Abstract
Worldwide colorectal cancer (CRC) was considered to be a primary health disease that leads to high morbidity and mortality in both developed and developing countries, which can be controlled with novel chemopreventive agents. In the past few decades, chemoprevention of several cancers by the use of natural flavonoids has become an attractive strategy. Bioactive polyphenols with a low molecular weight in the dietary supplements determine the fate of a cell by interacting at the molecular and cellular level. In the past few decades, several preclinical/clinical studies perceptibly figured the chemopreventive and cytotoxic effect of bioactive flavonoids on cancers. Flavonoids have been frequently considered as enzyme inhibitors and ligands of receptors involved in the signal transduction. There has been increasing interest in unrevealing the beneficial and chemoprotective potential of flavonoids that had encouraged in novel drug discovery for CRC. An elaborated molecular mechanism of phytocompounds against cancer treatment overlies with direct interactions between various types of genes and enzymes. It is well known that anticancer efficacy would include apoptosis, antiproliferation, antioxidation, cell cycle arrest, and reversal of multidrug resistance mechanism. This chapter highlights the cumulative research findings of plant-derived bioactive flavonoids against CRC treatment and provides novel insights into its molecular regulation and mechanism with protein interaction in anticancer drug discovery.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
2.1 Introduction
Bowel cancer has been diagnosed as the third most prevalent cancer worldwide. It is reported globally with a very high rate of prevalence and mortality in both sexes. Study literature pinpoints westernized life anticipation, unbalanced diet, uncooked food, processed and canned meats, change in environmental conditions due to urbanization, and synthetic mutagens due to pollution were thought to be a serious etiology of colorectal cancer (CRC). The key factors behind CRC etiology were denoted as oxidative stress caused by the generation of non-neutralizable reactive oxygen species (ROS) and free radicals, which can directly interact with the bio-molecules of living cells and induce spontaneous mutations in bowel cells that leads to colon cancer (Mariyappan et al. 2017). Subsequent accumulation of sequential genetic mutation in a colonic gene, in particular, oncogenes and tumor suppressor genes resulted in the stepwise progression of colon cancer that starts from bowl inflammation followed by the formation of adenomatous polyposis coli and finally leads to colorectal carcinogenesis (Fearon and Vogelstein 1990). Further, intensive study reports on colorectal cancer in rodent models have stated that microflora of animal guts have a major contribution to the development of colon cancer (Manju and Nalini 2005). Environmental pollutants, chemical toxicants in food, and modernized dietary food style have a direct impact on the microenvironment of gut microbes. Such uncomfortable gut environment resulted in improper metabolism and development of resistance against deadly pathogens and procarcinogens along with unbalanced homeostasis of increased immune response that had extensively known to regulate T cell development and Th17 responses that were denoted as key factors for colon cancer development (Kamada et al. 2013). As per the previous reseach study, various epidemiological case studies had evidently proved that daily diet accounts a dominant role in etiology of CRC; in particular, processed meat and red meat consumption had evidently increased the risk of colon carcinogenesis, whereas consumption of fiber-rich food like fruits, vegetables, and whole grains had highly decreased the colon cancer risk (McGirr et al. 2017). As a highlight of this chapter, consumption of a variety of green leaves, vegetables, and fruits with high fiber contents also includes a variety of naturally occurring bioactive molecules scientifically termed as dietary flavonoids that possess a strong chemoprotective effect against colon cancer and also have inverse action on colon carcinogenesis mechanism (Chang et al. 2018). For decades, scientists have been working with the isolation and characterization of a number of naturally occurring flavonoids and elucidated its pharmacological properties on various study models. Therefore, this chapter particularly provides a brief review of a few such flavonoids along with its biological properties and protective mechanism of action against colorectal cancer.
2.2 Bacterial Enzymes and Colon Cancer
The human digestive tract inhabits approximately about 400 species of bacteria, and it is a complex ecosystem of multiple microorganisms. The literature review provides evidence that invasion of pathogenic bacteria into the intestine was prevented by the presence of anaerobic gut bacteria which in turn reduce the threatened occurrence of CRC, and it is because of bacterial conversion of short-chain fatty acids by dietary fiber (Abeni et al. 2013). Consumption of high-fat and imbalanced diet alters the protective metabolism of intestinal microflora by increased bacterial enzyme activity, which resulted in a decreased host immune responses (Asha and Gayathri 2012). One of the best examples is β-glucuronidase, a common gut bacterial enzyme that produces the toxic metabolites with carcinogenic effect, which has a significant role in the progression of the tumor at secondary sites like large intestine, colon, etc. (Beaud et al. 2005). 1,2-Dimethylhydrazine (DMH) is an alkylating agent which stimulates the formation of methyl adducts with DNA bases that leads to point mutation. It has been well studied that β-glucuronidase enzymes catalysis the conversion of procarcinogen form of DMH into various metabolic intermediates which has a higher efficacy to interact with colon epithelium and act as potent carcinogens (Reddy et al. 1974). The accumulation of aglycones is due to the catalytic activity of β-glucosidase which synergistically hydrolyzes cellulose to lignocellulose (Sohail et al. 2009; Jeng et al. 2011). On the other hand, the permeability of the colon mucosal membrane was highly altered by the enzymatic catalysis of intestinal mucin by gut bacterial mucinase enzyme (Robertson et al. 1940). Along with other bacterial enzymes, nitroreductase also has a unique task in colon cancer development by producing amines in the colon tissues by catalytic conversion of procarcinogens such like nitropyrenes, dinitrotoluene, and nitrobenzenes (Facchini and Griffiths 1981).
2.3 Reactive Oxygen Species (ROS) and Cancer
Mitochondrial oxidative phosphorylation generates a large amount of ROS by impaired transfer of electrons between complexes I and III of the mitochondrial respiratory chain (MRC). ROS is a collective term that refers to highly reactive oxygen molecules that can be listed as hydroxyl radical (OH−) ions, hydrogen peroxide (H2O2), and superoxide anion (O2-) (Fulda et al. 2010). Naturally occurring antioxidant enzymes such as superoxide dismutase enzymes and catalase serve as defense system by catalytically naturalizing the generated reactive pro-oxidants. ROS may have beneficial effects on regulation of cell proliferation and gene expression, and increased ROS production results in oxidative stress-induced damage to proteins, lipids, and DNA, which has implications in diseases including diabetes, Parkinson’s disease, and cancer (Gogvadze et al. 2009). Studies of aging mechanism had revealed the role of oxidative stress in higher probability of risk for cancer development in old age. Generated reactive species or free radicals actively damage genomic or mitochondrial DNA that leads to sudden mutation implicated in the earlier stage of cancer development. Due to mutational genetic instability in DNA causes the modulations in the signal transduction or even induce error in replication would efficiently reflects in induction or repression of specific transcription of the genes related to carcinogenesis. The recent investigation also described that the increased level of intracellular ROS in cancer cells possess a constant and chronic oxidative stress, and thereby it provides a favorable microenvironment for tumor progression. Further, the p53 tumor suppressor was found to be mutated and accounts for up to approximately 50% of all human cancers and is a major activator of antioxidant defense mechanisms. With respect to its apoptosis-inducing capacity, molecules that promote ROS production may be novel therapeutics, proposed to selectively target cancer cells by elevating ROS levels beyond a tolerable threshold to induce mitochondrial outer membrane permeabilization (MOMP) and cell death (Graham et al. 2010).
2.4 Flavonoid
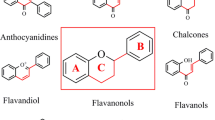
From the ancient years, our folk medicine has been made of different and variety of naturally occurring plant products which later we call as nutraceuticals. Flavonoids are polyphenols produced as secondary byproducts of plants, which were considered as main constituents of nutraceuticals. Hence, these flavonoids possess numerous pharmacological properties, and these polyphenols were consumed for hundreds of years as diet in form of fruits and vegetables; therefore these polyphenols were called as dietary flavonoids (Li et al. 2018). Chemically these flavonoids were characterized to have basic hydroxylated phenyl moieties with a number of phenolic rings and electron donation functional groups (Manach et al. 2004). Basically, these flavonoids are classified as polyphenols that are responsible for pigmentation in plants. Such polyphenols have a significant physiological function in plants to perform maturation of seeds, actively encounters biotic and abiotic stress of plants, maintains homeostasis of heat, freezing tolerance, act as a defense mechanism against pathogens and takes part in plants detoxification mechanism (Mierziak et al. 2014). Out of thousands of identified flavonoids, diphenyl propane skeleton (C6–C3–C6) with two phenyl ring and one heterocyclic ring form the basic structure of all the flavonoids (Kawser Hossain et al. 2016). Briefly, flavonoids are low molecular weight large group bioactive polyphenols with a basic structure of benzo-γ-pyrone and are widely found in a variety of plants. These flavonoids contain the number of hydroxyl group along with the catechol group at 3- and 5-positions at flavon ring that attributes its radical scavenging effect and antioxidant properties. Based on the structures, these flavonoids were classified into six major subclasses that can be listed as flavones, flavanones, flavanols, flavonols, isoflavones, and anthocyanins/anthocyanidins (Fig. 2.1). A large number of plant-derived bioactive polyphenols have been studied and evidently proved as efficient anti-carcinogenic agents. These flavonoids potentially block cancer development by interfering at multi-stages of carcinogenesis either at initiation, development, or progression of tumors; thereby these molecules have a significant inhibitory effect on proliferation and differentiation of cells and block angiogenesis and metastasis along with a decrease in tumor mass by induction of programmed cell death (Ramos 2007). Earlier research studies evidence that secondary metabolites include flavonoids which are phenolic in nature that possess several pharmacological activities which are structure-dependent (Mahomoodally et al. 2005). The specified quantity of hydroxylation, conjugations with other substitutional functional groups, and polymerization basically determines the structure and chemical nature of the flavonoids. It is well known that flavonoids were rich in hydroxyl groups and functionally act as scavengers to neutralize the free radicals and act as an antioxidant. It also has the capability to chelate metal ions. Previous studies evidenced that these flavonoids possess a chemoprotective effect against various degenerative and infectious diseases like cancer and cardiovascular diseases.
2.5 Functional Flavonoids as Antioxidants
Brief reviews from vast literature denoted that variety of flavonoids act as an antioxidant; in particular, it possesses reducing power to scavenge highly reactive radicals that are generated during human metabolic functions (Brunetti et al. 2013). Previous research investigations on antioxidant activity of these polyphenolic compounds have shown that flavonoids efficiently inhibit specific enzymes like xanthine oxidase and protein kinase C which produce active superoxide radicals (Hanasaki et al. 1994). Also, these bioactive compounds inhibit a wide range of enzymes like cyclooxygenase, lipoxygenase, microsomal monooxygenase, etc. that involves in lipid peroxidation metabolism. An extensive report on the relation between the structure of free radicals and the respective flavonoids had revealed the radical scavenging mechanism and demonstrated its antioxidant potentials (Rice-Evans et al. 1996). Numerous in vivo study models pictured that dietary flavonoids readily scavenge free radicals in the stomach, and thereby they reduce ROS and act as primary antioxidant defense system (Papas 1998). Green medicines always get more attention toward researchers and are an important tool to prevent, reverse, or delay the carcinogenesis. Therefore, prevention could be a possible option for expensive treatments for existing disease. There have been many more compounds that are in a trial which have been declared as prospect chemopreventive agents. It is well known that these bioactive compounds were commonly present in many food products, commercially available beverages, and many other dietary components. Consumption of these compounds directly or indirectly acts as inhibitory agents on cancer cell proliferation, and thereby these plant flavonoids in foods serve as preventive shield against human carcinogenesis implicated in the prevention of human carcinogenesis possibly through their inhibitory activities of cell proliferation or survival. Decades of research were interested to fix on the natural compounds that include any plant byproduct substances to unmask their anti-carcinogenic effect by tuning the level of intracellular lipid peroxidation and modulate the status of antioxidant that would be used as chemopreventive medicine in the treatment of colorectal cancer. The following are a short brief of few bioactive compounds which were recently studied for their anticancer activity against colorectal cancer.
2.5.1 Eriodictyol
Number of bioactive compounds that are in a trial which have been declared as prospect chemopreventive agents; one of the novel flavonoids recently studied was eriodictyol which comes under flavanone and has been proved to possess antioxidant effect by reducing the level of lipid peroxidation and oxidative stress. The flavonoid rich in citrus and lemons with chemical structure 5,7,3′,4′-tetrahydroxyflavanone was called eriodictyol, and it is naturally occurring in citrus fruits in glycoside form. Eriodictyol was previously considered as vitamin P that was found to be essential in normal small blood vessels (Horowitz 1958). Eriodictyol is an isomer of kaempferol and has been isolated from yerba santa (Eriodictyon californicum). Other major sources of eriodictyol were identified as citrus fruits including lemon, lime, and sour orange and green leaves like peppermint. A number of studies had reported few pharmacological properties such as antioxidant, anti-inflammatory, and anticancer activities. Hence the structural chemistry of eriodictyol consists of free electron-donating hydroxyl moieties, which would impact on potent free radicals’ scavenging activities which in turn reduces the oxidative stress. Previous experiments on supplementation of eriodictyol to rodent model bearing DMH-induced colon cancer had reported with a significant anti-carcinogenic efficacy against colon cancer models (Fig. 2.2). The study had described that eriodictyol primarily alters antioxidant status, gut microbial enzyme activity, and lipid oxidation status in cancer-bearing rats. Prolonged treatment with this flavonoid had intensively reduced pre-neoplastic lesions along with the decrease in aberrant crypt numbers that was reported. β-Glucuronidase suppressor would effectively reduce ACF formation that was early reported in past decades (Takada et al. 1982). Cordially another study also evidently pointed out that chronic treatment of eriodictyol had highly influenced the reduction of β-glucuronidase activity in DMH-induced colon cancer rat models. It is likely to be believed that oxidative stress and lipid peroxidation induce cancer cell initiation and proliferation (Peluso et al. 2011). In connection, extensive study with eriodictyol supplementation to colon cancer-bearing rats showed a notable decrease in lipid peroxidation and pro-oxidant status; thereby colon cancer progression and severity were highly reduced (Mariyappan et al. 2017). Highlights of eriodictyol on gut enzyme activity were reported as considerable inhibition of sulfo-conjugations; thereby nitroreductase sulfatase enzyme activities were thought to be decreased, and on the other hand it is well studied that high number of cancer was reported on increased activity of gut sulfatase and nitroreductase enzymes. As a pictorial proof, eriodictyol’s anti-carcinogenic effect was reported with the histological examination on eriodictyol-supplemented rat models, and the study had pictured the architecture of the colon cancer cells after treatment, and the abnormal changes were highly reduced with decreased dysplastic cells in the colon tissue of flavonoid-treated rats. As a whole, this review about eriodictyol had briefly elaborated the mechanism of antioxidant potential and anti-carcinogenic effect against cancer models.
2.5.2 Umbelliferone
Umbelliferone had been a known and well-studied coumarin, a flavonoid with benzopyrone in nature, and was widely spread in common plants and herbs like parsley sanicle, fennel, celery, giant hogweed, cumin, and asafoetida (Mazimba 2017). This particular bioactive coumarin compound was reported to possess an effective antioxidant role; hence it has been derived from plant polyphenols and consumed as diet in form of fruits and vegetables from ancient days (Hoult and Payá 1996). Here we concise a summarized view on antioxidant potential and anti-carcinogenic effect of pharmacologically active 7-hydroxycoumarin, i.e., umbelliferone. A recent research was highly considered that the use of potentially active phyto-flavonoids is a novel and efficient method to treat and eliminate colon cancer as illustrated in (Muthu et al. 2016). These bioactive compounds that include umbelliferone have been found to modulate the various signaling pathways; thereby it considerably inhibits the pathogenesis of colorectal cancer, and it is designated as a novel anticancer agent. One of the previous studies in our team had efficiently proved that umbelliferone had stiffly and strongly inhibited CRC in DMH-induced colon cancer rat models (Muthu et al. 2016). It is well studied that DMH induction in rats generates a large amount of ROS and causes genetic mutation in colon genes which would be resulted in an initial increase in aberrant crypt formation and later the pathogenesis that lead to colon epithelial carcinoma (Sengottuvelan et al. 2006). Earlier study on rats had reported that intraperitoneal administration of umbelliferone to CRC-bearing rats significantly inhibited and reduced the formation of a specified number of the aberrant crypt in colon epithelial at an early stage of colon cancer. It is to be highlighted that the study also confirmed with the histological pictures of reduced ACF in umbelliferone-treated CRC rat models. Further, the same study had stated that umbelliferone has efficacy to arrest cancer cell proliferation and the AgNOR quantification with image tools that had indexed the reduced cell activation in umbelliferone-treated to CRC-bearing rats (Sirri et al. 1995). Subsequently, mast cells provide a considerable microenvironment for cancer cells to proliferate and survive by means of increased angiogenesis (Oldford and Marshall 2015).
Further reports from previous literature had stated that umbelliferone-treated to CRC-bearing rats have a significant decrease in a number of mast cells, and it clearly indexed the anti-inflammatory effect of umbelliferone in carcinogenesis models. Extensive study reports of primary inflammatory markers like tumor necrosis factor-alpha (TNF-α), interleukin-1, cyclooxygenase (COX), and iNOS that were known to be activated by ROS on CRC models had indicated that umbelliferone significantly had changed the microenvironment of tumor cells by downregulating the expression of intestinal inflammatory marker in cancer-bearing rats (Takahashi et al. 2015). Thereby it has been evidently shown that umbelliferone modulates NF-kB signaling pathway.
Also, umbelliferone had been reported to induce apoptosis and DNA fragmentation by activation of Bax-dependent caspase pathway of apoptosis in CRC rats (Fig. 2.3). As a whole, this brief review about umbelliferone against CRC has clearly pictured the potential use of this particular flavonoid to eliminate colon cancer. Further intensive preclinical and clinical studies on cancer models would be helpful to unravel its complete mechanism of action and would be made commercially available for cancer patients.
2.5.3 Luteolin
Luteolin was classified under flavones of flavonoids based on its chemical structure. Chemically its architecture is of 3′,4′,5,7-tetrahydroxyflavone and was found widespread in most of the plants. Luteolin structure is composed of A and B, two benzene ring, one C ring with oxygen, and a double bond at 2–3 along with readily reducing several hydroxyl groups at 5-, 7-, 3′-, and 4′-positions as shown in (Ross and Kasum 2002). Abundant and naturally occurring sources of luteolin supplementation that were available such as parsley, onion leaves, broccoli, celery, cabbages, chrysanthemum flowers, carrots, peppers, and peanuts were listed (Pandurangan and Esa 2014). Hence it consists of a large number of reducing groups, and it can readily donate an electron to free radicals and scavenge generated ROS. Further literature studies have cumulatively accounted pharmacological properties such like cardio-protective (Madhesh and Vaiyapuri 2013), antioxidant (Ashokkumar and Sudhandiran 2008), anticancer (Manju and Nalini 2005) (Manju), and anti-inflammatory activities (Nishitani et al. 2013). Previous investigations had stated that luteolin possesses significant antiproliferative effect against various cancers that include breast, liver, prostate, esophageal, and lung cancers; thereby it plays a key role as strong chemoprotective agent (Pandurangan and Esa 2014). Any abnormal increase in neoplastic cells due to the absence of apoptosis leads to tumor mass progression with increased cell differentiates, angiogenesis, oxidative stress due to over metabolic process and resulted with metastasis of cancer. Overproduction of ROS due to increased metabolism is not only the factor for cancer progression; lack of sufficient antioxidant defense to scavenge the radicals generated by cancer cell plays a vital role in carcinogenesis (Cheng et al. 2012). Therefore normal cells require endogenous and supplemented antioxidants to fight against the oxidative stress generated by cancer cells. Hence, luteolin possesses more electron donor functional groups, and it serves as a rapid antioxidant to scavenge the unstable reactive free radicals to protect the cells from intracellular ROS lesions. In particular, luteolin induces cell cycle arrest and programmed cell death; thereby it efficiently blocks the invasion of cancer cells. Earlier research studies on cancer therapy had explained about the apoptosis mechanism and it was initiated by death receptors and sub-sequential activation of mitochondrial-dependent caspase pathway (Ma et al. 2018). Large number of bioactive compound has a potential chemoprotective effect to induce programmed cell death in cancer cells that were evaluated by a number of investigators in past decades, and also the previous study reports the anticancer efficacy of luteolin against human colon cancer cells that were sequenced by induced cell cycle arrest and followed with activation of caspase-dependent intrinsic apoptotic pathway (Pandurangan and Esa 2014). Pathogenesis of colon cancer in DMH rats involves lipid peroxidation to form active malondialdehyde, conjugated dienes, and lipid hydroperoxides, which causes considerable damage in cells. In our previous study using DMH-induced rate models, we had evidently pictured and elaborated mechanism of action on how the target flavonoid luteolin had significantly reduced the tumor cell number mediated by a decrease in lipid peroxidation and inversely increased endogenous enzyme-dependent antioxidant mechanism. For the past decades level of glycoprotein in serum was considered as one of the hallmark markers to assess cancer, hence this glycoprotein plays a key role in cell-cell recognition, cellular adhesion, binding, and cellular transport, during rapid proliferation and differentiation of cancer cells the level of this specific protein would be elevated and its server as valuable marker carcinogenic process. Luteolin-administered DMH rats found to have a lower level of these glycoproteins were reported (Pandurangan et al. 2012). Earlier literature reports on luteolin had stated to induce cell shrinkage followed by chromatin condensation and finally leads to DNA fragmentation, which was said to be the hallmark of apoptosis in colon cancer cells. Activation of effector caspases and Bax/Bcl2 ratio critically determine the fate of the cancer cell to induce apoptosis. This was followed by the successive release of mitochondrial cytochrome C conform the activation of mitochondrial-dependent intrinsic apoptosis and this hypothesis was tested with various cancer cell against luteolin treatment was reported (Attoub et al. 2011; Pandurangan and Ganapsam 2013). Use of such naturally occurring phytocompounds in the prevention of colon cancer was expected to be safe and avoid the adverse effect of anticancer drugs like synthetic chemotherapeutic compounds. This chapter also summarizes the ample of research evidence from the past study reports to show the chemopreventive potential of bioactive flavonoids in the prevention of colon carcinoma.
2.6 Conclusions
Consumption of dietary flavonoids in a consistent manner and an adequate amount has been proven to be a significant nutritional therapy to prevent and lower the risk of colorectal cancer, which could be implicated to enhance for cancer treatments. This chapter insensitively discussed about the flavonoids as chemopreventive drugs to treat cancer, and also elaborated the primary mechanisms of action of such bioactive flavonoids on chemically/carcinogen-induced oxidative stress. Efficacy of flavonoids to reduce the oxidative stress by scavenging ROS is considered as a principal factor to act as antioxidant and anti-inflammatory properties. Hence, colon cancer is a chronic disease, and its progression was brought by continuous bowel inflammation caused by oxidative stress and various other factors. Supplementation of flavonoids potentially prevents the inflammation, thus controlling colon cancer progression and invasion. Further, it modulate the expression of oncogenic and apoptotic proteins in cancer models, thereby activating Bax/Bcl2-mediated mitochondrial apoptotic pathway. A number of recent studies also have pinpointed the synergetic effects of flavonoids along with other anticancer drugs. Hence, the present review concludes that flavonoids have a significant anticancer potential against colon cancer, and it can be used as drug/supplements to prevent inflammation, and it helps to eradicate cancer diseases. However, the prospective cohort preclinical and clinical studies on flavonoids would help to better understand other mechanisms of dietary flavonoids for improved implication in the treatment of a number of cancer diseases.
References
Abeni C, Rota L, Ogliosi C, Bertocchi P, Centurini PB, Zaniboni A (2013) Correlation among Streptococcus bovis, endocarditis and septicemia in a patient with advanced colon cancer: a case report. J Med Case Rep 7(1):185
Asha A, Gayathri D (2012) Synergistic impact of Lactobacillus fermentum, Lactobacillus plantarum and vincristine on 1,2-dimethylhydrazine-induced colorectal carcinogenesis in mice. Exp Ther Med 3(6):1049–1054
Ashokkumar P, Sudhandiran G (2008) Protective role of luteolin on the status of lipid peroxidation and antioxidant defense against azoxymethane-induced experimental colon carcinogenesis. Biomed Pharmacother 62(9):590–597
Attoub S, Hassan AH, Vanhoecke B, Iratni R, Takahashi T, Gaben A-M, Bracke M, Awad S, John A, Kamalboor HA (2011) Inhibition of cell survival, invasion, tumor growth and histone deacetylase activity by the dietary flavonoid luteolin in human epithelioid cancer cells. Eur J Pharmacol 651(1–3):18–25
Beaud D, Tailliez P, Anba-Mondoloni J (2005) Genetic characterization of the β-glucuronidase enzyme from a human intestinal bacterium, Ruminococcus gnavus. Microbiology 151(7):2323–2330
Brunetti C, Di Ferdinando M, Fini A, Pollastri S, Tattini M (2013) Flavonoids as antioxidants and developmental regulators: relative significance in plants and humans. Int J Mol Sci 14(2):3540–3555
Chang H, Lei L, Zhou Y, Ye F, Zhao G (2018) Dietary flavonoids and the risk of colorectal cancer: an updated meta-analysis of epidemiological studies. Nutrients 10(7):950
Cheng Y-Y, Yang J-S, Tsai S-C, Liaw C-C, Chung J-G, Huang L-J, Lee K-H, Lu C-C, Chien H-C, Tsuzuki M (2012) The newly synthesized 2-(3-hydroxy-5-methoxyphenyl)-6,7-methylenedioxyquinolin-4-one triggers cell apoptosis through induction of oxidative stress and upregulation of the p38 MAPK signaling pathway in HL-60 human leukemia cells. Oncol Rep 28(4):1482–1490
Facchini V, Griffiths L (1981) The involvement of the gastro-intestinal microflora in nitro-compound-induced methaemoglobinaemia in rats and its relationship to nitrogroup reduction. Biochem Pharmacol 30(9):931–935
Fearon ER, Vogelstein B (1990) A genetic model for colorectal tumorigenesis. Cell 61(5):759–767
Fulda S, Gorman AM, Hori O, Samali A (2010) Cellular stress responses: cell survival and cell death. Int J Cell Biol 2010:1
Gogvadze V, Orrenius S, Zhivotovsky B (2009) Mitochondria as targets for chemotherapy. Apoptosis 14(4):624–640
Graham KA, Kulawiec M, Owens KM, Li X, Desouki MM, Chandra D, Singh KK (2010) NADPH oxidase 4 is an oncoprotein localized to mitochondria. Cancer Biol Ther 10(3):223–231
Hanasaki Y, Ogawa S, Fukui S (1994) The correlation between active oxygens scavenging and antioxidative effects of flavonoids. Free Radic Biol Med 16(6):845–850
Horowitz RM (1958) Process for production of eriodictyol. Google Patents
Hoult J, Payá M (1996) Pharmacological and biochemical actions of simple coumarins: natural products with therapeutic potential. Gen Pharmacol Vasc S 27(4):713–722
Jeng W-Y, Wang N-C, Lin M-H, Lin C-T, Liaw Y-C, Chang W-J, Liu C-I, Liang P-H, Wang AH-J (2011) Structural and functional analysis of three β-glucosidases from bacterium Clostridium cellulovorans, fungus Trichoderma reesei and termite Neotermes koshunensis. J Struct Biol 173(1):46–56
Kamada N, Seo S-U, Chen GY, Núñez G (2013) Role of the gut microbiota in immunity and inflammatory disease. Nat Rev Immunol 13(5):321
Kawser Hossain M, Abdal Dayem A, Han J, Yin Y, Kim K, Kumar Saha S, Yang G-M, Choi H, Cho S-G (2016) Molecular mechanisms of the anti-obesity and anti-diabetic properties of flavonoids. Int J Mol Sci 17(4):569
Li Y, Zhang T, Chen G (2018) Flavonoids and colorectal cancer prevention. Antioxidants 7(12):187
Ma Z-J, Lu L, Yang J-J, Wang X-X, Gang S, Wang Z-l, Chen G-h, Sun H-m, Wang M-y, Yang Y (2018) Lariciresinol induces apoptosis in HepG2 cells via mitochondrialmediated apoptosis pathway. Eur J Pharmacol 821:1–10
Madhesh M, Vaiyapuri M (2013) Luteolin a dietary flavonoid attenuates isoproterenol-induced myocardial oxidative stress in rat myocardium: an in vivo study. Biomed Prev Nutr 3(2):159–164
Mahomoodally MF, Gurib-Fakim A, Subratty AH (2005) Antimicrobial activities and phytochemical profiles of endemic medicinal plants of Mauritius. Pharm Biol 43(3):237–242
Manach C, Scalbert A, Morand C, Rémésy C, Jiménez L (2004) Polyphenols: food sources and bioavailability. Am J Clin Nutr 79(5):727–747
Manju V, Nalini N (2005) Chemopreventive efficacy of ginger, a naturally occurring anticarcinogen during the initiation, post-initiation stages of 1, 2 dimethylhydrazine-induced colon cancer. Clin Chim Acta 358(1–2):60–67
Mariyappan P, Kalaiyarasu T, Manju V (2017) Effect of eriodictyol on preneoplastic lesions, oxidative stress and bacterial enzymes in 1, 2-dimethyl hydrazine-induced colon carcinogenesis. Toxicol Res 6(5):678–692
Mazimba O (2017) Umbelliferone: sources, chemistry and bioactivities review. Bull Fac Pharm Cairo Univ 55(2):223–232
McGirr C, McEvoy CT, Woodside JV (2017) Vegetarian and vegan diets: weighing the claims. In: Nutrition guide for physicians and related healthcare professionals. Springer, pp 203–212
Mierziak J, Kostyn K, Kulma A (2014) Flavonoids as important molecules of plant interactions with the environment. Molecules 19(10):16240–16265
Muthu R, Selvaraj N, Vaiyapuri M (2016) Anti-inflammatory and proapoptotic effects of umbelliferone in colon carcinogenesis. Hum Exp Toxicol 35(10):1041–1054
Nishitani Y, Yamamoto K, Yoshida M, Azuma T, Kanazawa K, Hashimoto T, Mizuno M (2013) Intestinal anti-inflammatory activity of luteolin: role of the aglycone in NF-κB inactivation in macrophages co-cultured with intestinal epithelial cells. Biofactors 39(5):522–533
Oldford SA, Marshall JS (2015) Mast cells as targets for immunotherapy of solid tumors. Mol Immunol 63(1):113–124
Pandurangan AK, Esa NM (2014) Luteolin, a bioflavonoid inhibits colorectal cancer through modulation of multiple signaling pathways: a review. Asian Pac J Cancer Prev 15(14):5501–5508
Pandurangan AK, Ganapsam S (2013) Luteolin induces apoptosis in azoxymethane-induced colon carcinogenesis through the involvement of Bcl-2, Bax and Caspase-3
Pandurangan AK, Dharmalingam P, Sadagopan SKA, Ganapasam S (2012) Effect of luteolin on the levels of glycoproteins during azoxymethane-induced colon carcinogenesis in mice. Asian Pac J Cancer Prev 13(4):1569–1573
Papas AM (1998) Antioxidant status, diet, nutrition, and health, vol 9. CRC Press, Boca Raton, FL
Peluso M, Munnia A, Risso GG, Catarzi S, Piro S, Ceppi M, Giese RW, Brancato B (2011) Breast fine-needle aspiration malondialdehyde deoxyguanosine adduct in breast cancer. Free Radic Res 45(4):477–482
Ramos S (2007) Effects of dietary flavonoids on apoptotic pathways related to cancer chemoprevention. J Nutr Biochem 18(7):427–442
Reddy BS, Weisburger JH, Wynder EL (1974) Fecal bacterial β-glucuronidase: control by diet. Science 183(4123):416–417
Rice-Evans CA, Miller NJ, Paganga G (1996) Structure-antioxidant activity relationships of flavonoids and phenolic acids. Free Radic Biol Med 20(7):933–956
Robertson W, Ropes MW, Bauer W (1940) Mucinase: a bacterial enzyme which hydrolyzes synovial fluid Mucin and other Mucins. J Biol Chem 133:261–276
Ross JA, Kasum CM (2002) Dietary flavonoids: bioavailability, metabolic effects, and safety. Annu Rev Nutr 22(1):19–34
Sengottuvelan M, Senthilkumar R, Nalini N (2006) Modulatory influence of dietary resveratrol during different phases of 1, 2-dimethylhydrazine induced mucosal lipid-peroxidation, antioxidant status and aberrant crypt foci development in rat colon carcinogenesis. Biochim Biophys Acta 1760(8):1175–1183
Sirri V, Roussel P, Trere D, Derenzini M, Hernandez-Verdun D (1995) Amount variability of total and individual Ag-NOR proteins in cells stimulated to proliferate. J Histochem Cytochem 43(9):887–893
Sohail M, Siddiqi R, Ahmad A, Khan SA (2009) Cellulase production from Aspergillus niger MS82: effect of temperature and pH. New Biotechnol 25(6):437–441
Takada H, Hirooka T, Hiramatsu Y, Yamamoto M (1982) Effect of β-glucuronidase inhibitor on azoxymethane-induced colonic carcinogenesis in rats. Cancer Res 42(1):331–334
Takahashi H, Jin C, Rajabi H, Pitroda S, Alam M, Ahmad R, Raina D, Hasegawa M, Suzuki Y, Tagde A (2015) MUC1-C activates the TAK1 inflammatory pathway in colon cancer. Oncogene 34(40):5187
Author information
Authors and Affiliations
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2020 Springer Nature Singapore Pte Ltd.
About this chapter
Cite this chapter
Thimmarayan, S. et al. (2020). Plant Flavonoids Against Colorectal Cancer and Mechanisms of Action. In: Swamy, M. (eds) Plant-derived Bioactives. Springer, Singapore. https://doi.org/10.1007/978-981-15-2361-8_2
Download citation
DOI: https://doi.org/10.1007/978-981-15-2361-8_2
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-15-2360-1
Online ISBN: 978-981-15-2361-8
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)