
Xu N, Zhang J, Shen C, Luo Y, Xia L, Xue F, Xia Q (2012) Cisplatin-induced downregulation of miR-199a-5p increases drug resistance by activating autophagy in HCC cell. Biochem Biophys Res Commun 423(4):826–831
Xu Y, An Y, Wang Y, Zhang C, Zhang H, Huang C, Jiang H, Wang X, Li X (2013) miR-101 inhibits autophagy and enhances cisplatin-induced apoptosis in hepatocellular carcinoma cells. Oncol Rep 29:2019–2024
Xu Q, Meng S, Liu B, Li MQ, Li Y, Fang L, Li YG (2014) MicroRNA-130a regulates autophagy of endothelial progenitor cells through Runx3. Clin Exp Pharmacol Physiol 41:351–357
Xu R, Liu S, Chen H, Lao L (2016) MicroRNA-30a downregulation contributes to chemoresistance of osteosarcoma cells through activating Beclin-1-mediated autophagy. Oncol Rep 35:1757–1763
Xue H, Yuan G, Guo X, Liu Q, Zhang J, Gao X, Guo X, Xu S, Li T, Shao Q (2016) A novel tumor-promoting mechanism of IL6 and the therapeutic efficacy of tocilizumab: hypoxia-induced IL6 is a potent autophagy initiator in glioblastoma via the p-STAT3-MIR155-3p-CREBRF pathway. Autophagy 12:1129–1152
Yang X, Zhong X, Tanyi JL, Shen J, Xu C, Gao P, Zheng TM, DeMichele A, Zhang L (2013) mir-30d Regulates multiple genes in the autophagy pathway and impairs autophagy process in human cancer cells. Biochem Biophys Res Commun 431:617–622
Yi R, Qin Y, Macara IG, Cullen BR (2003) Exportin-5 mediates the nuclear export of pre-microRNAs and short hairpin RNAs. Genes Dev 17:3011–3016
Yi H, Liang B, Jia J, Liang N, Xu H, Ju G, Ma S, Liu X (2013) Differential roles of miR-199a-5p in radiation-induced autophagy in breast cancer cells. FEBS Lett 587:436–443
Yu Y, Yang L, Zhao M, Zhu S, Kang R, Vernon P, Tang D, Cao L (2012) Targeting microRNA-30a-mediated autophagy enhances imatinib activity against human chronic myeloid leukemia cells. Leukemia 26:1752–1760
Yun CW, Lee SH (2018) The roles of autophagy in cancer. Int J Mol Sci 19:3466
Zeng Y, Cullen BR (2004) Structural requirements for pre-microRNA binding and nuclear export by Exportin 5. Nucleic Acids Res 32:4776–4785
Zeng Y, Huo G, Mo Y, Wang W, Chen H (2015) MIR137 regulates starvation-induced autophagy by targeting ATG7. J Mol Neurosci 56:815–821
Zhai H, Song B, Xu X, Zhu W, Ju J (2013) Inhibition of autophagy and tumor growth in colon cancer by miR-502. Oncogene 32:1570–1579
Zhai H, Fesler A, Ba Y, Wu S, Ju J (2015) Inhibition of colorectal cancer stem cell survival and invasive potential by hsa-miR-140-5p mediated suppression of Smad2 and autophagy. Oncotarget 6:19735
Zhang H, Tang J, Li C, Kong J, Wang J, Wu Y, Xu E, Lai M (2015a) MiR-22 regulates 5-FU sensitivity by inhibiting autophagy and promoting apoptosis in colorectal cancer cells. Cancer Lett 356:781–790
Zhang X, Shi H, Lin S, Ba M, Cui S (2015b) MicroRNA-216a enhances the radiosensitivity of pancreatic cancer cells by inhibiting beclin-1-mediated autophagy. Oncol Rep 34:1557–1564
Zhang J-A, Zhou B-R, Xu Y, Chen X, Liu J, Gozali M, Wu D, Z-q Y, Luo D (2016) MiR-23a-depressed autophagy is a participant in PUVA-and UVB-induced premature senescence. Oncotarget 7:37420
Zhu H, Wu H, Liu X, Li B, Chen Y, Ren X, Liu C-G, Yang J-M (2009) Regulation of autophagy by a beclin 1-targeted microRNA, miR-30a, in cancer cells. Autophagy 5:816–823
Zou Z, Wu L, Ding H, Wang Y, Zhang Y, Chen X, Chen X, Zhang C-Y, Zhang Q, Zen K (2012) MicroRNA-30a sensitizes tumor cells to cis-platinum via suppressing beclin 1-mediated autophagy. J Biol Chem 287:4148–4156
Acknowledgments
Department of Science and Technology (DST), Govt. of India (EMR/2016/000048, EEQ/2016/000205 and DST/INSPIRE/Faculty award/ 2014/DST/INSPIRE/04/2014/01662) is acknowledged by R.D. for the generous financial support to his laboratory. R.D. is also thankful to the Ministry of Human Resource and Development (MHRD), Govt. of India for intramural support. The research fellowship by DST to A.B. and MHRD to A.M., A.K. and L.N. is deeply acknowledged. D.M. is thankful to DST for Kishore Vaigyanik Protsahan Yojana (KVPY) fellowship.
Declaration of competing interest: The authors declare that they have no known competing financial interests that could have appeared to influence the work reported in this paper.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2020 Springer Nature Singapore Pte Ltd.
About this chapter
Cite this chapter
Behura, A., Mishra, A., Kumar, A., Naik, L., Manna, D., Dhiman, R. (2020). miRNAs and Its Regulatory Role on Autophagy in Tumor Microenvironment. In: Bhutia, S.K. (eds) Autophagy in tumor and tumor microenvironment . Springer, Singapore. https://doi.org/10.1007/978-981-15-6930-2_4
Download citation
DOI: https://doi.org/10.1007/978-981-15-6930-2_4
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-15-6929-6
Online ISBN: 978-981-15-6930-2
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)