Abstract
Follistatin (FST), which was first found in the follicles of cattle and pigs, has been shown to be an essential regulator for muscle development. Mice that were genetically engineered to overexpress Fst specifically in muscle had at least twice the amount of skeletal muscle mass as controls; these findings are similar to earlier results obtained in myostatin-knockout mice. However, the role of follistatin in skeletal muscle development has yet to be clarified in livestock. Here, we describe transgenic Duroc pigs that exogenously express Fst specifically in muscle tissue. The transgenic pigs exhibited an increased proportion of skeletal muscle and a reduced proportion of body fat that were similar to those reported in myostatin-null cattle. The lean percentage of lean meat was significantly higher in the F1 generation of TG pigs (72.95 ± 1.0 %) than in WT pigs (69.18 ± 0.97 %) (N = 16, P < 0.05). Myofiber hypertrophy was also observed in the longissimus dorsi of transgenic pigs, possibly contributing to the increased skeletal muscle mass. Western blot analysis showed a significantly reduced level of Smad2 phosphorylation and an increased level of AktS473 phosphorylation in the skeletal muscle tissue of the transgenic pigs. Moreover, no cardiac muscle hypertrophy or reproductive abnormality was observed. These findings indicate that muscle-specific Fst overexpression in pigs enhances skeletal muscle growth, at least partly due to myofiber hypertrophy and providing a promising approach to increase muscle mass in pigs and other livestock.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Follistatin (FST) is essential for skeletal myogenesis, which enhances muscle fiber formation and growth (Iezzi et al. 2004; Medeiros et al. 2009). The targeted ablation of follistatin leads to perinatal lethality associated with impaired skeletal muscle development (Matzuk et al. 1995), and mice that are heterozygous for the follistatin (Fst+/−) gene exhibit haploinsufficiency and significantly reduced muscle mass (Lee et al. 2010).
Follistatin was originally found to antagonize activin A in reproductive tissues and was later shown to antagonize several other proteins within the transforming growth factor-beta (TGF-beta) superfamily (Abe et al. 2004; Amthor et al. 2002; Iemura et al. 1998; Thompson et al. 2005b; Wang et al. 2000). Myostatin (MSTN) is a member of the TGF-beta superfamily and is expressed specifically in developing and adult muscle tissues (McPherron et al. 1997). Myostatin binds to activin receptor II (ActRIIB) at the cell membrane to activate ALK4/5 (type I), which triggers the phosphorylation of smad2/3 (Kollias and McDermott 2008). Previous studies have demonstrated that this signal pathway plays an important role in the negative regulation of muscle development (Langley et al. 2002). Myostatin inhibition has been shown to decrease smad2/3 phosphorylation and stimulate Akt/mTOR signaling, thus promoting protein anabolism in muscle cells (Trendelenburg et al. 2009; Welle 2009). The reduction or loss of myostatin activity, either through inhibition or through naturally occurring mutations, significantly enhances skeletal muscle mass in several species, including mice, dogs, human and cattle (Kambadur et al. 1997; McPherron et al. 1997; Mosher et al. 2007; Williams 2004). Follistatin is capable of binding directly to myostatin to inhibit the receptor-binding activity according to in vitro reporter gene assays (Amthor et al. 2004). Moreover, follistatin also appears to be capable of blocking endogenous myostatin activity in vivo because transgenic mice with follistatin overexpression specifically in skeletal muscle exhibited dramatic increases in muscle growth that were comparable to increases observed in myostatin-knockout mice (Lee and McPherron 2001).
Follistatin has two precursor isoforms, FST317 (317aa) and FST344 (344aa), which are translated after the alternative splicing of follistatin precursor mRNA. Subsequent post-translational modifications of FST317 and FST344 create two mature forms of follistatin, FST288 (288aa) and FST315 (315aa), respectively (Patel 1998). FST315 is the major circulating isoform of follistatin, and FST288 is tissue-specific (Schneyer et al. 2004a). It is noteworthy that the increased FST344 expression was not detrimental to the reproductive function of treated mice (Haidet et al. 2008). In addition, the safety and effectiveness of long-term FST344 expression have also been evaluated in non-human primates. The long-term expression of AAV-mediated FST344 in the quadriceps muscles of cynomolgus macaque monkeys increased their muscle mass and strength without any deleterious effect on their critical organs or systems (Kota et al. 2009). This minimal off-target effect suggests that increasing FST344 expression specifically in muscles might be a useful strategy for enhancing muscle growth in livestock.
Pigs are important in agriculture as sources of animal protein for humans. Pigs are also good animal models for studying human diseases or tissue development because the anatomy and physiological properties of pigs are very similar to those of humans. The exact function of myostatin or follistatin during pig muscle development remains unknown. To address this question, we generated transgenic pigs by somatic cell nuclear transfer (SCNT) and the resulting animals expressed recombinant human follistatin-344 (rhFst344) specifically in their muscle tissues. We found that rhFst344 over-expression significantly increased skeletal muscle mass and reduced fat accumulation in transgenic pigs. The results presented in this study provide new insights into the role of follistatin in pig muscle development.
Results
Generation and characterization of transgenic pigs
To investigate the function of follistatin in pig skeletal muscle development, we expressed the long splice variant of human Fst344 (hFst344) in pig muscle tissues under the control of a pig skeletal alpha- actin (PSA) promoter. The transgene cassette contained the hFst344 CDS together with 11.5 kb of the 5′ upstream and 7.5 kb of the 3′ downstream regulatory elements from the PSA gene (Fig. 1a).
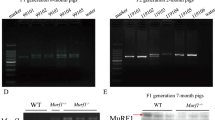
Identification of transgenic pigs. a The linearized transgene construct contains a 12-kb 5′ flanking region and an 8-kb 3′ flanking region from PSA. The coding sequence of PSA was replaced by human follistatin-344 cDNA starting from the initial codon. b 14 out of 16 male piglets were identified as positive for transgene integration based on genotyping PCR. The PCR primers are shown in (a). +, transgene construct DNA as a positive control; −, genomic DNA of wild-type pig as a negative control. c 16 male piglets were further screened using Southern blot analysis. Genomic DNA was digested using EcoRI. The EcoRI restriction sites and the location of the DNA probe in the transgene are indicated in (a). NC, wild-type pig; P1C, one copy of the transgene construct; P2C, 2 copies of the transgene construct; P10C, 10 copies of the transgene construct; piglets Nos. 273, 274 and 276 were derived from the No. 3 cell clone; piglets Nos. 277, 278, 279, 280, 281, 282, 284, 287, 288 and 289 were derived from the No. 24 cell clone; Piglets Nos. 291, 292 and 296 were derived from the No. 6 cell clone
Three positive cell clones (Nos. 3, 6 and 24) of transgenic fetal fibroblasts (♂) were generated and selected using the above-described method. Cells derived from cell clones Nos. 3, 6 and 24 were used as donor cells to produce cloned embryos using SCNT. 1200 cloned embryos at the 2- to 4-cell stages were implanted in the oviducts of 8 recipient sows. Two recipients had spontaneous abortions, and the fetuses of the remaining six recipients developed to full term. 16 cloned male piglets were obtained from 6 litters. Genomic PCR (Fig. 1b) and Southern blot analysis (Fig. 1c) identified the 16 surviving cloned piglets that 14 were positive and two (Nos. 282 and 284) were negative for the rhFst344 transgene.
Analyzing rhFst344 expression in transgenic pigs
Using RT-PCR, transgene expression was determined in the heart, liver, spleen, lung, kidney, tongue, stomach, intestine, brain, pituitary, testis and skeletal muscle tissues of rhFst344 transgenic pigs (Fig. 2a, b). As shown in Fig. 2b, rhFst344 transgene transcription was detected in the heart, lingualis and skeletal muscle, suggesting that transgene expression was specific to muscle tissues. The expression of follistatin in transgenic pig skeletal muscle was quantified using a human FST ELISA kit. The transgenic pig groups TG-3, TG-6 and TG-24 were derived from transgenic clones Nos. 3, 6 and 24, respectively. The follistatin concentrations in longissimus dorsi tissues from pigs in the TG-3 (N = 3), TG-6 (N = 3), TG-24 (N = 8) and wild-type (WT) (N = 3) groups were 38.15 ± 3.63, 49.21 ± 2.97, 165.77 ± 6.63 and 39.11 ± 0.43 pg/mg, respectively (Fig. 2c). Thus, the TG-24 transgenic pigs expressed the highest levels of rhFst344 transgene within the transgenic groups.
rhFst344 expression in transgenic pigs. a mRNA of the rhFst344 transgene. E1 and E2 represent exon 1 and exon 2 of PSA. b The mRNA expression of the rhFst344 transgene in various tissues of TG-24 transgenic pig was determined using RT-PCR. GAPDH was used as a reference gene. The location of the RT-PCR primers is shown in (a). The concentration of follistatin protein in the longissimus dorsi tissue (c) and serum (d) of pigs was quantified using ELISA. TG-3, a transgenic pig group derived from clone No. 3 (N = 3); TG-6, a transgenic pig group derived from clone No. 6 (N = 3); TG-24, a transgenic pig group derived from clone No. 24 (n = 8); WT, wild-type pig; *P < 0.05; **P < 0.01; ***P < 0.005
The FST315 (mature form of FST344) can circulate and diffuse from the original expression site into the blood (Haidet et al. 2008). To determine whether rhFst344 that had been specifically expressed in muscle would diffuse into the blood circulation of transgenic pigs, plasma concentrations of follistatin in transgenic pigs were quantified using ELISA. The follistatin concentration in pig serum obtained from pigs in the TG-6 and TG-24 groups (355.63 ± 11.94 and 632.69 ± 37.48 pg/mL, respectively) was much higher than that obtained from pigs in the WT group (297.17 ± 4.18 pg/mL); however, there was no significant difference between the plasma concentration of follistatin in TG-3 pigs (313.42 ± 8.85 pg/mL) and in the WT controls (Fig. 2d). These results might suggest that the increased follistatin concentration observed in the serum of transgenic pigs resulted from rhFst344 transgene expression.
Productive performance is improved in transgenic pigs expressing FST344 specifically in muscles
Transgenic pigs in the TG-24 group expressed the highest rhFst344 levels (Fig. 2c, d) and exhibited a significant increase in skeletal muscle mass with no other phenotype abnormalities (Fig. 3a). Therefore, we selected the TG-24 group for further characterization.
To investigate the productive performance of F1 generation transgenic pigs in the TG-24 group, we compared the carcass traits of transgenic (TG) pigs with that of their WT littermates. Sixteen TG pigs and 16 wild-type controls (8 males and 8 females in each group) were slaughtered at 165 ± 15 days. As shown in Table 1, the TG and WT pigs exhibited significant differences in their dressing percentage and lean percentage, longissimus dorsi muscle, semimembranosus muscle and leaf fat. The lean percentage of TG pigs (72.95 ± 1.0 %) was significantly higher than that of WT pigs (69.18 ± 0.97 %) (P = 0.0118). Moreover, the proportion of longissimus dorsi muscle mass was significantly higher in TG pigs (9.792 ± 0.407 %) than in WT pigs (7.749 ± 0.241 %) (P = 0.0002). Excitingly, the dressing percentage of TG pigs (75.90 ± 0.49 %) was also significantly higher than that of the WT controls (72.70 ± 0.39 %) (P < 0.0001). In contrast to the lean mass, fat accumulation in TG pigs was clearly lower; the leaf fat percentage was 0.4376 ± 0.2726 % in TG pigs and 0.5939 ± 0.5371 % in the WT controls (N = 16, P = 0.0145). These results demonstrate that the specific expression of the rhFst344 transgene in pig muscle tissue enhanced skeletal muscle mass while reducing fat accumulation.
Follistatin overexpression in pig muscles did not cause any abnormalities in cardiac muscle
There was some concern about whether the overexpression of follistatin would cause hypertrophy of the cardiac muscles. Therefore, we examined the cardiac muscle of transgenic pigs at the molecular and histological levels. Although the expression of rhFst344 mRNA was detected in the cardiac muscle of transgenic pigs (Fig. 2b), no obvious differences in the size or weight of their hearts, or in the diameter of cardiac myofibers was observed between transgenic and WT pigs; no pathological changes were identified in the hearts of the transgenic pigs (Fig. S2).
The myofiber in the skeletal muscles of transgenic pigs is hypertrophic
To characterize the skeletal muscle fiber in follistatin-overexpressing TG pigs, the longissimus dorsi muscles of TG and WT pigs were collected and processed. The longissimus muscle sections were stained with hematoxylin and eosin (H&E) for histological analysis (Fig. 4a). Approximately 1000 myofibers from each experimental group were randomly selected and measured, and the distribution frequency of the fiber diameters was plotted (Fig. 4b). The results indicated that the myofiber size in TG pigs is larger than that of WT pigs.
FST transgene expression caused myofiber hypertrophy. a Representative H&E-stained cross-sections of longissimus dorsi muscles from TG and WT pigs. b The distribution of myofiber diameter in the longissimus dorsi muscle in TG and WT pigs. The myofibers in TG pigs were hypertrophic when compared with the WT control littermates. The TG and WT pigs were 110 ± 10 kg at slaughter and the same age at slaughter, and 1000 myofibers were measured in each group (N = 3). The values are expressed as the means. WT, wild type pig; TG, transgenic pig
The expression of rhFst344 enhances the Akt signaling pathway in skeletal muscle of transgenic pigs
Lysates of the longissimus dorsi muscle from transgenic pigs and their wild-type littermates were prepared and analyzed for Smad2 and Akt using immunoblotting experiments (Fig. 5a, b). The ratio of phosphorylated Smad2 (P-Smad2) to total Smad2 was significantly lower in TG pigs than in WT pigs (Fig. 5a), and the phosphorylation of AktS473 was higher in TG pigs (Fig. 5b). In addition, no significant difference in the concentration of mature myostatin protein in longissimus dorsi muscle tissue was observed between TG pigs and WT pigs as quantified by ELISA (Fig. 5c). Based on these results, rhFst344 overexpression within skeletal muscle could inhibit the function of mature myostatin protein, which in turn could inhibit downstream Smad2/3 signaling. Because Akt signaling is regulated by myostatin and is important for skeletal muscle hypertrophy, it is reasonable to assume that the observed myofiber hypertrophy was caused by the overexpression of follistatin in the transgenic pigs and myostatin was functionally inhibited.
Analysis of Smad2, Akt and myostatin proteins. Immunoblot and ELISA analyses of lysates from the longissimus muscle in TG and WT littermate pigs. a Immunoblotting with antibodies against phosphorylated Smad2 (P-Smad2) and total Smad2. The P-Smad2/total Smad2 ratio was significantly reduced in TG pigs, suggesting that rhFst344 transgene expression greatly decreased the phosphorylation of Smad2 (N = 3). b Immunoblotting with anti-phosphorylated Akt (AktPser473) antibody and anti-Akt antibody. The P-Akt/total Akt ratio was significantly increased in TG pigs, indicating that transgene expression greatly enhanced the phosphorylation of Akt (N = 3). c The concentration of myostatin protein in longissimus dorsi tissue was quantified using ELISA, and no significant difference was observed between TG and WT pigs (N = 3, P = 0.6139). Wild-type (WT) pig; transgenic (TG) pig; *P < 0.05; ***P < 0.001
Discussion
In the present study, we generated transgenic pigs that over-expressed rhFst344 in their skeletal muscles under the control of the skeletal alpha-actin gene promoter. The transgenic pigs, which expressed a high level of the hFst344 transgene, exhibited significantly higher muscle mass than WT controls.
It is well known that Belgian Blue and Piedmontese cattle, in which myostatin is naturally mutated, show a dramatic and global increase in skeletal muscle mass, and these cattle are termed double-muscle cattle (Bellinge et al. 2005). The phenotype of the transgenic pigs examined here was similar to that of cattle exhibiting hypertrophic muscle tissue primarily in the proximal fore- and hindquarters and to those of cattle exhibiting a prominent muscular protrusion with intramuscular boundaries and grooves visible beneath the skin. A myostatin-null mutation in Belgian Blue cattle caused only a 20–30 % increase in the muscle mass, and this increase was much less than that observed in myostatin-deficient mice (200–300 %) (McPherron and Lee 1997). It was assumed that the cattle might be nearer the maximum limit for muscle size after many generations of selective breeding for large muscle mass when compared with mice. In fact, the intensity of selective breeding for larger muscle mass in Duroc pigs is higher than that in Belgian Blue cattle because the generational interval of pigs is much shorter than that of cattle. Therefore, it is reasonable that the muscle mass increased by only 3.8 % in the follistatin transgenic pigs in this study, unlike the findings in mice which have not been similarly selected.
The skeletal actin gene was primarily expressed in postnatal skeletal muscle tissue (Vandekerckhove et al. 1986); thus, the skeletal actin promoter was expected to drive expression of the hFst344 transgene in skeletal muscle fibers. In the rhFst344 muscle-specific expression vector, hFst344 replaced the PSA CDS from the initial ATG codon to the TAA stop codon. The transgenic vector also contained regulatory elements necessary for PSA expression in order to reduce the genomic position effect. As is known, the transgene expression is affected by the integration sites and the number of integrants in host genome. Generally, the more copy numbers of transgene, the higher expression levels of transgene. However, all transgenic pigs derived from cell clone Nos. 3, 6, 24 had 2 copies of exogenous transgene determined by quantitative RT-PCR (data not shown). Therefore, it was thought that the differential expression levels of transgene might result from different integration sites in transgenic pigs.
Follistatin can promote muscle mass growth, and the effect is dosage-dependent (Haidet et al. 2008; Lee et al. 2010). Muscle mass is enhanced when the level of follistatin expression in muscle tissue is increased, suggesting that follistatin can be used as a marker to facilitate livestock breeding to obtain a higher proportion of lean meat. Duroc pig exhibits a high proportion of lean meat and is usually used as the terminal male parent when improving pig breeds. Theoretically, it is more difficult to achieve a remarkable improvement in the lean meat proportion of Durocs than in other pig breeds, especially those with a low proportion of lean meat. However, the specific overexpression of follistatin in muscle tissue significantly increased the proportion of lean meat in the Duroc pigs studied here. Thus, follistatin could be one of the best candidates in transgenic breeding to improve the proportion of lean meat in pigs. In China, where production improvement is a priority, follistatin could be used to improve indigenous breeds. Our original aim of muscle-specific expression of follistatin was to promote muscle growth, and we were surprised when we found the dressing percentage of transgenic pigs was also significantly higher compared to WT controls suggesting the potential application of follistatin.
The structure and function of myostatin is highly conserved among mammalian species, and the bioactive mature peptide of pig myostatin shares 100 % identity with that of human myostatin. In mammals, several proteins have been identified to bind to MSTN and inhibit its association with MSTN receptor (Joulia-Ekaza and Cabello 2006). Follistatin is a robust antagonist of myostatin and has been shown to inhibit myostatin signaling by surrounding MSTN with two FST molecules, thereby blocking the MSTN receptor-binding site (Thompson et al. 2005a). Follistatin over-expression could inhibit myostatin action, reduces the phosphorylation level of smad2 and activates Akt signaling in these transgenic pigs, thereby promoting protein synthesis in the muscle tissue. Besides regulated by follistatin, myostatin regulates its own expression through a negative feedback loop (Forbes et al. 2006), it might result in no significant difference of myostatin expression between TG pigs and WT pigs. Follistatin was originally thought to mediate skeletal muscle hypertrophy by antagonizing MSTN (Amthor et al. 2004), but a recent finding suggests that the effect produced by follistatin might also be regulated by Smad3 and mTOR, independently of MSTN (Winbanks et al. 2012). A recent study showed that the heterozygous loss of FST (FST+/−) decreased muscle mass in both of MSTN+/− and MSTN−/− mice (Lee et al. 2010). Moreover, quadrupled muscle mass was observed in myostatin-null mice carrying a follistatin transgene, which had approximately twice the muscle mass as the MSTN−/− mice (Lee 2007). Follistatin was suggested to bind to other TGF-beta superfamily members in addition to myostatin, such as activin, which would increase muscle mass growth (Gilson et al. 2009; Lee et al. 2010). However, the molecular mechanisms of muscle hypertrophy caused by follistatin overexpression in muscle tissue still remain unclear and require further investigation.
As described earlier, the two isoforms of FST (FST315 and FST288) have completely different distributions in vivo. FST288 is tissue-specific but is undetectable in human serum (Schneyer et al. 2004b). FST315 has an additional 17 C-terminal residues compared with FST288, which decreases its affinity for heparin (Sugino et al. 1993). In vitro experiments have shown that both the FST288 and FST315 isoforms increase the cell-surface binding of myostatin and increase myostatin degradation (Cash et al. 2009). Although rhFst344 was engineered to be specifically expressed in muscle tissue, the follistatin concentration in the blood of transgenic pigs was significantly higher than that in control pigs, indicating that the over-expressed follistatin was also secreted into the blood circulation. Recent studies have suggested that FST288 might remain localized in the immediate vicinity of the cell where it is secreted (Winbanks et al. 2012). Thus, the muscle-specific overexpression of FST317 might affect other tissues less than FST344.
The size of the heart was not altered, and myocardial hypertrophy was not induced in the transgenic pigs despite the expression of rhFst344 in cardiac muscle, which is consistent with previous findings in mice and cynomolgus macaque monkeys (Haidet et al. 2008; Kota et al. 2009). More interestingly, the proportion of fat in the transgenic pig carcasses was significantly lower than that in the WT pigs, indicating that follistatin is also directly or indirectly involved in the regulation of fat metabolism. Based on the findings in this study, we suggest that the inhibition of myostatin by follistatin overexpression in skeletal muscle could be used to treat not only muscle diseases but also obesity.
In summary, this is the first time to demonstrate that rhFst344 overexpression can significantly increase muscle mass in pigs. We found that the expression of rhFst344 in our transgenic pigs mainly affected skeletal muscles, with no obvious effects on other tissues or organs. This study suggests a promising approach to increase muscle mass in livestock. In addition, because of the similarities between human and pig physiology, our results with the transgenic pig model also suggest that FST344 gene therapy is a safe treatment for human muscle diseases.
Materials and methods
Ethics statement
All the animal work in this study including the establishment of primary fetal fibroblasts were approved by the animal ethics committee of State Key Laboratory of Agrobiotechnology (license number SKLAB-2012-04-04). All experimental procedures were performed in accordance with the guidelines for the Care and Use of Laboratory Animals of China Agricultural University. All efforts were made to minimise suffering of the animals.
Construction of the muscle-specific rhFST344 expression vector
FSTF and FSTR primers contained DNA sequences that were homologous to the 5′ and 3′ flanking sequences (77 bp and 83 bp, respectively) of the pig skeletal muscle alpha-actin (PSA) gene (Table S1). Full-length FST cDNA from humans (344aa, Origen) was used as the PCR template. This DNA fragment was then subcloned into the PMD-T vector to generate the T-hFst344 vector. FZF and FZR primers containing a 5′ FRT sequence (Table S1) were used to amplify a zeor fragment. The T-hFst344 vector and the zeor fragment were ligated to generate the T-hFst344-zeor vector (Fig. S1). A 430 bp PSA 5′ homology arm (5′HA) and a 417-bp PSA 3′ homology arm (3′HA) were generated using PCR with primer pairs TYB1 and TYB2, respectively (Table S1). The 5′HA, pBR322-neo vector and 3′HA were ligated to generate a gap-repair plasmid (Fig. S1).
A PSA BAC vector was obtained from the BAC/PAC Resource Center at the Children’s Hospital Oakland Research Institute (CH242-54G23). The PSA gap-repair plasmid was linearized and electroporated into the PSA BAC-containing SW102 competent cells (1.8 kV, 2 μF; Bio-Rad electroporator) (Fig. S1). Positive clones (SW102-1) were then identified by restriction enzyme digestion. The T-rhFst344-zeor vector was digested using NheI, and the hFst344-zeor cassette was purified and electroporated into SW102-1 cells, and positive recombinants were selected with zeocin (25 μg/mL) followed by appropriate colony screening. The positive clone and the 707-FLPe tetR plasmid were then transformed together into DH5α cells inducing deletion of the zeor cassette to make the PSA-rhFst344 vector (Fig. S1).
Primary fibroblast transfection and screening
Primary fetal fibroblasts of Duroc pigs (n = 38, XY) were established and maintained in DMEM with 15 % fetal bovine serum (Gibco). All media were supplemented with 100 U/mL penicillin, 0.1 mg/mL streptomycin and all cell were maintained at 37 °C, 5 % CO2. The PSA-rhFST344 vector was linearized and 3 μg of the linearized vector DNA was introduced into 1 × 106 Duroc primary fetal fibroblasts by electroporation using the Nucleofector. After 48 h of incubation (37 °C and 5 % CO2), the transfected cells were selected using 400 μg/mL of G418. Two copies of each cell clone were prepared; one copy was used for PCR screening, and the second copy was used for cell expansion and subsequent cell freezing in DMEM (HyClone) containing 10 % dimethylsulfoxide (DMSO) and 30 % fetal bovine serum (HyClone).
SCNT and the transfer of reconstructed embryos
Integration of the PSA-rhFst344 cassette into transfected fibroblast clones was detected using PCR, and positive transgenic fibroblast clones were subsequently used as donor cells for SCNT. Single donor cells were directly injected into enucleated recipient oocytes, followed by electrical fusion and activation (Zhang et al. 2007). The activated embryos were cultured at 39 °C under 5 % CO2, 5 % O2, 90 % N2 and 100 % humidity. The following day, SCNT embryos were transferred into both uterine horns of recipient sows. After 21 days, the pregnancies were verified using ultrasonography and re-confirmed every 2 weeks. All experimental pigs were housed and cared for in accordance with the guidelines for animal research, as reviewed by the China Agricultural University.
Southern blot analysis and copy number determination
Genomic DNA was extracted from animal ear punch samples to verify that the vector DNA had been correctly integrated. Genomic DNA samples from transgenic and wild-type pigs were digested using EcoRI and analyzed using Southern blotting with a 680-bp DNA probe labeled using a PCR digoxigenin probe synthesis kit (Roche). Pre-hybridization and hybridization procedures were performed at 45 °C, and the wash steps used were carried out at 68 °C according to the previously described method. Quantitative real time-PCR was performed on each sample in triplicate using the Light Cycler 480 II (Roche). Each 10 μg qRT-PCR reaction mixture contained 5 μL SYBR Green I Master (Roche), 200 nM of primer Q1 and Q2 for rhFst344, and 1 μg DNA. Myostatin was used as the internal control with primer MF and MR. Copy number of transgenes was calculated as described previously (Liu et al. 2012).
Transcriptional analysis of transgenes in transgenic pigs
Total RNA was isolated separately from the heart, liver, spleen, lung, kidney and skeletal muscle tissues of transgenic pigs using Trizol reagent (Invitrogen). RNA samples were digested using DNaseI for 15 min and then quantified using a Nanodrop spectrophotometer (Thermo). Total RNA (500 ng) was reverse-transcribed using Moloney murine leukemia virus reverse transcriptase (MMLV-RT) and oligo (dT) 18 primer (Promega). To quantify rhFst344 expression at the transcriptional level, RT-PCR was performed using specific P1 primer on the 5′ homology arm of PSA and P2 primer on the rhFst344 CDS (Table S1). Porcine GAPDH served as the reference gene and was amplified using primers P3 and P4 (Table S1).
Translational analysis of the transgene in transgenic pigs using ELISA
The amount of rhFst315 (mature form of rhFst344 protein) in longissimus dorsi muscles and in serum from the transgenic pigs was evaluated using a human follistatin ELISA kit according to the manufacturer’s instructions (R&D Systems). A non-transgenic pig was used as the control. The concentrations of rhFst315 in longissimus dorsi muscles and in serum from the transgenic pigs were determined based on a standard curve that was determined using recombinant human follistatin provided by the manufacturer. The concentrations of myostatin protein in the longissimus dorsi muscle from the transgenic pigs were measured using a human myostatin ELISA kit according to the manufacturer’s instructions (Life Science Inc.).
Western blot analysis
Samples of longissimus dorsi muscle from the transgenic and age-matched WT control pigs were homogenized in IP lysis buffer and centrifuged at 10,000 g for 10 min at 4 °C. The supernatant was recovered as cell lysate, and 250 μg protein in lysate aliquots were resolved using SDS-PAGE and transferred onto polyvinylidene difluoride membranes. The blotted membranes were probed separately with anti-phosphorylated Smad2, anti-Smad2, anti-phosphorylated Akt (p473ser) and anti-Akt antibodies (Cell Signaling Technology, Beverly, MA, USA) and incubated with horseradish peroxidase-conjugated secondary antibodies. The immunoblots were developed using chemiluminescence, and images of the results were captured using a cooled CCD camera system.
Samples and carcass traits
Two groups of cross-bred F1 pigs (transgenic and non-transgenic groups, randomly selected) (N = 16) were fed with the same commercial diet and reared in the same pen. The pigs were slaughtered at a commercial slaughtering house at 165 ± 15 days old and at 100 ± 20 kg of weight according to standard procedures of the Chinese livestock production system. Carcass weights were measured immediately after the pigs were slaughtered. The skin, fat mass, lean mass and bone tissue were recovered and measured separately.
Hematoxylin and eosin staining
Muscle samples were collected from the longissimus dorsi muscle at the 5th thoracic vertebra oftransgenic pigs and WT control pigs within 1 h post-mortem.The muscle samples were fixed in formalin (10 %) for 10 h and the samples were then subjected to routine tissue processing and paraffin sectioning. The muscle sections were then stained with hematoxylin and eosin. Five representative images were captured from each muscle section (one at the center and four at the periphery). The myofiber diameter of each selected area was measured using ImageJ software based on three independent images. A distribution diagram of myofiber diameter (size) was generated based on the percentage of 1000 analyzed myofibers.
Statistical analysis
The experimental data were analyzed using an analysis of variance procedure using SAS software. Differences were considered as significant when P < 0.05.
References
Abe Y, Abe T, Aida Y, Hara Y, Maeda K (2004) Follistatin restricts bone morphogenetic protein (BMP)-2 action on the differentiation of osteoblasts in fetal rat mandibular cells. J Bone Min Res 19:1302–1307. doi:10.1359/JBMR.040408
Amthor H, Christ B, Rashid-Doubell F, Kemp CF, Lang E, Patel K (2002) Follistatin regulates bone morphogenetic protein-7 (BMP-7) activity to stimulate embryonic muscle growth. Dev Biol 243:115–127. doi:10.1006/dbio.2001.0555
Amthor H, Nicholas G, McKinnell I, Kemp CF, Sharma M, Kambadur R, Patel K (2004) Follistatin complexes myostatin and antagonises myostatin-mediated inhibition of myogenesis. Dev Biol 270:19–30. doi:10.1016/j.ydbio.2004.01.046
Bellinge RH, Liberles DA, Iaschi SP, O’Brien PA, Tay GK (2005) Myostatin and its implications on animal breeding: a review. Anim Genet 36:1–6. doi:10.1111/j.1365-2052.2004.01229.x
Cash JN, Rejon CA, McPherron AC, Bernard DJ, Thompson TB (2009) The structure of myostatin:follistatin 288: insights into receptor utilization and heparin binding. EMBO J 28:2662–2676. doi:10.1038/emboj.2009.205
Forbes D, Jackman M, Bishop A, Thomas M, Kambadur R, Sharma M (2006) Myostatin auto-regulates its expression by feedback loop through Smad7 dependent mechanism. J Cell Physiol 206(1):264–272
Gilson H, Schakman O, Kalista S, Lause P, Tsuchida K, Thissen JP (2009) Follistatin induces muscle hypertrophy through satellite cell proliferation and inhibition of both myostatin and activin. Am J Physiol Endocrinol Metab 297:E157–E164. doi:10.1152/ajpendo.00193.2009
Haidet AM et al (2008) Long-term enhancement of skeletal muscle mass and strength by single gene administration of myostatin inhibitors. Proc Natl Acad Sci USA 105:4318–4322. doi:10.1073/pnas.0709144105
Iemura S et al (1998) Direct binding of follistatin to a complex of bone-morphogenetic protein and its receptor inhibits ventral and epidermal cell fates in early Xenopus embryo. Proc Natl Acad Sci USA 95:9337–9342
Iezzi S et al (2004) Deacetylase inhibitors increase muscle cell size by promoting myoblast recruitment and fusion through induction of follistatin. Dev Cell 6:673–684
Joulia-Ekaza D, Cabello G (2006) Myostatin regulation of muscle development: molecular basis, natural mutations, physiopathological aspects. Exp Cell Res 312:2401–2414. doi:10.1016/j.yexcr.2006.04.012
Kambadur R, Sharma M, Smith TP, Bass JJ (1997) Mutations in myostatin (GDF8) in double-muscled Belgian Blue and Piedmontese cattle. Genome Res 7:910–916
Kollias HD, McDermott JC (2008) Transforming growth factor-beta and myostatin signaling in skeletal muscle. J Appl Physiol (1985) 104:579–587. doi:10.1152/japplphysiol.01091.2007
Kota J et al (2009) Follistatin gene delivery enhances muscle growth and strength in nonhuman primates. Sci Transl Med. doi:10.1126/scitranslmed.3000112
Langley B, Thomas M, Bishop A, Sharma M, Gilmour S, Kambadur R (2002) Myostatin inhibits myoblast differentiation by down-regulating MyoD expression. J Biol Chem 277:49831–49840. doi:10.1074/jbc.M204291200
Lee SJ (2007) Quadrupling muscle mass in mice by targeting TGF-beta signaling pathways. PLoS ONE 2:e789. doi:10.1371/journal.pone.0000789
Lee SJ, McPherron AC (2001) Regulation of myostatin activity and muscle growth. Proc Natl Acad Sci USA 98:9306–9311. doi:10.1073/pnas.151270098
Lee SJ et al (2010) Regulation of muscle mass by follistatin and activins. Mol Endocrinol 24:1998–2008. doi:10.1210/me.2010-0127
Liu S, Li X, Lu D, Shang S, Wang M, Zheng M, Zhang R, Tang B, Li Q, Dai Y, Li N (2012) High-level expression of bioactive recombinant human lysozyme in the milk of transgenic mice using a modified human lactoferrin BAC. Transgenic Res 21(2):407–414
Matzuk MM, Lu N, Vogel H, Sellheyer K, Roop DR, Bradley A (1995) Multiple defects and perinatal death in mice deficient in follistatin. Nature 374:360–363. doi:10.1038/374360a0
McPherron AC, Lee SJ (1997) Double muscling in cattle due to mutations in the myostatin gene. Proc Natl Acad Sci USA 94:12457–12461
McPherron AC, Lawler AM, Lee SJ (1997) Regulation of skeletal muscle mass in mice by a new TGF-beta superfamily member. Nature 387:83–90. doi:10.1038/387083a0
Medeiros EF, Phelps MP, Fuentes FD, Bradley TM (2009) Overexpression of follistatin in trout stimulates increased muscling. Am J Phys Regul Integr Comp Physiol 297:R235–R242. doi:10.1152/ajpregu.91020.2008
Mosher DS, Quignon P, Bustamante CD, Sutter NB, Mellersh CS, Parker HG, Ostrander EA (2007) A mutation in the myostatin gene increases muscle mass and enhances racing performance in heterozygote dogs. PLoS Genet 3:e79. doi:10.1371/journal.pgen.0030079
Patel K (1998) Follistatin. Int J Biochem Cell Biol 30:1087–1093
Schneyer AL, Wang Q, Sidis Y, Sluss PM (2004a) Differential distribution of follistatin isoforms: application of a new FS315-specific immunoassay. J Clin Endocrinol Metab 89:5067–5075. doi:10.1210/jc.2004-0162
Schneyer AL, Wang QF, Sidis Y, Sluss PM (2004b) Differential distribution of follistatin isoforms: application of a new FS315-specific immunoassay. J Clin Endocrinol Metab 89:5067–5075. doi:10.1210/Jc.2004-0162
Sugino K et al (1993) Molecular heterogeneity of follistatin, an activin-binding protein—higher affinity of the carboxyl-terminal truncated forms for heparan-sulfate proteoglycans on the ovarian granulosa-cell. J Biol Chem 268:15579–15587
Thompson TB, Lerch TF, Cook RW, Woodruff TK, Jardetzky TS (2005a) The structure of the follistatin: activin complex reveals antagonism of both type I and type II receptor binding. Dev Cell 9:535–543. doi:10.1016/j.devcel.2005.09.008
Thompson TB, Lerch TF, Cook RW, Woodruff TK, Jardetzky TS (2005b) The structure of the follistatin:activin complex reveals antagonism of both type I and type II receptor binding. Dev Cell 9:535–543. doi:10.1016/j.devcel.2005.09.008
Trendelenburg AU, Meyer A, Rohner D, Boyle J, Hatakeyama S, Glass DJ (2009) Myostatin reduces Akt/TORC1/p70S6 K signaling, inhibiting myoblast differentiation and myotube size. Am J Physiol Cell Physiol 296:C1258–C1270. doi:10.1152/ajpcell.00105.2009
Vandekerckhove J, Bugaisky G, Buckingham M (1986) Simultaneous expression of skeletal muscle and heart actin proteins in various striated muscle tissues and cells. A quantitative determination of the two actin isoforms. J Biol Chem 261:1838–1843
Wang Q, Keutmann HT, Schneyer AL, Sluss PM (2000) Analysis of human follistatin structure: identification of two discontinuous N-terminal sequences coding for activin A binding and structural consequences of activin binding to native proteins. Endocrinology 141:3183–3193
Welle SL (2009) Myostatin and muscle fiber size. Focus on “Smad2 and 3 transcription factors control muscle mass in adulthood” and “Myostatin reduces Akt/TORC1/p70S6 K signaling, inhibiting myoblast differentiation and myotube size”. Am J Physiol Cell Physiol 296:C1245–C1247. doi:10.1152/ajpcell.00154.2009
Williams MS (2004) Myostatin mutation associated with gross muscle hypertrophy in a child. N Engl J Med 351:1030–1031 (author reply 1030–1031)
Winbanks CE et al (2012) Follistatin-mediated skeletal muscle hypertrophy is regulated by Smad3 and mTOR independently of myostatin. J Cell Biol 197:997–1008. doi:10.1083/jcb.201109091
Zhang Y, Li J, Villemoes K, Pedersen AM, Purup S, Vajta G (2007) An epigenetic modifier results in improved in vitro blastocyst production after somatic cell nuclear transfer. Cloning Stem Cells 9:357–363. doi:10.1089/clo.2006.0090
Acknowledgments
We thank Qiuyan Li for technical assistance. This work was supported by funding from the National Basic Research Program of China (2015CB943103), National Transgenic Breeding Program (2014ZX08006 and 2013ZX08006-002) and National Scientific Foundation of China (81370840).
Author contributions
Rui Fang conceived and designed the experiments; Fei Chang, Meng Wang, Xin Zhao, Rui Fang and Wen Chang performed the experimental work; Zaihu Zhang managed the breeding and feeding of transgenic pigs; Rui Fang, Fei Chang, Meng Wang, Ning Li and Qingyong Meng wrote the manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
The authors declare no conflicts of interest.
Additional information
Fei Chang, Rui Fang and Meng Wang contributed equally to this work.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Chang, F., Fang, R., Wang, M. et al. The transgenic expression of human follistatin-344 increases skeletal muscle mass in pigs. Transgenic Res 26, 25–36 (2017). https://doi.org/10.1007/s11248-016-9985-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11248-016-9985-x