Abstract
Chronic kidney disease (CKD), a chronic catabolic condition, is characterized by muscle wasting and a decreased muscle endurance. Many insights have made into the molecular mechanisms of muscle atrophy in CKD. A persistent imbalance between protein synthesis and degradation causes a loss of muscle mass. A decrease in insulin/IGF-1-Akt-mTOR signaling and an increased ubiquitin-proteasome system (UPS) have emerged as inducers of muscle loss. During muscle wasting, abnormal levels of reactive oxygen species (ROS) and inflammatory cytokines are detected in skeletal muscle. These increased ROS and inflammatory cytokine levels induce the expression of myostatin. The binding of myostatin to its receptor ActRIIB stimulates the expression of Foxo-dependent atrogenes. An impaired mitochondrial function also contributes to reduced muscle endurance. Increased glucocorticoid, angiotensin II, parathyroid hormone, and protein-bound uremic toxin levels that are observed in CKD all have a negative effect on muscle mass and endurance. The loss of skeletal muscle mass during the progression of CKD further contributes to the development of renal failure. Some potential therapeutic approaches based on the molecular mechanisms of muscle wasting in CKD are currently in the testing stages using animal models and clinical settings.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
2.1 Introduction
Skeletal muscle atrophy, referred to as sarcopenia, and decreased exercise endurance are frequently observed in chronic kidney disease (CKD) and are correlated with the risk of morbidity and mortality in such patients [1,2,3,4,5]. Therefore, maintaining physical performance is considered to be an essential factor for improving the prognosis of CKD patients. Muscle tissue functions as a protein reservoir and a source of amino acids that can be used for energy production by various tissues during catabolic conditions. In catabolic conditions such as CKD, persistent imbalances between protein synthesis and degradation result in a substantial loss of muscular protein mass (cachexia). Impaired mitochondrial function also contributes to reducing muscle endurance. This chapter explores the available evidence for the molecular mechanism of muscle wasting and potential therapeutic agents that might be used to counteract muscle atrophy in CKD.
2.2 Molecular Mechanism of Muscle Atrophy in CKD
2.2.1 Protein Degradation in Muscle
2.2.1.1 Atrogenes: Atrogin-1, MuRF-1, and Autophagy-Related Genes
A balance between protein synthesis and degradation is important for the maintenance of muscle mass. Therefore, the decrease in muscle mass can be attributed to either an increase in protein degradation or a decrease in protein synthesis. Several molecular mechanisms have been proposed to explain CKD-induced skeletal muscle atrophy in which multiple intracellular signaling pathways stimulate the expression of atrogenes such as atrogen-1 (known as muscle atrophin F-box (MAFbx)) and muscle ring factor 1 (MuRF-1, known as TRIM63), a member of the muscle-specific ubiquitin ligase family, in addition to autophagy-related genes (Fig. 2.1) [6,7,8]. The increased expression of these atrogenes induces protein degradation via the activation of the ubiquitin-proteasome system (UPS) and autophagy. In a catabolic state such as CKD, increased oxidative stress, inflammation, the production of glucocorticoids, angiotensin II, parathyroid hormone, and defective insulin signaling can initiate these pathways [6,7,8,9]. Hemodialysis procedures can also reduce protein synthesis and stimulate protein degradation [10].
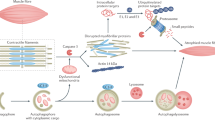
Proposed molecular mechanisms for the muscle atrophy that develops in CKD. In a catabolic condition such as CKD, increased myostatin, TGF-β, and glucocorticoid levels induce atrogenes such as atrogin-1, MuRF1, and autophagy-related genes. The reduction in insulin/IGF-1-Akt-mTOR activity then results in a decrease in protein synthesis
2.2.1.2 Myostatin and TGF-β
Myostatin, a member of the TGF-β family and an autocrine inhibitor of muscle growth, is produced predominantly in skeletal muscle and functions as a negative regulator of muscle growth [11, 12]. It binds to the activin A receptor type IIB (ActRIIB) followed by activation of the downstream pathway in which Smad2 and Smad3 are factors that mediate the effects of myostatin on muscle (Fig. 2.1) [13, 14]. In a study of the skeletal muscle of patients with CKD, Verzola et al. reported that the mRNA expression of myostatin were upregulated [15]. Zhang et al. reported that the expression of myostatin in muscle was also increased in five-sixth nephrectomized mice (CKD mice) as well as CKD patients [16], and that the administration of an anti-myostatin anti-peptide to these mice suppressed the reduction in muscle mass [17]. Myostatin expression is enhanced by oxidative stress, inflammation, and glucocorticoids [18,19,20] through the forkhead box protein O (Foxo), NF-κB [21], and Smad2/3.
TGF-β also functions as a potent inducer of muscle wasting. In fact, Mendias et al. reported that the administration of TGF- β induced muscle atrophy and fibrosis through the induction of atrogin-1 [22]. TGF-β binds to TGF-β type II and type I receptors, which activate the Smad2/3 and TAK1/p38 MAPK signaling pathways to induce atrogenes (Fig. 2.1).
2.2.2 Protein Synthesis in Muscle
2.2.2.1 Akt-mTOR Signaling and Foxo Activation
The insulin or insulin-like growth factor (IGF-1)-PI3K-Akt pathway plays important roles in skeletal muscle hypertrophy by increasing muscle protein synthesis via mTOR and decreasing protein degradation via the inactivation of the Foxo family [8, 23,24,25,26]. Lee et al. reported that muscle atrophy was increased under conditions where insulin responsiveness was impaired, and suppressing PI3K activity increased atrogin-1 activity [27]. Sandri et al. reported that a decrease in Akt activity led to the activation of Foxo transcription factors and atrogen-1 induction. In addition, an IGF-1 treatment or the overexpression of Akt suppressed the expression of Foxo and atrogen-1 [28]. In this scenario, the expression of atrogenes such as atrogin-1, MuRF-1, and autophagy-related genes is suppressed by Akt via the inactivation of Foxo, a negative regulator of transcriptional factors for atrogenes [26,27,28].
2.2.3 Mitochondria
It is well known that exercise capacity is strongly related to mitochondrial function in skeletal muscle [29]. The amount of mitochondria is regulated by both mitochondrial biosynthesis and degradation [30, 31]. Tamaki et al. recently reported that muscle mitochondria and running distance were decreased in the early-stage of CKD model mice and that it was correlated with increased oxidative stress and inflammatory responses [32]. In fact, oxidative stress and inflammation cause the expression of the peroxisome proliferator-activated receptor gamma coactivator 1α (PGC-1α), a master regulator for mitochondrial biosynthesis, to be reduced and to an increase in autophagy, a mitochondria degradation system. Interestingly, Brault et al. demonstrated that the overexpression of PGC-1α caused a resistance to muscle atrophy that was induced by denervation or fasting [33]. Similarly, Wenz et al. also showed that the overexpression of PGC-1α in mice prevented muscle atrophy, resulting in an extended life span [34]. In patients with stage 3~4 CKD, Balakrishnan et al. reported that the numbers of mitochondria in skeletal muscle were decreased [35]. They also demonstrated that the exercise increased the mitochondria content in skeletal muscle of CKD patients. Therefore, a decrease in the number of mitochondria in muscle appears to play a critical role in muscle endurance in CKD patients.
2.3 Initiating Factors Responsible for the Onset and Progression of Muscle Atrophy in CKD
2.3.1 Oxidative Stress and Inflammation
During muscle wasting, abnormally high levels of reactive oxygen species (ROS) and inflammatory cytokines are produced in skeletal muscle [21, 36]. Zhang et al. previously reported that an increase in ROS-induced TNF-α expression triggers myostatin production via a NF-κB dependent pathway, which further stimulates the production with the release of IL-6 in muscle tissue [16]. Sriram et al. also demonstrated that myostatin-induced TNF-α production via NF-κB signaling resulted in a further increase in ROS levels through the activation of NADPH oxidase [21]. Therefore, increased ROS production results in a feed forward loop that further increases the expression of myostatin via the NF-κB signaling of TNF-α.
Inflammatory cytokines such as TNF-α and IL-6, which were known to cause skeletal muscle breakdown, were also increased in muscle tissue of CKD mice [3, 37], whereas the inhibition of myostatin reduced the levels of these cytokines in the blood circulation [17]. In addition, Cheung et al. demonstrated that the infusion of TNF-α and IL-6 into mice resulted in the development of muscle atrophy, while it was attenuated by the neutralization of these cytokines [38]. Zhang et al. also reported that TNF-α activates myostatin, which further accelerates UPS-mediated catabolism [17]. Similar to myostatin, atrogin-1 was also found to be regulated by oxidative stress and inflammatory cytokines. These findings point to the conclusion that the development of skeletal muscle atrophy is mutually linked with myostatin, atrogenes, oxidative stress, and inflammation [21, 39,40,41,42,43].
2.3.2 Glucocorticoids
Increased levels of circulating glucocorticoids are associated with muscle atrophy. Watson et al. tested the direct contribution of a glucocorticoid receptor in skeletal atrophy by creating muscle-specific glucocorticoid receptor knockout mice. They subsequently showed that the knockout mice were resistance to glucocorticoid-induced muscle atrophy [44], suggesting that the glucocorticoid receptor was essential for muscle atrophy in response to glucocorticoids. Several reports have shown that myostatin expression is increased in the presence of glucocorticoids [45,46,47,48], thereby inducing protein breakdown by enhancing atrogenes (atrogine-1 and MuRF1) expression and decreasing protein synthesis by inhibiting the mTOR pathway. In particular, in the case of the IGF-1-PI3K-Akt-mTOR pathway, glucocorticoids were found to inhibit IGF-1 production [49, 50], accelerate the degradation of insulin receptor growth factor (IRS-1), followed by reducing PI3K activity [51,52,53,54]. Frost and Lang et al. showed that the constitutively activated form of Akt suppressed the negative effects of glucocorticoids on protein synthesis [55] and muscle mass [19]. Glucocorticoids also caused an increase in Foxo gene expression [46, 56]. It therefore appears that glucocorticoid receptors and Foxo synergistically contribute to the upregulation of atrogene expression [57]. Glucocorticoid-induced muscle atrophy is characterized by fast-twitch (type II muscle fiber) atrophy and reduced protein mass in muscle [58]. On the other hand, it was also reported that the administration of glucocorticoid paradoxically exerted a positive effect on muscle function, probably due to suppressing inflammatory cytokine expression [59].
2.3.3 Angiotensin II
Increased levels of circulating angiotensin II are associated with the loss of lean body mass in CKD. Brink et al. reported that angiotensin II infusion to rats induced cachexia [60]. They found that, when rats were infused with angiotensin II, muscle mass became decreased but kidney and left ventricular weights were increased (Brink [61]. In these experimental conditions, circulating IGF-1 levels were reduced by about 30% in angiotensin II-treated rats. Zhang et al. also demonstrated that the infusion of angiotensin II increased the levels of circulating IL-6 and its hepatic production [62]. In addition, the infusion of angiotensin II stimulates the suppressor of cytokine signaling (SOCS3) in muscle which led to a loss of the insulin receptor substrate 1 (IRS-1), thus impairing insulin/IGF-1 signaling [62]. Benigni et al. reported that the mouse homolog of antgiotensin II type 1 (AT1) knockout mice (agtr1a−/−) showed a decrease in oxidative stress and an increase in the number of mitochondria. In addition, the mice had a prolonged life span [63]. Yabumoto et al. recently reported that the administration of irbesartan, an AT1 receptor blocker, improved muscle repair and regeneration through the downregulation of the aging promoting C1q-Wnt/β-catenin signaling pathway [64]. These data indicate that angiotensin II can stimulate muscle atrophy through a defect in insulin/IGF-1 signaling and an inflammatory mechanism via an AT1 receptor.
2.4 Molecular Mechanism of Uremic Toxin-Induced Muscle Wasting
2.4.1 Uremic Toxin
Uremic toxins accumulate in the body under CKD conditions and exert biological actions. Among the uremic toxins, the presence of protein-bound uremic toxins, such as indoxyl sulfate, indole acetic acid, p-cresyl sulfate, hippuric acid, kynurenic acid, and 3-carboxy-4-methyl-5-propyl-2-furanpropanoic acid, etc., has been reported, due to the difficulty associated with their removal by hemodialysis because of their strong binding to serum albumin [65,66,67,68]. An accumulation of evidence has clarified that protein-bound uremic toxins are related to renal toxicity and CKD complications, including cardiovascular damage caused by enhanced oxidative stress and inflammation [69,70,71,72,73,74]. In addition, Tamaki et al. reported that feeding a high protein diet not only exacerbates impaired renal function but also reduces exercise endurance in CKD mice [75], which is accompanied by an increased production of protein-bound uremic toxins [76]. These bodies of experimental evidence led us to hypothesize that protein-bound uremic toxins play an important role in the muscle atrophy and reduced endurance.
Several mechanisms have been proposed to explain the harmful actions of protein-bound uremic toxins. For example, protein-bound uremic toxins enter the target cell via specific transporters, such as an organic anion transporter (OAT) [77,78,79,80,81,82,83], and they then exert their toxicity via the activation of cellular NADPH oxidase, which results in the overproduction of ROS and inflammatory cytokines [71,72,73]. In addition, recent reports have shown that indole containing toxins, especially indoxyl sulfate, act as aryl hydrocarbon receptor (AHR) ligands and exert their toxicity via AHR [84, 85]. Interestingly, Ohake et al. reported that AHR functions as a component of the ubiquitin ligase complex [86]. We recently demonstrated that, among the protein-bound uremic toxins, indole containing compounds, namely, indoxyl sulfate, contributed to skeletal muscle wasting [87, 88].
2.4.2 The Distribution of Indoxyl Sulfate in Muscle Tissue
OAT such as Oat1 and Oat3 is responsible for the uptake of indoxyl sulfate by cells [77,78,79]. Western blotting analyses showed the mouse Oat1 and Oat3 are expressed in C2C12 mouse myoblast cells. In addition, when half-nephrectomized mice are administered indoxyl sulfate, the indoxyl sulfate is distributed to skeletal muscle (gastrocnemius) [87]. At the same time, the pattern of the immunostaining image of indoxyl sulfate was similar to that for ROS production, suggesting that indoxyl sulfate induces ROS production in skeletal muscle in vivo (Fig. 2.2).
Proposed mechanism for indoxyl sulfate-induced muscle atrophy. Indoxyl sulfate accumulates in muscle cells via Oat where indoxyl sulfate activates the AHR pathway and NADPH oxidase to cause increased ROS production. The enhanced ROS production, in turn, triggers the production of inflammatory cytokines to induce the expression of myostatin and atrogin-1, which are involved in muscle wasting. Indoxyl sulfate also impairs mitochondrial function
2.4.3 Redox Properties of Indoxyl Sulfate in Skeletal Muscle
Indoxyl sulfate inhibits the proliferation and myotube formation in C2C12 myoblast cells. In addition, indoxyl sulfate caused an increased ROS production and inflammatory cytokine expression (TNF-α, IL-6, and TGF-β1) in C2C12 cells. It also enhances the expression of myostatin and atrogin-1. These effects which are induced by indoxyl sulfate were suppressed in the presence of an antioxidant, inhibitors of the Oat and AHR, or in the presence of siAHR. The chronic administration of indoxyl sulfate to half-nephrectomized mice significantly reduced their body weights and this reduction was accompanied by a loss in skeletal muscle weight. In these mice, indoxyl sulfate induced the expression of myostatin and atrogin-1, in addition to increasing the production of inflammatory cytokines by enhancing oxidative stress in skeletal muscle [87]. Indoxyl sulfate also induced mitochondrial dysfunction by decreasing the expression of PGC-1α and inducing autophagy (Fig. 2.2) [88].
2.4.4 Effect of p-Cresyl Sulfate on Insulin Signaling in Skeletal Muscle
Koppe et al. demonstrated that, when mice are treated with p-cresyl sulfate, insulin signaling is altered in skeletal muscle where p-cresyl sulfate inhibited insulin-stimulated glucose uptake and decreased insulin signaling pathways through the activation of the ERK kinase [89]. Regarding the downstream pathway of insulin signaling, p-cresyl sulfate suppressed the insulin-induced phosphorylation of Akt. Since indoxyl sulfate had no effect on Akt phosphorylation [87], the effect of indoxyl sulfate or p-cresyl sulfate on muscle atrophy appears to be independent of each other (Fig. 2.3).
2.5 Muscle–Kidney Crosstalk: Skeletal Muscle Affects the Renal Pathology
Hanatani and Izumiya et al. investigated the effects of muscle growth on kidney disease using muscle-specific Akt transgenic mice [90]. They showed that unilateral ureteral obstruction (UUO)-induced renal interstitial fibrosis was significantly diminished in Akt transgenic mice via mediation by an increased level of eNOS signaling in the kidney. In a recent study, Peng et al. reported that the overexpression of muscle-specific PGC-1α resulted in reduced kidney damage and fibrosis in a mouse model of kidney disease [91]. These data suggest that skeletal muscle loss during kidney disease can affect the further progress of renal failure [90,91,92].
2.6 Kidney–Fat–Muscle Crosstalk: Parathyroid Hormone (PTH) Contributes to Muscle Atrophy Via PTH Receptor Expressed in Fat Tissue
Kir et al. demonstrated that the parathyroid hormone (PTH) is involved in stimulating the expression of thermogenic gene, such as UCP1, in five-sixth nephrectomized CKD mice [9]. In this mouse model, the expression of the atrogine-1, MuRF1, and myostatin genes was increased in gastrocnemius muscle tissue, whereas IGF-1 expression was decreased. Interestingly, they also showed that the loss of PTH receptors in fat tissue blocked the upregulation of thermogenic genes and prevented muscle atrophy. These data indicate that PTH/PHR receptor signaling in fat tissue is an important player in muscle atrophy in CKD.
2.7 Potential Therapeutic Interventions for CKD-Associated Sarcopenia in the Animal Model
2.7.1 Blocking Myostatin-ActRIIB Signaling
Myostatins are negative regulators of skeletal muscle mass, which are known to signal via the ActRIIB receptor on skeletal muscle, thereby inducing muscle wasting [11]. Morvan et al. recently showed that bimagrumab, acting as a human dual-specific anti-ActRIIA/ActRIIB antibody, neutralized muscle atrophy [93]. The activin decoy receptor ActRIIB also prevented skeletal muscle pathophysiology [94, 95]. Endogenous circulating proteins such as follistatin and follistatin-like proteins are known to inhibit the binding of myostatin to ActRIIB [96, 97]. Lee et al. reported that transgenic mice expressing high levels of follistatin showed an increased muscle mass [98]. Chang et al. also demonstrated that the overexpression of muscle-specific follistatin enhanced skeletal muscle growth, due, at least in part, to myofiber hypertrophy [99]. Follistatin gene therapy against sporadic inclusion body myositis or facioscapulohumeral muscular dystrophy improved functional outcomes such as the distance traveled in a 6-min walk test [100]. Follistatin delivery systems such as nanoparticles and Fc fusion systems, etc. are under development in clinical settings [101,102,103]. In addition, the anti-myostatin peptibody that binds myostatin or blocks its receptor is also under development [17]. These data suggest that molecules that block myostatin-ActRIIB signaling would be potentially useful for enhancing muscle growth.
2.7.2 L-Carnitine
In CKD patients, restricted protein intake, decreased L-carnitine biosynthesis, and the easy removal of L-carnitine by dialysis result in an L-carnitine deficiency. Such a deficiency results in a decline in muscle power, the development of fatigue, non-ketotic hypoglycemia, or myocardial myopathy, while L-carnitine supplementation is effective for myopathy and for a decrease in muscle mass and power [104, 105]. An L-carnitine treatment ameliorates muscle atrophy and exercise capacity in CKD mice without affecting their renal function or the indoxyl sulfate levels in both plasma and muscle [88]. This can be attributed to the inhibition of mitochondrial dysfunction and decreased numbers of type I slow twitch fibers (Enoki [88].
2.7.3 DPP-4 Inhibitor
Teneligliptin, a dipeptidyl peptidase-4 (DPP-4) inhibitor, has therapeutic potential for the treatment of CKD-induced muscular dysfunction without causing changes in indoxyl sulfate accumulation [88]. The DPP-4 enzyme catalyzes the degradation of incretin hormones such as GLP-1 and glucose-dependent insulinotropic polypeptide [106]. Kang et al. recently reported that GLP-1 increased mitochondrial membrane potential and oxygen consumption in addition to increasing PGC-1α expression [107]. Fukuda-Tsuru et al. reported that a teneligliptin treatment suppressed mitochondrial dysfunction in the livers of mice that had been fed a high-fat diet [108]. GLP-1 also ameliorated insulin resistance via the activation of the PI3K-Akt signal pathway in skeletal muscle [109]. In addition, Kimura et al. reported that teneligliptin acts as a hydroxyl radical scavenger [110]. Using human proximal tubular cells, Wang et al. also reported that diportin, another DPP-4 inhibitor, inhibited cell injury via the inhibition of indoxyl sulfate-induced ROS/p38MAPK/ERK activity, and the recovery of the PI3K-Akt signaling pathway without involving the action of GLP-1[111]. Taking these findings into consideration, a DDP-4 inhibitor may exert cytoprotective activities not only indirectly via GLP-1 but also via its direct action against CKD-induced muscle atrophy.
2.7.4 AST-120
In clinical settings, AST-120 is used to suppress the progression of renal failure in CKD patients via inhibiting the accumulation of protein-bound uremic toxins. The administration of AST-120 to CKD mice resulted in a significant decrease in the plasma and muscular levels of indoxyl sulfate, which resulted in exercise capacity, muscle weight, and the number of type I slow twitch fibers to be restored and mitochondrial dysfunction was suppressed [88]. Nishikawa et al. also showed that the administration of AST-120 improved exercise capacity and mitochondrial biogenesis of skeletal muscle via reducing oxidative stress in CKD mice [112].
2.7.5 Ghrelin
Tamaki et al. reported that the administration of acylated ghrelin to five-sixth nephrectomized CKD mice increased muscle mass and muscle mitochondrial content through increasing PGC-1α expression [75, 113]. It has also been reported that the non-peptidergic ghrelin receptor agonist counteracts cachectic body weight loss under inflammatory conditions [114,115,116].
2.7.6 Blockade of Leptin Activity
Elevated serum leptin levels are correlated with changes in lean body mass in patients with CKD, suggesting that leptin signaling could be an important cause of CKD-induced muscle loss [3, 117]. Cheung et al. reported that a pegylated leptin receptor antagonist attenuated CKD-induced muscle loss [118]. Interestingly, they also found the pegylated leptin receptor antagonist was able to cross the blood–brain barrier.
2.7.7 Others
Increased miR27a/b was reported to negatively regulate the expression of myostatin [119]. Wang et al. investigated the role of miR-23a and miR-27a in the regulation of muscle mass. The injection of an adeno-virus encoding miR-23a and miR-27a or the overexpression of miR-23a and miR-27a in CKD mice suppressed muscle loss through increasing Akt phosphorylation [120]. miR1 is a muscle-specific microRNA which induces muscle atrophy by regulating HSA70. The antagonism of miR1 may be beneficial during muscle atrophy [121]. Hu et al. reported that a low frequency electrical stimulation ameliorates CKD-induced muscle atrophy by upregulating the IGF-1 signaling pathway through decreasing the expression of miR1 and miR206 [122]. Interestingly, low frequency electrical stimulation induced the activation of M2 macrophage [122].
2.8 Conclusions
This chapter summarizes the available evidence for the molecular mechanism of muscle wasting in CKD. It is noteworthy that oxidative stress and inflammation appear to be strong contributors to the muscle atrophy caused by a decrease in muscle mass and mitochondrial dysfunction. Increased levels of glucocorticoids, angiotensin II, parathyroid hormone, and uremic toxin also contribute to this type of muscle atrophy and reduced muscle endurance. These data point to the importance of developing potential therapeutic agents for counteracting the muscle atrophy that is associated with CKD.
References
Gracia-Iguacel C, González-Parra E, Pérez-Gómez MV, Mahíllo I, Egido J, Ortiz A, Carrero JJ. Prevalence of protein-energy wasting syndrome and its association with mortality in haemodialysis patients in a Centre in Spain. Nefrologia. 2013;33:495–505.
Kalantar-Zadeh K, Rhee C, Sim JJ, Stenvinkel P, Anker SD, Kovesdy CP. Why cachexia kills: examining the causality of poor outcomes in wasting conditions. J Cachexia Sarcopenia Muscle. 2013;4:89–94.
Mak RH, Ikizler AT, Kovesdy CP, Raj DS, Stenvinkel P, Kalantar-Zadeh K. Wasting in chronic kidney disease. J Cachexia Sarcopenia Muscle. 2011;2:9–25.
Sietsema KE, Amato A, Adler SG, Brass EP. Exercise capacity as a predictor of survival among ambulatory patients with end-stage renal disease. Kidney Int. 2004;65:719–24.
Wang AY, Sea MM, Tang N, Sanderson JE, Lui SF, Li PK, Woo J. Resting energy expenditure and subsequent mortality risk in peritoneal dialysis patients. J Am Soc Nephrol. 2004;15:3134–43.
Cohen S, Nathan JA, Goldberg AL. Muscle wasting in disease: molecular mechanisms and promising therapies. Nat Rev Drug Discov. 2015;14:58–74.
Gumucio JP, Mendias CL. Atrogin-1, MuRF-1, and sarcopenia. Endocrine. 2013;43:12–21.
Wang XH, Mitch WE. Mechanisms of muscle wasting in chronic kidney disease. Nat Rev Nephrol. 2014;10:504–16.
Kir S, Komaba H, Garcia AP, Economopoulos KP, Liu W, Lanske B, Hodin RA, Spiegelman BM. PTH/PTHrP receptor mediates Cachexia in models of kidney failure and Cancer. Cell Metab. 2016;23:315–23.
Crowe AV, McArdle A, McArdle F, Pattwell DM, Bell GM, Kemp GJ, Bone JM, Griffiths RD, Jackson MJ. Markers of oxidative stress in the skeletal muscle of patients on haemodialysis. Nephrol Dial Transplant. 2007;22:1177–83.
Han HQ, Zhou X, Mitch WE, Goldberg AL. Myostatin/activin pathway antagonism: molecular basis and therapeutic potential. Int J Biochem Cell Biol. 2013;45:2333–47.
McPherron AC, Lawler AM, Lee SJ. Regulation of skeletal muscle mass in mice by a new TGF-beta superfamily member. Nature. 1997;387:83–90.
Lokireddy S, McFarlane C, Ge X, Zhang H, Sze SK, Sharma M, Kambadur R. Myostatin induces degradation of sarcomeric proteins through a Smad3 signaling mechanism during skeletal muscle wasting. Mol Endocrinol. 2011;25:1936–49.
Sartori R, Milan G, Patron M, Mammucari C, Blaauw B, Abraham R, Sandri M. Smad2 and 3 transcription factors control muscle mass in adulthood. Am J Physiol Cell Physiol. 2009;296:C1248–57.
Verzola D, Procopio V, Sofia A, Villaggio B, Tarroni A, Bonanni A, Mannucci I, de Cian F, Gianetta E, Saffioti S, Garibotto G. Apoptosis and myostatin mRNA are upregulated in the skeletal muscle of patients with chronic kidney disease. Kidney Int. 2011;79:773–82.
Zhang L, Pan J, Dong Y, Tweardy DJ, Garibotto G, Mitch WE. Stat3 activation links a C/EBPδ to myostatin pathway to stimulate loss of muscle mass. Cell Metab. 2013;18:368–79.
Zhang L, Rajan V, Lin E, Hu Z, Han HQ, Zhou X, Song Y, Min H, Wang X, Du J, Mitch WE. Pharmacological inhibition of myostatin suppresses systemic inflammation and muscle atrophy in mice with chronic kidney disease. FASEB J. 2011;25:1653–63.
Lang CH, Silvis C, Nystrom G, Frost RA. Regulation of myostatin by glucocorticoids after thermal injury. FASEB J. 2001;15:1807–9.
Schakman O, Gilson H, Thissen JP. Mechanisms of glucocorticoid-induced myopathy. J Endocrinol. 2008;197:1–10.
Schakman O, Kalista S, Barbé C, Loumaye A, Thissen JP. Glucocorticoid-induced skeletal muscle atrophy. Int J Biochem Cell Biol. 2013;45:2163–72.
Sriram S, Subramanian S, Sathiakumar D, Venkatesh R, Salerno MS, McFarlane CD, Kambadur R, Sharma M. Modulation of reactive oxygen species in skeletal muscle by myostatin is mediated through NF-κB. Aging Cell. 2011;10:931–48.
Mendias CL, Gumucio JP, Davis ME, Bromley CW, Davis CS, Brooks SV. Transforming growth factor-beta induces skeletal muscle atrophy and fibrosis through the induction of atrogin-1 and scleraxis. Muscle Nerve. 2012;45:55–9.
Bodine SC, Latres E, Baumhueter S, Lai VK, Nunez L, Clarke BA, Poueymirou WT, Panaro FJ, Na E, Dharmarajan K, Pan ZQ, Valenzuela DM, DeChiara TM, Stitt TN, Yancopoulos GD, Glass DJ. Identification of ubiquitin ligases required for skeletal muscle atrophy. Science. 2001;294:1704–8.
Bonaldo P, Sandri M. Cellular and molecular mechanisms of muscle atrophy. Dis Model Mech. 2013;6:25–39.
Lai KM, Gonzalez M, Poueymirou WT, Kline WO, Na E, Zlotchenko E, Stitt TN, Economides AN, Yancopoulos GD, Glass DJ. Conditional activation of akt in adult skeletal muscle induces rapid hypertrophy. Mol Cell Biol. 2004;24:9295–304.
Stitt TN, Drujan D, Clarke BA, Panaro F, Timofeyva Y, Kline WO, Gonzalez M, Yancopoulos GD, Glass DJ. The IGF-1/PI3K/Akt pathway prevents expression of muscle atrophy-induced ubiquitin ligases by inhibiting FOXO transcription factors. Mol Cell. 2004;14:395–403.
Lee SW, Dai G, Hu Z, Wang X, Du J, Mitch WE. Regulation of muscle protein degradation: coordinated control of apoptotic and ubiquitin-proteasome systems by phosphatidylinositol 3 kinase. J Am Soc Nephrol. 2004;15:1537–45.
Sandri M, Sandri C, Gilbert A, Skurk C, Calabria E, Picard A, Walsh K, Schiaffino S, Lecker SH, Goldberg AL. Foxo transcription factors induce the atrophy-related ubiquitin ligase atrogin-1 and cause skeletal muscle atrophy. Cell. 2004;117:399–412.
Gamboa JL, Billings FT, Bojanowski MT, Gilliam LA, Yu C, Roshanravan B, Roberts LJ, Himmelfarb J, Ikizler TA, Brown NJ. Mitochondrial dysfunction and oxidative stress in patients with chronic kidney disease. Physiol Rep. 2016;4:e12780.
Arany Z, Lebrasseur N, Morris C, Smith E, Yang W, Ma Y, Chin S, Spiegelman BM. The transcriptional coactivator PGC-1beta drives the formation of oxidative type IIX fibers in skeletal muscle. Cell Metab. 2007;5:35–46.
Woldt E, Sebti Y, Solt LA, Duhem C, Lancel S, Eeckhoute J, Hesselink MK, Paquet C, Delhaye S, Shin Y, Kamenecka TM, Schaart G, Lefebvre P, Nevière R, Burris TP, Schrauwen P, Staels B, Duez H. Reverb-α modulates skeletal muscle oxidative capacity by regulating mitochondrial biogenesis and autophagy. Nat Med. 2013;19:1039–46.
Tamaki M, Miyashita K, Wakino S, Mitsuishi M, Hayashi K, Itoh H. Chronic kidney disease reduces muscle mitochondria and exercise endurance and its exacerbation by dietary protein through inactivation of pyruvate dehydrogenase. Kidney Int. 2014;85:1330–9.
Brault JJ, Jespersen JG, Goldberg AL. Peroxisome proliferator-activated receptor gamma coactivator 1alpha or 1beta overexpression inhibits muscle protein degradation, induction of ubiquitin ligases, and disuse atrophy. J Biol Chem. 2010;285:19460–71.
Wenz T, Rossi SG, Rotundo RL, Spiegelman BM, Moraes CT. Increased muscle PGC-1alpha expression protects from sarcopenia and metabolic disease during aging. Proc Natl Acad Sci U S A. 2009;106:20405–10.
Balakrishnan VS, Rao M, Menon V, Gordon PL, Pilichowska M, Castaneda F, Castaneda-Sceppa C. Resistance training increases muscle mitochondrial biogenesis in patients with chronic kidney disease. Clin J Am Soc Nephrol. 2010;5:996–1002.
Powers SK, Kavazis AN, DeRuisseau KC. Mechanisms of disuse muscle atrophy: role of oxidative stress. Am J Physiol Regul Integr Comp Physiol. 2005;288:R337–44.
Axelsson J, Heimbürger O, Stenvinkel P. Adipose tissue and inflammation in chronic kidney disease. Contrib Nephrol. 2006;151:165–74.
Cheung WW, Paik KH, Mak RH. Inflammation and cachexia in chronic kidney disease. Pediatr Nephrol. 2010;25:711–24.
Carrero JJ, Chmielewski M, Axelsson J, Snaedal S, Heimbürger O, Bárány P, Suliman ME, Lindholm B, Stenvinkel P, Qureshi AR. Muscle atrophy, inflammation and clinical outcome in incident and prevalent dialysis patients. Clin Nutr. 2008;27:557–64.
Derbre F, Ferrando B, Gomez-Cabrera MC, Sanchis-Gomar F, Martinez-Bello VE, Olaso-Gonzalez G, Diaz A, Gratas-Delamarche A, Cerda M, Viña J. Inhibition of xanthine oxidase by allopurinol prevents skeletal muscle atrophy: role of p38 MAPKinase and E3 ubiquitin ligases. PLoS One. 2012;7:e46668.
Hung AM, Ellis CD, Shintani A, Booker C, Ikizler TA. IL-1β receptor antagonist reduces inflammation in hemodialysis patients. J Am Soc Nephrol. 2011;22:437–42.
Semprun-Prieto LC, Sukhanov S, Yoshida T, Rezk BM, Gonzalez-Villalobos RA, Vaughn C, Michael Tabony A, Delafontaine P. Angiotensin II induced catabolic effect and muscle atrophy are redox dependent. Biochem Biophys Res Commun. 2011;409:217–21.
Sukhanov S, Semprun-Prieto L, Yoshida T, Michael Tabony A, Higashi Y, Galvez S, Delafontaine P. Angiotensin II, oxidative stress and skeletal muscle wasting. Am J Med Sci. 2011;342:143–7.
Watson ML, Baehr LM, Reichardt HM, Tuckermann JP, Bodine SC, Furlow JD. A cell-autonomous role for the glucocorticoid receptor in skeletal muscle atrophy induced by systemic glucocorticoid exposure. Am J Physiol Endocrinol Metab. 2012;302:E1210–20.
Artaza JN, Bhasin S, Mallidis C, Taylor W, Ma K, Gonzalez-Cadavid NF. Endogenous expression and localization of myostatin and its relation to myosin heavy chain distribution in C2C12 skeletal muscle cells. J Cell Physiol. 2002;190:170–9.
Gilson H, Schakman O, Combaret L, Lause P, Grobet L, Attaix D, Ketelslegers JM, Thissen JP. Myostatin gene deletion prevents glucocorticoid-induced muscle atrophy. Endocrinology. 2007;148:452–60.
Ma K, Mallidis C, Artaza J, Taylor W, Gonzalez-Cadavid N, Bhasin S. Characterization of 5′-regulatory region of human myostatin gene: regulation by dexamethasone in vitro. Am J Physiol Endocrinol Metab. 2001;281:E1128–36.
Ma K, Mallidis C, Bhasin S, Mahabadi V, Artaza J, Gonzalez-Cadavid N, Arias J, Salehian B. Glucocorticoid-induced skeletal muscle atrophy is associated with upregulation of myostatin gene expression. Am J Physiol Endocrinol Metab. 2003;285:E363–71.
Gayan-Ramirez G, Vanderhoydonc F, Verhoeven G, Decramer M. Acute treatment with corticosteroids decreases IGF-1 and IGF-2 expression in the rat diaphragm and gastrocnemius. Am J Respir Crit Care Med. 1999;159:283–9.
Inder WJ, Jang C, Obeyesekere VR, Alford FP. Dexamethasone administration inhibits skeletal muscle expression of the androgen receptor and IGF-1--implications for steroid-induced myopathy. Clin Endocrinol. 2010;73:126–32.
Koh A, Lee MN, Yang YR, Jeong H, Ghim J, Noh J, Kim J, Ryu D, Park S, Song P, Koo SH, Leslie NR, Berggren PO, Choi JH, Suh PG, Ryu SH. C1-ten is a protein tyrosine phosphatase of insulin receptor substrate 1 (IRS-1), regulating IRS-1 stability and muscle atrophy. Mol Cell Biol. 2013;33:1608–20.
Morgan SA, Sherlock M, Gathercole LL, Lavery GG, Lenaghan C, Bujalska IJ, Laber D, Yu A, Convey G, Mayers R, Hegyi K, Sethi JK, Stewart PM, Smith DM, Tomlinson JW. 11beta-hydroxysteroid dehydrogenase type 1 regulates glucocorticoid-induced insulin resistance in skeletal muscle. Diabetes. 2009;58:2506–15.
Nakao R, Hirasaka K, Goto J, Ishidoh K, Yamada C, Ohno A, Okumura Y, Nonaka I, Yasutomo K, Baldwin KM, Kominami E, Higashibata A, Nagano K, Tanaka K, Yasui N, Mills EM, Takeda S, Nikawa T. Ubiquitin ligase Cbl-b is a negative regulator for insulin-like growth factor 1 signaling during muscle atrophy caused by unloading. Mol Cell Biol. 2009;29:4798–811.
Zheng B, Ohkawa S, Li H, Roberts-Wilson TK, Price SR. FOXO3a mediates signaling crosstalk that coordinates ubiquitin and atrogin-1/MAFbx expression during glucocorticoid-induced skeletal muscle atrophy. FASEB J. 2010;24:2660–9.
Frost RA, Lang CH. Multifaceted role of insulin-like growth factors and mammalian target of rapamycin in skeletal muscle. Endocrinol Metab Clin N Am. 2012;41:297–322, vi.
Cho JE, Fournier M, Da X, Lewis MI. Time course expression of Foxo transcription factors in skeletal muscle following corticosteroid administration. J Appl Physiol (1985). 2010;108:137–45.
Waddell DS, Baehr LM, van den Brandt J, Johnsen SA, Reichardt HM, Furlow JD, Bodine SC. The glucocorticoid receptor and FOXO1 synergistically activate the skeletal muscle atrophy-associated MuRF1 gene. Am J Physiol Endocrinol Metab. 2008;295:E785–97.
Menconi MJ, Arany ZP, Alamdari N, Aversa Z, Gonnella P, O'Neal P, Smith IJ, Tizio S, Hasselgren PO. Sepsis and glucocorticoids downregulate the expression of the nuclear cofactor PGC-1beta in skeletal muscle. Am J Physiol Endocrinol Metab. 2010;299:E533–43.
Crossland H, Constantin-Teodosiu D, Greenhaff PL, Gardiner SM. Low-dose dexamethasone prevents endotoxaemia-induced muscle protein loss and impairment of carbohydrate oxidation in rat skeletal muscle. J Physiol. 2010;588:1333–47.
Brink M, Wellen J, Delafontaine P. Angiotensin II causes weight loss and decreases circulating insulin-like growth factor I in rats through a pressor-independent mechanism. J Clin Invest. 1996;97:2509–16.
Brink M, Price SR, Chrast J, Bailey JL, Anwar A, Mitch WE, Delafontaine P. Angiotensin II induces skeletal muscle wasting through enhanced protein degradation and down-regulates autocrine insulin-like growth factor I. Endocrinology. 2001;142:1489–96.
Zhang L, Du J, Hu Z, Han G, Delafontaine P, Garcia G, Mitch WE. IL-6 and serum amyloid A synergy mediates angiotensin II-induced muscle wasting. J Am Soc Nephrol. 2009;20:604–12.
Benigni A, Cassis P, Remuzzi G. Angiotensin II revisited: new roles in inflammation, immunology and aging. EMBO Mol Med. 2010;2:247–57.
Yabumoto C, Akazawa H, Yamamoto R, Yano M, Kudo-Sakamoto Y, Sumida T, Kamo T, Yagi H, Shimizu Y, Saga-Kamo A, Naito AT, Oka T, Lee JK, Suzuki J, Sakata Y, Uejima E, Komuro I. Angiotensin II receptor blockade promotes repair of skeletal muscle through down-regulation of aging-promoting C1q expression. Sci Rep. 2015;5:14453.
Duranton F, Cohen G, de Smet R, Rodriguez M, Jankowski J, Vanholder R, Argiles A, Group EUTW. Normal and pathologic concentrations of uremic toxins. J Am Soc Nephrol. 2012;23:1258–70.
Vanholder R, Schepers E, Pletinck A, Nagler EV, Glorieux G. The uremic toxicity of indoxyl sulfate and p-cresyl sulfate: a systematic review. J Am Soc Nephrol. 2014;25:1897–907.
Vanholder R, Pletinck A, Schepers E, Glorieux G. Biochemical and clinical impact of organic uremic retention solutes: a comprehensive update. Toxins (Basel). 2018;10:33.
Watanabe H, Miyamoto Y, Otagiri M, Maruyama T. Update on the pharmacokinetics and redox properties of protein-bound uremic toxins. J Pharm Sci. 2011;100:3682–95.
Miyamoto Y, Watanabe H, Otagiri M, Maruyama T. New insight into the redox properties of uremic solute indoxyl sulfate as a pro- and anti-oxidant. Ther Apher Dial. 2011b;15:129–31.
Miyamoto Y, Iwao Y, Mera K, Watanabe H, Kadowaki D, Ishima Y, Chuang VT, Sato K, Otagiri M, Maruyama T. A uremic toxin, 3-carboxy-4-methyl-5-propyl-2-furanpropionate induces cell damage to proximal tubular cells via the generation of a radical intermediate. Biochem Pharmacol. 2012;84:1207–14.
Niwa T. Role of indoxyl sulfate in the progression of chronic kidney disease and cardiovascular disease: experimental and clinical effects of oral sorbent AST-120. Ther Apher Dial. 2011;15:120–4.
Watanabe H, Miyamoto Y, Honda D, Tanaka H, Wu Q, Endo M, Noguchi T, Kadowaki D, Ishima Y, Kotani S, Nakajima M, Kataoka K, Kim-Mitsuyama S, Tanaka M, Fukagawa M, Otagiri M, Maruyama T. p-Cresyl sulfate causes renal tubular cell damage by inducing oxidative stress by activation of NADPH oxidase. Kidney Int. 2013a;83:582–92.
Watanabe H, Miyamoto Y, Enoki Y, Ishima Y, Kadowaki D, Kotani S, Nakajima M, Tanaka M, Matsushita K, Mori Y, Kakuta T, Fukagawa M, Otagiri M, Maruyama T. p-Cresyl sulfate, a uremic toxin, causes vascular endothelial and smooth muscle cell damages by inducing oxidative stress. Pharmacol Res Perspect. 2015;3:e00092.
Yamamoto S, Kazama JJ, Omori K, Matsuo K, Takahashi Y, Kawamura K, Matsuto T, Watanabe H, Maruyama T, Narita I. Continuous reduction of protein-bound uraemic toxins with improved oxidative stress by using the oral charcoal adsorbent AST-120 in haemodialysis patients. Sci Rep. 2015;5:14381.
Tamaki M, Hagiwara A, Miyashita K, Wakino S, Inoue H, Fujii K, Fujii C, Sato M, Mitsuishi M, Muraki A, Hayashi K, Doi T, Itoh H. Improvement of physical decline through combined effects of muscle enhancement and mitochondrial activation by a gastric hormone ghrelin in male 5/6Nx CKD model mice. Endocrinology. 2015;156:3638–48.
Poesen R, Mutsaers HA, Windey K, van den Broek PH, Verweij V, Augustijns P, Kuypers D, Jansen J, Evenepoel P, Verbeke K, Meijers B, Masereeuw R. The influence of dietary protein intake on mammalian tryptophan and phenolic metabolites. PLoS One. 2015;10:e0140820.
Deguchi T, Ohtsuki S, Otagiri M, Takanaga H, Asaba H, Mori S, Terasaki T. Major role of organic anion transporter 3 in the transport of indoxyl sulfate in the kidney. Kidney Int. 2002;61:1760–8.
Deguchi T, Kusuhara H, Takadate A, Endou H, Otagiri M, Sugiyama Y. Characterization of uremic toxin transport by organic anion transporters in the kidney. Kidney Int. 2004;65:162–74.
Deguchi T, Kouno Y, Terasaki T, Takadate A, Otagiri M. Differential contributions of rOat1 (Slc22a6) and rOat3 (Slc22a8) to the in vivo renal uptake of uremic toxins in rats. Pharm Res. 2005;22:619–27.
Miyamoto Y, Watanabe H, Noguchi T, Kotani S, Nakajima M, Kadowaki D, Otagiri M, Maruyama T. Organic anion transporters play an important role in the uptake of p-cresyl sulfate, a uremic toxin, in the kidney. Nephrol Dial Transplant. 2011a;26:2498.
Ohtsuki S, Asaba H, Takanaga H, Deguchi T, Hosoya K, Otagiri M, Terasaki T. Role of blood-brain barrier organic anion transporter 3 (OAT3) in the efflux of indoxyl sulfate, a uremic toxin: its involvement in neurotransmitter metabolite clearance from the brain. J Neurochem. 2002;83:57–66.
Tanaka H, Iwasaki Y, Yamato H, Mori Y, Komaba H, Watanabe H, Maruyama T, Fukagawa M. p-Cresyl sulfate induces osteoblast dysfunction through activating JNK and p38 MAPK pathways. Bone. 2013;56:347–54.
Watanabe H, Sakaguchi Y, Sugimoto R, Kaneko KI, Iwata H, Kotani S, Nakajima M, Ishima Y, Otagiri M, Maruyama T. Human organic anion transporters function as a high-capacity transporter for p-cresyl sulfate, a uremic toxin. Clin Exp Nephrol. 2014;18:814–20
Sallée M, Dou L, Cerini C, Poitevin S, Brunet P, Burtey S. The aryl hydrocarbon receptor-activating effect of uremic toxins from tryptophan metabolism: a new concept to understand cardiovascular complications of chronic kidney disease. Toxins (Basel). 2014;6:934–49.
Watanabe I, Tatebe J, Namba S, Koizumi M, Yamazaki J, Morita T. Activation of aryl hydrocarbon receptor mediates indoxyl sulfate-induced monocyte chemoattractant protein-1 expression in human umbilical vein endothelial cells. Circ J. 2013c;77:224–30.
Ohtake F, Baba A, Takada I, Okada M, Iwasaki K, Miki H, Takahashi S, Kouzmenko A, Nohara K, Chiba T, Fujii-Kuriyama Y, Kato S. Dioxin receptor is a ligand-dependent E3 ubiquitin ligase. Nature. 2007;446:562–6.
Enoki Y, Watanabe H, Arake R, Sugimoto R, Imafuku T, Tominaga Y, Ishima Y, Kotani S, Nakajima M, Tanaka M, Matsushita K, Fukagawa M, Otagiri M, Maruyama T. Indoxyl sulfate potentiates skeletal muscle atrophy by inducing the oxidative stress-mediated expression of myostatin and atrogin-1. Sci Rep. 2016;6:32084.
Enoki Y, Watanabe H, Arake R, Fujimura R, Ishiodori K, Imafuku T, Nishida K, Sugimoto R, Nagao S, Miyamura S, Ishima Y, Tanaka M, Matsushita K, Komaba H, Fukagawa M, Otagiri M, Maruyama T. Potential therapeutic interventions for chronic kidney disease-associated sarcopenia via indoxyl sulfate-induced mitochondrial dysfunction. J Cachexia Sarcopenia Muscle. 2017;8:735–47.
Koppe L, Pillon NJ, Vella RE, Croze ML, Pelletier CC, Chambert S, Massy Z, Glorieux G, Vanholder R, Dugenet Y, Soula HA, Fouque D, Soulage CO. p-Cresyl sulfate promotes insulin resistance associated with CKD. J Am Soc Nephrol. 2013;24:88–99.
Hanatani S, Izumiya Y, Araki S, Rokutanda T, Kimura Y, Walsh K, Ogawa H. Akt1-mediated fast/glycolytic skeletal muscle growth attenuates renal damage in experimental kidney disease. J Am Soc Nephrol. 2014;25:2800–11.
Peng H, Wang Q, Lou T, Qin J, Jung S, Shetty V, Li F, Wang Y, Feng XH, Mitch WE, Graham BH, Hu Z. Myokine mediated muscle-kidney crosstalk suppresses metabolic reprogramming and fibrosis in damaged kidneys. Nat Commun. 2017;8:1493.
Rondon-Berrios H, Wang Y, Mitch WE. Can muscle-kidney crosstalk slow progression of CKD? J Am Soc Nephrol. 2014;25:2681–3.
Morvan F, Rondeau JM, Zou C, Minetti G, Scheufler C, Scharenberg M, Jacobi C, Brebbia P, Ritter V, Toussaint G, Koelbing C, Leber X, Schilb A, Witte F, Lehmann S, Koch E, Geisse S, Glass DJ, Lach-Trifilieff E. Blockade of activin type II receptors with a dual anti-ActRIIA/IIB antibody is critical to promote maximal skeletal muscle hypertrophy. Proc Natl Acad Sci U S A. 2017;114:12448–53.
Bondulich MK, Jolinon N, Osborne GF, Smith EJ, Rattray I, Neueder A, Sathasivam K, Ahmed M, Ali N, Benjamin AC, Chang X, Dick JRT, Ellis M, Franklin SA, Goodwin D, Inuabasi L, Lazell H, Lehar A, Richard-Londt A, Rosinski J, Smith DL, Wood T, Tabrizi SJ, Brandner S, Greensmith L, Howland D, Munoz-Sanjuan I, Lee SJ, Bates GP. Myostatin inhibition prevents skeletal muscle pathophysiology in Huntington's disease mice. Sci Rep. 2017;7:14275.
Jeong Y, Daghlas SA, Kahveci AS, Salamango D, Gentry BA, Brown M, Rector RS, Pearsall RS, Phillips CL. Soluble activin receptor type IIB decoy receptor differentially impacts murine osteogenesis imperfecta muscle function. Muscle Nerve. 2018;57:294–304.
Gilson H, Schakman O, Kalista S, Lause P, Tsuchida K, Thissen JP. Follistatin induces muscle hypertrophy through satellite cell proliferation and inhibition of both myostatin and activin. Am J Physiol Endocrinol Metab. 2009;297:E157–64.
Kalista S, Schakman O, Gilson H, Lause P, Demeulder B, Bertrand L, Pende M, Thissen JP. The type 1 insulin-like growth factor receptor (IGF-IR) pathway is mandatory for the follistatin-induced skeletal muscle hypertrophy. Endocrinology. 2012;153:241–53.
Lee SJ, McPherron AC. Regulation of myostatin activity and muscle growth. Proc Natl Acad Sci U S A. 2001;98:9306–11.
Chang F, Fang R, Wang M, Zhao X, Chang W, Zhang Z, Li N, Meng Q. The transgenic expression of human follistatin-344 increases skeletal muscle mass in pigs. Transgenic Res. 2017;26:25–36.
Mendell JR, Sahenk Z, Al-Zaidy S, Rodino-Klapac LR, Lowes LP, Alfano LN, Berry K, Miller N, Yalvac M, Dvorchik I, Moore-Clingenpeel M, Flanigan KM, Church K, Shontz K, Curry C, Lewis S, McColly M, Hogan MJ, Kaspar BK. Follistatin gene therapy for sporadic inclusion body myositis improves functional outcomes. Mol Ther. 2017;25:870–9.
Castonguay R, Lachey J, Wallner S, Strand J, Liharska K, Watanabe AE, Cannell M, Davies MV, Sako D, Troy ME, Krishnan L, Mulivor AW, Li H, Keates S, Alexander MJ, Pearsall RS, Kumar R. Follistatin-288-fc fusion protein promotes localized growth of skeletal muscle. J Pharmacol Exp Ther. 2019;368:435–45.
Iskenderian A, Liu N, Deng Q, Huang Y, Shen C, Palmieri K, Crooker R, Lundberg D, Kastrapeli N, Pescatore B, Romashko A, Dumas J, Comeau R, Norton A, Pan J, Rong H, Derakhchan K, Ehmann DE. Myostatin and activin blockade by engineered follistatin results in hypertrophy and improves dystrophic pathology in mdx mouse more than myostatin blockade alone. Skelet Muscle. 2018;8:34.
Schumann C, Nguyen DX, Norgard M, Bortnyak Y, Korzun T, Chan S, Lorenz AS, Moses AS, Albarqi HA, Wong L, Michaelis K, Zhu X, Alani AWG, Taratula OR, Krasnow S, Marks DL, Taratula O. Increasing lean muscle mass in mice via nanoparticle-mediated hepatic delivery of follistatin mRNA. Theranostics. 2018;8:5276–88.
Levitan MD, Murphy JT, Sherwood WG, Deck J, Sawa GM. Adult onset systemic carnitine deficiency: favorable response to L-carnitine supplementation. Can J Neurol Sci. 1987;14:50–4.
Pistone G, Marino A, Leotta C, Dell’Arte S, Finocchiaro G, Malaguarnera M. Levocarnitine administration in elderly subjects with rapid muscle fatigue: effect on body composition, lipid profile and fatigue. Drugs Aging. 2003;20:761–7.
Hansotia T, Maida A, Flock G, Yamada Y, Tsukiyama K, Seino Y, Drucker DJ. Extrapancreatic incretin receptors modulate glucose homeostasis, body weight, and energy expenditure. J Clin Invest. 2007;117:143–52.
Kang MY, Oh TJ, Cho YM. Glucagon-like peptide-1 increases mitochondrial biogenesis and function in INS-1 rat insulinoma cells. Endocrinol Metab (Seoul). 2015;30:216–20.
Fukuda-Tsuru S, Kakimoto T, Utsumi H, Kiuchi S, Ishii S. The novel dipeptidyl peptidase-4 inhibitor teneligliptin prevents high-fat diet-induced obesity accompanied with increased energy expenditure in mice. Eur J Pharmacol. 2014;723:207–15.
Rupprecht LE, Mietlicki-Baase EG, Zimmer DJ, McGrath LE, Olivos DR, Hayes MR. Hindbrain GLP-1 receptor-mediated suppression of food intake requires a PI3K-dependent decrease in phosphorylation of membrane-bound Akt. Am J Physiol Endocrinol Metab. 2013;305:E751–9.
Kimura S, Inoguchi T, Yamasaki T, Yamato M, Ide M, Sonoda N, Yamada K, Takayanagi R. A novel DPP-4 inhibitor teneligliptin scavenges hydroxyl radicals: in vitro study evaluated by electron spin resonance spectroscopy and in vivo study using DPP-4 deficient rats. Metabolism. 2016;65:138–45.
Wang WJ, Chang CH, Sun MF, Hsu SF, Weng CS. DPP-4 inhibitor attenuates toxic effects of indoxyl sulfate on kidney tubular cells. PLoS One. 2014;9:e93447.
Nishikawa M, Ishimori N, Takada S, Saito A, Kadoguchi T, Furihata T, Fukushima A, Matsushima S, Yokota T, Kinugawa S, Tsutsui H. AST-120 ameliorates lowered exercise capacity and mitochondrial biogenesis in the skeletal muscle from mice with chronic kidney disease via reducing oxidative stress. Nephrol Dial Transplant. 2015;30:934–42.
Tamaki M, Miyashita K, Hagiwara A, Wakino S, Inoue H, Fujii K, Fujii C, Endo S, Uto A, Mitsuishi M, Sato M, Doi T, Itoh H. Ghrelin treatment improves physical decline in sarcopenia model mice through muscular enhancement and mitochondrial activation. Endocr J. 2017;64:S47–51.
Borner T, Loi L, Pietra C, Giuliano C, Lutz TA, Riediger T. The ghrelin receptor agonist HM01 mimics the neuronal effects of ghrelin in the arcuate nucleus and attenuates anorexia-cachexia syndrome in tumor-bearing rats. Am J Physiol Regul Integr Comp Physiol. 2016;311:R89–96.
Villars FO, Pietra C, Giuliano C, Lutz TA, Riediger T. Oral treatment with the ghrelin receptor agonist HM01 attenuates Cachexia in mice bearing Colon-26 (C26) tumors. Int J Mol Sci. 2017;18:E986.
Yoshimura M, Shiomi Y, Ohira Y, Takei M, Tanaka T. Z-505 hydrochloride, an orally active ghrelin agonist, attenuates the progression of cancer cachexia via anabolic hormones in Colon 26 tumor-bearing mice. Eur J Pharmacol. 2017;811:30–7.
Mak RH, Cheung WW, Solomon G, Gertler A. Preparation of potent leptin receptor antagonists and their therapeutic use in mouse models of uremic Cachexia and kidney fibrosis. Curr Pharm Des. 2018;24:1012–8.
Cheung WW, Ding W, Gunta SS, Gu Y, Tabakman R, Klapper LN, Gertler A, Mak RH. A pegylated leptin antagonist ameliorates CKD-associated cachexia in mice. J Am Soc Nephrol. 2014;25:119–28.
McFarlane C, Vajjala A, Arigela H, Lokireddy S, Ge X, Bonala S, Manickam R, Kambadur R, Sharma M. Negative auto-regulation of Myostatin expression is mediated by Smad3 and MicroRNA-27. PLoS One. 2014;9:e87687.
Wang B, Zhang C, Zhang A, Cai H, Price SR, Wang XH. MicroRNA-23a and MicroRNA-27a mimic exercise by ameliorating CKD-induced muscle atrophy. J Am Soc Nephrol. 2017;28:2631–40.
Kukreti H, Amuthavalli K, Harikumar A, Sathiyamoorthy S, Feng PZ, Anantharaj R, Tan SL, Lokireddy S, Bonala S, Sriram S, McFarlane C, Kambadur R, Sharma M. Muscle-specific microRNA1 (miR1) targets heat shock protein 70 (HSP70) during dexamethasone-mediated atrophy. J Biol Chem. 2013;288:6663–78.
Hu L, Klein JD, Hassounah F, Cai H, Zhang C, Xu P, Wang XH. Low-frequency electrical stimulation attenuates muscle atrophy in CKD--a potential treatment strategy. J Am Soc Nephrol. 2015;26:626–35.
Acknowledgement
We are grateful to Professor Masafumi Fukagawa, Division of Nephrology, Endocrinology and Metabolism, Tokai University School of Medicine, Kanagawa, Japan, Dr. Kazutaka Matsushita and Dr. Motoko Tanaka, Department of Nephrology, Akebono Clinic, Kumamoto, Japan for their valuable advice in the preparation of this manuscript. Our work was supported, in part, by a Grant-in-Aid for Scientific Research from the Japan Society for the Promotion of Science (JSPS) (KAKENHI 25460190; 16H05114), the Research Foundation for Pharmaceutical Sciences, Japan and The Nakatomi Foundation.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2020 Springer Nature Singapore Pte Ltd.
About this chapter
Cite this chapter
Watanabe, H., Enoki, Y., Maruyama, T. (2020). Molecular Mechanism of Muscle Wasting in CKD. In: Kato, A., Kanda, E., Kanno, Y. (eds) Recent Advances of Sarcopenia and Frailty in CKD. Springer, Singapore. https://doi.org/10.1007/978-981-15-2365-6_2
Download citation
DOI: https://doi.org/10.1007/978-981-15-2365-6_2
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-15-2364-9
Online ISBN: 978-981-15-2365-6
eBook Packages: MedicineMedicine (R0)